Abstract
Conditioning the heart to resist predictable and unpredictable ischaemia–reperfusion (IR) injury is one of the fastest growing areas of bench to bedside research within cardiology. Basic science has provided important insights into signalling pathways and protective mechanisms in the heart, and a growing number of clinical studies have, with important exceptions, shown the potential applicability and beneficial effect of various mechanical conditioning strategies achieved by intermittent short-lasting-induced ischaemia of the heart itself or a remote tissue. Remote ischaemic conditioning (RIC) in particular has been utilized in a number of clinical settings with promising results. However, while many novel ‘downstream’ mechanisms of RIC have been discovered, translation to pharmacological conditioning has not yet been convincingly demonstrated in clinical studies. One explanation for this apparent failure may be that most pharmacological approaches mimic a single instrument in a complex orchestra activated by mechanical conditioning. Recent studies, however, provide important insights into upstream events occurring in RIC, which may allow for development of drugs activating more complex systems of biological organ protection. With this review, we will systematically examine the first generation of pharmacological cardioprotection studies and then provide a summary of the recent discoveries in basic science that could illuminate the path towards more advanced approaches in the next generation of pharmacological agents that may work by reproducing the diverse effects of RIC, thereby providing protection against IR injury.
Tables of Links
| TARGETS | |
|---|---|
| GPCRsa | Enzymesd |
| Adenosine A1 receptors | Akt (protein kinase B) |
| Adenosine A3 receptors | Dipeptidyl peptidase 4 |
| CXCR4 | eNOS, endothelial NO synthase |
| GLP-1 receptors | GSK3, glycogen synthase kinase 3β |
| Nuclear receptorsb | JAK |
| Mineralocorticoid receptor | PI3K |
| Catalytic receptorsc | PKC-δ |
| Fibroblast growth factor receptor 2 | Ion channelse |
| Connexin (Cx) 43 | |
| Na+–H+ exchanger (SLC9) | |
| Insulin-like growth factor-1 receptor (IGFR1) | |
| TNF-α receptors |
| LIGANDS |
|---|
| Adenosine |
| Atorvastatin |
| CXCL12 (SDF1), |
| Cyclosporin A |
| Erythropoietin |
| Exenatide |
| GLP-1 |
| Metoprolol |
| Nicorandil |
| NO, nitric oxide |
| Rapamycin (sirolimus) |
| Spironolactone |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (a,b,c,d,eAlexander et al., 2013a,b,c,d,e,,,,)
Introduction
From acute events such as stroke and acute myocardial infarction (MI) to predictable circumstances such as elective surgery and angioplasty, the injury caused by ischaemia and reperfusion is a leading cause of death and disability (Murray and Lopez, 1997; Wang et al., 2012). Since 1990, more people have died from coronary heart disease than any other cause of death (Lloyd-Jones et al., 2009; 2010,). Ischaemia–reperfusion (IR) syndromes remain a major clinical challenge worldwide.
In acute coronary events, early and successful restoration of myocardial reperfusion following an ischaemic event is the most effective strategy to reduce final infarct size and improve clinical outcome, but reperfusion may induce further myocardial damage itself, so-called reperfusion injury (Murry et al., 1986). Although the process of myocardial reperfusion continues to improve with more timely and effective coronary intervention and antiplatelet and antithrombotic agents for maintaining the patency of the infarct-related artery, the development of effective drugs to treat the detrimental effects of reperfusion injury itself has proven to be a challenge. Indeed, several pharmacological strategies showing convincing effects in animal models of IR injury have failed to translate to clinical benefit.
Consequently, as the first generation of pharmacological conditioning studies {including large clinical trials such as AMISTAD-II (adenosine), APEX-MI (pexelizumab) and CREATE-ECLA [glucose-insulin-potassium (GIK) infusion]} (see Table 1) failed to show convincing effects, mechanical ischaemic conditioning strategies have dominated the more recent clinical trials.
Table 1.
Pharmacological adjunctive conditioning therapy in myocardial infarction
| Intervention | N | Clinical scenario | Outcome | |
|---|---|---|---|---|
| Adenosine | ||||
| Mahaffey et al. (1999) (AMISTAD) | Infusion of adenosine for 3 h | 236 | STEMI – thrombolysis | Reduction in infarct size |
| Kloner et al. (2006) (AMISTAD II) | Infusion of adenosine for 3 h | 2118 | STEMI – thrombolysis or primary PCI | No difference in death or HF |
| Atorvastatin | ||||
| Kim et al. (2010) (STATIN-STEMI) | Oral atorvastatin 80 mg before primary PCI | 171 | STEMI – primary PCI | No difference in death, revascularization or infarct size |
| Hahn et al. (2011b) | Oral atorvastatin 80 mg before primary PCI | 173 | STEMI – primary PCI | No difference in infarct size |
| Cyclosporin A | ||||
| Piot et al. (2008) | Cyclosporin infusion before primary PCI | 58 | STEMI – primary PCI | Reduction in infarct size |
| Erythropoietin | ||||
| Voors et al. (2010) | Single dose EPO | 529 | STEMI – primary PCI | No difference in LVEF or infarct size |
| Exenatide | ||||
| Lonborg et al. (2012b) | Infusion of exenatide for 6 h | 107 | STEMI – primary PCI | Reduction in infarct size |
| Glucose-insulin-potassium (GIK) | ||||
| Mehta et al. (2005) (CREATE-ECLA) | Infusion of GIK for 24 h | 20 201 | STEMI – thrombolysis | No difference in mortality |
| Selker et al. (2012) (IMMEDIATE) | Out-of-hospital infusion of GIK | 357 | STEMI – thrombolysis | Reduced mortality among patients with cardiac arrest |
| PKC-δ inhibitor | ||||
| Bates et al. (2008) | 2 doses of KAI 9803 | 154 | STEMI – primary PCI | No difference in infarct size |
HF, heart failure; LVEF, left ventricular ejection fraction.
Since its conceptual demonstration in 1986, local ischaemic preconditioning of the heart (and subsequently other organs) achieved by intermittent sub-lethal periods of ischaemia prior to a longer lasting ischaemic insult has evolved into the more clinically applicable methods of local ischaemic postconditioning and remote ischaemic conditioning (RIC) (see below), both of which have shown promising results in clinical trials (Staat et al., 2005; Hoole et al., 2009; Botker et al., 2010; Lonborg et al., 2010; Thielmann et al., 2010; 2013,; Davies et al., 2013; Sloth et al., 2014). The increasing insight into the mechanisms and pathways of ischaemic conditioning may pave the road for development of a new generation of cardioprotective drugs closely mimicking the powerful inherent protection afforded by ischaemic conditioning.
This review will focus upon novel advances in our understanding of the mechanisms involved in RIC and the scope for potential development of novel pharmacological approaches.
Remote ischaemic conditioning
‘Remote ischaemic conditioning’ (RIC), induced by repeated short-lasting ischaemia in a distant tissue – largely achieved by intermittent interruption of circulation in a limb – has recently emerged as a promising adjunctive therapy to avoid organ damage, thereby improving the outcomes of well-established therapies. From the site of the remote stimulus, through humoral (Shimizu et al., 2009) and neuronal (Loukogeorgakis et al., 2005; Lim et al., 2010) pathways, RIC activates several protective mechanisms in the target organ similar to those activated by local preconditioning. Furthermore, RIC modifies the systemic inflammatory response (Konstantinov et al., 2004; Shimizu et al., 2010), prevents endothelial dysfunction (Kharbanda et al., 2002) and platelet activation (Pedersen et al., 2011) following IR injury. In experimental studies, RIC has been shown to afford protection against IR in the liver (Kanoria et al., 2006; Lai et al., 2006), lung (Harkin et al., 2002; Jan et al., 2011), kidney (Ali et al., 2007), brain (Hahn et al., 2011a), heart (Kharbanda et al., 2002) and against cardiopulmonary bypass-induced neural, pulmonary and myocardial damage (Kharbanda et al., 2006).
Direct translation of RIC into clinical use
In proof-of-principle randomized clinical trials based upon surrogate endpoints such as biomarkers and imaging, RIC has been shown to protect against IR injury in the heart (Cheung et al., 2006; Hoole et al., 2009; Botker et al., 2010; Thielmann et al., 2010), brain (Hougaard et al., 2014), kidney (Lazaris et al., 2009) and lung (Cheung et al., 2006).
Three recent clinical follow-up studies indicate that RIC also confers prognostic benefit to the patient. An original study demonstrating reduced myocardial injury in patients treated with RIC prior to coronary artery by-pass surgery (Thielmann et al., 2010) in a subsequent follow-up study showed that cardiac mortality and major adverse cardiac and/or cerebrovascular event (MACCE) were reduced among RIC-treated patients (Thielmann et al., 2013). In a study investigating patients undergoing elective percutaneous coronary intervention (PCI), RIC not only reduced periprocedural myocardial injury (Hoole et al., 2009) but also MACCE rate up to 6 years after the coronary intervention (Davies et al., 2013). Finally, in a study utilizing RIC during ambulance transport to hospital in patients admitted with acute MI, RIC increased myocardial salvage (Botker et al., 2010), which, up to 4 years after the infarction, translated into a 35% reduction in MACCE and a 52% reduction in all-cause mortality (Sloth et al., 2014).
Although an increasing amount of evidence thus indicates that the cardioprotective effect of RIC can be translated into beneficial clinical effects, it is important to stress that properly sized studies with clinical endpoints, such as the ERICCA trial (ClinicalTrials.gov NCT01247545), RIP-Heart study (ClinicalTrials.gov NCT01067703) and the CONDI 2 trial (ClinicalTrials.gov NCT01857414), are needed.
Mechanisms of RIC
The organ-protective effects of RIC are at least partially mediated through the release of endogenous substances into the bloodstream, as plasma from RIC-treated animals is cardioprotective. Moreover, the same plasma can be dialysed and the dialysate applied to a naïve isolated heart to achieve cardioprotection equal in strength to cardioprotection in hearts from RIC-treated animals (Shimizu et al., 2009). Similarly, in a cardiac transplant model, RIC of a recipient animal reduces IR injury in the subsequently transplanted (denervated) donor heart, again suggesting the presence of a powerful humoral component to the RIC stimulus (Konstantinov et al., 2005a). Although the exact nature of the circulating effector(s) is not yet clear, the signalling cascade induced by RIC has similarities with local ischaemic preconditioning, there being a ‘window’ of cardioprotection lasting 2–6 h after the conditioning stimulus. Interestingly, RIC also induces a portfolio of myocardial gene expression responses associated with the stress-response and repair mechanisms (Konstantinov et al., 2005b). Consequently, RIC induces later ‘windows’ of protection (a second window re-emerging at 12–24 h, and other chronic effects when RIC is delivered for days after an event) (Wei et al., 2011), but their underlying biology is only partially understood and is beyond the scope of the current review.
As both local and remote ischaemic conditioning activate a multitude of pathways and mechanisms (Zhao et al., 2003), eventually resulting in a complex and powerful organ protection, and experimental models rarely allow for investigation of more than one or two systems, a complete understanding of the underlying protective mechanisms is still lacking. Furthermore, a wide range of animal models, conditioning protocols and variable endpoints add to the puzzle. Nevertheless, a consensus on how to categorize the components of cardioprotection is emerging and a distinction between the three levels of signal transduction – triggers, intracellular mediators and effectors – has been suggested by Heusch et al. (Heusch et al., 2008; Heusch, 2013) and Kharbanda et al. (2009).
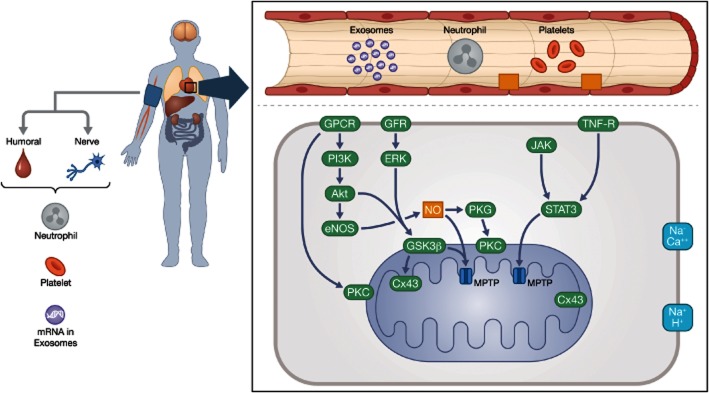
Triggers are substances that are released as an immediate response to the conditioning stimulus. These include adenosine, bradykinin and opioids that activate sarcolemmal receptors thereby initiating the intracellular mediators, mainly consisting of protein kinases that finally act on the effectors, which include mitochondria, connexins and cytoskeletal elements. In line with this conceptual understanding, three individual intracellular pathways have been identified; the nitric oxide-dependent GPCR–endothelial nitric oxide synthase (eNOS)–PKG pathway; the reperfusion-injury salvage kinase pathway based upon PI3K-Akt and glycogen synthase kinase 3β (GSK3β; Hausenloy et al., 2012b); and the survivor activating factor enhancement (Lecour, 2009; Tamareille et al., 2011; Heusch et al., 2012) signalling pathway involving the JAK-STAT system and TNF-α receptors (Heusch et al., 2008; Heusch, 2013), all eventually acting on the mitochondria to modify their energetic and membrane integrity (Figure 1).
Figure 1.
Simplified schematic presentation of the cytosolic signalling pathways that converge to prevent mitochondrial permeability transition pore (MPTP) opening in cardioprotection. eNOS/PGK: the nitric oxide-dependent GPCR–eNOS–PKG pathway; RISK: the reperfusion-injury salvage kinase pathway based upon PKB, PI3K-Akt and GSK3ß; and SAFE: the survivor activating factor enhancement signalling pathway involving the JAK-STAT system and TNF-α receptors. Proteins implicated in MPTP formation include the matrix cyclophilin D (CyD), the inner membrane adenine nucleotide translocase and the outer membrane voltage-dependent anion channel. Additional proteins such as the translocator protein 18 kDa (TSPO), located in the outer mitochondrial membrane, interact with proteins involved in MPTP formation. Under pathophysiological conditions, such as high Ca2+ concentration and increased oxidative stress, the complex forms an open pore between the inner and outer membranes that ultimately results in mitochondrial swelling, mitochondrial Ca2+ efflux and the release of apoptogenic proteins. Cyclosporin A targets matrix CyD, where Ca2+ overload triggers MPTP opening. TRO40303 binds to TSPO in the outer membrane. Other abbreviations: eNOS, endothelial nitric oxide synthase; GFR, growth factor receptors (insulin-like growth factor-1 and fibroblast growth factor-2); GSK3ß, glycogen synthase kinase 3ß; IMM, inner mitochondrial membrane; OMM, outer mitochondrial membrane; TNF-R, tumour necrosis factor receptor.
However appealing this schematic framework appears, we need to point out that it cannot be taken for a biological reality. Indeed, several recent studies indicate that the biological defence system activated by ischaemic conditioning may be far more complex in its nature. For example, the triggers mentioned earlier seem to act in concert with inhibition of platelet and neutrophil activation, and other anti-inflammatory and exosome-based microRNA signalling cascades (see below). Similarly, effector mechanisms may involve more complex long-term effects such as stimulating autophagy and modifying myocardial remodelling, later after the IR event.
First-generation pharmacological strategies and trials
The translation of mechanisms involved in mechanical conditioning into development of pharmacological agents has been attempted for decades.
The first clinical applications of pharmacological conditioning strategies reproduced a single step in cardioprotective signal transduction, mostly based upon findings concerning local ischaemic conditioning. Importantly, the majority of pharmacological cardioprotection trials are based upon experimental evidence of the effect of the drugs in question when administered prior to myocardial ischaemia, whereas the trials mostly tested their effect during evolving MI.
While many are negative, several of these trials have tested relevant putative agents and have provided important information and, hence, a condensed overview of the most prominent trials utilizing drugs sharing known or likely mechanistic properties with ischaemic conditioning will be provided. Several excellent and more comprehensive reviews covering pharmacological conditioning studies have been published recently (Gerczuk and Kloner, 2012; Hausenloy and Yellon, 2013; Heusch, 2013), and as the present paper primarily focuses upon the scope for new drug discovery, the summary below mainly serves as a preliminary to the discussion of potential future pharmacological conditioning.
Adenosine
Early discoveries of the importance of adenosine receptors in the upstream signalling of ischaemic conditioning led to intense investigation of the effect of adenosine itself or adenosine receptor agonists in various models and scenarios of IR injury. Several adenosine receptors are expressed in the myocardium and their individual role in the various forms of cardioprotection is not fully clarified, but stimulation of adenosine A1 and A3 receptors has repeatedly been shown to induce cardioprotection in experimental models (Toombs et al., 1992; Zhao et al., 1993). The protective effects appear to be mediated through PKC and ultimately achieved by inhibition of mitochondrial permeability transition pore (MPTP) formation (McIntosh and Lasley, 2012).
The AMISTAD-I trial (Mahaffey et al., 1999) was one of the first clinical trials investigating pharmacological conditioning as an adjunct to acute myocardial reperfusion therapy in patients admitted with ST-elevation myocardial infarction (STEMI). This randomized placebo-controlled multi-centre trial involving 236 patients showed that adenosine infusion in patients treated with thrombolytic therapy for anterior wall STEMI reduced infarct size up to 50%. The following large-scale AMISTAD II (>2000 patients, Table 1) investigated the effect of adenosine infusion in patients undergoing thrombolysis or primary PCI for anterior STEMI (Ross et al., 2005; Kloner et al., 2006). No difference in the primary clinical endpoint (death or heart failure) was achieved by adenosine, although a post hoc analysis showed that among patients who received early (<3.17 h from symptom onset) reperfusion therapy, those that also received adenosine infusion had lower mortality and fewer events (in-hospital congestive heart failure or rehospitalization for congestive heart failure).
Intracoronary bolus administration immediately prior to reperfusion has not convincingly shown clinical effect, but timing, route and duration of adenosine administration seem crucial, and the optimal therapeutic protocol may not yet have been discovered. Such a protocol may include pretreatment with isoprenaline.
Beta blockers
Cardioselective beta blockers (β-adrenoceptor antagonists) are among the oldest and best-known pharmacological agents used in the treatment of acute and chronic ischaemic heart disease (IHD). Although their position in the modern multi-drug treatment era is frequently discussed, few dispute their beneficial effects in the treatment of acute MI and chronic IHD. Their mechanism of action in chronic IHD is complex and is beyond the scope of this paper, and the acute effects are incompletely understood.
Nevertheless, beta blockers have regained attention lately after the preclinical and clinical studies by Ibanez and colleagues have shown that administration of i.v. metoprolol immediately prior to reperfusion decreased infarct size and improved left ventricular function. In the recent METOCARD-CNIC trial conducted by the same group, i.v. metoprolol administered prior to primary PCI not only reduced myocardial injury but also improved long-term ventricular function and resulted in fewer admissions due to heart failure (Ibanez et al., 2013). Whether these acute cardioprotective effects of beta blockers were achieved through activation of signalling pathways involved in ischaemic conditioning remains to be determined.
Mitochondria, cyclosporin A and the permeability transition pore
Mitochondria are generally considered among the most important final effectors in ischaemic conditioning, and in particular the MPTP has attracted much attention as it is closed during myocardial ischaemia but opens during reperfusion (Griffiths and Halestrap, 1995), which causes mitochondrial swelling, loss of function and, potentially, cellular necrosis. This opening of the MPTP can be modified by ischaemic postconditioning as described previously or by pharmacological intervention with, for example, cyclosporin A (Hausenloy et al., 2002; Waldmeier et al., 2002).
Encouraging experimental studies supporting this hypothesis facilitated translation into clinical trials. Cyclosporin A has been shown to be a promising candidate for pharmacological conditioning agents in clinical studies, most conspicuously in the treatment of acute MI. Notably, Piot et al. in a study of 58 patients admitted with STEMI, randomized subjects to receive a bolus of cyclosporin A or normal saline immediately prior to reperfusion treatment with primary PCI. The authors found that cyclosporin A reduced the release of troponin I and improved left ventricular remodelling compared with controls (Piot et al., 2008; Mewton et al., 2010). Thorough reviews of the MPTP as a pharmacological target and of cyclosporin A as a cardioprotective agent are provided by Halestrap (2010) and Hausenloy et al. (2012a). The CIRCUS (NCT01502774) trial is currently investigating the clinical effects of cyclosporin A in patients admitted with STEMI.
Other pharmacological approaches to prevent or modify the MPTP opening by, for example, volatile anaesthetics (Piriou et al., 2004) or metformin (Bhamra et al., 2008) have shown cardioprotective capacity in experimental studies, but remain to be tested systematically in clinical trials. Similarly, other tactics to manipulate mitochondrial function, for example, through the mitochondrial respiration and electron transport chain may have potential pharmacological perspectives (see below). Most recently, the MITOCARE study investigating the effect of TRO40303, another drug targeting the MPTP, was launched and first results are expected later this year (MITOCARE Study Group, 2012).
The pH hypothesis
Rapid restoration of intracellular pH during reperfusion after the severe acidosis occurring during ischaemia is afforded by washout of lactate and activation of the Na+–H+ exchanger. The resulting rapid increase in pH is believed to generate detrimental effects, including opening of the MPTP and myocardial hypercontracture, eventually resulting in lethal myocardial reperfusion injury (the ‘pH hypothesis’). Slowing down the pH restoration by infusion of an acidic buffer reduces IR injury, and a similar effect can be achieved by pharmacological inhibition of the Na+–H+ exchanger. It is likely that the cardioprotection associated with local postconditioning (stuttered reperfusion by intermittent coronary balloon occlusion), at least in part, is mediated via this effect (Lemasters et al., 1996).
Interestingly, RIC also attenuates hypercontracture during early reperfusion in a porcine model (Schmidt et al., 2007), indicating that RIC may also influence intracellular pH, although this finding may be indirect, via RIC's effect on the MPTP.
Pharmacological attempts to control or slow down the restoration of intracellular pH by inhibition of the Na+–H+ exchanger showed very promising cardioprotective effects in animal models. However, two large clinical trials (GUARDIAN and EXPEDITION) (Boyce et al., 2003; Mentzer et al., 2008) both exploiting the principle of inhibition of the Na+–H+ exchanger by cariporide failed to show clinical benefit of the treatment. Again, drug, timing and treatment protocol may not have been fully optimized to mimic the physiological concept of ‘gentle reperfusion’, and pharmacologically decelerated pH restoration may still find its way into clinical practice.
Insulin and glucagon-like peptide 1 (GLP-1) analogues
Metabolic support with glucose and insulin to alleviate substrate drought during ischaemia and reperfusion is a theoretically attractive principle that has been extensively investigated over several decades. Infusions of a combination of glucose and insulin and potassium salts (GIK) improved cardiac function, modified myocardial energetics and reduced infarct size in a wide range of experimental models of IR syndromes. Furthermore, recent animal studies show that metabolic support with glucose and insulin may be a prerequisite to achieve cardioprotection by RIC in the carbohydrate-dependent heart of the newborn (Schmidt et al., 2014).
Clinically, GIK infusion has been shown to improve myocardial recovery after revascularization surgery or angioplasty in both diabetic and non-diabetic populations (Majid et al., 1972; Coleman et al., 1989; Gradinac et al., 1989; Depre et al., 1999; Kjellman et al., 2000; Lazar et al., 2000), although the clinical relevance of the results have been debated (Bruemmer-Smith et al., 2002; Smith et al., 2002; Castro et al., 2003). Similarly, metabolic support based upon amino acid-enriched cardioplegic solutions was shown to afford cardioprotection in some early studies of MI (Bull et al., 1984; Robertson et al., 1984; Rosenkranz et al., 1984; 1986,; Julia et al., 1991; Kofsky et al., 1991), but later studies have not confirmed this effect (Uyar et al., 2005). GIK infusion during transport to primary PCI, however, may reduce IR injury (Selker et al., 2012).
Equally intriguing and potentially more easily clinically applicable is to use the gut incretin GLP-1, which, apart from its direct metabolic effects, activates anti-apoptotic signalling pathways such as PI3K and MAPK. GLP-1 receptors are present on cardiomyocytes, and GLP-1 and its analogues have been shown to protect against myocardial reperfusion injury (Bose et al., 2005; Zhao et al., 2006; Sonne et al., 2008). Among several GLP-1 analogues, exenatide has repeatedly been shown to provide cardioprotection in animal models (Sonne et al., 2008) and was recently shown to reduce myocardial injury in patients admitted for primary PCI when administered prior to reperfusion (Lonborg et al., 2012a,b,).
Exenatide represents a modern biomimetic drug that exerts complex biological actions by activating upstream events in several physiological signalling cascades. While the mechanisms behind the cardioprotective effect of exenatide may not yet be fully elucidated, they are believed to include the stimulation of glucose over fatty acid metabolism, resulting in more oxygen-efficient ATP production and activation of the PI3K/Akt system also involved in ischaemic conditioning.
Dipeptidyl-peptidase 4 inhibitors seem to have similar cardioprotective effects, at least in diabetic animals (Huisamen et al., 2011), and may prove to be of clinical importance in clinical cardiovascular disease (Hausenloy et al., 2013).
Connexin 43 (Cx43)
Connexins are transmembrane proteins that assemble into hexameric structures (hemichannels), which interconnect cells by the formation of gap junctions by docking of two hemichannels (Boengler et al., 2006). Connexin 43 (Cx43) is the predominant connexin isoform in the cardiomyocyte membrane and is also present in the inner cardiomyocyte mitochondrial membrane. During later phases of ischaemia, Cx43 levels are decreased, which leads to lowered gap junction communication. In contrast, Cx43 levels are increased by ischaemic preconditioning (Boengler et al., 2005), and reduction of Cx43 abolishes the cardioprotection by ischaemic preconditioning (Boengler et al., 2007) but not postconditioning (Schulz et al., 2007). Mitochondrial Cx43 is targeted by several protein kinases, including GSK3, which links Cx43 to one of the down-stream signalling pathways of ischaemic conditioning (Boengler et al., 2007; 2012,).
Apart from its anti-arrhythmic effect, metoprolol seems to increase Cx43 protein levels, independent of protein kinases (Salameh et al., 2010), which might indicate that metoprolol stabilizes Cx43 gap junctions or prevents Cx43 degradation, hence providing a mechanism for the benefits seen in the clinical trial (METOCARD-CNIC) of the acute effects of metoprolol in evolving MI.
Rotigaptide (ZP123) is one of the best-known modulators of Cx43 and has been shown in several experimental studies to protect against IR injury (Haugan et al., 2006; Hennan et al., 2006), although its mechanism of action is still not fully understood. While rotigaptide seems to prevent the ischaemia-related down-regulation/internalization of gap junctions, it seems to be its effect on the mitochondrial Cx43 that results in cardioprotection.
Clinically, limited data exist with rotigaptide and other connexin modulators. Preliminary data suggest a good clinical safety profile of rotigaptide (Kjolbye et al., 2007), but no publications showing cardioprotective benefit in patients were found at the time of writing. Other modulators of Cx43 showing cardioprotective effects in experimental studies, such as GAP-134 and ZP-1609 (danegaptide), are currently under consideration for clinical trials.
Other (failed) pharmacological strategies
In smaller clinical studies, pretreatment with HMG-CoA inhibitors (statins) prior to revascularization reduced myocardial injury (Pasceri et al., 2004), but later larger studies failed to show any clinically relevant effect of high dose atorvastatin prior to primary PCI (Kim et al., 2010; Hahn et al., 2011b; Post et al., 2012). Similarly, the anti-inflammatory drugs pexelizumab (Armstrong and Granger, 2007), erythropoietin (Voors et al., 2010), opioids and nicorandil (Kitakaze et al., 2007), despite promising preclinical data, all failed to afford clinically relevant cardioprotection in larger clinical trials.
An example of direct intervention on a pathway involved in ischaemic conditioning, the PKC-δ inhibitor delcasertib also failed to reduce myocardial injury in patients admitted with STEMI for primary PCI (Lincoff et al., 2014), despite PKC being one of the most intensively studied signalling steps in cardioprotection with several preclinical studies showing cardioprotection by pharmacological control of the PKC activity (Budas et al., 2007). Most recently, a clinical trial (NIAMI) investigating the effect of i.v. sodium nitrite prior to reperfusion in STEMI patients admitted for primary PCI was negative (Siddiqi et al., 2014). However, this was immediately followed by a study by Rassaf et al., showing that circulating nitrite derived from shear stress-dependent stimulation of eNOS at the remote site of RIC contributes to cardioprotection during IR injury, and again timing, dose and delivery site seem crucial (Rassaf et al., 2014a,b,).
Translating RIC into new pharmacological strategies
The search for new adjunctive pharmacological therapies of myocardial IR injury is probably among the most active areas of research today. The repeatedly convincing findings of cardioprotective effects of various forms of ischaemic conditioning therapies in relation to both elective and acute clinical procedures have strongly encouraged the pursuit of drugs that reproduce mechanical conditioning. Below, we will discuss how the most recent and exciting insights into the mechanisms of RIC may contribute to the development of new drugs.
Mimicking triggers and mediators of RIC
Intuitively, the higher up the signalling cascade of ischaemic conditioning a pharmacological agent acts, the closer it will reproduce the complexity of the endogenous signalling system. Consequently, the understanding of what sparks the initial events in, for example, RIC is fundamental in the search for more advanced biomimetic drugs. The signal transduction cascade in RIC begins with neural stimulation followed by release of cardioprotective substances. While the cardioprotective effect of RIC is reliant on intact neural pathways (Steensrud et al., 2010; Donato et al., 2013), it appears possible to mimic the neuronal trigger by acupuncture (Gao et al., 2006; Redington et al., 2013). Furthermore, nociceptor stimulation by topical application of the irritant capsaicin also induces cardioprotection mediated by circulating factors (Jones et al., 2009; Redington et al., 2012). Interestingly, adenosine and NO may also play a role in upstream signalling of RIC as intra-arterial adenosine administration evokes cardioprotection (Liem et al., 2002; Dong et al., 2004) by circulating humoral factors, and this effect is abolished by nerve transection (Steensrud et al., 2010). Although these findings are highly interesting, a larger pharmacological potential may lie in discovering the nature of the cardioprotective circulating humoral factor(s).
The elusive factor X: the Higgs boson of RIC?
Cardioprotective plasma from animals and humans exposed to RIC can be dialysed through a 12 kDa standard membrane, and the dialysate will subsequently protect naive hearts against IR injury (Shimizu et al., 2009; Lim et al., 2010; Jensen et al., 2012; Michelsen et al., 2012). The dialysate can even be frozen or its contents freeze-dried and remain cardioprotective. Furthermore, the eluate of a C18 column, over which cardioprotective dialysate has been passed, is also cardioprotective. Although poignantly termed ‘Factor X’, the small, hydrophobic, cardioprotective content of the dialysate most likely consists of several substances. Nevertheless, it is tempting to search for the trigger molecules in the dialysate as their identification could potentially lead to the development of pharmacological substitutes.
Recently, several studies have suggested putative candidates for factor(s) X. In a proteomic study, Hepponstall et al. identified a number of proteins (including α1-antitrypsin, apolipoproteins and haptoglobin) that were up-regulated in response to RIC (Hepponstall et al., 2012) but also observed a significant down-regulation of other (predominantly inflammatory) proteins, in accordance with a previous genomic study (Konstantinov et al., 2004). More direct evidence for a factor X comes from Davidson et al. They showed that the chemokine CXCL12 (SDF1) and its receptor CXCR4 were crucial to the cardioprotection afforded by RIC, as RIC increased levels of CXCL12, cardioprotection could be induced by administration of CXCL12, and the effect of RIC could be blocked by a CXCR4 antagonist, although only partly, suggesting that other factors may be at play, or that release of this chemokine was an epiphenomenon downstream of another effector.
MicroRNA and exosomes
RNA is usually rapidly degraded in the blood and mainly serves as an intracellular mediator of protein transcription, but recently small non-coding RNAs that circulate in a stable form in blood and regulate gene expression post-transcriptionally have been identified. Such microRNAs (miRNAs or miRs) seem to act as both mediators of and protection against disease, and furthermore may serve as biomarkers of activation of protective conditions or other biological reactions to external stimuli.
Recent studies suggest that miRNAs are also involved in the signalling cascade of IR injury and ischaemic conditioning. Indeed, miRs have been shown to be effector molecules in ischaemic events (Li et al., 2014; Varga et al., 2014), and in one study, limited to analysis of two miRs (miR-1 and miR-21), there was differential expression depending upon the type of stimulus (local vs. remote) (Kukreja et al., 2011).
miRs appear to be intimately involved in the RIC stimulus. In a recent study, myocardial miR-144 levels were shown to be markedly reduced by IR injury alone (Li et al., 2014). The RIC stimulus increased plasma levels of miR-144 in mice and human volunteers, and RIC markedly attenuated the reduction in myocardial miR-144 levels in mice subjected to IR injury. Furthermore, i.v. administration of the miR-144 antagonist (antagomiR-144) abolished the cardioprotection afforded by RIC. Perhaps even more exciting was the finding that i.v. administration of miR-144 not only induced early cardioprotection (<60 min) associated with induced autophagy and increased phospho-Akt, phospho-GSK and phospho-p44/42 MAPK signal but also a delayed window of protection at 24 h and 3 days after IR injury, including down-regulation of the mammalian target of rapamycin (mTor). mTor is a key negative regulator of autophagy and a known target gene of miR-144. Pharmacological stimulation of mTor using rapamycin has been shown to modify post-MI remodelling (and subsequent development of heart failure) in a mouse model, much as has been shown for RIC when administered daily for 28 days after MI in rats. Although not yet studied directly, treatment with miR-144 may therefore provide early, and later, cardioprotection against the acute and chronic effects of myocardial IR injury in patients.
There is growing evidence for the existence of small vesicles, termed exosomes, that act as key components in the transport of miRNAs or their precursors. Although direct evidence for exosome trafficking, containing a nucleic acid signalling cargo, to ischaemic sites and the fate of the exosomes is lacking, the discovery of exosomes has presented a fascinating signalling system in the body. Indeed, miRNAs have been shown to be effector molecules in ischaemic events (Kukreja et al., 2011; Bhalala et al., 2013), with compelling evidence for a prominent role of exosomes in distant cell–cell transport. Exosomes enriched with miR-22 and miR-451 from anoxic cultured muscle stem cells (Feng et al., 2014) and cardiomyocyte progenitor cells (Chen et al., 2013) reduced cardiac damage in mouse myocardial ischaemia models. In relation to ischaemic conditioning, Ferdinandy's group recently demonstrated increased exosome release in the coronary effluent of locally preconditioned hearts, and that those exosomes were cardioprotective (Giricz et al., 2014). Furthermore, in this study of miR-144 biology in relation to RIC, the double-stranded hairpin precursor of miR-144 was increased over fourfold in the exosomes of animals subjected to RIC, using transient lower limb ischaemia.
Based upon conditioning-specific miRNA signatures, it may be possible to develop an exosome-based delivery system with a multifaceted RNAi payload capable of either sequestering using miRNA sponges or antagomiRs targeted at detrimental miRNAs associated with disease progression or introduction of beneficial miRNAs to alleviate disease burden, ultimately leading to the utilization of exosomes as nanocarriers of nucleic acid-based therapeutics in personalized treatment.
Mimicking effectors of ischaemic conditioning within the cell
The intensive studies of the effector mechanisms of ischaemic conditioning have identified a number of potential targets for pharmacological intervention. Of these, the MPTP and other mitochondrial signalling systems appear to be the most promising candidates for pharmacological intervention.
DJ-1
DJ-1 is a widely expressed and conserved mitochondrial protein that has been implicated in numerous pathologies, most notably in neurodegeneration where mutations in DJ-1 ultimately result in an early onset, familial form of Parkinson's disease (Bonifati et al., 2003). In relation to IR injury, DJ-1 has interesting properties, including oxidative stress sensing and reactive oxygen species scavenging. In a recent study by Dongworth et al. in a murine model of IR injury, overexpression of DJ-1 delayed MPTP opening and reduced cell death, whereas DJ-1 deficiency increased cell death and mitochondrial fragmentation (Dongworth et al., 2014). DJ-1 can be up-regulated by sodium 4-phenylbutyrate and may therefore represent a new therapeutic target.
Mineralocorticoid receptor antagonists
It is well established that the mineralocorticoid receptor antagonist spironolactone improves prognosis in patients with chronic heart failure (Pitt et al., 1999). Paradoxically, high plasma levels of aldosterone are associated with increased mortality in acute cardiovascular disease (Beygui et al., 2006).
Nonetheless, administration of mineralocorticoid receptor antagonists before ischaemia (Chai et al., 2005; 2006,) or at time of reperfusion (Schmidt et al., 2010) reduces myocardial IR injury in animal models, and this effect appears to be independent of aldosterone itself and shares mechanisms with ischaemic conditioning. For a thorough review, please see van den Berg et al. (2014).
Currently, two large trials [ALBATROSS (NCT01059136) and REMINDER (NCT01176968)] are investigating the effects of mineralocorticoid receptor antagonists in patients admitted with acute coronary syndrome.
The heart outside the cardiomyocyte
RIC attenuates IR injury-related endothelial dysfunction through mechanisms that are generally believed to be NO dependent (Heusch et al., 2008) and the well-described cardioprotective effects of the NO donor S-nitroso-N-acetylpenicillamine may also be exerted through preservation of endothelial function. Similarly, microvascular obstruction is also important in relation to myocardial IR injury, and new imaging techniques allow for visualization of capillary function (Ostergaard et al., 2014) and better understanding of microvascular dysfunction. Hence, vascular dysfunction after ischaemia may prove to be another important part of IR injury that can be targeted by pharmacological intervention, albeit beyond the scope of the current article.
Although IR injury is partly caused by an inflammatory response to ischaemia, pharmacological anti-inflammatory agents such as pexelizumab (antibody to the C5 component of the complement system) have not shown clinical effect in larger trials (Armstrong and Granger, 2007). Another anti-inflammatory agent, the naturally occurring fibrin-derived peptide FX06, reduced infarct size in patients treated with primary PCI for STEMI (Atar et al., 2009) in a proof-of-concept study but has not yet been tested in a large-scale trial.
Summary
The increasing insight into the mechanisms behind the cardioprotective effects of RIC has uncovered several targets for pharmacological intervention that potentially may partly reproduce the effects of mechanical conditioning. While the first generation of drugs used for adjunctive therapy against IR injury has been disappointing, newer trials investigating more advanced treatment strategies with GLP-1 analogues and cyclosporin A are more promising.
Importantly, the recent discoveries of microRNA signalling and exosome-based transport may open for a completely new field of pharmacological cardioprotection, potentially closely reproducing the biological effects of RIC.
As RIC induces a wide range of systemic effects including multi-organ protection, anti-inflammatory, reduced platelet activation and increased exercise performance, perhaps indicating that this inherent protection system in mammalian species is a fundamental and complex part of the biological response to stress, it may yet prove too complex to be fully reproduced by a single pharmacological intervention. Future work should consider combination therapies or ‘up-stream’ intervention, which may mimic the signalling systems sufficiently to achieve clinically relevant organ protection comparable to the effects of remote and local ischaemic conditioning.
Acknowledgments
This work was supported by the Danish Council for Strategic Research (11-115818), The Danish Research Council (11-108354) and Fondation Leducq (06CVD).
Glossary
- Cx43
connexin 43
- eNOS
endothelial nitric oxide synthase
- GIK
glucose-insulin-potassium
- GLP-1
glucagon-like peptide 1
- IHD
ischaemic heart disease
- IR
ischaemia–reperfusion
- MACCE
major adverse cardiac and/or cerebrovascular event
- MI
myocardial infarction
- miR
microRNA
- MPTP
mitochondrial permeability transition pore
- PCI
percutaneous coronary intervention
- RIC
remote ischaemic conditioning
- STEMI
ST-elevation myocardial infarction
Conflict of interest
M. R. S., A. R. and H. E. B. are shareholders of CellAegis Inc.
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL. Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G Protein-Coupled Receptors. Br J Pharmacol. 2013a;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL. Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Nuclear Hormone Receptors. Br J Pharmacol. 2013b;170:1652–1675. doi: 10.1111/bph.12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL. Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Catalytic Receptors. Br J Pharmacol. 2013c;170:1676–1705. doi: 10.1111/bph.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL. Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol. 2013d;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Catterall WA, et al. The Concise Guide to PHARMACOLOGY 2013/14: Ion Channels. Br J Pharmacol. 2013e;170:1607–1651. doi: 10.1111/bph.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali ZA, Callaghan CJ, Lim E, Ali AA, Nouraei SA, Akthar AM, et al. Remote ischemic preconditioning reduces myocardial and renal injury after elective abdominal aortic aneurysm repair: a randomized controlled trial. Circulation. 2007;116:105–198. doi: 10.1161/circulationaha.106.679167. [DOI] [PubMed] [Google Scholar]
- Armstrong PW, Granger CB. Pexelizumab and the APEX AMI trial. JAMA. 2007;297:1881. doi: 10.1001/jama.297.17.1881-b. , author reply 1881–1882. [DOI] [PubMed] [Google Scholar]
- Atar D, Petzelbauer P, Schwitter J, Huber K, Rensing B, Kasprzak JD, et al. Effect of intravenous FX06 as an adjunct to primary percutaneous coronary intervention for acute ST-segment elevation myocardial infarction results of the F.I.R.E. (Efficacy of FX06 in the Prevention of Myocardial Reperfusion Injury) trial. J Am Coll Cardiol. 2009;53:720–729. doi: 10.1016/j.jacc.2008.12.017. [DOI] [PubMed] [Google Scholar]
- Bates E, Bode C, Costa M, Gibson CM, Granger C, Green C, et al. Intracoronary KAI-9803 as an adjunct to primary percutaneous coronary intervention for acute ST-segment elevation myocardial infarction. Circulation. 2008;117:886–896. doi: 10.1161/CIRCULATIONAHA.107.759167. [DOI] [PubMed] [Google Scholar]
- van den Berg TN, Rongen GA, Frohlich GM, Deinum J, Hausenloy DJ, Riksen NP. The cardioprotective effects of mineralocorticoid receptor antagonists. Pharmacol Ther. 2014;142:72–87. doi: 10.1016/j.pharmthera.2013.11.006. [DOI] [PubMed] [Google Scholar]
- Beygui F, Collet JP, Benoliel JJ, Vignolles N, Dumaine R, Barthelemy O, et al. High plasma aldosterone levels on admission are associated with death in patients presenting with acute ST-elevation myocardial infarction. Circulation. 2006;114:2604–2610. doi: 10.1161/CIRCULATIONAHA.106.634626. [DOI] [PubMed] [Google Scholar]
- Bhalala OG, Srikanth M, Kessler JA. The emerging roles of microRNAs in CNS injuries. Nat Rev Neurol. 2013;9:328–339. doi: 10.1038/nrneurol.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhamra GS, Hausenloy DJ, Davidson SM, Carr RD, Paiva M, Wynne AM, et al. Metformin protects the ischemic heart by the Akt-mediated inhibition of mitochondrial permeability transition pore opening. Basic Res Cardiol. 2008;103:274–284. doi: 10.1007/s00395-007-0691-y. [DOI] [PubMed] [Google Scholar]
- Boengler K, Dodoni G, Rodriguez-Sinovas A, Cabestrero A, Ruiz-Meana M, Gres P, et al. Connexin 43 in cardiomyocyte mitochondria and its increase by ischemic preconditioning. Cardiovasc Res. 2005;67:234–244. doi: 10.1016/j.cardiores.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Boengler K, Schulz R, Heusch G. Connexin 43 signalling and cardioprotection. Heart. 2006;92:1724–1727. doi: 10.1136/hrt.2005.066878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boengler K, Konietzka I, Buechert A, Heinen Y, Garcia-Dorado D, Heusch G, et al. Loss of ischemic preconditioning's cardioprotection in aged mouse hearts is associated with reduced gap junctional and mitochondrial levels of connexin 43. Am J Physiol Heart Circ Physiol. 2007;292:H1764–H1769. doi: 10.1152/ajpheart.01071.2006. [DOI] [PubMed] [Google Scholar]
- Boengler K, Ruiz-Meana M, Gent S, Ungefug E, Soetkamp D, Miro-Casas E, et al. Mitochondrial connexin 43 impacts on respiratory complex I activity and mitochondrial oxygen consumption. J Cell Mol Med. 2012;16:1649–1655. doi: 10.1111/j.1582-4934.2011.01516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset Parkinsonism. Science. 2003;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- Bose AK, Mocanu MM, Carr RD, Brand CL, Yellon DM. Glucagon-like peptide 1 can directly protect the heart against ischemia/reperfusion injury. Diabetes. 2005;54:146–151. doi: 10.2337/diabetes.54.1.146. [DOI] [PubMed] [Google Scholar]
- Botker HE, Kharbanda R, Schmidt MR, Bottcher M, Kaltoft AK, Terkelsen CJ, et al. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet. 2010;375:727–734. doi: 10.1016/S0140-6736(09)62001-8. [DOI] [PubMed] [Google Scholar]
- Boyce SW, Bartels C, Bolli R, Chaitman B, Chen JC, Chi E, et al. Impact of sodium-hydrogen exchange inhibition by cariporide on death or myocardial infarction in high-risk CABG surgery patients: results of the CABG surgery cohort of the GUARDIAN study. J Thorac Cardiovasc Surg. 2003;126:420–427. doi: 10.1016/s0022-5223(03)00209-5. [DOI] [PubMed] [Google Scholar]
- Bruemmer-Smith S, Avidan MS, Harris B, Sudan S, Sherwood R, Desai JB, et al. Glucose, insulin and potassium for heart protection during cardiac surgery. Br J Anaesth. 2002;88:489–495. doi: 10.1093/bja/88.4.489. [DOI] [PubMed] [Google Scholar]
- Budas GR, Churchill EN, Mochly-Rosen D. Cardioprotective mechanisms of PKC isozyme-selective activators and inhibitors in the treatment of ischemia-reperfusion injury. Pharmacol Res. 2007;55:523–536. doi: 10.1016/j.phrs.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Bull C, Cooper J, Stark J. Cardioplegic protection of the child's heart. J Thorac Cardiovasc Surg. 1984;88:287–293. [PubMed] [Google Scholar]
- Castro PF, Larrain G, Baeza R, Corbalan R, Nazzal C, Greig DP, et al. Effects of glucose-insulin-potassium solution on myocardial salvage and left ventricular function after primary angioplasty. Crit Care Med. 2003;31:2152–2155. doi: 10.1097/01.CCM.0000079604.46997.7B. [DOI] [PubMed] [Google Scholar]
- Chai W, Garrelds IM, Arulmani U, Schoemaker RG, Lamers JM, Danser AH. Genomic and nongenomic effects of aldosterone in the rat heart: why is spironolactone cardioprotective? Br J Pharmacol. 2005;145:664–671. doi: 10.1038/sj.bjp.0706220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai W, Garrelds IM, de Vries R, Danser AH. Cardioprotective effects of eplerenone in the rat heart: interaction with locally synthesized or blood-derived aldosterone? Hypertension. 2006;47:665–670. doi: 10.1161/01.HYP.0000205831.39339.a5. [DOI] [PubMed] [Google Scholar]
- Chen L, Wang Y, Pan Y, Zhang L, Shen C, Qin G, et al. Cardiac progenitor-derived exosomes protect ischemic myocardium from acute ischemia/reperfusion injury. Biochem Biophys Res Commun. 2013;431:566–571. doi: 10.1016/j.bbrc.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung MM, Kharbanda RK, Konstantinov IE, Shimizu M, Frndova H, Li J, et al. Randomized controlled trial of the effects of remote ischemic preconditioning on children undergoing cardiac surgery: first clinical application in humans. J Am Coll Cardiol. 2006;47:2277–2282. doi: 10.1016/j.jacc.2006.01.066. [DOI] [PubMed] [Google Scholar]
- Coleman GM, Gradinac S, Taegtmeyer H, Sweeney M, Frazier OH. Efficacy of metabolic support with glucose-insulin-potassium for left ventricular pump failure after aortocoronary bypass surgery. Circulation. 1989;80:I91–I96. [PubMed] [Google Scholar]
- Davies WR, Brown AJ, Watson W, McCormick LM, West NE, Dutka DP, et al. Remote ischemic preconditioning improves outcome at 6 years after elective percutaneous coronary intervention: the CRISP stent trial long-term follow-up. Circ Cardiovasc Interv. 2013;6:246–251. doi: 10.1161/CIRCINTERVENTIONS.112.000184. [DOI] [PubMed] [Google Scholar]
- Depre C, Vanoverschelde JL, Taegtmeyer H. Glucose for the heart. Circulation. 1999;99:578–588. doi: 10.1161/01.cir.99.4.578. [DOI] [PubMed] [Google Scholar]
- Donato M, Buchholz B, Rodriguez M, Perez V, Inserte J, Garcia-Dorado D, et al. Role of the parasympathetic nervous system in cardioprotection by remote hindlimb ischaemic preconditioning. Exp Physiol. 2013;98:425–434. doi: 10.1113/expphysiol.2012.066217. [DOI] [PubMed] [Google Scholar]
- Dong JH, Liu YX, Ji ES, He RR. Limb ischemic preconditioning reduces infarct size following myocardial ischemia-reperfusion in rats. Sheng Li Xue Bao. 2004;56:41–46. [PubMed] [Google Scholar]
- Dongworth RK, Mukherjee UA, Hall AR, Astin R, Ong SB, Yao Z, et al. DJ-1 protects against cell death following acute cardiac ischemia-reperfusion injury. Cell Death Dis. 2014;5:e1082. doi: 10.1038/cddis.2014.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Huang W, Wani M, Yu X, Ashraf M. Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting Mecp2 via miR-22. PLoS ONE. 2014;9:e88685. doi: 10.1371/journal.pone.0088685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Fu W, Jin Z, Yu X. A preliminary study on the cardioprotection of acupuncture pretreatment in rats with ischemia and reperfusion: involvement of cardiac beta-adrenoceptors. J Physiol Sci. 2006;56:275–279. doi: 10.2170/physiolsci.RP006606. [DOI] [PubMed] [Google Scholar]
- Gerczuk PZ, Kloner RA. An update on cardioprotection: a review of the latest adjunctive therapies to limit myocardial infarction size in clinical trials. J Am Coll Cardiol. 2012;59:969–978. doi: 10.1016/j.jacc.2011.07.054. [DOI] [PubMed] [Google Scholar]
- Giricz Z, Varga ZV, Baranyai T, Sipos P, Paloczi K, Kittel A, et al. Cardioprotection by remote ischemic preconditioning of the rat heart is mediated by extracellular vesicles. J Mol Cell Cardiol. 2014;68:75–78. doi: 10.1016/j.yjmcc.2014.01.004. [DOI] [PubMed] [Google Scholar]
- Gradinac S, Coleman GM, Taegtmeyer H, Sweeney MS, Frazier OH. Improved cardiac function with glucose-insulin-potassium after aortocoronary bypass grafting. Ann Thorac Surg. 1989;48:484–489. doi: 10.1016/s0003-4975(10)66844-0. [DOI] [PubMed] [Google Scholar]
- Griffiths EJ, Halestrap AP. Mitochondrial non-specific pores remain closed during cardiac ischaemia, but open upon reperfusion. Biochem J. 1995;307(Pt 1):93–98. doi: 10.1042/bj3070093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn CD, Manlhiot C, Schmidt MR, Nielsen TT, Redington AN. Remote ischemic per-conditioning: a novel therapy for acute stroke? Stroke. 2011a;42:2960–2962. doi: 10.1161/STROKEAHA.111.622340. [DOI] [PubMed] [Google Scholar]
- Hahn JY, Kim HJ, Choi YJ, Jo SH, Kim HJ, Lee S, et al. Effects of atorvastatin pretreatment on infarct size in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am Heart J. 2011b;162:1026–1033. doi: 10.1016/j.ahj.2011.08.011. [DOI] [PubMed] [Google Scholar]
- Halestrap AP. A pore way to die: the role of mitochondria in reperfusion injury and cardioprotection. Biochem Soc Trans. 2010;38:841–860. doi: 10.1042/BST0380841. [DOI] [PubMed] [Google Scholar]
- Harkin DW, Barros D, Sa AA, McCallion K, Hoper M, Campbell FC. Ischemic preconditioning before lower limb ischemia–reperfusion protects against acute lung injury. J Vasc Surg. 2002;35:1264–1273. doi: 10.1067/mva.2002.121981. [DOI] [PubMed] [Google Scholar]
- Haugan K, Marcussen N, Kjolbye AL, Nielsen MS, Hennan JK, Petersen JS. Treatment with the gap junction modifier rotigaptide (ZP123) reduces infarct size in rats with chronic myocardial infarction. J Cardiovasc Pharmacol. 2006;47:236–242. doi: 10.1097/01.fjc.0000200990.31611.6e. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest. 2013;123:92–100. doi: 10.1172/JCI62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausenloy DJ, Maddock HL, Baxter GF, Yellon DM. Inhibiting mitochondrial permeability transition pore opening: a new paradigm for myocardial preconditioning? Cardiovasc Res. 2002;55:534–543. doi: 10.1016/s0008-6363(02)00455-8. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Boston-Griffiths EA, Yellon DM. Cyclosporin A and cardioprotection: from investigative tool to therapeutic agent. Br J Pharmacol. 2012a;165:1235–1245. doi: 10.1111/j.1476-5381.2011.01700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausenloy DJ, Iliodromitis EK, Andreadou I, Papalois A, Gritsopoulos G, Anastasiou-Nana M, et al. Investigating the signal transduction pathways underlying remote ischemic conditioning in the porcine heart. Cardiovasc Drugs Ther. 2012b;26:87–93. doi: 10.1007/s10557-011-6364-y. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Whittington HJ, Wynne AM, Begum SS, Theodorou L, Riksen N, et al. Dipeptidyl peptidase-4 inhibitors and GLP-1 reduce myocardial infarct size in a glucose-dependent manner. Cardiovasc Diabetol. 2013;12:154. doi: 10.1186/1475-2840-12-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennan JK, Swillo RE, Morgan GA, Keith JC, Jr, Schaub RG, Smith RP, et al. Rotigaptide (ZP123) prevents spontaneous ventricular arrhythmias and reduces infarct size during myocardial ischemia/reperfusion injury in open-chest dogs. J Pharmacol Exp Ther. 2006;317:236–243. doi: 10.1124/jpet.105.096933. [DOI] [PubMed] [Google Scholar]
- Hepponstall M, Ignjatovic V, Binos S, Monagle P, Jones B, Cheung MH, et al. Remote ischemic preconditioning (RIPC) modifies plasma proteome in humans. PLoS ONE. 2012;7:e48284. doi: 10.1371/journal.pone.0048284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heusch G. Cardioprotection: chances and challenges of its translation to the clinic. Lancet. 2013;381:166–175. doi: 10.1016/S0140-6736(12)60916-7. [DOI] [PubMed] [Google Scholar]
- Heusch G, Boengler K, Schulz R. Cardioprotection: nitric oxide, protein kinases, and mitochondria. Circulation. 2008;118:1915–1919. doi: 10.1161/CIRCULATIONAHA.108.805242. [DOI] [PubMed] [Google Scholar]
- Heusch G, Musiolik J, Kottenberg E, Peters J, Jakob H, Thielmann M. STAT5 activation and cardioprotection by remote ischemic preconditioning in humans. Circ Res. 2012;110:111–115. doi: 10.1161/CIRCRESAHA.111.259556. [DOI] [PubMed] [Google Scholar]
- Hoole SP, Heck PM, Sharples L, Khan SN, Duehmke R, Densem CG, et al. Cardiac Remote Ischemic Preconditioning in Coronary Stenting (CRISP Stent) Study: a prospective, randomized control trial. Circulation. 2009;119:820–827. doi: 10.1161/CIRCULATIONAHA.108.809723. [DOI] [PubMed] [Google Scholar]
- Hougaard KD, Hjort N, Zeidler D, Sorensen L, Norgaard A, Hansen TM, et al. Remote ischemic perconditioning as an adjunct therapy to thrombolysis in patients with acute ischemic stroke: a randomized trial. Stroke. 2014;45:159–167. doi: 10.1161/STROKEAHA.113.001346. [DOI] [PubMed] [Google Scholar]
- Huisamen B, Genis A, Marais E, Lochner A. Pre-treatment with a DPP-4 inhibitor is infarct sparing in hearts from obese, pre-diabetic rats. Cardiovasc Drugs Ther. 2011;25:13–20. doi: 10.1007/s10557-010-6271-7. [DOI] [PubMed] [Google Scholar]
- Ibanez B, Macaya C, Sanchez-Brunete V, Pizarro G, Fernandez-Friera L, Mateos A, et al. Effect of early metoprolol on infarct size in ST-segment-elevation myocardial infarction patients undergoing primary percutaneous coronary intervention: the Effect of Metoprolol in Cardioprotection During an Acute Myocardial Infarction (METOCARD-CNIC) trial. Circulation. 2013;128:1495–1503. doi: 10.1161/CIRCULATIONAHA.113.003653. [DOI] [PubMed] [Google Scholar]
- Jan WC, Chen CH, Tsai PS, Huang CJ. Limb ischemic preconditioning mitigates lung injury induced by haemorrhagic shock/resuscitation in rats. Resuscitation. 2011;82:760–766. doi: 10.1016/j.resuscitation.2011.02.010. [DOI] [PubMed] [Google Scholar]
- Jensen RV, Stottrup NB, Kristiansen SB, Botker HE. Release of a humoral circulating cardioprotective factor by remote ischemic preconditioning is dependent on preserved neural pathways in diabetic patients. Basic Res Cardiol. 2012;107:285. doi: 10.1007/s00395-012-0285-1. [DOI] [PubMed] [Google Scholar]
- Jones WK, Fan GC, Liao S, Zhang JM, Wang Y, Weintraub NL, et al. Peripheral nociception associated with surgical incision elicits remote nonischemic cardioprotection via neurogenic activation of protein kinase C signaling. Circulation. 2009;120:S1–S9. doi: 10.1161/CIRCULATIONAHA.108.843938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julia PL, Buckberg GD, Acar C, Partington MT, Sherman MP. Studies of controlled reperfusion after ischemia. XXI. Reperfusate composition: superiority of blood cardioplegia over crystalloid cardioplegia in limiting reperfusion damage – importance of endogenous oxygen free radical scavengers in red blood cells. J Thorac Cardiovasc Surg. 1991;101:303–313. [PubMed] [Google Scholar]
- Kanoria S, Jalan R, Davies NA, Seifalian AM, Williams R, Davidson BR. Remote ischaemic preconditioning of the hind limb reduces experimental liver warm ischaemia-reperfusion injury. Br J Surg. 2006;93:762–768. doi: 10.1002/bjs.5331. [DOI] [PubMed] [Google Scholar]
- Kharbanda RK, Mortensen UM, White PA, Kristiansen SB, Schmidt MR, Hoschtitzky JA, et al. Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation. 2002;106:2881–2883. doi: 10.1161/01.cir.0000043806.51912.9b. [DOI] [PubMed] [Google Scholar]
- Kharbanda RK, Li J, Konstantinov IE, Cheung MM, White PA, Frndova H, et al. Remote ischaemic preconditioning protects against cardiopulmonary bypass-induced tissue injury: a preclinical study. Heart. 2006;92:1506–1511. doi: 10.1136/hrt.2004.042366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharbanda RK, Nielsen TT, Redington AN. Translation of remote ischaemic preconditioning into clinical practice. Lancet. 2009;374:1557–1565. doi: 10.1016/S0140-6736(09)61421-5. [DOI] [PubMed] [Google Scholar]
- Kim JS, Kim J, Choi D, Lee CJ, Lee SH, Ko YG, et al. Efficacy of high-dose atorvastatin loading before primary percutaneous coronary intervention in ST-segment elevation myocardial infarction: the STATIN STEMI trial. JACC Cardiovasc Interv. 2010;3:332–339. doi: 10.1016/j.jcin.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Kitakaze M, Asakura M, Kim J, Shintani Y, Asanuma H, Hamasaki T, et al. Human atrial natriuretic peptide and nicorandil as adjuncts to reperfusion treatment for acute myocardial infarction (J-WIND): two randomised trials. Lancet. 2007;370:1483–1493. doi: 10.1016/S0140-6736(07)61634-1. [DOI] [PubMed] [Google Scholar]
- Kjellman UW, Bjork K, Dahlin A, Ekroth R, Kirno K, Svensson G, et al. Insulin(GIK) improves myocardial metabolism in patients during blood cardioplegia. Scand Cardiovasc J. 2000;34:321–330. doi: 10.1080/713783123. [DOI] [PubMed] [Google Scholar]
- Kjolbye AL, Haugan K, Hennan JK, Petersen JS. Pharmacological modulation of gap junction function with the novel compound rotigaptide: a promising new principle for prevention of arrhythmias. Basic Clin Pharmacol Toxicol. 2007;101:215–230. doi: 10.1111/j.1742-7843.2007.00123.x. [DOI] [PubMed] [Google Scholar]
- Kloner RA, Forman MB, Gibbons RJ, Ross AM, Alexander RW, Stone GW. Impact of time to therapy and reperfusion modality on the efficacy of adenosine in acute myocardial infarction: the AMISTAD-2 trial. Eur Heart J. 2006;27:2400–2405. doi: 10.1093/eurheartj/ehl094. [DOI] [PubMed] [Google Scholar]
- Kofsky E, Julia P, Buckberg GD, Young H, Tixier D. Studies of myocardial protection in the immature heart. V. Safety of prolonged aortic clamping with hypocalcemic glutamate/aspartate blood cardioplegia. J Thorac Cardiovasc Surg. 1991;101:33–43. [PubMed] [Google Scholar]
- Konstantinov IE, Arab S, Kharbanda RK, Li J, Cheung MM, Cherepanov V, et al. The remote ischemic preconditioning stimulus modifies inflammatory gene expression in humans. Physiol Genomics. 2004;19:143–150. doi: 10.1152/physiolgenomics.00046.2004. [DOI] [PubMed] [Google Scholar]
- Konstantinov IE, Li J, Cheung MM, Shimizu M, Stokoe J, Kharbanda RK, et al. Remote ischemic preconditioning of the recipient reduces myocardial ischemia-reperfusion injury of the denervated donor heart via a Katp channel-dependent mechanism. Transplantation. 2005a;79:1691–1695. doi: 10.1097/01.tp.0000159137.76400.5d. [DOI] [PubMed] [Google Scholar]
- Konstantinov IE, Arab S, Li J, Coles JG, Boscarino C, Mori A, et al. The remote ischemic preconditioning stimulus modifies gene expression in mouse myocardium. J Thorac Cardiovasc Surg. 2005b;130:1326–1332. doi: 10.1016/j.jtcvs.2005.03.050. [DOI] [PubMed] [Google Scholar]
- Kukreja RC, Yin C, Salloum FN. MicroRNAs: new players in cardiac injury and protection. Mol Pharmacol. 2011;80:558–564. doi: 10.1124/mol.111.073528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai IR, Chang KJ, Chen CF, Tsai HW. Transient limb ischemia induces remote preconditioning in liver among rats: the protective role of heme oxygenase-1. Transplantation. 2006;81:1311–1317. doi: 10.1097/01.tp.0000203555.14546.63. [DOI] [PubMed] [Google Scholar]
- Lazar HL, Chipkin S, Philippides G, Bao Y, Apstein C. Glucose-insulin-potassium solutions improve outcomes in diabetics who have coronary artery operations. Ann Thorac Surg. 2000;70:145–150. doi: 10.1016/s0003-4975(00)01317-5. [DOI] [PubMed] [Google Scholar]
- Lazaris AM, Maheras AN, Vasdekis SN, Karkaletsis KG, Charalambopoulos A, Kakisis JD, et al. Protective effect of remote ischemic preconditioning in renal ischemia/reperfusion injury, in a model of thoracoabdominal aorta approach. J Surg Res. 2009;154:267–273. doi: 10.1016/j.jss.2008.06.037. [DOI] [PubMed] [Google Scholar]
- Lecour S. Activation of the protective survivor activating factor enhancement (SAFE) pathway against reperfusion injury: does it go beyond the RISK pathway? J Mol Cell Cardiol. 2009;47:32–40. doi: 10.1016/j.yjmcc.2009.03.019. [DOI] [PubMed] [Google Scholar]
- Lemasters JJ, Bond JM, Chacon E, Harper IS, Kaplan SH, Ohata H, et al. The pH paradox in ischemia-reperfusion injury to cardiac myocytes. EXS. 1996;76:99–114. doi: 10.1007/978-3-0348-8988-9_7. [DOI] [PubMed] [Google Scholar]
- Li J, Rohalia S, Gelber N, Rutka J, Sabah N, Gladstone R, et al. MicroRNA-144 is a circulating effector of remote ischemic preconditioning. Basic Res Cardiol. 2014;109:423. doi: 10.1007/s00395-014-0423-z. [DOI] [PubMed] [Google Scholar]
- Liem DA, Verdouw PD, Ploeg H, Kazim S, Duncker DJ. Sites of action of adenosine in interorgan preconditioning of the heart. Am J Physiol Heart Circ Physiol. 2002;283:H29–H37. doi: 10.1152/ajpheart.01031.2001. [DOI] [PubMed] [Google Scholar]
- Lim SY, Yellon DM, Hausenloy DJ. The neural and humoral pathways in remote limb ischemic preconditioning. Basic Res Cardiol. 2010;105:651–655. doi: 10.1007/s00395-010-0099-y. [DOI] [PubMed] [Google Scholar]
- Lincoff AM, Roe M, Aylward P, Galla J, Rynkiewicz A, Guetta V, et al. Inhibition of delta-protein kinase C by delcasertib as an adjunct to primary percutaneous coronary intervention for acute anterior ST-segment elevation myocardial infarction: results of the PROTECTION AMI Randomized Controlled Trial. Eur Heart J. 2014;35:2516–2523. doi: 10.1093/eurheartj/ehu177. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. Heart disease and stroke statistics – 2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–e181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. Executive summary: heart disease and stroke statistics – 2010 update: a report from the American Heart Association. Circulation. 2010;121:948–954. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- Lonborg J, Kelbaek H, Vejlstrup N, Jorgensen E, Helqvist S, Saunamaki K, et al. Cardioprotective effects of ischemic postconditioning in patients treated with primary percutaneous coronary intervention, evaluated by magnetic resonance. Circ Cardiovasc Interv. 2010;3:34–41. doi: 10.1161/CIRCINTERVENTIONS.109.905521. [DOI] [PubMed] [Google Scholar]
- Lonborg J, Kelbaek H, Vejlstrup N, Botker HE, Kim WY, Holmvang L, et al. Exenatide reduces final infarct size in patients with ST-segment-elevation myocardial infarction and short-duration of ischemia. Circ Cardiovasc Interv. 2012a;5:288–295. doi: 10.1161/CIRCINTERVENTIONS.112.968388. [DOI] [PubMed] [Google Scholar]
- Lonborg J, Vejlstrup N, Kelbaek H, Botker HE, Kim WY, Mathiasen AB, et al. Exenatide reduces reperfusion injury in patients with ST-segment elevation myocardial infarction. Eur Heart J. 2012b;33:1491–1499. doi: 10.1093/eurheartj/ehr309. [DOI] [PubMed] [Google Scholar]
- Loukogeorgakis SP, Panagiotidou AT, Broadhead MW, Donald A, Deanfield JE, MacAllister RJ. Remote ischemic preconditioning provides early and late protection against endothelial ischemia-reperfusion injury in humans: role of the autonomic nervous system. J Am Coll Cardiol. 2005;46:450–456. doi: 10.1016/j.jacc.2005.04.044. [DOI] [PubMed] [Google Scholar]
- Mahaffey KW, Puma JA, Barbagelata NA, DiCarli MF, Leesar MA, Browne KF, et al. Adenosine as an adjunct to thrombolytic therapy for acute myocardial infarction: results of a multicenter, randomized, placebo-controlled trial: the Acute Myocardial Infarction STudy of ADenosine (AMISTAD) trial. J Am Coll Cardiol. 1999;34:1711–1720. doi: 10.1016/s0735-1097(99)00418-0. [DOI] [PubMed] [Google Scholar]
- Majid PA, Sharma B, Meeran MK, Taylor SH. Insulin and glucose in the treatment of heart-failure. Lancet. 1972;2:937–941. doi: 10.1016/s0140-6736(72)92468-3. [DOI] [PubMed] [Google Scholar]
- McIntosh VJ, Lasley RD. Adenosine receptor-mediated cardioprotection: are all 4 subtypes required or redundant? J Cardiovasc Pharmacol Ther. 2012;17:21–33. doi: 10.1177/1074248410396877. [DOI] [PubMed] [Google Scholar]
- Mehta SR, Yusuf S, Diaz R, Zhu J, Pais P, Xavier D, et al. Effect of glucose-insulin-potassium infusion on mortality in patients with acute ST-segment elevation myocardial infarction: the CREATE-ECLA randomized controlled trial. JAMA. 2005;293:437–446. doi: 10.1001/jama.293.4.437. [DOI] [PubMed] [Google Scholar]
- Mentzer RM, Jr, Bartels C, Bolli R, Boyce S, Buckberg GD, Chaitman B, et al. Sodium-hydrogen exchange inhibition by cariporide to reduce the risk of ischemic cardiac events in patients undergoing coronary artery bypass grafting: results of the EXPEDITION study. Ann Thorac Surg. 2008;85:1261–1270. doi: 10.1016/j.athoracsur.2007.10.054. [DOI] [PubMed] [Google Scholar]
- Mewton N, Croisille P, Gahide G, Rioufol G, Bonnefoy E, Sanchez I, et al. Effect of cyclosporine on left ventricular remodeling after reperfused myocardial infarction. J Am Coll Cardiol. 2010;55:1200–1205. doi: 10.1016/j.jacc.2009.10.052. [DOI] [PubMed] [Google Scholar]
- Michelsen MM, Stottrup NB, Schmidt MR, Lofgren B, Jensen RV, Tropak M, et al. Exercise-induced cardioprotection is mediated by a bloodborne, transferable factor. Basic Res Cardiol. 2012;107:260. doi: 10.1007/s00395-012-0260-x. [DOI] [PubMed] [Google Scholar]
- MITOCARE Study Group. Rationale and design of the ‘MITOCARE’ Study: a phase II, multicenter, randomized, double-blind, placebo-controlled study to assess the safety and efficacy of TRO40303 for the reduction of reperfusion injury in patients undergoing percutaneous coronary intervention for acute myocardial infarction. Cardiology. 2012;123:201–207. doi: 10.1159/000342981. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet. 1997;349:1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- Ostergaard L, Kristiansen SB, Angleys H, Frokiaer J, Michael Hasenkam J, Jespersen SN, et al. The role of capillary transit time heterogeneity in myocardial oxygenation and ischemic heart disease. Basic Res Cardiol. 2014;109:409. doi: 10.1007/s00395-014-0409-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasceri V, Patti G, Nusca A, Pristipino C, Richichi G, Di Sciascio G, et al. Randomized trial of atorvastatin for reduction of myocardial damage during coronary intervention: results from the ARMYDA (Atorvastatin for Reduction of MYocardial Damage during Angioplasty) study. Circulation. 2004;110:674–678. doi: 10.1161/01.CIR.0000137828.06205.87. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledge base of drug targets and their ligands. Nucl Acids Res. 2014;42(Database Issue):D1098-1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen CM, Cruden NL, Schmidt MR, Lau C, Botker HE, Kharbanda RK, et al. Remote ischemic preconditioning prevents systemic platelet activation associated with ischemia-reperfusion injury in humans. J Thromb Haemost. 2011;9:404–407. doi: 10.1111/j.1538-7836.2010.04142.x. [DOI] [PubMed] [Google Scholar]
- Piot C, Croisille P, Staat P, Thibault H, Rioufol G, Mewton N, et al. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med. 2008;359:473–481. doi: 10.1056/NEJMoa071142. [DOI] [PubMed] [Google Scholar]
- Piriou V, Chiari P, Gateau-Roesch O, Argaud L, Muntean D, Salles D, et al. Desflurane-induced preconditioning alters calcium-induced mitochondrial permeability transition. Anesthesiology. 2004;100:581–588. doi: 10.1097/00000542-200403000-00018. [DOI] [PubMed] [Google Scholar]
- Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- Post S, Post MC, van den Branden BJ, Eefting FD, Goumans MJ, Stella PR, et al. Early statin treatment prior to primary PCI for acute myocardial infarction: REPERATOR, a randomized placebo-controlled pilot trial. Catheter Cardiovasc Interv. 2012;80:756–765. doi: 10.1002/ccd.23449. [DOI] [PubMed] [Google Scholar]
- Rassaf T, Ferdinandy P, Schulz R. Nitrite in organ protection. Br J Pharmacol. 2014a;171:1–11. doi: 10.1111/bph.12291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassaf T, Totzeck M, Hendgen-Cotta UB, Shiva S, Heusch G, Kelm M. Circulating nitrite contributes to cardioprotection by remote ischemic preconditioning. Circ Res. 2014b;114:1601–1610. doi: 10.1161/CIRCRESAHA.114.303822. [DOI] [PubMed] [Google Scholar]
- Redington KL, Disenhouse T, Strantzas SC, Gladstone R, Wei C, Tropak MB, et al. Remote cardioprotection by direct peripheral nerve stimulation and topical capsaicin is mediated by circulating humoral factors. Basic Res Cardiol. 2012;107:241. doi: 10.1007/s00395-011-0241-5. [DOI] [PubMed] [Google Scholar]
- Redington KL, Disenhouse T, Li J, Wei C, Dai X, Gladstone R, et al. Electroacupuncture reduces myocardial infarct size and improves post-ischemic recovery by invoking release of humoral, dialyzable, cardioprotective factors. J Physiol Sci. 2013;63:219–223. doi: 10.1007/s12576-013-0259-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson JM, Vinten-Johansen J, Buckberg GD, Rosenkranz ER, Maloney JV., Jr Safety of prolonged aortic clamping with blood cardioplegia. I. Glutamate enrichment in normal hearts. J Thorac Cardiovasc Surg. 1984;88:395–401. [PubMed] [Google Scholar]
- Rosenkranz ER, Okamoto F, Buckberg GD, Vinten-Johansen J, Robertson JM, Bugyi H. Safety of prolonged aortic clamping with blood cardioplegia. II. Glutamate enrichment in energy-depleted hearts. J Thorac Cardiovasc Surg. 1984;88:402–410. [PubMed] [Google Scholar]
- Rosenkranz ER, Okamoto F, Buckberg GD, Robertson JM, Vinten-Johansen J, Bugyi HI. Safety of prolonged aortic clamping with blood cardioplegia. III. Aspartate enrichment of glutamate-blood cardioplegia in energy-depleted hearts after ischemic and reperfusion injury. J Thorac Cardiovasc Surg. 1986;91:428–435. [PubMed] [Google Scholar]
- Ross AM, Gibbons RJ, Stone GW, Kloner RA, Alexander RW, Investigators A-I. A randomized, double-blinded, placebo-controlled multicenter trial of adenosine as an adjunct to reperfusion in the treatment of acute myocardial infarction (AMISTAD-II) J Am Coll Cardiol. 2005;45:1775–1780. doi: 10.1016/j.jacc.2005.02.061. [DOI] [PubMed] [Google Scholar]
- Salameh A, Blanke K, Dhein S, Janousek J. Cardiac gap junction channels are upregulated by metoprolol: an unexpected effect of beta-blockers. Pharmacology. 2010;85:203–210. doi: 10.1159/000276982. [DOI] [PubMed] [Google Scholar]
- Schmidt K, Tissier R, Ghaleh B, Drogies T, Felix SB, Krieg T. Cardioprotective effects of mineralocorticoid receptor antagonists at reperfusion. Eur Heart J. 2010;31:1655–1662. doi: 10.1093/eurheartj/ehp555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt MR, Smerup M, Konstantinov IE, Shimizu M, Li J, Cheung M, et al. Intermittent peripheral tissue ischemia during coronary ischemia reduces myocardial infarction through a KATP-dependent mechanism: first demonstration of remote ischemic perconditioning. Am J Physiol Heart Circ Physiol. 2007;292:H1883–H1890. doi: 10.1152/ajpheart.00617.2006. [DOI] [PubMed] [Google Scholar]
- Schmidt MR, Stottrup NB, Contractor H, Hyldebrandt JA, Johannsen M, Pedersen CM, et al. Remote ischemic preconditioning with – but not without – metabolic support protects the neonatal porcine heart against ischemia-reperfusion injury. Int J Cardiol. 2014;170:388–393. doi: 10.1016/j.ijcard.2013.11.020. [DOI] [PubMed] [Google Scholar]
- Schulz R, Boengler K, Totzeck A, Luo Y, Garcia-Dorado D, Heusch G. Connexin 43 in ischemic pre- and postconditioning. Heart Fail Rev. 2007;12:261–266. doi: 10.1007/s10741-007-9032-3. [DOI] [PubMed] [Google Scholar]
- Selker HP, Beshansky JR, Sheehan PR, Massaro JM, Griffith JL, D'Agostino RB, et al. Out-of-hospital administration of intravenous glucose-insulin-potassium in patients with suspected acute coronary syndromes: the IMMEDIATE randomized controlled trial. JAMA. 2012;307:1925–1933. doi: 10.1001/jama.2012.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu M, Tropak M, Diaz RJ, Suto F, Surendra H, Kuzmin E, et al. Transient limb ischaemia remotely preconditions through a humoral mechanism acting directly on the myocardium: evidence suggesting cross-species protection. Clin Sci (Lond) 2009;117:191–200. doi: 10.1042/CS20080523. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Saxena P, Konstantinov IE, Cherepanov V, Cheung MM, Wearden P, et al. Remote ischemic preconditioning decreases adhesion and selectively modifies functional responses of human neutrophils. J Surg Res. 2010;158:155–161. doi: 10.1016/j.jss.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Siddiqi N, Neil C, Bruce M, MacLennan G, Cotton S, Papadopoulou S, et al. Intravenous sodium nitrite in acute ST-elevation myocardial infarction: a randomized controlled trial (NIAMI) Eur Heart J. 2014;35:1255–1262. doi: 10.1093/eurheartj/ehu096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloth AD, Schmidt MR, Munk K, Kharbanda RK, Redington AN, Schmidt M, et al. Improved long-term clinical outcomes in patients with ST-elevation myocardial infarction undergoing remote ischaemic conditioning as an adjunct to primary percutaneous coronary intervention. Eur Heart J. 2014;35:168–175. doi: 10.1093/eurheartj/eht369. [DOI] [PubMed] [Google Scholar]
- Smith A, Grattan A, Harper M, Royston D, Riedel BJ. Coronary revascularization: a procedure in transition from on-pump to off-pump? The role of glucose-insulin-potassium revisited in a randomized, placebo-controlled study. J Cardiothorac Vasc Anesth. 2002;16:413–420. doi: 10.1053/jcan.2002.125151. [DOI] [PubMed] [Google Scholar]
- Sonne DP, Engstrom T, Treiman M. Protective effects of GLP-1 analogues exendin-4 and GLP-1(9–36) amide against ischemia-reperfusion injury in rat heart. Regul Pept. 2008;146:243–249. doi: 10.1016/j.regpep.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Staat P, Rioufol G, Piot C, Cottin Y, Cung TT, L'Huillier I, et al. Postconditioning the human heart. Circulation. 2005;112:2143–2148. doi: 10.1161/CIRCULATIONAHA.105.558122. [DOI] [PubMed] [Google Scholar]
- Steensrud T, Li J, Dai X, Manlhiot C, Kharbanda RK, Tropak M, et al. Pretreatment with the nitric oxide donor SNAP or nerve transection blocks humoral preconditioning by remote limb ischemia or intra-arterial adenosine. Am J Physiol Heart Circ Physiol. 2010;299:H1598–H1603. doi: 10.1152/ajpheart.00396.2010. [DOI] [PubMed] [Google Scholar]
- Tamareille S, Mateus V, Ghaboura N, Jeanneteau J, Croue A, Henrion D, et al. RISK and SAFE signaling pathway interactions in remote limb ischemic perconditioning in combination with local ischemic postconditioning. Basic Res Cardiol. 2011;106:1329–1339. doi: 10.1007/s00395-011-0210-z. [DOI] [PubMed] [Google Scholar]
- Thielmann M, Kottenberg E, Boengler K, Raffelsieper C, Neuhaeuser M, Peters J, et al. Remote ischemic preconditioning reduces myocardial injury after coronary artery bypass surgery with crystalloid cardioplegic arrest. Basic Res Cardiol. 2010;105:657–664. doi: 10.1007/s00395-010-0104-5. [DOI] [PubMed] [Google Scholar]
- Thielmann M, Kottenberg E, Kleinbongard P, Wendt D, Gedik N, Pasa S, et al. Cardioprotective and prognostic effects of remote ischaemic preconditioning in patients undergoing coronary artery bypass surgery: a single-centre randomised, double-blind, controlled trial. Lancet. 2013;382:597–604. doi: 10.1016/S0140-6736(13)61450-6. [DOI] [PubMed] [Google Scholar]
- Toombs CF, McGee S, Johnston WE, Vinten-Johansen J. Myocardial protective effects of adenosine. Infarct size reduction with pretreatment and continued receptor stimulation during ischemia. Circulation. 1992;86:986–994. doi: 10.1161/01.cir.86.3.986. [DOI] [PubMed] [Google Scholar]
- Uyar I, Mansuroglu D, Kirali K, Erentug V, Bozbuga NU, Uysal G, et al. Aspartate and glutamate-enriched cardioplegia in left ventricular dysfunction. J Card Surg. 2005;20:337–344. doi: 10.1111/j.1540-8191.2005.200355.x. [DOI] [PubMed] [Google Scholar]
- Varga ZV, Zvara A, Faragó N, Kocsis GF, Pipicz M, Gáspár R, et al. MicroRNAs associated with ischemia-reperfusion injury and cardioprotection by ischemic pre- and postconditioning: protectomiRs. Am J Physiol Heart Circ Physiol. 2014;307:H216–H227. doi: 10.1152/ajpheart.00812.2013. [DOI] [PubMed] [Google Scholar]
- Voors AA, Belonje AM, Zijlstra F, Hillege HL, Anker SD, Slart RH, et al. A single dose of erythropoietin in ST-elevation myocardial infarction. Eur Heart J. 2010;31:2593–2600. doi: 10.1093/eurheartj/ehq304. [DOI] [PubMed] [Google Scholar]
- Waldmeier PC, Feldtrauer JJ, Qian T, Lemasters JJ. Inhibition of the mitochondrial permeability transition by the nonimmunosuppressive cyclosporin derivative NIM811. Mol Pharmacol. 2002;62:22–29. doi: 10.1124/mol.62.1.22. [DOI] [PubMed] [Google Scholar]
- Wang H, Dwyer-Lindgren L, Lofgren KT, Rajaratnam JK, Marcus JR, Levin-Rector A, et al. Age-specific and sex-specific mortality in 187 countries, 1970–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2071–2094. doi: 10.1016/S0140-6736(12)61719-X. [DOI] [PubMed] [Google Scholar]
- Wei M, Xin P, Li S, Tao J, Li Y, Li J, et al. Repeated remote ischemic postconditioning protects against adverse left ventricular remodeling and improves survival in a rat model of myocardial infarction. Circ Res. 2011;108:1220–1225. doi: 10.1161/CIRCRESAHA.110.236190. [DOI] [PubMed] [Google Scholar]
- Zhao T, Parikh P, Bhashyam S, Bolukoglu H, Poornima I, Shen YT, et al. Direct effects of glucagon-like peptide-1 on myocardial contractility and glucose uptake in normal and postischemic isolated rat hearts. J Pharmacol Exp Ther. 2006;317:1106–1113. doi: 10.1124/jpet.106.100982. [DOI] [PubMed] [Google Scholar]
- Zhao ZQ, McGee S, Nakanishi K, Toombs CF, Johnston WE, Ashar MS, et al. Receptor-mediated cardioprotective effects of endogenous adenosine are exerted primarily during reperfusion after coronary occlusion in the rabbit. Circulation. 1993;88:709–719. doi: 10.1161/01.cir.88.2.709. [DOI] [PubMed] [Google Scholar]
- Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, et al. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285:H579–H588. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]