Abstract
Key points
The G-protein-mediated metabotropic glutamate receptor 6 (mGluR6) signalling cascade is critical for light responses in all retinal ON bipolar cells. The present study evaluates the role of G-protein subunit Gγ13 in ON bipolar cells by deleting the mouse Gng13 gene.
Gγ13 is a partner of Gβ3 in all types of retinal ON bipolar cells, and contributes to generating the light response.
Gγ13 also contributes to maintenance because, in its absence, the light response decreases with age.
Three independent functionality assessments (i.e. light response as measured by electroretinogram b-waves, localization of GTPase activating proteins and deterioration of the light response with age) show that the contribution of Gγ13 is much greater in rod bipolar cells than in ON cone bipolar cells.
Our data also suggest that the contribution of Gγ13 to coupling mGluR6 to its effector is smaller than its contribution to maintaining GTPase activating protein localization.
Abstract
Heterotrimeric G-proteins (comprising Gα and Gβγ subunits) are critical for coupling of metabotropic receptors to their downstream effectors. In the retina, glutamate released from photoreceptors in the dark activates metabotropic glutamate receptor 6 (mGluR6) receptors in ON bipolar cells; this leads to activation of Go, closure of transient receptor potential melastatin 1 channels and hyperpolarization of these cells. Go comprises Gαo, Gβ3 and a Gγ. The best Gγ candidate is Gγ13, although functional data to support this are lacking. Thus, we tested Gγ13 function by generating Gng13−/− knockout (KO) mice, recording electroretinograms (ERG) and performing immunocytochemical staining. The amplitude of scotopic ERG b-waves in KO mice was lower than in wild-type (WT) mice. Furthermore, in both KO and WT mice, the ERG b-wave decreased with age; this decrease was much more pronounced in KO mice. By contrast, the photopic ERG b-waves in KO mice were hardly affected at any age. In KO mice retinas, immunostaining for Gβ3 and for the GTPase activating proteins RGS7, RGS11, R9AP and Gβ5 decreased significantly in rod bipolar cells but not in ON cone bipolar cells. Staining for Gαo and certain other cascade elements decreased only slightly. Analysis of our ON bipolar cDNA library showed that these cells express mRNAs for Gγ5, Gγ10 and Gγ11. Quantitative RT-PCR of retinal cDNA showed greater values for these transcripts in retinas of KO mice, although the difference was not significant. Our results suggest that Gγ13 contributes to mGluR6 signalling in rod bipolar cells more than in ON cone bipolar cells, and that this contribution includes both coupling the receptor and maintaining a stable localization of the mGluR6-related cascade elements.
Introduction
The key function of heterotrimeric G-proteins (comprising Gα, Gβ and Gγ subunits) is the coupling of metabotropic receptors to their downstream effectors. These downstream effectors (enzymes or channels) modulate diverse processes, ranging from growth and differentiation to normal physiological functions. Although most classical cascades, such as phototransduction and hormonal signalling, use the α-subunit of the G-protein as the signal carrier, numerous cascades use Gβγ to directly gate a channel or modulate enzymatic activity (Logothetis et al. 1987; Boyer et al. 1992; Camps et al. 1992; Herlitze et al. 1996; Smrcka, 2008). Gβγ also contributes to the assembly and trafficking of receptor based signalling complexes, vastly expanding the range of signalling repertoires that are covered by G-proteins (Bomsel & Mostov, 1992; Dupre et al. 2009; Dhingra et al. 2012; Nikonov et al. 2013; Sulaiman et al. 2013).
In the retina, an important G-protein-mediated signalling cascade in the ON class of bipolar cells is initiated by metabotropic glutamate receptor 6 (mGluR6). This cascade is essential for night vision and for detecting light increments in daylight vision. This cascade is of particular interest because it inverts the photoreceptor's hyperpolarizing light signal to a depolarizing response (Nawy & Jahr, 1990; Shiells & Falk, 1990). In the dark, glutamate released from photoreceptors binds mGluR6, activating a G-protein cascade that closes the non-selective cation channel transient receptor potential melastatin 1 (TRPM1) and thereby hyperpolarizes the ON bipolar cells (Masu et al. 1995; Koike et al. 2010a; Morgans et al. 2010). Light hyperpolarizes the photoreceptors, reducing glutamate release and thus opening the TRPM1 channels and depolarizing the ON bipolar cells. Mutations in several of the proteins participating in the ON bipolar cell G-protein cascade lead to complete congenital stationary night blindness (Zeitz et al. 2005; McCall & Gregg, 2008; Audo et al. 2009).
ON bipolar cells express Gαo, Gβ3 and Gγ13 (Vardi et al. 1993; Huang et al. 2003; Ritchey et al. 2010). Deleting Gnao (i.e. the gene encoding for the two splice variants of Gαo) eliminates the light ON response (Dhingra et al. 2000, 2002; Okawa et al. 2010). By contrast, deleting Gnb3 (i.e. the gene encoding Gβ3) greatly reduces light sensitivity but leaves a residual response (Dhingra et al. 2012). Interestingly, deleting Gnb3 also mislocalizes many cascade components, suggesting that it has additional functions beyond coupling. To determine whether Gγ13 has similar functions, we tested the effect of its absence by generating and examining the Gng13 (the gene encoding Gγ13) knockout (KO) mouse. In rod bipolar cells (RBCs), but not in ON cone bipolar cells (CBCs), the absence of Gγ13 decreases the light response in an age-dependent manner. Moreover, Gγ13 deletion greatly reduces immunostaining for Gβ3 and for the GTPase activating proteins (GAPs) in rod bipolar dendritic tips but scarcely affects this immunostaining in ON cone bipolar dendrites. Taken together, these results indicate differential roles for Gγ13 in rod bipolar and ON CBCs, and suggest non-identical contributions of Gβ3 and Gγ13 to coupling and maintenance of synaptic chemical composition.
Methods
Ethical approval
Procedures involving animals were performed in accordance with National Institute of Health guidelines and the protocol was reviewed and approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. The total number of animals used in the present study was 22 wild-type (WT) mice and 27 Gng13 KO mice. The earliest age tested was 4 weeks old; both male and female mice were used.
Generation of Gng13−/− mice
Targeted ES cell clones with replacement of the entire coding region of Gng13 (after the ATG codon) and part of the 3′ UTR (Fig. 1A) with a LacZ containing cassette were obtained from Knockout Mouse Project (KOMP, University of California, Davis, CA, USA; Clone 13525B-A10). The chromosome count was conducted by KOMP (passed, 90% euploid). The line was expanded by Penn Gene Targeting Core (PGT) and was injected at the Transgenic and Chimeric mouse facility (University of Pennsylvania, Philadelphia, PA, USA). The mouse blastocysts used for injection were derived from C57BL/6J females mated with C57BL/6J males (Charles River) and were implanted in CD-1 pseudo-pregnant females. Pups were screened by conventional PCR on genomic DNA from mouse tail using primers based on a KOMP allele genotyping strategy (Fig. 1A and B). NCBI gene ID: 64337; TUF, forward position (25855690), (CCTCAAGTACCAACTGGCC TTC); TUR, reverse (25855764), (TGGCTGGGCAGGTAAATGAC); NeoInF, forward (TTCGG CTATGACTGGGCAC-AACAG); NeolnR, reverse (TACTTTCTCGGCAGGAG-CAAGGTG). Mouse colonies were maintained by breeding heterozygous mice; KO mice were infertile. In general, Gng13 KO young mice were smaller than their WT littermates, although they eventually gained weight.
Figure 1.
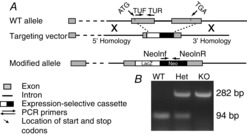
Strategy for generating and genotyping Gng13 KO mice
ES cells carrying the Gng13-null allele were obtained from KOMP. A, homologous recombination in ES cells using a bacterial artificial chromosome based construct with a Gng13 gene specific deletion and an expression selective cassette containing the LacZ reporter. The final allele lacks the entire coding region of Gng13 and part of the 3′ UTR. The positions of various primers used for genotyping are indicated. B, PCR products run on an agarose gel show the expected size products for different genotypes: TUF and TUR, 94 bp (WT and heterozygous, Het); NeoInf and NeoInR, 282 bp (KO and Het).
Immunofluorescence of retinal cryosections
Mice were deeply anaesthetized by being injected i.p. with ketamine and xylazine [100 and 10 μg g−1 bodyweight]. Eyes were enucleated and incised just below the lens, and the mice were killed with an anaesthetic overdose (300 μg ketamine g−1 bodyweight and 300 μg xylazine g−1 bodyweight). Eyeballs were fixed in 4% paraformaldehyde for 10 min (for most stainings) or 60 min (for Gβ3), rinsed in PB, soaked overnight at 4°C in 0.1 m PB containing 30% sucrose for cryoprotection and embedded in a mixture of two parts 20% sucrose in PB and one part optimal cutting temperature compound (Tissue Tek, Electron Microscopy Sciences, Hatfield, PA, USA). Radial sections (15–18 μm) were cut on a cryostat (Leica Biosystems, Buffalo Grove, IL, USA) at –20°C and sections were collected on a superfrost plus glass slide (Fisher Scientific, Pittsburgh, PA, USA). Retinal cryosections were permeabilized and blocked with 10% normal goat serum, 5% sucrose and 0.5% Triton X-100 in PB for 1 h at 20°C. Sections were incubated in primary antibodies (diluted in blocking solution) at 4°C overnight or occasionally for 3 days. For some of the sections, peanut agglutinin (PNA) conjugated to fluorescein isothiocyanate (Vector Laboratories, Burlingame, CA, USA) was added along with the primary antibody. Primary antibodies were washed 3 times in PB at room temperature. Sections were then incubated in secondary antibodies at room temperature for 3 h, washed, mounted with Vectashield mounting medium (Vector Laboratories), and coverslipped.
Imaging and quantification
Sections were photographed with a confocal laser scanning microscope (Olympus Fluoview 1000, Center Valley, PA, USA) under a ×60 oil-immersion objective. Immunostainings for KO and WT littermate (or occasionally age-matched) retinas were performed and imaged in parallel under the same conditions and settings. Each pair of WT and KO retinas that were processed together constitutes a set. Regions of interest (ROI) were drawn around different retinal layers, and the average intensity measurement for each ROI was taken from z-stacks (same thickness for WT and KO mice) using Volocity software (Improvision, PerkinElmer, Waltham, MA, USA). Staining intensities of KO retinas were compared with those of WT retinas with Student's t test. The data are displayed more concisely as KO/WT ratios where each set gave one data point, although the statistics were performed by comparing KO and WT intensities. To discriminate between RBCs and CBCs, we drew ROIs around the cone bipolar dendritic tips below each cone pedicle (identified by PNA stain). ROIs to quantify staining in rod bipolar dendritic tips included the whole outer plexiform layer (OPL) but excluded the region of PNA staining. The mean intensity per pixel for rod bipolar and CBC dendrites was computed as the mean intensity per pixel of the corresponding ROI minus the mean background intensity per pixel (measured from the outer nuclear layer). To further analyse differences between rod bipolar and cone bipolar dendrites, we compared their staining intensities in the same image. For all data, P < 0.05 indicates a significant difference between the average staining intensities and P < 0.01 indicates a highly significant difference.
Antibodies
Rabbit anti-Gγ13 (dilution 1:500; a gift from R. Margolskee, Monell Institute, Philadelphia, PA, USA); rabbit anti-Gβ3 (dilution 1:300; HPA005645; Sigma, St Louis, MO, USA); guinea pig anti-mGluR6 (dilution 1:100; Neuromics, Edina, MN, USA); mouse anti-Gαo (dilution 1:100; MAB 3073; Millipore, Billerica, MA, USA); rabbit anti-TRPM1 (dilution 1:100; a gift from T. Furukawa, Osaka Bioscience Institute, Osaka, Japan) (Koike et al. 2010b, 2011; Peachey et al. 2012); rabbit anti-Gβ5 (dilution 1:500) and rabbit anti-RGS11 (dilution 1:1000; gifts from T. Wensel, Baylor College of Medicine, Houston, TX, USA); rabbit anti-RGS9 anchor protein (R9AP) (dilution 1:300; a gift from V. Arshavsky, Duke University, Durham, NC, USA); rabbit anti-RGS7 (dilution 1:200; a gift from K. Martemyanov, Scripps Research Institute, Jupiter, FL, USA): rabbit anti-protein kinase C (PKC) (dilution 1:1000; Sigma; P4334); mouse anti-PKC (dilution 1:40; Sigma; P5704); monoclonal mouse anti-β-galactosidase (dilution 1:200; Promega, Madison, WI, USA; Z3781); mouse anti-kinesin (monoclonal clone K2.4; Covance, Princeton, NJ, USA MMS-198P); polyclonal anti-Gγ5 (S-14) (dilution 1:500; Santa Cruz Biotechnology, Dallas, TX, USA; SC-376); and PNA-fluorescein isothiocyanate (dilution 1:100; Vector Laboratories; FL-1071).
Electroretinogram
The electroretinogram (ERG) recording set-up and methods have been described previously (Lyubarsky et al. 1999, 2000; Ng et al. 2010). In short, mice were dark-adapted, deeply anaesthetized i.p. under dim red light with ketamine/xylazine/urethane [20 μg, 8 μg and 800 μg g–1 bodyweight, respectively] and were placed on a platform maintained at 38°C. Pupils were dilated with 1% tropicamide saline solution (Mydriacyl, Alconox, New York, NY, USA). A platinum electrode was inserted into the mouth to serve as the reference and ground electrode, and another platinum electrode was placed on the cornea. Mice were placed inside a light proof Ganzfeld Faraday cage. ERG recordings from KO mice and from their WT (or age matched) littermates were performed on the same day under the same settings and conditions. Light stimuli were either 4 ms flashes produced by a light-emitting diode or <1 ms flashes produced by a Xenon tube delivered in the Ganzfeld (Espion Electrophysiology System; Diagnosys). For scotopic ERG, the intensities of light flashes were converted to the estimated number of photoisomerizations (R*) per rod, as described previously (Lyubarsky et al. 2004). For photopic ERG, light intensity was converted to number of photons μm–2 at the cornea, as described previously (Lyubarsky et al. 1999, 2000). For photopic stimuli, a rod-suppressing step of light (30 scot cd m−2) was given every 5.5 s and a light flash was superimposed on this 2 s after the onset of the step. ERGs were recorded from both eyes using differential amplifiers with a bandwidth of 0.1 Hz to 1 kHz, and were sampled at 1 ms intervals. Depending on the signal-to-noise ratio, a typical record was an average of 3 to 25 individual responses. At the end of the recording session, animals were removed from the chamber, returned to their cage and observed until they fully recovered. These animals were either killed the next day to obtain their retinas, or left in the cage for more than 1 week for an additional ERG recording session.
The amplitude of the ERG b-wave was quantified by subtracting the peak of the a-wave from the peak of the b wave. The implicit times of the a- and b-waves were calculated from time of flash to the peak of the a- and b-waves, respectively. The functional characteristics of phototransduction in rods in WT and KO mice were determined from the ERG a-wave by fitting the rising phase of the a-wave with the transduction cascade activation model (Breton et al. 1994; Lyubarsky & Pugh, 1996):
| 1 |
where F(t) is the a-wave amplitude at time t after a brief flash (normalized to the saturated amplitude); Φ is the number of photoisomerizations per rod; teff is a brief delay; and A is the amplification constant whose value was estimated by the best fit.
The average values of individual ERG characteristics from KO and WT mice were calculated and compared using an unpaired two tailed Student's t test. To determine the effect of age on ERG characteristics, we used the Wald test (with the generalized estimated equation) to account for the inter-eye correlation (performed by a Univeristy of Pennylvania Vision Core statistics consultant).
PCR
PCR was performed on the ON bipolar cDNA library with gene specific primers on a Perkin Elmer thermocycler (Branchburg, NJ, USA). Thirty-five PCR cycles were performed; each cycle included denaturing at 95°C for 15 s, annealing at 60°C for 15 s and extension at 72°C for 30 s. Mouse retina was used as a positive control to test all primers. PCR products of the correct size were verified by sequencing. For quantitative real-time PCR, total RNA from mouse retina was isolated with a Nucleospin RNA II kit (Macherey-Nagel, Bethlehem, PA, USA). cDNA was synthesized with a high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA, USA). Quantitative real-time PCR was performed with a Power SYBR Green kit (Applied Biosystems) on an Applied Biosystems 7500 Fast Real-Time PCR System. Each sample was tested in triplicate. Melt curve analysis verified the specificity of the product. GAPDH was used as a reference gene for normalization, and analysis of relative gene expression was performed using the relative standard curve method in accordance with the manufacturer's instructions.
Results
Gγ13 is probably the main partner of Gβ3 in ON bipolar cells
Previous studies showed that immunostaining for Gγ13 labels all types of ON bipolar cells (Huang et al. 2003). In the present study, we found that this staining is specific because ON bipolar cell staining in KO retina was eliminated (Fig. 2A to C). Furthermore, we have localized Gγ13 indirectly by staining for the reporter LacZ, which replaces Gng13 in the KO mouse line. Staining in KO was observed in the OPL, inner nuclear layer (INL) and in the ON sublaminas of the inner plexiform layer (IPL) (strongly in sublamina 5, where axon terminals of RBCs reside, and weakly in sublaminas 3 and 4, where axon terminals of ON CBCs reside); there was no staining in photoreceptors, amacrine cells or ganglion cells (Fig. 2D). The localization of LacZ gene product, β-galactosidase, is a reliable indicator of Gγ13 expression because there was no staining in WT mice (Fig. 2E).
Figure 2.
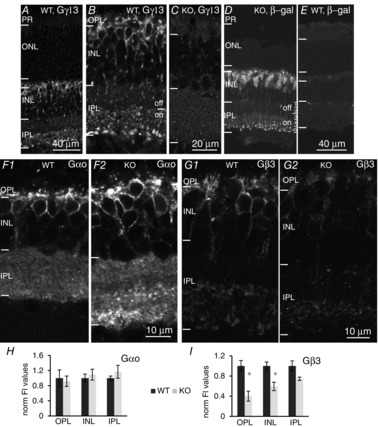
Absence of Gγ13 greatly reduces expression of Gβ3 in ON bipolar cells
A and B, staining for Gγ13 in WT retina (lower and higher magnifications) shows its localization in dendrites, somas and axon terminals of all ON bipolar cells, and its absence elsewhere. Note staining is throughout the ON sublamina. C, staining for Gγ13 in Gng13 KO retina is absent. D, staining for the reporter LacZ in KO retina shows the exact same localization as Gγ13 in WT mice; staining is present throughout the sublaminas of the ON sublayer, although it is strongest in sublamina 5. E, staining for LacZ is absent in WT mice. F, Staining for Gαo in KO mice (F2) closely resembles staining in WT mice (F1) (5 sets). G, staining for Gβ3 in KO mice (G2) is much fainter than in WT mice (G1) (3 sets). H and I, averaged normalized pixel intensity (± SEM) for Gαo (H) and Gβ3 (I) in the OPL, INL and IPL. Values were normalized to WT average intensity. *Statistically significant difference between staining for Gβ3 in WT mice and Gng13 KO mice (in both OPL and INL but not IPL). ONL, outer nuclear layer.
Expression of the three subunits of heterotrimeric G-proteins is often coordinated and interdependent. For example, ON bipolar cells lacking Gβ3 show reduced staining for Gαo and Gγ13 (Dhingra et al. 2012). To determine whether this relationship is reciprocal, we examined whether the absence of Gγ13 affects the expression of its suspected partners Gαo and Gβ3. The proteins were quantified by immunostaining rather than by western blotting because the former can reveal staining localized only to ON bipolar cells, whereas the latter sums the protein of interest in the entire retina. Because Gβ3 is heavily expressed in cones (Ritchey et al. 2010; Nikonov et al. 2013) and Gαo is also localized to amacrine cells in the IPL (Vardi et al. 1993; Vardi, 1998), western blotting would miss or underestimate the differences in protein levels between WT and KO ON bipolar cells. While staining for Gαo was not significantly affected in Gng13 KO (Fig. 2F and H), staining for Gβ3 was significantly reduced to almost 40% in the OPL and to 60% in the INL (P < 0.05; Student's t test) (Fig. 2G and I). This reduction in Gβ3 expression in ON bipolar cells suggests that it cooperates with Gγ13 to form the heterotrimer Go. Co-immunoprecipitation could not be used to show that these proteins are in the same complex because native Gγ13 is barely detectable in the retina by western blotting. However, the localization dependency that we report in the present study when Gγ13 is absent, as well as that shown by Dhingra et al. (2012) when Gβ3 is absent, combined with previous evidence that Gβ3 dimerizes with Gγ13 efficiently in several systems, suggests that Gβ3γ13 is a dimer in ON bipolar cells (Huang et al. 1999; Blake et al. 2001; Li et al. 2006; Kerr et al. 2008; Liu et al. 2012).
Deleting Gng13 reduces scotopic but not photopic ERG b-waves
To determine the contribution of Gγ13 to the light response of ON bipolar cells, we performed ERG recordings under dark-adapted and light-adapted conditions. Animals were dark-adapted and stimulated with dim flashes in the scotopic range to generate a scotopic b-wave that reflects the responses of RBCs. For this set of experiments, records were averaged from 4- to 13-week-old mice. Under these scotopic conditions, Gng13 KO mice produced b-waves whose amplitudes were 30–80% of WT b-waves depending on the light intensity (Fig. 3A and B; 31–40 records for WT mice and 35–48 for KO mice; for all intensities P < 0.001; Student's t test). In WT mice, the implicit time (time to peak) of the b-wave depended on stimulus intensity: values were similar up to 10 R* per rod, and reduced with higher light intensities (i.e. responses became faster with increasing intensities). In KO, at lower intensities (0.2 and 0.6 R* per rod), the b-wave reached its peak 20 ms after the WT b-wave did (P < 0.001; Student's t test) but, at higher intensities, this difference diminished (Fig. 3C). Stronger flashes under dark-adapted conditions elicited mixed rod and cone responses (Fig. 3E). Under these conditions, the b-wave in KO mice was reduced on average by 23% (P < 0.001 for all intensities; Fig.3E); which represents a lower reduction than that observed in response to dim stimuli.
Figure 3.
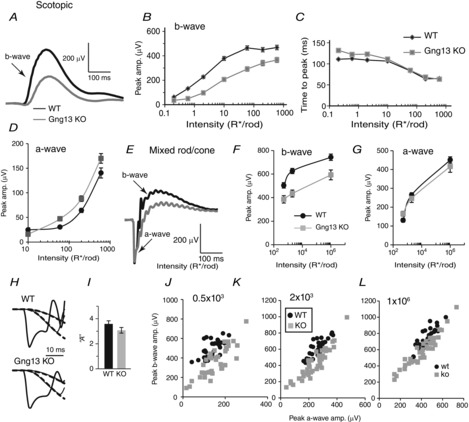
Absence of Gγ13 reduces scotopic ERG b-waves
Black lines and symbols indicate WT mice and grey lines and symbols indicate KO mice. A to D, scotopic conditions (dark-adapted, rod only stimulation). A, an example of the ERG responses of littermate WT and Gng13 KO mice to 10 R* per rod (flash in the scotopic range). B, average peak amplitude (amp.) (± SEM) of scotopic b-wave vs. flash intensity; responses in KO mice are reduced (depending on intensity; for WT mice, the number of records was 31–40 and, for KO mice, it was 35–48. Note that SEM for several intensities was smaller than the symbol size). C, average time to peak (± SEM) of b-wave for the same data as in B. D, peak a-wave amplitude vs. intensity for WT and KO mice; the difference in amplitude between WT and KO mice for most responses was highly significant. E to L, Mixed rod/cone input (dark-adapted, bright light stimulates both rods and cones). Data are from 24 records from 13 WT mice and 21 records from 16 KO mice. E, representative examples of ERG responses of WT and KO littermates to saturated bright light under dark-adapted conditions representing mixed rod and cone responses. F, peak b-wave amplitude vs. 3 rod-saturating intensities for WT and KO mice. The difference in amplitude between responses for WT and KO mice was highly significant. G, peak a-wave amplitude vs. 3 rod-saturating intensities for WT and KO mice; no significant difference was found. H and I, Lamb and Pugh analysis of the amplification factor ‘A’. H, examples of expanded normalized a-wave responses and their fits (dashed lines) for WT and KO mice. I, the resulting average ‘A’ values for WT and KO mice. J to L, correlograms of a- and b-waves for the three strong stimuli (indicated on the graphs in R*/rod). Each dot represents data from one ERG recording. Note that, for any a-wave amplitude, the corresponding b-wave amplitude in WT mice is greater than that in KO mice.
To test the possibility that photoreceptors in Gng13 KO mice were also affected and might have contributed to the reduced b-wave, we measured several parameters of the a-wave. First, we looked at the a-wave amplitude. For the strongest flash, this amplitude in KO was 8% lower than the amplitude in WT mice (450 ± 16 μV in WT mice vs. 415 ± 31 μV in KO mice), although this difference was not statistically significant (Fig. 3G). Because the a-wave response to a strong stimulus receives contribution from the inner retina (Xu et al. 2003; Sharma et al. 2005; Mojumder et al. 2008; Shirato et al. 2008; Dhingra et al. 2014), we also examined it under dim stimuli (which is typically very small). Under these scotopic stimuli, the a-wave in KO mice was consistently larger than that in WT mice, showing a high significance for most intensities (Fig. 3D). The Lamb and Pugh amplification constant A was computed from the a-wave (Fig.3H). The average values for KO (A = 3.1 ± 0.2) and WT (A = 3.6 ± 0.2) mice were not statistically different (P = 0.15; Student's t test) (Fig. 3I). Finally, to investigate how the b-wave behaves when the same a-wave amplitude is recorded in KO and WT mice, for each ERG trace, its b-wave vs. a-wave amplitudes were plotted (Fig. 3J to L). The graphs show that, for any given a-wave amplitude, the corresponding b-wave in KO is smaller than that in WT mice. Note that the difference is greater at the lower intensities; note also that the variability in KO mice is greater than in WT mice. For both KO and WT mice, the correlation between a-waves and b-waves is greater at the stronger stimuli (indicating that the saturated response is less variable within a genotype than the unsaturated response). The implicit times of the a-waves of KO and WT mice were practically identical in both genotypes (7.3 ± 0.5 ms in WT mice vs. 7.1 ± 0.4 ms in KO mice).
The photopic ERG (bright flashes under light-adapted conditions) was tested with bright white light (1000 scot cd s m−2; Fig. 4B) and with lower intensities of UV and green light (Fig. 4C and D). For the bright saturating white light, the b-wave amplitudes of KO mice were 83% that of WT mice (P < 0.05; Student's t test). The KO b-wave amplitudes of UV and green stimuli were also slightly lower, although the difference was not significant. Similarly, the implicit times of the b-waves (Fig. 4B, E and F), and the cone-generated a-waves were similar in Gng13 KO and WT mice. Thus, the contribution of Gγ13 to light responses in RBCs differs from its contribution to those in ON CBCs.
Figure 4.
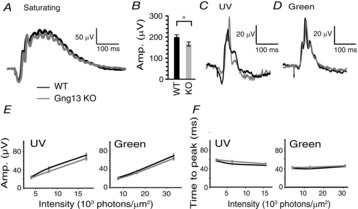
Absence of Gγ13 hardly affects the photopic ERG
A, C and D, examples of ERG responses under light-adapted conditions representing purely cone-generated responses. A, responses to bright (saturating) photopic stimulation (1000 scot cd s m−2). B, average amplitude (Amp.) of b-wave (± SEM) in response to the saturated flash (26 records from 14 WT mice and 35 records from 18 KO mice). C, responses to a UV flash (0.0035 cd s m−2 or 3.6 × 103 photons μm–2). D, responses to a green light (4 cd s m−2 or 7.2 × 103 photons μm–2). E, average amplitude of b-wave (± SEM) vs. flash intensity for UV and green lights. F, time to peak of b-wave using same records as in E. Average responses (Amp.) under photopic conditions were similar for WT and KO mice.
Absence of Gγ13 greatly reduces staining for the GAP complex in dendritic tips of RBCs but not in tips of ON cone bipolar cells
The expression and function of several of the molecular elements involved in the mGluR6 cascade in ON bipolar cells are interdependent, such that the absence of one of these proteins affects the expression and/or localization of the other proteins involved in the cascade. For example, absence of Gβ3 causes mislocalization of TRPM1, mGluR6 and the GAP complex (Dhingra et al. 2012); and absence of (or mutation in) mGluR6 affects GAP proteins (Cao et al. 2009; Xu et al. 2012). If Gγ13 is an obligatory subunit of the Gβγ dimer of Go in ON bipolar cells, similar deficiencies would be expected in the absence of Gγ13. This issue was addressed by immunostaining for several proteins that are involved in the mGluR6 cascade. First, immunostaining was conducted for mGluR6 and TRPM1 in WT and Gng13 KO mice (Fig. 5A and B; 4 sets each) and the average intensity per pixel in the OPL and the INL was analysed (Fig. 5D). In the OPL, the intensity in KO mice for both proteins was approximately 80% that of WT mice, although this difference was not statistically significant. In the INL, the average TRPM1 intensity was slightly higher than in WT mice but, again, the difference was not statistically significant. Similarly, PKC staining (n = 3 sets) was slightly but not significantly reduced in the OPL, and did not change in the INL or IPL (Fig. 5C and E).
Figure 5.
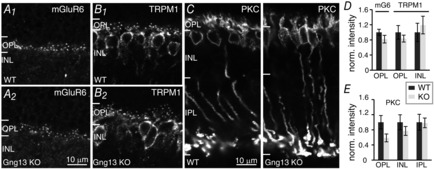
Absence of Gγ13 slightly but not significantly reduces staining intensity for mGluR6, TRPM1, and PKCα
A to C, immunostaining of wild type (WT) and Gng13 KO retinas as indicated. The scale bar in A applies also to B. D, average normalized (norm.) pixel intensity (± SEM) for mGluR6 staining in the OPL (mG6, n = 5 sets) and for TRPM1 staining in the OPL and INL (n = 4 sets); TRPM1 staining in INL remains unchanged but both stains in OPL are slightly reduced. E, average normalized pixel intensity for PKC staining in the OPL, INL and IPL (n = 3 sets); staining in OPL is slightly reduced.
Next, we examined proteins that inactivate Go. There exist at least two GAP complexes that accelerate the hydrolysis of GTP on Gαo: one obligatory complex contains Gβ5, RGS11 and R9AP, and the other complex comprises Gβ5, RGS7 and GPR179 (Jeffrey et al. 2010; Orlandi et al. 2012, 2013; Vardi & Dhingra, 2014). Immunostaining for Gβ5, RGS11 and R9AP showed that the absence of Gγ13 produced an overall reduction in staining of the ON bipolar cell dendritic tips (Fig. 6A to C; left). However, careful examination of the OPL showed that rows of stained puncta persisted in the lower part of this layer, apparently labelling ON cone bipolar dendrites. To confirm the identity of these stained structures, we incubated sections stained for GAP proteins with fluorescently labelled peanut agglutinin (PNA; Fig. 6A to C, middle), a marker for the synaptic cleft between cone terminals and ON cone bipolar dendrites. PNA staining did indeed colocalize with the strong staining for GAP proteins (Fig. 6A and B; right; 6C middle). To quantify the difference in staining intensity between RBC and ON CBC dendritic tips, we drew ROIs around the PNA stained structures (CBC tips) and around the OPL that did not include PNA staining (RBC tips). Next, the average pixel intensity was computed for each region of interest, the intensities for rod bipolar and ON CBCs were averaged across animals, and the reduction of staining in KO relative to WT mice in the two regions was compared. The KO/WT ratio for RBCs was 0.44 (>50% reduction; averaged across Gβ5, RGS11 and R9AP) and that for ON CBCs was 0.82 (a moderate reduction of ∼20%) (Fig. 6D). When compared statistically with WT mice, the staining intensities of the 3 GAP proteins in the Gng13 KO mice showed a highly significant decrease in RBCs (P < 0.01 for all; Student's t test; 6–8 sets) but not in CBCs. The same data set was also examined looking at the ratio of RBC to CBC for each image, and then averaging these ratios over many images. This approach directly compares RBC to CBC in each image. In WT mice, RBC/CBC for Gβ5, RGS11 and R9AP ranged from 0.56–0.66, with an average of 0.62; in Gng13 KO mice, it ranged from 0.25–0.42 with an average of 0.34 (Fig. 6E). The difference between the ratios in WT and KO mice for each protein was highly significant (P < 0.002; Student's t test). Note that the higher intensities obtained for ON cone bipolar dendrites relative to rod bipolar dendrites in WT mice do not indicate stronger staining. This is because the intensity of rod bipolar puncta is underestimated when its value is computed from the whole OPL as a result of this region of interest also including unstained structures (such as rod terminals). However, because the dilution factor in WT and KO mice is the same, this does not affect the comparison between their values.
Figure 6.
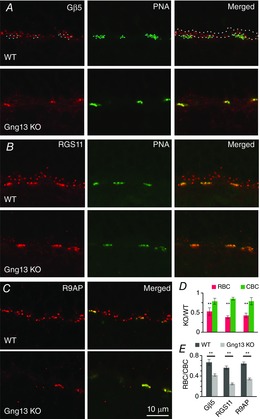
Absence of Gγ13 significantly reduces Gβ5, RGS11 and R9AP staining intensity in the dendritic tips of RBCs but not in those of ON CBCs
A to C, immunostaining for GAP proteins Gβ5 (A), RGS11 (B) and R9AP (C) in WT and in littermate Gng13 KO mice. PNA staining identifies the location of dendritic tips of ON CBCs in apposition to cone pedicles. The white dotted outline in A (right) shows an example of a ROI taken to quantify staining in dendritic tips of RBCs; blue dotted lines show ROIs drawn to quantify dendritic tips of CBCs (CBC). D, staining intensity for Gβ5, RGS11 and R9AP in KO mice relative to that in WT for RBC (red) and CBC (green) dendritic tips. For RBCs, but not for CBCs, staining intensities in KO mice showed a highly significant decrease from intensities in WT. E, same data as in D recalculated to show the staining intensities for Gβ5, RGS11 and R9AP in RBCs relative to these intensities in CBCs in the same sections. The average ratio (± SEM) for 6 (Gβ5), 7 (RGS11) and 8 (R9AP) sets is shown. For each protein, the ratio in KO mice was significantly (P < 0.01) lower than that in WT mice, indicating a greater reduction of staining intensity in the rod bipolar dendritic tips than in the cone bipolar dendritic tips.
Addressing the second GAP complex, we immunostained for RGS7. For this protein, we also found that the absence of Gγ13 reduced immunostaining in the OPL (Fig. 7A to D). Because RGS7 is also expressed in two bands in the IPL, we could test whether the reduction in staining is specific to the loss of Gγ13 (not expressed in these bands). There was no difference in staining intensities between KO and WT mice in the IPL (Fig. 7A, B and E). Furthermore, as with RGS11, staining for RGS7 was more affected in the rod bipolar dendrites than in the ON cone bipolar dendrites (Fig. 7C to F). In this experiment, we also stained for kinesin to determine the distribution of presynaptic ribbons in rods and cones; this distribution was normal in KO mice (Fig. 7C4 and D4). Clearly, the absence of Gγ13 has a greater effect on GAP proteins in the RBCs than in the ON CBCs.
Figure 7.
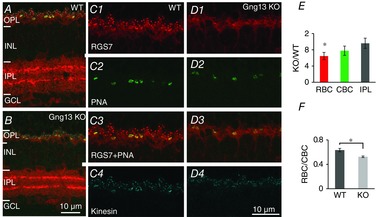
Absence of Gγ13 significantly reduces RGS7 staining intensity in the dendritic tips of RBCs, but not in those of ON CBCs or in the IPL
A and B, retinas of WT (A) and KO (B) mice triple stained for RGS7 (red), PNA (green) and kinesin (blue). Note reduced staining for RGS7 in OPL but not in IPL. C and D, the above staining in OPL is shown at higher magnifications for each protein as indicated. E, staining intensity in KO mice relative to WT mice for rod bipolar dendrites (RBC, red), ON cone bipolar dendrites as identified using PNA staining (CBC, green) and IPL (grey). The difference between KO and WT mice intensities in RBCs was significant, whereas that in CBCs was not. F, staining in rod bipolar dendrites relative to cone bipolar dendrites for WT and KO mice. Staining is significantly lower in KO than in WT mice (Student's t test; 3 sets).
Absence of Gγ13 has a greater effect on Gβ3 expression in RBCs than in ON CBCs
The differential effect of Gγ13 on GAP protein localization in rod bipolar vs. ON CBCs prompted us to determine whether the reduction in Gβ3 staining was also different between these two cell classes. Thus, we identified the somas of RBCs by staining for PKC and the ON cone bipolar dendritic tips by staining with PNA (Fig. 8A and B). Staining in the dendrites and somas of rod bipolar and CBCs was quantified by drawing 4 regions of interest. (1) For cone bipolar dendrites, the region was drawn around the PNA staining. (2) For rod bipolar dendrites, the region was drawn around the upper part of the OPL (not including PNA-stained regions). (3) For rod bipolar somas, the region was drawn around somas stained for PKC. (4) For ON cone bipolar somas, the region was drawn around somas stained for Gβ3 but not for PKC (see example in Fig. 8A). Gβ3 staining intensities in KO and WT mice were then computed. In rod bipolar dendrites and somas, staining intensities in KO mice were significantly lower than those in WT mice. By contrast, in both ON cone bipolar dendrites and somas, staining intensities between KO and WT mice were not significantly different (Student's t test, 4 sets; Fig. 8C). Next, we calculated the intensity of RBC/CBC for all images of the WT and KO retinas. For dendritic staining, this ratio was 0.60 in WT and 0.41 in KO (Fig. 8D, left) mice. For somatic staining, it was 1.45 in WT and 0.91 in KO (Fig 8D, right bars) mice. For both the dendritic and somatic staining, the differences between WT and KO ratios were highly significant (P < 0.01; Student's t test). As explained with respect to GAP complex staining, the dendritic RBC/CBC ratio (<1) does not indicate a lower intensity in a rod bipolar dendritic tip than in a cone bipolar dendritic tip because of the way the regions of interest were drawn. However, ratio >1 for somatic staining correctly represents a higher intensity in WT RBCs (relative to CBCs) because the soma size is the same and the dilution factor introduced by drawing the regions of interest around the somas is similar. Thus, the absence of Gγ13 is more detrimental in RBCs than in ON CBCs for all the tested cascade elements. This finding may explain why ERGs in ON CBCs remain close to normal, and it suggests either that WT ON CBCs use another Gγ or that Gγ13-null CBCs upregulate another Gγ.
Figure 8.
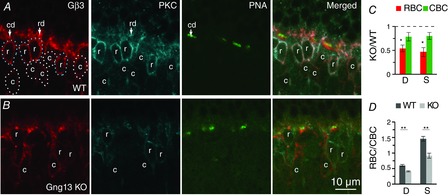
Absence of Gγ13 reduces Gβ3 staining in RBCs more than in ON CBCs
Triple labelling for Gβ3, PKC and PNA in WT (A) and Gng13 KO (B) retinas. PNA staining locates cone bipolar dendritic tips (cd); the rest of the OPL staining was considered as rod bipolar dendrites (rd). Somas stained for PKC are rod bipolar somas (r); somas stained for Gβ3 and not for PKC are cone bipolar somas (c). ROIs for rod bipolar somas (dotted outlines) and ON cone bipolar somas (white dotted outlines) are shown (A). Note that, in WT mice, the rod bipolar somas display brighter Gβ3 staining than the cone bipolar somas and, in KO mice, the cone bipolar somas have brighter staining. C and D, quantitative analysis of staining intensity. Average intensities per pixel were measured for the four different ROIs (rod bipolar dendrites, cone bipolar dendrites, rod bipolar somas and cone bipolar somas). C, staining intensities in dendrites (D) and somas (S) of KO RBCs (but not ON CBCs) are significantly different from that in WT cells. D, same data set as in C recalculated to show the average ratio (RBC/CBC) of Gβ3 staining intensity in the cell dendrites (D) and somas (S) in WT and KO retinas. For both dendrites and somas, the differences between these ratios for WT and KO are highly significant (4 sets of animals).
ON bipolar cells express transcripts for several Gγ subunits
To determine whether WT mice use a different Gγ, we tested mRNA expression for 11 Gγ subunits in our ON bipolar cDNA library (Table1). The library production has been previously described in detail; cDNA derived from FACS-sorted ON bipolar cells were inserted into pEXP1 and the purity of cDNA was confirmed by testing for the presence of messages unique to photoreceptors (Dhingra et al. 2008). Using this cDNA library, we found transcripts for Gng13, Gng5, Gng10 and Gng11. Testing for Gγ5 expression with a commercially available antibody revealed no staining in ON bipolar cells (not shown); we had no antibodies for Gγ10 and Gγ11. Total RNA from mouse retina was then used for quantitative RT-PCR to test for upregulation of these genes in the KO retina. For all three transcripts, the ratio of KO/WT was greater than 1 (Gng5: 1.63 ± 0.35; Gng10: 1.36 ± 0.14; and Gng11: 1.51 ± 0.26), although the difference between KO and WT mice did not reach significance (7 retinal sets).
Table 1.
PCR primers
| Gene | Accession number | 5′ Forward position and primer | 5′ Reverse position and primer |
|---|---|---|---|
| Gng2 variant 1 | NM_010315.4 | (3372) AGCTGCTCCTGCAAATGGCGA | (3792) ACCGTTCATGTGGGGTAAAGATGCG |
| Gng3 | NM_010316.3 | (1206) AATGGCGGTCCCCAGCGTTC | (1547) GGGCTTCTTGGGTCCCAGTCC |
| Gng4 | NM_010317.2 | (2339) CCCACCCCTGCCACTCTACCA | (2749) GCCAGGCCTGTGCTGTATGG |
| Gng5 | NM_010318.2 | (177) CGCGTGAAGGTTTCCCAGGCA | (442) AGGACTGACGAGCAGAAGATAGTGT |
| Gng7 variant 1 | NM_001038655.1 | (19) TGCAGGAGAGCGGTGCCTGA | (976) GTGCATTGTGTGTGCCCCGC |
| Gng7 variant 2 | NM_010319.3 | (29) TGCGTTCGGCTGTGCGGCT | (1216) GCCACAGTCACGATGTGGGACG |
| Gng8 | NM_010320.3 | (563) TGTGACAAGCGAGACTGTGGGC | (817) CGGAAGGGATTCTCGGCGGC |
| Gng10 | NM_025277.3 | (506) GCCCTGTGTGGGATAGAGGCA | (765) TTGCCAAGGAGCAGCGCCCA |
| Gng11 | NM_025331 | (517) CCTTCAAAGAAAAGGGCAGCTGT | (785) AGTGGGGGAGCCATGCCTTCAT |
| Gng12 variant 1 | NM_001177560.1 | (3678) TGGTCAGTCCTGTGCCGGGT | (4068) TGCTGGATGGACAGCCGAAGA |
| Gng13 | NM_022422 | (143) GGAGGAGTGGGATGTGCCCCA | (403) GGACTAAAGCAGAGACACCCGGA |
| Gng5 | NM_010318.2 | (170) GCTCAACCGCGTGAAGGTTT | (259) ACTCCAGTCAGCAGAGGGTC |
| Gng10 | NM_025277.3 | (151) GGAGAGGATCAAGGTCTCCCA | (222) GCGTCCTTGCATGCATTCTG |
| Gng11 | NM_025331.2 | (407) CAAGTTGCAGAGACAACAGGTATC | (503) TGGGATTCCCTTTACCAGAGGA |
| GAPDH | NM_008084 | (5) ACGGCCGCATCTTCTTGTGCA | (85) ATACGGCCA AATCCGTTCACACCG |
Specific primers used to amplify cDNA from the ON bipolar library or for quantitative RT-PCR comparing WT and Gng13 KO retinas (last four).
Scotopic but not photopic ERG b-waves in Gng13 KO mice decrease with age
Given that deleting Gng13 reduced the expression of (and also mislocalized) several proteins of the cascade, we aimed to determine whether the deficit in the ERG b-wave in KO stays the same with age or whether it changes slowly as a result of additional effects that either compensate or aggravate the situation. Therefore, scotopic and photopic ERGs were acquired at various postnatal days up to 130 days in a total of 12 WT and 17 KO mice. Of these, 4 WT and 7 KO mice were tested at more than one time point. Although all but one WT mouse showed reduced b-wave amplitudes at older ages, KO mice displayed a much greater reduction in scotopic b-wave amplitude at older ages (Fig. 9A). For the purpose of analysis, we grouped all tested mice into 4 age groups (Table2): ages 31–42 days (median 35); 52–59 days (median 55); 68–77 days (median 73); and 97–130 days (median 113). For each group, we plotted the average scotopic b-wave amplitude vs. flash intensity (Fig. 9B). For two intensities, we have also plotted the average b-wave amplitude against age (Fig. 9C); these plots show that, although the b-wave amplitude in WT mice decreased slightly with age, this amplitude in KO mice decreased much more. To better demonstrate the difference in the age effect between WT and KO mice, we normalized the b-wave amplitude to the youngest group and plotted the normalized values against age (Fig. 9D). In WT mice, the oldest group tested showed 90% of the amplitude of the youngest group (averaged over all intensities), whereas, in KO mice, it showed 50%. The difference across ages in WT mice was significant only for one intensity; the difference across ages in KO mice was highly significant for all intensities with typical P < 0.0001 (Wald test). The reduction of the scotopic b-wave with age suggests that Gγ13 contributes not only to coupling mGluR6 to TRPM1, but also to cellular processes required for the maintenance of RBCs.
Figure 9.
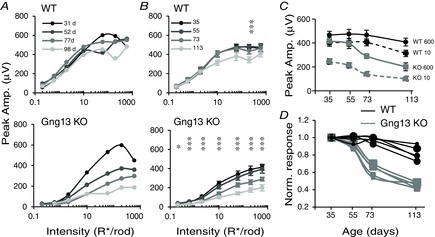
Scotopic light responses in Gng13 KO mice decrease with age
A, amplitude (Amp.) of ERG b-wave vs. flash intensity of a single WT mouse and a single littermate Gng13 KO for 4 time points (in days) as indicated. With age, response curves decreased slightly for WT mice, and dramatically for KO mice. B, population average of ERG b-wave amplitudes (± SEM) for the different intensities. Twelve WT and 17 KO mice were grouped into 4 age groups. The age (in days) indicates the median of the group. A star and 3 vertical stars indicate a significant (P < 0.05) and highly significant (P < 0.0001) decline over age. C, scotopic b-wave amplitudes for two flash intensities (10, and 600 R* per rod) are plotted against age. With age, these amplitudes decline more steeply in KO mice (grey) than in WT mice (black). D, data for the 5 highest intensities in B were normalized to the youngest age and plotted against age for WT mice (black) and KO mice (grey).
Table 2.
Number of ERG records used to determine the age effect
| Scotopic | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | 35 | 55 | 73 | 113 | |||||||||
| Number of records | WT-15; KO-19 | WT-8; KO-9 | WT-7; KO-11 | WT-7; KO-10 | |||||||||
| Days | 31 | 34 | 35 | 38 | 42 | 52 | 59 | 68 | 77 | 97 | 98 | 113 | 130 |
| WT | 4 | 1 | 4 | 4 | 2 | 6 | 2 | 1 | 6 | 2 | 1 | 2 | 2 |
| KO | 3 | 1 | 9 | 2 | 4 | 7 | 2 | 2 | 9 | 2 | 2 | 4 | 2 |
| Photopic | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | 35 | 55 | 73 | 105 | |||||||||
| Number of records | WT-15; KO-19 | WT-7; KO-11 | WT-5; KO-8 | WT-4; KO-6 | |||||||||
| Days | 31 | 34 | 35 | 38 | 42 | 52 | 59 | 68 | 77 | 97 | 98 | 113 | 130 |
| WT | 4 | 2 | 3 | 6 | 0 | 5 | 2 | 1 | 4 | 2 | 0 | 2 | 0 |
| KO | 3 | 1 | 9 | 6 | 0 | 9 | 2 | 2 | 6 | 2 | 0 | 4 | 0 |
For each section, the top row (group) shows the median day for each age group; the second row shows the number of records for each group; the third row (day) shows the days that were combined into each group; and the fourth and fifth rows show the number of records for the different postnatal days for WT and KO mice. The total number of mice used was 12 WT and 17 KO; several mice were used for more than one time point.
When similar grouping and analysis were performed for the three photopic stimulus types (UV, green and saturating flashes) under light adapted conditions, the general photopic b-wave did not consistently change with age (Fig. 10A to C). Out of the 7 different intensities tested (3 UV, 3 green and 1 saturating), the Wald test showed a significant P-value for age effect only for 1 intensity in WT (the lowest green; P < 0.05), and for 3 intensities in KO (highest UV and middle and highest green; P < 0.01). To simplify the data, we normalized the results for each intensity level for each group to the youngest group (i.e. the values for the young group average at 1). The normalized responses of all 7 conditions tested were averaged and the normalized response vs. age was plotted (Fig. 10D). There was little change in WT over age and a slight deterioration in KO. Combining the two oldest groups, we found that, in WT mice, the b-wave amplitude dropped to 95% of the youngest group and, in KO mice, it dropped to 86%. Overall, we conclude that there may be a slight reduction in the photopic response with age, and that this reduction appeared larger in KO. This drop with age is clearly much less severe under photopic conditions than under scotopic conditions where the b-wave dropped to approximately 50%. Thus, Gγ13 contributes to the light responses of RBCs much more than to those of ON CBCs.
Figure 10.
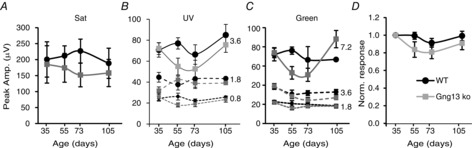
Photopic light responses in Gng13 KO mice are not affected by age
A to C, peak amplitudes of the b-wave for WT (black) and KO (grey) are plotted against age for the saturated flash (A) (Sat; 1000 scot cd s m–2), for the 3 intensities of UV flashes (B), and for the 3 intensities of the green flashes (C); intensities for UV and green flashes are indicated on the right side of the graphs in 103 photons μm−2. D, average normalized responses against age. All responses were normalized for each intensity level to the youngest group and were then averaged across all 7 conditions (3 intensities for UV, 3 for green light, as well as a saturating stimulus). Responses in KO are reduced with age by less than 15%.
Retina structure is largely intact in old Gng13 KO mice
In Gnb3 KO, the rod to rod-bipolar synapse is greatly compromised, with fewer invaginating RBCs observed in the rod spherule. This raises the question of whether the deletion of Gng13 causes similar structural deficiencies. Because the ERG b-wave was affected much more in older mice, we considered that an older retina is more likely to show a deficit than a younger one. Therefore, WT and KO retinas of ∼9-month-old mice were fixed and processed for electron microscopy. Examining semi-thin plastic sections with light microscopy, we found that the general structure of the retina in WT and KO mice was very similar, with a normal thickness for all of the layers (Fig. 11A and B). Examining ultra-thin sections under the electron microscope also revealed a close to normal structure (Fig. 11C and D). The number of profiles in which we could observe a ribbon, at least one horizontal cell process and at least one bipolar dendrite was counted. Comparing WT and KO mice showed that the percentage profiles with bipolar cells went down by ∼10% (from 43% to 33%), although the difference did not reach significance (Student's t test, 778 profiles from 5 WT mice and 847 profiles from 3 Gng13 KO mice) (Fig. 11E). In comparison, in 1- to 2-month-old Gnb3 KOs, the percentage of profiles with bipolar cells was only 24%; which is significantly different from WT mice (P < 0.01; 404 profiles from 3 Gnb3 KO mice).
Figure 11.
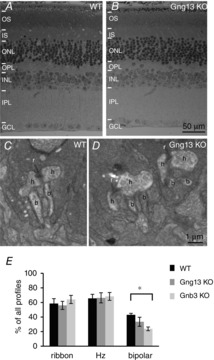
Retinal structure in the old Gng13 KO mouse is normal
A and B, semi-thin (0.5 μm thick) plastic retinal sections of 8.5-month-old WT and KO mice stained with toluidine blue; all layers appear normal. OS, outer segments; IS, inner segments; ONL, outer nuclear layer; GCL, ganglion cell layer. C and D, electron micrographs of WT mice (C) and KO mice (D) show the rod to rod bipolar synapse with a ribbon (r), two lateral horizontal cell processes (h) and the invaginating bipolar dendrites (b). In most images, only one bipolar cell is seen; profiles with 2 invaginating bipolar dendrites are shown because they could also be seen in KO. E, percentages of profiles in which at least one ribbon, one horizontal cell process (Hz) or one bipolar dendrite (bipolar) was seen for WT mice (black), Gng13 KO mice (grey) and Gnb3 KO mice (light grey). For bipolar cells, the differences between WT mice and Gng13 KO mice and that between Gng13 KO mice and Gnb3 KO mice did not reach statistical significance, although the difference between WT mice and Gnb3 KO mice did (Student's t test). Number of profiles analysed: WT, 778 from 5 mice; Gng13 KO, 847 from 3 mice; Gnb3 KO, 404 from 3 mice.
Absence of Gβ3 differentially affects staining for GAP complex in rod bipolar and ON cone bipolar dendritic tips
Because the lack of Gγ13 differentially affects the two cell classes, the absence of its partner Gβ3 might also produce a similar effect. Therefore, to investigate this, Gnb3 KO retina were stained for Gβ5, RGS11, RGS7 and R9AP together with PNA (Fig. 12A to D). Staining for RGS11, RGS7 and R9AP in the dendritic tips of ON CBCs was less affected than this staining in the dendritic tips of RBCs, whereas staining for Gβ5 was equally reduced in both cell classes (Fig. 12E and F).
Figure 12.
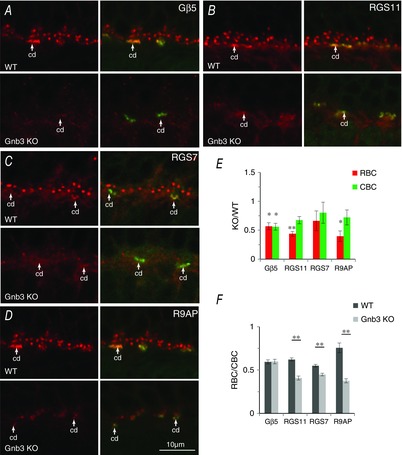
Absence of Gβ3 reduces GAP staining in the dendritic tips of RBCs more than in the tips of ON CBCs
A to D, immunostaining for Gβ5 (A), RGS11 (B), RGS7 (C) or R9AP (D) (red) in WT mice and in a littermate Gnb3 KO mouse. PNA staining (green) locates the dendritic tips of ON CBCs. Cd, cone bipolar dendritic tips. E, staining intensity in KO mice relative to WT mice in RBCs (red) and CBCs (green) (3 sets for RGS7; 4 sets for the rest). For RGS11 and R9AP, staining in KO RBCs is significantly lower than WT cells, whereas staining in KO CBCs is similar to that for WT; for RGS7, the trend is similar, although the difference does not reach significance; for Gβ5, RBCs and ON CBCs decrease similarly in staining and both cell types in KO mice show staining that is significantly lower than that in WT mice. F, ratio of staining intensity for Gβ5, RGS11, RGS7 and R9AP in dendritic tips of RBCs relative to that in tips of CBCs in the same sections. For RGS11, RGS7 and R9AP, RBC/CBC is significantly lower in KO mice than in WT mice, indicating a greater reduction of staining in the rod bipolar dendritic tips.
Discussion
Four important findings are reported. (1) ON bipolar cells express Gγ13 that contributes greatly to scotopic light responses but only slightly to photopic responses. (2) In correlations, Gγ13 contributes to the maintenance of the mGluR6-cascade elements in RBCs much more than in ON CBCs. (3) As is the case in the absence of Gγ13, in the absence of Gβ3 (which contributes much more than Gγ13 to the light response), the mGluR6-related GAP proteins in rod bipolar dendritic tips are affected more than those in ON cone bipolar dendritic tips. (4) In the absence of Gγ13, the scotopic ERG b-wave deteriorates with age.
Gγ13 is a player in the mGluR6 cascade in RBCs
The first piece of evidence suggesting that Gγ13 is a player is straightforward: the scotopic light responses in Gng13 KO mice are reduced. The second piece of evidence relies on the localization study. The expression and localization of the three subunits in a heterotrimeric G-protein are often interdependent (Bomsel & Mostov, 1992; Marrari et al. 2007; and Dupre et al. 2009). Consistent with this, we find that staining for Gβ3 decreases greatly in the absence of Gγ13. Interestingly, in RBCs, the punctate expression of RGS11, RGS7, Gβ5 and R9AP (GAP proteins) also decreases greatly. This general phenomenon showing that elimination of one cascade element affects the expression or localization of its interactors has been reported in numerous studies. For example, deleting Gγ13 from olfactory neurons not only diminishes the response to numerous odours, but also dramatically reduces the expression of several interactors in these cells, including Gαolf, RIC-8B and CEP260 (Li et al. 2013). In retinal ON bipolar cells, eliminating almost any protein in the mGluR6 cascade (e.g. mGluR6, GPR179, Gβ3 or Gβ5) reduces the expression of many others, especially the GAPs (Rao et al. 2007; Cao et al. 2009, 2010; Dhingra et al. 2012; Orlandi et al. 2012; Xu et al. 2012; Orlandi et al. 2013). Thus, the reduced scotopic light response in Gng13 KO combined with the effect of the absence of Gγ13 on the mGluR6 cascade components shows that Gγ13 is part of the cascade in RBCs.
Gγ13 contributes to maintaining the rod bipolar postsynaptic machinery
Although it is clear that Gγ13 is a player in the mGluR6 cascade in RBCs, and although it is highly probable that its main function is to couple mGluR6 to its effector, it is not clear to what extent the loss in the light response is a result of coupling inefficiency and to what extent it is a result of disorganization of the mGluR6-associated proteins. The fact that the ERG b-wave was not totally eliminated in the young Gng13 KO and progressively declines with age indicates a limited role of this subunit in coupling, as well as a significant role in maintaining synaptic integrity. It is documented that Gβγ dimers have additional functions beyond coupling G-protein coupled receptors to their effectors. Studies on G-protein subunits in photoreceptors support a function in trafficking (or maintaining complex stability) because, in cones lacking Gβ3, Gαt2 is homogenously distributed throughout the cone (Nikonov et al. 2013). Similarly, in rods lacking Gγ1, Gαt1 is not well localized to the outer segment (Lobanova et al. 2008; Kolesnikov et al. 2011). More dramatic effects are seen in ON bipolar cells lacking Gβ3, where most cascade elements including Gαo are mislocalized (Dhingra et al. 2012). Evidence for the role of Gβγ in trafficking (or maintenance) is not limited to KO studies because, in expression systems, the expression of exogenous Gβγ affects the surface localization of several proteins including the Kir2.4 channel (Sulaiman et al. 2013). Thus, Gγ13 has two main functions: coupling and maintenance.
Is there another Gβγ dimer in ON bipolar cells?
Although Gγ13 contributes to the light response and to the postsynaptic chemical structure, its contribution to both is smaller than that of Gβ3. In Gng13 KO mice, the scotopic b-wave at young ages at high intensity has a peak amplitude that reaches approximately 80% of the WT amplitude but, in Gnb3 KO mice, this peak amplitude reaches less than 30% of the WT amplitude (Dhingra et al. 2012). Also, the synaptic structure in 9-month-old Gng13 KOs is close to normal, whereas that in young Gnb3 KO adults mostly shows rod terminal profiles with no visible bipolar dendrites. There are three possible explanations for this unexpectedly mild effect in Gng13 KO: first, a different Gγ subunit is expressed in WT ON bipolar cells; second, Gγ13-null ON bipolar cells upregulate another Gγ to compensate for the loss of Gγ13; and third, Gγ13 is the sole Gγ in ON bipolar cells but Gβ3, on its own, can assume the role of the dimer. At present, we cannot favour any one of these explanations. However, we did study the possibility that another Gγ is expressed or upregulated, and found transcripts for three other Gγ subunits in ON bipolar cells (Gγ5, Gγ10 and Gγ11) that all appeared to increase in the Gng13 KO retina. Although we could not show protein expression or significant transcript elevation, further experiments with new reagents may detect some of these proteins in ON bipolar cells.
Differences between the mGluR6 machinery of rod and ON CBCs
Using the Gng13 KO, we have shown that deleting Gγ13 has vastly different effects on the rod and ON CBCs. Although RBCs lose 20–80% of their response (depending on age and flash intensity) and approximately 50% of their GAP staining, ON CBCs largely retain their responses at all ages and may lose approximately 20% of their GAP staining. The present study also reports a similar differential reduction of GAP staining in the Gnb3 KO, suggesting that the mGluR6 machinery in ON CBCs is not identical to that in RBCs. Current knowledge about the cascade in the two cell classes indicates that the basic machinery is very similar, and only little is known about the differences. Assuming that a Gγ subunit is necessary for coupling, the strong ERG responses in ON bipolar cells suggest that they may use more than just one Gγ. Furthermore, the robustness of the photopic relative to scotopic responses, as well as the greater persistence of Gβ3 staining in ON CBCs, suggests that ON CBCs rely more heavily on the non-Gγ13 species. Other differences that may fine tune the responses of different types of ON bipolar cells have been noted. (1) ON CBCs may express an additional channel beside TRPM1 (Morgans et al. 2009). (2) RBCs appear to express more RGS7 than ON CBCs (Mojumder et al. 2009). (3) Different types of ON bipolar cells express different amounts of retinal Purkinje cell protein 2 (Xu et al. 2008; Sulaiman et al. 2010). (4) Unlike ON CBCs, RBCs express PKC, which may modulate the TRPM1 current (Rampino & Nawy, 2011). (5) All RBCs express Gαo2 splice variant, whereas only certain ON CBCs appear to express this variant (Dhingra et al. 2002; Okawa et al. 2010). (6) The synaptic cleft of ON CBCs contains a glycoprotein that is not present in the extracellular matrix of the rod to rod bipolar synapse (i.e. the glycoprotein that binds peanut agglutinin) (Kawano et al. 1984). Except for retinal Purkinje cell protein 2, it is still unclear how differences in the machinery affect the normal cell response. In particular, it is difficult to suggest why Gγ13 is less effective in ON CBCs but, if Gβγ gates the TRPM1 channel, as reported previously (Shen et al. 2012), then the different Gβγ dimers suggested in the present study may endow the TRPM1 channel with different gating properties, or perhaps serve the additional channel that may be present in ON CBCs (Morgans et al. 2009).
Acknowledgments
We thank Drs Takahisa Furukawa, Robert Margolskee, Theodore G. Wensel, Vadim Y Arshavsky and Kirill Martemyanov for donating the antibodies used in the study. We also thank Drs Sergei Nikonov, Robert Smith and Michael Freed for multiple discussions, as well as Dr Gui-Shuang Ying for help with processing the statistical tests. The submitted manuscript was edited by Mirotznik Editing Services. All authors declare that they have no conflicts of interest.
Glossary
Abbreviations
- CBC
cone bipolar cell
- ERG
electroretinogram
- GAP
GTPase activating protein
- INL
inner nuclear layer
- IPL
inner plexiform layer
- KO
knockout
- mGluR6
metabotropic glutamate receptor 6
- OPL
outer plexiform layer
- PKC
protein kinase C
- PNA
peanut agglutinin
- RBC
rod bipolar cell
- ROI
regions of interest
- TRPM1
transient receptor potential melastatin 1
- WT
wild-type
Additional information
Competing interests
The authors declare that no conflicts of interest exist.
Author contributions
H.R. was responsible for the study design, and acquisition of all types of data; analysis and interpretation of data; as well as drafting and revising the article. A.D. was responsible for the study design, and acquisition of ERG data; analysis and interpretation of all data types; as well as revising the article. S.T. was responsible for the study design, and acquisition and analysis of immunostaining data. M.F. was responsible for acquisition and analysis of immunostaining data. J.L. was responsible for the acquisition and analysis of EM data. A.L. was responsible for designing the ERG protocol, and acquisition and help in analysis of the ERG. N.V. conceived and designed the experiments, and was responsible for data analysis, interpretation, and writing and revising the article. All authors approved the version submitted for publication.
Funding
This work was supported by National Institutes of Health Grants EY11105 (NV) and NEI P30 EY01583 (Vision Research Core of the University of Pennsylvania).
References
- Audo I, Kohl S, Leroy BP, Munier FL, Guillonneau X, Mohand-Said S, Bujakowska K, Nandrot EF, Lorenz B, Preising M, Kellner U, Renner AB, Bernd A, Antonio A, Moskova-Doumanova V, Lancelot ME, Poloschek CM, Drumare I, Defoort-Dhellemmes S, Wissinger B, Leveillard T, Hamel CP, Schorderet DF, De Baere E, Berger W, Jacobson SG, Zrenner E, Sahel JA, Bhattacharya SS. Zeitz C. TRPM1 is mutated in patients with autosomal-recessive complete congenital stationary night blindness. Am J Human Gen. 2009;85:720–729. doi: 10.1016/j.ajhg.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake BL, Wing MR, Zhou JY, Lei Q, Hillmann JR, Behe CI, Morris RA, Harden TK, Bayliss DA, Miller RJ. Siderovski DP. G beta association and effector interaction selectivities of the divergent G gamma subunit G gamma(13) J Biolog Chem. 2001;276:49267–49274. doi: 10.1074/jbc.M106565200. [DOI] [PubMed] [Google Scholar]
- Bomsel M. Mostov K. Role of heterotrimeric G proteins in membrane traffic. Mol Biol Cell. 1992;3:1317–1328. doi: 10.1091/mbc.3.12.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer JL, Waldo GL. Harden TK. Beta gamma-subunit activation of G-protein-regulated phospholipase C. J Biol Chem. 1992;267:25451–25456. [PubMed] [Google Scholar]
- Breton ME, Schueller AW, Lamb TD. Pugh EN., Jr Analysis of ERG a-wave amplification and kinetics in terms of the G-protein cascade of phototransduction. Invest Ophthalmol Vis Sci. 1994;35:295–309. [PubMed] [Google Scholar]
- Camps M, Carozzi A, Schnabel P, Scheer A, Parker PJ. Gierschik P. Isozyme-selective stimulation of phospholipase C-beta 2 by G protein beta gamma-subunits. Nature. 1992;360:684–686. doi: 10.1038/360684a0. [DOI] [PubMed] [Google Scholar]
- Cao Y, Kolesnikov AV, Masuho I, Kefalov VJ. Martemyanov KA. Membrane anchoring subunits specify selective regulation of RGS9.Gbeta5 GAP complex in photoreceptor neurons. J Neurosci. 2010;30:13784–13793. doi: 10.1523/JNEUROSCI.1191-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Masuho I, Okawa H, Xie K, Asami J, Kammermeier PJ, Maddox DM, Furukawa T, Inoue T, Sampath AP. Martemyanov KA. Retina-specific GTPase accelerator RGS11/G beta 5S/R9AP is a constitutive heterotrimer selectively targeted to mGluR6 in ON-bipolar neurons. J Neurosci. 2009;29:9301–9313. doi: 10.1523/JNEUROSCI.1367-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra A, Jiang M, Wang TL, Lyubarsky A, Savchenko A, Bar-Yehuda T, Sterling P, Birnbaumer L. Vardi N. Light response of retinal ON bipolar cells requires a specific splice variant of Galpha(o) J Neurosci. 2002;22:4878–4884. doi: 10.1523/JNEUROSCI.22-12-04878.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra A, Lyubarsky A, Jiang M, Pugh EN, Jr, Birnbaumer L, Sterling P. Vardi N. The light response of ON bipolar neurons requires G[alpha]o. J Neurosci. 2000;20:9053–9058. doi: 10.1523/JNEUROSCI.20-24-09053.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra A, Ramakrishnan H, Neinstein A, Fina ME, Xu Y, Li J, Chung DC, Lyubarsky A. Vardi N. Gbeta3 is required for normal light on responses and synaptic maintenance. J Neurosci. 2012;32:11343–11355. doi: 10.1523/JNEUROSCI.1436-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra A, Sulaiman P, Xu Y, Fina ME, Veh RW. Vardi N. Probing neurochemical structure and function of retinal ON bipolar cells with a transgenic mouse. J Comp Neurol. 2008;510:484–496. doi: 10.1002/cne.21807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra A, Tummala SR, Lyubarsky A. Vardi N. PDE9A is expressed in the inner retina and contributes to the normal shape of the photopic ERG waveform. Front Mol Neurosci. 2014;7:60. doi: 10.3389/fnmol.2014.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupre DJ, Robitaille M, Rebois RV. Hebert TE. The role of Gbetagamma subunits in the organization, assembly, and function of GPCR signaling complexes. Annu Rev Pharmacol Toxicol. 2009;49:31–56. doi: 10.1146/annurev-pharmtox-061008-103038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlitze S, Garcia DE, Mackie K, Hille B, Scheuer T. Catterall WA. Modulation of Ca2+ channels by G-protein beta gamma subunits. Nature. 1996;380:258–262. doi: 10.1038/380258a0. [DOI] [PubMed] [Google Scholar]
- Huang L, Max M, Margolskee RF, Su H, Masland RH. Euler T. G protein subunit G gamma 13 is coexpressed with G alpha o, G beta 3, and G beta 4 in retinal ON bipolar cells. J Comp Neurol. 2003;455:1–10. doi: 10.1002/cne.10396. [DOI] [PubMed] [Google Scholar]
- Huang L, Shanker YG, Dubauskaite J, Zheng JZ, Yan W, Rosenzweig S, Spielman AI, Max M. Margolskee RF. Ggamma13 colocalizes with gustducin in taste receptor cells and mediates IP3 responses to bitter denatonium. Nat Neurosci. 1999;2:1055–1062. doi: 10.1038/15981. [DOI] [PubMed] [Google Scholar]
- Jeffrey BG, Morgans CW, Puthussery T, Wensel TG, Burke NS, Brown RL. Duvoisin RM. R9AP stabilizes RGS11-G beta5 and accelerates the early light response of ON-bipolar cells. Vis Neurosci. 2010;27:9–17. doi: 10.1017/S0952523809990319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano K, Uehara F, Sameshima M. Ohba N. Binding sites of peanut agglutinin in mammalian retina. Jpn J Ophthalmol. 1984;28:205–214. [PubMed] [Google Scholar]
- Kerr DS, Von Dannecker LE, Davalos M, Michaloski JS. Malnic B. Ric-8B interacts with G alpha olf and G gamma 13 and co-localizes with G alpha olf, G beta 1 and G gamma 13 in the cilia of olfactory sensory neurons. Mol Cell Neurosci. 2008;38:341–348. doi: 10.1016/j.mcn.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Koike C, Numata T, Ueda H, Mori Y. Furukawa T. TRPM1: a vertebrate TRP channel responsible for retinal ON bipolar function. Cell Calcium. 2010a;48:95–101. doi: 10.1016/j.ceca.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Koike C, Obara T, Uriu Y, Numata T, Sanuki R, Miyata K, Koyasu T, Ueno S, Funabiki K, Tani A, Ueda H, Kondo M, Mori Y, Tachibana M. Furukawa T. TRPM1 is a component of the retinal ON bipolar cell transduction channel in the mGluR6 cascade. Proc Natl Acad Sci USA. 2010b;107:332–337. doi: 10.1073/pnas.0912730107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesnikov AV, Rikimaru L, Hennig AK, Lukasiewicz PD, Fliesler SJ, Govardovskii VI, Kefalov VJ. Kisselev OG. G-protein betagamma-complex is crucial for efficient signal amplification in vision. J Neurosci. 2011;31:8067–8077. doi: 10.1523/JNEUROSCI.0174-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo M, Sanuki R, Ueno S, Nishizawa Y, Hashimoto N, Ohguro H, Yamamoto S, Machida S, Terasaki H, Adamus G. Furukawa T. Identification of autoantibodies against TRPM1 in patients with paraneoplastic retinopathy associated with ON bipolar cell dysfunction. PLoS One. 2011;6:e19911. doi: 10.1371/journal.pone.0019911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Ponissery-Saidu S, Yee KK, Wang H, Chen ML, Iguchi N, Zhang G, Jiang P, Reisert J. Huang L. Heterotrimeric G protein subunit Ggamma13 is critical to olfaction. J Neurosci. 2013;33:7975–7984. doi: 10.1523/JNEUROSCI.5563-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Benard O. Margolskee RF. Ggamma13 interacts with PDZ domain-containing proteins. J Biol Chem. 2006;281:11066–11073. doi: 10.1074/jbc.M600113200. [DOI] [PubMed] [Google Scholar]
- Liu Z, Fenech C, Cadiou H, Grall S, Tili E, Laugerette F, Wiencis A, Grosmaitre X. Montmayeur JP. Identification of new binding partners of the chemosensory signaling protein Ggamma13 expressed in taste and olfactory sensory cells. Front Cell Neurosci. 2012;6:26. doi: 10.3389/fncel.2012.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobanova ES, Finkelstein S, Herrmann R, Chen YM, Kessler C, Michaud NA, Trieu LH, Strissel KJ, Burns ME. Arshavsky VY. Transducin gamma-subunit sets expression levels of alpha- and beta-subunits and is crucial for rod viability. J Neurosci. 2008;28:3510–3520. doi: 10.1523/JNEUROSCI.0338-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis DE, Kurachi Y, Galper J, Neer EJ. Clapham DE. The beta gamma subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature. 1987;325:321–326. doi: 10.1038/325321a0. [DOI] [PubMed] [Google Scholar]
- Lyubarsky AL, Chen C, Simon MI. Pugh EN., Jr Mice lacking G-protein receptor kinase 1 have profoundly slowed recovery of cone-driven retinal responses. J Neurosci. 2000;20:2209–2217. doi: 10.1523/JNEUROSCI.20-06-02209.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyubarsky AL, Daniele LL. Pugh EN., Jr From candelas to photoisomerizations in the mouse eye by rhodopsin bleaching in situ and the light-rearing dependence of the major components of the mouse ERG. Vis Res. 2004;44:3235–3251. doi: 10.1016/j.visres.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Lyubarsky AL, Falsini B, Pennesi ME, Valentini P. Pugh EN., Jr UV- and midwave-sensitive cone-driven retinal responses of the mouse: a possible phenotype for coexpression of cone photopigments. J Neurosci. 1999;19:442–455. doi: 10.1523/JNEUROSCI.19-01-00442.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyubarsky AL. Pugh EN., Jr Recovery phase of the murine rod photoresponse reconstructed from electroretinographic recordings. J Neurosci. 1996;16:563–571. doi: 10.1523/JNEUROSCI.16-02-00563.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrari Y, Crouthamel M, Irannejad R. Wedegaertner PB. Assembly and trafficking of heterotrimeric G proteins. Biochemistry. 2007;46:7665–7677. doi: 10.1021/bi700338m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masu M, Iwakabe H, Tagawa Y, Miyoshi T, Yamashita M, Fukuda Y, Sasaki H, Hiroi K, Nakamura Y, Shigemoto R, et al. Specific deficit of the ON response in visual transmission by targeted disruption of the mGluR6 gene. Cell. 1995;80:757–765. doi: 10.1016/0092-8674(95)90354-2. [DOI] [PubMed] [Google Scholar]
- McCall MA. Gregg RG. Comparisons of structural and functional abnormalities in mouse b-wave mutants. J Physiol. 2008;586:4385–4392. doi: 10.1113/jphysiol.2008.159327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojumder DK, Qian Y. Wensel TG. Two R7 regulator of G-protein signaling proteins shape retinal bipolar cell signaling. J Neurosci. 2009;29:7753–7765. doi: 10.1523/JNEUROSCI.1794-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojumder DK, Sherry DM. Frishman LJ. Contribution of voltage-gated sodium channels to the b-wave of the mammalian flash electroretinogram. J Physiol. 2008;586:2551–2580. doi: 10.1113/jphysiol.2008.150755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgans CW, Brown RL. Duvoisin RM. TRPM1: the endpoint of the mGluR6 signal transduction cascade in retinal ON-bipolar cells. BioEssays: News and Reviews in Molecular, Cellular and Developmental Biology. 2010;32:609–614. doi: 10.1002/bies.200900198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgans CW, Zhang J, Jeffrey BG, Nelson SM, Burke NS, Duvoisin RM. Brown RL. TRPM1 is required for the depolarizing light response in retinal ON-bipolar cells. Proc Natl Acad Sci USA. 2009;106:19174–19178. doi: 10.1073/pnas.0908711106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawy S. Jahr CE. Time-dependent reduction of glutamate current in retinal bipolar cells. Neurosci Lett. 1990;108:279–283. doi: 10.1016/0304-3940(90)90654-r. [DOI] [PubMed] [Google Scholar]
- Ng L, Lyubarsky A, Nikonov SS, Ma M, Srinivas M, Kefas B, St Germain DL, Hernandez A, Pugh EN., Jr Forrest D. Type 3 deiodinase, a thyroid-hormone-inactivating enzyme, controls survival and maturation of cone photoreceptors. J Neurosci. 2010;30:3347–3357. doi: 10.1523/JNEUROSCI.5267-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikonov SS, Lyubarsky A, Fina ME, Nikonova ES, Sengupta A, Chinniah C, Ding XQ, Smith RG, Pugh EN, Jr, Vardi N. Dhingra A. Cones respond to light in the absence of transducin beta subunit. J Neurosci. 2013;33:5182–5194. doi: 10.1523/JNEUROSCI.5204-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okawa H, Pahlberg J, Rieke F, Birnbaumer L. Sampath AP. Coordinated control of sensitivity by two splice variants of Galpha(o) in retinal ON bipolar cells. J Gen Physiol. 2010;136:443–454. doi: 10.1085/jgp.201010477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlandi C, Cao Y. Martemyanov KA. Orphan Receptor GPR179 Forms Macromolecular Complexes With Components of Metabotropic Signaling Cascade in Retina ON-Bipolar Neurons. Invest Ophthalmol Visual Sci. 2013;54:7153–7161. doi: 10.1167/iovs.13-12907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlandi C, Posokhova E, Masuho I, Ray TA, Hasan N, Gregg RG. Martemyanov KA. GPR158/179 regulate G protein signaling by controlling localization and activity of the RGS7 complexes. J Cell Biol. 2012;197:711–719. doi: 10.1083/jcb.201202123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peachey NS, Pearring JN, Bojang P, Jr, Hirschtritt ME, Sturgill-Short G, Ray TA, Furukawa T, Koike C, Goldberg AF, Shen Y, McCall MA, Nawy S, Nishina PM. Gregg RG. Depolarizing bipolar cell dysfunction due to a Trpm1 point mutation. J Neurophysiol. 2012;108:2442–2451. doi: 10.1152/jn.00137.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampino MA. Nawy SA. Relief of Mg(2)(+)-dependent inhibition of TRPM1 by PKCalpha at the rod bipolar cell synapse. J Neurosci. 2011;31:13596–13603. doi: 10.1523/JNEUROSCI.2655-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A, Dallman R, Henderson S. Chen CK. Gbeta5 is required for normal light responses and morphology of retinal ON-bipolar cells. J Neurosci. 2007;27:14199–14204. doi: 10.1523/JNEUROSCI.4934-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchey ER, Bongini RE, Code KA, Zelinka C, Petersen-Jones S. Fischer AJ. The pattern of expression of guanine nucleotide-binding protein beta3 in the retina is conserved across vertebrate species. Neuroscience. 2010;169:1376–1391. doi: 10.1016/j.neuroscience.2010.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Ball SL. Peachey NS. Pharmacological studies of the mouse cone electroretinogram. Visual Neurosci. 2005;22:631–636. doi: 10.1017/S0952523805225129. [DOI] [PubMed] [Google Scholar]
- Shen Y, Rampino MA, Carroll RC. Nawy S. G-protein-mediated inhibition of the Trp channel TRPM1 requires the Gbetagamma dimer. Proc Natl Acad Sci USA. 2012;109:8752–8757. doi: 10.1073/pnas.1117433109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiells RA. Falk G. Glutamate receptors of rod bipolar cells are linked to a cyclic GMP cascade via a G-protein. Proc R Soc Biol Sci. 1990;242:91–94. doi: 10.1098/rspb.1990.0109. [DOI] [PubMed] [Google Scholar]
- Shirato S, Maeda H, Miura G. Frishman LJ. Postreceptoral contributions to the light-adapted ERG of mice lacking b-waves. Exp Eye Res. 2008;86:914–928. doi: 10.1016/j.exer.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smrcka AV. G protein betagamma subunits: central mediators of G protein-coupled receptor signaling. Cell Mol Life Sci: CMLS. 2008;65:2191–2214. doi: 10.1007/s00018-008-8006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulaiman P, Fina M, Feddersen R. Vardi N. Ret-PCP2 colocalizes with protein kinase C in a subset of primate ON cone bipolar cells. J Comp Neurol. 2010;518:1098–1112. doi: 10.1002/cne.22266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulaiman P, Xu Y, Fina ME, Tummala SR, Ramakrishnan H, Dhingra A. Vardi N. Kir2.4 surface expression and basal current are affected by heterotrimeric G-proteins. J Biol Chem. 2013;288:7420–7429. doi: 10.1074/jbc.M112.412791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardi N. Alpha subunit of Go localizes in the dendritic tips of ON bipolar cells. J Comp Neurol. 1998;395:43–52. [PubMed] [Google Scholar]
- Vardi N. Dhingra A. In: Identification and Function of G-proteins in Retinal ON bipolar Cells. Sampath, editor; Martemyanov, editor. G-proteins in Retina; 2014. (in press) [Google Scholar]
- Vardi N, Matesic DF, Manning DR, Liebman PA. Sterling P. Identification of a G-protein in depolarizing rod bipolar cells. Visual Neurosci. 1993;10:473–478. doi: 10.1017/s0952523800004697. [DOI] [PubMed] [Google Scholar]
- Xu L, Ball SL, Alexander KR. Peachey NS. Pharmacological analysis of the rat cone electroretinogram. Visual Neurosci. 2003;20:297–306. doi: 10.1017/s0952523803203084. [DOI] [PubMed] [Google Scholar]
- Xu Y, Dhingra A, Fina ME, Koike C, Furukawa T. Vardi N. mGluR6 deletion renders the TRPM1 channel in retina inactive. J Neurophysiol. 2012;107:948–957. doi: 10.1152/jn.00933.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Sulaiman P, Feddersen RM, Liu J, Smith RG. Vardi N. Retinal ON bipolar cells express a new PCP2 splice variant that accelerates the light response. J Neurosci. 2008;28:8873–8884. doi: 10.1523/JNEUROSCI.0812-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitz C, van Genderen M, Neidhardt J, Luhmann UF, Hoeben F, Forster U, Wycisk K, Matyas G, Hoyng CB, Riemslag F, Meire F, Cremers FP. Berger W. Mutations in GRM6 cause autosomal recessive congenital stationary night blindness with a distinctive scotopic 15-Hz flicker electroretinogram. Invest Ophthalmol Visual Sci. 2005;46:4328–4335. doi: 10.1167/iovs.05-0526. [DOI] [PubMed] [Google Scholar]


