Abstract
The development of novel adjuvant strategies capable of attenuating myocardial ischaemia-reperfusion injury and reducing infarct size remains a major, unmet clinical need. A wealth of preclinical evidence has established that ischaemic ‘conditioning’ is profoundly cardioprotective, and has positioned the phenomenon (in particular, the paradigms of postconditioning and remote conditioning) as the most promising and potent candidate for clinical translation identified to date. However, despite this preclinical consensus, current phase II trials have been plagued by heterogeneity, and the outcomes of recent meta-analyses have largely failed to confirm significant benefit. As a result, the path to clinical application has been perceived as ‘disappointing’ and ‘frustrating’. The goal of the current review is to discuss the pitfalls that may be stalling the successful clinical translation of ischaemic conditioning, with an emphasis on concerns regarding: (i) appropriate clinical study design and (ii) the choice of the ‘right’ preclinical models to facilitate clinical translation.
Tables of Links
| LIGANDS | |
|---|---|
| Adenosine | Glimepiride |
| Bradykinin | Insulin |
| Glibenclamide | Propofol |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guideto PHARMACOLOGY 2013/14 (a,b,cAlexander et al., 2013a,b,c,,).
Coronary heart disease, culminating in the acute obstruction of one or more coronary arteries and the death or infarction of cardiomyocytes distal to the site of occlusion, remains the leading cause of mortality in the United States and Europe (Nichols et al., 2013; Go et al., 2014). The current standard of care for the treatment of acute myocardial infarction (MI) is prompt restoration of blood flow to the ischaemic territory in an effort to curtail cardiomyocyte death and thus limit infarct size. However, although reperfusion is a de facto requirement for the salvage of ischaemic myocardium, the benefits of early reperfusion are in part undermined by the phenomenon of lethal reperfusion injury, or paradoxical death (rather than rescue) of myocytes in response to the reintroduction of oxygen (Braunwald and Kloner, 1985; Yellon and Hausenloy, 2007; Sanada et al., 2011; Heusch, 2013; Przyklenk, 2013).
Considerable effort has been devoted throughout the past four decades to the investigation of novel adjuvant therapies aimed at mitigating myocardial ischaemia-reperfusion injury, reducing infarct size and thereby improving patient outcomes post-MI (Lefer and Bolli, 2011; Longacre et al., 2011; Hausenloy et al., 2013b). Among the host of potential candidates that have been identified, the intervention that has, without question, shown the greatest preclinical potential to achieve this elusive goal is ischaemic conditioning, encompassing the paradigms of preconditioning, postconditioning and remote conditioning (Murry et al., 1986; Przyklenk et al., 1993; Zhao et al., 2003; Heusch, 2013; Przyklenk, 2013). Nonetheless, despite the consensus that, in experimental models, ischaemic conditioning has a profound infarct-sparing effect, ‘the outcome of attempting to effect the translation of this most potent and basic cardioprotective response to the clinical environment’ has been described as ‘somewhere between frustrating and disappointing’ (Schevchuck and Laskey, 2013). The goals of the current review are to discuss the issues contributing to this frustration and disappointment, and identify the pitfalls that may be stalling the successful translation of ischaemic conditioning from preclinical and clinical trials to clinical practice.
Basic principles: the ‘what’ and ‘how’ of ischaemic conditioning
Ischaemic conditioning is the phenomenon whereby one or more episodes of brief ischaemia (too brief in themselves to cause tissue necrosis) render the heart resistant to ischaemia-reperfusion injury and reduce myocardial infarct size (Heusch, 2013; Przyklenk, 2013). Three basic variants of conditioning have been identified – ischaemic preconditioning, postconditioning and remote conditioning (Murry et al., 1986; Przyklenk et al., 1993; Zhao et al., 2003), each of which are distinct in terms of the timing and site at which the protective stimulus is applied (Figure 1). Ischaemic preconditioning, as described in the seminal report by Murry et al. (1986), is by definition a pretreatment: a conditioning stimulus typically composed of two to four 5 min episodes of transient ischaemia, each interspersed with 5 min of intervening reperfusion, is applied to the heart before the onset of the sustained ischaemic insult. In contrast, postconditioning, as first discovered by Zhao et al. (2003), is a derivative of the concept of ‘gentle’, graded and controlled reperfusion described nearly two decades earlier (Okamoto et al., 1986): relief of sustained ischaemia is achieved by reintroduction of blood flow to the ischaemic myocardium in a stuttered or staccato manner [with typical algorithms consisting of three to six cycles of (10–30 s of reflow + 10–30 s of reocclusion)] before instituting full and complete reperfusion. The phenomenon of remote conditioning is uniquely different from both pre- and postconditioning in that the brief cardioprotective stimulus is applied either in a separate myocardial vascular territory [intra-cardiac conditioning, as first demonstrated by Przyklenk et al. (1993)] or, of greater practical relevance, in a distant tissue or organ such as skeletal muscle [inter-organ conditioning (Kharbanda et al., 2002)]. Indeed, protection can be achieved by simple inflation/deflation of a blood pressure cuff positioned on an arm or leg, initiated either before the onset of sustained myocardial ischaemia (remote preconditioning), during the sustained ischaemic insult (remote perconditioning) or at reperfusion (remote postconditioning) (Vinten-Johansen and Shi, 2011a; Przyklenk and Whittaker, 2013; Wider and Przyklenk, 2014) (Figure 1).
Figure 1.
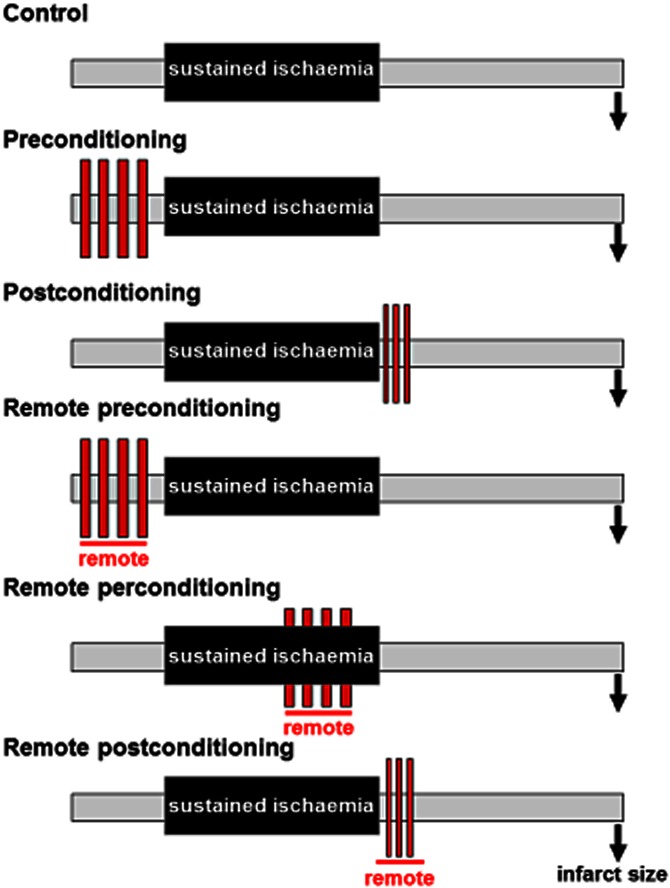
Schematic diagram illustrating the key temporal aspects of preconditioning, postconditioning, and remote pre-, per- and postconditioning. Red boxes denote the conditioning stimulus. Reprinted with permission from Wider et al. (2014).
Common themes
Despite these logistic differences among the three conditioning paradigms, all share a common fundamental theme: the undisputed hallmark of pre-, post- and remote conditioning is attenuation of myocardial ischaemia-reperfusion injury and reduction of infarct size beyond that achieved by reperfusion alone (Heusch, 2013; Przyklenk, 2013; Wider and Przyklenk, 2014). In addition, all forms of conditioning appear to share commonalities in terms of molecular mechanisms, with the general scenario of: (i) GPCR stimulation (by adenosine, bradykinin, opioids and other autocoids) on cardiomyocyte membranes; (ii) post-receptor cardiac up-regulation of multiple kinases [including key components of the reperfusion injury salvage kinase (RISK) and survival activating factor enhancement (SAFE) pathways]; and, ultimately (iii) stabilization of mitochondria (including closure of the mitochondrial permeability transition pore), proposed to play a role in establishing a conditioned phenotype (reviewed in Heusch et al., 2008; Murphy and Steenbergen, 2008; Hausenloy and Yellon, 2009; Cohen and Downey, 2011; 2015,; Hausenloy et al., 2011a; Heusch, 2013; Przyklenk, 2013; Przyklenk and Whittaker, 2013; Kleinbongard and Heusch, 2015; Schmidt et al., 2015; Wider and Przyklenk, 2014).
A notable exception to this concept of commonality among conditioning paradigms is the added mechanistic component inherent in remote conditioning: that is, the communication or transfer of the protective stimulus from the remote organ to the heart. Details regarding the identity of the protective factor(s) and route(s) of communication remain incompletely resolved. However, there is compelling evidence in support of two primary hypotheses: remote conditioning may be triggered by: (i) one or more blood- or perfusate-borne humoral factors released during the remote ischaemic stimulus (with potential candidates including adenosine, bradykinin, opioids, cytokines and an elusive small <15 kDa hydrophobic molecule) or (ii) stimulation of sensory neurons at the site of the remote stimulus and subsequent activation of autonomic reflex pathways (Dickson et al., 2001; Serejo et al., 2007; Shimizu et al., 2009; Saxena et al., 2010; Przyklenk and Whittaker, 2011; 2013,; Mastitskaya et al., 2012; Albrecht et al., 2013; Donato et al., 2013; Przyklenk, 2013; Gourine and Gourine, 2014; Schmidt et al., 2015; Wider and Przyklenk, 2014). Moreover, this communication phase may be complex and, in some in vivo models, require an amalgam of both humoral and neuronal components (Lim et al., 2010; Steensrud et al., 2010; Przyklenk and Whittaker, 2011; 2013,; Redington et al., 2012; Przyklenk, 2013; Schmidt et al., 2015; Wider and Przyklenk, 2014).
Early phase versus late phase of ischaemic conditioning
All of the aforementioned discussion has focused the so-called classic or early phase of ischaemic conditioning, in which the protective stimulus is applied within minutes of the sustained ischaemic insult (Figure 1). For ischaemic preconditioning (and, possibly, remote conditioning): (i) prolongation of the time interval between the brief ischaemic stimulus and the onset of sustained ischaemia to a period of ∼2 to 4 h results in a loss in cardioprotective efficacy, while (ii) a further extension to ∼12 to 24 h initiates a second and distinct, delayed or late phase of protection that persists for ∼3 to 4 days (Bolli, 2000; Kanoria et al., 2007; Huffmyer and Raphael, 2009). Late phase ischaemic preconditioning differs from the classic, early phase of the phenomenon in terms of both timing and mechanisms: that is, late phase conditioning is reportedly a consequence of kinase-mediated up-regulation of transcription factors responsible for the coordinated activation of multiple stress-responsive genes and subsequent increased expression of inducible proteins including (but not limited to) inducible NOS and COX-2 (Bolli, 2000; Kanoria et al., 2007). Although the emphasis of the current review is on the clinical translation of early phase ischaemic conditioning, the temporal gap in efficacy between the early and late phases of conditioning-induced cardioprotection is clearly an important consideration in both the design and interpretation of conditioning protocols.
The path to clinical translation
With rare exceptions (Nakano et al., 2002; Schwartz and Lagranha, 2006; Dow and Kloner, 2007; Sachdeva et al., 2014), there has been unprecedented agreement among preclinical studies that all forms of ischaemic conditioning render the heart resistant to ischaemia-reperfusion injury and reduce myocardial infarct size. However, despite this near consensus among experimental models, and the enthusiasm that the concept of endogenous cardioprotection has generated among investigators, progress in capitalizing on this wealth of evidence and translating ischaemic conditioning into clinical practice has been neither rapid nor smooth (Cohen and Downey, 2011; Heusch, 2013; Schevchuck and Laskey, 2013).
During the past decade, attention has largely focused on the investigation of postconditioning and remote conditioning (which, in contrast to preconditioning, do not require subjecting the heart to an antecedent ischaemic stimulus) in three clinical settings: percutaneous coronary intervention (PCI) for the treatment of ST segment elevation myocardial infarction (STEMI), non-emergent PCI and cardiac surgery. In multiple landmark studies, encouraging successes were achieved. For example, the first trial that sought to apply postconditioning to improve outcome in STEMI patients documented a significant reduction in creatine kinase release, a surrogate biomarker for infarct size, in patients randomized to receive four 1 min cycles of angioplasty balloon inflation/deflation begun within the first minute after establishing reflow (Staat et al., 2005). More importantly, this was followed by evidence of long-term benefit [i.e. persistent reduction of infarct size, as quantified by single photon emission computed tomography (SPECT), at 6 months post-MI] in the postconditioned cohort versus patients who received standard PCI (Thibault et al., 2008). Similar observations – acute and presumably favourable attenuation of cardiac enzyme release, and subsequent longer term evidence of improvement in hard clinical end points – have been reported with remote conditioning in patients undergoing coronary artery bypass graft (CABG) surgery (Thielmann et al., 2010; 2013,), patients undergoing elective PCI (Hoole et al., 2009; Davies et al., 2013) and STEMI patients undergoing primary PCI (Botker et al., 2010; Sloth et al., 2014). However, when all current phase II trials are considered, outcomes with postconditioning and remote conditioning have been mixed, ranging from protective to statistically neutral to, in some instances, deleterious with significant exacerbation of cardiac injury (Iliodromitis et al., 2006; Ovize et al., 2010; 2013,; Rahman et al., 2010; Freixa et al., 2012; Carrasco-Chinchilla et al., 2013; Heusch, 2013; Limalanathan et al., 2014). Indeed, recent systematic reviews and meta-analyses of randomized controlled trials were unable to confirm a significant benefit of postconditioning in patients undergoing PCI for the treatment of acute MI (Schevchuck and Laskey, 2013; Abdelnoor et al., 2014) and unable to establish a significant effect of remote preconditioning on clinical end points in adult surgical patients (Healy et al., 2014) (Table 1).
Table 1.
Recent meta-analyses of randomized clinical trials on postconditioning and remote conditioning
| Author | Intervention | Indication | Outcome | No. of trials | Total number of subjects | Effect estimate | 95% CI | |
|---|---|---|---|---|---|---|---|---|
| Abdelnoor et al. (2014) | Postconditioning | STEMI | Infarct size estimated by biomarkers | 18 | 1275 | Standardized mean difference: –0.58 | –0.96 to –0.19 | P = 0.003‡ |
| Abdelnoor et al. (2014) | Postconditioning | STEMI | Infarct size estimated by CMR | 8 | 586 | Standardized mean difference: –0.062 | –0.34 to 0.21 | P = 0.625 |
| Schevchuck and Laskey (2013) | Postconditioning | STEMI | Infarct size estimated by biomarkers | 14 | 765 | Standardized mean difference: –0.402 | –0.842 to 0.037 | P = 0.072 |
| Schevchuck and Laskey (2013) | Pooled postconditioning + remote postconditioning | STEMI | Infarct size estimated by biomarkers | 16 | 970 | Standardized mean difference: –0.370 | –0.775 to –0.014 | P = 0.042 |
| Healy et al. (2014) | Remote preconditioning | Adult cardiovascular surgery | Risk of perioperative MI | 17 | 1777 | Risk ratio: 0.69 | 0.34–1.40 | P = 0.31 |
| D'Ascenzo et al. (2014) | Remote preconditioning | Elective PCI | Incidence of peri-procedural MI | 5 | 731 | Odds ratio: 0.58 | 0.36–0.93 | P = 0.02 |
P-value calculated from the reported values of standardized mean difference and 95% CI using equations provided by Altman and Bland (2011): How to obtain the P-value from a confidence interval. BMJ 343:d2304.
How can this apparent dichotomy between preclinical success and clinical capriciousness be explained? The answer may lie, at least in part, in the inherent complexities involved in adapting the carefully orchestrated and time phenomenon of ischaemic conditioning and its gold standard end point (reduction of infarct size) from the controlled environment of the laboratory to the heterogeneity and uncertainty of the clinical setting. Two facets of this issue warrant consideration: (i) clinical study design (i.e. are the ‘right’ patients being enrolled in appropriately designed trials that heed the lessons learned from preclinical studies?) and (ii) choice of experimental models (have we used the ‘right’ models to facilitate clinical translation?).
Clinical study design: the recurring theme of heterogeneity
Postconditioning and remote conditioning as adjunct treatment in acute MI
The undisputed hallmark of ischaemic conditioning is reduction of myocardial infarct size. The primary determinants of infarct size are well established and include the volume of at-risk myocardium, together with the duration and severity of ischaemia. In addition, the ability to conclude with confidence that ischaemic conditioning (or, indeed any intervention) is cardioprotective requires the accurate quantification of infarct size and risk region, and the consideration of area at risk, duration of coronary artery occlusion and severity of ischaemia (i.e. magnitude of collateral blood flow) as covariates in the analysis (Reimer et al., 1977; Reimer and Jennings, 1979; Ovize et al., 2013). For studies conducted in preclinical models, the meticulous control of these variables is de rigueur. Assessment of these end points in STEMI patients is, however, challenging (Ovize et al., 2013). Lastly, logic would suggest that clinical trials aimed at investigating the efficacy of infarct size reduction with postconditioning or remote conditioning should be designed to follow, as closely as possible, the temporal and physiological criteria for cardioprotection elucidated in the preclinical models, including the appropriate timing of the conditioning stimulus. However, a recurring theme in recent meta-analyses is heterogeneity, as reflected in terms of both its statistical definition (Higgins et al., 2003; Schevchuck and Laskey, 2013; Abdelnoor et al., 2014) and, as discussed below, protocol design.
Methodological issues include variation among trials in the details and timing of the conditioning algorithms, ischaemic duration, and the techniques used to assess infarct size or myocardial salvage, as well as scatter within study cohorts in ischaemic times, risk regions and collateral blood flow (Ovize et al., 2013; Schevchuck and Laskey, 2013; Abdelnoor et al., 2014). For example, clinical postconditioning protocols have utilized two to four cycles of stuttered reflow, with the durations of balloon inflation and deflation ranging from 10 to 90 s and 30–300 s, respectively (Heusch, 2013; Schevchuck and Laskey, 2013). Moreover, repeated balloon inflation-deflations have been applied after stent placement in some protocols (Staat et al., 2005; Thibault et al., 2008; Thuny et al., 2012), before stent placement in others (Laskey et al., 2008; Xue et al., 2010; Garcia et al., 2011; Limalanathan et al., 2014) and, in at least one study, timing was mixed and at the discretion of the operator (Hahn et al., 2013). The optimal postconditioning stimulus has not however been identified (Przyklenk, 2013), and it is unclear whether these differences contribute importantly to the disparate outcomes among studies. However, preclinical evidence has demonstrated that a delay of only 1 min in the initiation of stuttered reflow compromises the infarct-sparing effect of postconditioning (Kin et al., 2004).
An additional technical issue that, to date, has not been considered is whether introduction of the guide wire or balloon catheter through the culprit coronary lesion in some instances initiates gradual, gentle reperfusion that may, in itself, be protective (Okamoto et al., 1986). If so, this may further undermine the magnitude of benefit that can be achieved by the subsequent postconditioning algorithm.
Finally, when studies were stratified according to the method used to assess myocardial salvage, the estimated treatment effect achieved significance in protocols in which cardiac enzyme release served as the primary end point, but was not significant in trials in which infarct size was quantified by cardiac magnetic resonance or SPECT. The reasons for this apparent disagreement are unknown (Schevchuck and Laskey, 2013; Abdelnoor et al., 2014) (Table 1).
While the consequences of the aforementioned sources of variability remain poorly defined, heterogeneity in the primary determinants of infarct size, both among and within studies, may be of particular relevance. For example, patients with small risk regions, extensive collateral perfusion and/or spontaneous reperfusion before PCI will develop small infarcts irrespective of treatment and thus will gain minimal benefit from ischaemic conditioning. A similar paradigm will be manifest in patients with prolonged ischaemic times: if reperfusion is initiated at ∼8 to 12 h after the onset of symptoms and collateral flow is negligible, evolution of the infarct may be complete, and, in the absence of salvageable myocardium, conditioning will again be of negligible benefit (Botker et al., 2010; Ovize et al., 2013; Schevchuck and Laskey, 2013; Roubille et al., 2014). Thus, failure to consider the determinants of infarct size in the enrollment criteria, and the ensuing heterogeneity within and among protocols, may mask the ability to reliably discern an infarct-sparing effect of postconditioning and remote conditioning in the subset of STEMI patients that may benefit most: that is, cohorts with large risk regions and minimal collateral blood flow, in whom reperfusion is initiated within an appropriate window of ischaemic times (Botker et al., 2010; Ovize et al., 2013; Schevchuck and Laskey, 2013).
Remote conditioning and cardiovascular surgery
The majority of trials investigating the efficacy of remote ischaemic conditioning have been conducted in patients undergoing CABG and other cardiovascular surgical procedures. Conclusions regarding benefit are again confounded by heterogeneity within and among studies (Heusch, 2013; Ovize et al., 2013; Przyklenk, 2013; Healy et al., 2014).
Sources of variation in the surgical setting include, first and foremost, differences among protocols in the timing and site of the conditioning algorithm: remote stimuli have encompassed three to four cycles of limb ischaemia/reperfusion, achieved by inflation/deflation of a blood pressure cuff for durations of 4–10 min, on either an arm or leg. As with postconditioning, there is no consensus on the optimal remote conditioning protocol, and it has in fact been proposed that an excessive conditioning stimulus (termed ‘hyperconditioning’) may be deleterious rather than protective (Ovize et al., 2013; Przyklenk, 2013; Healy et al., 2014; Whittaker and Przyklenk, 2014). A second issue that may be of critical importance is the heterogeneity among studies in the precise time at which the remote preconditioning stimulus was administered: in most protocols, brief limb ischaemia was applied after induction of anaesthesia but before commencement of surgery, while in others the remote trigger was initiated after making the first incision (Rahman et al., 2010; Young et al., 2012; Ovize et al., 2013; Healy et al., 2014). Additional sources of variability that are unique to surgical trials and may confound outcomes include differences among protocols in the types of surgeries and patients that were included, as well as the choice of cardioplegic agents and anaesthetic regimens. For example, some studies focused exclusively on stable patients undergoing CABG, while others included valve surgeries, patients with unstable coronary disease and, in some instances, high-risk patients undergoing multiple procedures. In terms of anaesthesia, the use of propofol is problematic in that the agent reportedly mitigates conditioning-induced cardioprotection, while volatile anaesthetics and opioids are cardioprotective per se and thus may provide limited scope for added benefit (Kottenberg et al., 2012; 2014,; Heusch, 2013; Ovize et al., 2013; Przyklenk, 2013; Healy et al., 2014; Zaugg et al., 2014).
Interestingly, despite the aforementioned heterogeneity within and among surgical trials, systematic reviews and meta-analyses focused on cardiac enzyme release have consistently concluded that remote preconditioning has a favourable effect on outcome (reviewed in Healy et al., 2014). In contrast, in the only meta-analysis to date that focused exclusively on hard clinical end points (including death, perioperative MI, cerebrovascular accident and length of hospital stay), no significant beneficial effect of remote ischaemic conditioning was discerned (Healy et al., 2014) (Table 1). Definitive evidence supporting (or refuting) a clinical benefit of remote conditioning in cardiac surgery will presumably emerge upon the highly anticipated completion of two ongoing large-scale phase III trials (Hausenloy et al., 2011b; Meybohm et al., 2012; Healy et al., 2014).
Remote conditioning in elective PCI
There has also been interest (albeit limited) in the possibility of utilizing remote preconditioning to attenuate peri-procedural MI in patients undergoing non-emergent elective PCI. Among the six randomized and controlled studies conducted to date, most have reported cardiac enzyme release as the primary end point, and the outcomes appear even more variable than those for conditioning applied in the setting of primary PCI and cardiac surgery (Schevchuck and Laskey, 2013). There is evidence from one trial of both short-term and long-term benefits (Hoole et al., 2009; Davies et al., 2013), an outcome that may be considered surprising given the fact that the remote conditioning stimulus was applied ∼1 h in advance of the procedure and thus may be predicted to fall within the temporal gap in efficacy between early phase and late phase conditioning. Moreover, the observation that remote conditioning attenuated peri-procedural cardiac enzyme release in this cohort in which the magnitude of biomarker release was, as expected, small, appears to contradict the aforementioned premise that patients with small infarcts will obtain minimal benefit from ischaemic conditioning. Nonetheless, despite these conceptual issues, the heterogeneity among studies and the resultant wide confidence intervals, systematic review and meta-analysis of cardiac enzyme release encompassing data from five studies concluded that remote preconditioning significantly reduced the incidence of MI following elective PCI (D'Ascenzo et al., 2014) (Table 1).
Choice of the ‘right’ preclinical models: confounders and comorbidities
The wealth of data obtained in preclinical models demonstrating infarct size reduction with ischaemic conditioning has provided the groundwork – and the impetus – for clinical translation of these cardioprotective strategies. However, of the >2500 experimental studies indexed to date in PubMed that have investigated conditioning-induced cardioprotection and included myocardial infarct size as a primary end point, the overwhelming majority (>95%) has been conducted using healthy juvenile or adult animal models (Ferdinandy et al., 2007; 2014,; Przyklenk, 2011; 2013,; Vinten-Johansen et al., 2011b; Miki et al., 2012; Wider and Przyklenk, 2014). Accordingly, our standard models: (i) do not reflect the fact that cardiovascular disease and acute MI are typically manifest in middle-aged and elderly populations, and (ii) do not incorporate the well-established risk factors and comorbidities (including, most notably, type-2 diabetes) seen in an increasing proportion of patients (Whiting et al., 2011; Go et al., 2014; Gregg et al., 2014). This is potentially problematic given the growing evidence that aged and diabetic models display defects in cardiac expression and phosphorylation of multiple kinases implicated in cardioprotection, including isoforms of PKC as well as PI3K/Akt, ERK, p70S6 kinase, glycogen synthase kinase 3β (GSK-3β), JAK and STAT (i.e. key constituents of the RISK and SAFE signalling pathways) (Boengler et al., 2009; Przyklenk, 2011; 2013,; Vinten-Johansen et al., 2011b; Miki et al., 2012; Wider and Przyklenk, 2014). These issues raise the question: have we used the ‘right’ preclinical models to provide the foundation for the clinical translation of ischaemic conditioning?
Ageing
The prevalence of cardiovascular disease and acute MI are well recognized to increase with increasing age: that is, in men, the annual rate for a first cardiovascular event is 0.3% in cohorts aged 35–44 years and escalates to 7.4% at age 85–94 years (Go et al., 2014). It is therefore not surprising that, in conditioning trials conducted to date, the patient populations are not young adults, but rather are typically middle aged (mean of ∼55 to 65 years) (Schevchuck and Laskey, 2013; D'Ascenzo et al., 2014; Healy et al., 2014).
Initial insights into the possible consequences of increasing age on conditioning-induced cardioprotection were obtained in rat models of classic ischaemic preconditioning: the infarct-sparing effect of preconditioning was attenuated in so-called middle-aged rats (aged 9–12 months) and lost in cohorts of 18- to 22-month-old rats (Fenton et al., 2000; Schulman et al., 2001). This concept of an age-associated decline in the efficacy of preconditioning has, with few exceptions (Dai et al., 2009), been corroborated in subsequent studies conducted in rats and mice (Boengler et al., 2007; Adam et al., 2013), and has been attributed to defects in adenosine receptor-mediated cardioprotective signalling (including impaired activation of PKC) and age-associated alterations in mitochondrial end effectors [including reduced mitochondrial connexin 43 content and failed GSK-3β-mediated modulation of mitochondrial stability (Schulman et al., 2001; Boengler et al., 2007; Zhu et al., 2013)] (reviewed in Boengler et al., 2009; Abete et al., 2010; Przyklenk, 2011).
In contrast, protocols conducted in models with a longer lifespan have yielded more complex results. Sustained preconditioning-induced cardioprotection was documented in both ‘senescent’ 5- to 8-year-old sheep (Burns et al., 1996) and ‘aged’ 4-year-old rabbits (Przyklenk et al., 2001; 2003,). The outcome in the sheep model was arguably predictable given their >20 year life expectancy coupled with the lack of molecular evidence of cardiac senescence in this cohort (Burns et al., 1996; Barja and Herrero, 2000; Boengler et al., 2009). However, the observation of a persistent infarct-sparing effect of preconditioning in 4-year-old rabbits is noteworthy, in that despite their lengthy (∼13 years) maximum lifespan (Barja and Herrero, 2000; Boengler et al., 2009), the ‘aged’ 4-year-old cohort was confirmed to display unambiguous hallmarks of cardiovascular ageing (Przyklenk et al., 2001). Moreover, there was an apparent age-associated change in cardioprotective signalling (in particular, a diminished role of the ε-isoform of PKC) in old versus adult animals (Przyklenk et al., 2003). Taken together, these data raise the intriguing but as-yet unexplored possibility that, if there is indeed an age-associated loss in preconditioning-induced cardioprotection, this may include a temporal phase of plasticity in survival kinase signalling during which redundant or alternative mediators are recruited (Przyklenk et al., 2003; Przyklenk, 2011).
For the more clinically relevant forms of conditioning – postconditioning and remote conditioning – only three studies have quantified infarct size in ageing cohorts, and all used postconditioning as the protective stimulus (Table 2A). Among these protocols, a spectrum of results was obtained. Postconditioning failed to reduce infarct size in 2-year-old mice, an effect attributed to a defect in ERK phosphorylation and possibly caused by an age-associated up-regulation in one or more MAPK phosphatases (Przyklenk et al., 2008). For 13-month-old mice, the efficacy of postconditioning-induced cardioprotection was reportedly attenuated: that is, an amplified stimulus was required to evoke a reduction in infarct size possibly due to an age-related attenuation in STAT3 phosphorylation (Boengler et al., 2008). Despite the differences in proposed mechanisms, the outcomes of these two studies are consistent with the concept that, at least in the mouse model, the infarct-sparing effect of postconditioning may wane with increasing age. However, in apparent contrast, 16- to 18-month-old rats showed no deficit in cardioprotective signalling and no loss in the ability of postconditioning to reduce infarct size (Yin et al., 2009). The current paucity of data, and complete absence of data from large animal models that might arguably be considered more clinically relevant, precludes the ability to draw meaningful conclusions on whether the infarct-sparing effect of postconditioning is compromised with increasing age. No data are available, and thus no conclusions can be made, on whether infarct size reduction with remote conditioning is mitigated versus maintained in ageing cohorts (Table 2).
Table 2.
Efficacy of infarct size reduction with postconditioning and remote conditioning in preclinical models of ageing and type-2 diabetes
| Author | Model | Reduction in infarct size? | Comments/mechanistic insights? |
|---|---|---|---|
| A. Ageing | |||
| Postconditioning | |||
| Przyklenk et al. (2008) | Mouse: 20–24 months | No | Impaired ERK phosphorylation |
| Boengler et al. (2008) | Mouse: >13 months | Attenuated | Efficacy attenuated; amplified postconditioning stimulus required to achieve protection |
| Attenuated STAT3 phosphorylation | |||
| Yin et al. (2009) | Rat: 16–18 months | Yes | Persistent PI3K/Akt-GSK-3β signalling in the old cohort |
| Remote conditioning | |||
| No published studies | |||
| B. Type-2 diabetes | |||
| Postconditioning | |||
| Wagner et al. (2008) | Rat: WOKW | No | Impaired ERK, GSK-3β phosphorylation |
| Bouhidel et al. (2008) | Mouse: ob/ob | No | Impaired Akt, ERK, p70S6 kinase, AMPK phosphorylation |
| Przyklenk et al. (2011) | Mouse: db/db | No | Impaired ERK phosphorylation |
| Zhu et al. (2012) | Mouse: db/db | No | Loss of protection associated with differential regulation of mitochondrial proteome |
| Oosterlinck et al. (2013) | Mouse: ob/ob | Attenuated | No mechanism proposed |
| Remote conditioning | |||
| No published studies | |||
WOKW, Wistar Ottawa Karlsburg W rat.
Type-2 diabetes
The development of novel clinical strategies to attenuate myocardial ischaemia-reperfusion injury and reduce infarct size is of particular importance to patients with type-2 diabetes. The urgency and scope of this issue is exemplified by: (i) the >2-fold greater incidence of cardiovascular disease and acute MI in diabetic versus non-diabetic cohorts; (ii) the two- to fourfold greater incidence of cardiovascular disease-related deaths in diabetic patients; and (iii) the predicted escalation in the incidence of type-2 diabetes during the next 20–30 years (Haffner et al., 1998; Murcia et al., 2004; Alegria et al., 2007; Krempf et al., 2010; Sarwar et al., 2010; Go et al., 2014; Gregg et al., 2014; Wider and Przyklenk, 2014).
The first studies aimed at investigating conditioning-induced cardioprotection in the setting of type-2 diabetes again (as with ageing) utilized classic ischaemic preconditioning as the protective stimulus. All protocols were conducted using either Zucker fatty or Goto-Kakizaki rats, and there was a consensus that the efficacy of infarct size reduction with preconditioning was either attenuated (with an amplified stimulus required to evoke protection) or lost in the diabetic cohorts (Kristiansen et al., 2004; Tsang et al., 2005; Katakam et al., 2007; Hausenloy et al., 2013a) (reviewed in Wider and Przyklenk, 2014). Moreover, in one particularly innovative study, the combined effects of diabetes and ageing were investigated and the general theme of ‘waning cardioprotection’ was recapitulated: young (3- to 8-month-old) Goto-Kakizaki rats remained responsive to an augmented preconditioning stimulus whereas 12- to 18-month-old diabetic rats were refractory to the infarct-sparing effect of ischaemic preconditioning (Whittington et al., 2013).
In the five studies published to date in which postconditioning was used as the protective trigger in models of type-2 diabetes, four were conducted in mouse strains (db/db or ob/ob) and one utilized the Wistar Ottawa Karlsburg W rat (Table 2B). Outcomes were similar to those obtained with ischaemic preconditioning: infarct size reduction with postconditioning was either absent (Bouhidel et al., 2008; Wagner et al., 2008; Przyklenk et al., 2011; Zhu et al., 2012) or, in one study, diminished (Oosterlinck et al., 2013) in diabetic animals when compared with normoglycaemic controls (reviewed in Wider and Przyklenk, 2014). Moreover, as with ageing, the loss in efficacy of postconditioning seen in the diabetic models was, perhaps not surprisingly, ascribed to defects in one or more components of RISK or SAFE signalling, including impaired ERK, PI3K/Akt, p70S6 kinase and/or GSK-3β phosphorylation, as well as possible abnormalities in mitochondrial end effectors (Bouhidel et al., 2008; Wagner et al., 2008; Przyklenk et al., 2011; Zhu et al., 2012; Wider and Przyklenk, 2014) (Table 2B). Finally, it is interesting to note that: (i) a diminished responsiveness to postconditioning was observed before the onset of significant hyperglycaemia in Zucker fatty rats (Katakam et al., 2007), and (ii) similar findings [loss of postconditioning-induced (and preconditioning-induced) cardioprotection] have been reported in models of type 1 diabetes (reviewed in Ferdinandy et al., 2007; 2014,; Przyklenk, 2011; 2013,; Vinten-Johansen et al., 2011b; Miki et al., 2012; Wider and Przyklenk, 2014). These latter data imply that the apparent diabetes-associated impairment in survival signalling is not a simple consequence of hyperglycaemia, hyperinsulinaemia, hyperlipidaemia or obesity.
Taken together, there is general agreement among the small number of published preclinical studies: type-2 diabetes appears to have a confounding effect on the infarct-sparing effect of postconditioning. This conclusion is, however, based exclusively on data obtained in rodent models. Whether infarct size reduction with remote conditioning is similarly compromised in diabetic cohorts is currently unknown (Table 2B).
Clinical relevance of comorbid models?
The preceding discussion raises an important conceptual question: can the results obtained from rat and mouse models of ageing and type-2 diabetes be considered clinically meaningful? That is, are these in fact the ‘right’ models (or more relevant models) to guide the clinical translation of postconditioning and remote conditioning?
It could be argued that the concept of ageing and possible (albeit inconclusive) evidence of an age-associated loss in conditioning-induced cardioprotection should be translatable among models and species, including man. However, ageing is a continuum and attempts to define ‘old’ cohorts based on chronological age and lifespan are, without question, subjective and may not correlate with cardiovascular indices of ageing on the organ, cellular, biochemical or molecular level (Przyklenk et al., 2001; 2003; 2008,,; Przyklenk, 2011; Vinten-Johansen et al., 2011b). This may in part underlie the variability in outcomes among experimental studies in which postconditioning was used as the protective stimulus (Table 2A), and apparent discrepancies among the small number of clinical conditioning studies (including pre- and postconditioning protocols) in which post hoc subset analyses based on age were performed (Abete et al., 1997; Kloner et al., 1998; Jimenez-Navarro et al., 2001; Wu et al., 2001; Vinten-Johansen et al., 2011b). For example, in two similarly designed analyses assessing the effect of pre-infarct angina (a presumptive preconditioning stimulus) on the incidence of in-hospital death, congestive heart failure and/or shock, one reported persistent benefit in patients >60 years of age (Kloner et al., 1998), while the second concluded that the favourable effects of pre-infarct angina were lost in ‘senescent’, >65-year-old patients (Abete et al., 1997). Only one post hoc analysis has been reported to date in which outcome (in this case, cardiac enzyme release) in response to postconditioning was stratified according to age: in the subset of patients >65 years of age, no significant infarct-sparing effect of postconditioning was observed (Darling et al., 2007; Vinten-Johansen et al., 2011b).
A second consideration is the fact that all preclinical models are simplistic; even novel ageing models displaying a second concomitant comorbidity (Ebrahim et al., 2007; Dai et al., 2009; Whittington et al., 2013) do not mimic the complexities of patients with cardiovascular disease. This question of complexity extends beyond the issue of multiple comorbidities. First, extrapolation of results obtained from animals enrolled days-weeks after the onset of diabetes, and typically characterized by extreme hyperglycaemia [>5.00 mg ml−1 (Przyklenk et al., 2011)], to patients with long-standing disease may be problematic. A second and related corollary is that preclinical studies do not typically incorporate the pharmacological therapies routinely administered to patients as standard of care for the management of diabetes and, in many instances, other attendant comorbidities. For example, there is evidence that nitrates, statins, opioids and anti-platelet agents are cardioprotective per se and mimic the benefits of ischaemic conditioning (reviewed in Przyklenk, 2011; Heusch, 2013; Ferdinandy et al., 2014). Sulfonylurea agents administered for glycaemic control in patients with type-2 diabetes can purportedly have complex and opposing consequences: glibenclamide has a well-documented, antagonistic effect and prevents conditioning-induced cardioprotection via inhibition of cardiac ATP-sensitive potassium channels, while glimepiride, although not cardioprotective when administered alone, has been shown to facilitate and re-establish the efficacy of ischaemic preconditioning in the Goto-Kakizaki rat model of type-2 diabetes (Hausenloy et al., 2013a; Ferdinandy et al., 2014). Interestingly, potentiation of the infarct-sparing effect of preconditioning with glimepiride could not be attributed to reductions in blood glucose concentration, but rather may be due to a proposed up-regulation in survival kinase signalling (Hausenloy et al., 2013a). Finally, effective treatment of the comorbid condition may favourably modulate the response to ischaemic conditioning. This latter concept is illustrated by the finding that restoration of plasma insulin concentrations in the mouse model of streptozotocin-induced type 1 diabetes (achieved by islet cell transplantation) re-established the infarct-sparing effect of postconditioning, thereby suggesting that the cardiac signalling defect(s) responsible for preventing postconditioning-induced cardioprotection in this model are labile and reversible (Przyklenk et al., 2011). Whether similar results are obtained with appropriate pharmacological management of type-2 diabetes that does not involve the use of sulfonylureas is currently unknown.
Given these caveats, the question remains: does the concept of a loss in efficacy of conditioning-induced cardioprotection derived from rodent models of type-2 diabetes have translational relevance? Subset analyses of diabetic cohorts have been reported for two clinical trials in which cardiac enzyme release following acute MI served as the primary end point: preconditioning (triggered by preinfarct angina) had no beneficial effect while postconditioning tended to exacerbate biomarker release (and, presumably, infarct size) in the diabetic subgroup (Ishihara et al., 2001; Yetgin et al., 2014). Similarly, a third study concluded that remote conditioning, administered after non-emergent PCI, increased the incidence of peri-procedural MI in patients with versus without diabetes (Carrasco-Chinchilla et al., 2013). These three observations appear to corroborate the concept of failed conditioning-induced cardioprotection seen in preclinical diabetic models. In contrast, the recent systemic review and meta-analysis of five randomized trials assessing the effect of remote preconditioning in the setting of elective PCI yielded the opposite conclusion: meta-regression revealed no interaction between diabetes or age on the rate of peri-procedural MI. That is, there was no apparent loss in efficacy of remote conditioning in these patient cohorts (D'Ascenzo et al., 2014).
Conclusions and future directions
There is no question that: (i) the development and implementation of novel adjuvant strategies to attenuate myocardial ischaemia-reperfusion injury and reduce infarct size remains a major, unmet clinical need; (ii) a wealth of preclinical data has positioned ischaemic conditioning (in particular, postconditioning and remote conditioning) to fulfil this need; yet (iii) the translation of ischaemic conditioning from preclinical models to clinical practice has been stalled. The slow progress in realizing the clinical potential of ischaemic conditioning, and the attendant frustration and disappointment, may be due in part to the heterogeneity within and among the currently completed phase II clinical trials, together with the still-limited body of evidence that the infarct-sparing effect of ischaemic conditioning may be compromised in diabetic (and, possibly, aging) cohorts. Robust evidence supporting (or refuting) the clinical efficacy of postconditioning and remote conditioning will require large-scale trials that have been designed and executed to heed the lessons learned from preclinical studies, including consideration of the determinants of myocardial infarct size, the importance of the timing of treatment and enrollment of the ‘right’ patient cohorts. It is hoped that the outcomes of the current and highly anticipated phase III trials will facilitate – and accelerate – the successful clinical translation of this most promising cardioprotective strategy.
Acknowledgments
K. P. is supported in part by the National Institutes of Health (NIH-HL072684).
Glossary
- CABG
coronary artery bypass graft
- GSK-3β
glycogen synthase kinase 3β
- MI
myocardial infarction
- PCI
percutaneous coronary intervention
- RISK
reperfusion injury salvage kinase
- SAFE
survival activating factor enhancement
- SPECT
single-photon emission computed tomography
- STEMI
ST segment elevation myocardial infarction
Conflict of interest
K. P. serves as a member of the Scientific Advisory Board of Infarct Reduction Technologies, Inc.
References
- Abdelnoor M, Sandven I, Limalanathan S, Eritsland J. Postconditioning in ST-elevation myocardial infarction: a systematic review, critical appraisal, and meta-analysis of randomized clinical trials. Vasc Health Risk Manag. 2014;10:477–491. doi: 10.2147/VHRM.S67154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abete P, Ferrara N, Cacciatore F, Madrid A, Bianco S, Calabrese C, et al. Angina-induced protection against myocardial infarction in adult and elderly patients: a loss of preconditioning mechanism in the aging heart? J Am Coll Cardiol. 1997;30:947–954. doi: 10.1016/s0735-1097(97)00256-8. [DOI] [PubMed] [Google Scholar]
- Abete P, Cacciatore F, Testa G, Della-Morte D, Galizia G, de Santis D, et al. Ischemic preconditioning in the aging heart: from bench to bedside. Ageing Res Rev. 2010;9:153–162. doi: 10.1016/j.arr.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Adam T, Sharp S, Opie LH, Lecour S. Loss of cardioprotection with ischemic preconditioning in aging hearts: role of sirtuin 1? J Cardiovasc Pharmacol Ther. 2013;18:46–53. doi: 10.1177/1074248412458723. [DOI] [PubMed] [Google Scholar]
- Albrecht M, Zitta K, Bein B, Wennemuth G, Broch O, Renner J, et al. Remote ischemic preconditioning regulates HIF-1alpha levels, apoptosis and inflammation in heart tissue of cardiosurgical patients: a pilot experimental study. Basic Res Cardiol. 2013;108:314. doi: 10.1007/s00395-012-0314-0. [DOI] [PubMed] [Google Scholar]
- Alegria JR, Miller TD, Gibbons RJ, Yi QL, Yusuf S. Infarct size, ejection fraction, and mortality in diabetic patients with acute myocardial infarction treated with thrombolytic therapy. Am Heart J. 2007;154:743–750. doi: 10.1016/j.ahj.2007.06.020. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G protein-coupled receptors. Br J Pharmacol. 2013a;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Ion channels. Br J Pharmacol. 2013b;170:1607–1651. doi: 10.1111/bph.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol. 2013c;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman DG, Bland JM. How to obtain the P value from a confidence interval. BMJ. 2011;343:d2304. doi: 10.1136/bmj.d2304. [DOI] [PubMed] [Google Scholar]
- Barja G, Herrero A. Oxidative damage to mitochondrial DNA is inversely related to maximum life span in the heart and brain of mammals. FASEB J. 2000;14:312–318. doi: 10.1096/fasebj.14.2.312. [DOI] [PubMed] [Google Scholar]
- Boengler K, Konietzka I, Buechert A, Heinen Y, Garcia-Dorado D, Heusch G, et al. Loss of ischemic preconditioning's cardioprotection in aged mouse hearts is associated with reduced gap junctional and mitochondrial levels of connexin 43. Am J Physiol Heart Circ Physiol. 2007;292:H1764–H1769. doi: 10.1152/ajpheart.01071.2006. [DOI] [PubMed] [Google Scholar]
- Boengler K, Buechert A, Heinen Y, Roeskes C, Hilfiker-Kleiner D, Heusch G, et al. Cardioprotection by ischemic postconditioning is lost in aged and STAT3-deficient mice. Circ Res. 2008;102:131–135. doi: 10.1161/CIRCRESAHA.107.164699. [DOI] [PubMed] [Google Scholar]
- Boengler K, Schulz R, Heusch G. Loss of cardioprotection with ageing. Cardiovasc Res. 2009;83:247–261. doi: 10.1093/cvr/cvp033. [DOI] [PubMed] [Google Scholar]
- Bolli R. The late phase of preconditioning. Circ Res. 2000;87:972–983. doi: 10.1161/01.res.87.11.972. [DOI] [PubMed] [Google Scholar]
- Botker HE, Kharbanda R, Schmidt MR, Bottcher M, Kaltoft AK, Terkelsen CJ, et al. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet. 2010;375:727–734. doi: 10.1016/S0140-6736(09)62001-8. [DOI] [PubMed] [Google Scholar]
- Bouhidel O, Pons S, Souktani R, Zini R, Berdeaux A, Ghaleh B. Myocardial ischemic postconditioning against ischemia-reperfusion is impaired in ob/ob mice. Am J Physiol Heart Circ Physiol. 2008;295:H1580–H1586. doi: 10.1152/ajpheart.00379.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunwald E, Kloner RA. Myocardial reperfusion: a double-edged sword? J Clin Invest. 1985;76:1713–1719. doi: 10.1172/JCI112160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns PG, Krunkenkamp IB, Calderone CA, Kirvaitis RJ, Gaudette GR, Levitsky S. Is the preconditioning response conserved in senescent myocardium? Ann Thorac Surg. 1996;61:925–929. doi: 10.1016/0003-4975(95)01188-9. [DOI] [PubMed] [Google Scholar]
- Carrasco-Chinchilla F, Munoz-Garcia AJ, Dominguez-Franco A, Millan-Vazquez G, Guerrero-Molina A, Ortiz-Garcia C, et al. Remote ischaemic postconditioning: does it protect against ischaemic damage in percutaneous coronary revascularisation? Randomised placebo-controlled clinical trial. Heart. 2013;99:1431–1437. doi: 10.1136/heartjnl-2013-304172. [DOI] [PubMed] [Google Scholar]
- Cohen MV, Downey JM. Is it time to translate ischemic preconditioning's mechanism of cardioprotection into clinical practice? J Cardiovasc Pharmacol Ther. 2011;16:273–280. doi: 10.1177/1074248411407071. [DOI] [PubMed] [Google Scholar]
- Cohen MV, Downey JM. Signaling pathways and mechanisms of protection in pre- and postconditioning: historical perspective and lessons for the future. Br J Pharmacol. 2009 doi: 10.1111/bph.12903. . doi: 10.1111/bph.12903. [Epub ahead of print: Sept 10 2014.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai W, Simkhovich BZ, Kloner RA. Ischemic preconditioning maintains cardioprotection in aging normotensive and spontaneously hypertensive rats. Exp Gerontol. 2015;44:344–349. doi: 10.1016/j.exger.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Darling CE, Solari PB, Smith CS, Furman MI, Przyklenk K. Postconditioning’ the human heart: multiple balloon inflations during primary angioplasty may confer cardioprotection. Basic Res Cardiol. 2007;102:274–278. doi: 10.1007/s00395-007-0643-6. [DOI] [PubMed] [Google Scholar]
- Davies WR, Brown AJ, Watson W, McCormick LM, West NE, Dutka DP, et al. Remote ischemic preconditioning improves outcome at 6 years after elective percutaneous coronary intervention: the CRISP stent trial long-term follow-up. Circ Cardiovasc Interv. 2013;6:246–251. doi: 10.1161/CIRCINTERVENTIONS.112.000184. [DOI] [PubMed] [Google Scholar]
- D'Ascenzo F, Moretti C, Omede P, Cerrato E, Cavallero E, Er F, et al. Cardiac remote ischaemic preconditioning reduces periprocedural myocardial infarction for patients undergoing percutaneous coronary interventions: a meta-analysis of randomised clinical trials. EuroIntervention. 2014;9:1463–1471. doi: 10.4244/EIJV9I12A244. [DOI] [PubMed] [Google Scholar]
- Dickson EW, Blehar DJ, Carraway RE, Heard SO, Steinberg G, Przyklenk K. Naloxone blocks transferred preconditioning in isolated rabbit hearts. J Mol Cell Cardiol. 2001;33:1751–1756. doi: 10.1006/jmcc.2001.1436. [DOI] [PubMed] [Google Scholar]
- Donato M, Buchholz B, Rodriguez M, Perez V, Inserte J, Garcia-Dorado D, et al. Role of the parasympathetic nervous system in cardioprotection by remote hindlimb ischaemic preconditioning. Exp Physiol. 2013;98:425–434. doi: 10.1113/expphysiol.2012.066217. [DOI] [PubMed] [Google Scholar]
- Dow J, Kloner RA. Postconditioning does not reduce myocardial infarct size in an in vivo regional ischemia rodent model. J Cardiovasc Pharmacol Ther. 2007;12:153–163. doi: 10.1177/1074248407300897. [DOI] [PubMed] [Google Scholar]
- Ebrahim Z, Yellon DM, Baxter GF. Ischemic preconditioning is lost in aging hypertensive rat heart: independent effects of aging and longstanding hypertension. Exp Gerontol. 2007;42:807–814. doi: 10.1016/j.exger.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Fenton RA, Dickson EW, Meyer TE, Dobson JG., Jr Aging reduces the cardioprotective effect of ischemic preconditioning in the rat heart. J Mol Cell Cardiol. 2000;32:1371–1375. doi: 10.1006/jmcc.2000.1189. [DOI] [PubMed] [Google Scholar]
- Ferdinandy P, Schulz R, Baxter GF. Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning. Pharmacol Rev. 2007;59:418–458. doi: 10.1124/pr.107.06002. [DOI] [PubMed] [Google Scholar]
- Ferdinandy P, Hausenloy DJ, Heusch G, Baxter GF, Schulz R. Interaction of risk factors, comorbidities, and comedications with ischemia/reperfusion injury and cardioprotection by preconditioning, postconditioning, and remote conditioning. Pharmacol Rev. 2014;66:1142–1174. doi: 10.1124/pr.113.008300. [DOI] [PubMed] [Google Scholar]
- Freixa X, Bellera N, Ortiz-Perez JT, Jimenez M, Pare C, Bosch X, et al. Ischaemic postconditioning revisited: lack of effects on infarct size following primary percutaneous coronary intervention. Eur Heart J. 2012;33:103–112. doi: 10.1093/eurheartj/ehr297. [DOI] [PubMed] [Google Scholar]
- Garcia S, Henry TD, Wang YL, Chavez IJ, Pedersen WR, Lesser JR, et al. Long-term follow-up of patients undergoing postconditioning during ST-elevation myocardial infarction. J Cardiovasc Transl Res. 2011;4:92–98. doi: 10.1007/s12265-010-9252-0. [DOI] [PubMed] [Google Scholar]
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine A, Gourine AV. Neural mechanisms of cardioprotection. Physiology (Bethesda) 2014;29:133–140. doi: 10.1152/physiol.00037.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg EW, Zhuo X, Cheng YL, Albright AL, Narayan KMV, Thompson TJ. Trends in lifetime risk and years of life lost due to diabetes in the USA, 1985—2011: a modelling study. Lancet Diabetes Endocrinol. 2014;14:70161–70165. doi: 10.1016/S2213-8587(14)70161-5. [DOI] [PubMed] [Google Scholar]
- Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- Hahn JY, Song YB, Kim EK, Yu CW, Bae JW, Chung WY, et al. Ischemic postconditioning during primary percutaneous coronary intervention: the effects of postconditioning on myocardial reperfusion in patients with ST-segment elevation myocardial infarction (POST) randomized trial. Circulation. 2013;128:1889–1896. doi: 10.1161/CIRCULATIONAHA.113.001690. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Yellon DM. Preconditioning and postconditioning: underlying mechanisms and clinical application. Atherosclerosis. 2009;204:334–341. doi: 10.1016/j.atherosclerosis.2008.10.029. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Lecour S, Yellon DM. Reperfusion injury salvage kinase and survivor activating factor enhancement prosurvival signaling pathways in ischemic postconditioning: two sides of the same coin. Antioxid Redox Signal. 2011a;14:893–907. doi: 10.1089/ars.2010.3360. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Candilio L, Laing C, Kunst G, Pepper J, Kolvekar S, et al. Effect of remote ischemic preconditioning on clinical outcomes in patients undergoing coronary artery bypass graft surgery (ERICCA): rationale and study design of a multi-centre randomized double-blinded controlled clinical trial. Clin Res Cardiol. 2011b;101:339–348. doi: 10.1007/s00392-011-0397-x. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Wynne AM, Mocanu MM, Yellon DM. Glimepiride treatment facilitates ischemic preconditioning in the diabetic heart. J Cardiovasc Pharmacol Ther. 2013a;18:263–269. doi: 10.1177/1074248412468945. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Botker EH, Condorelli G, Ferdinandy P, Garcia-Dorado D, Heusch G, et al. Translating cardioprotection for patient benefit: position paper from the Working Group of Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc Res. 2013b;98:7–27. doi: 10.1093/cvr/cvt004. [DOI] [PubMed] [Google Scholar]
- Healy DA, Khan WA, Wong CS, Moloney MC, Grace PA, Coffey JC, et al. Remote preconditioning and major clinical complications following adult cardiovascular surgery: systematic review and meta-analysis. Int J Cardiol. 2014;176:20–31. doi: 10.1016/j.ijcard.2014.06.018. [DOI] [PubMed] [Google Scholar]
- Heusch G. Cardioprotection: chances and challenges of its translation to the clinic. Lancet. 2013;381:166–175. doi: 10.1016/S0140-6736(12)60916-7. [DOI] [PubMed] [Google Scholar]
- Heusch G, Boengler K, Schulz R. Cardioprotection: nitric oxide, protein kinases, and mitochondria. Circulation. 2008;118:1915–1919. doi: 10.1161/CIRCULATIONAHA.108.805242. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoole SP, Heck PM, Sharples L, Khan SN, Duehmke R, Densem CG, et al. Cardiac Remote Ischemic Preconditioning in Coronary Stenting (CRISP Stent) Study: a prospective, randomized control trial. Circulation. 2009;119:820–827. doi: 10.1161/CIRCULATIONAHA.108.809723. [DOI] [PubMed] [Google Scholar]
- Huffmyer J, Raphael J. Physiology and pharmacology of myocardial preconditioning and postconditioning. Semin Cardiothorac Vasc Anesth. 2009;13:5–18. doi: 10.1177/1089253208330709. [DOI] [PubMed] [Google Scholar]
- Iliodromitis EK, Kyrzopoulos S, Paraskevaidis IA, Kolocassides KG, Adamopoulos S, Karavolias G, et al. Increased C reactive protein and cardiac enzyme levels after coronary stent implantation. Is there protection by remote ischaemic preconditioning? Heart. 2006;92:1821–1826. doi: 10.1136/hrt.2006.089060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara M, Inoue I, Kawagoe T, Shimatani Y, Kurisu S, Nishioka K, et al. Diabetes mellitus prevents ischemic preconditioning in patients with a first acute anterior wall myocardial infarction. J Am Coll Cardiol. 2001;38:1007–1011. doi: 10.1016/s0735-1097(01)01477-2. [DOI] [PubMed] [Google Scholar]
- Jimenez-Navarro M, Gomez-Doblas JJ, Alonso-Briales J, Hernandez Garcia JM, Gomez G, Alcantara AG, et al. Does angina the week before protect against first myocardial infarction in elderly patients? Am J Cardiol. 2001;87:11–15. doi: 10.1016/s0002-9149(00)01264-9. [DOI] [PubMed] [Google Scholar]
- Kanoria S, Jalan R, Seifalian AM, Williams R, Davidson BR. Protocols and mechanisms for remote ischemic preconditioning: a novel method for reducing ischemia reperfusion injury. Transplantation. 2007;84:445–458. doi: 10.1097/01.tp.0000228235.55419.e8. [DOI] [PubMed] [Google Scholar]
- Katakam PV, Jordan JE, Snipes JA, Tulbert CD, Miller AW, Busija DW. Myocardial preconditioning against ischemia-reperfusion injury is abolished in Zucker obese rats with insulin resistance. Am J Physiol Regul Integr Comp Physiol. 2007;292:R920–R926. doi: 10.1152/ajpregu.00520.2006. [DOI] [PubMed] [Google Scholar]
- Kharbanda RK, Mortensen UM, White PA, Kristiansen SB, Schmidt MR, Hoschtitzky JA, et al. Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation. 2002;106:2881–2883. doi: 10.1161/01.cir.0000043806.51912.9b. [DOI] [PubMed] [Google Scholar]
- Kin H, Zhao ZQ, Sun HY, Wang NP, Corvera JS, Halkos ME, et al. Postconditioning attenuates myocardial ischemia-reperfusion injury by inhibiting events in the early minutes of reperfusion. Cardiovasc Res. 2004;62:74–85. doi: 10.1016/j.cardiores.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Kleinbongard P, Heusch G. Extracellular signaling molecules in the ischaemic/reperfused heart – druggable and translatable for cardioprotection? Br J Pharmacol. 2015 doi: 10.1111/bph.12902. . doi: 10.1111/bph.12902. [Epub ahead of print: Sept 10 2014] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloner RA, Przyklenk K, Shook T, Cannon CP. Protection conferred by preinfarct angina is manifest in the aged heart: evidence from the TIMI 4 trial. J Thromb Thrombolysis. 1998;6:89–92. doi: 10.1023/A:1008833101817. [DOI] [PubMed] [Google Scholar]
- Kottenberg E, Thielmann M, Bergmann L, Heine T, Jakob H, Heusch G, et al. Protection by remote ischemic preconditioning during coronary artery bypass graft surgery with isoflurane but not propofol – a clinical trial. Acta Anaesthesiol Scand. 2012;56:30–38. doi: 10.1111/j.1399-6576.2011.02585.x. [DOI] [PubMed] [Google Scholar]
- Kottenberg E, Musiolik J, Thielmann M, Jakob H, Peters J, Heusch G. Interference of propofol with signal transducer and activator of transcription 5 activation and cardioprotection by remote ischemic preconditioning during coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2014;147:376–382. doi: 10.1016/j.jtcvs.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Krempf M, Parhofer KG, Steg PG, Bhatt DL, Ohman EM, Rother J, et al. Cardiovascular event rates in diabetic and nondiabetic individuals with and without established atherothrombosis (from the REduction of the rothrombosis for Continued Health [REACH] Registry) Am J Cardiol. 2010;105:667–671. doi: 10.1016/j.amjcard.2009.10.048. [DOI] [PubMed] [Google Scholar]
- Kristiansen SB, Lofgren B, Stottrup NB, Khatir D, Nielsen-Kudsk JE, Nielsen TT, et al. Ischaemic preconditioning does not protect the heart in obese and lean animal models of type 2 diabetes. Diabetologia. 2004;47:1716–1721. doi: 10.1007/s00125-004-1514-4. [DOI] [PubMed] [Google Scholar]
- Laskey WK, Yoon S, Calzada N, Ricciardi MJ. Concordant improvements in coronary flow reserve and ST-segment resolution during percutaneous coronary intervention for acute myocardial infarction: a benefit of postconditioning. Catheter Cardiovasc Interv. 2008;72:212–220. doi: 10.1002/ccd.21583. [DOI] [PubMed] [Google Scholar]
- Lefer DJ, Bolli R. Development of an NIH consortium for preclinicAl AssESsment of CARdioprotective therapies (CAESAR): a paradigm shift in studies of infarct size limitation. J Cardiovasc Pharmacol Ther. 2011;16:332–339. doi: 10.1177/1074248411414155. [DOI] [PubMed] [Google Scholar]
- Lim SY, Yellon DM, Hausenloy DJ. The neural and humoral pathways in remote limb ischemic preconditioning. Basic Res Cardiol. 2010;105:651–655. doi: 10.1007/s00395-010-0099-y. [DOI] [PubMed] [Google Scholar]
- Limalanathan S, Andersen GO, Klow NE, Abdelnoor M, Hoffmann P, Eritsland J. Effect of ischemic postconditioning on infarct size in patients with ST-elevation myocardial infarction treated by primary PCI results of the POSTEMI (POstconditioning in ST-Elevation Myocardial Infarction) randomized trial. J Am Heart Assoc. 2014;3:e000679. doi: 10.1161/JAHA.113.000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longacre LS, Kloner RA, Arai AE, Baines CP, Bolli R, Braunwald E, et al. New horizons in cardioprotection recommendations from the 2010 national heart, lung, and blood institute workshop. Circulation. 2011;124:1172–1179. doi: 10.1161/CIRCULATIONAHA.111.032698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastitskaya S, Marina N, Gourine A, Gilbey MP, Spyer KM, Teschemacher AG, et al. Cardioprotection evoked by remote ischaemic preconditioning is critically dependent on the activity of vagal pre-ganglionic neurones. Cardiovasc Res. 2012;95:487–494. doi: 10.1093/cvr/cvs212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meybohm P, Zacharowski K, Cremer J, Roesner J, Kletzin F, Schaelte G, et al. Remote ischaemic preconditioning for heart surgery. The study design for a multi-center randomized double-blinded controlled clinical trial–the RIPHeart-Study. Eur Heart J. 2012;33:1423–1426. [PubMed] [Google Scholar]
- Miki T, Itoh T, Sunaga D, Miura T. Effects of diabetes on myocardial infarct size and cardioprotection by preconditioning and postconditioning. Cardiovasc Diabetol. 2012;11:67. doi: 10.1186/1475-2840-11-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murcia AM, Hennekens CH, Lamas GA, Jimenez-Navarro M, Rouleau JL, Flaker GC, et al. Impact of diabetes on mortality in patients with myocardial infarction and left ventricular dysfunction. Arch Intern Med. 2004;164:2273–2279. doi: 10.1001/archinte.164.20.2273. [DOI] [PubMed] [Google Scholar]
- Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev. 2008;88:581–609. doi: 10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- Nakano A, Heusch G, Cohen MV, Downey JM. Preconditioning one myocardial region does not necessarily precondition the whole rabbit heart. Basic Res Cardiol. 2002;97:35–39. doi: 10.1007/s395-002-8385-3. [DOI] [PubMed] [Google Scholar]
- Nichols M, Townsend N, Scarborough P, Rayner M. Cardiovascular disease in Europe: epidemiological update. Eur Heart J. 2013;34:3028–3034. doi: 10.1093/eurheartj/eht356. [DOI] [PubMed] [Google Scholar]
- Okamoto F, Allen BS, Buckberg GD, Bugyi H, Leaf J. Reperfusion conditions: importance of ensuring gentle versus sudden reperfusion during relief of coronary occlusion. J Thorac Cardiovasc Surg. 1986;92(3 Pt 2):613–620. [PubMed] [Google Scholar]
- Oosterlinck W, Dresselaers T, Geldhof V, Nevelsteen I, Janssens S, Himmelreich U, et al. Diabetes mellitus and the metabolic syndrome do not abolish, but might reduce, the cardioprotective effect of ischemic postconditioning. J Thorac Cardiovasc Surg. 2013;145:1595–1602. doi: 10.1016/j.jtcvs.2013.02.016. [DOI] [PubMed] [Google Scholar]
- Ovize M, Baxter GF, Di Lisa F, Ferdinandy P, Garcia-Dorado D, Hausenloy DJ, et al. Postconditioning and protection from reperfusion injury: where do we stand? Position paper from the Working Group of Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc Res. 2010;87:406–423. doi: 10.1093/cvr/cvq129. [DOI] [PubMed] [Google Scholar]
- Ovize M, Thibault H, Przyklenk K. Myocardial conditioning: opportunities for clinical translation. Circ Res. 2013;113:439–450. doi: 10.1161/CIRCRESAHA.113.300764. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledgebase of drug targets and their ligands. Nucleic Acids Res. 2014;42(Database Issue):D1098–D1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przyklenk K. Efficacy of cardioprotective ‘conditioning’ strategies in aging and diabetic cohorts: the co-morbidity conundrum. Drugs Aging. 2011;28:331–343. doi: 10.2165/11587190-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Przyklenk K. Reduction of myocardial infarct size with ischemic ‘conditioning’: physiologic and technical considerations. Anesth Analg. 2013;117:891–901. doi: 10.1213/ANE.0b013e318294fc63. [DOI] [PubMed] [Google Scholar]
- Przyklenk K, Whittaker P. Remote ischemic preconditioning: current knowledge, unresolved questions, and future priorities. J Cardiovasc Pharmacol Ther. 2011;16:255–259. doi: 10.1177/1074248411409040. [DOI] [PubMed] [Google Scholar]
- Przyklenk K, Whittaker P. Genesis of remote conditioning: action at a distance–‘hypotheses non fingo. J Cardiovasc Med (Hagerstown) 2013;14:180–186. doi: 10.2459/JCM.0b013e328358c8eb. [DOI] [PubMed] [Google Scholar]
- Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P. Regional ischemic ‘preconditioning’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation. 1993;87:893–899. doi: 10.1161/01.cir.87.3.893. [DOI] [PubMed] [Google Scholar]
- Przyklenk K, Li G, Whittaker P. No loss in the in vivo efficacy of ischemic preconditioning in middle-aged and old rabbits. J Am Coll Cardiol. 2001;38:1741–1747. doi: 10.1016/s0735-1097(01)01603-5. [DOI] [PubMed] [Google Scholar]
- Przyklenk K, Li G, Simkhovich BZ, Kloner RA. Mechanisms of myocardial ischemic preconditioning are age related: PKC-epsilon does not play a requisite role in old rabbits. J Appl Physiol. 2003;95:2563–2569. doi: 10.1152/japplphysiol.00404.2003. [DOI] [PubMed] [Google Scholar]
- Przyklenk K, Maynard M, Darling CE, Whittaker P. Aging mouse hearts are refractory to infarct size reduction with post-conditioning. J Am Coll Cardiol. 2008;51:1393–1398. doi: 10.1016/j.jacc.2007.11.070. [DOI] [PubMed] [Google Scholar]
- Przyklenk K, Maynard M, Greiner DL, Whittaker P. Cardioprotection with postconditioning: loss of efficacy in murine models of type-2 and type-1 diabetes. Antioxid Redox Signal. 2011;14:781–790. doi: 10.1089/ars.2010.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman IA, Mascaro JG, Steeds RP, Frenneaux MP, Nightingale P, Gosling P, et al. Remote ischemic preconditioning in human coronary artery bypass surgery: from promise to disappointment? Circulation. 2010;122(11 Suppl):S53–S59. doi: 10.1161/CIRCULATIONAHA.109.926667. [DOI] [PubMed] [Google Scholar]
- Redington KL, Disenhouse T, Strantzas SC, Gladstone R, Wei C, Tropak MB, et al. Remote cardioprotection by direct peripheral nerve stimulation and topical capsaicin is mediated by circulating humoral factors. Basic Res Cardiol. 2012;107:241. doi: 10.1007/s00395-011-0241-5. [DOI] [PubMed] [Google Scholar]
- Reimer KA, Jennings RB. The ‘wavefront phenomenon’ of myocardial ischemic cell death. II. Transmural progression of necrosis within the framework of ischemic bed size (myocardium at risk) and collateral flow. Lab Invest. 1979;40:633–644. [PubMed] [Google Scholar]
- Reimer KA, Lowe JE, Rasmussen MM, Jennings RB. The wavefront phenomenon of ischemic cell death. 1. Myocardial infarct size vs duration of coronary occlusion in dogs. Circulation. 1977;56:786–794. doi: 10.1161/01.cir.56.5.786. [DOI] [PubMed] [Google Scholar]
- Roubille F, Mewton N, Elbaz M, Roth O, Prunier F, Cung TT, et al. No post-conditioning in the human heart with thrombolysis in myocardial infarction flow 2–3 on admission. Eur Heart J. 2014;35:1675–1682. doi: 10.1093/eurheartj/ehu054. [DOI] [PubMed] [Google Scholar]
- Sachdeva J, Dai W, Gerczuk PZ, Kloner RA. Combined remote perconditioning and postconditioning failed to attenuate infarct size and contractile dysfunction in a rat model of coronary artery occlusion. J Cardiovasc Pharmacol Ther. 2014;19:567–573. doi: 10.1177/1074248413518967. [DOI] [PubMed] [Google Scholar]
- Sanada S, Komuro I, Kitakaze M. Pathophysiology of myocardial reperfusion injury: preconditioning, postconditioning, and translational aspects of protective measures. Am J Physiol Heart Circ Physiol. 2011;301:H1723–H1741. doi: 10.1152/ajpheart.00553.2011. [DOI] [PubMed] [Google Scholar]
- Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena P, Newman MA, Shehatha JS, Redington AN, Konstantinov IE. Remote ischemic conditioning: evolution of the concept, mechanisms, and clinical application. J Card Surg. 2010;25:127–134. doi: 10.1111/j.1540-8191.2009.00820.x. [DOI] [PubMed] [Google Scholar]
- Schevchuck A, Laskey WK. Ischemic conditioning as an adjunct to percutaneous coronary intervention. Circ Cardiovasc Interv. 2013;6:484–492. doi: 10.1161/CIRCINTERVENTIONS.113.000146. [DOI] [PubMed] [Google Scholar]
- Schmidt MR, Redington A, Botker HE. Remote conditioning the heart overview – translatability and mechanism. Br J Pharmacol. 2015 doi: 10.1111/bph.12933. . doi: 10.1111/bph.12933. [Epub ahead of print: Sept 14 2014] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman D, Latchman DS, Yellon DM. Effect of aging on the ability of preconditioning to protect rat hearts from ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2001;281:H1630–H1636. doi: 10.1152/ajpheart.2001.281.4.H1630. [DOI] [PubMed] [Google Scholar]
- Schwartz LM, Lagranha CJ. Ischemic postconditioning during reperfusion activates Akt and ERK without protecting against lethal myocardial ischemia-reperfusion injury in pigs. Am J Physiol Heart Circ Physiol. 2006;290:H1011–H1018. doi: 10.1152/ajpheart.00864.2005. [DOI] [PubMed] [Google Scholar]
- Serejo FC, Rodrigues LF, Jr, da Silva Tavares KC, de Carvalho AC, Nascimento JH. Cardioprotective properties of humoral factors released from rat hearts subject to ischemic preconditioning. J Cardiovasc Pharmacol. 2007;49:214–220. doi: 10.1097/FJC.0b013e3180325ad9. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Tropak M, Diaz RJ, Suto F, Surendra H, Kuzmin E, et al. Transient limb ischaemia remotely preconditions through a humoral mechanism acting directly on the myocardium: evidence suggesting cross-species protection. Clin Sci (Lond) 2009;117:191–200. doi: 10.1042/CS20080523. [DOI] [PubMed] [Google Scholar]
- Sloth AD, Schmidt MR, Munk K, Kharbanda RK, Redington AN, Schmidt M, et al. Improved long-term clinical outcomes in patients with ST-elevation myocardial infarction undergoing remote ischaemic conditioning as an adjunct to primary percutaneous coronary intervention. Eur Heart J. 2014;35:168–175. doi: 10.1093/eurheartj/eht369. [DOI] [PubMed] [Google Scholar]
- Staat P, Rioufol G, Piot C, Cottin Y, Cung TT, L'Huillier I, et al. Postconditioning the human heart. Circulation. 2005;112:2143–2148. doi: 10.1161/CIRCULATIONAHA.105.558122. [DOI] [PubMed] [Google Scholar]
- Steensrud T, Li J, Dai X, Manlhiot C, Kharbanda RK, Tropak M, et al. Pretreatment with the nitric oxide donor SNAP or nerve transection blocks humoral preconditioning by remote limb ischemia or intra-arterial adenosine. Am J Physiol Heart Circ Physiol. 2010;299:H1598–H1603. doi: 10.1152/ajpheart.00396.2010. [DOI] [PubMed] [Google Scholar]
- Thibault H, Piot C, Staat P, Bontemps L, Sportouch C, Rioufol G, et al. Long-term benefit of postconditioning. Circulation. 2008;117:1037–1044. doi: 10.1161/CIRCULATIONAHA.107.729780. [DOI] [PubMed] [Google Scholar]
- Thielmann M, Kottenberg E, Boengler K, Raffelsieper C, Neuhaeuser M, Peters J, et al. Remote ischemic preconditioning reduces myocardial injury after coronary artery bypass surgery with crystalloid cardioplegic arrest. Basic Res Cardiol. 2010;105:657–664. doi: 10.1007/s00395-010-0104-5. [DOI] [PubMed] [Google Scholar]
- Thielmann M, Kottenberg E, Kleinbongard P, Wendt D, Gedik N, Pasa S, et al. Cardioprotective and prognostic effects of remote ischaemic preconditioning in patients undergoing coronary artery bypass surgery: a single-centre randomised, double-blind, controlled trial. Lancet. 2013;382:597–604. doi: 10.1016/S0140-6736(13)61450-6. [DOI] [PubMed] [Google Scholar]
- Thuny F, Lairez O, Roubille F, Mewton N, Rioufol G, Sportouch C, et al. Post-conditioning reduces infarct size and edema in patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2012;59:2175–2181. doi: 10.1016/j.jacc.2012.03.026. [DOI] [PubMed] [Google Scholar]
- Tsang A, Hausenloy DJ, Mocanu MM, Carr RD, Yellon DM. Preconditioning the diabetic heart: the importance of Akt phosphorylation. Diabetes. 2005;54:2360–2364. doi: 10.2337/diabetes.54.8.2360. [DOI] [PubMed] [Google Scholar]
- Vinten-Johansen J, Shi W. Perconditioning and postconditioning: current knowledge, knowledge gaps, barriers to adoption, and future directions. J Cardiovasc Pharmacol Ther. 2011a;16:260–266. doi: 10.1177/1074248411415270. [DOI] [PubMed] [Google Scholar]
- Vinten-Johansen J, Granfeldt A, Mykytenko J, Undyala VV, Dong Y, Przyklenk K. The multidimensional physiological responses to postconditioning. Antioxid Redox Signal. 2011b;14:791–810. doi: 10.1089/ars.2010.3396. [DOI] [PubMed] [Google Scholar]
- Wagner C, Kloeting I, Strasser RH, Weinbrenner C. Cardioprotection by postconditioning is lost in WOKW rats with metabolic syndrome: role of glycogen synthase kinase 3beta. J Cardiovasc Pharmacol. 2008;52:430–437. doi: 10.1097/FJC.0b013e31818c12a7. [DOI] [PubMed] [Google Scholar]
- Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- Whittaker P, Przyklenk K. From ischemic conditioning to ‘hyperconditioning’: clinical phenomenon and basic science opportunity. Dose Response. 2014;12:650–663. doi: 10.2203/dose-response.14-035.Whittaker. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington HJ, Harding I, Stephenson CI, Bell R, Hausenloy DJ, Mocanu MM, et al. Cardioprotection in the aging, diabetic heart: the loss of protective Akt signalling. Cardiovasc Res. 2013;99:694–704. doi: 10.1093/cvr/cvt140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wider J, Przyklenk K. Ischemic conditioning: the challenge of protecting the diabetic heart. Cardiovasc Diagn Ther. 2014;4:383–396. doi: 10.3978/j.issn.2223-3652.2014.10.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu ZK, Pehkonen E, Laurikka J, Kaukinen L, Honkonen EL, Kaukinen S, et al. The protective effects of preconditioning decline in aged patients undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2001;122:972–978. doi: 10.1067/mtc.2001.117279. [DOI] [PubMed] [Google Scholar]
- Xue F, Yang X, Zhang B, Zhao C, Song J, Jiang T, et al. Postconditioning the human heart in percutaneous coronary intervention. Clin Cardiol. 2010;33:439–444. doi: 10.1002/clc.20796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- Yetgin T, Magro M, Manintveld OC, Nauta ST, Cheng JM, den Uil CA, et al. Impact of multiple balloon inflations during primary percutaneous coronary intervention on infarct size and long-term clinical outcomes in ST-segment elevation myocardial infarction: real-world postconditioning. Basic Res Cardiol. 2014;109:403. doi: 10.1007/s00395-014-0403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z, Gao H, Wang H, Li L, Di C, Luan R, et al. Ischaemic post-conditioning protects both adult and aged Sprague-Dawley rat heart from ischaemia-reperfusion injury through the phosphatidylinositol 3-kinase-AKT and glycogen synthase kinase-3beta pathways. Clin Exp Pharmacol Physiol. 2009;36:756–763. doi: 10.1111/j.1440-1681.2009.05148.x. [DOI] [PubMed] [Google Scholar]
- Young PJ, Dalley P, Garden A, Horrocks C, La Flamme A, Mahon B, et al. A pilot study investigating the effects of remote ischemic preconditioning in high-risk cardiac surgery using a randomised controlled double-blind protocol. Basic Res Cardiol. 2012;107:256. doi: 10.1007/s00395-012-0256-6. [DOI] [PubMed] [Google Scholar]
- Zaugg M, Lucchinetti E, Behmanesh S, Clanachan AS. Anesthetic cardioprotection in clinical practice from proof-of-concept to clinical applications. Curr Pharm Des. 2014;20:5706–5726. doi: 10.2174/1381612820666140204120829. [DOI] [PubMed] [Google Scholar]
- Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, et al. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285:H579–H588. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
- Zhu J, Rebecchi MJ, Glass PS, Brink PR, Liu L. Interactions of GSK-3beta with mitochondrial permeability transition pore modulators during preconditioning: age-associated differences. J Gerontol A Biol Sci Med Sci. 2013;68:395–403. doi: 10.1093/gerona/gls205. [DOI] [PubMed] [Google Scholar]
- Zhu SG, Xi L, Kukreja RC. Type 2 diabetic obese db/db mice are refractory to myocardial ischaemic post-conditioning in vivo: potential role for Hsp20, F1-ATPase delta and Echs1. J Cell Mol Med. 2012;16:950–958. doi: 10.1111/j.1582-4934.2011.01376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


