Abstract
The pharmacological conditioning of the heart with anaesthetics, such as volatile anaesthetics or opioids, is a phenomenon whereby a transient exposure to an anaesthetic agent protects the heart from the harmful consequences of myocardial ischaemia and reperfusion injury. The cellular and molecular mechanisms of anaesthetic conditioning appear largely to mimic those of ischaemic pre- and post-conditioning. Progress has been made on the understanding of the underlying mechanisms although the order of events and the specific targets of anaesthetics that trigger protection are not always clear. In the laboratory, the protection afforded by certain anaesthetics against cardiac ischaemia and reperfusion injury is powerful and reproducible but this has not necessarily translated into similarly robust clinical benefits. Indeed, clinical studies and meta-analyses delivered variable results when comparing in the laboratory setting protective and non-protective anaesthetics. Reasons for this include underlying conditions such as age, obesity and diabetes. Animal models for disease or ageing, human cardiomyocytes derived from stem cells of patients and further clinical studies are employed to better understand the underlying causes that prevent a more robust protection in patients.
Tables of Links
| LIGANDS |
|---|
| Adenosine |
| Desflurane |
| Glibenclamide |
| Halothane |
| Isoflurane |
| Morphine |
| Propofol |
| Sevoflurane |
| Vascular endothelial growth factor |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (a,b,c,dAlexander et al., 2013a,b,c,d).
Introduction
There is an increased risk of adverse cardiac events, such as ischaemia and infarction, congestive heart failure and arrhythmias, during and after cardiac and other surgery (Tuman, 1991; London, 2009). Early on it was reported that halothane, a volatile anaesthetic, decreased the severity of experimentally induced myocardial ischaemia in the non-failing canine heart whereby ischaemia was produced by repeated reversible occlusions of a coronary artery (Bland and Lowenstein, 1976). Volatile anaesthetic exposure was also associated with a mild depression of heart rate and systemic arterial pressure, suggesting decreased oxygen consumption. Similarly, the recovery of contractile function after a short period of ischaemia and reperfusion (‘stunned’ myocardium without tissue necrosis) was enhanced by isoflurane, along with preserved levels of ATP (Warltier et al., 1988; Kanaya et al., 1995). Later, it was discovered that volatile anaesthetics protect the heart in vivo and in vitro (Langendorff model) when applied shortly before a period of prolonged coronary artery occlusion (Cason et al., 1997; Cope et al., 1997; Kersten et al., 1997) and discontinued for occlusion, creating a memory effect similar to the very powerful endogenous cardioprotective mechanism of ischaemic preconditioning (Murry et al., 1986). This phenomenon was called anaesthetic preconditioning.
It was shown early on during the discovery of anaesthetic preconditioning, based on the effect of pharmacological inhibitors, that ATP-sensitive potassium (KATP) channels, PKC and adenosine receptors were involved in protection, again similarly to ischaemic preconditioning (Cope et al., 1997; Kersten et al., 1997). Volatile anaesthetics can also protect the heart when applied 24 to 72 h before a prolonged ischaemic event (‘delayed’ preconditioning or ‘second window’ of preconditioning) (Tonkovic-Capin et al., 2002; Pagel and Hudetz, 2011). Potentially clinically most relevant for non-surgery cardiac infarct patients, volatile anaesthetics decreased infarct size when administered immediately after the ischaemic event at the onset of reperfusion (Chiari et al., 2005; Pagel, 2008). This phenomenon is named anaesthetic post-conditioning. Extensive research in various laboratories throughout the world has advanced our understanding of the mechanisms of cardioprotection by volatile anaesthetics. Many pathways are shared with ischaemic preconditioning and preconditioning by other pharmacological agents such as adenosine or opioids. Here, we will provide a summary of the mechanism involved in anaesthetic cardioprotection, review its translation into the clinical setting and discuss the limitations of anaesthetic-induced protection in the clinical practice.
Protocols and mechanisms of anaesthetic-induced protection
The underlying mechanisms of anaesthetic-induced protection have been investigated on the level of isolated proteins, cellular organelles, cardiomyocytes, isolated hearts and whole animals. Proteins, organelles and cardiomyocytes have been isolated from various animal models and also from humans. In vivo models are utilized to establish the best choice, dosage, timing and frequency of anaesthetic administration for optimal protection against ischaemia and reperfusion injury. When tissue samples are obtained, punctual information about mechanisms, such as alterations of proteins or protein levels at a specific time point can be gathered. Studying the isolated heart or cardiomyocytes additionally allows following online parameters such as ion concentration, redox state or mitochondrial metabolism.
Choice and timing of anaesthetic agent
While volatile anaesthetics are most frequently studied, other anaesthetics have also been implicated in the protection against cardiac injury. As a general anaesthetic agent, propofol is used widely for induction and maintenance of anaesthesia because of its rapid onset and short recovery period. Due to its phenol-based structure, propofol has free radical scavenging properties that might be beneficial against cardiac ischaemia and reperfusion injury. In addition, propofol acts as a Ca2+ channel antagonist by inhibition of cardiac L-type calcium channels which may prevent harmful cellular Ca2+ overload. Indeed, propofol improved functional and metabolic recovery in the ischaemic and reperfused isolated rat hearts when applied either before ischaemia and during reperfusion or only during reperfusion (Kokita et al., 1998). However, in an isolated rat heart model of regional ischaemia, propofol applied 5 min before reperfusion for 20 min provided no protective effect against myocardial reperfusion injury (Ebel et al., 1999). Similarly, propofol did not reduce the infarct size after 30 min coronary artery occlusion and 3 h reperfusion on the in vivo rabbit heart (Cope et al., 1997). Similarly, contradicting results were also found when propofol was administered in humans (Conzen et al., 2003; Xia et al., 2006; Ballester et al., 2011). While the beneficial effect of propofol particularly at the time of reperfusion is not firmly established and seems to depend on experimental model of injury and other variables, it seems clear that propofol has no preconditioning properties. When administered before ischaemia and followed by a memory period, the myocardial infarct size in rabbits was not reduced by propofol compared with its solvent intralipid alone. Moreover, propofol abolished the protection that was provided by the volatile anaesthetic desflurane (Smul et al., 2011). This might be explained by the radical scavenging properties as the presence of reactive oxygen species (ROS) is a necessary part of the preconditioning signalling cascade (see ‘Mechanisms of anaesthetic-induced protection’).
Less is known about the cardioprotective properties of other non-opioid i.v. anaesthetics. The short-acting barbiturate amobarbital reduces cardiac injury and improves mitochondrial function when present during ischaemia (Chen et al., 2006; Aldakkak et al., 2008). When administered during early reperfusion, amobarbital also decreased infarct size compared with untreated hearts (Stewart et al., 2009). The dissociative anaesthetic ketamine, on the other hand, has not been associated with cardioprotection but rather blocks ischaemic preconditioning in rabbit hearts in vivo (Mullenheim et al., 2001).
Opioids are widely used as analgesics alone and along with other agents such as i.v. non-opioid or inhalational anaesthetics. Morphine, through opioid receptor stimulation, causes a reduction in infarct size in rats similar to that produced by ischaemic preconditioning when applied intermittently before myocardial ischaemia (Schultz et al., 1996). These findings were confirmed and extended to other opioids, animal models, isolated heart and cardiomyocytes (Miki et al., 1998; Liang and Gross, 1999; Zhang et al., 2004). When applied 24 or 48 h before ischaemia, opioids also induced delayed cardioprotection in rats subjected to 30 min ischaemia and 2 h reperfusion (Fryer et al., 1999). Accumulating data demonstrate that opioids confer cardioprotection when applied at reperfusion as well (Weihrauch et al., 2014; Jang et al., 2008; Kim et al., 2010). This was extended to humans where morphine-induced post-conditioning reduced ischaemia and reperfusion injury in patients undergoing corrections of Tetralogy of Fallot (Zhang et al., 2013b).
Inhalational anaesthetics that have well-described cardioprotective properties include volatile anaesthetics and noble gases. In healthy, non-aged animal models, they protect against cardiac reperfusion injury as preconditioning, delayed preconditioning and post-conditioning agents. A notable exception is nitrous oxide (60%) that when administered for three 5 min periods before ischaemia and reperfusion in rats did not have an effect on infarct size (Weber et al., 2013b). In head-to-head comparisons of volatile anaesthetics, some differences in protective efficacy were found but those were minor and not consistent in different animals and protocols (Piriou et al., 2002; Redel et al., 2009). Overall, no ‘favourite’ volatile anaesthetic for cardioprotection has been identified. Noble gases with (Xe) (Weber et al., 2013c) and without (He, Ne, Ar) (Pagel et al., 2007) anaesthetic properties similarly reduce injury from ischaemic injury, suggesting that neither anaesthetic properties nor effects on haemodynamics are crucial for protective efficacy.
Dose-dependence has been described both for anaesthetic preconditioning (Riess et al., 2002) and post-conditioning (Ge et al., 2010). In general, clinically relevant anaesthetic concentrations were found to be protective. The duration of anaesthetic exposure in pre- and post-conditioning protocols varies between 5 and 30 min but no direct comparison between different exposure lengths has been reported so far. It is possible that anaesthetic preconditioning, similar to ischaemic preconditioning (Sandhu et al., 1997), can be enhanced by administering the volatile anaesthetic repeatedly interspersed with its washout. Data from the isolated guinea pig heart model suggest that this may be the case (Riess et al., 2004). A second in vivo administration of desflurane in rats accounted for more protein alterations in the myocardial proteome compared with only one (Dyballa-Rukes et al., 2014), and sevoflurane preconditioning is strengthened by multiple preconditioning cycles (Fradorf et al., 2010). Importantly, two periods of sevoflurane preconditioning were required to significantly reduce cellular damage in patients having coronary artery bypass, using troponin I as marker or cardiac damage (Frassdorf et al., 2009).
Mechanisms of anaesthetic-induced protection
Overall, the mechanisms responsible for anaesthetic-induced conditioning of the heart closely resemble those implicated in ischaemic conditioning. However, gene expression profiles in cardiac preconditioning demonstrated partly distinct genetic programmes in ischaemic and anaesthetic preconditioning (Sergeev et al., 2004). For example, there appears to be a divergent role for MAPKs in the signal transduction underlying ischaemic and anaesthetic preconditioning. MAPKs may act as triggers and mediators in ischaemic preconditioning, while they do not trigger, but may have mediator effects in anaesthetic preconditioning (da Silva et al., 2004). There are several excellent older and more recent articles that review various aspects related to the mechanisms of anaesthetic-induced cardioprotection, particularly in respect of the relevant signalling pathways (Zaugg et al., 2003; De Hert et al., 2005; Pagel, 2008; Weber and Schlack, 2013a; Pagel and Hudetz, 2011; Van Allen et al., 2012; Alvarez et al., 2014). Up to now, how and where small pharmacologic agents such as the practically inert volatile anaesthetics or noble gases exert their direct action that lead to protection against ischaemia and reperfusion injury remains largely unresolved. It is unclear whether the agents interact with proteins directly or, through their lipophilic nature, indirectly through membranes. For example, the effect of isoflurane on sarcolemmal KATP channels could be well mediated through direct interaction with the sulfonylurea receptor, the regulatory subunit of this channel. Single amino acid mutations in the nucleotide binding domain of the sulfonylurea receptor blunted the activation of KATP channels that was observed in a patch-clamp study on excised patches at a mildly acidic pH, comparable to that achieved during ischaemia (Bienengraeber et al., 2006). While an indirect effect involving changes in KATP channel regulating nucleotides cannot be entirely ruled out, these results point towards a direct effect of isoflurane on the channel.
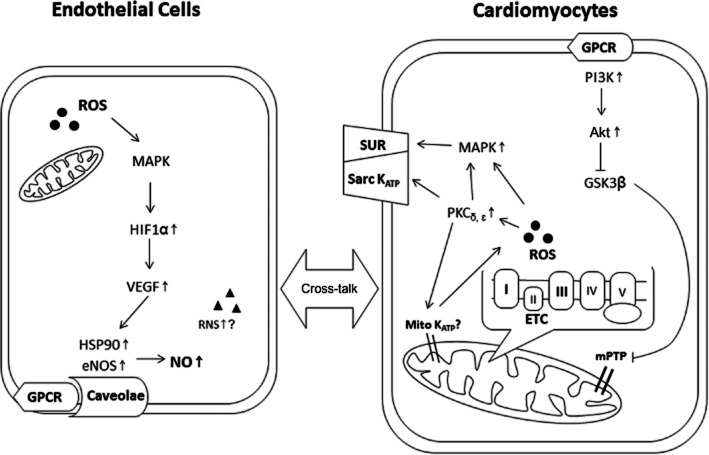
GPCRs, intracellular signalling kinases, ROS and nitrogen species, caveolae, sarcolemmal and mitochondrial potassium channels and mitochondrial metabolism are some of the main players in the mechanism of anaesthetic-induced protection (Figure 1). Originally, pharmacological inhibition was the main method for determining the molecular components required for protection. However, the non-specific action of some inhibitors may have led to a misplaced emphasis on, for example, the role of mitochondrial KATP channels in anaesthetic-induced protection (Hanley and Daut, 2005). In a more general approach, the impact of anaesthetic preconditioning on the myocardial proteome has been studied. Particularly, proteins associated with stress and mitochondrial metabolism were found to be up- or down-regulated after early and delayed anaesthetic preconditioning (Xiao et al., 2011; Bienengraeber et al., 2013; Dyballa-Rukes et al., 2014). In future studies, proteomics will serve as a useful tool to study post-translational modifications associated with anaesthetic-induced protection. Phosphorylation of signalling proteins, such as PKB/Akt (Chiari et al., 2005), glycogen synthase kinase 3β (GSK3β) (Feng et al., 2005) or endothelial nitric oxide synthase (eNOS) (Lamberts et al., 2009) but also of the adenine nucleotide carrier in mitochondria (Feng et al., 2008), have already been discovered. Most certainly, there are many more post-translational modifications, such as nitrosation of cysteine residues, that are waiting to be identified.
Figure 1.
Scheme depicting key elements of the pathways activated in anaesthetic-induced protection as described in the text. ETC, electron transport chain; HIF1α, hypoxia-inducible factor 1α; HSP90, heat shock protein 90; Mito KATP, mitochondrial ATP-sensitive potassium channels; mPTP, mitochondrial permeability transition pore; RNS, reactive nitrogen species; Sarc KATP, sarcolemmal ATP-sensitive potassium channels; SUR, sulfonylurea receptor.
The mechanistic endpoints of the different protocols of anaesthetic-induced protection (early and delayed preconditioning, post-conditioning) are similar: decreased cell death from necrosis and apoptosis along with prevention or delay of permeability transition pore opening (Piriou et al., 2004; Pravdic et al., 2009) and preserved mitochondrial function (Riess et al., 2002; Mio et al., 2009). There is ample evidence that mitochondria are not only endpoints but also act as triggers of protection and are direct targets of the anaesthetic agents. During preconditioning, low levels of ROS are generated, probably through direct or indirect interaction of the anaesthetic agent with complex I and/or III of the electron transport chain (Sedlic et al., 2010; Hirata et al., 2011; Agarwal et al., 2014). A moderate activation of ROS is required for activation of protective intracellular signalling pathways (Ludwig et al., 2004). The burst of ROS generated at the onset of reperfusion contributes to myocardial injury. Nevertheless, anaesthetic post-conditioning also depends on the presence of ROS as protection is abolished in the presence of radical scavengers (Tsutsumi et al., 2007; Yao et al., 2010). Whether there is a specific source and species of ROS that is required during anaesthetic post-conditioning is so far not known. Anaesthetics may have other direct effect on mitochondria during post-conditioning that contribute to protection, such as decreasing toxic ROS generation by reversible inhibition of complex I and decreasing mitochondrial matrix pH that could directly inhibit opening of the permeability transition pore (Hirata et al., 2011; Pravdic et al., 2012).
Aside from mitochondria, GPCRs (specifically, adenosine and opioid receptors) (Weihrauch et al., 2014; Bonney et al., 2014) participate in anaesthetic-induced protection and activate multiple protective signalling pathways. The participation of specific PK isoforms, their role (trigger or effector) and temporal relationship are dependent on species, experimental protocol and detection method (e.g. pharmacological inhibitors vs. Western blotting). Activation and/or translocation of PKs that were firmly associated with anaesthetic-induced protection include PKs Cδ and Cε, members of the MAPK family (Erk1/2 and p38) and the PI3K–Akt–GSK3β cascade (Uecker et al., 2003; Toma et al., 2004; Feng et al., 2005). Downstream of PK activation, there is up-regulation of hypoxia-inducible factor 1α, vascular endothelial growth factor, heat shock protein 90 and eNOS whereby a cross-talk between endothelial cells and cardiomyocytes enhances the protection (Wang et al., 2006; Amour et al., 2009; Leucker et al., 2011). Targeted intracellular signalling, for example towards mitochondria, is supported by spatially organized complexes of signalling molecules in lipid-rich microdomains of the plasma membrane known as caveolae. The number of caveolae and the interaction of signalling proteins with the scaffolding domain of caveolin are enhanced by volatile anaesthetics (Roth and Patel, 2011).
Translation to human tissues
Although most studies on mechanisms related to anaesthetic-induced protection of the heart were performed in animals, it seems likely that the same or similar pathways are involved in protection of the human heart as well. Trabeculae, cardiomyocytes and mitochondria were isolated from human right atrial appendages to confirm the presence and underlying mechanisms of anaesthetic-induced protection (Mio et al., 2008; Lemoine et al., 2011). Those studies confirmed the presence of anaesthetic-induced protection in human tissue. However, obvious obstacles are associated with the investigation of patient tissue, particularly when studying the mechanisms that are involved in protection and also those that may blunt protection. Among those obstacles are technical ones such as unpredictability of tissue availability and variable time lapse from tissue harvest to laboratory experiment and patient-related ones such as variations in age, underlying disease and medication history.
Thus, in the search for alternative source of human tissue, the use of cardiomyocytes that were derived from human embryonic stem cells and induced pluripotent stem cells (iPSCs) presents a novel approach. These cardiomyocytes can be generated without limit and carry the patient's genetic information. This is of particular interest in the case of iPSCs as these cells offer a model to dissect the roles of environmental factors and individual genetic background on the efficacy of anaesthetic-induced protection. Indeed, anaesthetic preconditioning protects human embryonic stem cell-derived cardiomyocytes against oxidative stress, triggers ROS generation and delays permeability transition pore opening, suggesting the feasibility to use these cells as a model for studying anaesthetic preconditioning (Sepac et al., 2010). When using cardiomyocytes derived from iPSCs from healthy and type 2 diabetic patients, elevated glucose levels disrupted anaesthetic-mediated protection present at normal glucose level for both cell lines (Canfield et al., 2012). Eventually, cardiomyocytes derived from healthy donors and patients with a specific disease open possibilities in studying genotype- and phenotype-related pathologies in a human-relevant model. However, challenges remain in regard to reproducibility and efficiency, as well as variability in maturity and cardiac subpopulations when generating cardiomyocytes either from the same or from different iPSC lines. For progressive complex multifactorial diseases such as diabetes, the disease phenotype may not be present in cardiomyocytes differentiated from iPSCs from a type 1 or type 2 diabetic donor. A large number of cell lines from both disease and healthy donors will be required to account for line-to-line and intra-line variability.
Apart from mechanistic studies, one of the main goals is to use stem cells and/or stem cell-derived cardiomyocytes for therapy, specifically for the infarcted heart, by injecting cells in or around the infarcted area. One great challenge for stem cell therapy of an infarcted heart is the limited survival of injected or endogenously activated cells in and around the ischaemic zone of the infarct. While sevoflurane inhalation did not increase the number of circulating endothelial progenitor cells in volunteers, in vitro sevoflurane preconditioning promoted proliferation of human endothelial progenitors (Lucchinetti et al., 2009). In this way, anaesthetic exposure may be used to promote perioperative vascular healing and to support cell replacement therapies. Being more resistant against ischaemic stress, stem cell-derived cardiomyocytes may exhibit better survival when injected into the infarcted zone.
Clinical studies
Anaesthetics, and in particular, volatile anaesthetics appear to evoke powerful protection in various injury models. This includes human tissue as well as cardiomyocytes derived from human tissue or stem cells. Obviously, of most relevance is the question whether those findings from the laboratory translate into significant improvement in the clinical outcome in patients that are either at risk for myocardial ischaemia or actually suffer from it. The American Heart Association stated in its guideline for coronary artery bypass graft surgery that volatile-based anaesthesia can be useful in reducing the risk of perioperative myocardial ischaemia and infarction (Hillis et al., 2012). Recent excellent reviews summarized clinical studies related to anaesthetic-induced protection during surgery (Pagel, 2013; Ishii, 2014); so we will only provide a short summary and report on the most recent studies. Overall, the outcome of clinical studies is highly variable and sometimes contradictory. Frequently, improvements in secondary endpoints such as cardiac troponin I release were detected but not in primary clinical outcome (such as mortality, heart function, myocardial infarction). Even meta-analyses where the outcome of cardiac surgery with volatile versus i.v. anaesthesia was compared and that included largely the same clinical studies sometimes lead to different conclusions. In two of the earlier meta-analyses analysing coronary artery bypass graft surgery, 2979 patients in 27 clinical trials (Symons and Myles, 2006) and 2841 patients in 32 clinical trials (Yu and Beattie, 2006) were examined. In both analyses, volatile anaesthetics reduced postoperative troponin I release when compared with i.v. anaesthetics. However, while sevoflurane-mediated reduction in cardiac troponin was associated with improved long-term outcomes in individual studies, these meta-analyses were not able to confirm that positive effects on troponin were translated into improved clinical outcomes. In contrast, in another meta-analysis, a year later, that included 22 studies with a total of 1922 patients undergoing cardiac surgery, a significant reduction of myocardial infarctions and mortality was found (Landoni et al., 2007).
More recently, the cardioprotective properties of isoflurane versus any non-volatile anaesthetics were investigated in both cardiac (16 studies) and non-cardiac (21 studies) surgery with a total of 3539 patients in terms of the rate of myocardial infarction and overall mortality. While mortality was reduced in the isoflurane group when only studies with a low risk of bias were included in the analyses, overall no differences in the rates of mortality and myocardial infarction were noted (Bignami et al., 2013). The same research group reported later that specifically desflurane and sevoflurane reduce mortality after cardiac surgery when compared with i.v. anaesthesia (Landoni et al., 2013). Similarly mixed are the results of the more recent individual clinical studies. In a prospective, randomized study with 193 patients, sevoflurane-based anaesthesia did not even reduce troponin T release after elective abdominal aortic surgery, compared with total i.v. anaesthesia (Lindholm et al., 2013). On the other hand, a small proof-of-concept study suggested that intramyocardial or systematic delivery of sevoflurane attenuated the systemic inflammatory response after cardiopulmonary bypass but without reducing postoperative markers of myocardial cell damage such as troponin T (Kortekaas et al., 2014). Some clinical studies result in a follow-up discussion in regard of data evaluation. In a randomized three-centre study, 385 patients with cardiovascular risk that underwent non-cardiac surgery received either sevoflurane or propofol for anaesthesia. Sevoflurane did not reduce the incidence of myocardial ischaemia in high-risk patients undergoing major non-cardiac surgery (Lurati Buse et al., 2012). However, the study was considered underpowered by others and the endpoints as well as the timing of endpoint measurements were criticized (Zaugg and Lucchinetti, 2013; Zhang et al., 2013a).
In the majority of clinical studies that investigate potential protective effects of anaesthetics against cardiac injury, the agent was present throughout injury. Fewer studies with low participant numbers investigated pre- and post-conditioning effects of anaesthetics on the outcome of surgery. In one direct comparison between only propofol anaesthesia, continuous sevoflurane exposure and sevoflurane pre- and post-conditioning (50 patients each), troponin I exposure was decreased in all sevoflurane groups, but hospital length of stay was only decreased when sevoflurane was administered throughout the operation (De Hert et al., 2004). Children seem susceptible to anaesthetic preconditioning: 80 children that underwent ventricular septal defect closure were assigned to preconditioning for 5 min with either isoflurane, sevoflurane, desflurane or placebo (oxygen-air mixture). Volatile anaesthetics were associated with significantly decreased postoperative release of creatine kinase (MB isoform) and with a trend towards shorter duration of inotropic support, mechanical ventilation and length of intensive care unit stay (Singh et al., 2013). Thus, compared with the cardioprotection by volatile anaesthetics unequivocally found in laboratory settings, the findings are more variable in the clinical setting. More studies will be required to find a more definitive answer whether and in what setting certain anaesthetics are more useful than others in providing protection against myocardial injury.
Underlying conditions
The reasons why reproducible cardioprotection for various anaesthetics observed in the laboratory setting does not translate into robust and reproducible clinical benefits are likely to be multifaceted. By and large, patients that are undergoing cardiac or non-cardiac surgery are elderly and/or suffer from underlying conditions such as obesity and diabetes. Indeed, in a study with 784 patients scheduled for arterial vascular surgery and a history of at least three of risk factors such as age, diabetes, prior myocardial infarction and hypertension, inhalational anaesthetics failed to reduce the incidence of postoperative cardiac events and troponin I release when compared with non-inhalational anaesthetics (De Hert et al., 2008). In the laboratory, anaesthetic-induced preconditioning with isoflurane decreases stress-induced cell death and preserves mitochondrial respiratory function to a greater degree in cardiomyocytes isolated from right atrial appendages from middle-aged patients than in those isolated from elderly patients (Mio et al., 2008). In aged rats, the lack of preconditioning was associated with disruption of well-described protective pathways such as volatile anaesthetic-induced ROS generation (Nguyen et al., 2008), activation and translocation of GSK3β and delay of permeability transition pore opening (Zhu et al., 2013).
Similarly, hyperglycaemia and diabetes in various animal and cell models attenuated or abolished anaesthetic-induced protection against myocardial injury. Isoflurane-induced pre- and post-conditioning was abolished in animal models of type 1 and 2 diabetes as well as hyperglycaemia (Tanaka et al., 2002; Huhn et al., 2008; Matsumoto et al., 2009; Muravyeva et al., 2014). While signalling kinase pathways seem to be at least partially intact after sevoflurane-induced preconditioning during hyperglycaemia (Weber and Schlack, 2013a; Weber et al., 2013d), coupling of eNOS through its interaction with heat shock protein 90 was disrupted after anaesthetic exposure (Amour et al., 2010). It is likely that diabetes-induced alterations in metabolism such as a switch in substrate preference affect anaesthetic-induced protection (van den Brom et al., 2013). However, desflurane was able to post-condition the right atrial trabeculae isolated from diabetic human myocardium (Lemoine et al., 2010). Factors such as glucose level and length of hyperglycaemia, degree of insulin insensitivity, obesity and hyperlipidaemia are all likely to affect the cardioprotective capacity of anaesthetics.
In addition to underlying disease, medications may influence the cardioprotective action of anaesthetics. As a case in point, patients with coronary artery disease that undergo surgery are frequently treated with β-adrenoceptor antagonists (β-blockers) for perioperative cardioprotection. In an in vivo rabbit model, the protection afforded by desflurane and sevoflurane was blunted upon inhibition of the β1-adrenoceptor pathway (Lange et al., 2006). Similarly, the glucose-lowering drug and KATP channel inhibitor glibenclamide, blocks anaesthetic-induced protection (Toller et al., 2000). Interestingly, a perioperative shift from glibenclamide to insulin restores reduction in troponin I release in diabetic patients undergoing coronary artery bypass surgery in the presence of isoflurane (Forlani et al., 2004). It will be interesting to further assess the ability of interventions with other glucose-lowering drugs such as metformin to restore or improve protection in patients. Metformin alone provides cardioprotection against ischaemic injury in hearts from diabetic db/db mice (Calvert et al., 2008), but the combination of volatile anaesthetics and metformin has not been systematically studied yet.
Outlook
Similar to ischaemic pre- and post-conditioning, certain anaesthetics provide powerful protection in defined models of ischaemia and reperfusion injury. As it avoids any mechanical intervention, the use of volatile anaesthetics is considered safer for clinical application as compared with the short ischaemic episodes during ischaemic conditioning. Nevertheless, anaesthetic-induced pre- and post-conditioning is currently met with scepticism at funding agencies, pointing out that the research had made little progress in regard of clinical implementation of over the last years. In order to see robust clinical benefits, conditions that hamper preconditioning, such as underlying diseases, and how to overcome them require further laboratory investigations and clinical studies. The rather high severity of ischaemia and reperfusion injury in experimental models may not compare well with the area of risk during coronary artery bypass graft surgery that is frequently examined in clinical studies of anaesthetic-induced protection (Pagel, 2013). In this regard, one potentially interesting application of anaesthetic-induced post-conditioning might be during cardiopulmonary resuscitation. Out-of-hospital cardiac arrests have a very low, regionally variable survival rate (Nichol et al., 2008; Hasegawa et al., 2013). In a recent study on rats, cardiopulmonary resuscitation was performed after electrically induced ventricular fibrillation. Rats received sevoflurane for 5 min either before resuscitation, during resuscitation or after resuscitation. While sevoflurane treatment did not affect the survival rate, all treatment protocols resulted in an improved contractility 24 h after restoration of spontaneous circulation, together with a higher ejection fraction (Knapp et al., 2013). In a pig model of cardiopulmonary resuscitation, sevoflurane post-conditioning reduced myocardial damage and dysfunction after cardiopulmonary resuscitation in the early post-resuscitation period, along with reduced apoptosis and myocardial proinflammatory cytokine expression (Meybohm et al., 2011). Sevoflurane did not improve the neurological recovery which might be explained by the rather late start of sevoflurane administration after recovery of spontaneous circulation. Ischaemic post-conditioning (two 20 s breaks during cardiopulmonary resuscitation) after 15 min of untreated cardiac arrest in pigs was neuroprotective and improved 48 h survival dramatically (Yannopoulos et al., 2013). Potential applications like this suggest that further research in anaesthetic-induced protection is warranted and clinically relevant. Understanding and overcoming obstacles related to concomitant conditions of ischaemia and reperfusion injury and a well-targeted patient population seem to be the key for successfully implementing anaesthetic-induced protection further into the clinical practice.
Acknowledgments
This work was supported in part by the National Institutes of Health (R01 HL098490).
Glossary
- eNOS
endothelial nitric oxide synthase
- GSK3β
glycogen synthase kinase 3β
- iPSCs
induced pluripotent stem cells
- KATP channel
ATP-sensitive potassium channel
- ROS
reactive oxygen species
Conflict of interest
None.
References
- Agarwal B, Dash RK, Stowe DF, Bosnjak ZJ, Camara AK. Isoflurane modulates cardiac mitochondrial bioenergetics by selectively attenuating respiratory complexes. Biochim Biophys Acta. 2014;1837:354–365. doi: 10.1016/j.bbabio.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldakkak M, Stowe DF, Chen Q, Lesnefsky EJ, Camara AK. Inhibited mitochondrial respiration by amobarbital during cardiac ischaemia improves redox state and reduces matrix Ca2+ overload and ROS release. Cardiovasc Res. 2008;77:406–415. doi: 10.1016/j.cardiores.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL. Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol. 2013a;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Catterall WA, et al. The Concise Guide to PHARMACOLOGY 2013/14: Ion Channels. Br J Pharmacol. 2013b;170:1607–1651. doi: 10.1111/bph.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL. Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G Protein-Coupled Receptors. Br J Pharmacol. 2013c;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL. Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Transporters. Br J Pharmacol. 2013d;170:1706–1796. doi: 10.1111/bph.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez P, Tapia L, Mardones LA, Pedemonte JC, Farias JG, Castillo RL. Cellular mechanisms against ischemia reperfusion injury induced by the use of anesthetic pharmacological agents. Chem Biol Interact. 2014;218:89–98. doi: 10.1016/j.cbi.2014.04.019. [DOI] [PubMed] [Google Scholar]
- Amour J, Brzezinska AK, Weihrauch D, Billstrom AR, Zielonka J, Krolikowski JG, et al. Role of heat shock protein 90 and endothelial nitric oxide synthase during early anesthetic and ischemic preconditioning. Anesthesiology. 2009;110:317–325. doi: 10.1097/ALN.0b013e3181942cb4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amour J, Brzezinska AK, Jager Z, Sullivan C, Weihrauch D, Du J, et al. Hyperglycemia adversely modulates endothelial nitric oxide synthase during anesthetic preconditioning through tetrahydrobiopterin- and heat shock protein 90-mediated mechanisms. Anesthesiology. 2010;112:576–585. doi: 10.1097/ALN.0b013e3181cded1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballester M, Llorens J, Garcia-De-La-Asuncion J, Perez-Griera J, Tebar E, Martinez-Leon J, et al. Myocardial oxidative stress protection by sevoflurane vs. propofol: a randomised controlled study in patients undergoing off-pump coronary artery bypass graft surgery. Eur J Anaesthesiol. 2011;28:874–881. doi: 10.1097/EJA.0b013e32834bea2a. [DOI] [PubMed] [Google Scholar]
- Bienengraeber M, Warltier DC, Bosnjak ZJ, Stadnicka A. Mechanism of cardiac sarcolemmal adenosine triphosphate-sensitive potassium channel activation by isoflurane in a heterologous expression system. Anesthesiology. 2006;105:534–540. doi: 10.1097/00000542-200609000-00017. [DOI] [PubMed] [Google Scholar]
- Bienengraeber M, Pellitteri-Hahn M, Hirata N, Baye TM, Bosnjak ZJ, Olivier M. Quantitative characterization of changes in the cardiac mitochondrial proteome during anesthetic preconditioning and ischemia. Physiol Genomics. 2013;45:163–170. doi: 10.1152/physiolgenomics.00117.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bignami E, Greco T, Barile L, Silvetti S, Nicolotti D, Fochi O, et al. The effect of isoflurane on survival and myocardial infarction: a meta-analysis of randomized controlled studies. J Cardiothorac Vasc Anesth. 2013;27:50–58. doi: 10.1053/j.jvca.2012.06.007. [DOI] [PubMed] [Google Scholar]
- Bland JH, Lowenstein E. Halothane-induced decrease in experimental myocardial ischemia in the non-failing canine heart. Anesthesiology. 1976;45:287–293. doi: 10.1097/00000542-197609000-00006. [DOI] [PubMed] [Google Scholar]
- Bonney S, Hughes K, Eckle T. Anesthetic cardioprotection: the role of adenosine. Curr Pharm Des. 2014;20:5690–5695. doi: 10.2174/1381612820666140204102524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Brom CE, Bulte CS, Loer SA, Bouwman RA, Boer C. Diabetes, perioperative ischaemia and volatile anaesthetics: consequences of derangements in myocardial substrate metabolism. Cardiovasc Diabetol. 2013;12:42–54. doi: 10.1186/1475-2840-12-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert JW, Gundewar S, Jha S, Greer JJ, Bestermann WH, Tian R, et al. Acute metformin therapy confers cardioprotection against myocardial infarction via AMPK-eNOS-mediated signaling. Diabetes. 2008;57:696–705. doi: 10.2337/db07-1098. [DOI] [PubMed] [Google Scholar]
- Canfield SG, Sepac A, Sedlic F, Muravyeva MY, Bai X, Bosnjak ZJ. Marked hyperglycemia attenuates anesthetic preconditioning in human-induced pluripotent stem cell-derived cardiomyocytes. Anesthesiology. 2012;117:735–744. doi: 10.1097/ALN.0b013e3182655e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cason BA, Gamperl AK, Slocum RE, Hickey RF. Anesthetic-induced preconditioning: previous administration of isoflurane decreases myocardial infarct size in rabbits. Anesthesiology. 1997;87:1182–1190. doi: 10.1097/00000542-199711000-00023. [DOI] [PubMed] [Google Scholar]
- Chen Q, Moghaddas S, Hoppel CL, Lesnefsky EJ. Reversible blockade of electron transport during ischemia protects mitochondria and decreases myocardial injury following reperfusion. J Pharmacol Exp Ther. 2006;319:1405–1412. doi: 10.1124/jpet.106.110262. [DOI] [PubMed] [Google Scholar]
- Chiari PC, Bienengraeber MW, Pagel PS, Krolikowski JG, Kersten JR, Warltier DC. Isoflurane protects against myocardial infarction during early reperfusion by activation of phosphatidylinositol-3-kinase signal transduction: evidence for anesthetic-induced postconditioning in rabbits. Anesthesiology. 2005;102:102–109. doi: 10.1097/00000542-200501000-00018. [DOI] [PubMed] [Google Scholar]
- Conzen PF, Fischer S, Detter C, Peter K. Sevoflurane provides greater protection of the myocardium than propofol in patients undergoing off-pump coronary artery bypass surgery. Anesthesiology. 2003;99:826–833. doi: 10.1097/00000542-200310000-00013. [DOI] [PubMed] [Google Scholar]
- Cope DK, Impastato WK, Cohen MV, Downey JM. Volatile anesthetics protect the ischemic rabbit myocardium from infarction. Anesthesiology. 1997;86:699–709. doi: 10.1097/00000542-199703000-00023. [DOI] [PubMed] [Google Scholar]
- De Hert SG, Van Der Linden PJ, Cromheecke S, Meeus R, Nelis A, Van Reeth V, et al. Cardioprotective properties of sevoflurane in patients undergoing coronary surgery with cardiopulmonary bypass are related to the modalities of its administration. Anesthesiology. 2004;101:299–310. doi: 10.1097/00000542-200408000-00009. [DOI] [PubMed] [Google Scholar]
- De Hert SG, Turani F, Mathur S, Stowe DF. Cardioprotection with volatile anesthetics: mechanisms and clinical implications. Anesth Analg. 2005;100:1584–1593. doi: 10.1213/01.ANE.0000153483.61170.0C. [DOI] [PubMed] [Google Scholar]
- De Hert SG, Longrois D, Yang H, Fleisher LA. Does the use of a volatile anesthetic regimen attenuate the incidence of cardiac events after vascular surgery? Acta Anaesthesiol Belg. 2008;59:19–25. [PubMed] [Google Scholar]
- Dyballa-Rukes N, Schuh C, Vogt H, Toma O, Schlack WS, Weber NC, et al. In vivo desflurane preconditioning evokes dynamic alterations of metabolic proteins in the heart–proteomic insights strengthen the link between bioenergetics and cardioprotection. Cell Physiol Biochem. 2014;33:967–981. doi: 10.1159/000358668. [DOI] [PubMed] [Google Scholar]
- Ebel D, Schlack W, Comfere T, Preckel B, Thamer V. Effect of propofol on reperfusion injury after regional ischaemia in the isolated rat heart. Br J Anaesth. 1999;83:903–908. doi: 10.1093/bja/83.6.903. [DOI] [PubMed] [Google Scholar]
- Feng J, Lucchinetti E, Ahuja P, Pasch T, Perriard JC, Zaugg M. Isoflurane postconditioning prevents opening of the mitochondrial permeability transition pore through inhibition of glycogen synthase kinase 3beta. Anesthesiology. 2005;103:987–995. doi: 10.1097/00000542-200511000-00013. [DOI] [PubMed] [Google Scholar]
- Feng J, Zhu M, Schaub MC, Gehrig P, Roschitzki B, Lucchinetti E, et al. Phosphoproteome analysis of isoflurane-protected heart mitochondria: phosphorylation of adenine nucleotide translocator-1 on Tyr194 regulates mitochondrial function. Cardiovasc Res. 2008;80:20–29. doi: 10.1093/cvr/cvn161. [DOI] [PubMed] [Google Scholar]
- Forlani S, Tomai F, De Paulis R, Turani F, Colella DF, Nardi P, et al. Preoperative shift from glibenclamide to insulin is cardioprotective in diabetic patients undergoing coronary artery bypass surgery. J Cardiovasc Surg (Torino) 2004;45:117–122. [PubMed] [Google Scholar]
- Fradorf J, Huhn R, Weber NC, Ebel D, Wingert N, Preckel B, et al. Sevoflurane-induced preconditioning: impact of protocol and aprotinin administration on infarct size and endothelial nitric-oxide synthase phosphorylation in the rat heart in vivo. Anesthesiology. 2010;113:1289–1298. doi: 10.1097/ALN.0b013e3181f97fec. [DOI] [PubMed] [Google Scholar]
- Frassdorf J, Borowski A, Ebel D, Feindt P, Hermes M, Meemann T, et al. Impact of preconditioning protocol on anesthetic-induced cardioprotection in patients having coronary artery bypass surgery. J Thorac Cardiovasc Surg. 2009;137:1436–1442. doi: 10.1016/j.jtcvs.2008.04.034. , 1442 e1–2. [DOI] [PubMed] [Google Scholar]
- Fryer RM, Hsu AK, Eells JT, Nagase H, Gross GJ. Opioid-induced second window of cardioprotection: potential role of mitochondrial KATP channels. Circ Res. 1999;84:846–851. doi: 10.1161/01.res.84.7.846. [DOI] [PubMed] [Google Scholar]
- Ge ZD, Pravdic D, Bienengraeber M, Pratt PF, Jr, Auchampach JA, Gross GJ, et al. Isoflurane postconditioning protects against reperfusion injury by preventing mitochondrial permeability transition by an endothelial nitric oxide synthase-dependent mechanism. Anesthesiology. 2010;112:73–85. doi: 10.1097/ALN.0b013e3181c4a607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley PJ, Daut J. K(ATP) channels and preconditioning: a re-examination of the role of mitochondrial K(ATP) channels and an overview of alternative mechanisms. J Mol Cell Cardiol. 2005;39:17–50. doi: 10.1016/j.yjmcc.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Hasegawa K, Tsugawa Y, Camargo CA, Jr, Hiraide A, Brown DF. Regional variability in survival outcomes of out-of-hospital cardiac arrest: the All-Japan Utstein Registry. Resuscitation. 2013;84:1099–1107. doi: 10.1016/j.resuscitation.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Hillis LD, Smith PK, Anderson JL, Bittl JA, Bridges CR, Byrne JG, et al. 2011 ACCF/AHA guideline for coronary artery bypass graft surgery: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Thorac Cardiovasc Surg. 2012;143:4–34. doi: 10.1016/j.jtcvs.2011.10.015. [DOI] [PubMed] [Google Scholar]
- Hirata N, Shim YH, Pravdic D, Lohr NL, Pratt PF, Jr, Weihrauch D, et al. Isoflurane differentially modulates mitochondrial reactive oxygen species production via forward versus reverse electron transport flow: implications for preconditioning. Anesthesiology. 2011;115:531–540. doi: 10.1097/ALN.0b013e31822a2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhn R, Heinen A, Weber NC, Hollmann MW, Schlack W, Preckel B. Hyperglycaemia blocks sevoflurane-induced postconditioning in the rat heart in vivo: cardioprotection can be restored by blocking the mitochondrial permeability transition pore. Br J Anaesth. 2008;100:465–471. doi: 10.1093/bja/aen022. [DOI] [PubMed] [Google Scholar]
- Ishii H. Cardioprotection with opioids- trusted old friends -clinical science. Curr Pharm Des. 2014;20:5794–5798. doi: 10.2174/1381612820666140204112011. [DOI] [PubMed] [Google Scholar]
- Jang Y, Xi J, Wang H, Mueller RA, Norfleet EA, Xu Z. Postconditioning prevents reperfusion injury by activating delta-opioid receptors. Anesthesiology. 2008;108:243–250. doi: 10.1097/01.anes.0000299437.93898.4a. [DOI] [PubMed] [Google Scholar]
- Kanaya N, Kobayashi I, Nakayama M, Fujita S, Namiki A. ATP sparing effect of isoflurane during ischaemia and reperfusion of the canine heart. Br J Anaesth. 1995;74:563–568. doi: 10.1093/bja/74.5.563. [DOI] [PubMed] [Google Scholar]
- Kersten JR, Schmeling TJ, Pagel PS, Gross GJ, Warltier DC. Isoflurane mimics ischemic preconditioning via activation of K(ATP) channels: reduction of myocardial infarct size with an acute memory phase. Anesthesiology. 1997;87:361–370. doi: 10.1097/00000542-199708000-00024. [DOI] [PubMed] [Google Scholar]
- Kim HS, Cho JE, Hong SW, Kim SO, Shim JK, Kwak YL. Remifentanil protects myocardium through activation of anti-apoptotic pathways of survival in ischemia-reperfused rat heart. Physiol Res. 2010;59:347–356. doi: 10.33549/physiolres.931772. [DOI] [PubMed] [Google Scholar]
- Knapp J, Bergmann G, Bruckner T, Russ N, Bottiger BW, Popp E. Pre- and postconditioning effect of Sevoflurane on myocardial dysfunction after cardiopulmonary resuscitation in rats. Resuscitation. 2013;84:1450–1455. doi: 10.1016/j.resuscitation.2013.04.012. [DOI] [PubMed] [Google Scholar]
- Kokita N, Hara A, Abiko Y, Arakawa J, Hashizume H, Namiki A. Propofol improves functional and metabolic recovery in ischemic reperfused isolated rat hearts. Anesth Analg. 1998;86:252–258. doi: 10.1097/00000539-199802000-00006. [DOI] [PubMed] [Google Scholar]
- Kortekaas KA, van der Baan A, Aarts LP, Palmen M, Cobbaert CM, Verhagen JC, et al. Cardiospecific sevoflurane treatment quenches inflammation but does not attenuate myocardial cell damage markers: a proof-of-concept study in patients undergoing mitral valve repair. Br J Anaesth. 2014;112:1005–1014. doi: 10.1093/bja/aet588. [DOI] [PubMed] [Google Scholar]
- Lamberts RR, Onderwater G, Hamdani N, Vreden MJ, Steenhuisen J, Eringa EC, et al. Reactive oxygen species-induced stimulation of 5'AMP-activated protein kinase mediates sevoflurane-induced cardioprotection. Circulation. 2009;120:S10–S15. doi: 10.1161/CIRCULATIONAHA.108.828426. [DOI] [PubMed] [Google Scholar]
- Landoni G, Biondi-Zoccai GG, Zangrillo A, Bignami E, D'avolio S, Marchetti C, et al. Desflurane and sevoflurane in cardiac surgery: a meta-analysis of randomized clinical trials. J Cardiothorac Vasc Anesth. 2007;21:502–511. doi: 10.1053/j.jvca.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Landoni G, Greco T, Biondi-Zoccai G, Nigro Neto C, Febres D, Pintaudi M, et al. Anaesthetic drugs and survival: a Bayesian network meta-analysis of randomized trials in cardiac surgery. Br J Anaesth. 2013;111:886–896. doi: 10.1093/bja/aet231. [DOI] [PubMed] [Google Scholar]
- Lange M, Smul TM, Blomeyer CA, Redel A, Klotz KN, Roewer N, et al. Role of the beta1-adrenergic pathway in anesthetic and ischemic preconditioning against myocardial infarction in the rabbit heart in vivo. Anesthesiology. 2006;105:503–510. doi: 10.1097/00000542-200609000-00014. [DOI] [PubMed] [Google Scholar]
- Lemoine S, Durand C, Zhu L, Ivasceau C, Lepage O, Babatasi G, et al. Desflurane-induced postconditioning of diabetic human right atrial myocardium in vitro. Diabetes Metab. 2010;36:21–28. doi: 10.1016/j.diabet.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Lemoine S, Zhu L, Buleon C, Massetti M, Gerard JL, Galera P, et al. Mechanisms involved in the desflurane-induced post-conditioning of isolated human right atria from patients with type 2 diabetes. Br J Anaesth. 2011;107:510–518. doi: 10.1093/bja/aer201. [DOI] [PubMed] [Google Scholar]
- Leucker TM, Bienengraeber M, Muravyeva M, Baotic I, Weihrauch D, Brzezinska AK, et al. Endothelial-cardiomyocyte crosstalk enhances pharmacological cardioprotection. J Mol Cell Cardiol. 2011;51:803–811. doi: 10.1016/j.yjmcc.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang BT, Gross GJ. Direct preconditioning of cardiac myocytes via opioid receptors and KATP channels. Circ Res. 1999;84:1396–1400. doi: 10.1161/01.res.84.12.1396. [DOI] [PubMed] [Google Scholar]
- Lindholm EE, Aune E, Noren CB, Seljeflot I, Hayes T, Otterstad JE, et al. The anesthesia in abdominal aortic surgery (ABSENT) study: a prospective, randomized, controlled trial comparing troponin T release with fentanyl-sevoflurane and propofol-remifentanil anesthesia in major vascular surgery. Anesthesiology. 2013;119:802–812. doi: 10.1097/ALN.0b013e31829bd883. [DOI] [PubMed] [Google Scholar]
- London MJ. Cardiovascular problems in noncardiac surgery. Curr Opin Crit Care. 2009;15:333–341. doi: 10.1097/MCC.0b013e32832e4795. [DOI] [PubMed] [Google Scholar]
- Lucchinetti E, Zeisberger SM, Baruscotti I, Wacker J, Feng J, Zaugg K, et al. Stem cell-like human endothelial progenitors show enhanced colony-forming capacity after brief sevoflurane exposure: preconditioning of angiogenic cells by volatile anesthetics. Anesth Analg. 2009;109:1117–1126. doi: 10.1213/ANE.0b013e3181b5a277. [DOI] [PubMed] [Google Scholar]
- Ludwig LM, Weihrauch D, Kersten JR, Pagel PS, Warltier DC. Protein kinase C translocation and Src protein tyrosine kinase activation mediate isoflurane-induced preconditioning in vivo: potential downstream targets of mitochondrial adenosine triphosphate-sensitive potassium channels and reactive oxygen species. Anesthesiology. 2004;100:532–539. doi: 10.1097/00000542-200403000-00011. [DOI] [PubMed] [Google Scholar]
- Lurati Buse GA, Schumacher P, Seeberger E, Studer W, Schuman RM, Fassl J, et al. Randomized comparison of sevoflurane versus propofol to reduce perioperative myocardial ischemia in patients undergoing noncardiac surgery. Circulation. 2012;126:2696–2704. doi: 10.1161/CIRCULATIONAHA.112.126144. [DOI] [PubMed] [Google Scholar]
- Matsumoto S, Cho S, Tosaka S, Ureshino H, Maekawa T, Hara T, et al. Pharmacological preconditioning in type 2 diabetic rat hearts: the roles of mitochondrial ATP-sensitive potassium channels and the phosphatidylinositol 3-kinase-Akt pathway. Cardiovasc Drugs Ther. 2009;23:263–270. doi: 10.1007/s10557-009-6184-5. [DOI] [PubMed] [Google Scholar]
- Meybohm P, Gruenewald M, Albrecht M, Muller C, Zitta K, Foesel N, et al. Pharmacological postconditioning with sevoflurane after cardiopulmonary resuscitation reduces myocardial dysfunction. Crit Care. 2011;15:R241. doi: 10.1186/cc10496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T, Cohen MV, Downey JM. Opioid receptor contributes to ischemic preconditioning through protein kinase C activation in rabbits. Mol Cell Biochem. 1998;186:3–12. [PubMed] [Google Scholar]
- Mio Y, Bienengraeber MW, Marinovic J, Gutterman DD, Rakic M, Bosnjak ZJ, et al. Age-related attenuation of isoflurane preconditioning in human atrial cardiomyocytes: roles for mitochondrial respiration and sarcolemmal adenosine triphosphate-sensitive potassium channel activity. Anesthesiology. 2008;108:612–620. doi: 10.1097/ALN.0b013e318167af2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mio Y, Shim YH, Richards E, Bosnjak ZJ, Pagel PS, Bienengraeber M. Xenon preconditioning: the role of prosurvival signaling, mitochondrial permeability transition and bioenergetics in rats. Anesth Analg. 2009;108:858–866. doi: 10.1213/ane.0b013e318192a520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullenheim J, Frassdorf J, Preckel B, Thamer V, Schlack W. Ketamine, but not S(+)-ketamine, blocks ischemic preconditioning in rabbit hearts in vivo. Anesthesiology. 2001;94:630–636. doi: 10.1097/00000542-200104000-00017. [DOI] [PubMed] [Google Scholar]
- Muravyeva M, Baotic I, Bienengraeber M, Lazar J, Bosnjak ZJ, Sedlic F, et al. Cardioprotection during diabetes: the role of mitochondrial DNA. Anesthesiology. 2014;120:870–879. doi: 10.1097/ALN.0000000000000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- Nguyen LT, Rebecchi MJ, Moore LC, Glass PS, Brink PR, Liu L. Attenuation of isoflurane-induced preconditioning and reactive oxygen species production in the senescent rat heart. Anesth Analg. 2008;107:776–782. doi: 10.1213/ane.0b013e318180419d. [DOI] [PubMed] [Google Scholar]
- Nichol G, Thomas E, Callaway CW, Hedges J, Powell JL, Aufderheide TP, et al. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA. 2008;300:1423–1431. doi: 10.1001/jama.300.12.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagel PS. Postconditioning by volatile anesthetics: salvaging ischemic myocardium at reperfusion by activation of prosurvival signaling. J Cardiothorac Vasc Anesth. 2008;22:753–765. doi: 10.1053/j.jvca.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Pagel PS. Myocardial protection by volatile anesthetics in patients undergoing cardiac surgery: a critical review of the laboratory and clinical evidence. J Cardiothorac Vasc Anesth. 2013;27:972–982. doi: 10.1053/j.jvca.2012.10.011. [DOI] [PubMed] [Google Scholar]
- Pagel PS, Hudetz JA. Delayed cardioprotection by inhaled anesthetics. J Cardiothorac Vasc Anesth. 2011;25:1125–1140. doi: 10.1053/j.jvca.2010.09.017. [DOI] [PubMed] [Google Scholar]
- Pagel PS, Krolikowski JG, Shim YH, Venkatapuram S, Kersten JR, Weihrauch D, et al. Noble gases without anesthetic properties protect myocardium against infarction by activating prosurvival signaling kinases and inhibiting mitochondrial permeability transition in vivo. Anesth Analg. 2007;105:562–569. doi: 10.1213/01.ane.0000278083.31991.36. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledge base of drug targets and their ligands. Nucl. Acids Res. 2014;42(Database Issue):D1098–1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piriou V, Chiari P, Lhuillier F, Bastien O, Loufoua J, Raisky O, et al. Pharmacological preconditioning: comparison of desflurane, sevoflurane, isoflurane and halothane in rabbit myocardium. Br J Anaesth. 2002;89:486–491. [PubMed] [Google Scholar]
- Piriou V, Chiari P, Gateau-Roesch O, Argaud L, Muntean D, Salles D, et al. Desflurane-induced preconditioning alters calcium-induced mitochondrial permeability transition. Anesthesiology. 2004;100:581–588. doi: 10.1097/00000542-200403000-00018. [DOI] [PubMed] [Google Scholar]
- Pravdic D, Sedlic F, Mio Y, Vladic N, Bienengraeber M, Bosnjak ZJ. Anesthetic-induced preconditioning delays opening of mitochondrial permeability transition pore via protein Kinase C-epsilon-mediated pathway. Anesthesiology. 2009;111:267–274. doi: 10.1097/ALN.0b013e3181a91957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravdic D, Hirata N, Barber L, Sedlic F, Bosnjak ZJ, Bienengraeber M. Complex I and ATP synthase mediate membrane depolarization and matrix acidification by isoflurane in mitochondria. Eur J Pharmacol. 2012;690:149–157. doi: 10.1016/j.ejphar.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redel A, Stumpner J, Tischer-Zeitz T, Lange M, Smul TM, Lotz C, et al. Comparison of isoflurane-, sevoflurane-, and desflurane-induced pre- and postconditioning against myocardial infarction in mice in vivo. Exp Biol Med (Maywood) 2009;234:1186–1191. doi: 10.3181/0902-RM-58. [DOI] [PubMed] [Google Scholar]
- Riess ML, Camara AK, Chen Q, Novalija E, Rhodes SS, Stowe DF. Altered NADH and improved function by anesthetic and ischemic preconditioning in guinea pig intact hearts. Am J Physiol Heart Circ Physiol. 2002;283:H53–H60. doi: 10.1152/ajpheart.01057.2001. [DOI] [PubMed] [Google Scholar]
- Riess ML, Kevin LG, Camara AK, Heisner JS, Stowe DF. Dual exposure to sevoflurane improves anesthetic preconditioning in intact hearts. Anesthesiology. 2004;100:569–574. doi: 10.1097/00000542-200403000-00016. [DOI] [PubMed] [Google Scholar]
- Roth DM, Patel HH. Role of caveolae in cardiac protection. Pediatr Cardiol. 2011;32:329–333. doi: 10.1007/s00246-010-9881-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu R, Diaz RJ, Mao GD, Wilson GJ. Ischemic preconditioning: differences in protection and susceptibility to blockade with single-cycle versus multicycle transient ischemia. Circulation. 1997;96:984–995. doi: 10.1161/01.cir.96.3.984. [DOI] [PubMed] [Google Scholar]
- Schultz JE, Hsu AK, Gross GJ. Morphine mimics the cardioprotective effect of ischemic preconditioning via a glibenclamide-sensitive mechanism in the rat heart. Circ Res. 1996;78:1100–1104. doi: 10.1161/01.res.78.6.1100. [DOI] [PubMed] [Google Scholar]
- Sedlic F, Pravdic D, Hirata N, Mio Y, Sepac A, Camara AK, et al. Monitoring mitochondrial electron fluxes using NAD(P)H-flavoprotein fluorometry reveals complex action of isoflurane on cardiomyocytes. Biochim Biophys Acta. 2010;1797:1749–1758. doi: 10.1016/j.bbabio.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepac A, Sedlic F, Si-Tayeb K, Lough J, Duncan SA, Bienengraeber M, et al. Isoflurane preconditioning elicits competent endogenous mechanisms of protection from oxidative stress in cardiomyocytes derived from human embryonic stem cells. Anesthesiology. 2010;113:906–916. doi: 10.1097/ALN.0b013e3181eff6b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeev P, Da Silva R, Lucchinetti E, Zaugg K, Pasch T, Schaub MC, et al. Trigger-dependent gene expression profiles in cardiac preconditioning: evidence for distinct genetic programs in ischemic and anesthetic preconditioning. Anesthesiology. 2004;100:474–488. doi: 10.1097/00000542-200403000-00005. [DOI] [PubMed] [Google Scholar]
- da Silva R, Grampp T, Pasch T, Schaub MC, Zaugg M. Differential activation of mitogen-activated protein kinases in ischemic and anesthetic preconditioning. Anesthesiology. 2004;100:59–69. doi: 10.1097/00000542-200401000-00013. [DOI] [PubMed] [Google Scholar]
- Singh P, Chauhan S, Jain G, Talwar S, Makhija N, Kiran U. Comparison of cardioprotective effects of volatile anesthetics in children undergoing ventricular septal defect closure. World J Pediatr Congenit Heart Surg. 2013;4:24–29. doi: 10.1177/2150135112457580. [DOI] [PubMed] [Google Scholar]
- Smul TM, Stumpner J, Blomeyer C, Lotz C, Redel A, Lange M, et al. Propofol inhibits desflurane-induced preconditioning in rabbits. J Cardiothorac Vasc Anesth. 2011;25:276–281. doi: 10.1053/j.jvca.2010.07.018. [DOI] [PubMed] [Google Scholar]
- Stewart S, Lesnefsky EJ, Chen Q. Reversible blockade of electron transport with amobarbital at the onset of reperfusion attenuates cardiac injury. Transl Res. 2009;153:224–231. doi: 10.1016/j.trsl.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Symons JA, Myles PS. Myocardial protection with volatile anaesthetic agents during coronary artery bypass surgery: a meta-analysis. Br J Anaesth. 2006;97:127–136. doi: 10.1093/bja/ael149. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Kehl F, Gu W, Krolikowski JG, Pagel PS, Warltier DC, et al. Isoflurane-induced preconditioning is attenuated by diabetes. Am J Physiol Heart Circ Physiol. 2002;282:H2018–H2023. doi: 10.1152/ajpheart.01130.2001. [DOI] [PubMed] [Google Scholar]
- Toller WG, Gross ER, Kersten JR, Pagel PS, Gross GJ, Warltier DC. Sarcolemmal and mitochondrial adenosine triphosphate- dependent potassium channels: mechanism of desflurane-induced cardioprotection. Anesthesiology. 2000;92:1731–1739. doi: 10.1097/00000542-200006000-00033. [DOI] [PubMed] [Google Scholar]
- Toma O, Weber NC, Wolter JI, Obal D, Preckel B, Schlack W. Desflurane preconditioning induces time-dependent activation of protein kinase C epsilon and extracellular signal-regulated kinase 1 and 2 in the rat heart in vivo. Anesthesiology. 2004;101:1372–1380. doi: 10.1097/00000542-200412000-00018. [DOI] [PubMed] [Google Scholar]
- Tonkovic-Capin M, Gross GJ, Bosnjak ZJ, Tweddell JS, Fitzpatrick CM, Baker JE. Delayed cardioprotection by isoflurane: role of K(ATP) channels. Am J Physiol Heart Circ Physiol. 2002;283:H61–H68. doi: 10.1152/ajpheart.01040.2001. [DOI] [PubMed] [Google Scholar]
- Tsutsumi YM, Yokoyama T, Horikawa Y, Roth DM, Patel HH. Reactive oxygen species trigger ischemic and pharmacological postconditioning: in vivo and in vitro characterization. Life Sci. 2007;81:1223–1227. doi: 10.1016/j.lfs.2007.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuman KJ. Perioperative myocardial infarction. Semin Thorac Cardiovasc Surg. 1991;3:47–52. [PubMed] [Google Scholar]
- Uecker M, Da Silva R, Grampp T, Pasch T, Schaub MC, Zaugg M. Translocation of protein kinase C isoforms to subcellular targets in ischemic and anesthetic preconditioning. Anesthesiology. 2003;99:138–147. doi: 10.1097/00000542-200307000-00023. [DOI] [PubMed] [Google Scholar]
- Van Allen NR, Krafft PR, Leitzke AS, Applegate RL, 2nd, Tang J, Zhang JH. The role of volatile anesthetics in cardioprotection: a systematic review. Med Gas Res. 2012;2:22. doi: 10.1186/2045-9912-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Weihrauch D, Schwabe DA, Bienengraeber M, Warltier DC, Kersten JR, et al. Extracellular signal-regulated kinases trigger isoflurane preconditioning concomitant with upregulation of hypoxia-inducible factor-1alpha and vascular endothelial growth factor expression in rats. Anesth Analg. 2006;103:281–288. doi: 10.1213/01.ane.0000226094.94877.98. , table of contents. [DOI] [PubMed] [Google Scholar]
- Warltier DC, Al-Wathiqui MH, Kampine JP, Schmeling WT. Recovery of contractile function of stunned myocardium in chronically instrumented dogs is enhanced by halothane or isoflurane. Anesthesiology. 1988;69:552–565. doi: 10.1097/00000542-198810000-00016. [DOI] [PubMed] [Google Scholar]
- Weber NC, Schlack W. Inhalational anaesthetics and cardioprotection. Handb Exp Pharmacol. 2008;182:187–207. doi: 10.1007/978-3-540-74806-9_9. [DOI] [PubMed] [Google Scholar]
- Weber NC, Toma O, Awan S, Frassdorf J, Preckel B, Schlack W. Effects of nitrous oxide on the rat heart in vivo: another inhalational anesthetic that preconditions the heart? Anesthesiology. 2005a;103:1174–1182. doi: 10.1097/00000542-200512000-00011. [DOI] [PubMed] [Google Scholar]
- Weber NC, Toma O, Wolter JI, Obal D, Mullenheim J, Preckel B, et al. The noble gas xenon induces pharmacological preconditioning in the rat heart in vivo via induction of PKC-epsilon and p38 MAPK. Br J Pharmacol. 2005b;144:123–132. doi: 10.1038/sj.bjp.0706063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber NC, Goletz C, Huhn R, Grueber Y, Preckel B, Schlack W, et al. Blockade of anaesthetic-induced preconditioning in the hyperglycaemic myocardium: the regulation of different mitogen-activated protein kinases. Eur J Pharmacol. 2008;592:48–54. doi: 10.1016/j.ejphar.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Weihrauch D, Krolikowski JG, Bienengraeber M, Kersten JR, Warltier DC, Pagel PS. Morphine enhances isoflurane-induced postconditioning against myocardial infarction: the role of phosphatidylinositol-3-kinase and opioid receptors in rabbits. Anesth Analg. 2005;101:942–949. doi: 10.1213/01.ane.0000171931.08371.a2. , table of contents. [DOI] [PubMed] [Google Scholar]
- Xia Z, Huang Z, Ansley DM. Large-dose propofol during cardiopulmonary bypass decreases biochemical markers of myocardial injury in coronary surgery patients: a comparison with isoflurane. Anesth Analg. 2006;103:527–532. doi: 10.1213/01.ane.0000230612.29452.a6. [DOI] [PubMed] [Google Scholar]
- Xiao YY, Chang YT, Ran K, Liu JP. Delayed preconditioning by sevoflurane elicits changes in the mitochondrial proteome in ischemia-reperfused rat hearts. Anesth Analg. 2011;113:224–232. doi: 10.1213/ANE.0b013e3182239b71. [DOI] [PubMed] [Google Scholar]
- Yannopoulos D, Segal N, Matsuura T, Sarraf M, Thorsgard M, Caldwell E, et al. Ischemic post-conditioning and vasodilator therapy during standard cardiopulmonary resuscitation to reduce cardiac and brain injury after prolonged untreated ventricular fibrillation. Resuscitation. 2013;84:1143–1149. doi: 10.1016/j.resuscitation.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao YT, Li LH, Chen L, Wang WP, Li LB, Gao CQ. Sevoflurane postconditioning protects isolated rat hearts against ischemia-reperfusion injury: the role of radical oxygen species, extracellular signal-related kinases 1/2 and mitochondrial permeability transition pore. Mol Biol Rep. 2010;37:2439–2446. doi: 10.1007/s11033-009-9755-4. [DOI] [PubMed] [Google Scholar]
- Yu CH, Beattie WS. The effects of volatile anesthetics on cardiac ischemic complications and mortality in CABG: a meta-analysis. Can J Anaesth. 2006;53:906–918. doi: 10.1007/BF03022834. [DOI] [PubMed] [Google Scholar]
- Zaugg M, Lucchinetti E. Letter by Zaugg and Lucchinetti regarding article, ‘Randomized comparison of sevoflurane versus propofol to reduce perioperative myocardial ischemia in patients undergoing noncardiac surgery’. Circulation. 2013;127:e875. doi: 10.1161/CIRCULATIONAHA.112.000201. [DOI] [PubMed] [Google Scholar]
- Zaugg M, Lucchinetti E, Uecker M, Pasch T, Schaub MC. Anaesthetics and cardiac preconditioning. Part I. Signalling and cytoprotective mechanisms. Br J Anaesth. 2003;91:551–565. doi: 10.1093/bja/aeg205. [DOI] [PubMed] [Google Scholar]
- Zhang JL, Hua D, Yang JP. Letter by Zhang et al regarding article, ‘Randomized comparison of sevoflurane versus propofol to reduce perioperative myocardial ischemia in patients undergoing noncardiac surgery’. Circulation. 2013a;127:e876. doi: 10.1161/CIRCULATIONAHA.112.000832. [DOI] [PubMed] [Google Scholar]
- Zhang R, Shen L, Xie Y, Gen L, Li X, Ji Q. Effect of morphine-induced postconditioning in corrections of tetralogy of fallot. J Cardiothorac Surg. 2013b;8:76. doi: 10.1186/1749-8090-8-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Irwin MG, Wong TM. Remifentanil preconditioning protects against ischemic injury in the intact rat heart. Anesthesiology. 2004;101:918–923. doi: 10.1097/00000542-200410000-00017. [DOI] [PubMed] [Google Scholar]
- Zhu J, Rebecchi MJ, Glass PS, Brink PR, Liu L. Interactions of GSK-3β with mitochondrial permeability transition pore modulators during preconditioning: age-associated differences. J Gerontol A Biol Sci Med Sci. 2013;68:395–403. doi: 10.1093/gerona/gls205. [DOI] [PubMed] [Google Scholar]