Abstract
Key points
The nucleus tractus solitarii (NTS) integrates visceral afferent information essential for cardiovascular haemostasis.
Using fast-cyclic voltammetry in anaesthetized rats, 5-HT (serotonin) release was detected in NTS in response to activation of these afferents.
Removal of 5-HT from the extracellular space is usually regulated by the low-capacity, high-affinity 5-HT transporter (5-HTT/SERT).
The present data demonstrate that 5-HT removal in the NTS is regulated by the plasma membrane monoamine transporter (PMAT), a high-capacity, low-affinity transporter.
The present data also demonstrate that the 5-HT released by afferent activation comes from at least two different sources. It is suggested that one of these sources is the afferents themselves.
These results demonstrate a physiological role for the low-affinity uptake transporter in the regulation of 5-HT concentration in NTS.
Abstract
The nucleus tractus solitarii (NTS) integrates inputs from cardiovascular afferents and thus is crucial for cardiovascular homeostasis. These afferents primarily release glutamate, although 5-HT has also been shown to play a role in their actions. Using fast-cyclic voltammetry, an increase in 5-HT concentrations (range 12–50 nm) could be detected in the NTS in anaesthetized rats in response to electrical stimulation of the vagus and activation of cardiopulmonary, chemo- and baroreceptor reflexes. This 5-HT signal was not potentiated by the serotonin transporter (SERT) or the noradrenaline transporter (NET) inhibitors citalopram and desipramine (1 mg kg−1). However, decynium-22 (600 μg kg−1), an organic cation 3 transporter (OCT3)/plasma membrane monoamine transporter (PMAT) inhibitor, increased the 5-HT signal by 111 ± 21% from 29 ± 10 nm. The effectiveness of these inhibitors was tested against the removal time of 5-HT and noradrenaline applied by microinjection to the NTS. Citalopram and decynium-22 attenuated the removal of 5-HT but not noradrenaline, whereas desipramine had the reverse action. The OCT3 inhibitor corticosterone (10 mg kg−1) had no effect. Blockade of glutamate receptors with topical kynurenate (10–50 nm) reduced the vagally evoked 5-HT signal by 50%, indicating that this release was from at least two sources. It is concluded that vagally evoked 5-HT release is under the regulation of the high-capacity, low-affinity transporter PMAT, not the low-capacity, high-affinity transporter SERT. This is the first demonstration that PMAT may be playing a physiological role in the regulation of 5-HT transmission and this could indicate that 5-HT is acting, in part, as a volume transmitter within the NTS.
Introduction
The nucleus tractus solitarii (NTS), located near the dorsal surface of the brainstem, receives sensory information from arterial baroreceptors and chemoreceptors and other receptors in the cardiopulmonary region (see Andresen & Kunze, 1994). Such sensory information is crucial for cardiovascular homeostasis. 5-Hydroxytryptamine (5-HT; serotonin) is one of the many transmitters that have been identified to play an important role in this regulation (see Ramage & Villalón, 2008), as might be expected given the rich innervation of the NTS by 5-HT (Steinbusch, 2002), some of which originates centrally (Schaffar et al. 1988; Sim & Joseph, 1992) and some from vagal (Sykes et al. 1981; Gaudin-Chazal et al. 1982; Nosjean et al. 1990; Thor et al. 1992) and petrosal afferents (Thor et al. 1992). The NTS is known to contain many 5-HT receptor subtypes, namely 5-HT1A and 5-HT1B (Thor et al. 1988; Manaker & Verderame, 1990), 5-HT2A and 5-HT2C (Pompeiano et al. 1994; Wright et al. 1995; Cornea-Hébert et al. 1999), 5-HT3 (Leslie et al. 1990), 5-HT5A (Oliver et al. 2000) and 5-HT7 (To et al. 1995; Gustafson et al. 1996). However, only 5-HT3 (Jeggo et al. 2005) and 5-HT7 receptors (Oskutyte et al. 2009) have so far been shown to play a physiological role in these reflex pathways. In contrast, 5-HT2A receptors have been implicated in baroreflex regulation by higher centres (Sévoz-Couche et al. 2006).
Overall, these data indicate that activation of cardiovascular afferent input should cause the release of 5-HT in the NTS. This release could come from vagal and glossopharyngeal afferents and/or from the centrally originating 5-HT-containing nerve terminals. In this respect, it was recently reported, using the technique of fast-cyclic voltammetry (see Millar & Pelling, 2001), that 5-HT could be detected in the NTS in response to electrical stimulation of the vagus (Millar et al. 2009). The present experiments were carried out firstly to refine and characterize these observations and to determine whether physiological activation of various cardiovascular reflexes would also cause 5-HT release in NTS, and secondly, to establish whether this release could be modified by blockade of the 5-HT high-affinity, low-capacity reuptake transporter, known as the serotonin transporter (SERT), with citalopram (see Hyttel, 1977) and/or the low-affinity, high-capacity transporters, the organic cation 3 transporter (OCT3) and plasma membrane monoamine transporter (PMAT), with decynium-22 (D-22; Hayer-Zillgen et al. 2002). Preliminary communication of some of these data has been given previously (Hosford et al. 2011, 2012).
Methods
General preparation
All the experiments were carried out under the Animals (Scientific Procedures) Act 1986. At the end of each experiment, all animals were humanely killed by an overdose of sodium pentobarbital (30 mg kg−1, i.v.).
Experiments were performed on 147 male Sprague–Dawley rats (250–350 g). Anaesthesia was induced by isoflurane (2.5% in oxygen) and maintained with α-chloralose (100 mg kg−1, i.v.). Supplementary doses of α-chloralose (10–20 mg kg−1, i.v.) were given as required. The depth of anaesthesia was assessed by the stability of cardiovascular and respiratory variables being recorded. The right femoral artery was cannulated for the measurement of blood pressure and for sampling arterial blood for analysis of pH and blood gases. Blood pressure was measured using a pressure transducer (Gould Statham P23XL), and heart rate was derived electronically from the blood pressure signal. The right jugular vein was cannulated for drug administration. In some experiments, in which the cardiopulmonary reflex was activated with phenylbiguanide (PBG), this cannula was inserted so that the tip lay within or close to the right atrium. Between drug administrations, an infusion (6 ml kg−1 h−1) of a solution consisting of 10 ml distilled water, 10 ml plasma substitute (Gelofusine), 168 mg sodium bicarbonate and 36 mg glucose was given. This helped to maintain blood volume and prevent metabolic acidosis. The trachea was cannulated and the animals were artificially ventilated (rate of 60 strokes min−1; stroke volume of the pump was set at 8 ml kg−1) with oxygen-enriched room air by the use of a positive pressure pump (Harvard Rodent Ventilator 683) and subsequent induction of neuromuscular block by a single dose of α-bungarotoxin (140 μg kg−1, i.v.). During neuromuscular block, the depth of anaesthesia was assessed by monitoring the stability of the arterial blood pressure and heart rate and the cardiovascular responses to pinching of the paw. Additional anaesthetic agent was administered if required. At regular intervals, arterial blood samples were collected in heparinized capillary tubes and analysed using a pH/blood gas analyser (Siemens Rapidlab® 248). Arterial blood gases were maintained at 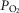 90–120 mmHg,
90–120 mmHg, 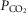 40–50 mmHg and pH at 7.3–7.4, by adjusting the rate and/or stroke volume of the ventilator. Body temperature was monitored via a rectal probe and maintained at 37–38°C with a Homeothermic Blanket Control Unit (Harvard).
40–50 mmHg and pH at 7.3–7.4, by adjusting the rate and/or stroke volume of the ventilator. Body temperature was monitored via a rectal probe and maintained at 37–38°C with a Homeothermic Blanket Control Unit (Harvard).
The rats were placed in a stereotaxic frame, and in vagal stimulation experiments the left cervical vagus nerve was dissected free from the sympathetic trunk using a dorsolateral approach low in the neck. The vagus was placed on bipolar silver wire electrodes for electrical stimulation (50–500 μA, 1 ms, 1–50 Hz), using a constant-current isolated stimulator (Digitimer DS3) triggered by the 1401 plus. Distal to this stimulating site, the vagus was crushed and tied. The exposed length of nerve was embedded in dental impression material (Super-Dent light body dental polyvinylsiloxane; Carlisle Laboratories). In all experiments, the dorsal surface of the caudal brainstem was exposed by removing the nuchal muscles from the back of the neck and the occipital bone. The dura overlying the brainstem was cut and reflected laterally.
Recording
Two types of electrodes for voltammetric recording were constructed in the laboratory using the methods described by Millar & Pelling (2001). One was constructed by bonding two glass capillaries filled with a carbon fibre (7 μm in diameter) separated by two further empty glass capillaries (four barrels). The tips of the carbon fibres were ∼10 μm apart and protruding 50–100 μm from the ends of the glass. These were used to measure endogenous release of 5-HT, with one of carbon fibres used as a working electrode and the other recording the local tissue voltage. The second was a composite electrode consisting of a single-barrel carbon-fibre electrode glued to a three-barrel micropipette. There was 100–150 μm separation between the tip of the carbon fibre and the three-barrel micropipette. This arrangement was used to measure exogenously applied monoamines.
The carbon-fibre electrodes were connected to the head stage of a Millar Voltammeter (PD Systems, East Molesey, UK) along with an Ag–AgCl2 reference electrode, which was placed on the surface of the brainstem. The working electrode was placed 0.8–1 mm lateral to the mid-line and within 1–2 mm caudal to the obex, an area known to contain cardiopulmonary afferent input (Hines et al. 1994). The electrode was switched to record extracellular spike activity and advanced through the gracile nucleus until evoked activity from stroking the hindlimb was no longer detected. Electrical stimulation of the vagus was then commenced (1 Hz, 100 μA, 1 ms) and the electrode advanced until an evoked vagal afferent volley was maximal at the C fibre latency. This occurred at a depth of 400–600 μm. The threshold for evoked activity was determined and the current adjusted to give a response 10 times threshold because this had been determined to give the most stable levels of release. The carbon-fibre electrode was then switched to voltammetry.
Voltammetric scans were triggered at a rate of 2 Hz and the electrode left in place for at least 10 min for the background current to stabilize before beginning the experiment. At the end of each experiment, Pontamine Sky Blue dye (1% in saline) was ejected from one barrel of the electrode and/or a DC current of +500 nA was applied to the carbon-fibre microelectrode in situ. The brainstem was then removed and fixed in 10% formal saline. Frozen sections (50 μm) were cut and the location of the recording sites visualized and mapped onto standard sections of a rat brainstem (Paxinos & Watson, 1998).
Fast-cyclic voltammetry detection of 5-HT and noradrenaline
For in vivo detection of 5-HT, differential scan fast-cyclic voltammetry was used. In this technique (Millar & Williams, 1990), two separate ramps (Fig. 1A) are applied in rapid succession to the carbon-fibre electrode. The first ramp is the ‘active’ scan as it ramps from 0 to 500 mV and then back to −200 mV. Using this waveform, 5-HT generates an oxidation current peak starting at ∼350 mV and a re-reduction peak at ∼100 mV. The second ramp follows the first after 20 ms. This ramp is identical in shape to the first but is offset negatively by 400 mV. No 5-HT oxidation (or re-reduction) will occur on this second ramp. During these scans, the total current through the electrode has two components, the voltammetric oxidation/reduction peaks plus a background current. The background current is the current that flows simply as a result of the electrode impedance in the solution. This background current depends mainly on the rate of change in voltage (dV/dt) (Fox et al. 1980), so it is similar in both ramps. The voltammeter electronically superimposes and subtracts the currents during the two ramps (Fig. 1A). This process removes most of the background current from the signal, leaving the oxidation and reduction signals intact. Variations in background current due to changes in tissue impedance are one of the main sources of noise in in vivo voltammetry, so this process reduces noise and improves the signal-to-noise ratio of the recording. During the experiments, a background-subtracted signal from a pair of scans was obtained in the tissue immediately before each experimental test, such as vagal stimulation. This formed a reference signal. During and after the stimulation, this reference signal was automatically subtracted from the ongoing signal to form the final voltammetric signal. In the present experiments, the technique was modified slightly by using trapezoidal (‘flat-top’) rather than triangular oxidizing voltage ramps (Fig. 1Aa–c). Use of these ramps was found to reduce the background current significantly and thus again improve the signal-to-noise ratio of the voltammetric signal.
Figure 1.
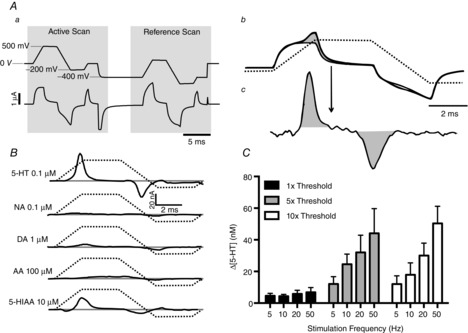
Identification and characterization of 5-HT release using voltammetry
Aa shows the voltammetric scan waveform used. Upper trace, voltage applied to electrode tip; lower trace, background current in saline. Ab shows the current from two scans, with and without 5-HT present. The shaded area is the increase in faradaic current caused by oxidation/reduction of 5-HT. Ac shows, at higher gain, the signal following subtraction of the background current, leaving only the faradaic current. B shows the signals from other electroactive substances known to be found in the brain; the voltammograms are recorded in PBS. Active scan voltage ramp is shown by dashed line. Abbreviations: AA, ascorbic acid; DA, dopamine; 5-HIAA, 5-hydroxyindoleacetic acid; and NA, noradrenaline. C, histograms (n = 5) showing the maximal increase of 5-HT in nucleus tractus solitarii (NTS) following electrical stimulation of the central end of the left vagus (1 ms pulse width, 10 s duration). Stimuli were applied at 3 min intervals at different frequencies and thresholds (threshold range was between 70 and 110 μA). The error bars show the SEM.
By comparing the (in vitro) signal obtained from the expected confounding substances, 5-hydroxyindoleacetic acid (5-HIAA), noradrenaline (NA), dopamine (DA) and ascorbic acid (AA), the selectivity of the trapezoidal waveform for 5-HT could be estimated (Fig. 1B). It was found that only 5-HIAA would significantly confound the 5-HT signal (Fig. 1B). Before each experiment the electrodes were calibrated with a standard concentration of 5-HT (100 nm) before use.
In the experiments where 5-HT or noradrenaline was microinjected, the voltammetry drive waveform used for their detection was a single triangular wave ramped from 0 to +1.2 V then to −500 mV at a rate of 400 Vs−1. This waveform was chosen because it generates the maximal voltammetric signal from both 5-HT and noradrenaline, and in these experiments, distinguishing between 5-HT and NA was not necessary because they were exogenously applied.
Experimental protocols
Once the microelectrode was in place in the NTS, voltammetry scans were started (at 2 Hz) and monitored along with blood pressure and heart rate until the signal was stable; this usually took 10–15 min.
Electrical stimulation of the vagus
A standard stimulus of 20 Hz at 10 times threshold (threshold was between 70 and 110 μA at 1 ms) for 10 s was applied to the vagus at 3 min intervals until the evoked release of putative 5-HT stabilized. This usually occurred by the fourth stimulation. Once stabilized, a further three stimulations were carried out and then the experimental challenge was carried out 1 min later. This was repeated if necessary at 3 min intervals. The mean changes caused by the three vagal stimulations before the experimental challenge were taken as the baseline values with which the effects of the test substances were compared.
Activation of cardiopulmonary afferents, baro- and chemoreflexes
In all these experiments, both vagi were intact. Activation of the cardiopulmonary reflex was carried out by intra-atrial (i.a.) injections of phenylbiguanide (PBG). For the dose–response curve experiments, doses (3, 10 and 30 μg per animal) were administered randomly between experiments, and changes were measured from the baseline evoked 5-HT value (mean value over 3 min) immediately before the first injection. For uptake studies, the middle dose (10 μg per animal) was given and thereafter, once the variables had returned to a stable baseline (3–5 min), injections of citalopram, decynium-22 or vehicle (DMSO; 0.1 ml kg−1). Ninety seconds after administration of these test solutions, this dose of PBG was re-injected. Changes in the amount of PBG-evoked 5-HT in the presence of the drug were expressed as the percentage change from the amount of 5-HT released by this dose of PBG given before the injection of the test solutions. All changes were measured at maximal change.
In a separate group of experiments, the baro- and chemoreflexes were tested to determine their effects on 5-HT release. This was carried out by giving injections of noradrenaline (0.5, 1 and 5 μg kg−1) and sodium nitroprusside (5, 10 and 50 μg kg−1, i.v.) and sodium cyanide (50, 100 and 200 μg per animal, i.a.). It should be noted that these methods for activating these particular reflexes are indirect; thus, it is possible that some of the effects observed are due to actions at other sites within the circulation. The effects of each reflex activation were allowed to dissipate fully before the next activation, which usually took between 3 and 5 min. Again, changes were measured from the baseline evoked 5-HT value (mean value over 3 min) immediately before the first injection.
Kynurenate experiments
This protocol started as for vagal stimuli alone, but 90 s after the third stable response to vagal stimulation, vehicle PBS (30 μl) or 10 or 50 mm kynurenate (KYN) was applied topically to the NTS, followed 90 s later by vagal stimulation. Changes in variables caused by vehicle or kynurenate application were compared with an average of the three predrug/vehicle stimulations. In experiments where D-22 was given the protocol was similar, but vagal stimulation was repeated twice after application of 10 mm kynurenate followed 90 s later by i.v. injection of D-22. Ninety seconds after administration, vagal stimulation was repeated. In separate experiments, time-matched controls were carried out, in which DMSO was given i.v. instead of D-22.
Pressure microinjection of 5-HT and noradrenaline into the NTS
Multibarrelled electrodes were used as described above (see ‘Recording’), with one barrel loaded with 5-HT (0.1 mm) or noradrenaline (1.0 mm). The electrodes were placed in the NTS (see ‘Recording’). The voltammetric scans (2 s−1) were then started, and the electrode was left for at least 5 min for the background current to stabilize. Once the current was stable, 5-HT or NA microinjections were made. The pressure and time (normally 17.2 kPa for 1–3 s) of the injections were adjusted so that the peak concentration of the detected monoamine was ∼1 μm. Microinjections were then applied at 5 min intervals. This timing allowed the evoked signal to decay completely before the next application. At least three stable applications of 5-HT or NA were required before administration of the test drug or vehicle was attempted.
Data analysis
The amount of putative 5-HT release was measured as the maximal change in the voltammetric signal relative to the baseline value. The concentration of 5-HT was calculated from this signal using the in vitro calibration values for that electrode. The 5-HT clearance was measured as the time taken for the signal to decline to 20% of its peak amplitude (T80; see Daws & Toney, 2007). Mean arterial pressure and heart rate were computed from the instantaneous values averaged over 1 s. Changes in mean arterial pressure and heart rate quoted in the results were the maximal changes observed. When comparing treatment groups, statistical analysis was performed using a one-way ANOVA to compare drug-treated experimental groups with the time-matched control group. Post hoc analysis was performed using Fisher's least significant difference (LSD) test, to calculate significant differences between means of drug-treated and control groups. In some experiments, statistical analysis was performed using a two-way ANOVA where multiple observations needed to be compared. Post hoc analysis was performed, in this case, using the Bonferroni correction. For all statistical analysis, differences between groups were considered significant when P < 0.05. These data are presented as means ± SEM.
Drugs and solutions
Drugs and chemicals were obtained from the following sources: 5-hydroxytryptamine creatinine sulphate, decynium-22 (D-22), noradrenaline bitartrate, fluoxetine, citalopram, cadmium chloride, α-bungarotoxin, sodium nitroprusside, phenylbiguanide (PBG), polyethylene glycol and DMSO from Sigma Aldrich Chemicals, Poole, UK; chlorisondamine diiodide from Tocris Bioscience, Bristol, UK; α-chloralose from Vickers Laboratories Ltd, Pudsey, UK; sodium chloride, d-glucose, sodium cyanide and sodium bicarbonate from BDH, Poole, UK; isoflurane (Aerrane®) from Baxter Healthcare Ltd, Thetford, UK; pentobarbitone sodium (Sagatal®) from Rhône-Mérieux Ltd, Harlow, UK; Gelofusine® from Braun Medical Ltd, Sheffield, UK; and heparin from CP Pharmaceuticals, Wrexham, UK. All chemicals were dissolved as their salts in either 0.9% saline or DMSO. Decynium-22 was dissolved 100% DMSO to give a stock solution of 10 mg ml−1. The maximal volume given i.v. was 0.1 ml, while for topical application it was 30 μl. The concentrations of 5-HT and NA in the injection barrels were 0.1 and 1 mm, respectively.
Results
Evoked release of 5-HT in the NTS in response to electrical stimulation of vagal afferents
The threshold for detection of afferent volleys and extracellular spike activity in the NTS varied between 40 and 100 μA in different preparations. At threshold stimulation, small increases in 5-HT release could be detected (n = 5) but were not frequency dependent. At five times threshold (n = 5) and 10 times threshold (n = 5) there was a frequency-dependent increase in 5-HT, ranging from 12 ± 5 to 44 ± 16 and from 12 ± 6 to 50 ± 11 nm, respectively. From these data, a standard stimulus for electrical stimulation of vagal afferents was chosen of 20 Hz at 10 times threshold for 10 s. This gave a submaximal but reliable response in all preparations (see Fig. 1C).
Effect of uptake blockers on evoked 5-HT release
The selective 5-HT transporter inhibitor citalopram (1 mg kg−1, i.v.; n = 6; Fig. 2A and D) had no significant effect on the vagally evoked (20 Hz, 10 times threshold for 10 s) release of 5-HT in the NTS. Citalopram caused a decrease of 1 ± 5% in the detected concentration of 5-HT, which was 28 ± 4 nm (n = 6). In control experiments, saline caused a change of 3 ± 3% in the detected concentration of 5-HT, which was 25 ± 9 nm (n = 6). Citalopram also had no effect on the bradycardia (40 ± 3 beats min−1) and hypotension (37 ± 5 mmHg) resulting from the vagal stimulation. Vehicle control (DMSO; 0.1 ml kg−1, i.v.; n = 6) likewise had no effect on vagally evoked bradycardia (47 ± 4 beats min−1) or hypotension (37 ± 4 mmHg). Resting blood pressure and heart rate were unaffected by either citalopram or DMSO. Data from a typical experiment are shown in Fig. 2A. In three experiments, the effect of citalopram was followed for 30 min and no overt effects were observed on vagally evoked changes (data not analysed).
Figure 2.
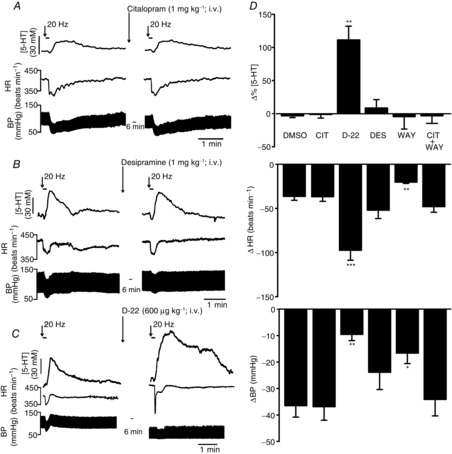
Effect of the monoamine uptake blockers on vagally evoked release of 5-HT in the NTS
Experimental traces showing the effects of citalopram (1 mg kg−1, i.v.; A), desipramine (1 mg kg−1; B) and decynium-22 (D-22; 600 μg kg−1; C) on the 5-HT signal and the decreases in blood pressure (BP) and heart rate (HR) evoked by electrical stimulation (20 Hz, 1 ms, 10 s at 10 times threshold) of the central end of the left vagus. D shows bar graphs of the combined data comparing the effects of vehicle (DMSO, 0.1 ml kg−1; n = 6), citalopram (CIT; n = 6), D-22 (n = 6); desipramine (DES; n = 6) and WAY-100635 (WAY; 1 mg kg−1; n = 4). Also shown is WAY-100635 given 3 min after citalopram (CIT + WAY; n = 5). The error bars show the SEM. These changes were compared with control values using a one-way ANOVA and the means compared with Fisher's LSD test. *P < 0.05, **P < 0.01 and ***P < 0.001.
The selective 5-HT1A antagonist WAY-100635 (1 mg kg−1; n = 4; Fig. 2D) also had no significant effect on the vagally evoked release of 5-HT, causing a change of −5 ± 9% on a release of 28 ± 4 nm. However, WAY-100635 did significantly attenuate the vagally evoked bradycardia (20 ± 1 beats min−1) and hypotension (17 ± 4 mmHg), when compared with DMSO (47 ± 4 beats min−1 and 37 ± 4 mmHg, respectively). Likewise, WAY-100635 given 3 min after citalopram (1 mg kg−1; n = 5) had no effect (−3 ± 5%) on the vagally evoked increase in extracellular 5-HT, although WAY-100635 now no longer blocked the vagally evoked bradycardia and hypotension (Fig. 2D).
Desipramine (1 mg kg−1; n = 6; Fig. 2B and D), the selective NA transporter inhibitor, had no significant effect on the vagally evoked release of 5-HT, causing an increase of 9 ± 13% from a baseline of 38 ± 5 nm, when compared with DMSO. There was also no effect on the evoked bradycardia and hypotension from a baseline of −52 ± 9 beats min−1 and −23 ± 6 mmHg. Desipramine differed from citalopram in that it caused a significant increase in the baseline blood pressure of 20 ± 4 mmHg when compared with DMSO. A trace of one of these experiments is shown in Fig. 2B.
Decynium-22 (600 μg kg−1; n = 6; Fig. 2C and D), the OCT3/PMAT inhibitor, caused a significant increase in the vagally evoked release of 5-HT by 111 ± 21% from a baseline level of 29 ± 10 nm. Decynium-22 also significantly increased the bradycardia caused by vagal stimulation of 98 ± 11 beats min−1 compared with vehicle (DMSO; 0.1 ml kg−1; n = 6) of 47 ± 4 beats min−1. The hypotension evoked by vagal stimulation was reduced in the presence of D-22 to 9 ± 2 mmHg compared with 37 ± 5 mmHg. Decynium-22 alone caused a significant reduction in baseline blood pressure of 49 ± 7 mmHg, which was associated with a significant tachycardia of 86 ± 15 beats min−1. A trace from one of these experiments is shown in Fig. 2C.
In a separate set of experiments, the decrease in blood pressure was mimicked by i.v. injection of the ganglion blocker chlorisondamine (1 mg kg−1; n = 3). This caused a decrease in blood pressure of 44 ± 8 mmHg, but the evoked increase in extracellular 5-HT was only increased by 5 ± 10%.
Cardiovascular reflexes and release of 5-HT concentration in the NTS
Cardiopulmonary reflex (Fig. 3A)
Figure 3.
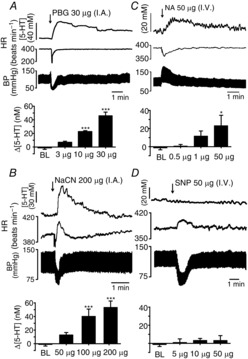
The effect of activation of the cardiovascular reflexes on 5-HT release in the NTS
The effect of activation of the cardiovascular reflexes on 5-HT release in the NTS Traces showing the effects of activation of the cardiopulmonary reflex with intra-atrial (i.a.) phenylbiguanide (PBG; 30 μg in 10 μl; A), the chemoreflex with i.v. NaCN (200 μg in 10 μl; B), the depressor reflex with i.v. noradrenaline (NA; 50 μg; C) and the pressor reflex with i.v. sodium nitroprusside (SNP; D) on the 5-HT signal, blood pressure (BP) and heart rate (HR). Histograms of the combined data (n = 4 or 5) for each reflex using different levels of activation of these receptors are shown below each trace. Doses of reflex activators were randomized between experiments. The error bars show the SEM. Changes were compared with the baseline (BL) values obtained before the first reflex evoked by the above substances using a one-way ANOVA and the means compared with Fisher's LSD test. *P < 0.05 and ***P < 0.001.
Activation of this reflex by increasing doses (3, 10 and 30 μg, i.a.) of PBG caused a release of 5-HT in the NTS of 7 ± 2 (n = 4), 23 ± 2 (n = 4) and 46 ± 5 nm (n = 5), respectively. For doses of 10 and 30 μg, the increases in extracellular 5-HT were significantly different from baseline values of −1 ± 2 nm. Mean changes from baseline are shown in Fig. 3A.
Chemoreflex reflex (Fig. 3B)
Activation of this reflex by increasing doses (50, 100 and 200 μg, i.v.) of sodium cyanide (n = 4) caused a release of 5-HT of 13 ± 4, 40 ± 11 and 54 ± 9 nm, respectively. For doses of 100 and 200 μg, the increases in extracellular 5-HT were significantly different from the baseline value of −1 ± 2 nm. Mean changes over baseline are shown in Fig. 3B.
Depressor reflex (Fig. 3C)
Activation of this reflex by increasing doses (0.5, 10 and 50 μg, i.v.; n = 4) of NA caused increases in extracellular 5-HT of 0.7 ± 1.7, 12 ± 6 and 23 ± 12 nm, respectively. Mean changes over baseline (−1.3 ± 2 nm) are shown in Fig. 3C.
Pressor reflex (Fig. 3D)
Activation of this reflex by increasing doses (0.5, 10 and 50 μg, i.v.; n = 4) of sodium nitroprusside caused no significant changes in extracellular 5-HT of 1 ± 4, 4 ± 2 and 3 ± 5 nm, respectively, compared with baseline (1 ± 2 nm). Mean changes over baseline are shown in Fig. 3D.
Effect of re-uptake inhibitors on the cardiopulmonary reflex
Citalopram (1 mg kg−1; n = 4; Fig. 4) had no significant effect when compared with vehicle control (DMSO, 0.1 ml kg−1; n = 6) on the increases in extracellular 5-HT of 25 ± 6 and 18 ± 5 nm, respectively, evoked by PBG (10 μg). It caused a change of only −26 ± 12% compared with vehicle control, which caused a change of −9 ± 8%. There were also no significant effects on the bradycardia and hypotension caused by PBG in the presence of citalopram.
Figure 4.
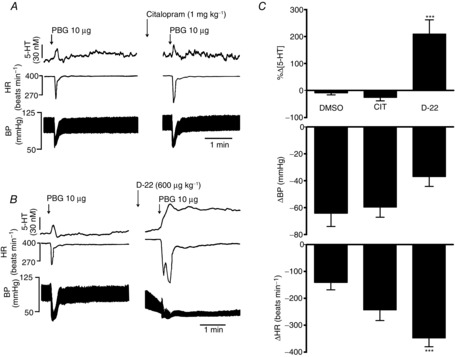
Effects of blockade of serotonin transporter (SERT) by citalopram and blockade of organic cation 3 transporter (OCT3)/plasma monoamine transporter (PMAT) by decynium-22 (D-22) on the cardiopulmonary reflex
The reflex was activated by intra-atrial (i.a.) phenylbiguanide (PBG; 30 μg in 10 μl). Left-hand traces in A and B show controls. Right-hand traces show the effect of administration i.v. of citalopram (1 mg kg−1; dissolved in DMSO; A) and D-22 (B) on the 5-HT signal, heart rate (HR) and blood pressure (BP). C, bar graphs of the effect of DMSO (0.1 ml kg−1; n = 6), citalopram (CIT; n = 4) and D-22 (n = 5) on the cardiopulmonary reflex. The error bars show the SEM. These changes were compared with vehicle (DMSO) using a one-way ANOVA and the means compared using Fisher's LSD test. ***P < 0.001.
Decynium-22 (600 μg kg−1; n = 5; Fig. 4) caused a significant increase in the release of 5-HT (25 ± 6 nm) evoked by PBG (10 μg) by 210 ± 53% compared with vehicle. The PBG-evoked bradycardia was also significantly potentiated from 141 ± 28 to 348 ± 32 beats min−1, while the PBG-evoked hypotension was unaffected, at 37 ± 7 mmHg compared with the control value of 64 ± 10 mmHg.
Effect of kynurenate (30 μl) topically applied to the NTS
Topically applied KYN (10 and 50 mm; n = 6 and 7; Fig. 5) significantly attenuated the vagally evoked release of 5-HT in the NTS by 52 ± 5 and 51 ± 5% from baseline values of 33 ± 9 and 28 ± 4 nm, respectively, compared with vehicle control (PBS; 0.01 m, n = 5). Kynurenate at 10 mm (Fig. 5A) had no effect on vagally evoked bradycardia and hypotension, which were 52 ± 9 beats min−1 and 23 ± 6 mmHg. Kynurenate at 50 mm (Fig. 5B) reversed these effects to a small tachycardia of 6 ± 4 beats min−1 and a rise in blood pressure of 12 ± 1 mmHg. Both doses of KYN caused significant increases in resting blood pressure of 12 ± 1 and 29 ± 2 mmHg and heart rate of 10 ± 2 and 26 ± 8 beats min−1, respectively.
Figure 5.
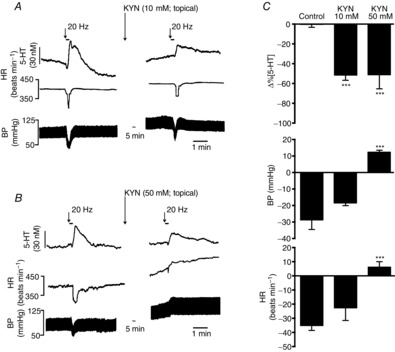
Effects of topical application of kynurenate (KYN) to the NTS on vagally evoked release of 5-HT
On the left are traces showing the effect of application of 10 (A) and 50 mm KYN (B) on changes in the 5-HT signal, heart rate (HR) and blood pressure (BP) evoked by electrical stimulation (20 Hz, 1 ms, 3 s at 10 times threshold) of the central end of the left vagus. C shows histograms of the combined data on the these effects. The error bars show the SEM. These changes caused by kynurenate [10 (n = 6) and 50 mm (n = 7)] were compared with control (PBS; 0.01 m; n = 5) using a one-way ANOVA and the means compared with Fisher's LSD test. ***P < 0.001.
Effect of decynium-22 on the vagally evoked increase in 5-HT after topical application of kynurenate
Kynurenate (10 mm; Fig. 6), after 3 min, caused a significant reduction in the vagally evoked release of 5-HT both in the D-22 group (600 μg kg−1, i.v.; n = 5) of 35 ± 9% and in the vehicle group (DMSO; 0.1 ml kg−1, i.v.; n = 5) of 41 ± 6% from baseline values of 28 ± 5 and 27 ± 3 nm, respectively. By 6 min, this had reached 57 ± 6 and 60 ± 3%, respectively. Decynium-22 given i.v. 2 min later reversed the reduction in the vagally evoked release of 5-HT by kynurenate to the pre-KYN control levels (5 ± 17%). Vehicle (DMSO; i.v.) had no significant effect on the reduction in the vagally evoked release of 5-HT caused by kynurenate, which was still 53 ± 5% of pre-KYN control levels.
Figure 6.
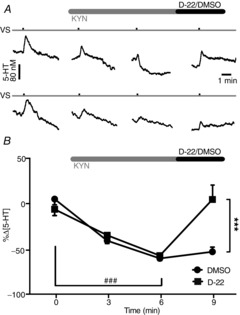
Effect of decynium-22 on the reduction in vagally evoked 5-HT release in the NTS caused by topical application of kynurenate
A, traces comparing the effect of D-22 (600 μg kg−1, i.v.; top traces) with that of vehicle (DMSO; 0.1 ml kg−1, i.v.; bottom traces) after kynurenate (KYN; 30 μl, 10 mm; applied topically to the NTS). The tick on time traces indicates the start of the vagal stimulation (20 Hz, 1 ms pulse for 3 s at 10 times threshold). B, the mean peak percentage change in the 5-HT concentration following vagal stimulation before and at 3 and 6 min after application of 10 mm KYN followed by the effect of D-22 (600 μg kg−1, i.v.; n = 5) or vehicle (DMSO; 0.1 ml kg−1; n = 5). The error bars show the SEM. A comparison between the data was carried out using one- and two-way ANOVA and a post hoc analysis was performed using the Bonferroni correction. ###P < 0.01 and ***P < 0.01 (one-way ANOVA).
Effect of uptake blockers on the clearance of exogenous 5-HT and NA microinjected into the NTS
5-Hydroxytryptamine
Citalopram (1 mg kg−1, i.v.; n = 4; Fig. 7A and E) significantly increased (+37 ± 5%) the time taken for 5-HT microinjected into the NTS to be cleared to 80% of its peak value (T80). A time-matched saline control (0.1 ml kg−1; n = 6; Fig. 7E) changed the time by −3 ± 3%. Likewise, D-22 (600 μg kg−1, i.v.; n = 5; Fig. 7C and E) also significantly increased T80 (+76 ± 13%) compared with time-matched vehicle (DMSO; 0.1 ml kg−1; n = 7; Fig. 7E; +2 ± 1.6%). Desipramine (1 mg kg−1, i.v.; n = 4; Fig. 7E) caused a non-significant change of 5 ± 8% in T80. Likewise, corticosterone (10 mg kg−1, i.v.; n = 5) also had no significant effect on T80, causing a change of only 3 ± 2%. The control T80 changed by only 1.7 ± 1.6% (n = 8).
Figure 7.
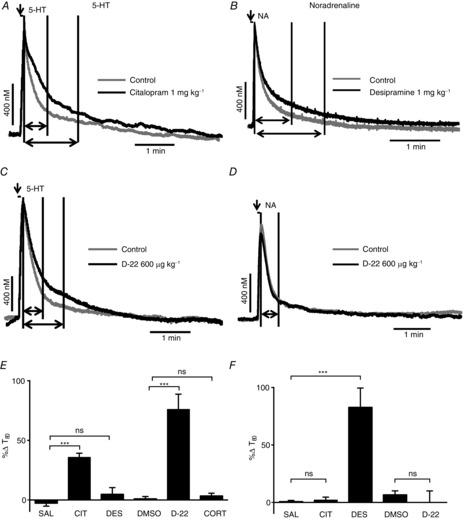
Effect of uptake inhibitors on exogenously applied 5-HT (left column) or noradrenaline (NA; right column) into the NTS
Voltammetric oxidation currents produced in response to local pressure ejection of 5-HT (A and C; 0.1 mm) and NA (B and D; 1 mm) into the NTS. Predrug/vehicle controls (light grey) are overlaid in black with citalopram (1 mg kg−1, i.v.; A), desipramine (1 mg kg−1; B) and D-22 (600 μg kg−1; C and D). The first vertical line on the traces indicates time 0, the second shows T80 for the control and the third T80 for the drug, although in D only a second line is shown because the T80 for control and D-22 where identical. The horizontal arrows show the distance between time 0 and T80 on the traces, while the vertical arrow shows when 5-HT or NA is microinjected. E and F show bar graphs of mean percentage changes in the time taken for the electrochemical signal for 5-HT and noradrenaline to decay to 80% of its peak value (T80) in the presence of saline (SAL; n = 8/6) citalopram (CIT; n = 4/5), desipramine (n = 4/5), DMSO (0.1 ml kg−1; n = 7/4), D-22 (n = 5/5), corticosterone (CORT; 1 mg kg−1; n = 5) from pre-drug/vehicle. The n numbers given for each substance apply for both bar graphs E and F. The error bars show the SEM. These changes were compared with vehicle control using a one-way ANOVA and the means compared with Fisher's LSD test. ***P < 0.001.
Noradrenaline
Citalopram (1 mg kg−1, i.v.; n = 4; Fig. 7F) and D-22 (600 μg kg−1, i.v.; n = 5; Fig. 7D and F) had no significant effect on the T80 of NA microinjected into the NTS. They caused changes of 2 ± 3 and 0 ± 10%, respectively, compared with time-matched saline controls (1 ml kg−1; n = 6) and DMSO controls (0.1 ml kg−1; n = 4), which were 1 ± 1.2 and 7 ± 4%, respectively. However, desipramine (1 mg kg−1, i.v.; n = 5; Fig. 7B and F) increased T80 significantly by 83 ± 17% compared with the saline control.
Discussion
The present data demonstrate that activation of visceral sensory afferents (cardiopulmonary, chemo- and baroreceptors), either electrically or physiologically, increases the level of extracellular 5-HT in the NTS of anaesthetized rats, in a frequency-dependent manner. This is consistent with the ability of 5-HT antagonists to attenuate reflex activation of NTS neurones (see Ramage & Villalón, 2008).
Surprisingly, the 5-HT release was not increased by blockade of the high-affinity, low-capacity 5-HT transporter (SERT) with citalopram (Hyttel, 1977). Citalopram was clearly active in the NTS, because the same dose was able to decrease the rate of removal of extracellular 5-HT directly applied in the NTS by microinjection. It could be argued from this observation that although the voltammetric scan was optimized for 5-HT it was in fact detecting NA or a mixture of NA and 5-HT. Oxidation peaks from both amines are difficult to distinguish in fast-cyclic voltammetry methodology. Noradrenaline-containing terminals and neuronal cell bodies (the A2 group) are found in the NTS (Fuxe, 1965; Takahashi et al. 1980; Kalia et al. 1985; Rinaman, 2011), and vagal afferents make synaptic connections with the A2 group (Sumal et al. 1994) and can activate these neurones (Appleyard et al. 2007). In the present study, however, the selective NA uptake inhibitor desipramine (Glowinski & Axelrod, 1964), at a dose that selectively decreased the removal of NA when applied to the NTS by microinjection, failed to affect the putative 5-HT signal.
In contrast, blockade of the two low-affinity, high-capacity transporters, OCT3 and PMAT, with D-22 (Schömig et al. 1993; Duan & Wang, 2010) increased the evoked 5-HT signal and decreased the rate of removal of microinjected 5-HT but not NA. The PMAT is ∼70% more effective in transporting 5-HT than NA, while OCT3 is 23% more effective in transporting noradrenaline than 5-HT (Duan & Wang, 2010). Corticosterone (Engel & Wang, 2005), even at high doses, failed to affect the removal of microinjected 5-HT into the NTS. This supports the view that PMAT, not OCT3, is involved in the removal of 5-HT in the NTS. This is consistent with the observation that the levels of OCT3 in the NTS are low and that PMAT mRNA is nearly four times more abundant in the medulla oblongata than OCT3 mRNA (Duan & Wang, 2010). Thus, it can be concluded that the optimized voltammetric scan for 5-HT was detecting 5-HT rather than NA. This conclusion is further supported by brainstem slice experiments showing that solitary tract-evoked glutamatergic EPSCs recorded from NTS neurones are attenuated by blockade of 5-HT3 receptors (Wan & Browning, 2008; Hosford et al. 2012) but not by blockade of α1-adrenoceptors (Zhang & Mifflin, 2007). Overall, the present results support the view that 5-HT, not NA, is released in the NTS by vagal stimulation and that the removal of this released 5-HT is regulated by PMAT, not SERT.
The ability of D-22 to potentiate the evoked release of 5-HT in the NTS by blocking PMAT was associated with a corresponding increase by the drug of the evoked bradycardia, which again is consistent with an excitatory role of 5-HT in these pathways (Ramage & Villalón, 2008). The failure to see any potentiation of the reflex reduction in blood pressure evoked by electrical stimulation of the vagus or cardiopulmonary afferent activation is presumably due to the result of the hypotension caused by i.v. D-22 owing to α1-adrenoceptor blockade (Russ et al. 1996). It should be noted that this hypotensive action does not play a role in the ability of D-22 to potentiate the release of 5-HT in the NTS, because a similar reduction in blood pressure caused by the ganglion blocker chlorisondamine did not affect the 5-HT signal. Furthermore, the failure to see any increases in 5-HT in the NTS in response to activation of the pressor reflex by nitroprusside is consistent with this reflex reducing afferent traffic into the NTS.
The observation that this 5-HT release is regulated by PMAT differs from that reported in the substantia nigra reticulata. In substantia nigra reticulata, a voltammetrically measured increase in 5-HT evoked by stimulating the dorsal raphé was found to be regulated by SERT (Hashemi et al. 2009). The evoked increase in extracellular 5-HT in substantia nigra reticulata was in a similar range to that recorded in the present study. Possible reasons for this difference may be that in the NTS the 5-HT release is evoked by visceral afferent fibres, whereas in substantia nigra reticulata it is evoked by stimulation of central 5-HT neurones. It could also be that in the NTS 5-HT release involves volume/paracrine transmission rather than classical synaptic transmission (see Ramage, 2009; Fuxe et al. 2013), and this is further supported by the observation that many of the presynaptic terminals that express 5-HT3 receptors in the NTS were without SERT immunoreactivity (Huang et al. 2004).
Although the above data indicate that vagally evoked release of 5-HT in the NTS is regulated by PMAT, the experiments where 5-HT was added exogenously indicate that SERT also has a functional role in this region. A possible explanation for failure, in the present experiments, to see an effect of SERT on the evoked release of 5-HT could be that which is used to explain the delay in the onset of the therapeutic effect of antidepressants in man. In this model, although 5-HT uptake is blocked immediately, the activation of inhibitory 5-HT1A receptors by excess perisynaptic 5-HT causes a paradoxical reduction in the overall release of 5-HT (see Artigas, 1996). In brainstem slice experiments (Hosford et al. 2014), it has been demonstrated that citalopram uncovers a 5-HT1A receptor-mediated inhibition of glutamatergic miniature excitatory postsynaptic currents. However, in the present experiments, pretreatment with the 5-HT1A antagonist WAY-100635 (Forster et al. 1995) did not uncover any effect of citalopram, although WAY-100635 alone significantly decreased the bradycardia and hypotension evoked by electrical stimulation of the vagus. The failure of WAY-100635 to have any effects is consistent with the recent observation that 5-HT1A receptors are not autoreceptors but heteroreceptors located on the cell bodies/dendrites and not on synaptic or vagal afferent terminals in NTS neurones (Ostrowski et al. 2014). The effects of WAY-100635 on vagally evoked changes in heart rate and blood pressure were consistent with the known role of 5-HT1A receptors in the excitatory regulation of cardiac vagal preganglionic neurones (Bogle et al. 1990; Wang & Ramage, 2001; Hildreth & Goodchild, 2010). The site of this action is probably at the level of nucleus ambiguus, where 5-HT1A receptors are known to be involved in regulating the excitation of cardiac vagal preganglionic neurones by the cardiovascular afferents (Wang & Ramage, 2001; Hildreth & Goodchild, 2010) and suggests that 5-HT actions in the nucleus ambiguus in these reflex pathways could be regulated by SERT. It should be noted that there is evidence against a role for 5-HT1A receptors in the NTS in cardiovascular reflex regulation (Oskutyte et al. 2009).
Glutamate is considered to be the major transmitter released by cardiovascular/visceral afferents in the NTS (see Talman, 1997; Baude et al. 2009). In the present experiments, blockade of glutamate receptors with kynurenate (see Stone & Addae, 1983) indicated that only approximately half of the 5-HT released by vagal afferent activation was associated with glutamate release. It may be argued that not all of the glutamate receptors were blocked; however, increasing the dose of kynurenate 10-fold failed to have any further effect on the evoked 5-HT signal. This ‘kynurenate-resistant’ signal could be potentiated by D-22. It is possible that this glutamate-independent release of 5-HT could come directly from vagal afferent terminals (see Introduction) and/or involve the release of another transmitter from the vagal afferents, such as substance P, acting on NTS cells (see Helke & Seagard, 2004). However, all that can be concluded at present is that ∼50% of the 5-HT that is released by the afferent activation is independent of ionotropic glutamate receptor activation.
Overall, the present data demonstrate that in the anaesthetized rat stimulation of vagal cardiovascular/visceral afferents evokes release of 5-HT in the NTS and this increase is dependent in part on the simultaneous release of glutamate, presumably also from vagal afferents. Other data indicate that 5-HT release within the NTS itself can cause the release of glutamate via activation of 5-HT3 receptors (Jeggo et al. 2005; Wan & Browning, 2008; Hosford et al. 2014). The source of this 5-HT release has not been determined; it may be from vagal afferent terminals and/or 5-HT terminals within the NTS originating from other brain areas. This evoked-5-HT release, surprisingly, is regulated by the high-capacity, low-affinity transporter PMAT, not the low-capacity, high-affinity transporter SERT. This is the first demonstration that PMAT may be playing a physiological role in the regulation of 5-HT transmission in the NTS. Interestingly, in the present experiments in vivo and those in the slice (Hosford et al. 2014), these data indicate that SERT does function in the NTS, but is not involved in vagally evoked release of 5-HT. This implies that PMAT must be located much nearer to the site of this evoked 5-HT release than SERT. This arrangement might be due to the fact that 5-HT is acting, in part, as a volume transmitter within the NTS. Overall, these data support the view that 5-HT is an important transmitter in cardiovascular homeostasis at the level of the NTS.
Glossary
- AA
ascorbic acid
- CIT
citalopram
- CORT
corticosterone
- D-22
decynium-22
- DA
dopamine
- DES
desipramine
- DMSO
dimethyl sulfoxide
- 5-HIAA
5-hydroxyindoleacetic acid
- 5-HTT
5-HT transporter
- i.a.
intra-atrial
- KYN
kynurenate
- NA
noradrenaline
- NTS
nucleus tractus solitarii
- OCT3
organic cation 3 transporter
- PBG
phenylbiguanide
- PMAT
plasma monoamine transporter
- SERT
serotonin transporter
Additional information
Competing interests
None declared.
Author contributions
Conception and design of the experiments, analysis and interpretation of data and drafting and revising the manuscript: P.S.H., J.M. and A.G.R. Collection of data: P.S.H. All authors approved the final version. All experiments were carried out at University College London.
Funding
This work was funded in part by a Biotechnology and Biological Sciences Research Council studentship (grant BB/F017146/1) to P.S.H. and in part by a British Heart Foundation Project grant (no. PG/13/79/30429).
References
- Andresen MC. Kunze DL. Nucleus tractus solitarius—gateway to neural circulatory control. Annu Rev Physiol. 1994;56:93–116. doi: 10.1146/annurev.ph.56.030194.000521. [DOI] [PubMed] [Google Scholar]
- Appleyard SM, Marks D, Kobayashi K, Okano H, Low MJ. Andresen MC. Visceral afferents directly activate catecholamine neurons in the solitary tract nucleus. J Neurosci. 2007;27:13292–13302. doi: 10.1523/JNEUROSCI.3502-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artigas F, Romero L, de Montigny C. Blier P. Acceleration of the effect of selected antidepressant drugs in major depression by 5-HT1A antagonists. Trends Neurosci. 1996;19:378–383. doi: 10.1016/S0166-2236(96)10037-0. [DOI] [PubMed] [Google Scholar]
- Baude A, Strube C, Tell T. Kessler J-P. Glutamatergic neurotransmission in the nucleus tractus solitarii: structural and functional characteristics. J Chem Neuroanat. 2009;38:145–153. doi: 10.1016/j.jchemneu.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Bogle RG, Pires JGP. Ramage AG. Evidence that central 5-HT1A-receptors play a role in the von Bezold-Jarisch reflex in the rat. Br J Pharmacol. 1990;100:757–760. doi: 10.1111/j.1476-5381.1990.tb14088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornea-Hébert V, Riad M, Wu C, Singh SK. Descarries L. Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. J Comp Neurol. 1999;409:187–209. doi: 10.1002/(sici)1096-9861(19990628)409:2<187::aid-cne2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Daws LC. Toney GM. Voltammetric methods to study kinetics and mechanisms for serotonin clearance in vivo. In: Nicolelis MAL, editor; Michael AC, Simon SA, editors. Electrochemical Methods in Neuroscience for Methods and New Frontiers in Neuroscience. CRC Press; 2007. pp. 63–81. Boca Raton, FL, USA. [Google Scholar]
- Duan H. Wang J. Selective transport of monoamine neurotransmitters by human plasma membrane monoamine transporter and organic cation transporter 3. J Pharmacol Exp Ther. 2010;335:743–753. doi: 10.1124/jpet.110.170142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel K. Wang J. Interaction of organic cations with a newly identified plasma membrane monoamine transporter. Mol Pharmacol. 2005;68:1397–1407. doi: 10.1124/mol.105.016832. [DOI] [PubMed] [Google Scholar]
- Forster EA, Cliffe IA, Bill DJ, Dover GM, Jones D, Reilly Y. Fletcher A. A pharmacological profile of the selective silent 5-HT1A receptor antagonist, WAY-100635. Eur J Pharmacol. 1995;281:81–88. doi: 10.1016/0014-2999(95)00234-c. [DOI] [PubMed] [Google Scholar]
- Fox K, Armstrong-James M. Millar J. The electrical characteristics of carbon fibre microelectrodes. J Neurosci Methods. 1980;3:37–48. doi: 10.1016/0165-0270(80)90032-1. [DOI] [PubMed] [Google Scholar]
- Fuxe K. Evidence for the existence of monoamine containing neurons in the central nervous system. IV. The distribution of monoamine terminals in the central nervous system. Acta Physiol Scand. 1965;64(Suppl 24):39–85. [PubMed] [Google Scholar]
- Fuxe K, Borroto-Escuela DO, Romero-Fernandez W, Zhang WB. Agnati LF. Volume transmission and its different forms in the central nervous system. Chin J Integr Med. 2013;19:323–329. doi: 10.1007/s11655-013-1455-1. [DOI] [PubMed] [Google Scholar]
- Gaudin-Chazal G, Portalier P, Barrit MC. Puizillout JJ. Serotonin-like immunoreactivity in paraffin-sections of the nodose ganglia of the cat. Neurosci Lett. 1982;33:169–172. doi: 10.1016/0304-3940(82)90246-4. [DOI] [PubMed] [Google Scholar]
- Glowinski J. Axelrod J. Inhibition of uptake of tritiated-noradrenaline in the intact rat brain by imipramine and structurally related compounds. Nature. 1964;204:1318–1319. doi: 10.1038/2041318a0. [DOI] [PubMed] [Google Scholar]
- Gustafson EL, Durkin MM, Bard JA, Zgombick J. Branchek TA. A receptor autoradiographic and in situ hybridization analysis of the distribution of the 5-ht7 receptor in rat brain. Br J Pharmacol. 1996;117:657–666. doi: 10.1111/j.1476-5381.1996.tb15241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemi P, Dankoski EC, Petrovic J, Keithley RB. Wightman RM. Voltammetric detection of 5-hydroxytryptamine release in the rat brain. Anal Chem. 2009;81:9462–9471. doi: 10.1021/ac9018846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayer-Zillgen M, Brüss M. Bönisch H. Expression and pharmacological profile of the human organic cation transporters hOCT1, hOCT2 and hOCT3. Br J Pharmacol. 2002;136:829–836. doi: 10.1038/sj.bjp.0704785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helke CJ. Seagard J. Substance P in the baroreceptor reflex: 25 years. Peptides. 2004;25:413–423. doi: 10.1016/j.peptides.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Hildreth CM. Goodchild AK. Role of ionotropic GABA, glutamate and glycine receptors in the tonic and reflex control of cardiac vagal outflow in the rat. BMC Neurosci. 2010;11:128. doi: 10.1186/1471-2202-11-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines T, Toney GM. Mifflin SW. Responses of neurons in the nucleus tractus solitarius to stimulation of heart and lung receptors in the rat. Circ Res. 1994;74:1188–1196. doi: 10.1161/01.res.74.6.1188. [DOI] [PubMed] [Google Scholar]
- Hosford PS, Millar JD. Ramage AG. Characterization of vagal afferent-evoked 5-HT release detected by fast-cyclic voltammetry in the nucleus tractus solitarius (NTS) of the anaesthetised male rat. Proc Physiol Soc. 2011:PC39. http://www.physoc.org/proceedings/abstract/Proc%20Physiol%20Soc%2023PC39. [Google Scholar]
- Hosford P, Mifflin S. Ramage AG. 5-Hydroxytryptamine-mediated neurotransmission modulates spontaneous and vagal evoked glutamate release in the nucleus of the solitary tract (NTS) effect of uptake blockade. J Pharmacol Exp Ther. 2014;349:288–296. doi: 10.1124/jpet.113.211334. [DOI] [PubMed] [Google Scholar]
- Hosford PS, Millar JD. Ramage AG. Organic cation transporter 3 and plasma membrane monoamine transporter, not SERT, remove 5-HT released in the NTS by vagal stimulation as measured by in vivo fast cyclic voltammetry. New Orleans: Society for Neuroscience; 2012. . Online http://www.abstractsonline.com/.../ViewAbstract.aspx?cKey=8e5f3d3c-2cd8-4342-...". [Google Scholar]
- Huang J, Spier AD. Pickel VM. 5-HT3A receptor subunits in the rat medial nucleus of the solitary tract: subcellular distribution and relation to the serotonin transporter. Brain Res. 2004;1028:156–169. doi: 10.1016/j.brainres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Hyttel J. Neurochemical characterization of a new potent and selective serotonin uptake inhibitor: Lu 10-171. Psychopharmacology (Berl) 1977;51:225–233. doi: 10.1007/BF00431629. [DOI] [PubMed] [Google Scholar]
- Jeggo RD, Kellett DO, Wang Y, Ramage AG. Jordan D. The role of central 5-HT3 receptors in cardiopulmonary reflex inputs to neurones in the nucleus tractus solitarius of anaesthetized rats. J Physiol. 2005;566:939–953. doi: 10.1113/jphysiol.2005.085845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia M, Fuxe K. Goldstein M. Rat medulla oblongata. II. Dopaminergic, noradrenergic (A1 and A2) and adrenergic neurons, nerve fibers, and presumptive terminal processes. J Comp Neurol. 1985;233:308–332. doi: 10.1002/cne.902330303. [DOI] [PubMed] [Google Scholar]
- Leslie RA, Reynolds DJM, Andrews PLR, Grahame-Smith DG, Davis CJ. Harvey JM. Evidence for presynaptic 5-hydroxytryptamine3 recognition sites on vagal afferent terminals in the brainstem of the ferret. Neuroscience. 1990;38:667–673. doi: 10.1016/0306-4522(90)90060-h. [DOI] [PubMed] [Google Scholar]
- Manaker S. Verderame HM. Organization of serotonin 1A and 1B receptors in the nucleus of the solitary tract. J Comp Neurol. 1990;301:535–553. doi: 10.1002/cne.903010405. [DOI] [PubMed] [Google Scholar]
- Millar J, Oskutyte D. Ramage AG. Using fast cyclic voltammetry, 5-HT increases can be detected in the nucleus tractus solitarius (NTS) in response to vagal afferent stimulation in anaesthetized rats. Proc Physiol Soc. 2009;15:PC79. http://www.physoc.org/proceedings/abstract/Proc%20Physiol%20Soc%2015PC79. [Google Scholar]
- Millar J. Pelling CW. Improved methods for construction of carbon fibre electrodes for extracellular spike recording. J Neurosci Methods. 2001;110:1–8. doi: 10.1016/s0165-0270(01)00411-3. [DOI] [PubMed] [Google Scholar]
- Millar J. Williams GV. Fast differential ramp voltammetry: a new voltammetric technique designed specifically for use in neuronal tissue. J Electroanal Chem. 1990;282:33–49. [Google Scholar]
- Nosjean A, Compoint C, Buisseret-Delmas C, Orer HS, Merahi N, Puizillout JJ. Laguzzi R. Serotonergic projections from the nodose ganglia to the nucleus tractus solitarius: an immunohistochemical and double labeling study in the rat. Neurosci Lett. 1990;114:22–26. doi: 10.1016/0304-3940(90)90422-6. [DOI] [PubMed] [Google Scholar]
- Oliver KR, Kinsey AM, Wainwright A. Sirinathsinghji DJS. Localization of 5-ht5A receptor-like immunoreactivity in the rat brain. Brain Res. 2000;867:131–142. doi: 10.1016/s0006-8993(00)02273-3. [DOI] [PubMed] [Google Scholar]
- Oskutyte D, Jordan D. Ramage AG. Evidence that 5-hydroxytryptamine7 receptors play a role in the mediation of afferent transmission within the nucleus tractus solitarius in anaesthetized rats. Br J Pharmacol. 2009;158:1387–1394. doi: 10.1111/j.1476-5381.2009.00410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski TD, Ostrowski D, Hasser EM. Kline DD. Depressed GABA and glutamate synaptic signaling by 5-HT1A receptors in the nucleus tractus solitarii and their role in cardiorespiratory function. J Neurophysiol. 2014;111:2493–2504. doi: 10.1152/jn.00764.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G. Watson C. The Rat Brain in Stereotaxic Coordinates. 1998 doi: 10.1016/0165-0270(80)90021-7. , 4th edn. Academic Press, New York. [DOI] [PubMed] [Google Scholar]
- Pompeiano M, Palacios JM. Mengod G. Distribution of the serotonin 5-HT2 receptor family mRNAs: comparison between 5-HT2A and 5-HT2C receptors. Mol Brain Res. 1994;23:163–178. doi: 10.1016/0169-328x(94)90223-2. [DOI] [PubMed] [Google Scholar]
- Ramage AG. Serotonin (5-hydroxtryptamine; 5-HT): neurotransmission and neuromodulation. In: Squire L, editor. Encyclopedia of Neuroscience. Vol. 8. Oxford: Academic Press; 2009. pp. 705–710. [Google Scholar]
- Ramage AG. Villalón CB. 5-Hydroxytryptamine (serotonin) and cardiovascular regulation. Trends Pharmacol Sci. 2008;29:472–481. doi: 10.1016/j.tips.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Rinaman L. Hindbrain noradrenergic A2 neurons: diverse roles in autonomic, endocrine, cognitive, and behavioral functions. Am J Physiol Regul Integr Comp Physiol. 2011;300:R222–R235. doi: 10.1152/ajpregu.00556.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ H, Friedgen B, Königs B, Schumacher C, Graefe KH. Schömig E. Pharmacokinetic and α1-adrenoceptor antagonistic properties of two cyanine-type inhibitors of extraneuronal monoamine transport. Naunyn Schmiedebergs Arch Pharmacol. 1996;354:268–274. doi: 10.1007/BF00171057. [DOI] [PubMed] [Google Scholar]
- Schaffar N, Kessler JP, Bosler O. Jean A. Central serotonergic projections to the nucleus tractus solitarii: evidence from a double labeling study in the rat. Neuroscience. 1988;26:951–958. doi: 10.1016/0306-4522(88)90111-x. [DOI] [PubMed] [Google Scholar]
- Schömig E, Babin-Ebell J. Russ H. 1,1′-Diethyl-2,2′-cyanine (decynium22) potently inhibits the renal transport of organic cations. Naunyn Schmiedebergs Arch Pharmacol. 1993;347:379–383. doi: 10.1007/BF00165387. [DOI] [PubMed] [Google Scholar]
- Sévoz-Couche C, Comet MA, Bernard JF, Hamon M. Laguzzi R. Cardiac baroreflex facilitation evoked by hypothalamus and prefrontal cortex stimulation: role of the nucleus tractus solitarius 5-HT2A receptors. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1007–R1015. doi: 10.1152/ajpregu.00052.2006. [DOI] [PubMed] [Google Scholar]
- Sim LJ. Joseph SA. Efferent projections of the nucleus raphe magnus. Brain Res Bull. 1992;28:679–682. doi: 10.1016/0361-9230(92)90246-t. [DOI] [PubMed] [Google Scholar]
- Steinbusch HW. Distribution of serotonin-immunoreactivity in the central nervous system of the rat—cell bodies and terminals. Neuroscience. 1981;6:557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- Stone TW. Addae JI. The pharmacological manipulation of glutamate receptors and neuroprotection. Eur J Pharmacol. 2002;447:285–296. doi: 10.1016/s0014-2999(02)01851-4. [DOI] [PubMed] [Google Scholar]
- Sumal KK, Blessing WW, Joh TH, Reis DJ. Pickel VM. Synaptic interaction of vagal afferents and catecholaminergic neurons in the rat nucleus tractus solitarius. Brain Res. 1983;277:31–40. doi: 10.1016/0006-8993(83)90904-6. [DOI] [PubMed] [Google Scholar]
- Sykes RM, Spyer KM. Izzo PN. Central distribution of substance P, calcitonin gene-related peptide and 5-hydroxytryptamine in vagal sensory afferents in the rat dorsal medulla. Neuroscience. 1994;59:195–210. doi: 10.1016/0306-4522(94)90110-4. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Tohyama M, Satoh K, Sakumoto T, Kashiba A. Shimizu N. Fine structure of noradrenaline nerve terminals in the dorsomedial portion of the nucleus tractus solitarii as demonstrated by a modified potassium permanganate method. J Comp Neurol. 1980;189:525–535. doi: 10.1002/cne.901890306. [DOI] [PubMed] [Google Scholar]
- Talman WT. Glutamatergic transmission in the nucleus tractus solitarii: from server to peripherals in the cardiovascular information superhighway. Braz J Med Biol Res. 1997;30:1–7. doi: 10.1590/s0100-879x1997000100001. [DOI] [PubMed] [Google Scholar]
- Thor KB, Blitz-Siebert A. Helke CJ. Autoradiographic localization of 5HT1 binding sites in autonomic areas of the rat dorsomedial medulla oblongata. Synapse. 1992;10:217–227. doi: 10.1002/syn.890100305. [DOI] [PubMed] [Google Scholar]
- Thor KB, Hill KM, Harrod C. Helke CJ. Immunohistochemical and biochemical analysis of serotonin and substance P colocalization in the nucleus tractus solitarii and associated afferent ganglia of the rat. Synapse. 1988;2:225–231. doi: 10.1002/syn.890020309. [DOI] [PubMed] [Google Scholar]
- To ZP, Bonhaus DW, Eglen RM. Jakeman LB. Characterization and distribution of putative 5-ht7 receptors in guinea-pig brain. Br J Pharmacol. 1995;115:107–116. doi: 10.1111/j.1476-5381.1995.tb16327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan S. Browning KN. Glucose increases synaptic transmission from vagal afferent central nerve terminals via modulation of 5-HT3 receptors. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1050–G1057. doi: 10.1152/ajpgi.90288.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. Ramage AG. The role of central 5-HT1A receptors in the control of B-fibre cardiac and bronchoconstrictor vagal preganglionic neurones in anaesthetized cats. J Physiol. 2001;536:753–767. doi: 10.1111/j.1469-7793.2001.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DE, Seroogy KB, Lundgren KH, Davis BM. Jennes L. Comparative localization of serotonin1A1C, and 2 receptor subtype mRNAs in rat brain. J Comp Neurol. 1995;351:357–373. doi: 10.1002/cne.903510304. [DOI] [PubMed] [Google Scholar]
- Zhang W. Mifflin SW. Modulation of synaptic transmission to second-order peripheral chemoreceptor neurons in caudal nucleus tractus solitarius by α1-adrenoreceptors. J Pharmacol Exp Ther. 2007;320:670–677. doi: 10.1124/jpet.106.114033. [DOI] [PubMed] [Google Scholar]


