Abstract
Several noble gases, although classified as inert substances, exert a tissue-protective effect in different experimental models when applied before organ ischaemia as an early or late preconditioning stimulus, after ischaemia as a post-conditioning stimulus or when given in combination before, during and/or after ischaemia. A wide range of organs can be protected by these inert substances, in particular cardiac and neuronal tissue. In this review we summarize the data on noble gas-induced cardioprotection, focusing on the underlying protective mechanisms. We will also look at translatability of experimental data to the clinical situation.
Tables of Links
| TARGETS | |
|---|---|
| Other protein targetsa | Enzymesf |
| HSP70 | Akt (PKB) |
| GRPCRsb | Caspase-3 |
| 5-HT3 receptor | COX-2 |
| Opioid receptors | Endothelial (e) NOS |
| Ligand-gated ion channelsc | ERK |
| Nicotinic ACh receptors | GSK3β |
| NMDA receptors | Haem oxygenase 1 |
| Ion channelsd | Inducible (i) NOS |
| KATP channel | JNK |
| KCa channel | MAPKAPK-2 |
| K2P2.1 (TREK-1) channel | MEK1 |
| Catalytic receptorse | Neuronal (n) NOS |
| CD11b | P38 MAPK |
| CD18 | PDK1 |
| PKCα | |
| PKCδ | |
| PKCε | |
| Tissue plasminogen activator |
| LIGANDS | |
|---|---|
| ACh | LPS |
| ADP | Morphine |
| ATP | Propofol |
| Cyclosporin A | Sevoflurane |
| Desflurane | Staurosporine |
| Glycine | TNF-α |
| ICAM-1 | Urate oxidase |
| L-NAME | VCAM-1 |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (
a,b,c,d,,e,fAlexander et al., 2013a,b,c,d,e,f,,,,,).
Introduction
The noble gases helium, neon, argon, krypton, xenon and radon are odourless, colourless, monatomic gases that are characterized by a filled outer shell of valence electrons, making them ‘inert’ or at least less capable of interaction with other compounds. However, some of these gases are already frequently used in medicine, for example, helium is applied to patients with severe airway disease because of its very low density (Rodrigo et al., 2002; Vorwerk and Coats, 2010). Xenon has been shown to act at the NMDA receptor (Franks et al., 1998), thereby inducing anaesthesia under normobaric conditions (Rossaint et al., 2003).
In recent years, several investigators demonstrated a tissue-protective effect of noble gases in different animal species as well as in humans (Preckel et al., 2006; Oei et al., 2010; Pagel, 2010; Deng et al., 2014). This protection was shown for various periods of ischaemia reperfusion, for example, when the gas was applied before organ ischaemia as an early or late preconditioning (LPC) stimulus (Weber et al., 2005a; Heinen et al., 2008; Huhn et al., 2009a), after ischaemia as a post-conditioning stimulus (Weber et al., 2008a; Huhn et al., 2009b) or when given in combination before, during and/or after ischaemia (Oei et al., 2012b). A wide range of organs can be protected by these inert substances, in particular the heart and neuronal tissue (Preckel et al., 2006; Oei et al., 2010; Coburn et al., 2012). This article will summarize the current knowledge on noble gas-induced cardioprotection and will focus on the mechanisms of protection and a possible translatability to the clinical situation.
Xenon
Cardioprotection by xenon
Anaesthetic properties of xenon have been described as early as 1951 (Cullen and Gross, 1951), and during the last two decades numerous studies evaluated molecular properties (Preckel et al., 2006) and the clinical benefits (Harris and Barnes, 2008) of xenon as an anaesthetic agent. With regard to the ongoing discussion on anaesthesia-induced post-operative cognitive dysfunction in elderly surgical patients, xenon might be advantageous compared with commonly used inhalational anaesthetics (Bronco et al., 2010; Stuttmann et al., 2010), although this protective effect has been challenged by other investigators (Rasmussen et al., 2006; Höcker et al., 2009).
By far the most information on organ protection by noble gases comes from studies using xenon as an inhalational agent. Experimental studies have clearly shown that xenon protects the brain (Limatola et al., 2010), spinal cord (Yamamoto et al., 2010; Yang et al., 2013), kidney (Ma et al., 2009), heart (Weber et al., 2005a,b,) and vascular endothelium (Weber et al., 2008c) from ischaemia reperfusion injury.
Applying the noble gas at the end of ischaemia and during the first minutes of reperfusion might also have protective effects leading to a reduction in infarct size by post-conditioning. In rabbits subjected to 30 min of coronary artery occlusion followed by 120 min of reperfusion, inhalation of xenon (70%) at the very end of regional myocardial ischaemia and during the first 15 min of reperfusion (post-conditioning) reduced infarct size (Preckel et al., 2000). Post-conditioning by sub-anaesthetic concentrations of xenon (20%) combined with mild hypothermia during early reperfusion also reduced myocardial damage in rats in vivo (Schwiebert et al., 2010).
In comparison to its use for post-conditioning, much more information is available for myocardial preconditioning with xenon. Cardioprotection by xenon might be established if the gas is given as an early (within 2–3 h) or late (within 12–24 h) preconditioning stimulus before organ ischaemia occurs: in rats subjected to 25 min of regional myocardial ischaemia followed by 2 h of reperfusion, xenon inhalation for three times 5 min before myocardial ischaemia significantly reduced infarct size from 51% of the area at risk to 28% (Weber et al., 2005a).
Mechanisms of xenon-induced cardioprotection
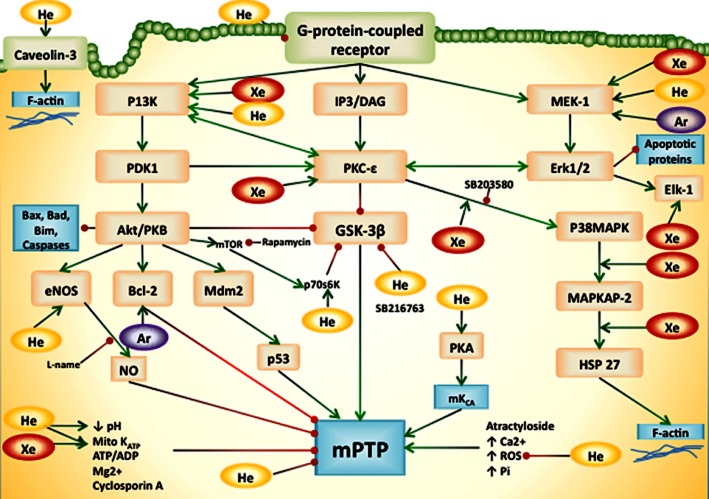
There are numerous enzymes and cellular structures involved in mediating the organ-protective effects of conditioning. Namely the survivor activating factor enhancement (SAFE) pathway and the reperfusion injury salvage kinase (RISK) pathway have been suggested to play significant roles in mediating tissue protection (Hausenloy et al., 2011). The SAFE pathway is influenced by, for example, the JAK, STAT pathways and the mitochondrial permeability transition pore (mPTP). The RISK pathway involves numerous intracellular mediators, such as PI3K, PKC, MAPK, glycogen synthase kinase 3β (GSK3β) and ERK (Hausenloy et al., 2011). It is likely that these pathways, which have mainly been described for ischaemic conditioning (Przyklenk, 2013), also play a significant role in pharmacological conditioning by noble gases. Figure 1 summarizes the possible mechanisms of noble gas-induced cardioprotection.
Figure 1.
Schematic diagram showing mechanisms underlying the protective effects of noble gases. This figure is a summary of the mechanisms that may contribute to organ protection by noble gases, mainly via the RISK pathway. Gas names are shown in circles (He, Ar, Xe). Green arrows indicate an activating or up-regulatory effect, whereas red dots indicate a suppressive or down-regulatory effect. Bcl-2, B-cell lymphoma-2; DAG, diacylglycerol; IP3, inositol triphosphate-3; Mdm2, murine double minute-2; mKCa, mitochondrial Ca2+-sensitive potassium channel; p38MAPK, p38 MAPK; PDK-1, phosphoinositide-dependent PK-1; Pi, inorganic phosphate; ROS, reactive oxygen species.
Mechanism of xenon early preconditioning
The infarct size reduction by xenon preconditioning (Weber et al., 2005a) was completely blocked by infusion of a PKC inhibitor or a p38 MAPK inhibitor, demonstrating that these enzymes play a significant role in xenon-induced preconditioning. Xenon significantly increased phosphorylation of the isoform PKCε, and this effect was blocked by the PKC inhibitor but not by the MAPK inhibitor. These data show that p38 MAPK is located downstream of PKC in the signalling cascade of xenon-induced preconditioning. Further experiments revealed that p38 MAPK is directly activated by xenon (an effect again blocked by a PKC inhibitor) and that the downstream target of p38 MAPK, the MAPK-activated PK2 (MAPKAPK-2), is also phosphorylated after xenon preconditioning (Weber et al., 2005b). Using immunofluorescence staining, it was shown that xenon induced a translocation of PKCε from the cytosol to the membrane fraction of myocardial tissue. To summarize, xenon reduced infarct size by a PKCε and MAPK-dependent mechanism whereby the PKCε-dependent mechanism is mediated by a translocation of PKCε from the cytosol to the membrane fraction of the cardiomyocytes.
In addition to MAPKAPK-2, the small heat shock protein (HSP) 27 also plays an important role in the reorganization of the actin cytoskeleton network of the cell. HSP27 was phosphorylated and translocated to the particulate fraction of cell homogenates after xenon inhalation. From looking at the actin cytoskeleton, xenon was found to increase the polymerization of F-actin fibres, and these fibres were co-localized with the phosphorylated (p) HSP27 (Weber et al., 2005b).
A central role for PKC in mediating the protective effects of different kinds of preconditioning has been confirmed in different animal species as well as in humans (Simkhovich et al., 2013). PKC can be activated by translocation, by phosphorylation of a threonine or serine residue, mediated by the 3′phosphatidylinositol-dependent kinase-1 (PDK-1), as well as by free radical release induced by activation of the mitochondrial ATP-dependent potassium channel (KATP channel). Inhibitors of both target pathways blocked xenon-induced infarct size reduction as well as the phosphorylation of PKC (Weber et al., 2006b). The absence of PKC activation in the presence of a mitochondrial KATP channel blocker within the signalling cascade of xenon preconditioning makes it likely that the opening of the KATP channel takes place upstream of PKC activation. PDK-1 was time-dependently activated by xenon before PKCε was activated.
There are also other isoforms of PKC, namely PKCδ and PKCα. However, these isoforms were not involved in xenon preconditioning, suggesting an isoform-specific activation of PKCε by xenon (Weber et al., 2006a,b,). Similarly, other MAPKs, such as ERK (p44/42 MAPK) and the stress-activated p54/46 MAPK, are critically involved in cell differentiation, cell survival as well as in cellular apoptosis. Although ERK is involved in xenon-induced tissue protection and an inhibitor of ERK blocked the cardioprotective actions of xenon, blocking of JNK1/2 and JNK3 had no effect on xenon-induced infarct size reduction (Weber et al., 2006a). These data suggest a specific regulation of different kinases by xenon preconditioning in the heart (Weber et al., 2006a).
Mechanism of xenon-induced late preconditioning LPC
A second window of protection (LPC) occurs 12–24 h after application of a preconditioning stimulus and lasts up to 72 h. Xenon-induced LPC reduced infarct size in rat hearts subjected to regional ischaemia and reperfusion from 64 to 31% of the area at risk (Weber et al., 2008a). Co-administration of an inhibitor of COX-2 completely blocked this infarct size reduction, although there was no direct increase in COX-2 mRNA or COX-2 protein expression observed after xenon preconditioning.
Endothelial protection by xenon
An endothelial layer covers all vessels in the body, including coronary artery vessels. Cardioprotective effects of noble gases might therefore be mediated via changes within the endothelium. The endothelial cell surface is relatively non-adhesive for macromolecular structures, but this physiological property might be significantly altered after ischaemia and reperfusion. Increased pro-inflammatory cytokines such as TNF-α will increase the expression of cell adhesion molecules on the endothelial layer, thereby recruiting circulating leukocytes to the site of inflammation. In cultured HUVEC, TNF-α was applied to induce cell damage, leading to increased expression of intracellular (ICAM-1) and vascular (VCAM-1) adhesion molecules (Weber et al., 2008c). Pretreatment of the cells with xenon as a preconditioning stimulus (three times 5 min) reduced the expression of the mRNA and protein of ICAM-1 and VCAM-1, but had no effect on a third adhesion molecule, E-selectin. In addition, xenon prevented the TNF-α-induced increase in NF-κB transcriptional activity. Xenon thus most likely confers preconditioning via an ICAM-1- and VCAM-1-mediated pathway that includes inhibition of NF-κB activity.
Xenon blocks the calcium-dependent calcium influx in endothelial cells (Petzelt et al., 1999), thereby affecting mechanisms regulating the calcium release-activated calcium channel of the plasma membrane. Taken together, these data show that xenon might significantly alter endothelial function and, therefore, some of the organ-protective properties of xenon in different organs might be mediated by changes within the endothelium.
The organ-protective effects of xenon might also be caused by modulation of inflammatory reactions, which have been demonstrated in neuronal tissue (Fahlenkamp et al., 2010) as well as in blood (de Rossi et al., 2004). However, xenon had no effect on the inflammatory response to cardiopulmonary bypass (CPB) as measured by cytokine, pro-inflammatory IL-6 and anti-inflammatory IL-10, levels in a rat model of CPB (Clark et al., 2005). These data confirmed previous findings from in vitro experiments using human blood showing that xenon had no effect on the inflammatory response to CBP (Bedi et al., 2002).
How might inert gases be able to induce cellular changes?
Although xenon – like other noble gases – is supposed to be inert, it is obvious from the aforementioned experimental data that xenon is able to produce biological changes within different cells. Using X-ray crystallography studies, it has been suggested that xenon may disrupt conformational changes of the proteins urate oxidase, an intracellular globular protein with large hydrophobic cavities, and annexin V, a protein with a hydrophilic pore inside supposed to bind to cell membranes by a calcium-dependent action (Colloc'h et al., 2007). Binding sites of xenon within the respective proteins are flexible gas cavities with no water inside.
A series of cell membrane receptors has been shown to be influenced by xenon, for example, the NMDA receptors (Franks et al., 1998), the two-pore domain potassium channel TREK-1 (Gruss et al., 2004), the plasmalemmal KATP channel (Bantel et al., 2009), the nicotinic ACh receptor (Yamakura and Harris, 2000) as well as the 5-HT3 receptor (Suzuki et al., 2002). Most of this knowledge comes from neuronal cells. Whether these cellular effects also play a role in myocardial protection by xenon remain unclear. However, the KATP channel, at least the mitochondrial KATP channel, has been demonstrated to be critically involved in preconditioning of the heart (O'Rourke, 2004), and xenon-induced cardioprotection might be mediated partly via this channel. Regarding the previously mentioned actions on cerebral NMDA receptors, it has been shown that xenon competes with the co-agonist glycine at the glycine site of the NMDA receptor (Dickinson et al., 2007).
Translatability of xenon-related organ protection
The protective effects of xenon have been investigated in human tissue in vitro. Xenon limited cell loss and decreased caspase-3 activity in cultured human osteosarcoma cells, indicating an anti-apoptotic effect (Spaggiari et al., 2013). In cultured renal tubular cells (HK-2 cells) subjected to oxygen and glucose deprivation, xenon was the only noble gas with cell-protective properties (Rizvi et al., 2010). In this cell type subjected to hypoxia–hypothermia, xenon limited cell loss and promoted cell expression of HSP70 and haemoxygenase-1 (Zhao et al., 2013). In an isolated CPB system filled with blood from healthy human volunteers, xenon had no effect on cellular markers of inflammation caused by the extracorporeal circulation (Saravanan et al., 2009). Fahlenkamp et al. (2014) compared the effects of xenon and sevoflurane anaesthesia on leukocyte function in surgical patients. Leukocyte subpopulations were not different, and phagocytosis and oxidative burst of granulocytes were reduced to the same extent in both groups (Fahlenkamp et al., 2014). After ex vivo LPS stimulation, pro-inflammatory cytokine release was not affected by xenon. These data show that xenon has a minimal influence on inflammatory activity in humans.
Xenon has virtually no direct influence on myocardial blood flow and global haemodynamics in healthy and diseased hearts (Preckel et al., 2002a,b,). Therefore, the use of xenon has been advocated in cardiac-compromised patients (Baumert et al., 2005; 2008,; Bein et al., 2005), and several studies have demonstrated intraoperative preservation of myocardial contractility and stable haemodynamics (Coburn et al., 2005; Wappler et al., 2007; Schaefer et al., 2011). Despite the huge amount of experimental studies on xenon conditioning of the heart (Preckel et al., 2006) and neuronal tissue (Deng et al., 2014), there are, as yet, no studies clearly translating the conditioning properties of xenon found in experimental studies to humans. One reason might be the limited availability of xenon, leading to very high costs of this noble gas. Another limitation for using xenon in the clinical ischaemia reperfusion situation might have been the lack of suitable anaesthesia machines to safely and cost-effectively deliver xenon. More recently, some new machines have become available for xenon ventilation, and advances in recovery and recycling of xenon might further help to make use of this substance economically more viable (Dingley and Mason, 2007; Rawat and Dingley, 2010). Because xenon has many fewer haemodynamic side effects than routinely used volatile anaesthetics, such as sevoflurane and desflurane, it might be advantageous to investigate the possible beneficial effects of this noble gas in clinical studies on pre- and post-conditioning.
Lockwood et al. (2006) performed a feasibility and safety study, applying xenon to patients undergoing coronary artery bypass grafting using extracorporeal CPB. Although the study was not randomized and does not allow firm conclusions on myocardial injury, there was a tendency towards reduced troponin release in patients receiving 20–50% xenon compared with patients ventilated without any xenon. In contrast, in a randomized trial in 30 patients undergoing cardiac surgery there was no difference in post-operative troponin release when using xenon compared with sevoflurane anaesthesia (Stoppe et al., 2013). Bein et al. (2005) determined troponin and creatine kinase-muscle/brain release in high-risk surgical patients subjected to aortic surgery under inhalational anaesthesia with xenon or total i.v. anaesthesia with propofol. The authors found very low levels of myocardial damage markers, with no significant differences between groups, although three patients in the propofol group had elevated troponin values in the post-operative period compared with zero patients in the xenon group. All clinical studies included only few patients and, therefore, do not allow any firm conclusion to be drawn about cardioprotection of xenon in humans. A summary of the clinical studies concerning cardioprotection by xenon is given in Table 1. Recently, a clinical trial (NCT01294163) finished, which included more than 500 patients and compared the effects of xenon, propofol or sevoflurane-based anaesthesia on post-operative myocardial damage after coronary artery bypass graft surgery (Hofland, 2014). The results of this study should provide us with a definitive answer to the question of whether the beneficial cardioprotective effects of xenon observed in numerous animal studies are translatable to the clinical situation in humans.
Table 1.
Table summarizing available data of noble gas induced protection in human tissue
| Injury | Noble gas | Type | Method | Outcome | Reference |
|---|---|---|---|---|---|
| Staurosporine, mitochondrial toxins | He, Ne, Ar, Kr, Xe (75%) | Osteosarcoma cells | Continuous | Xe and Ar limited cell loss and decreased caspase-3 activation | Spaggiari et al., 2013 |
| Oxygen glucose deficiency | He, Ne, Ar, Kr, Xe (75%) | Renal tubular cells (HK-2) | Preconditioning | Xe protects against cell death, He is cytotoxic, other gases had no effect | Rizvi et al., 2010 |
| None | Ar (50%) | Astroglial cells | Continuous | Ar enhanced ERK1/2 activity in microglia via the upstream kinase MEK | Fahlenkamp et al., 2012 |
| Microglial cells (BV-2) | |||||
| Oxygen glucose deficiency | Xe (80%) | Neuronal glial cells | Preconditioning | Xe limited cell loss via K-ATP channel activation | Bantel et al., 2009 |
| Hypothermia–hypoxia | Xe (70%) | Renal tubular cells (HK-2) | Preconditioning | Xe limited cell loss and promoted cell expression of HSP70 and haemoxygenase-1 | Zhao et al., 2013 |
| Post-conditioning | |||||
| LPS lipoteichoic acid anti-CD3/anti-CD28 | He (79%) | Blood from healthy volunteers | Preconditioning | 30 and 60 min of helium inhalation does not affect immune system function | Oei et al., 2012a |
| LPS | Xe (60%) | Blood from patients undergoing elective abdominal surgery | Continuous | Xe provides modest anti-inflammatory and no pro-inflammatory effect. ERK1/2 phosphorylation in leukocytes was reduced after 1 h of Xe anaesthesia | Fahlenkamp et al., 2014 |
| Cardiopulmonary bypass | Xe (50%) | Blood from healthy volunteers | Continuous | Xe had no effect on CPB induced leukocyte and platelet activation after CPB | Saravanan et al., 2009 |
| Cardiopulmonary bypass | Xe (70%) | Blood from healthy volunteers | Continuous | Xe had no effect on cytokine (IL-8, IL-10, TNF) and adhesion molecule expression L-selectin, CD18, CD11b) after CPB | Bedi et al., 2002 |
| Forearm I/R 15 min | He (50%) | Human volunteers | Continuous | He had no effect on endothelium, but decreased expression of CD11b and ICAM on leukocytes ad CD42b and PSGL-1 on platelets | Lucchinetti et al., 2009 |
| Forearm I/R 20 min | He (79%) | Human volunteers | Preconditioning | He protects post-ischaemic endothelial function | Smit et al., 2013 |
| Blocking eNOS did not abolish this effect | |||||
| None | Xe (59%) | Healthy volunteers | Continuous | Xenon had minimal effects on coronary flow dynamics | Schaefer et al., 2011 |
| None | Xe (65%) | CAD patients undergoing non-cardiac surgery | Continuous | Xe anaesthesia has higher mean arterial blood pressure and better left ventricle ejection fraction | Baumert et al., 2008 |
| Out-of-hospital cardiac arrest | Xe (47%) | Out-of-hospital cardiac arrest patients | Post-conditioning | Xe + mild hypothermia is feasible and favourable with decreased troponin T release | Arola et al., 2013 |
| CABG | Xe (45–50%) | CABG | Continuous | Balanced xenon anaesthesia is feasible and safe | Stoppe et al., 2013 |
| Aortic surgery | Xe (60%) | Aortic surgery | Continuous | Xe does not improve haemodynamic parameters or troponin release compared with total venous anaesthesia | Bein et al., 2008 |
| CABG | Xe (20, 35, 50%) | CABG | Continuous | Xe was safely and efficiently delivered to CABG patients while on CPB | Lockwood et al., 2006 |
| None | Xe (60–65%) | Intracardiac device implantation | Continuous | Xe preserves mean arterial blood pressure and left ventricle ejection fraction compared with propofol | Baumert et al., 2005 |
Injury, injury type against which protection was induced; noble gas, type and concentration of gas used; type, type of patients or cell type; method, type of stimulus used; outcome, short summary of results; reference, reference of original paper.
CABG, coronary artery bypass grafting; CAD, coronary artery disease.
In a feasibility and safety study in adults with out-of-hospital cardiac arrest, xenon (at least 40%) was given for 24 h during mild hypothermia (Arola et al., 2013), initiated at the moment of intensive care unit admission. Because these patients have both cardiac as well as cerebral damage, xenon may be particularly advantageous in this patient population. The post-arrival incremental change of troponin T from baseline to 24, 48 and 72 h post-resuscitation was significantly lower in patients treated with xenon + mild hypothermia compared with patients treated solely with mild hypothermia. There was no safety issue observed in these post-cardiac arrest patients ventilated with a xenon–oxygen mixture. Although these data indicate that xenon might be a suitable treatment addition in patients with cardiac arrest, we have to take into account that all clinical studies only determine indirect parameters (e.g. enzyme release) for organ protection and that up to now no clinical outcome studies are available allowing a strong conclusion on xenon-induced organ protection in a clinical ischaemia reperfusion situation. With regard to neuroprotection, several small studies were not able to demonstrate any positive effect of xenon on incidence of post-operative cognitive dysfunction in the elderly (Rasmussen et al., 2006; Coburn et al., 2007; Höcker et al., 2009; Cremer et al., 2011). A clinical challenge might be the experimental finding that any kind of pre- and post-conditioning can be negatively influenced by co-morbidities such as hypertension (Oei et al., 2012b), diabetes (Weber et al., 2008b) or simply senescence (Heinen et al., 2008). As yet, no clinical data are available demonstrating a conditioning effect of xenon in co-morbid or elderly patients, although this group of patients is likely to be the most pertinant population for organ protection.
Helium
In contrast to xenon, the noble gas helium has no anaesthetic properties, and might therefore be used in awake patients subjected to ischaemia reperfusion situations, for example, during percutaneous coronary interventions in patients with myocardial infarction.
Helium has not been found to have any side effects on global or regional haemodynamics. Because helium has a low density, it reduces the energy needed to breathe and is, therefore, used in patients with airway diseases. Ventilators allowing application of helium by invasive and non-invasive ventilation strategies are available and, therefore, helium might also be used during heart or vascular surgery or in patients undergoing organ transplantation. Because this noble gas is much less expensive than xenon, helium might be an excellent alternative for organ protection in clinical ischaemia reperfusion situations.
Mechanisms of cardioprotection induced by helium
Preconditioning by helium
Besides xenon, the non-anaesthetic noble gas helium also exerts profound organ-protective effects (Oei et al., 2010). Three times 5 min inhalation of 70% helium before 30 min of coronary artery occlusion followed by 3 h of reperfusion significantly reduced infarct size in rabbit hearts (Pagel et al., 2007). These data show that the noble gas helium induces preconditioning of the heart.
Administration of a PI3K antagonist, a mitogen/ERK 1 (MEK1) inhibitor or an inhibitor of the 70 kDa ribosomal protein s6 kinase (p70s6kinase) abolished this helium-induced preconditioning, indicating a role for the so-called RISK pathway in helium-induced cardioprotection. As these pro-survival kinases inhibit GSK3β and apoptotic protein p53 degradation, Pagel et al. (2008a) investigated whether inhibition of GSK3β and p53 lowers the threshold of helium-induced protection. The authors could indeed demonstrate that with these pharmacological interventions the protection of helium could be facilitated, an effect that was also observed after morphine application (Pagel et al., 2009). These data indicate an opioid receptor-mediated mechanism in helium-induced preconditioning, which might play a significant role in patients subjected to ischaemia reperfusion as morphine is a routinely used opioid analgesic applied in these clinical situations.
Opening of the mPTP leads to mitochondrial dysfunction, and preconditioning might be beneficial by preserving cardiac mitochondrial function. The mPTP is postulated to be a possible end-effector for myocardial necrosis and apoptosis after ischaemia/reperfusion (I/R) injury, and prosurvival kinases (PI3K, ERK1/2) and their downstream targets (endothelial NOS, p53, GSK3β) all prevent opening of the mPTP (Hausenloy et al., 2009). Application of a selective mPTP opener abolished helium-induced early preconditioning (Pagel et al., 2008a), indicating that helium eventually inhibits mPTP opening. Prolongation of post-ischaemic acidosis has been shown to reduce myocardial infarct size (Preckel et al., 1998), and correction of acidic pH after restoration of blood flow can cause mPTP opening (Cohen et al., 2007), thereby inducing tissue damage. Transient alkalosis during early reperfusion abolished helium-induced cardioprotection, but the protection was restored by the mPTP inhibitor cyclosporine A (Pagel and Krolikowski, 2009). These data suggest that helium prevents mPTP opening by maintaining intracellular acidosis during early reperfusion. The effect of radical oxygen species and the mitochondrial ATP-regulated potassium channel (KATP) in helium preconditioning was investigated using the reactive oxygen species scavengers N-acetylcysteine and N-2-mercaptopropionyl glycine or the KATP channel blocker 5-hydroxydeconate respectively (Pagel et al., 2008b). All blockers completely abolished helium-induced cardioprotection indicating radical oxygen scavengers, and that KATP channels mediate helium preconditioning. In addition, infusion of the non-selective NOS inhibitor N-nitro-L-arginine methyl ester during helium preconditioning abolished cardioprotection, whereas infusion of a selective inducible NOS inhibitor or a selective neuronal NOS inhibitor had no effect, indicating that protection by helium is mediated by NO generated by endothelial NOS (Pagel et al., 2008c).
Cardiac mitochondrial function can be further analysed by the rate of oxygen consumption of isolated mitochondria after administration of a complex 2 substrate (state 2), ADP (state 3) and after complete phosphorylation of ADP to ATP (state 4) respectively. Preconditioning by helium increased state 4 respiration, thereby reducing the respiratory control index and suggesting a mild mitochondrial uncoupling. This effect was blocked by a selective Ca2+-sensitive potassium channel blocker iberiotoxin (Heinen et al., 2008). More recent data show that the activation of Ca2+-sensitive potassium channels by helium preconditioning is mediated via PKA (Huhn et al., 2012).
Interestingly, in helium-induced organ protection the involvement of various enzymes and kinases has only been shown by use of specific or non-specific blockers. Until now, for helium – in contrast to xenon – no significant up- or down-regulation or phosphorylation of the different kinases or its products has been demonstrated (Huhn et al., 2009b).
Post-conditioning by helium
To gain more mechanistic insights into the protective effect of helium, the expression of genes involved in cell death and survival pathways was investigated after helium post-conditioning in male rats subjected to ischaemia, I/R or I/R and 15 min of 70% helium at the onset of reperfusion (Oei et al., 2013). Helium post-conditioning caused the up-regulation of genes involved in necrosis (17 of 23) and pro-apoptosis (18 of 25). Simultaneously, 4 of 23 (necrosis) and 7 of 25 genes (pro-apoptosis) were down-regulated. The majority of anti-apoptotic genes (9 of 11) and genes involved in autophagy (24 of 32) were up-regulated after helium post-conditioning. These data suggest that helium post-conditioning at least partly prevents the execution of cell death programmes, thereby reducing myocardial infarct size.
Most of the aforementioned influences of helium on the heart were investigated in healthy myocardium. However, pathophysiological changes, for example, hypertension, diabetes mellitus or ageing, may block any conditioning effect. Helium conditioning is more difficult to obtain in diseased animals: it is abolished in aged rats (Heinen et al., 2008; Huhn et al., 2012) as well as in diabetic, Zucker obese rats (Huhn et al., 2009b). The combination of both, helium pre- with post-conditioning, was protective against infarct size development in spontaneous hypertensive rats, whereas each stimulus alone was not able to induce cardioprotection in the hypertensive rat heart (Oei et al., 2012b).
Effect of helium on caveolae and caveolins
Caveolae are cholesterol and sphingolipid-enriched invaginations of the plasma membrane. Caveolins, the structural proteins essential for caveolae formation, are critically involved in anaesthetic-induced cardioprotection (Horikawa et al., 2008). Helium inhalation decreased caveolin-1 and 3 expressions after 24 h (Weber et al., 2012). Buoyant caveolin-enriched fractions, indicative of increased caveolin formation, supported the results showing lower caveolin-1 and 3 levels in cytosolic and mitochondrial fractions, whereas caveolin-1/3 were accumulated in serum of mice 24 h after exposure to helium. These data indicate that caveolin-1 and 3 are secreted into the blood after helium inhalation (Weber et al., 2013) and support the hypothesis that circulating factors in the blood stream may be involved in inducing organ protection. This is in accord with other means of conditioning (Rassaf et al., 2014).
Translatability of helium-related organ protection
Similar to xenon, the tremendous bulk of data on helium-induced organ protection in the heart (Oei et al., 2010) was not definitively translated to the clinical situation.
Helium pre- and post-conditioning of the heart was investigated in patients undergoing coronary artery bypass grafting surgery. Patients were ventilated with a gas mixture of helium (70%) for 3 × 5 min before the start of the CPB or at the moment of coronary reperfusion after declamping the aorta. In contrast to what would be expected from experimental data, neither helium pre- or post-conditioning nor a combination of pre- and post-conditioning had any protective effect on post-operative troponin release (Smit et al., 2012).
Helium conditioning was investigated in healthy human volunteers subjected to forearm ischaemia and reperfusion. Using venous occlusion plethysmography to measure forearm blood flow responses to ACh before and after 20 min of forearm I/R, we recently demonstrated that three times 5 min of 79% helium inhalation prevented post-ischaemic endothelial dysfunction (Smit et al., 2013). A similar protection was observed 24 h after helium inhalation, demonstrating an early as well as a late endothelial preconditioning effect of helium in humans in vivo. Even after blocking endothelial NOS during helium inhalation, the endothelial protection was maintained (Smit et al., 2013), meaning that the involvement of NOS found in previous animal studies (Pagel et al., 2008c) could not be reconfirmed in humans.
Lucchinetti et al. (2009) applied 50% helium before, during and after ischaemia to healthy human volunteers and used post-ischaemic reactive hyperaemia to assess endothelial function before and after 15 min of forearm ischaemia. An increase in the pro-inflammatory marker CD11b and ICAM-1 on leukocytes and an attenuated expression of the pro-coagulant markers CD42b and PSGL-1 on platelets was observed. However, no changes in the post-occlusive hyperaemic reaction were determined. Although Smit et al. (2013) used 70% helium, Lucchinetti and colleagues applied 50% helium. These differences in helium concentration might have influenced the results, although in animal experiments helium as low as 30% induced myocardial protection (while 10% helium was not protective) (Huhn et al., 2009a). The discrepancies of the results between the two studies might also be due to the different protocols of helium administration, as it was previously shown that continuous administration of a pharmacological agent, for example, a volatile anaesthetic, does not induce cardioprotection, whereas intermitted application with more than one cycle of inhalation of a volatile anaesthetic did protect the human heart (Bein et al., 2008; Frässdorf et al., 2009). In contrast to the study by Lucchinetti et al. (2009), another study in human volunteers did not show any effect of helium on the responsiveness of the innate and early adaptive immune system after 30 and 60 min of helium inhalation (Oei et al., 2012a).
Other noble gases
Argon, neon and krypton
The noble gas argon has anaesthetic properties under hyperbaric conditions and is mainly investigated for its neuroprotective effects. After occlusion of the middle cerebral artery in rats, administration of 50% argon reduced cerebral infarct size (Ryang et al., 2011). In a model using cardiac arrest in rats, post-conditioning with 70% argon administered after resuscitation reduced histopathological damage of the neocortex and hippocampus. The mechanism underlying this neuroprotective effect includes up-regulation of ERK1/2 via MEK (Fahlenkamp et al., 2012). Unlike neuroprotection by xenon, there seems no role for the NMDA receptor in argon-induced organ protection (Harris et al., 2013). A recent study implicated a direct and concentration-dependent modulating effect of argon on enzymatic and thrombolytic effect of tissue plasminogen activator (David et al., 2012): a low concentration of argon (25%) blocked and high concentrations (75%) increased enzymatic and thrombolytic efficiency of tissue plasminogen activator. This might be interesting not only in patients with ischaemic stroke but also in patients with myocardial infarction. With respect to cardioprotection, three cycles of 70% argon inhalation interspersed with washout periods reduced infarct size after regional myocardial ischaemia in rabbits (Pagel et al., 2007). As yet, no clinical studies investigating organ-protective effects of argon are available. In human cultured osteosarcoma cells, argon (similar to xenon) limited the cell loss induced by the broad spectrum tyrosine kinase inhibitor staurosporine and several other mitochondrial toxins. In addition, argon inhibited the apoptotic activation of caspase-3 (Spaggiari et al., 2013).
Neon reduced infarct size after regional myocardial ischaemia in rabbits to the same extent as helium and argon (Pagel et al., 2007). No data on the underlying mechanisms are available. Neon and krypton had no neuroprotective effect in cortical neuronal cell cultures subjected to oxygen and glucose deprivation (Jawad et al., 2009). Interestingly, in this model helium had detrimental effects on the cells and increased cell damage. In human cultured renal tubular cells (HK2) neon, argon and krypton showed no protection from cell injury provoked again by oxygen and glucose deprivation (Rizvi et al., 2010). No clinical studies are available investigating possible beneficial effects of neon or krypton in ischaemia reperfusion situations in humans.
Conclusion
In addition to neuro- and cardioprotection, helium and xenon exert beneficial effects in the lung, kidney and liver. However, after summarizing the promising experimental data on tissue protection, it remains to be proven whether this beneficial effect can be translated to the clinical situation. At this moment, there are not enough clinical data to allow any conclusion, although a lot of studies have been initiated with protocols published in trial registrations. The recently finished study on cardioprotection by xenon in coronary artery bypass graft surgery patients will answer the question whether xenon protects against ischaemic cardiac damage after cardiac surgery. However, in this study, the most relevant group of patients, namely those patients with severely reduced myocardial function and very high risk for ischaemic damage (e.g. combined coronary artery and valve surgery), was excluded. Thus, future studies will have to include high-risk patients in order to show any beneficial effect on organ protection, thereby translating the promising experimental results from various models of organ damage to the clinical situation. A recent feasibility study has already demonstrated that high-risk patients can be safely ventilated with up to 50% of helium after cardiac arrest and resuscitation (Brevoord et al., 2012).
Conflict of interest
None of the authors has competing interests to be disclosed.
Glossary
- CPB
cardiopulmonary bypass
- GSK
glycogen synthase kinase
- HSP
heat shock protein
- I/R
ischaemia/reperfusion
- ICAM
intracellular cell adhesion molecules
- KATP
ATP-dependent potassium
- LPC
late preconditioning
- MAPKAPK-2
MAPK-activated PK2
- MEK
mitogen/ERK
- mPTP
mitochondrial permeability transition pore
- PDK-1
3′phosphatidylinositol-dependent kinase-1
- RISK
reperfusion injury salvage kinase
- SAFE
survivor activating factor enhancement
- VCAM
vascular cell adhesion molecule
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, McGrath JC, et al. The Concise Guide to PHARMACOLOGY 2013/14: Overview. Br J Pharmacol. 2013a;170:1449–1458. [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G protein-coupled receptors. Br J Pharmacol. 2013b;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Ligand-gated ion channels. Br J Pharmacol. 2013c;170:1582–1606. doi: 10.1111/bph.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Ion channels. Br J Pharmacol. 2013d;170:1607–1651. doi: 10.1111/bph.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Catalytic receptors. Br J Pharmacol. 2013e;170:1676–1705. doi: 10.1111/bph.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol. 2013f;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arola OJ, Laitio RM, Roine RO, Grönlund J, Saraste A, Pietilä M, et al. Feasibility and cardiac safety of inhaled xenon in combination with therapeutic hypothermia following out-of-hospital cardiac arrest. Crit Care Med. 2013;41:2116–2124. doi: 10.1097/CCM.0b013e31828a4337. [DOI] [PubMed] [Google Scholar]
- Bantel C, Maze M, Trapp S. Neuronal preconditioning by inhalational anesthetics: evidence for the role of plasmalemmal adenosine triphosphate-sensitive potassium channels. Anesthesiology. 2009;110:986–995. doi: 10.1097/ALN.0b013e31819dadc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumert J-H, Hein M, Hecker KE, Satlow S, Neef P, Rossaint R. Xenon or propofol anaesthesia for patients at cardiovascular risk in non-cardiac surgery. Br J Anaesth. 2008;100:605–611. doi: 10.1093/bja/aen050. [DOI] [PubMed] [Google Scholar]
- Baumert JH, Falter F, Eletr D, Hecker KE, Reyle-Hahn M, Rossaint R. Xenon anaesthesia may preserve cardiovascular function in patients with heart failure. Acta Anaesthesiol Scand. 2005;49:743–749. doi: 10.1111/j.1399-6576.2005.00662.x. [DOI] [PubMed] [Google Scholar]
- Bedi A, McBride WT, Armstrong MA, Murray JM, Fee JPH. Xenon has no effect on cytokine balance and adhesion molecule expression within an isolated cardiopulmonary bypass system. Br J Anaesth. 2002;89:546–550. doi: 10.1093/bja/aef232. [DOI] [PubMed] [Google Scholar]
- Bein B, Turowski P, Renner J, Hanss R, Steinfath M, Scholz J, et al. Comparison of xenon-based anaesthesia compared with total intravenous anaesthesia in high risk surgical patients. Anaesthesia. 2005;60:960–967. doi: 10.1111/j.1365-2044.2005.04326.x. [DOI] [PubMed] [Google Scholar]
- Bein B, Renner J, Caliebe D, Hanss R, Bauer M, Fraund S, et al. The effects of interrupted or continuous administration of sevoflurane on preconditioning before cardio-pulmonary bypass in coronary artery surgery: comparison with continuous propofol. Anaesthesia. 2008;63:1046–1055. doi: 10.1111/j.1365-2044.2008.05563.x. [DOI] [PubMed] [Google Scholar]
- Brevoord D, Beurskens C, Juffermans N, van den Bergh W, Preckel B, Horn J. Helium ventilation is safe and feasible in ICU patients admitted after cardiac arrest. Eur J Anaesthesiol. 2012;29(Suppl):192. [Google Scholar]
- Bronco A, Ingelmo PM, Aprigliano M, Turella M, Sahillioğlu E, Bucciero M, et al. Xenon anaesthesia produces better early postoperative cognitive recovery than sevoflurane anaesthesia. Eur J Anaesthesiol. 2010;27:912–916. doi: 10.1097/EJA.0b013e32833b652d. [DOI] [PubMed] [Google Scholar]
- Clark JA, Ma D, Homi HM, Maze M, Grocott HP. Xenon and the inflammatory response to cardiopulmonary bypass in the rat. J Cardiothorac Vasc Anesth. 2005;19:488–493. doi: 10.1053/j.jvca.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Coburn M, Kunitz O, Baumert J-H, Hecker K, Haaf S, Zühlsdorff A, et al. Randomized controlled trial of the haemodynamic and recovery effects of xenon or propofol anaesthesia. Br J Anaesth. 2005;94:198–202. doi: 10.1093/bja/aei023. [DOI] [PubMed] [Google Scholar]
- Coburn M, Baumert J-H, Roertgen D, Thiel V, Fries M, Hein M, et al. Emergence and early cognitive function in the elderly after xenon or desflurane anaesthesia: a double-blinded randomized controlled trial. Br J Anaesth. 2007;98:756–762. doi: 10.1093/bja/aem103. [DOI] [PubMed] [Google Scholar]
- Coburn M, Sanders RD, Ma D, Fries M, Rex S, Magalon G, et al. Argon: the ‘lazy’ noble gas with organoprotective properties. Eur J Anaesthesiol. 2012;29:549–551. doi: 10.1097/EJA.0b013e328357bfdd. [DOI] [PubMed] [Google Scholar]
- Cohen MV, Yang X-M, Downey JM. The pH hypothesis of postconditioning: staccato reperfusion reintroduces oxygen and perpetuates myocardial acidosis. Circulation. 2007;115:1895–1903. doi: 10.1161/CIRCULATIONAHA.106.675710. [DOI] [PubMed] [Google Scholar]
- Colloc'h N, Sopkova-de Oliveira Santos J, Retailleau P, Vivarès D, Bonneté F, Langlois d'Estainto B, et al. Protein crystallography under xenon and nitrous oxide pressure: comparison with in vivo pharmacology studies and implications for the mechanism of inhaled anesthetic action. Biophys J. 2007;92:217–224. doi: 10.1529/biophysj.106.093807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer J, Stoppe C, Fahlenkamp AV, Schälte G, Rex S, Rossaint R, et al. Early cognitive function, recovery and well-being after sevoflurane and xenon anaesthesia in the elderly: a double-blinded randomized controlled trial. Med Gas Res. 2011;1:9. doi: 10.1186/2045-9912-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen SC, Gross EG. The anesthetic properties of xenon in animals and human beings, with additional observations on krypton. Science. 1951;113:580–582. doi: 10.1126/science.113.2942.580. [DOI] [PubMed] [Google Scholar]
- David HN, Haelewyn B, Risso J-J, Abraini JH. Modulation by the noble gas argon of the catalytic and thrombolytic efficiency of tissue plasminogen activator. Naunyn Schmiedebergs Arch Pharmacol. 2012;386:91–95. doi: 10.1007/s00210-012-0809-0. [DOI] [PubMed] [Google Scholar]
- Deng J, Lei C, Chen Y, Fang Z, Yang Q, Zhang H, et al. Neuroprotective gases – fantasy or reality for clinical use? Prog Neurobiol. 2014;115:210–245. doi: 10.1016/j.pneurobio.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Dickinson R, Peterson BK, Banks P, Simillis C, Martin JCS, Valenzuela CA, et al. Competitive inhibition at the glycine site of the N-methyl-D-aspartate receptor by the anesthetics xenon and isoflurane: evidence from molecular modeling and electrophysiology. Anesthesiology. 2007;107:756–767. doi: 10.1097/01.anes.0000287061.77674.71. [DOI] [PubMed] [Google Scholar]
- Dingley J, Mason RS. A cryogenic machine for selective recovery of xenon from breathing system waste gases. Anesth Analg. 2007;105:1312–1318. doi: 10.1213/01.ane.0000278148.56305.72. [DOI] [PubMed] [Google Scholar]
- Fahlenkamp AV, Coburn M, Haase H, Kipp M, Ryang Y-M, Rossaint R, et al. Xenon enhances LPS-induced IL-1β expression in microglia via the extracellular signal-regulated kinase 1/2 pathway. J Mol Neurosci. 2010;45:48–59. doi: 10.1007/s12031-010-9432-z. [DOI] [PubMed] [Google Scholar]
- Fahlenkamp AV, Rossaint R, Haase H, Al Kassam H, Ryang Y-M, Beyer C, et al. The noble gas argon modifies extracellular signal-regulated kinase 1/2 signaling in neurons and glial cells. Eur J Pharmacol. 2012;674:104–111. doi: 10.1016/j.ejphar.2011.10.045. [DOI] [PubMed] [Google Scholar]
- Fahlenkamp AV, Coburn M, Rossaint R, Stoppe C, Haase H. Comparison of the effects of xenon and sevoflurane anaesthesia on leucocyte function in surgical patients: a randomized trial. Br J Anaesth. 2014;112:272–280. doi: 10.1093/bja/aet330. [DOI] [PubMed] [Google Scholar]
- Franks NP, Dickinson R, De Sousa S, Hall NA, Lieb WR. How does xenon produce anaesthesia? Nature. 1998;396:324. doi: 10.1038/24525. [DOI] [PubMed] [Google Scholar]
- Frässdorf J, Borowski A, Ebel D, Feindt P, Hermes M, Meemann T, et al. Impact of preconditioning protocol on anesthetic-induced cardioprotection in patients having coronary artery bypass surgery. J Thorac Cardiovasc Surg. 2009;137:1436–1442. doi: 10.1016/j.jtcvs.2008.04.034. [DOI] [PubMed] [Google Scholar]
- Gruss M, Bushell TJ, Bright DP, Lieb WR, Mathie A, Franks NP. Two-pore-domain K+ channels are a novel target for the anesthetic gases xenon, nitrous oxide, and cyclopropane. Mol Pharmacol. 2004;65:443–452. doi: 10.1124/mol.65.2.443. [DOI] [PubMed] [Google Scholar]
- Harris K, Armstrong SP, Campos-Pires R, Kiru L, Franks NP, Dickinson R. Neuroprotection against traumatic brain injury by xenon, but not argon, is mediated by inhibition at the N-Methyl-D-Aspartate receptor glycine site. Anesthesiology. 2013;119:1137–1148. doi: 10.1097/ALN.0b013e3182a2a265. [DOI] [PubMed] [Google Scholar]
- Harris PD, Barnes R. The uses of helium and xenon in current clinical practice. Anaesthesia. 2008;63:284–293. doi: 10.1111/j.1365-2044.2007.05253.x. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Ong S-B, Yellon DM. The mitochondrial permeability transition pore as a target for preconditioning and postconditioning. Basic Res Cardiol. 2009;104:189–202. doi: 10.1007/s00395-009-0010-x. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Lecour S, Yellon DM. Reperfusion injury salvage kinase and survivor activating factor enhancement prosurvival signaling pathways in ischemic postconditioning: two sides of the same coin. Antioxid Redox Signal. 2011;14:893–907. doi: 10.1089/ars.2010.3360. [DOI] [PubMed] [Google Scholar]
- Heinen A, Huhn R, Smeele KMA, Zuurbier CJ, Schlack W, Preckel B, et al. Helium-induced preconditioning in young and old rat heart: impact of mitochondrial Ca(2+)-sensitive potassium channel activation. Anesthesiology. 2008;109:830–836. doi: 10.1097/ALN.0b013e3181895aa0. [DOI] [PubMed] [Google Scholar]
- Hofland J. 2014. Xenon compared to sevoflurane and total intravenous anaesthesia for coronary artery bypass graft surgery. ClinicalTrials.gov. NCT01294163: 1–3.
- Horikawa YT, Patel HH, Tsutsumi YM, Jennings MM, Kidd MW, Hagiwara Y, et al. Caveolin-3 expression and caveolae are required for isoflurane-induced cardiac protection from hypoxia and ischemia/reperfusion injury. J Mol Cell Cardiol. 2008;44:123–130. doi: 10.1016/j.yjmcc.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höcker J, Stapelfeldt C, Leiendecker J, Meybohm P, Hanss R, Scholz J, et al. Postoperative neurocognitive dysfunction in elderly patients after xenon versus propofol anesthesia for major noncardiac surgery: a double-blinded randomized controlled pilot study. Anesthesiology. 2009;110:1068–1076. doi: 10.1097/ALN.0b013e31819dad92. [DOI] [PubMed] [Google Scholar]
- Huhn R, Heinen A, Weber NC, Hieber S, Hollmann MW, Schlack W, et al. Helium-induced late preconditioning in the rat heart in vivo. Br J Anaesth. 2009a;102:614–619. doi: 10.1093/bja/aep042. [DOI] [PubMed] [Google Scholar]
- Huhn R, Heinen A, Weber NC, Kerindongo RP, Oei GTML, Hollmann MW, et al. Helium-induced early preconditioning and postconditioning are abolished in obese Zucker rats in vivo. J Pharmacol Exp Ther. 2009b;329:600–607. doi: 10.1124/jpet.108.149971. [DOI] [PubMed] [Google Scholar]
- Huhn R, Weber NC, Preckel B, Schlack W, Bauer I, Hollmann MW, et al. Age-related loss of cardiac preconditioning: impact of protein kinase A. Exp Gerontol. 2012;47:116–121. doi: 10.1016/j.exger.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Jawad N, Rizvi M, Gu J, Adeyi O, Tao G, Maze M, et al. Neuroprotection (and lack of neuroprotection) afforded by a series of noble gases in an in vitro model of neuronal injury. Neurosci Lett. 2009;460:232–236. doi: 10.1016/j.neulet.2009.05.069. [DOI] [PubMed] [Google Scholar]
- Limatola V, Ward P, Cattano D, Gu J, Giunta F, Maze M, et al. Xenon preconditioning confers neuroprotection regardless of gender in a mouse model of transient middle cerebral artery occlusion. Neuroscience. 2010;165:874–881. doi: 10.1016/j.neuroscience.2009.10.063. [DOI] [PubMed] [Google Scholar]
- Lockwood GG, Franks NP, Downie NA, Taylor KM, Maze M. Feasibility and safety of delivering xenon to patients undergoing coronary artery bypass graft surgery while on cardiopulmonary bypass: phase I study. Anesthesiology. 2006;104:458–465. doi: 10.1097/00000542-200603000-00012. [DOI] [PubMed] [Google Scholar]
- Lucchinetti E, Wacker J, Maurer C, Keel M, Härter L, Zaugg K, et al. Helium breathing provides modest antiinflammatory, but no endothelial protection against ischemia-reperfusion injury in humans in vivo. Anesth Analg. 2009;109:101–108. doi: 10.1213/ane.0b013e3181a27e4b. [DOI] [PubMed] [Google Scholar]
- Ma D, Lim T, Xu J, Tang H, Wan Y, Zhao H, et al. Xenon preconditioning protects against renal ischemic-reperfusion injury via HIF-1alpha activation. J Am Soc Nephrol. 2009;20:713–720. doi: 10.1681/ASN.2008070712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oei G, Heger M, van Golen RF, Preckel B, Hollmann MW, Weber NC. Transcriptional regulation of cardiac cell death and survival signaling by helium postconditioning in a rat model of regional cardiac ischemia/reperfusion. FASEB J. 2013;27(Meeting Abstract Suppl):lb623. [Google Scholar]
- Oei GTML, Smit KF, vander Vondervoort D, Brevoord D, Hoogendijk A, Wieland CW, et al. Effects of helium and air inhalation on the innate and early adaptive immune system in healthy volunteers ex vivo. J Transl Med. 2012a;10:201. doi: 10.1186/1479-5876-10-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oei GTML, Weber NC, Hollmann MW, Preckel B. Cellular effects of helium in different organs. Anesthesiology. 2010;112:1503–1510. doi: 10.1097/ALN.0b013e3181d9cb5e. [DOI] [PubMed] [Google Scholar]
- Oei GTML, Huhn R, Heinen A, Hollmann MW, Schlack WS, Preckel B, et al. Helium-induced cardioprotection of healthy and hypertensive rat myocardium in vivo. Eur J Pharmacol. 2012b;684:125–131. doi: 10.1016/j.ejphar.2012.03.045. [DOI] [PubMed] [Google Scholar]
- O'Rourke B. Evidence for mitochondrial K+ channels and their role in cardioprotection. Circ Res. 2004;94:420–432. doi: 10.1161/01.RES.0000117583.66950.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagel PS. Cardioprotection by noble gases. J Cardiothorac Vasc Anesth. 2010;24:143–163. doi: 10.1053/j.jvca.2009.03.016. [DOI] [PubMed] [Google Scholar]
- Pagel PS, Krolikowski JG. Transient metabolic alkalosis during early reperfusion abolishes helium preconditioning against myocardial infarction: restoration of cardioprotection by cyclosporin A in rabbits. Anesth Analg. 2009;108:1076–1082. doi: 10.1213/ane.0b013e318193e934. [DOI] [PubMed] [Google Scholar]
- Pagel PS, Krolikowski JG, Shim YH, Venkatapuram S, Kersten JR, Weihrauch D, et al. Noble gases without anesthetic properties protect myocardium against infarction by activating prosurvival signaling kinases and inhibiting mitochondrial permeability transition in vivo. Anesth Analg. 2007;105:562–569. doi: 10.1213/01.ane.0000278083.31991.36. [DOI] [PubMed] [Google Scholar]
- Pagel PS, Krolikowski JG, Pratt PF, Jr, Shim YH, Amour J, Warltier DC, et al. Inhibition of glycogen synthase kinase or the apoptotic protein p53 lowers the threshold of helium cardioprotection in vivo: the role of mitochondrial permeability transition. Anesth Analg. 2008a;107:769–775. doi: 10.1213/ane.0b013e3181815b84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagel PS, Krolikowski JG, Pratt PF, Shim YH, Amour J, Warltier DC, et al. Reactive oxygen species and mitochondrial adenosine triphosphate-regulated potassium channels mediate helium-induced preconditioning against myocardial infarction in vivo. J Cardiothorac Vasc Anesth. 2008b;22:554–559. doi: 10.1053/j.jvca.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagel PS, Krolikowski JG, Pratt PF, Shim YH, Amour J, Warltier DC, et al. The mechanism of helium-induced preconditioning: a direct role for nitric oxide in rabbits. Anesth Analg. 2008c;107:762–768. doi: 10.1213/ane.0b013e3181815995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagel PS, Krolikowski JG, Amour J, Warltier DC, Weihrauch D. Morphine reduces the threshold of helium preconditioning against myocardial infarction: the role of opioid receptors in rabbits. J Cardiothorac Vasc Anesth. 2009;23:619–624. doi: 10.1053/j.jvca.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledgebase of drug targets and their ligands. Nucl. Acids Res. 2014;42:D1098–1106. doi: 10.1093/nar/gkt1143. (Database Issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzelt C, Taschenberger G, Schmehl W, Kox WJ. Xenon-induced inhibition of Ca2+-regulated transitions in the cell cycle of human endothelial cells. Pflugers Arch. 1999;437:737–744. doi: 10.1007/s004240050840. [DOI] [PubMed] [Google Scholar]
- Preckel B, Schlack W, Obal D, Barthel H, Ebel D, Grunert S, et al. Effect of acidotic blood reperfusion on reperfusion injury after coronary artery occlusion in the dog heart. J Cardiovasc Pharmacol. 1998;31:179–186. doi: 10.1097/00005344-199802000-00002. [DOI] [PubMed] [Google Scholar]
- Preckel B, Müllenheim J, Moloschavij A, Thämer V, Schlack W. Xenon administration during early reperfusion reduces infarct size after regional ischemia in the rabbit heart in vivo. Anesth Analg. 2000;91:1327–1332. doi: 10.1097/00000539-200012000-00003. [DOI] [PubMed] [Google Scholar]
- Preckel B, Ebel D, Müllenheim J, Frässdorf J, Thämer V, Schlack W. The direct myocardial effects of xenon in the dog heart in vivo. Anesth Analg. 2002a;94:545–551. doi: 10.1097/00000539-200203000-00012. [DOI] [PubMed] [Google Scholar]
- Preckel B, Schlack W, Heibel T, Rütten H. Xenon produces minimal haemodynamic effects in rabbits with chronically compromised left ventricular function. Br J Anaesth. 2002b;88:264–269. doi: 10.1093/bja/88.2.264. [DOI] [PubMed] [Google Scholar]
- Preckel B, Weber NC, Sanders RD, Maze M, Schlack W. Molecular mechanisms transducing the anesthetic, analgesic, and organ-protective actions of xenon. Anesthesiology. 2006;105:187–197. doi: 10.1097/00000542-200607000-00029. [DOI] [PubMed] [Google Scholar]
- Przyklenk K. Reduction of myocardial infarct size with ischemic ‘conditioning’. Anesth Analg. 2013;117:891–901. doi: 10.1213/ANE.0b013e318294fc63. [DOI] [PubMed] [Google Scholar]
- Rasmussen LS, Schmehl W, Jakobsson J. Comparison of xenon with propofol for supplementary general anaesthesia for knee replacement: a randomized study. Br J Anaesth. 2006;97:154–159. doi: 10.1093/bja/ael141. [DOI] [PubMed] [Google Scholar]
- Rassaf T, Totzeck M, Hendgen-Cotta UB, Shiva S, Heusch G, Kelm M. Circulating nitrite contributes to cardioprotection by remote ischemic preconditioning. Circ Res. 2014;114:1601–1610. doi: 10.1161/CIRCRESAHA.114.303822. [DOI] [PubMed] [Google Scholar]
- Rawat S, Dingley J. Closed-circuit xenon delivery using a standard anesthesia workstation. Anesth Analg. 2010;110:101–109. doi: 10.1213/ANE.0b013e3181be0e17. [DOI] [PubMed] [Google Scholar]
- Rizvi M, Jawad N, Li Y, Vizcaychipi MP, Maze M, Ma D. Effect of noble gases on oxygen and glucose deprived injury in human tubular kidney cells. Exp Biol Med (Maywood) 2010;235:886–891. doi: 10.1258/ebm.2010.009366. [DOI] [PubMed] [Google Scholar]
- Rodrigo G, Pollack C, Rodrigo C, Rowe B. Heliox for treatment of exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2002;(2) doi: 10.1002/14651858.CD003571. CD003571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossaint R, Reyle-Hahn M, Schulte Am Esch J, Scholz J, Scherpereel P, Vallet B, et al. Multicenter randomized comparison of the efficacy and safety of xenon and isoflurane in patients undergoing elective surgery. Anesthesiology. 2003;98:6–13. doi: 10.1097/00000542-200301000-00005. [DOI] [PubMed] [Google Scholar]
- de Rossi LW, Brueckmann M, Rex S, Barderschneider M, Buhre W, Rossaint R. Xenon and isoflurane differentially modulate lipopolysaccharide-induced activation of the nuclear transcription factor KB and production of tumor necrosis factor-alpha and interleukin-6 in monocytes. Anesth Analg. 2004;98:1007–1012. doi: 10.1213/01.ANE.0000106860.27791.44. [DOI] [PubMed] [Google Scholar]
- Ryang Y-M, Fahlenkamp AV, Rossaint R, Wesp D, Loetscher PD, Beyer C, et al. Neuroprotective effects of argon in an in vivo model of transient middle cerebral artery occlusion in rats. Crit Care Med. 2011;39:1448–1453. doi: 10.1097/CCM.0b013e31821209be. [DOI] [PubMed] [Google Scholar]
- Saravanan P, Exley AR, Valchanov K, Casey ND, Falter F. Impact of xenon anaesthesia in isolated cardiopulmonary bypass on very early leucocyte and platelet activation and clearance: a randomized, controlled study. Br J Anaesth. 2009;103:805–810. doi: 10.1093/bja/aep297. [DOI] [PubMed] [Google Scholar]
- Schaefer W, Meyer PT, Rossaint R, Baumert JH, Coburn M, Fries M, et al. Myocardial blood flow during general anesthesia with xenon in humans: a positron emission tomography study. Anesthesiology. 2011;114:1373–1379. doi: 10.1097/ALN.0b013e3182137d9c. [DOI] [PubMed] [Google Scholar]
- Schwiebert C, Huhn R, Heinen A, Weber NC, Hollmann MW, Schlack W, et al. Postconditioning by xenon and hypothermia in the rat heart in vivo. Eur J Anaesthesiol. 2010;27:734–739. doi: 10.1097/EJA.0b013e328335fc4c. [DOI] [PubMed] [Google Scholar]
- Simkhovich BZ, Przyklenk K, Kloner RA. Role of protein kinase C in ischemic ‘conditioning’: from first evidence to current perspectives. J Cardiovasc Pharmacol Ther. 2013;18:525–532. doi: 10.1177/1074248413494814. [DOI] [PubMed] [Google Scholar]
- Smit KF, Brevoord D, De Hert SG, Hollmann MW, Weber NC, Preckel B. Helium induced pre- and postconditioning in patients subjected to coronary artery bypass graft (CABG) surgery. Eur J Anaesthesiol. 2012;29(Suppl):53. [Google Scholar]
- Smit KF, Oei GTML, Brevoord D, Stroes ES, Nieuwland R, Schlack WS, et al. Helium induces preconditioning in human endothelium in vivo. Anesthesiology. 2013;118:95–104. doi: 10.1097/ALN.0b013e3182751300. [DOI] [PubMed] [Google Scholar]
- Spaggiari S, Kepp O, Rello-Varona S, Chaba K, Adjemian S, Pype J, et al. Antiapoptotic activity of argon and xenon. Cell Cycle. 2013;12:2636–2642. doi: 10.4161/cc.25650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppe C, Fahlenkamp AV, Rex S, Veeck NC, Gozdowsky SC, Schälte G, et al. Feasibility and safety of xenon compared with sevoflurane anaesthesia in coronary surgical patients: a randomized controlled pilot study. Br J Anaesth. 2013;111:406–416. doi: 10.1093/bja/aet072. [DOI] [PubMed] [Google Scholar]
- Stuttmann R, Jakubetz J, Schultz K, Schäfer C, Langer S, Ullmann U, et al. Recovery index, attentiveness and state of memory after xenon or isoflurane anaesthesia: a randomized controlled trial. BMC Anesthesiol. 2010;10:5. doi: 10.1186/1471-2253-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Koyama H, Sugimoto M, Uchida I, Mashimo T. The diverse actions of volatile and gaseous anesthetics on human-cloned 5-hydroxytryptamine 3 receptors expressed in xenopus oocytes. Anesthesiology. 2002;96:699–704. doi: 10.1097/00000542-200203000-00028. [DOI] [PubMed] [Google Scholar]
- Vorwerk C, Coats T. Heliox for croup in children. Cochrane Database Syst Rev. 2010;(2) doi: 10.1002/14651858.CD006822.pub2. CD006822. [DOI] [PubMed] [Google Scholar]
- Wappler F, Rossaint R, Baumert J, Scholz J, Tonner PH, van Aken H, et al. Multicenter randomized comparison of xenon and isoflurane on left ventricular function in patients undergoing elective surgery. Anesthesiology. 2007;106:463–471. doi: 10.1097/00000542-200703000-00010. [DOI] [PubMed] [Google Scholar]
- Weber NC, Toma O, Wolter JI, Obal D, Müllenheim J, Preckel B, et al. The noble gas xenon induces pharmacological preconditioning in the rat heart in vivo via induction of PKC-epsilon and p38 MAPK. Br J Pharmacol. 2005a;144:123–132. doi: 10.1038/sj.bjp.0706063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber NC, Toma O, Wolter JI, Wirthle NM, Schlack W, Preckel B. Mechanisms of xenon- and isoflurane-induced preconditioning – a potential link to the cytoskeleton via the MAPKAPK-2/HSP27 pathway. Br J Pharmacol. 2005b;146:445–455. doi: 10.1038/sj.bjp.0706324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber NC, Stursberg J, Wirthle NM, Toma O, Schlack W, Preckel B. Xenon preconditioning differently regulates p44/42 MAPK (ERK 1/2) and p46/54 MAPK (JNK 1/2 and 3) in vivo. Br J Anaesth. 2006a;97:298–306. doi: 10.1093/bja/ael153. [DOI] [PubMed] [Google Scholar]
- Weber NC, Toma O, Damla H, Wolter JI, Schlack W, Preckel B. Upstream signaling of protein kinase C-epsilon in xenon-induced pharmacological preconditioning. Implication of mitochondrial adenosine triphosphate dependent potassium channels and phosphatidylinositol-dependent kinase-1. Eur J Pharmacol. 2006b;539:1–9. doi: 10.1016/j.ejphar.2006.03.054. [DOI] [PubMed] [Google Scholar]
- Weber NC, Frässdorf J, Ratajczak C, Grueber Y, Schlack W, Hollmann MW, et al. Xenon induces late cardiac preconditioning in vivo: a role for cyclooxygenase 2? Anesth Analg. 2008a;107:1807–1813. doi: 10.1213/ane.Ob013e31818874bf. [DOI] [PubMed] [Google Scholar]
- Weber NC, Goletz C, Huhn R, Grueber Y, Preckel B, Schlack W, et al. Blockade of anaesthetic-induced preconditioning in the hyperglycaemic myocardium: the regulation of different mitogen-activated protein kinases. Eur J Pharmacol. 2008b;592:48–54. doi: 10.1016/j.ejphar.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Weber NC, Kandler J, Schlack W, Grueber Y, Frädorf J, Preckel B. Intermitted pharmacologic pretreatment by xenon, isoflurane, nitrous oxide, and the opioid morphine prevents tumor necrosis factor alpha-induced adhesion molecule expression in human umbilical vein endothelial cells. Anesthesiology. 2008c;108:199–207. doi: 10.1097/01.anes.0000299441.32091.ed. [DOI] [PubMed] [Google Scholar]
- Weber NC, vander Vondervoort D, Niesman IR, Saldana M, Roth DM, Preckel B, et al. Effects of noble gas conditioning on caveolin expression in the rat heart in vivo. FASEB J. 2012;28:1114.17. [Google Scholar]
- Weber NC, Schilling JM, Finley JC, Irvine M, Kellerhals SE, Niesman IR, et al. Helium inhalation induces caveolin secretion to blood. FASEB J. 2013;27(Meeting Abstract Suppl):1089.3. [Google Scholar]
- Yamakura T, Harris RA. Effects of gaseous anesthetics nitrous oxide and xenon on ligand-gated ion channels. Comparison with isoflurane and ethanol. Anesthesiology. 2000;93:1095–1101. doi: 10.1097/00000542-200010000-00034. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Kawaguchi M, Kurita N, Kakimoto M, Inoue S, Furuya H. Effects of xenon on ischemic spinal cord injury in rabbits: a comparison with propofol. Acta Anaesthesiol Scand. 2010;54:337–342. doi: 10.1111/j.1399-6576.2009.02111.x. [DOI] [PubMed] [Google Scholar]
- Yang YW, Cheng WP, Lu JK, Dong XH, Wang CB, Zhang J, et al. Timing of xenon-induced delayed postconditioning to protect against spinal cord ischaemia-reperfusion injury in rats. Br J Anaesth. 2013;113:168–176. doi: 10.1093/bja/aet352. [DOI] [PubMed] [Google Scholar]
- Zhao H, Yoshida A, Xiao W, Ologunde R, O'Dea KP, Takata M, et al. Xenon treatment attenuates early renal allograft injury associated with prolonged hypothermic storage in rats. FASEB J. 2013;27:4076–4088. doi: 10.1096/fj.13-232173. [DOI] [PubMed] [Google Scholar]