Abstract
Key points
In the human, sensorimotor integration can be investigated using combined sensory and transcranial magnetic stimulation (TMS).
Short latency afferent inhibition (SAI) refers to motor cortical inhibition 20–25ms after median nerve stimulation.
We investigated the influence of SAI on a local excitatory interneuronal motor cortical circuit known as short-interval intracortical facilitation (SICF) and found that, contrary to expectations, SICF was facilitated in the presence of SAI (SICFSAI); this effect is specific to SICF since there was no effect in control conditions in which SICF was not elicited, and the facilitatory SICFSAI interaction increased with increasing strength of SICF or SAI.
The influence of sensory input on excitatory motor cortical circuitry was similar across different bodily regions, different circuits within motor cortex and across functional states, suggesting that this interaction may have general applicability in sensorimotor integration and motor control.
SAI and SICF were found to correlate between individuals in that those with high SAI were found to have high SICF, and this relationship was maintained when SICF was delivered in the presence of SAI, suggesting an intrinsic relationship between SAI and SICF; these findings are compatible with brain-slice studies of sensorimotor circuitry and add to our understanding of sensorimotor integration.
Abstract
In human, sensorimotor integration can be investigated by combining sensory input and transcranial magnetic stimulation (TMS). Short latency afferent inhibition (SAI) refers to motor cortical inhibition 20–25 ms after median nerve stimulation. We investigated the interaction between SAI and short-interval intracortical facilitation (SICF), an excitatory motor cortical circuit. Seven experiments were performed. Contrary to expectations, SICF was facilitated in the presence of SAI (SICFSAI). This effect is specific to SICF since there was no effect at SICF trough 1 when SICF was absent. Furthermore, the facilitatory SICFSAI interaction increased with stronger SICF or SAI. SAI and SICF correlated between individuals, and this relationship was maintained when SICF was delivered in the presence of SAI, suggesting an intrinsic relationship between SAI and SICF in sensorimotor integration. The interaction was present at rest and during muscle contraction, had a broad degree of somatotopic influence and was present in different interneuronal SICF circuits induced by posterior–anterior and anterior–posterior current directions. Our results are compatible with the finding that projections from sensory to motor cortex terminate in both superficial layers where late indirect (I-) waves are thought to originate, as well as deeper layers with more direct effect on pyramidal output. This interaction is likely to be relevant to sensorimotor integration and motor control.
Introduction
Transcranial magnetic stimulation (TMS) provides a non-invasive method to study neurophysiology at the systems level in the human brain. Previous work has identified a range of inhibitory and facilitatory intracortical circuits using conditioning–test stimulus protocols. However, these circuits do not exist in isolation, and recent studies of their interactions using multi-pulse protocols have advanced our insight into fundamental organisation of these networks (for review see Ni et al. 2011c). For example the strength and time course of presynaptic GABAB receptor-mediated disinhibition can be measured by the reduction in short-interval intracortical inhibition (SICI) during long-interval intracortical inhibition (LICI) (Sanger et al. 2001; Chu et al. 2008; Cash et al. 2010; Ni et al. 2011b). These interactions are important not only in understanding normal physiology of motor control but also in studying the pathophysiology of neurological disorders (Li et al. 2007; Chu et al. 2009; Silbert et al. 2011). For example, pre- and postsynaptic GABAB-mediated inhibition have been shown to be abnormal in Parkinson's disease (Chu et al. 2009). More recently such studies have been used as a guide to develop plasticity protocols of increased efficacy (Cash et al. 2014).
The circuitry underlying sensorimotor integration is of particular interest. The primary motor cortex receives strong monosynaptic inputs from the primary sensory cortex (Rocco-Donovan et al. 2011). Inactivation of these inputs causes disruption of motor acts such as fine motor coordination, sustained muscle contractions and appropriate grip force (Hikosaka et al. 1985; Brochier et al. 1999; Rocco-Donovan et al. 2011) and impedes the learning of new motor skills (Pavlides et al. 1993). In the human, sensorimotor integration and plasticity can be investigated using TMS, and were found to be abnormal in several movement and cognitive disorders (Battaglia et al. 2007; Quartarone et al. 2009; Player et al. 2013). Previous studies demonstrated that afferent input from peripheral nerve stimulation inhibits the motor output elicited by TMS delivered 20–25 ms later, known as short latency afferent inhibition (SAI; Delwaide & Olivier, 1990; Mariorenzi et al. 1991; Classen et al. 2000; Tokimura et al. 2000).
TMS activates both inhibitory and excitatory circuits and the motor evoked potential (MEP) from TMS represents the net pyramidal output. Interestingly, studies using multi-pulse stimulation demonstrated that local inhibitory circuits (SICI, LICI and the cortical silent period) are disinhibited by afferent input from SAI (Hess et al. 1999; Sailer et al. 2002; Stefan et al. 2002; Alle et al. 2009; Udupa et al. 2009, 2014). In the present study, we investigate in detail the physiological influence of SAI on an excitatory motor cortex circuit known as short-interval intracortical facilitation (SICF). SICF occurs when TMS test (TS) and conditioning (CS) stimuli are delivered at three peak inter-stimulus intervals (ISIs) of ∼1.5, 3.0 and 4.5 ms (SICF1, SICF2 and SICF3, respectively), while there is little or no facilitation at intermediate intervals (Tokimura et al. 1996; Ziemann et al. 1998; Ziemann & Rothwell, 2000). The periodicity of SICF peaks closely conforms to the ∼1.5 ms periodicity of descending indirect (I-) waves evoked by TMS, which are understood to arise from the trans-synaptic (indirect) activation of pyramidal neurons through excitatory interneuronal connections (Patton & Amassian, 1954; Amassian et al. 1987), and the magnitude of SICF peaks is considered to reflect the strength of excitatory synaptic interactions in primary motor cortex (M1) (Ziemann & Rothwell, 2000).
SAI has been shown to be mediated primarily by GABAergic and cholinergic systems (Di Lazzaro et al. 2000, 2005b, 2007; Fujiki et al. 2006). Decreased SAI may reflect cholinergic dysfunction in cognitive disorders including Alzheimer's disease (Di Lazzaro et al. 2002, 2005a).
Sensorimotor circuitry is a central element of normal motor control and abnormalities of sensorimotor integration are thought to play a role in neurological disorders including dystonia and Parkinson's disease (Abbruzzese & Berardelli, 2003; Sailer et al. 2003, 2007; Quartarone et al. 2008). An understanding of sensorimotor integration in healthy individuals is a prerequisite for better understanding changes that occur in diseases. We carried out seven experiments in order to comprehensively investigate the influence of SAI on SICF in healthy subjects. Previous studies have explored the influence of sensory input on single pulse TMS and on inhibitory intracortical circuits, but the influence on intracortical excitatory circuitry remains unknown. We hypothesised that SICF would be inhibited in the presence of SAI, as it is known that descending I-waves are reduced during SAI. We anticipated that if the interactions were specific to these circuits, it would occur in a ‘dose-dependent’ fashion. That is, the interactions would be strongest when SAI and SICF were strongest.
Methods
Subjects
We recruited a total of 24 right-handed healthy volunteers (12 women, mean age 28 ± 2 years, range 20–51 years). Handedness was confirmed using the Edinburgh Handedness Inventory (Oldfield, 1971). All participants provided written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the University Health Network (Toronto) Research Ethics Board.
Surface electromyography (EMG) recording
Surface electromyography (EMG) was recorded from the relaxed right first dorsal interosseous (FDI) and was simultaneously monitored in abductor pollicis brevis (APB) muscle with disposable surface Ag–AgCl electrodes in a tendon–belly arrangement. The signal was amplified 1000× (Model 2024F; Intronix Technologies Corp., Bolton, Ontario, Canada), filtered (bandpass 20 Hz–2.5 kHz), digitised at 5 kHz (Micro 1401; Cambridge Electronic Design, Cambridge, UK) and stored in a laboratory computer for off-line analysis.
Median nerve stimulation (MNS)
Electrical stimulation was applied to the right median nerve at the wrist by a DS7A constant-current stimulator (pulse width 0.2 ms; Digitimer, Welwyn Garden City, UK,) with standard bar electrodes, with the cathode positioned proximally. Sensory threshold (ST) was defined as the lowest MNS intensity felt by the subject. Except for Expt 5, in which the effect of MNS intensity was investigated, MNS intensity in all other experiments was adjusted to produce a small twitch in APB which was monitored throughout the experiment (Tokimura et al. 2000; Abbruzzese et al. 2001; Sailer et al. 2003; Kessler et al. 2005; Ni et al. 2011a). SAI of similar strength can be evoked in FDI and APB muscle with stimulation of the median nerve (cf. Fig. 1C in Tokimura et al. 2000), but by targeting median nerve and recording from FDI the effect of direct peripheral muscle activation is avoided. This MNS intensity was equal to 3.2 ± 0.3 ST, and thus comparable to stimulation strength used in previous studies to evoke SAI.
Figure 1.
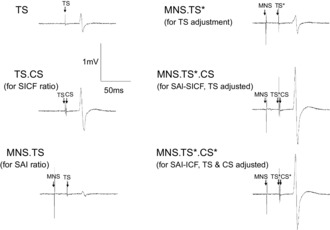
MEP amplitudes from one subject illustrating short-interval intracortical facilitation (SICF), short latency afferent inhibition (SAI) and SICF delivered in the presence of SAI
These traces illustrate the reduction in TS MEP amplitude during SAI (compare TS and MNS.TS), the increase of TS MEP amplitude during SICF (peak 1, compare TS.CS with TS) and the successful adjustment of TS (compare MNS.TS* and TS amplitude). SICF is facilitated in the presence of SAI with TS adjusted (MNS.TS*.CS) and with TS and CS adjusted (MNS.TS*.CS*, compare both with MNS.TS*).
Somatosensory evoked potentials (SSEP)
Median nerve somatosensory evoked potentials were reco-rded (active electrode 3 cm posterior to C3, reference electrode over Fz, the 10–20 International EEG system, bandpass filter 3 Hz–2 kHz, rate of stimulation 3.3 Hz, average of 200 trials). N20 is the first cortical component of the SSEP. An interval of N20 + 2 ms was used for SAI because it results in reliable inhibition of the test MEP (Tokimura et al. 2000; Alle et al. 2009; Fischer & Orth, 2011).
Transcranial magnetic stimulation (TMS)
TMS was performed with a figure-of-eight shaped coil (central diameter of each loop was 7 cm) and four Magstim 200 stimulators (Magstim, Whitland, Dyfed, UK) connected via a custom connector box (Magstim, Whitland, Dyfed, UK), generating monophasic current.
Posterior to anterior (PA) induced current was used except in Expt 2 in which the induced current direction was anterior to posterior (AP) following the methodology described previously (Ni et al. 2011a). Resting motor threshold (RMT) was tested by the relative frequency method (Groppa et al. 2012) and was defined as the lowest intensity eliciting MEPs >50 μV peak-to-peak amplitude in at least 5 of 10 trials and was determined separately for PA and AP current directions. Active motor threshold (AMT) was defined as the lowest intensity eliciting MEPs of 100 μV peak-to-peak amplitude in at least 5 of 10 trials during slight isometric contraction of the FDI muscle (10% of maximum voluntary contraction) (Vahabzadeh-Hagh, 2014). This intensity was chosen in order to avoid a significant contribution of CS amplitude to the MEP. The level of contraction was continuously monitored by audiovisual feedback of the EMG signal. TS 0.5 mV was defined as the lowest TS intensity to the nearest 1% maximum stimulator output (MSO) that generated an average MEP of 0.5 mV in the right FDI muscle and was determined for each current direction with the hand muscles completely relaxed. A low TS amplitude of 0.5 mV enabled similar MEPs to be generated in both AP and PA current directions. TS 1 mV and TS 2 mV were determined in an equivalent manner. For each stimulus combination (Table1), 10 stimuli (unless otherwise stated) were delivered at 6 ± 1.5 s intervals and the sequence of stimulation conditions was pseudo-randomised. When TS intensity was adjusted in the presence of SAI to match the amplitude of TS alone, this is referred to as MNS.TS*, while TS* represents the amplitude of adjusted TS without MNS.
Table 1.
Overview of stimulation conditions used in Expts 1–5
| Condition | MNS | TS | CS | Description |
|---|---|---|---|---|
| A | • | TS alone | ||
| B | • | • | SICF | |
| C | • | • | SAI | |
| D | • | • | • | SICFSAI |
| E | •* | TS* | ||
| F | • | •* | SAI (TS*) | |
| G | • | •* | • | SICFSAI (TS*) |
| H | • | •* | •* | SICFSAI (TS*.CS*) |
| I | •* | • | SICF (TS*, trough 1) |
MNS, median nerve stimulus; TS, test stimulus; CS conditioning stimulus for SICF, asterisks represent conditions in which TS intensity was adjusted to generate the same MEP or CS was adjusted to compensate for changes in RMT in the presence of SAI. The nature and specificity of the interaction were explored in various ways. In Expts 1 and 2, the influence of SAI on SICF peaks was explored in PA and AP orientation; in Expts 3, 4 and 5 the influence of TS, CS and MNS intensity, respectively, on SICFSAI was investigated. Expts 6 and 7 explored the influence of isometric contraction and the somatotopic specificity of the interaction. See Methods for full details.
SICF
Except in Expts 3 and 4, which investigated the influence of TMS intensity, SICF was elicited using TS that generated 0.5 mV MEP when delivered alone, followed by CS at RMT at ISIs corresponding to I-wave periodicity. This protocol reliably generates SICF peaks 1, 2 and 3 (Chen & Garg, 2000). SICF was calculated from the MEP amplitude ratio [TS.CS/(TS + CS)]. SICF in the presence of SAI (SICFSAI) was calculated as [MNS.TS.CS/(MNS.TS + MNS.CS)].
The order of experiments was pseudo-randomised between individuals, except for the identification of optimal ISIs which was performed prior to other experiments.
Experiment 1: influence of SAI on SICF: PA current direction
Determining the individualized optimal ISIs for SICF
Twelve subjects participated. In order to account for inter-individual variability, ensure optimal SICFSAI interaction and avoid contamination by SICI (Peurala et al. 2008), the optimal ISIs in each individual for evoking SICF peaks 1, 2, 3 and trough 1 (T1) were determined by generating an I-wave facilitation curve. Paired stimuli were delivered at a total of 12 ISIs (1.3, 1.5, 1.7, 1.9, 2.1, 2.3, 2.7, 2.9, 3.1, 4.5, 4.7, 4.9 ms) together with unconditioned TS alone (Table1, conditions A and B). Eight stimulus pairs were delivered per ISI.
Interaction between SAI and SICF, TS unadjusted
TS alone, MNS.TS, TS.CS and MNS.TS.CS were delivered at each of the 4 ISIs (SICF peaks 1, 2, 3 and T1) in one run (Table1, conditions A–D). Ten trials were performed each of the ten conditions (100 trials).
Interaction between SAI and SICF, with adjusted TS and CS
As SAI reduces the MEP amplitude produced by TS, TS intensity was adjusted such that MEP amplitude, conditioned by the preceding MNS (i.e. MNS.TS*), was equal to 0.5 mV. Since SAI may reduce the effectiveness of the CS, we also tested the interaction with a stronger CS. For this we determined the RMT at N20 + 2 ms after MNS and is referred to as the adjusted CS (CS*) (Tergau et al. 1999; Sanger et al. 2001). The experiment consisted of 11 conditions: TS*, MNS.TS*, MNS.TS*.CS, MNS.TS*.CS* at each the 4 ISIs (total 110 trials). Also in this run TS*.CS was delivered at T1 ISI to ensure SICF at T1 was not significant at this intensity (Table1, conditions E–I).
Correlational analyses
The intrinsic relationship between SAI, SICF and the relationship between these circuits and SICFSAI was further explored by correlational analysis.
Experiment 2: influence of SAI on SICF: AP current direction
Optimal ISIs
Nine subjects participated. The optimal ISI for AP SICF peak 1 was determined in each individual. Eight stimulus pairs were delivered at each of three ISIs (1.3, 1.5, 1.7 ms) intermixed with eight TS alone (32 trials in total; Table1, conditions A and B). The intensity for TS was 0.5 mV and CS was 1 × RMT (both determined in AP orientation).
Interaction between SAI and SICF, unadjusted and adjusted conditions
These experiments were performed in the same manner as described for PA orientation except that only the AP-SICF1 peak was investigated and that intensities were determined in the AP orientation. Unadjusted conditions included TS alone, TS.CS, MNS.TS and MNS.TS.CS (total 40 trials) (Table1, conditions A–D). The adjusted conditions were TS*, MNS.TS*, MNS.TS*.CS and MNS.TS*.CS* for a total 40 trials (Table1, conditions E–H).
Correlational analyses
The relationship between SICF activated in AP and PA current orientations, and its modulation in the presence of SAI (SICFSAI) was explored by correlational analysis in seven participants who completed both conditions.
Experiment 3: influence of TS intensity on SICFSAI
In 10 subjects, the influence of TS intensity on the SICFSAI interaction, SAI and SICF was compared. TS intensities of 0.5, 1 and 2 mV were studied in separate runs. SICF1 was studied henceforth because the greatest SICFSAI interaction was observed at this peak in Expt 1. Each unadjusted TS run consisted of TS alone, TS.CS, MNS.TS and MNS.TS.CS (40 trials at each TS intensity; Table1, conditions A–D). Each adjusted TS run consisted of MNS.TS* and MNS.TS*.CS (20 trials at each TS* intensity; Table1, conditions F and G). The intensity of CS was not adjusted as Expt 1 demonstrated similarity between unadjusted and adjusted CS conditions.
Experiment 4: influence of CS intensity on SICFSAI
In 11 subjects three CS intensities were investigated: 0.5, 1 and 1.2 RMT. With TS of 0.5 mV, CS intensity of 0.5 RMT was likely to be close to the threshold for SICF (Ilic et al. 2002; Ortu et al. 2008), RMT should give close to maximal SICF while 1.2 RMT should evoke suboptimal SICF (Ilic et al. 2002; Ortu et al. 2008). In the first run, TS was unadjusted and the test conditions included TS alone, TS.CS, MNS.TS and MNS.TS.CS with CS at intensities of 0.5, 1 and 1.2 RMT (total 80 trials; Table1, conditions A–D)). This was possible with the use of four magnetic stimulators. The adjusted condition consisted of MNS.TS*, MNS.TS*.CS (at the three CS intensities), as well as CS alone (1.2 RMT) and MNS.CS (1.2 RMT) as the influence of CS amplitude becomes significant at 1.2 RMT (total 60 trials; Table1, conditions F and G).
Experiment 5: influence of MNS intensity on SICFSAI
The influence of three MNS intensities (0.5, 3, 6 × ST) on SICFSAI interaction was studied in ten subjects. Each MNS intensity was investigated in a separate run. In the TS unadjusted condition, each run consisted of TS 0.5 mV alone, TS.CS, MNS.TS and MNS.TS.CS (Table1, conditions A–D). Adjusted runs consisted of TS*, MNS.TS*, MNS.TS*.CS with TS* adjusted according to the strength of SAI at each MNS intensity (Table1, conditions E–H).
Experiment 6: influence of isometric contraction on SICFSAI
Ten subjects were tested during slight contraction of the right FDI muscle (10% of maximal voluntary contraction, MVC). SICF peak 1 and tough 1 were studied to ensure specificity of the interaction to SICF. The optimal ISIs for SICF peak 1 and trough 1 were determined in each individual during contraction (ISI curve 1.1 – 2.3 ms; 0.2 ms intervals; 8 stimuli per ISI). CS intensity was 95% of active motor threshold (AMT). The intensity of peripheral stimulation was adjusted to ensure that SAI occurred for the interaction. Unadjusted collections included conditions A, B, C, D and adjusted collections included conditions E–H (Table1).
Correlational analyses
The relationship between SICF at rest and during contraction, and its modulation in the presence of SAI was explored in nine participants who completed both conditions.
Experiment 7: somatotopic specificity
The previous Expts 1–6 investigated the heterotopic interaction in order to avoid direct stimulation of the target muscle by peripheral nerve stimulation. For this experiment, three sub-studies were performed in ten participants to compare homo- and heterotopic interactions. In each case EMG was recorded from both the FDI and abductor pollicis brevis (APB) muscles, TMS was delivered to the cortical representation of the target muscle and TS MEP amplitude was 0.5 mV in target muscle. RMT was determined for each target muscle. The experiments included: (a) homotopic interaction: MNS with APB as target muscle; (b) homotopic interaction: ulnar nerve stimulation with FDI as target muscle; (c) heterotopic interaction: MNS with FDI as target muscle. The optimal ISI for PA SICF peak 1 was determined in each individual for each muscle (ISI curve: 1.3, 1.5, 1.7 ms; 8 stimuli per ISI). For testing the interaction, unadjusted collections included conditions A–D and adjusted collections included conditions E–H (Table1).
Data analysis
A linear mixed model (LMM) with random effect of individual was used for data analysis. Restricted maximum likelihood estimation was used in all models. If Kolmogorov–Smirnov test indicated non-normality of data distribution, the analysis was carried out on log transformed data. Statistical significance was defined as P < 0.05 and tested using post hoc two-tailed t tests for paired samples with Bonferroni's correction for multiple comparisons. The LMM is more powerful than the analysis of variance and takes into account the random effect of individual. Degrees of freedom were the number of conditions – 1. All data are given as means ± SEM. Pearson's correlation coefficient was determined using linear regression analysis and data were log transformed. SPSS version 16.0 was used for statistical analysis.
MNS reduced the TS MEP to very small values in the unadjusted conditions resulting in very high SICFSAI percentages, sometimes greater than 1000% with large variations. Due to the high variability with the unadjusted SICF, and in line with previous interaction studies (Muller-Dahlhaus et al. 2008; Alle et al. 2009), we focus on the adjusted TS data.
Results
Experiment 1: influence of SAI on SICF
Optimal ISIs
TS alone generated MEP of 0.42 ± 0.04 mV. RMT was 50.6 ± 3.5% of maximum stimulator output (MSO). LMM of MEP amplitude as a percentage of baseline (TS alone) revealed a significant effect of ISI (F(11,23.6) = 6.1, P < 0.01). As anticipated, SICF peaks 1, 2, 3 (SICF1, SICF2 and SICF3) were maximal at ISIs of 1.3, 2.9 and 4.7 (415 ± 79, 423 ± 72 and 266 ± 52% of TS alone), while trough 1 (T1) was minimal at 2.1 ms (134 ± 25% of TS alone; Fig. 2A). At all ISIs studied except for 2.1 ms, SICF was significant (P < 0.05).
Figure 2.
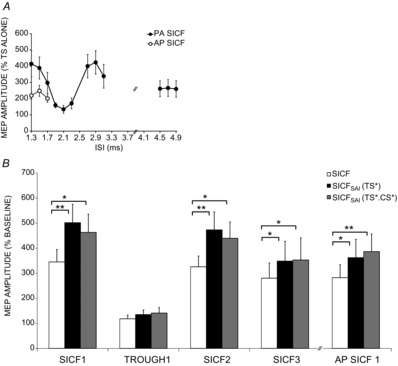
The influence of SAI on SICF peaks and trough 1
A, I-wave facilitation curve. SICF peaks 1, 2 and 3 (PA) were maximal at ISIs of 1.3, 2.9 and 4.7, while SICF was minimal at 2.1 ms (trough 1). SICF was significant at all ISIs except for 2.1 ms, (P < 0.05). B, SICF (PA) was significant at peaks 1, 2 and 3 but not at trough1 and was significant at SICF (AP) peak 1. SICFSAI (PA and AP) was significantly higher than SICF alone at SICF peaks but not at SICF (PA) trough 1. SICFSAI was similar with TS adjusted or TS and CS both adjusted. SICF and SICFSAI at AP SICF1 were comparable to SICFSAI at PA peak 3. Adjusted TS data shown; for unadjusted TS data refer to text. Asterisks indicate significance (*P < 0.05, **P < 0.01).
SICFSAI interaction, TS unadjusted
The N20 latency was 19.4 ± 0.2 ms (mean ± SD) and accordingly the average ISI used for SAI was 21.4 ms. TS alone generated MEP of 0.56 ± 0.06 mV. SICF was significant at peaks 1, 2, 3 (all P < 0.01) but not T1 and was 345 ± 50, 326 ± 43, 280 ± 60 and 118 ± 14% of TS alone, respectively. SAI was 32 ± 7% of TS alone, equivalent to MEP of 0.20 ± 0.06 mV. This small amplitude denominator resulted in large SICF in the presence of SAI (SICFSAI) values with TS unadjusted. SICFSAI values were 1900 ± 567, 1796 ± 563, 1872 ± 765 and 325 ± 100% of MNS.TS at SICF peaks 1, 2, 3 and T1, respectively, and were significantly greater than SICF alone at all peaks (each P < 0.05), but not SICF T1.
Interactions between SAI and SICF, with adjusted TS and CS
Adjusted TS MEP amplitude in the presence of SAI (MNS.TS*) was 0.50 ± 0.03 mV, which was not different to TS alone (above) indicating that TS adjustment was effective. TS alone was 1.6 ± 0.27 mV. SAI was significant (P < 0.01; 43 ± 7% of TS alone). RMT was significantly higher (2.9 ± 3.8% MSO) in the presence of SAI (P < 0.01).
The results for a representative subject are shown in Fig. 1 and the group results are shown in Fig. 2B. LMM analysis revealed significant effects of SICF ISI (F(3,21.3) = 12.9; P < 0.01) and test condition (SICF alone, SICFSAI with TS unadjusted, SICFSAI with TS adjusted, SICFSAI with TS and CS adjusted; F(3,51.5) = 10.8; P < 0.01) on SICF. Pairwise comparisons revealed that compared to SICF alone, there was increased SICF with (i) SICFSAI with TS unadjusted (P < 0.01), (ii) SICFSAI with TS adjusted (P < 0.01) and (iii) SICFSAI with TS and CS adjusted (P < 0.01), with no difference between the latter two conditions. Post hoc paired t tests indicated that with adjusted TS, SICFSAI was significantly higher than SICF alone at SICF peaks 1 (502 ± 73%, P < 0.01), 2 (473 ± 72, P < 0.01) and 3 (349 ± 79, P < 0.05) but not at T1 (135 ± 18% of MNS.TS*). With adjusted TS, there was no SICF alone at T1 (99 ± 7.5% of adjusted TS).
With TS and CS both adjusted (TS* and CS*), SICF in the presence of SAI was significantly greater than SICF alone at peaks 1, 2, 3 (463 ± 72, 440 ± 65 and 353 ± 88% of MNS.TS* respectively; each P < 0.05) but not at T1 (141 ± 22% of MNS.TS*, Fig. 2B).
Experiment 2: influence of SAI on SICF peak 1 with AP current direction
Optimal ISIs
TS alone with AP current direction resulted in MEP amplitude of 0.49 ± 0.13 mV. SICF was significant at ISIs 1.3, 1.5, 1.7 (219 ± 23, 248 ± 34, 201 ± 24% of TS alone, P < 0.05). AP SICF1 was not significantly different from PA SICF3 but was showed significantly lower facilitation than PA SICF peaks 1 and 2 (two-tailed paired t test, P < 0.05; Fig. 2A and B).
Interaction between SAI and SICF with TS unadjusted
TS alone was 0.50 ± 0.09 mV. AP SICF1 was 283 ± 48% of TS alone (P < 0.05). SAI was 28 ± 7% of TS alone (P < 0.01) and MNS reduced TS amplitude to 0.13 ± 0.04 mV. SICF in the presence of SAI was 1724 ± 480% of MNS.TS and was significantly greater than SICF alone (P < 0.05).
Interaction between SAI and SICF, with adjusted TS and CS
The MEP amplitude of TS adjusted for SAI (MNS.TS*) was 0.45 ± 0.05 mV and was similar to TS alone (see above), indicating that the TS adjustment was successful. The amplitude of TS* alone was 0.87 mV. SAI with TS* was 54 ± 8%. The results are shown in Fig. 2B. LMM analysis revealed a significant effect of condition (SICF alone, SICFSAI with TS unadjusted, SICFSAI with TS adjusted, SICFSAI with TS and CS adjusted; F(3,30) = 6.9; P < 0.01) on SICF. SICF in the presence of SAI with TS unadjusted was 1724 ± 480% of MNS.TS, SICFSAI with TS adjusted was 362 ± 68% of baseline MNS.TS* and SICFSAI with TS and CS both adjusted was 387 ± 65% of baseline MNS.TS*. All conditions were significantly greater than SICF alone (P = 0.01, P = 0.02, P = 0.01, respectively). SICFSAI (TS*) and SICFSAI (TS*CS*) were not significantly different.
Correlational analysis
Intrinsic relationship between SICF and SAI and the relationship to SICFSAI. SICF correlated positively with SICFSAI, whereby greater SICF predicted greater SICFSAI (Fig. 8A, Table2A). Although we focused our results on SICFSAI with TS adjusted (Fig. 8A), the relationship was similar for SICFSAI with TS unadjusted or TS and CS both adjusted (Table2A). SAI correlated with SICF between individuals, whereby individuals with greater SICF also had stronger SAI (Fig. 8B and Table2B). This relationship was maintained between SAI and SICFSAI with TS and CS unadjusted or adjusted (Fig. 8B and Table2B). The relationship was weaker with the AP current orientation, possibly due to greater inter-individual variability or a lower number of participants. However, the same pattern was evident. Correlation coefficients, significance levels and regression line equations are summarised in Table2. All the correlations between SICF and SICFSAI remained significant with correction for multiple comparisons, except those for SICF T1 (Table2A). Although the correlation between SAI and SICF1 (P = 0.038) and SICF2 (P = 0.039) was not significant when corrected for multiple comparisons (Table2B and Fig. 8B), the correlation between SAI and SICF3 was significant. Given that all SAI–SICF correlations showed a similar pattern and direction (Table2B), it is likely that there is a true correlation between SAI and SICF1 and SICF2.
Figure 8.
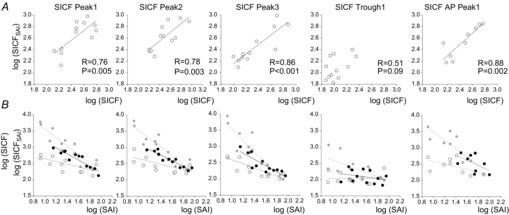
Main correlations between SAI, SICF and SICFSAI
Correlations are displayed for SICF peaks 1–3, SICF trough 1 (PA current direction) and SICF peak 1 (AP current direction). All data are log transformed. Each data point is from a separate participant. SICFSAI conditions with adjusted TS are correlated with SAI with adjusted TS. Pearson's correlation coefficient (R) and statistical significance values are indicated where space permits or presented in Table2. P < 0.05 indicates a significant correlation without correction for multiple comparisons; P < 0.01 indicates a significant correlation with correction for multiple (5) comparisons in each condition. A, SICF in the presence of SAI (SICFSAI) with TS adjusted correlated positively and strongly with SICF alone in all conditions except at SICF trough 1 at which there was usually no SICF. B, there was a significant correlation between SAI and SICF (open circles, dotted trend line), whereby participants with greater SICF were also found to have stronger SAI. This relationship was maintained between SAI and SICFSAI with TS unadjusted (grey circles, grey trend line) or adjusted (black circles, black trend line). The relationship was weaker with the AP current orientation, but the same pattern was evident, with non-significant trend lines indicated by a dotted line.
Table 2.
Correlation coefficients, significance levels and regression line equations for the relationships between SICF, SAI and SICFSAI
| A. Correlation between SICF and SICFSAI | ||||
|---|---|---|---|---|
| Pearson correlation | Regression line | |||
| SICF vs. | coefficient (R) | P-value | equation | |
| Not shown | SICFSAI (TS unadjusted) | |||
| SICF1SAI (TS) | 0.84 | 0.001 | y = 1.73x – 1.24 | |
| SICF T1SAI (TS) | 0.28 | 0.384 | y = 0.60x + 1.13 | |
| SICF2SAI (TS) | 0.75 | 0.005 | y = 1.50x – 0.71 | |
| SICF3SAI (TS) | 0.90 | <0.001 | y = 1.98x – 1.80 | |
| AP SICF1SAI (TS) | 0.85 | 0.004 | y = 1.72x – 1.17 | |
| Fig. 8A | SICFSAI (TS adjusted) | |||
| SICF1SAI (TS*) | 0.76 | 0.005 | y = 0.85x + 0.53 | |
| SICF T1SAI (TS*) | 0.51 | 0.088 | y = 0.63x + 0.80 | |
| SICF2SAI (TS*) | 0.78 | 0.003 | y = 0.75x + 0.74 | |
| SICF3SAI (TS*) | 0.86 | <0.001 | y = 0.83x + 0.49 | |
| AP SICF1SAI (TS*) | 0.88 | 0.002 | y = 0.98x + 0.15 | |
| Not shown | SICFSAI (TS and CS adjusted) | |||
| SICF1SAI (TS* CS*) | 0.76 | 0.004 | y = 0.85x + 0.49 | |
| SICF T1SAI (TS* CS*) | 0.45 | 0.147 | y = 0.72x + 0.62 | |
| SICF2SAI (TS* CS*) | 0.75 | 0.005 | y = 0.69x + 0.86 | |
| SICF3SAI (TS* CS*) | 0.87 | <0.001 | y = 1.01x + 0.04 | |
| AP SICF1SAI (TS* CS*) | 0.89 | 0.001 | y = 0.82x + 0.57 | |
| B. Correlation between SAI and SICF or SICFSAI | ||||
|---|---|---|---|---|
| Pearson correlation | Regression line | |||
| SICF vs. | coefficient (R) | P-value | equation | |
| Fig. 8B | SICF | |||
| SICF1 | −0.60 | 0.038 | y = −0.42x + 3.06 | |
| SICF T1 | 0.02 | 0.949 | y = 0.01x + 2.03 | |
| SICF2 | −0.60 | 0.039 | y = −0.43x + 3.09 | |
| SICF3 | −0.72 | 0.009 | y = −0.63x + 3.23 | |
| AP SICF1 | −0.53 | 0.142 | y = −0.40x + 2.98 | |
| Fig. 8B | SICFSAI (TS unadjusted) | |||
| SICF1SAI (TS) | −0.91 | <0.001 | y = −1.32x + 4.88 | |
| SICF T1SAI (TS) | −0.61 | 0.036 | y = −0.64x + 3.24 | |
| SICF2SAI (TS) | −0.91 | <0.001 | y = −1.30x + 4.83 | |
| SICF3SAI (TS) | −0.92 | <0.001 | y = −1.79x + 5.34 | |
| AP SICF1SAI (TS) | −0.85 | 0.004 | y = −1.30x + 4.79 | |
| Fig. 8B | SICFSAI (TS adjusted) | |||
| SICF1SAI (TS*) | −0.92 | <0.001 | y = −0.95x + 4.12 | |
| SICF T1SAI (TS*) | −0.29 | 0.367 | y = −0.23x + 2.45 | |
| SICF2SAI (TS*) | −0.94 | <0.001 | y = −0.85x + 3.96 | |
| SICF3SAI (TS*) | −0.85 | <0.001 | y = −0.96x + 3.95 | |
| AP SICF1SAI (TS*) | −0.53 | 0.145 | y = −0.78x + 3.86 | |
| Not shown | SICFSAI (TS and CS adjusted) | |||
| SICF1SAI (TS* CS*) | −0.97 | <0.001 | y = −0.99x + 4.14 | |
| SICF T1SAI (TS* CS*) | −0.30 | 0.341 | y = −0.31x + 2.57 | |
| SICF2SAI (TS* CS*) | −0.94 | <0.001 | y = −0.83x + 3.89 | |
| SICF3SAI (TS* CS*) | −0.76 | 0.004 | y = −0.63x + 3.23 | |
| AP SICF1SAI (TS* CS*) | −0.52 | 0.153 | y = −0.63x + 3.66 | |
A, strong correlations were observed between SICF and SICFSAI in adjusted and unadjusted conditions. B, SAI and SICF correlated at baseline and this relationship was maintained when SICF was delivered in the presence of SAI. Correlations were strongest at SICF peaks. SICF1, SICF2 and SICF3, SICF peaks 1, 2, 3; T1, trough 1. P < 0.05 indicates a significant correlation without correction for multiple comparisons; P < 0.01 indicates a significant correlation with correction for multiple (5) comparisons in each condition.
Relationship between SICF circuits activated in AP and PA current orientations, and their modulation in the presence of SAI
The correlations are indicated in Table3. SICF (AP) correlated strongly with SICF (PA) at all peaks, (all R ≈ 0.9; all P < 0.01). The correlations for SICFSAI were also significant (all P < 0.05). No significant correlations were observed at T1. For SICFSAI, the correlation between AP SICF1 and PA SICF2 (P = 0.022) and SICF3 (P = 0.013) was not significant after correction for multiple comparisons while the relationship between AP SICF1 and PA SICF1 remained significant. Nevertheless, the same pattern was evident across all three SICF peaks.
Table 3.
Relationship between AP and PA SICF circuitry and its modulation in the presence of SAI
| A. Correlation between SICF (AP) and SICF (PA) | |||
|---|---|---|---|
| SICF (AP) vs. | Pearson correlation coefficient (R) | P-value | Regression line equation |
| PA SICF1 | 0.90 | 0.006 | y = 0.90x + 0.29 |
| PA SICF2 | 0.93 | 0.003 | y = 0.98x + 0.08 |
| PA SICF3 | 0.90 | 0.006 | y = 0.70x + 0.80 |
| PA SICF T1 | 0.55 | 0.204 | y = 0.88x + 0.66 |
| B. Correlation between SICFSAI (AP) and SICFSAI (PA) | |||
|---|---|---|---|
| SICFSAI (AP) vs. | Pearson correlation coefficient (R) | P-value | Regression line equation |
| PA SICF1SAI | 0.89 | 0.008 | y = 0.71x + 0.72 |
| PA SICF2SAI | 0.83 | 0.022 | y = 0.71x + 0.70 |
| PA SICF3SAI | 0.86 | 0.013 | y = 0.72x + 0.80 |
| PA SICF T1SAI | 0.58 | 0.175 | y = 0.66x + 1.18 |
A, there is a high degree of correlation between AP SICF1 and all PA SICF peaks. B, this relationship remains strong in the presence of SAI. SICF1, SICF2 and SICF3, SICF peaks 1, 2, 3; T1, trough 1. P < 0.05 indicates a significant correlation without correction for multiple comparisons; P < 0.0125 indicates a significant correlation with correction for multiple (4) comparisons in each condition.
Experiment 3: influence of TS intensity on SICFSAI
The MEP amplitudes of unadjusted TS 0.5, 1 and 2 were 0.52 ± 0.06, 0.98 ± 0.10 and 2.00 ± 0.16 mV, respectively. MEP amplitudes of MNS.TS* at TS 0.5, 1 and 2 were 0.47 ± 0.04, 1.04 ± 0.06 and 1.92 ± 0.07 mV respectively, indicating that amplitude matching was successful. As expected, SICF decreased significantly with increasing TS intensity (LMM: F(2,12.1) = 8.6, P < 0.01). SICF was significant at TS 0.5, 1 and 2 mV facilitating MEP amplitude to 400 ± 74 (P < 0.01), 253 ± 27 (P < 0.01) and 158 ± 18% (P < 0.01) of baseline TS MEP amplitude, respectively (Fig. 3). Likewise SAI decreased with increasing TS intensity (LMM: F(2,16.6) = 4.0, P < 0.05) and was 27 ± 7, 40 ± 8 and 49 ± 9% of TS alone at TS 0.5, 1 and 2 mV, respectively, reducing TS amplitude to 0.15 ± 0.05, 0.42 ± 11 and 1.00 ± 0.22 mV and reaching significance at all TS intensities (P < 0.01). Post hoc analysis revealed that inhibition was greater at TS 0.5 mV compared to TS 1 mV or TS 2 mV (both P < 0.05), while there was no significant difference at higher TS intensities between SAI at TS 1 mV and 2 mV.
Figure 3.
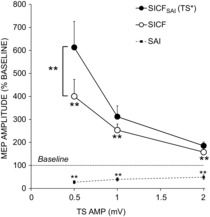
Influence of TS intensity on SICFSAI
SICF and SAI both decreased significantly with increasing TS amplitude, but were significant at each TS intensity tested. Likewise, SICF in the presence of SAI decreased with increasing intensity and was significantly increased relative to SICF alone at TS 0.5 mV, at which SAI and SICF were strongest, but the interaction did not reach significance at higher TS intensities in adjusted TS data. For unadjusted TS data, refer to text. Asterisks indicate significant difference in SAI and SICF compared to TS baseline, or significant facilitation of SICFSAI relative to SICF (*P < 0.05, **P < 0.01).
LMM revealed significant effects of condition (SICF alone, SICFSAI with TS unadjusted, SICFSAI with TS adjusted (F(2,22.7) = 9.6; P < 0.01) and TS intensity (F(2,21.6) = 4.1; P < 0.05) on SICF. SICFSAI (TS unadjusted) decreased with increasing TS intensity (LMM: F(2,10.3) = 6.1, P < 0.05) to 1852 ± 537% (P < 0.01), 1136 ± 255% (P < 0.05) and 739 ± 158% (n.s.) of MNS.TS at TS 0.5, 1 and 2 mV, respectively. Likewise, SICFSAI (TS adjusted) decreased with increasing intensity (LMM: F(2,10.2) = 8.7, P < 0.01) and was 613 ± 113, 289 ± 43 and 185 ± 17% of MNS.TS*. Post hoc paired t tests revealed that SICFSAI was significantly increased relative to SICF alone at TS 0.5 mV (P < 0.01), at which SAI and SICF were strongest, but the interaction was not significant at the higher intensities of TS 1 mV or TS 2 mV (Fig. 3).
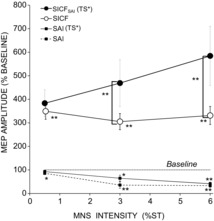
Experiment 4: influence of CS intensity on SICFSAI
TS evoked a MEP of 0.61 ± 0.06 mV. SAI was significant and reduced MEP amplitude to 37 ± 7% of TS alone (0.22 ± 0.05 mV, P < 0.01). LMM revealed a significant effect of condition (SICF alone, SICFSAI with TS unadjusted, SICFSAI with TS adjusted (F(2,15.2) = 7.0; P < 0.01) and of CS intensity (F(2,16.5) = 13.4; P < 0.01) on SICF. SICF was largest at 1 × RMT. With CS at 0.5, 1 and 1.2 RMT, SICF was significant and was 159 ± 13 (P < 0.01), 395 ± 76 (P < 0.01), 126 ± 10% (P < 0.01) of TS alone respectively.
SICFSAI with TS unadjusted was 193 ± 24, 1883 ± 491 and 375 ± 79% of baseline with CS 0.5, 1 and 1.2 RMT, respectively, and was significantly greater than SICF alone at 1 RMT (P < 0.01) and 1.2 RMT (P < 0.01), but not at 0.5 RMT. With suprathreshold CS of 1.2RMT, the MEP amplitude evoked by CS alone (1.37 ± 0.24 mV) and MNS.CS (0.63 ± 0.15 mV) was factored into the calculation of SICF and SICF in the presence of SAI, respectively. MNS.TS* evoked MEP of 0.54 ± 0.04 mV, indicating successful adjustment. With TS adjusted, SICFSAI was significantly increased compared to SICF alone with CS at RMT (586 ± 107%, P < 0.01) and 1.2RMT (222 ± 28%, P < 0.01) but not at CS 0.5RMT (145 ± 22% of baseline) (Fig. 4).
Figure 4.
Influence of CS intensity on SICFSAI
With CS at 0.5, 1 and 1.2 RMT, SICF was significant and was maximal at 1 RMT. SAI reduced MEP amplitude to 37 ± 7% of TS alone (shaded area represents SEM). SICF in the presence of SAI was also maximal at 1 RMT, and was significantly increased compared to SICF alone when CS was 1 RMT and 1.2 RMT, but not at CS 0.5 RMT. Adjusted TS data shown; for unadjusted TS data refer to text. Asterisks indicate significant difference in SAI and SICF compared to TS baseline, or significant facilitation of SICFSAI relative to SICF (*P < 0.05, **P < 0.01).
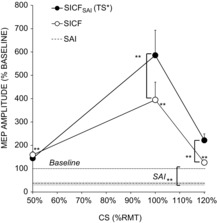
Experiment 5: influence of MNS intensity on SICFSAI
The MEP for TS alone was recorded separately for each run (0.5, 3, 6 ST) and generated MEP of 0.64 ± 0.1, 0.62 ± 0.1, and 0.55 ± 0.08 mV respectively. As expected, SAI became stronger with increasing MNS intensity, with a significant effect of MNS intensity (P < 0.01). SAI was weaker in the adjusted condition where TS amplitude was higher (significant effect of TS adjustment on SAI, LMM: (F(1,31.9) = 4.2, P < 0.05). SAI with TS unadjusted was significant at 0.5 ST (86 ± 5%, P < 0.05), 3 ST (36 ± 12%, P < 0.01) and 6 ST (33 ± 7%, P < 0.01). SAI with TS adjusted was not significant at 0.5 ST (93 ± 6%), but was significant at 3 ST and 6 ST (65 ± 12% (P < 0.05) and 41 ± 6% (P < 0.01), respectively).
The adjustment of TS was successful and MNS.TS* evoked MEPs of similar amplitude with 0.62 ± 0.1, 0.60 ± 0.1, 0.59 ± 0.1 mV at MNS intensities of 0.5, 3 and 6 ST respectively. LMM revealed significant effects of condition (SICF alone, SICFSAI with TS unadjusted, SICFSAI with TS adjusted (F(2,20.3) = 11.9; P < 0.01) and SAI intensity (F(3,19.0) = 6.5; P < 0.01) on SICF (Fig. 5). SICF alone (without MNS) was recorded in each run for 0.5, 3 and 6 ST and was 349 ± 34%, 305 ± 34% and 331 ± 39%, respectively. With TS unadjusted, SICF in the presence of SAI was 406 ± 59, 1171 ± 287 and 1088 ± 199% at 0.5, 3 and 6 ST respectively, and was significantly greater than SICF alone at 3ST and 6ST (P = 0.01 and P < 0.01 respectively), but not at 0.5ST. With TS adjusted, SICFSAI was 383 ± 58, 469 ± 99 and 584 ± 126% of baseline at MNS intensities of 0.5, 3 and 6 ST, and was significantly greater than SICF at 3 ST and 6 ST (both P < 0.05), but not at 0.5 ST.
Figure 5.
Influence of MNS intensity on SICFSAI
SAI became significantly stronger with increasing MNS intensity (P < 0.001). SICF alone was recorded in each run for 0.5, 3 and 6 ST and did not differ significantly between conditions. With TS adjusted, SICFSAI was significantly greater than SICF alone at 3 ST and 6 ST, but not at 0.5 ST. SAI was weaker in the adjusted condition at which TS amplitude was higher, and SAI with both TS unadjusted and adjusted are shown, with the latter of greater relevance here. Adjusted TS data shown; for unadjusted TS data refer to text. Asterisks indicate significance difference in SAI and SICF compared o baseline, and significant facilitation of SICFSAI relative to SICF (*P < 0.05, **P < 0.01).
Experiment 6: influence of muscle contraction on SICFSAI
Optimal ISIs
The effect of ISI on SICF during muscle contraction was significant (LMM: F(6,8.2) = 7.0; P < 0.01). SICF peak 1 occurred at ISI 1.3 ms (428 ± 94% of baseline; P < 0.05) and T1 occurred at ISI at 2.1 ms (126 ± 12%; n.s.). SICF was 428 ± 94% of baseline at peak 1 (P < 0.05) and 126 ± 12% at the trough (n.s.).
Interactions
There was a significant effect of stimulation condition (SICF alone, SICFSAI with TS unadjusted or adjusted) on SICF at SICF peak 1 (LMM: F(2,12.2) = 3.7; P < 0.05) but not at T1 (LMM: F(2,17.7) = 0.08; P < 0.99), indicating specificity of the interaction to the SICF peak during voluntary muscle contraction. TS alone evoked MEPs of 0.63 ± 0.1 mV, and SAI reduced TS amplitude to 63% of baseline. SICF1 was 528 ± 131% and unadjusted SICFSAI was significantly greater at 835 ± 265% (P < 0.05). SICF at T1 was 125 ± 9% and unadjusted SICFSAI was 137 ± 15%. Adjustment of TS intensity in the presence of SAI gave MEP 0.66 ± 0.09 mV. With TS adjusted, SICFSAI at peak 1 was 680 ± 120% of baseline and was significantly increased compared to SICF alone (P < 0.05). SICFSAI at T1 was 154 ± 16%.) (Fig. 6A).
Figure 6.
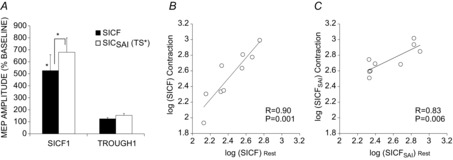
Influence of contraction on SICFSAI
The SICFSAI interaction was tested during isometric contraction to investigate the influence of the functional state. A, SICFSAI was tested at both SICF (PA) peak 1 and trough 1 to ensure specificity to SICF. SICF was significant at peak1 but not trough 1. SICF was significantly facilitated in the presence of SAI at peak 1, but not at trough 1 for both unadjusted (not shown) and adjusted TS intensity conditions. Asterisks indicate significant difference in SICF compared to TS baseline, and significant facilitation of SICFSAI relative to SICF (*P < 0.05, **P < 0.01). B, SICF correlated positively between rest and contraction conditions. C, SICFSAI was also highly correlated between these conditions.
Correlational analysis
SICF correlated positively betw-een rest and contraction conditions (R = 0.90, P < 0.01; Fig. 6B). SICFSAI was also highly correlated between these conditions (R = 0.83, P < 0.01; Fig. 6C).
Experiment 7: somatotopic specificity
Homotopic MNS with APB target muscle
TS MEP amplitude was 0.60 ± 0.08 mV in the APB and was 0.52 ± 0.1 mV in the FDI muscle. SAI was 27 ± 5% and 27 ± 6% for APB and FDI muscles, and was significant for both muscles (P < 0.01). SICF for APB and FDI muscles was significant for both muscles at 247 ± 32% and 293 ± 46% of baseline, respectively (both P < 0.01), and unadjusted SICFSAI was increased to 1502 ± 315% and 1560 ± 644% of baseline. Adjusted TS MEP amplitude in the presence of SAI was well matched and was 0.61 ± 0.08 mV for APB and 0.50 ± 0.09 mV for FDI muscle. The effects of SAI on SICF are shown in Fig. 7A. LMM showed that the effect of condition was significant (SICF alone, SICFSAI with TS unadjusted, SICFSAI with TS adjusted; F(2,12.2) = 9.7, P < 0.01) but the effect of muscle and the interaction between muscle and condition were not significant. Post hoc testing showed increased SICF both in SICFSAI with TS unadjusted (P < 0.01) and with TS adjusted (P < 0.05) compared to SICF alone, and SICFSAI with TS unadjusted was higher than SICFSAI with TS adjusted (P < 0.05).
Figure 7.
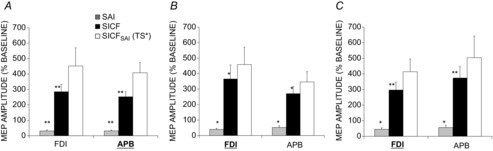
Somatotopy of SICFSAI interaction
Hetero- and homotopic interactions and somatotopic specificity were tested with EMG recorded from the FDI and APB muscles (target muscle indicated in bold and underlined). Similar levels of SAI (grey), SICF (black) and SICFSAI (white) were observed for all interactions tested. The conditions tested were: homotopic interaction: median nerve stimulation with APB as target muscle (A); homotopic interaction: ulnar nerve stimulation with FDI as target muscle (B); heterotopic interaction: median nerve stimulation with FDI as target muscle (C). SICF was significantly facilitated in the presence of SAI with unadjusted (not shown) and adjusted TS and to similar degrees for both the homotopic and heterotopic muscles. This is confirmed by linear mixed model analysis which showed no significant effect of muscle or muscle × condition interaction. Asterisks indicate significant difference in SAI and SICF relative to TS baseline (*P < 0.05, **P < 0.01).
Homotopic ulnar nerve stimulation with FDI as target muscle
TS alone gave 0.65 ± 0.07 mV for the FDI and 0.77 ± 0.18 mV for the APB muscle. SICF was 366 ± 90 of baseline for FDI and 272 ± 45% for APB (both P < 0.05). SAI was 40 ± 7% of TS alone for FDI and 52 ± 11% for APB (both P < 0.05). Unadjusted SICFSAI was 1338 ± 421% of baseline for FDI and 1170 ± 510% of baseline for APB. Adjusted TS in the presence of SAI was 0.59 ± 0.05 mV for FDI and 0.83 ± 0.23 mV for APB and was not significantly different to TS alone. The effects of SAI on SICF are shown in Fig. 7B. The effect of condition was significant (SICF alone, SICFSAI with TS unadjusted, SICFSAI with TS adjusted; F(2,26.3) = 4.2, P < 0.05) but the effect of muscle and the interaction between muscle and condition were not significant. Post hoc testing indicated increased SICF both in SICFSAI with TS unadjusted (P < 0.01) and with TS adjusted (P < 0.01) compared to SICF alone, and SICFSAI with TS unadjusted was higher than SICFSAI with TS adjusted (P < 0.05).
Heterotopic MNS with FDI as target muscle
TS evoked MEPs of 0.60 ± 0.06 mV in the FDI and 0.63 mV in the APB muscle. SICF was 297 ± 50% of baseline in FDI and 375 ± 72% in APB muscle (both P < 0.01). SAI reduced MEP amplitude to 45 ± 9% of TS alone for FDI (P < 0.01) and 56 ± 15% (P < 0.05) for APB muscle. Unadjusted SICFSAI was 803 ± 182% of baseline for FDI and 613 ± 106% for APB. Adjusted TS in the presence of SAI gave 0.61 ± 0.07 mV in the FDI and 0.59 ± 0.33 mV in the APB muscle. LMM revealed a significant effect of condition (SICF alone, SICFSAI with TS unadjusted and TS adjusted; F(2,22.2) = 4.5, P < 0.05) but not muscle and there was no interaction between muscle and condition. Post hoc testing showed increased SICF both in SICFSAI with TS unadjusted (P < 0.01) and with TS adjusted (P < 0.05) compared to SICF alone, and SICFSAI with TS unadjusted was higher than SICFSAI with TS adjusted (P < 0.05).
Discussion
SAI facilitates SICF
The main finding in the present study is that SICF is facilitated in the presence of SAI. This effect is specific to SICF and SAI since there was no effect at SICF T1 and because the interaction increased with increasing strength of SICF or SAI and was absent at intensities that were suboptimal for either circuit. Across a range of experiments the behaviour of the interaction can be briefly summarised as being strongest in conditions where its components, SAI or SICF, were also strongest. The interaction was maintained during contraction and occurred in both homotopic and heterotopic muscles. Furthermore, SAI and SICF were found to correlate among individuals, and this relationship was maintained when SICF was delivered in the presence of SAI, suggesting an intrinsic relationship between SAI and SICF that may be relevant to sensorimotor integration.
Physiology of SAI and SICF and their interaction
TS intensity
The interaction between SAI and SICF was strongest at low TS intensity (Fig. 3). This is in agreement with the finding that both SAI and SICF increase in strength with decreasing TS intensity. One interpretation of this is that both may act preferentially on lower threshold corticospinal neurons (CSNs) (Ilic et al. 2002; Wagle-Shukla et al. 2009; Ni et al. 2011a). Another interpretation relates to the idea that TS alone depolarises a fraction of CSNs to firing threshold while others are subliminally activated and do not fire. When a subsequent CS is applied, temporal summation occurs and the subliminally depolarised neurons reach their threshold leading to MEP facilitation (Ziemann & Rothwell, 2000; Hanajima et al. 2002). With increasing TS intensity, more CSNs are discharged by TS, meaning that the population that can be recruited by CS is smaller leading to reduced facilitation. This would imply that SICF does not target low threshold neurons per se, but that at increased TS intensity the remaining population that can be recruited by CS declines, leading to lower SICF. With regard to the decrease in SAI with increasing TS amplitude, inhibition of CSNs by afferent input may be progressively overcome by greater excitation of CSNs with increasing TS intensity. Regardless of the mechanisms, the results indicate that when both phenomena are strongest, the interaction is likewise strongest.
CS intensity
SICF is strongest with a CS intensity of around 90–100% RMT (Tokimura et al. 1996; Ziemann et al. 1998; Chen & Garg, 2000; Wagle-Shukla et al. 2009). Here we investigated SICFSAI with CS intensities of 50 and 120% RMT to further determine specificity to SICF as CS at 50% RMT is around the threshold for SICF, and 120% RMT is suboptimal for SICF (Ilic et al. 2002). The results indicated that the facilitation of SICF in the presence of SAI was also maximal at a CS intensity of 100%, and that SICFSAI interaction follows a similar behaviour and recruitment pattern to that of SICF alone (Fig. 4).
SAI intensity
SAI with unadjusted TS started to plateau between 3× and 6× ST (similar to Ni et al. 2011a). However, SAI (with TS adjusted) increased in strength in a linear manner as MNS intensity was increased. There was also a linear increase in the strength of the SICFSAI interaction with increasing MNS intensity (Fig. 5). These results indicate a linear relationship between SAI strength and the SICFSAI interaction. Most likely, as more sensorimotor projections are recruited by increasing MNS intensity, the cortical effects on SICF and SAI increase.
I-wave circuits involved in the interaction
The finding that there was no facilitation at trough 1 where SICF was absent indicates that the effect of SAI was specific to I-wave circuitry and periodicity. The level of SICF declines from PA SICF1 to SICF3, most likely due to a progressive reduction in temporal overlap between I-waves, and as a consequence the extent to which SAI can interact with SICF is also reduced. Accordingly, the strength of interaction likewise declined from PA SICF1 to SICF3. This is consistent with our finding that the interaction was strongest when SICF was strongest and also the strong correlation between SICF and SICFSAI.
In line with previous studies, the level of SICF1 was lower in the AP orientation compared to the PA orientation, and was similar between AP SICF1 and PA SICF3. The degrees of interaction with SAI were also similar for these two conditions. Several studies have suggested that PA SICF3 and AP SICF1 target similar I-wave circuits, although recent studies have indicated some differences in the neuronal mechanisms underlying late I-waves in PA and AP orientation (Ni et al. 2011a; Delvendahl et al. 2014). Interestingly, we found that the strength of AP SICF1 was strongly and significantly correlated with all PA SICF peaks, suggesting that these circuits are highly related although the similar levels of SICF may still reflect similar levels of I-wave composition. The strength of this correlation was not significant at the SICF trough. Furthermore, SICFSAI also correlated between AP and PA orientations indicating that the behaviour of AP and PA SICF circuits in the presence of SAI is similar. It was also interesting that the degree of correlation with AP SICF1 was similar for PA SICF 1, 2 and 3 (Table3) and did not indicate a specific relationship to SICF1 or SICF3. The similar modulation of AP SICF1 and PA SICF3 in the presence of SAI may be related to similar I-wave circuitry, or similar levels of SICF, or both.
Somatotopic specificity and functional state
Similar facilitation of SICF in the presence of SAI was observed during heterotopic and homotopic stimulation. This suggests that the effect of SAI on SICF is not related to the muscle innervation of the peripheral nerve used to produce SAI. This is consistent with previous studies which have shown that similar levels of SAI may be evoked in FDI and APB regardless of whether ulnar or median nerve was stimulated (Tokimura et al. 2000; Alle et al. 2009; Fischer & Orth, 2011), and that SICF is evident in hand muscles surrounding the target muscle (Chen & Garg, 2000; Cash et al. 2014). This allows for a wide-spread influence of sensory input on local M1 excitatory circuitry and on sensorimotor integration.
In addition we found that the interaction was present at rest as well as during voluntary muscle contraction. This indicates that the interaction has relevance across different functional states and persists during active motor control. A strong positive correlation amongst individuals was observed between SICF at rest and during contraction, and this was maintained in the presence of SAI. This finding suggests that highly related if not identical circuits are involved at rest and during isometric contraction. The approach of testing during slight muscle contraction in interaction studies of healthy individuals has been adopted elsewhere as it has the advantage of allowing for a more direct comparison of sensorimotor integration in future studies of patients with movement disorders in whom complete relaxation may not be possible (Tokimura et al. 2000; Kessler et al. 2005; Alle et al. 2009).
Sensorimotor circuitry
We showed that SAI has two distinct effects on the motor cortex: inhibition of corticospinal output resulting in MEP inhibition and facilitation of SICF. What are the circuits mediating these different effects? The idea that SAI is likely to be mediated by projections from sensory to motor cortex circuitry is supported by the finding that continuous theta burst stimulation of the sensory cortex modulates SAI (Jacobs et al. 2014). These projections tend to be excitatory rather than inhibitory (Ferezou et al. 2007; Aronoff et al. 2010). A common sensorimotor afferent input that regulates SAI-mediated corticospinal inhibition and modulates SICF would be compatible with the observed correlation between SICF and SAI. Thus, one possibility is that these excitatory afferent inputs project onto the excitatory interneurons underlying SICF in M1, thereby facilitating SICF, in addition to activation of inhibitory GABAergic cells that mediate SAI (Di Lazzaro et al. 2000, 2005b, 2007; Fujiki et al. 2006). We found that the modulation of SICF in the presence of SAI was specific to SICF peaks (Figs 2B and 5), indicating specificity of the interaction to SICF. Thus, it appears likely that the afferent input that influences SICF projects to circuits mediating I-waves rather than directly to corticospinal neurons. Indeed the present findings would support recent models which have hypothesised the existence of direct sensory afferent excitatory inputs to I-wave circuitry (Di Lazzaro & Ziemann, 2013; Weise et al. 2013).
Previous studies have suggested that the pharmac-ological profile of MEP inhibition mediated by SAI is most compatible with that of basket cells which, like SAI, are sensitive to drugs acting on GABAA-α1 subtype and acetylcholine receptors. These synapse at the perisomatic region of pyramidal cells and thus have a privileged position in blocking the propagation of dendritic spikes and shunting cell firing (Di Lazzaro et al. 2006, 2007; Spruston, 2008; Teo et al. 2009; Zhang et al. 2013). Indeed there is evidence from human (Albuquerque et al. 2000; Alkondon et al. 2000) and rodent cortex (Kimura, 2000; Alkondon & Albuquerque, 2001) of cholinergically modulated GABAergic input at the soma including circuitry models that would be compatible with the interactions reported here between SAI and SICF and previously between SAI and SICI (Alle et al. 2009). Because the ultimate effect of SAI is reduced rather than increased MEP, this would indeed be compatible with the notion that SAI reduces MEP amplitude by inhibitory shunting of pyramidal output close to the soma, thus masking the facilitatory and disinhibitory effects on interneuronal inputs from more superficial layers. One proposed function of shunting at the soma is to allow a brief time window for the cooperative integration of multiple synaptic inputs at the pyramidal neuron (Isaacson & Scanziani, 2011).
Rodent studies indicate that sensorimotor projections terminate in layers 2/3 where late I-wave inputs are thought to originate (Amassian et al. 1987) as well as layers 5/6 where corticospinal pyramidal cells are located (Ferezou et al. 2007; Aronoff et al. 2010). These data are compatible with the present findings, whereby an excitatory sensorimotor input to layers 2/3 could evoke facilitation of I-waves while facilitation of inhibitory input to layer 5 could inhibit firing of pyramidal neurons. Layer 2/3 neurons are known to play a critical role in motor skill learning (Pavlides et al. 1993; Rioult-Pedotti et al. 1998, 2000). It is conceivable that facilitation of excitatory I-wave circuitry also plays a role in a sensorimotor plasticity protocol known as paired associative stimulation (PAS), and could provide the necessary excitatory input for spike timing -dependent plasticity. However, the precise relationships between SAI, SICF and PAS need to be examined in future studies.
In Parkinson's disease patients, dopaminergic medications lead to a reduction in SAI (Sailer et al. 2003; Wagle Shukla et al. 2013) that is accompanied by a reduction of excessive SICF (Ni et al. 2013). In light of the association between SAI and SICF presented here, future studies might explore whether the direct influence of sensory input on specific local motor cortical circuits is abnormal in movement disorders and whether this is normalised by treatment in line with the notion that sensory deficiencies contribute to motor deficits (Moore, 1987; O'Suilleabhain et al. 2001).
Limitations
TS intensity was increased in the TS adjusted condition to compensate for the reduction in I-waves by the inhibitory conditioning MNS (Tokimura et al. 2000), such that these are at least partially available to be recruited by CS (Thickbroom, 2010). Two recent interaction studies in which descending I-wave volleys were recorded directly via epidural recordings demonstrated that I-waves can at least be partially restored by adjusting TS intensity (Ni et al. 2011b; Weise et al. 2013). In the TS unadjusted and adjusted conditions, SICF was facilitated in the presence of SAI. Another possibility is that increasing intensity might favour the recruitment of higher threshold CSNs. In that case, as SAI, SICF and their interaction decreased in strength with increasing TS intensity (Fig. 3), the adjusted TS data might understate the strength of the interaction.
We cannot completely exclude a spinal contribution here. However SICF and SAI are both considered to be cortical phenomena (Tokimura et al. 1996, 2000; Di Lazzaro et al. 1999), and the timing of TMS was targeted to the arrival of afferent stimuli at the cortex rather than spinal cord. In addition we accounted for changes in corticospinal excitability in the adjusted TS condition in line with previous interaction studies (Muller-Dahlhaus et al. 2008; Alle et al. 2009).
Conclusions
The presents study indicates that SICF is facilitated in the presence of SAI, the effect is specific to SICF and occurs in a dose-dependent manner. The influence of sensory input on excitatory motor cortical circuitry appears to be similar across somatotopic regions, intracortical circuitries (circuits activated by PA and AP induced current) and functional states suggesting that this interaction may have general applicability in sensorimotor integration and motor control. The correlation between SAI and SICF at baseline, which was maintained during the interaction, suggests an intrinsic relationship between SAI and SICF in sensorimotor integration. The multiple inhibitory, disinhibitory and facilitatory effects of afferent stimulation on M1 circuitry may add to the flexibility of cortical circuits and appear to be compatible with animal models of cellular circuitry.
Acknowledgments
We thank Gary Thickbroom for helpful discussions during the planning of this study.
Glossary
- AP
anterior to posterior induced current direction
- APB
abductor pollicis brevis
- CS(*)
conditioning stimulus (adjusted)
- FDI
first dorsal interosseous
- ISI
inter-stimulus interval
- LICI
long interval intracortical inhibition
- LMM
linear mixed model
- MEP
motor-evoked potential
- MNS
median nerve stimulation
- PA
posterior to anterior induced current direction
- RMT
resting motor threshold
- SAI
short latency afferent inhibition
- SICI
short-interval intracortical inhibition
- SICF
short-interval intracortical facilitation
- SSEP
somatosensory evoked potentials
- ST
sensory threshold
- T1
trough 1
- TMS
transcranial magnetic stimulation
- TS(*)
test stimulus (adjusted)
Additional information
Competing interests
All authors declare that they do not have any conflicts of interest.
Author contributions
R.F.H.C., Z.N. and R.C. designed the study; R.F.H.C., R.I. and C.A.G. collected research data; R.F.H.C., Z.N. and R.C. analysed data; R.F.H.C. and R.C. wrote the manuscript; all authors revised and approved the manuscript.
Funding
This work was supported by Canadian Institutes of Health Rese-arch (grant number MOP62917).
References
- Abbruzzese G. Berardelli A. Sensorimotor integration in movement disorders. Mov Disord. 2003;18:231–240. doi: 10.1002/mds.10327. [DOI] [PubMed] [Google Scholar]
- Abbruzzese G, Marchese R, Buccolieri A, Gasparetto B. Trompetto C. Abnormalities of sensorimotor integration in focal dystonia: a transcranial magnetic stimulation study. Brain. 2001;124:537–545. doi: 10.1093/brain/124.3.537. [DOI] [PubMed] [Google Scholar]
- Albuquerque EX, Pereira EF, Mike A, Eisenberg HM, Maelicke A. Alkondon M. Neuronal nicotinic receptors in synaptic functions in humans and rats: physiological and clinical relevance. Behav Brain Res. 2000;113:131–141. doi: 10.1016/s0166-4328(00)00208-4. [DOI] [PubMed] [Google Scholar]
- Alkondon M. Albuquerque EX. Nicotinic acetylcholine receptor α7 and α4β2 subtypes differentially control GABAergic input to CA1 neurons in rat hippocampus. J Neurophysiol. 2001;86:3043–3055. doi: 10.1152/jn.2001.86.6.3043. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Eisenberg HM. Albuquerque EX. Nicotinic receptor activation in human cerebral cortical interneurons: a mechanism for inhibition and disinhibition of neuronal networks. J Neurosci. 2000;20:66–75. doi: 10.1523/JNEUROSCI.20-01-00066.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alle H, Heidegger T, Krivanekova L. Ziemann U. Interactions between short-interval intracortical inhibition and short-latency afferent inhibition in human motor cortex. J Physiol. 2009;587:5163–5176. doi: 10.1113/jphysiol.2009.179820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amassian VE, Stewart M, Quirk GJ. Rosenthal JL. Physiological basis of motor effects of a transient stimulus to cerebral cortex. Neurosurgery. 1987;20:74–93. [PubMed] [Google Scholar]
- Aronoff R, Matyas F, Mateo C, Ciron C, Schneider B. Petersen CC. Long-range connectivity of mouse primary somatosensory barrel cortex. Eur J Neurosci. 2010;31:2221–2233. doi: 10.1111/j.1460-9568.2010.07264.x. [DOI] [PubMed] [Google Scholar]
- Battaglia F, Wang HY, Ghilardi MF, Gashi E, Quartarone A, Friedman E. Nixon RA. Cortical plasticity in Alzheimer's disease in humans and rodents. Biol Psychiatry. 2007;62:1405–1412. doi: 10.1016/j.biopsych.2007.02.027. [DOI] [PubMed] [Google Scholar]
- Brochier T, Boudreau MJ, Pare M. Smith AM. The effects of muscimol inactivation of small regions of motor and somatosensory cortex on independent finger movements and force control in the precision grip. Exp Brain Res. 1999;128:31–40. doi: 10.1007/s002210050814. [DOI] [PubMed] [Google Scholar]
- Cash RF, Murakami T, Chen R, Thickbroom GW. Ziemann U. Augmenting plasticity induction in human motor cortex by disinhibition stimulation. Cereb Cortex. 2014 doi: 10.1093/cercor/bhu176. DOI: 10.1093/cercor/bhu176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash RF, Ziemann U, Murray K. Thickbroom GW. Late cortical disinhibition in human motor cortex: a triple-pulse transcranial magnetic stimulation study. J Neurophysiol. 2010;103:511–518. doi: 10.1152/jn.00782.2009. [DOI] [PubMed] [Google Scholar]
- Chen R. Garg R. Facilitatory I wave interaction in proximal arm and lower limb muscle representations of the human motor cortex. J Neurophysiol. 2000;83:1426–1434. doi: 10.1152/jn.2000.83.3.1426. [DOI] [PubMed] [Google Scholar]
- Chu J, Gunraj C. Chen R. Possible differences between the time courses of presynaptic and postsynaptic GABAB mediated inhibition in the human motor cortex. Exp Brain Res. 2008;184:571–577. doi: 10.1007/s00221-007-1125-7. [DOI] [PubMed] [Google Scholar]
- Chu J, Wagle-Shukla A, Gunraj C, Lang AE. Chen R. Impaired presynaptic inhibition in the motor cortex in Parkinson disease. Neurology. 2009;72:842–849. doi: 10.1212/01.wnl.0000343881.27524.e8. [DOI] [PubMed] [Google Scholar]
- Classen J, Steinfelder B, Liepert J, Stefan K, Celnik P, Cohen LG, Hess A, Kunesch E, Chen R, Benecke R. Hallett M. Cutaneomotor integration in humans is somatotopically organized at various levels of the nervous system and is task dependent. Exp Brain Res. 2000;130:48–59. doi: 10.1007/s002210050005. [DOI] [PubMed] [Google Scholar]
- Delvendahl I, Lindemann H, Jung NH, Pechmann A, Siebner HR. Mall V. Influence of waveform and current direction on short-interval intracortical facilitation: a paired-pulse TMS study. Brain Stimul. 2014;7:49–58. doi: 10.1016/j.brs.2013.08.002. [DOI] [PubMed] [Google Scholar]
- Delwaide PJ. Olivier E. Conditioning transcranial cortical stimulation (TCCS) by exteroceptive stimulation in parkinsonian patients. Adv Neurol. 1990;53:175–181. [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Marra C, Ghirlanda S, Ranieri F, Gainotti G. Tonali P. Neurophysiological predictors of long term response to AChE inhibitors in AD patients. J Neurol Neurosurg Psychiatry. 2005;76:1064–1069. doi: 10.1136/jnnp.2004.051334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Profice P, Pennisi MA, Di Giovanni S, Zito G, Tonali P. Rothwell JC. Muscarinic receptor blockade has differential effects on the excitability of intracortical circuits in the human motor cortex. Exp Brain Res. 2000;135:455–461. doi: 10.1007/s002210000543. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Tonali PA, Marra C, Daniele A, Profice P, Saturno E, Pilato F, Masullo C. Rothwell JC. Noninvasive in vivo assessment of cholinergic cortical circuits in AD using transcranial magnetic stimulation. Neurology. 2002;59:392–397. doi: 10.1212/wnl.59.3.392. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Dileone M, Profice P, Ranieri F, Ricci V, Bria P, Tonali PA. Ziemann U. Segregating two inhibitory circuits in human motor cortex at the level of GABAA receptor subtypes: a TMS study. Clin Neurophysiol. 2007;118:2207–2214. doi: 10.1016/j.clinph.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Dileone M, Ranieri F, Ricci V, Profice P, Bria P, Tonali PA. Ziemann U. GABAA receptor subtype specific enhancement of inhibition in human motor cortex. J Physiol. 2006;575:721–726. doi: 10.1113/jphysiol.2006.114694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Dileone M, Tonali PA. Ziemann U. Dissociated effects of diazepam and lorazepam on short-latency afferent inhibition. J Physiol. 2005;569:315–323. doi: 10.1113/jphysiol.2005.092155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Rothwell JC, Oliviero A, Profice P, Insola A, Mazzone P. Tonali P. Intracortical origin of the short latency facilitation produced by pairs of threshold magnetic stimuli applied to human motor cortex. Exp Brain Res. 1999;129:494–499. doi: 10.1007/s002210050919. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V. Ziemann U. The contribution of transcranial magnetic stimulation in the functional evaluation of microcircuits in human motor cortex. Front Neural Circuits. 2013;7:18. doi: 10.3389/fncir.2013.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferezou I, Haiss F, Gentet LJ, Aronoff R, Weber B. Petersen CC. Spatiotemporal dynamics of cortical sensor-imotor integration in behaving mice. Neuron. 2007;56:907–923. doi: 10.1016/j.neuron.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Fischer M. Orth M. Short-latency sensory afferent inhibition: conditioning stimulus intensity, recording site, and effects of 1 Hz repetitive TMS. Brain Stimul. 2011;4:202–209. doi: 10.1016/j.brs.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Fujiki M, Hikawa T, Abe T, Ishii K. Kobayashi H. Reduced short latency afferent inhibition in diffuse axonal injury patients with memory impairment. Neurosci Lett. 2006;405:226–230. doi: 10.1016/j.neulet.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Groppa S, Oliviero A, Eisen A, Quartarone A, Cohen LG, Mall V, Kaelin-Lang A, Mima T, Rossi S, Thickbroom GW, et al. A practical guide to diagnostic transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol. 2012;123:858–882. doi: 10.1016/j.clinph.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Terao Y, Enomoto H, Shiio Y, Mochizuki H, Furubayashi T, Uesugi H, Iwata NK. Kanazawa I. Mechanisms of intracortical I-wave facilitation elicited with paired-pulse magnetic stimulation in humans. J Physiol. 2002;538:253–261. doi: 10.1113/jphysiol.2001.013094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess A, Kunesch E, Classen J, Hoeppner J, Stefan K. Benecke R. Task-dependent modulation of inhibitory actions within the primary motor cortex. Exp Brain Res. 1999;124:321–330. doi: 10.1007/s002210050629. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Tanaka M, Sakamoto M. Iwamura Y. Deficits in manipulative behaviors induced by local injections of muscimol in the first somatosensory cortex of the conscious monkey. Brain Res. 1985;325:375–380. doi: 10.1016/0006-8993(85)90344-0. [DOI] [PubMed] [Google Scholar]
- Ilic TV, Meintzschel F, Cleff U, Ruge D, Kessler KR. Ziemann U. Short-interval paired-pulse inhibition and facilitation of human motor cortex: the dimension of stimulus intensity. J Physiol. 2002;545:153–167. doi: 10.1113/jphysiol.2002.030122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson JS. Scanziani M. How inhibition shapes cortical activity. Neuron. 2011;72:231–243. doi: 10.1016/j.neuron.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs MF, Tsang P, Lee KG, Asmussen MJ, Zapallow CM. Nelson AJ. 30 Hz theta-burst stimulation over primary somatosensory cortex modulates corticospinal output to the hand. Brain Stimul. 2014;7:269–274. doi: 10.1016/j.brs.2013.12.009. [DOI] [PubMed] [Google Scholar]
- Kessler KR, Ruge D, Ilic TV. Ziemann U. Short latency afferent inhibition and facilitation in patients with writer's cramp. Mov Disord. 2005;20:238–242. doi: 10.1002/mds.20295. [DOI] [PubMed] [Google Scholar]
- Kimura F. Cholinergic modulation of cortical function: a hypothetical role in shifting the dynamics in cortical network. Neurosci Res. 2000;38:19–26. doi: 10.1016/s0168-0102(00)00151-6. [DOI] [PubMed] [Google Scholar]
- Li JY, Espay AJ, Gunraj CA, Pal PK, Cunic DI, Lang AE. Chen R. Interhemispheric and ipsilateral connections in Parkinson's disease: relation to mirror movements. Mov Disord. 2007;22:813–821. doi: 10.1002/mds.21386. [DOI] [PubMed] [Google Scholar]
- Mariorenzi R, Zarola F, Caramia MD, Paradiso C. Rossini PM. Non-invasive evaluation of central motor tract excitability changes following peripheral nerve stimulation in healthy humans. Electroencephalogr Clin Neurophysiol. 1991;81:90–101. doi: 10.1016/0168-5597(91)90002-f. [DOI] [PubMed] [Google Scholar]
- Moore AP. Impaired sensorimotor integration in parkinsonism and dyskinesia: a role for corollary discharges? J Neurol Neurosurg Psychiatry. 1987;50:544–552. doi: 10.1136/jnnp.50.5.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Dahlhaus JFM, Liu Y. Ziemann U. Inhibitory circuits and the nature of their interactions in the human motor cortex a pharmacological TMS study. J Physiol. 2008;586:495–514. doi: 10.1113/jphysiol.2007.142059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Z, Bahl N, Gunraj CA, Mazzella F. Chen R. Increased motor cortical facilitation and decreased inhibition in Parkinson disease. Neurology. 2013;80:1746–1753. doi: 10.1212/WNL.0b013e3182919029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Z, Charab S, Gunraj C, Nelson AJ, Udupa K, Yeh I-J. Chen R. Transcranial magnetic stimulation in different current directions activates separate cortical circuits. J Neurophysiol. 2011;105:749–756. doi: 10.1152/jn.00640.2010. [DOI] [PubMed] [Google Scholar]
- Ni Z, Gunraj C, Wagle-Shukla A, Udupa K, Mazzella F, Lozano AM. Chen R. Direct demonstration of inhibitory interactions between long interval intracortical inhibition and short interval intracortical inhibition. J Physiol. 2011;589:2955–2962. doi: 10.1113/jphysiol.2011.207928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Z, Muller-Dahlhaus F, Chen R. Ziemann U. Triple-pulse TMS to study interactions between neural circuits in human cortex. Brain Stimul. 2011;4:281–293. doi: 10.1016/j.brs.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Oldfield R. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Ortu E, Deriu F, Suppa A, Tolu E. Rothwell JC. Effects of volitional contraction on intracortical inhibition and facilitation in the human motor cortex. J Physiol. 2008;586:5147–5159. doi: 10.1113/jphysiol.2008.158956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Suilleabhain P, Bullard J. Dewey RB. Proprioception in Parkinson's disease is acutely depressed by dopaminergic medications. J Neurol Neurosurg Psychiatry. 2001;71:607–610. doi: 10.1136/jnnp.71.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton HD. Amassian VE. Single and multiple-unit analysis of cortical stage of pyramidal tract activation. J Neurophysiol. 1954;17:345–363. doi: 10.1152/jn.1954.17.4.345. [DOI] [PubMed] [Google Scholar]
- Pavlides C, Miyashita E. Asanuma H. Projection from the sensory to the motor cortex is important in learning motor skills in the monkey. J Neurophysiol. 1993;70:733–741. doi: 10.1152/jn.1993.70.2.733. [DOI] [PubMed] [Google Scholar]
- Peurala SH, Muller-Dahlhaus JF, Arai N. Ziemann U. Interference of short-interval intracortical inhibition (SICI) and short-interval intracortical facilitation (SICF) Clin Neurophysiol. 2008;119:2291–2297. doi: 10.1016/j.clinph.2008.05.031. [DOI] [PubMed] [Google Scholar]
- Player MJ, Taylor JL, Weickert CS, Alonzo A, Sachdev P, Martin D, Mitchell PB. Loo CK. Neuroplasticity in depressed individuals compared with healthy controls. Neuropsychopharmacology. 2013;38:2101–2108. doi: 10.1038/npp.2013.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quartarone A, Morgante F, Sant'angelo A, Rizzo V, Bagnato S, Terranova C, Siebner HR, Berardelli A. Girlanda P. Abnormal plasticity of sensorimotor circuits extends beyond the affected body part in focal dystonia. J Neurol Neurosurg Psychiatry. 2008;79:985–990. doi: 10.1136/jnnp.2007.121632. [DOI] [PubMed] [Google Scholar]
- Quartarone A, Rizzo V, Terranova C, Morgante F, Schneider S, Ibrahim N, Girlanda P, Bhatia KP. Rothwell JC. Abnormal sensorimotor plasticity in organic but not in psychogenic dystonia. Brain. 2009;132:2871–2877. doi: 10.1093/brain/awp213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rioult-Pedotti MS, Friedman D. Donoghue JP. Learning-induced LTP in neocortex. Science. 2000;290:533–536. doi: 10.1126/science.290.5491.533. [DOI] [PubMed] [Google Scholar]
- Rioult-Pedotti MS, Friedman D, Hess G. Donoghue JP. Strengthening of horizontal cortical connections following skill learning. Nat Neurosci. 1998;1:230–234. doi: 10.1038/678. [DOI] [PubMed] [Google Scholar]
- Rocco-Donovan M, Ramos RL, Giraldo S. Brumberg JC. Characteristics of synaptic connections between rodent primary somatosensory and motor cortices. Somatosens Mot Res. 2011;28:63–72. doi: 10.3109/08990220.2011.606660. [DOI] [PubMed] [Google Scholar]
- Sailer A, Cunic DI, Paradiso GO, Gunraj CA, Wagle-Shukla A, Moro E, Lozano AM, Lang AE. Chen R. Subthalamic nucleus stimulation modulates afferent inhibition in Parkinson disease. Neurology. 2007;68:356–363. doi: 10.1212/01.wnl.0000252812.95774.aa. [DOI] [PubMed] [Google Scholar]
- Sailer A, Molnar GF, Cunic DI. Chen R. Effects of peripheral sensory input on cortical inhibition in humans. J Physiol. 2002;544:617–629. doi: 10.1113/jphysiol.2002.028670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailer A, Molnar GF, Paradiso G, Gunraj CA, Lang AE. Chen R. Short and long latency afferent inhibition in Parkinson's disease. Brain. 2003;126:1883–1894. doi: 10.1093/brain/awg183. [DOI] [PubMed] [Google Scholar]
- Sanger TD, Garg RR. Chen R. Interactions between two different inhibitory systems in the human motor cortex. J Physiol. 2001;530:307–317. doi: 10.1111/j.1469-7793.2001.0307l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbert BI, Heaton A, Dunne JW, Lawn ND, Silbert PL, Mastaglia FL. Thickbroom GW. Long-interval cortical facilitation is reduced in idiopathic generalised epilepsy. 2011. Brisbane Abstract book of the Epilepsy Society of Australia Annual Scientific Meeting.
- Spruston N. Pyramidal neurons: dendritic structure and synaptic integration. Nat Rev Neurosci. 2008;9:206–221. doi: 10.1038/nrn2286. [DOI] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Benecke R, Cohen LG. Classen J. Mechanisms of enhancement of human motor cortex excitability induced by interventional paired associative stimulation. J Physiol. 2002;543:699–708. doi: 10.1113/jphysiol.2002.023317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo JT, Terranova C, Swayne O, Greenwood RJ. Rothwell JC. Differing effects of intracortical circuits on plasticity. Exp Brain Res. 2009;193:555–563. doi: 10.1007/s00221-008-1658-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tergau F, Wanschura V, Canelo M, Wischer S, Wassermann EM, Ziemann U. Paulus W. Complete suppression of voluntary motor drive during the silent period after transcranial magnetic stimulation. Exp Brain Res. 1999;124:447–454. doi: 10.1007/s002210050640. [DOI] [PubMed] [Google Scholar]
- Thickbroom GW. A model of the contribution of late I-waves to α-motoneuronal activation: implications for paired-pulse TMS. Brain Stim. 2010;4:77–83. doi: 10.1016/j.brs.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Tokimura H, Di Lazzaro V, Tokimura Y, Oliviero A, Profice P, Insola A, Mazzone P, Tonali P. Rothwell JC. Short latency inhibition of human hand motor cortex by somatosensory input from the hand. J Physiol. 2000;523:503–513. doi: 10.1111/j.1469-7793.2000.t01-1-00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokimura H, Ridding MC, Tokimura Y, Amassian VE. Rothwell JC. Short latency facilitation between pairs of threshold magnetic stimuli applied to human motor cortex. Electroencephalogr Clin Neurophysiol. 1996;101:263–272. doi: 10.1016/0924-980x(96)95664-7. [DOI] [PubMed] [Google Scholar]
- Udupa K, Ni Z, Gunraj C. Chen R. Interactions between short latency afferent inhibition and long interval intracortical inhibition. Exp Brain Res. 2009;199:177–183. doi: 10.1007/s00221-009-1997-9. [DOI] [PubMed] [Google Scholar]
- Udupa K, Ni Z, Gunraj C. Chen R. Effects of short-latency afferent inhibition on short-interval intracortical inhibition. J Neurophysiol. 2014;111:1350–1361. doi: 10.1152/jn.00613.2013. [DOI] [PubMed] [Google Scholar]
- Vahabzadeh-Hagh A. Paired-pulse transcranial magnetic stimulation (TMS) protocols. In: Pascual-Leone A, editor; Rotenberg A, Horvath JC, editors. Transcranial Magnetic Stimulation. New York: Springer; 2014. pp. 117–127. [Google Scholar]
- Wagle Shukla A, Moro E, Gunraj C, Lozano A, Hodaie M, Lang A. Chen R. Long-term subthalamic nucleus stimulation improves sensorimotor integration and proprioception. J Neurol Neurosurg Psychiatry. 2013;84:1020–1028. doi: 10.1136/jnnp-2012-304102. [DOI] [PubMed] [Google Scholar]
- Wagle-Shukla A, Ni Z, Gunraj CA, Bahl N. Chen R. Effects of short interval intracortical inhibition and intracortical facilitation on short interval intracortical facilitation in human primary motor cortex. J Physiol. 2009;587:5665–5678. doi: 10.1113/jphysiol.2009.181446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weise D, Mann J, Ridding M, Eskandar K, Huss M, Rumpf JJ, Di Lazzaro V, Mazzone P, Ranieri F. Classen J. Microcircuit mechanisms involved in paired associative stimulation-induced depression of corticospinal excitability. J Physiol. 2013;591:4903–4920. doi: 10.1113/jphysiol.2013.253989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Li Y, Rasch MJ. Wu S. Nonlinear multiplicative dendritic integration in neuron and network models. Front Comput Neurosci. 2013;7:56. doi: 10.3389/fncom.2013.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U. Rothwell JC. I-waves in motor cortex. J Clin Neurophysiol. 2000;17:397–405. doi: 10.1097/00004691-200007000-00005. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Tergau F, Wassermann EM, Wischer S, Hildebrandt J. Paulus W. Demonstration of facilitatory I wave interaction in the human motor cortex by paired transcranial magnetic stimulation. J Physiol. 1998;511:181–190. doi: 10.1111/j.1469-7793.1998.181bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


