Abstract
The identification of key tumorigenic events in Sonic Hedgehog subgroup medulloblastomas (MBSHH) will be essential for the development of individualized therapies and improved outcomes. However, beyond confirmation of characteristic SHH-pathway mutations, recent genome-wide sequencing studies have not revealed commonly-mutated genes with widespread relevance as potential therapeutic targets. We therefore examined any role for epigenetic DNA methylation events in MBSHH using a cross-species approach to candidate identification, prioritization and validation. MBSHH–associated DNA methylation events were first identified in 216 subgrouped human medulloblastomas (50 MBSHH, 28 WNT, 44 Group 3, 94 Group 4) and their conservation then assessed in tumors arising from four independent murine models of Shh medulloblastoma, alongside any role in tumorigenesis using functional assessments in mouse and human models. This strategy identified widespread regional CpG hypo-methylation of VAV1, leading to its elevated expression, as a conserved aberrant epigenetic event which characterizes the majority of MBSHH tumors in both species, and is associated with a poor outcome in MBSHH patients. Moreover, direct modulation of VAV1 in mouse and human models revealed a critical role in tumor maintenance, and its abrogation markedly reduced medulloblastoma growth. Further, Vav1 activity regulated granule neuron precursor (GNP) germinal zone exit and migration initiation in an ex vivo model of early post-natal cerebellar development. These findings establish VAV1 as a critical epigenetically-regulated oncogene with a key role in MBSHH maintenance, and highlight its potential as a validated therapeutic target and prognostic biomarker for the improved therapy of medulloblastoma.
Keywords: DNA methylation, epigenetics, medulloblastoma, sonic hedgehog, VAV1
Introduction
Medulloblastoma, the most common malignant brain tumor of childhood, comprises four consensus molecular subtypes [Wnt/Wingless (WNT), Sonic Hedgehog (SHH), Group 3 and Group 4] characterized by distinct clinical, molecular and pathological features, and by different postulated cells of origin.1-4 These advances are rapidly informing targeted therapeutic strategies and the design of individualized clinical trials aimed at increased cure rates and reduced long-term treatment side-effects.5
Targeted therapies for MBSHH (~25% of patients) are currently most advanced. MBSHH (~70% five-year survival) peak in infancy, but also occur throughout childhood and into adulthood.2 Tumors are characterized by SHH signaling activation via mechanisms including pathway mutations (PTCH1 (~45% of MBSHH), SUFU (~8%) and SMO (~14%)) and gene amplifications (GLI2 (~8%), MYCN (~13%)).6 Early clinical trials of SHH pathway inhibitors (targeting SMO) show promise,7, 8 but significant challenges are predicted in their application, with reported developmental toxicities, 9 acquired drug resistance, 10 and intrinsic insensitivity in tumors with pathway activation downstream of SMO (e.g. SUFU mutation, GLI2 and MYCN amplification).6 Thus, there is clear need to identify additional targetable genes and pathways in MBSHH, to support additional or alternative targeted therapeutic strategies.
The identification of therapeutically exploitable ‘driver’ events from the many aberrations identified in contemporary genome-wide genetic and epigenetic investigations represents a major challenge. Recent genome-wide mutational screens in MBSHH have revealed notable genetic diversity, however the most frequent novel coding mutations characterize small patient groups (e.g. MLL2 (~16% of MBSHH) and DDX3X (~26%)) and most mutations are found singly in individual tumors.6, 11 Significantly, a prominent role for epigenetic alterations is also emerging.6, 12-14 Our recent DNA methylation profiling study of 230 medulloblastomas showed MBSHH harbor specific DNA methylation events which characterize significant proportions of tumors and identify candidate disease driver genes.15 However, their functional relevance and contribution to tumor development is unknown.
Genetically-engineered mouse models, which give rise to medulloblastoma with constitutive SHH pathway activation, support granule neuron precursors (GNPs) of the developing cerebellum as their cells of origin4, 16-19 and provide opportunities to systematically interrogate epigenetic events in the mouse medulloblastoma genome. It may be reasoned that any conservation of aberrant DNA methylation events between MBSHH and SHH-driven mouse medulloblastomas could identify events important in tumorigenesis and provide faithful model systems for their functional investigation.
Here we report a cross-species investigation of DNA methylation events in medulloblastomas from 230 human patients (216 with subgroup assigned (50 MBSHH, 28 WNT, 44 Group 3, 94 Group 4)) and four independent SHH mouse models. Using this approach, we identify regional hypo-methylation of VAV1, leading to its elevated expression, as a conserved and widespread epigenetic event in both species, affecting the majority of MBSHH tumors. We further show (i) VAV1 status predicts poor treatment outcome within the MBSHH subgroup and (ii) VAV1 plays a critical functional role in medulloblastoma maintenance, highlighting its clear potential as a biomarker and validated drug target in MBSHH. Finally, we provide evidence that Vav1 is expressed in early cerebellar GNPs, where its activity regulates their development. The roles characterized for VAV1 in MBSHH, and in cerebellar development, demonstrate the potential of comparative tumor epigenomics using genetically-engineered mice to identify, prioritize and functionally analyze critical molecular events in human tumorigenesis.
Results
Human MBSHH tumors harbor distinct and characteristic DNA methylation events
We first sought to identify DNA methylation events which may contribute to human MBSHH. We have previously shown the four major medulloblastoma molecular disease subgroups -WNT, SHH, Group3 and Group 4 - may be distinguished based on their DNA methylation patterns (Supplementary Figure S1A).15 Comparison of tumor DNA methylation patterns of subgrouped medulloblastomas (n=216) with the normal cerebellum (n=21) showed normal cerebella form a distinct group, which is most closely related to MBSHH (Supplementary Figure S1B).
Statistical analysis identified 15 genes (associated with 18 CpG sites) whose methylation status significantly distinguished MBSHH from other medulloblastomas, and from the normal cerebellum (Figure 1A). Both hypo- (n=6) and hyper-methylation (n=9) events were observed. In addition, the methylation status of RASSF1A and COL1A2 CpG sites, two of the most frequent hyper-methylation events reported in medulloblastoma,12, 20 was assessed and showed expected frequent and tumor-specific methylation which affected all tumor subgroups (Figure 1A). In summary, these investigations show MBSHH harbor distinct DNA methylation patterns and identify a series of gene-specific DNA methylation events for further analysis.
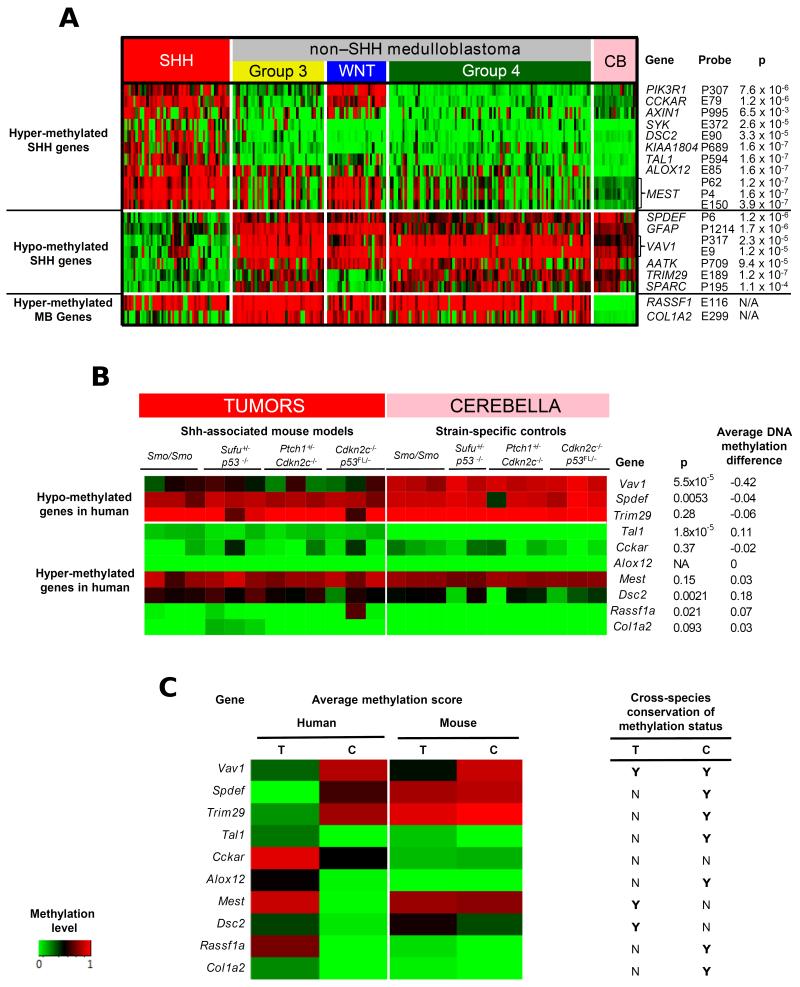
Figure 1. Identification of DNA methylation events associated with human MBSHH and their analysis in mouse tumors and cerebella identify VAV1/Vav1 hypo-methylation as a conserved cross-species epigenetic event in SHH-associated medulloblastomas.
A. Heatmap showing the most differentially methylated CpG sites between human primary MBSHH tumors (n=50) and (i) non-MBSHH tumors (n=166) and (ii) cerebellar samples (n=21). Heatmap shows methylation scores (green (unmethylated) to red (fully-methylated)). All CpG sites selected showed a statistically significant difference in average methylation status between groups (mean β difference >0.34 and adjusted p<0.05 following correction for multiple testing). B. and C. The DNA methylation status (methylation score) of orthologous regions in the mouse and human genomes (mean, based on all CpG sites) was calculated for each gene of interest by bisulfite sequencing of 12 spontaneous medulloblastomas arising from four independent Shh-associated mouse models (genotypes indicated) and 11 strain-matched normal cerebella, in comparison with 6 MBSHH human tumors and 6 human cerebella. B. Heatmap shows methylation scores, from green (unmethylated) to red (fully-methylated) for individual mouse tumors and cerebella. ‘p’ indicates significance following Mann-Whitney tests. The difference between the average DNA methylation score for mouse cerebella, and the average methylation score for mouse tumor samples, is shown. C. Heatmap comparing average methylation scores in all analyzed tumors (T) and cerebella (C) from mice and humans. Conservation of overall methylation status between species (average methylation score difference ≤0.2) is indicated (Y, Yes; N, No).
Identification of conserved DNA methylation events in mouse models of SHH-driven medulloblastoma
We next assessed the DNA methylation status of regions orthologous to those encompassing the CpG sites identified in human MBSHH tumors, in medulloblastomas from four independent SHH-associated mouse models, alongside strain-matched normal cerebella of different developmental stages. Eight orthologous regions surrounding CpG sites which showed MBSHH-specific methylation, alongside regions surrounding RASSF1A and COL1A2, were selected for analysis by direct bisulfite sequencing (Figure1, Supplementary Table S1 and Figure S2A, B). The overall cerebellar methylation status was conserved in the majority of regions assessed (7/10; Figure 1C); cerebella from different mouse strains, including immature and mature mice, showed equivalent patterns (Figure 1B). Consistent methylation profiles were also observed across tumors from the different SHH-associated mouse models (Figure 1B).
Most strikingly, VAV1 showed widespread tumor-specific patterns of hypo-methylation across the regions examined which were of equivalent extent, magnitude and frequency in human MBSHH and SHH mouse tumor models, compared to cerebellar controls. In all mouse tumors, the majority of CpG sites across the Vav1 region were hypo-methylated (Figure 1B, C; Supplementary Figure S2C), while in humans, VAV1 hypo-methylation encompassed all assessed CpG sites (Supplementary Figures S2B and S3A, B, C). Tal1, Dsc2, Spdef and Rassf1a also showed statistically significant methylation differences, but these represented methylation changes in individual samples or CpG sites and were not comparable to differences seen between human MBSHH tumors and cerebella (Figure 1B, C). These findings identify regional VAV1 hypo-methylation as a frequent evolutionarily conserved epigenetic event in human MBSHH, for further analysis.
VAV1 hypo-methylation characterizes the majority of MBSHH and is associated with a poor outcome
VAV1 hypo-methylation was a frequent region-wide event in human MBSHH (70% (36/50)), affecting only occasional tumors (2% (4/166)) within the other molecular subgroups (Figure 2A, B; Supplementary Figure S3C). In whole-cohort analysis (n=230), VAV1 hypo-methylation was significantly correlated with desmoplastic nodular (DN) pathology (p=2.3×10−11, Fisher’s exact test), infant patients (<3 years at diagnosis; p=0.003)) and MYCN gene amplification (p=0.02) (Figure 2C), consistent with the established associations of these features with MBSHH in this cohort 15.
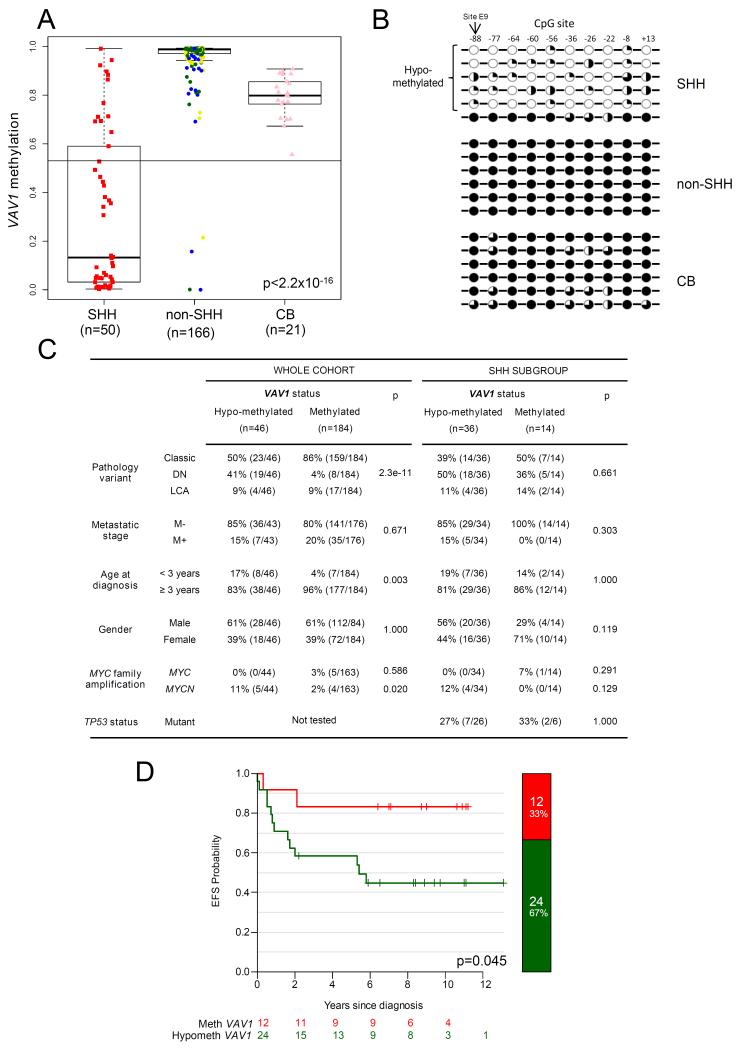
Figure 2. VAV1 hypo-methylation in MBSHH: Incidence, regional distribution, and association with clinical, pathological and molecular disease features.
A. VAV1 methylation levels in our cohort of 216 primary medulloblastomas with subgroup classification. Box plots show methylation levels (β-value, probe E9) in the MBSHH subgroup (n=50) compared to non-MBSHH tumors (n=166) and normal cerebella (CB; n=21). The cerebellar methylation distribution (mean-3SD; cut-off marked) was used to distinguish hypo-methylated tumors (β<0.53) for subsequent assessment of clinical, pathological and molecular associations. ‘p’ value, Kruskal-Wallis test. non-MBSHH tumors are shown colored by subgroup (Group 3, yellow; WNT, blue; Group 4, green). B. Methylation levels at adjacent CpG sites to probe E9, following analysis by bisulfite sequencing in representative MBSHH, non-MBSHH tumors and normal cerebella samples showing region-wide hypomethylation of CpG sites in the majority of MBSHH tumors. CpG sites are labelled by their position relative to the translational start site of VAV1. Black circles, ≥80% methylation; three-quarter black circles, ≥60%; half-black circles, ≥40%; quarter-black circles, ≥20% methylation; white circles, <20% methylation. C. Clinical, pathological and molecular features of VAV1 methylated and hypo-methylated tumors. ‘p’ values, X2 or Fisher’s exact test, as appropriate. D. Kaplan-Meier survival curves show event-free survival (EFS) for VAV1 hypo-methylated and methylated tumors (β-value, probe E9) in a sub-cohort of 36 primary MBSHH medulloblastomas comprising patients with available survival information, aged ≥3 and <16 years old at diagnosis, treated with standard upfront radiotherapy and chemotherapy 15. ‘p’ value, log-rank test.
Within the MBSHH subgroup, VAV1 hypo-methylation was observed across tumors of all key clinical, pathological and molecular demographics, and did not associate with any particular medulloblastoma disease feature; it is however notable that all MYCN amplifications occurred in VAV1 hypo-methylated tumors (Figure 2C). Associations were assessed using the CpG site recognized by the VAV1_e9 probe which lies within the region conserved between humans and mice. Notably, VAV1 hypo-methylation was associated with a poor outcome amongst MBSHH tumors (Figure 2D), in an analysis of 36 patients aged ≥3 years at diagnosis, who received standard upfront radiotherapy and chemotherapy and were derived predominantly from the PNET3 clinical trial.15, 21 The rarity of VAV1 hypo-methylation in non-MBSHH precluded the analysis of any relationships to survival in these subgroups. Thus, VAV1 hypo-methylation occurs frequently within the MBSHH subgroup and is associated with specific disease features and poor outcomes.
VAV1 hypo-methylation is associated with elevated expression in human and mouse SHH medulloblastomas
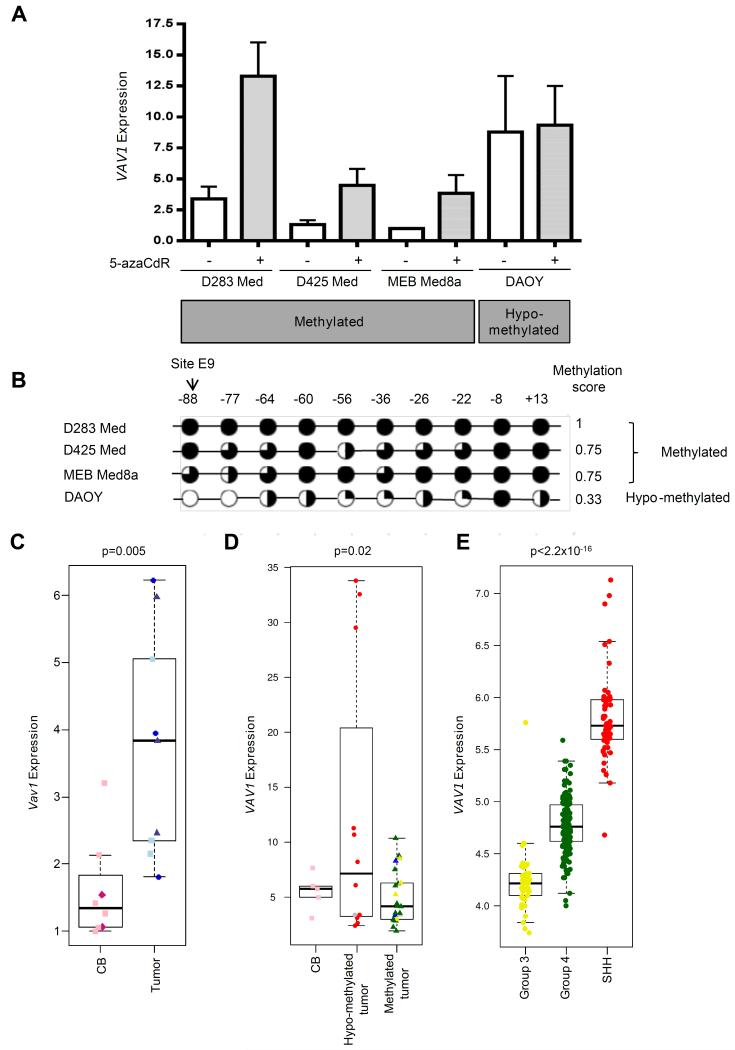
VAV1 showed a direct methylation-dependent relationship between methylation status and expression in human medulloblastoma cell lines treated with the DNA methyltransferase inhibitor, 5′-Aza-2′-deoxycytidine (5-azaCdR) (Figure 3A, B). All VAV1-methylated cell lines (D283Med, MEB-Med8a and D425Med) had low VAV1 expression which was upregulated following 5-azaCdR de-methylation. In contrast, VAV1 expression was higher in hypo-methylated cells (DAOY) and was unaffected by 5-azaCdR. Consistent with this, Vav1 expression was significantly increased in mouse tumors (all hypo-methylated) compared to normal adult cerebella (all methylated) (Figure 3C; Supplementary Figure S2C), and in VAV1 hypo-methylated human tumors compared to VAV1 methylated tumors or the normal human cerebellum (all methylated) (Figure 3D). Finally, VAV1 expression was highest in MBSHH tumors (associated with VAV1 hypo-methylation) compared to the other medulloblastoma molecular subgroups (Figure 3E; data from published dataset22). These demonstrated relationships between VAV1 methylation and expression are consistent with the hypo-methylation-dependent elevation of VAV1 expression in human and mouse SHH-subgroup medulloblastomas.
Figure 3. VAV1/Vav1 hypo-methylation is associated with elevated expression.
A. RT-PCR analysis of VAV1 mRNA expression in human medulloblastoma cell lines before (−) and after (+) treatment with the DNA methyltransferase inhibitor, 5 AzaCdr. Mean VAV1 expression (+SE) is shown based on three independent replicates. B. DNA methylation levels at individual CpG sites in the assessed VAV1 region in human cell lines following analysis by bisulfite sequencing shown alongside the methylation score. Black circles, ≥80% methylation; three-quarter black circles, ≥60%; half-black circles, ≥40%; quarter-black circles, ≥20%; white circles, <20% methylation. C. RT-PCR analysis of Vav1 mRNA expression in Vav1 hypo-methylated mouse tumors (n=9; 3 each of Smo/Smo (blue circles), Ptch1+/− Cdkn2c−/− (light-blue squares) and Cdkn2c−/− p53FL/−, Nestin-Cre+ (grey-blue triangles) mice) and strain-matched Vav1 methylated normal mouse cerebella (CB; n=9; Smo/Smo matched strain, dark pink diamonds; Cdkn2c−/− strains, pink squares). D. RT-PCR analysis of VAV1 methylated (n=22) and hypo-methylated (n=12) human tumors and methylated human normal cerebella (CB; n=5). VAV1 methylation status was determined as described in Figure 2. E. VAV1 mRNA expression in a cohort of 255 primary human medulloblastomas previously described by 22 containing no WNT subgroup tumors. D and E. Molecular subgroup status is shown for human medulloblastomas (MBSHH, red; WNT, blue; Group 3, yellow; Group 4, green; non-classified, grey), and ‘p’ values (C, t-test; D, E, ANOVA) are indicated.
VAV1 expression is pro-proliferative in human medulloblastoma cells
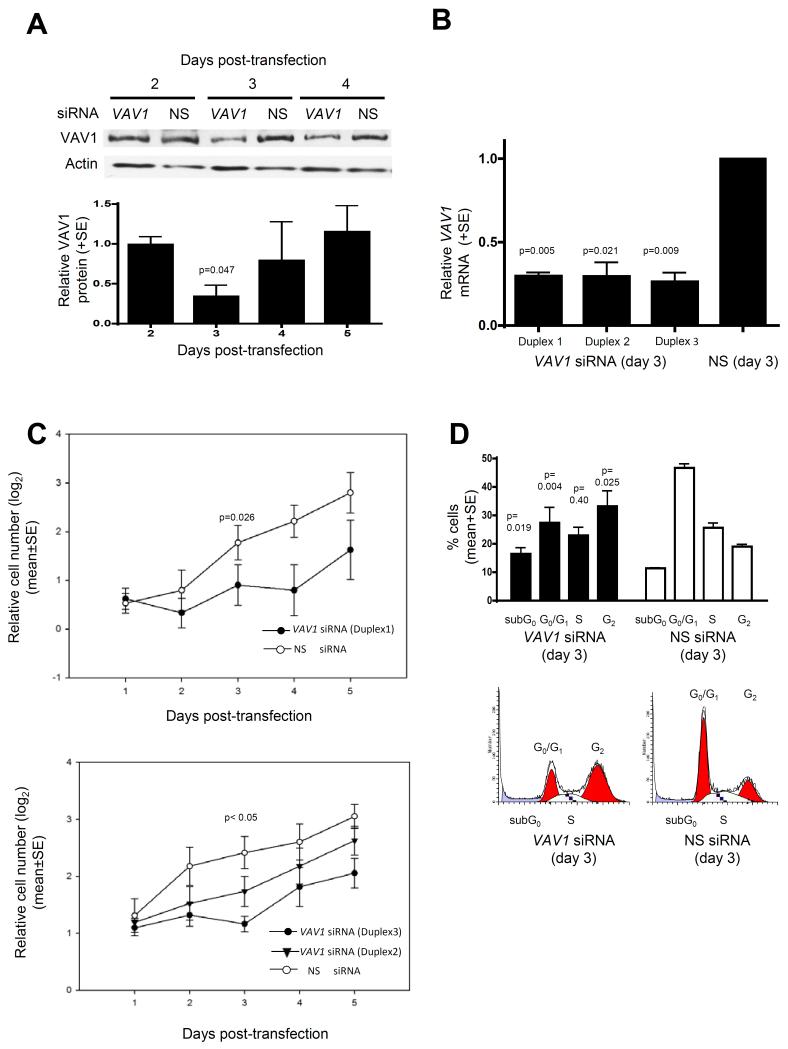
To investigate the functional consequences of VAV1 hypo-methylation and expression in human medulloblastoma, we modelled the effect of silencing VAV1 over-expression in hypo-methylated human medulloblastoma cells. VAV1 silencing using siRNA (60-80% knockdown, day 3; Figure 4A, B) significantly decreased proliferation (Figure 4C) and altered cell cycle distribution; a pronounced G2 arrest and increased G0/sub-G1 fraction occurred following VAV1 knockdown (Figure 4D). These data support a pro-proliferative role for the elevated VAV1 expression observed in VAV1 hypo-methylated human medulloblastoma cells.
Figure 4. VAV1 expression promotes proliferation in VAV1 hypo-methylated human medulloblastoma cells.
A,B. Consistent reduction of VAV1 expression (60-70%) in VAV1 hypo-methylated DAOY medulloblastoma cells (Figure 3) at 3 days post-transfection with 3 anti-VAV1 siRNA duplexes, compared to non-silencing (NS) siRNA. A. Western blot shows representative VAV1 knockdown and graph shows the mean reduction in VAV1 expression (+SE; based on three independent replicates) caused by anti-VAV1 siRNA (duplex 1) relative to NS siRNA controls, following normalization to actin protein levels. B. RT-PCR analysis of VAV1 mRNA expression at 3 days post-transfection with anti-VAV1 siRNA (duplexes 1-3). Graph shows mean reduction in VAV1 expression (+SE; based on four independent replicates) caused by VAV1 siRNA relative to NS siRNA controls, following normalization to the expression of a control gene (TBP). C. Cell proliferation following transfection with anti-VAV1 siRNA or NS siRNA, determined by XTT assay. Graphs show log2 number of cells at 1 to 5 days post-transfection relative to day 0 (before addition of siRNA). Results shown represent 4 independent replicates (+ SE). D. Fluorescence-activated cell sorting (FACS) analysis of cell cycle distribution at 3 days post-transfection (duplex 1). Graph shows mean (+SE) percentage of cells in each cell cycle phase, based on 4 independent replicates. Representative cell cycle distribution plots are shown for each siRNA. All ‘p’ values shown represent paired t-tests.
Dominant-negative Vav1 suppresses medulloblastoma maintenance in the Ptch1+/− Cdkn2c−/− mouse model
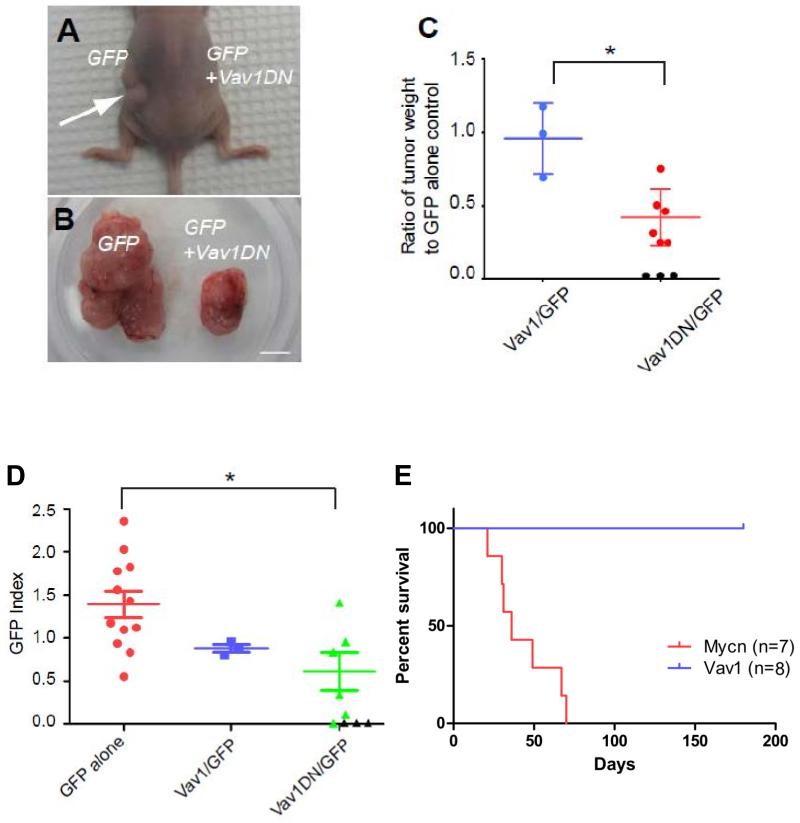
To address whether Vav1 was required for tumor growth in a mouse SHH medulloblastoma model, we purified tumor cells from spontaneously occurring Vav1 hypomethylated/overexpressing medulloblastomas arising in Ptch1+/− Cdkn2c−/− mice (Figures 1,3C, Supplementary Figure S2C) and infected them with retroviruses carrying GFP alone, wild-type Vav1/GFP or a dominant-negative form of Vav1 (Vav1DN/GFP 23, 24), an isolated amino terminal fragment (residues 1-186), which acts in trans to inhibit the GEF function of native Vav1. Cells from each infected pool were transplanted in the flanks of recipient nude mice and tumor formation assessed.
Approximately two months post-implantation, tumors appeared on the flanks of all mice transplanted with tumor cells marked with GFP only or with Vav1/GFP viruses. These tumors harbored similar weights (Figure 5C). In contrast, tumors that developed after the transplant of tumor cells infected with Vav1DN-carrying viruses were smaller than those derived from GFP only-infected cells and only 6 out of 9 transplants induced secondary tumors (Figure 5A-C). Thus, enforced expression of a Vav1 dominant-negative mutant inhibits tumor maintenance in our model. Ectopic over-expression of wild-type Vav1 did not impact, suggesting interference with the endogenous Vav1 expression associated with its hypo-methylation is important for the inhibition of tumor growth.
Figure 5. Inhibition of SHH tumor maintenance by a dominant-negative form of Vav1.
A-D. In each experiment Ptch1+/− Cdkn2c−/−mouse medulloblastoma cells infected with a GFP only virus were transplanted into the left side of recipient nude mice, whereas tumor cells infected with Vav1 or Vav1DN viruses were transplanted on the right side. Parallel FACS analysis revealed that approximately 20-40% tumor cells were infected. A. A representative mouse transplanted with tumor cells infected with GFP- (left) or Vav1DN/GFP- (right) carrying retroviruses. A significantly larger tumor appeared on the left side from GFP alone-infected cells (white arrow). B. Surgically removed tumors from a mouse in which tumors developed on both sides. Scale bar, 5mm. C. Quantification of the weight ratio between Vav1- or Vav1DN-expressing tumors compared to control GFP alone-expressing tumors. *p<0.05. Black circles represent transplanted mice that did not develop tumors. D. Contribution of either Vav1-or Vav1DN-expressing cells to tumor maintenance. The GFP index is defined as the ratio of the percentage of GFP-positive tumor cells in the secondary tumors compared to the percentage of GFP-positive infected tumor cells before transplant. Note that 3 recipient mice did not develop secondary tumors from Vav1DN-infected tumor cells (black triangles). *p<0.05. E. Kaplan-Meier survival curve of mice following stereotactic cortical transplantation of GNPs, infected with Vav1/GFP (n=8) or Mycn /GFP GNPs (n=7) retroviruses.
To examine the contribution of retrovirally-infected cells to tumor development, we analyzed by FACS the percentage of infected GFP-positive tumor cells before transplant and in each secondary tumor. On average, we found a ~40% reduction of Vav1DN-infected GFP+ cells in secondary tumors compared to the initially infected pool (Figure 5D). In contrast, when tumor cells were infected with GFP-only retroviruses, the percentage of GFP positive cells increased by ~ 20% in the secondary tumors (Figure 5D). Consistent with the tumor weight, enforced wild-type Vav1 expression did not affect the percentage of GFP+ cells in the secondary flank tumors (Figure 5D).
In contrast, enforced wild–type Vav1 expression did not appear to contribute significantly to medulloblastoma initiation in the Ptch1+/− Cdkn2c−/− model. Cerebellar granule neuron progenitors (GNPs), the cell type of origin of MBSHH, were enriched from the cerebella of postnatal (P) day 7, Ptch1+/− Cdkn2c−/− pups, then infected with retroviruses carrying GFP-alone or co-expressing wild-type Vav1. Mycn-carrying retroviruses were used as a positive control. 25 Average infection efficiencies were comparable (30.86% for GFP, 35.34% for Vav1/GFP and 40.28% for Mycn/GFP). Tumors failed to develop more than 6 months after stereotactic transplantation of GNPs, infected with GFP only containing virus (n=7) or co-expressing wild-type Vav1 (n=8), into the cerebral cortex. GNPs infected with a Mycn containing retrovirus induced medulloblastomas within 2 months as previously shown (n=7/725) (Figure 5E). In summary, dominant-negative Vav1 suppressed medulloblastoma maintenance in our Ptch1+/− Cdkn2c−/− mouse model.
Vav1 activity regulates granule neuron precursor (GNP) germinal zone exit and migration initiation in normal cerebellar development
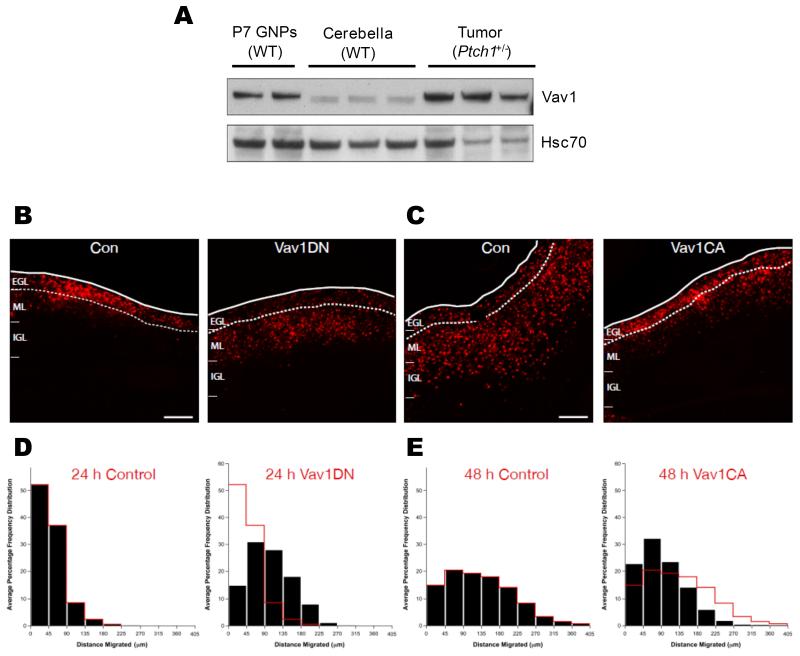
We next examined whether Vav1 activity had any effect on the development of GNPs and postmitotic cerebellar granule neurons (CGNs). GNPs are located near the cerebellar surface in a germinal zone (GZ) called the external germinal layer (EGL), whereas after cell cycle exit and neuronal differentiation, CGNs exit their GZ by migrating away from the EGL to cross the molecular layer (ML) and eventually reside within the internal granule layer (IGL).26 GNP or CGN position with the developing cerebellar cortex thus represent an excellent model to examine neuronal progenitor maturation, as cells of the CGN lineage occupy stereotyped positions in the developing cerebellar cortex dependent upon their differentiation status.26 We first found Vav1 was expressed at elevated levels in developing (P7) GNPs compared to cerebella from wild-type mice (Figure 6A). Then, we used electroporation of early post-natal mouse cerebellum followed by ex vivo culture of organotypic cerebellar slices as an assay to examine GNP germinal zone (GZ) exit and migration initiation.27 Expression constructs for Vav1DN and the fluorescent nuclear reporter H2B-mCherry were co-electroporated into the cerebellar cortices of P7 mice and cerebellar slices were cultured ex vivo. While control GNPs remained within the EGL after 24 hours, cells expressing elevated Vav1DN entered the ML and IGL (Figure 6B, D), suggesting that inhibition of Vav1 activity is sufficient to induce precocious GZ exit.
Figure 6. Vav1 regulates GNP germinal zone exit and migration initiation in cerebellar development.
A. Vav1 is expressed in purified P7 GNPs from wild-type (WT) mice and in Ptch1+/− tumors, relative to the normal cerebellum from one-month old mice. Western blot shows Vav1 and control protein (Hsc70) expression in independent samples. B E. P7 EGL was co-electroporated with the indicated expression constructs and H2B-mCherry. After 24 (B, D) or 48 (C, E) hours of ex vivo culture, the migration distance of H2B-labeled CGN from the pial layer (outer dashed line) was analyzed. EGL boundary is between zero and 45 μm (first bin in histograms); red overlay indicates the average migration distribution of control cells at each time point. B. Most control cells remain within the EGL (dashed lines) at 24 h, while Vav1DN over-expressing cells have prematurely entered the ML and IGL. D. Migration distance versus frequency histogram of control neurons expressing only H2B-mCherry (n=967 cells) or Vav1DN over-expressing (n=2119 cells) CGNs. Control vs. Vav1DN, p=1.08×10−67 (Chi2 test). C. While control cells expressing only H2B-mCherry entered the ML and IGL after 48 h, Vav1CA over-expressing cells remained in the EGL. E. Migration distance vs. frequency histogram of control (n=2252 cells) or Vav1CA over-expressing (n=2570 cells) CGNs. Control vs. Vav1CA, p= 0.0008 (Chi2 test). Scale bar, 100 μm.
Given that Vav1 inhibition appeared to regulate GNP GZ exit and migration initiation, we next evaluated whether Vav1 activity could regulate GNP’s GZ exit using a gain-of-function approach. Expression vectors for a constitutively active Vav1 lacking the auto-inhibitory amino terminal domain (Vav1CA28 ) were electroporated into P7 EGL. After 2 days of ex vivo culture, control CGNs initiated migration correctly and had entered the molecular layer and IGL while Vav1CA expressing CGNs remained within the EGL (Figure 6C, E), suggesting that activation of Vav-1 activity blocks GZ exit. Finally, we examined the morphology of Vav1DN and Vav1CA over expressing cells via time-lapse microscopy of in vitro CGN cultures. Whilst control neurons and Vav1DN expressing neurons elaborated the long neurites of mature CGNs, Vav1CA expressing retained the short processes which are the characteristic of immature CGNs (See Supplementary Movies 1-3).
Together, these data show Vav1 activity blocks GNP exit from the developing cerebellar germinal zone and indicate a role in normal cerebellar development.
Discussion
Our investigations of DNA methylation events in human MBSHH and four independent SHH mouse medulloblastoma models have identified VAV1 hypo-methylation as a conserved and defining MBSHH event which affects around 70% of human tumors, more common than any mutated gene (PTCH1 (~45%) and TERT (~30%) are most frequent),6, 29, 30 and which describes MBSHH of all key clinical, pathological and molecular demographics. Its complete regional de-methylation (Supplementary Figure S3), coupled with an absence of significant copy number alterations or mutations in large cohorts [data from11, 22] indicates hypomethylation is its major mechanism of alteration. VAV1 methylation status is intimately related to its expression; VAV1 expression is controlled by methylation in human cell lines and its hypo-methylation, in both mouse and human tumors, is significantly associated with increased expression. Consistent with this, the region of uniform hypo-methylation we have characterized overlaps with the minimal region shown in mutagenesis studies to be sufficient to drive VAV1 expression.31
Modelling VAV1 epigenetic silencing in mouse and human tumor models validated a role for VAV1 expression in SHH-associated tumor growth and maintenance. Introduction of dominant-negative Vav1 into tumor cells from established hypo-methylated Ptch1+/− Cdkn2c−/− mouse tumors significantly reduced tumor re-establishment and growth. In contrast, enforced expression of wild-type Vav1 did not further increase tumorigenic potential, indicating endogenous Vav1 levels in hypo-methylated cells are sufficient to promote tumorigenesis. Further, the impact of VAV1 silencing on proliferation and cell cycle arrest in VAV1 hypo-methylated human cells is consistent with VAV1-dependent effects in other cancer cell types, 24, 32 while the identification of Vav1 mutation as a SHH-cooperating event in a murine insertional mutagenesis screen 33 provides further genetic evidence to substantiate its role in MBSHH. Any role for VAV1 in tumor initiation is less clear; enforced Vav1 expression in engrafted Ptch1+/− Cdkn2c−/− GNPs did not cause tumor development, in contrast to Mycn controls. 25 However, given Ptch1’s established role in initiation, 34 these results do not preclude Vav1 involvement. Future strategies to examine any interaction between Ptch1 and Vav1 in spontaneous SHH-tumor formation, which account for endogenous Vav1 expression35 should prove informative.
The role of VAV1 in the majority of MBSHH tumors, together with the inhibition of tumor growth following its silencing, identify and validate VAV1 and its dependent pathways as important potential therapeutic targets. In particular, its homogeneous mechanism of activation and SHH-independence highlight potential applications for alternative or second-line / combination therapies with SHH pathway inhibitors. Consistent with its protumorigenic role, VAV1 hypo-methylation was associated with a poor outcome in clinical trial-derived MBSHH patients,15, 21 supporting its utility as both a prognostic and predictive biomarker for use in stratified therapies. Multivariate survival analysis, validation in alternative cohorts, and investigations in all disease demographic groups, are now essential to confirm reproducibility of these initial findings prior to any clinical application.
Vav1 is expressed in GNPs [the proposed cells of origin for MBSHH36] during early cerebellar development. Our data show its activity blocks GNP exit from the developing cerebellar germinal zone, suggesting a mechanism by which disruption of these processes by Vav1 hypo-methylation and expression may contribute to medulloblastoma tumorigenesis. These findings further emphasize the intimate links between cerebellar development and medulloblastoma, and evidence that temporally/spatially restricted pathways in normal cerebellar development become constitutively activated during tumorigenesis.36 Coupled with the established roles of SHH pathway activation in GNP proliferation 34 and MBSHH, our data also suggest common functions for Vav1 and SHH in both processes, and provide a basis for the investigation of any functional interaction. Interestingly, Vav3 plays roles in the migration of GNPs from the EGL to the IGL, and in the survival of GCNs in the IGL37, implicating the involvement of multiple Vav family members in cerebellar development. Consideration of developmental toxicities will thus be essential in developing targeted therapies against VAV1, however that Vav1−/− mice are viable and healthy apart from impaired T-cell development 38 is encouraging.
How might VAV1 exert its function? VAV1 expression is normally confined to the hematopoietic system, where its best characterized function is as a GDP/GTP nucleotide exchange factor for the Rho/Rac family of GTPases. However, roles in multiple diverse processes including activation of the JNK, ERK, Ras, NF-kB, and NFAT pathways, cytoskeleton organization, migration and TP53-mediated apoptosis have also been described.32, 39, 40 Direct small molecule VAV1 inhibitors are not widely reported, however strategies against many of its candidate downstream pathways are in development.41 A detailed exploration of the pathways impacted by VAV1 in MBSHH and normal cerebellar development will therefore now be essential to understand their mechanistic basis and to enable therapeutic targeting.
Finally, available evidence suggests an emerging widespread relevance for VAV1 in tumor development. In addition to its expression in hematopoiesis and hematological malignancies,42 VAV1 expression has been reported in solid tumors including neuroblastomas32 and pancreatic ductal adenocarcinomas, where expression was associated with a poor survival outcome, and with demethylation of the VAV1 promoter in tumor cell lines. 24 In preliminary investigations of other pediatric brain tumor types, VAV1 hypo-methylation at the CpG residues involved in MBSHH is also a feature of a proportion of posterior fossa ependymomas, high-grade gliomas and CNS-PNETs, as well as occasional non-MBSHH medulloblastomas (Supplementary Figure S4; Figure 2), suggesting potential roles in their development for further investigation.
Materials and Methods
Patient samples, DNA methylation profiling and subgroup status
DNA methylation profiling and consensus clustering of molecular subgroups was performed as previously described15 on 230 primary medulloblastomas (of which 216 were confidently assigned to a subgroup), alongside 21 normal non-neoplastic cerebella (representing fetal, infant and adult samples43), using the Illumina GoldenGate Cancer Panel I microarray. 44 The DNA methylation status of 1505 CpG sites (807 genes) was represented as a β-score from zero (unmethylated) to one (methylated). Subgroup status was additionally assessed using mRNA signature,45 immunohistochemistry (IHC) 46 and mutational (CTNNB1) 45 biomarker assays.
5-azaCdR treatment of human cell lines
Four human medulloblastoma cell lines (D283Med, MEB-Med8a, D425Med and DAOY) were grown in the presence or absence of the demethylating agent 5-azaCdR as previously described.12 Cell lines were validated by confirmation of published karyotypes. 47
Mouse tumor and tissue samples
Mouse medulloblastomas from Smo/Smo mice 18, Ptch1+/− Cdkn2c−/− mice 16, Cdkn2c−/− p53FL/−, Nestin-Cre+ mice irradiated at P7 16 and Sufu+/− p53 −/− mice 17 (all n=3) were assessed alongside control cerebella from strain-matched non-transgenic mice.
Percoll-enriched GNPs from post-natal P7 cerebellum and GNP-like tumor cells from spontaneously-arisen medulloblastomas were purified from Ptch1+/− Cdkn2c−/− mice. 16 P7 cerebellar GNPs were also purified from wild-type mice. CD-1 nu/nu mice (Charles River Laboratories, Wilmington, MA) were used for cortical or flank transplantation. Wild type C57BL/6 mice (Charles River Laboratories, Wilmington, MA) were used for ex vivo cerebellar slice assays. All animal experiments were approved by and conducted in accordance with St. Jude Children’s Research Hospital Animal Care and Use Committee guidelines.
Nucleic acid extraction
DNA and RNA were extracted from frozen tumors, tissues and cell lines using Trizol (Invitrogen, Carlsbad, CA, USA), and DNA from FFPE samples using a Qiagen DNeasy kit (Qiagen, Valencia, CA, USA), according to manufacturer’s instructions.
Identification of differentially methylated CpG sites
CpG sites showing significant differences between the SHH and other medulloblastoma subgroups (combined) were identified (Mann-Whitney U test, retaining probes significant (p<0.05) after Benjamini-Hochberg false discovery rate (FDR) correction). All CpG sites identified showed an absolute change in average (mean) β≥0.34 between these groups. Differences in CpG methylation between the SHH subgroup and normal cerebella were assessed equivalently.
Analysis of DNA methylation status by bisulfite sequencing
Bisulfite DNA treatment was carried out using the Epitect bisulfite kit (Qiagen Ltd. Valencia, CA, USA), and PCR using standard reaction conditions (Supplementary Table S2). Purified products were directly sequenced with a CEQ DTCS kit (Beckman Coulter, High Wycombe, UK) and analyzed on a CEQ 2000XL DNA analysis system (Beckman Coulter). The methylation status at each CpG residue was determined by assessment of relative peak intensities, as previously described.12 The DNA methylation status (methylation score) of orthologous regions in the mouse and human genomes (mean, based on all sequenced CpG sites) was subsequently calculated for each region of interest.
cDNA synthesis and quantitative RT-PCR
cDNA was synthesized from total RNA using a Biorad iScript™ cDNA synthesis kit (Biorad, Irvine, CA, USA) according to manufacturer’s instructions. Measurements of VAV1 transcript levels in mouse and human samples were determined using SYBR Green on the ABI PRISM 7900HT Detection System (Applied Biosystems, Foster City, CA, USA), using the comparative Ct relative quantification method (Supplementary Table S2).
RNA interference
Cells were seeded in 6- or 96-well plates and allowed to adhere for 24 hours, then transfected with 40nM small interfering RNA (siRNA) duplexes to VAV1 (sense strands: duplex1 CGUCGAGGUCAAGCACAUUdTdT;48 duplex2 GCCAUCAGCAUUAAAUAUAdTdT, duplex3 UCAAAUACAAGGAGAGGUUdTdT (DharmaconGE, Layfayette, CO, USA) or negative control siRNA duplexes (sense UUCUCCGAACGUGUCACGUdTdT (Qiagen)) using Lipofectamine™ RNAiMAX (Invitrogen), following the manufacturer’s instructions.
Growth assays
Cells were seeded at 1 ×103 in 96-well plates and allowed to adhere for 24 hours. The number of viable cells at day 0 (prior to siRNA addition) and days 1-5 (after addition) was quantified using an XTT cell proliferation assay kit (Roche, Hertfordshire, UK) according to the manufacturer’s instructions. Experiments were performed in quadruplicate.
Flow cytometry
Adherent cells were harvested with trypsin 36 and 48 hours after siRNA transfection, fixed in ice-cold 70% ethanol/30% phosphate buffered saline (PBS) solution, then resuspended in 40 mg/ml propidium iodide/0.1 mg/ml RNAse A (Sigma-Aldrich, Gillingham, UK) at 37°C for 30 minutes, and assessed using a FACScan (Becton Dickinson Oxford, UK) and Modfit software (Verity software, Maine, USA). Experiments were performed in quadruplicate.
Retrovirus production
Plasmids used for retrovirus production were generated using the mouse stem cell virus LTR (MSCV) backbone expressing the green fluorescent protein (GFP) downstream of the internal ribosomal entry site (IRES) pMSCV-IRES-GFP. The full-length Vav1 cDNA (amino acids 1-845 (pMSCV-Vav1-IRES-GFP)) and the N-terminal region (amino acids 1-186 (pMSCV-Vav1DN-IRES-GFP)) were inserted downstream of the MSCV LTR in the pMSCV-IRES-GFP plasmid. The pMSCV-Mycn-IRES-GFP construct and generation of retroviral stocks were previously described. 25
Mouse cerebellar and tumor cell purification, virus infection and orthotopic transplantations
GNP-enriched mouse cerebellar and medulloblastoma cells were purified by percoll gradient. 25, 49 Purified cells were plated on Matrigel-coated dishes and infected with retroviruses (GFP alone, Vav1-IRES-GFP, Vav1DN-IRES-GFP and Mycn-IRES-GFP). 2 days after infection, cells were harvested, re-suspended in Matrigel (BD Biosciences, San Jose, CA, USA) and transplanted into CD-1 nu/nu mice (2.0 × 106 cells/injection) as previously described. 25 GNPs infected with GFP alone, Mycn-IRES-GFP or Vav1-IRES-GFP retroviruses were transplanted into the cortices of recipient mice. Virally-infected tumor cells were transplanted in the flank of naïve recipient animals. Cortically-transplanted animals were sacrificed, when neurological symptoms were confirmed. Flank injections of virally-marked tumor cells were performed on both sides of each mouse; in the left side, 2.0×106 tumor cells infected with GFP and on the other side, the same number of tumor cells infected with either Vav1/GFP or Vav1DN/GFP retroviruses. In parallel, a subset of infected cells was analyzed by FACS to calculate the efficiency of infection reflected by the percentage of GFP+ cells. Transplanted mouse recipients were monitored daily; tumors were harvested and weighed, and tumor cells purified by Percoll gradient and analyzed by FACS for GFP expression as previously described.3
Protein lysate preparation and immunoblotting
Whole-cell extracts were prepared from human cell lines, wild-type 1 month-old whole cerebella, Percoll-purified GNPs from P7 cerebella, and mouse Ptch+/− medulloblastomas. 25-35 mg protein/sample was separated by gel electrophoresis and immunoblotted with defined antibodies as previously described.12 Primary antibodies were VAV1 (C14; Santa Cruz, Santa Cruz, CA, USA), Actin (Sigma-Aldrich) and HSC70 (K19; Santa Cruz). Appropriate secondary antibodies with horse radish peroxidise conjugates (Dakocymation Ltd, Glostrup, Denmark) were bound to the primary antibodies, followed by incubation with enhanced chemiluminescence (ECL or Dura) substrate (Pierce, Rockford, IL, USA), visualization by autoradiography, and densitometry using a Fuji-Las (Fujifilm Corporation, Tokyo, Japan) camera and the AIDA image analyzer program (Raytek, Sheffield, UK).
Ex-vivo cerebellar electroporation, organotypic slice culture and imaging
Plasmids for ex-vivo cerebellar slice experiments were generated using the pCIG2 vector, which contains a CAG promoter for high-level expression in CGNs and GNPs. The dominant-negative form of Vav1 (Vav1DN), the constitutively active form of Vav1 (Vav1CA) or H2B-mCherry cDNAs were inserted downstream of the CAG promoter in the pCIG2 expression vector.
Ex-vivo cerebellar electroporation and organotypic slice culture was performed on P7 mouse cerebella as described previously. 27 Migration distance measurements of fixed slices were performed by marking the centre of individual cell nuclei marked by mCherry-H2B using the pen function of SlideBook (Intelligent Imaging Innovations, Denver, CO, USA), exporting the cellular coordinates and calculating the distance between the center of each cell and the cerebellar surface using a custom written algorithm in Igor Pro (Wavemetrics Inc, Lake Oswego, OR, USA). All measurements were analyzed statistically using Microsoft Excel and graphed using Kaleidagraph v4.03 (Synergy software systems, Reading, PA, USA).
Statistical analysis
All array-based bioinformatic and statistical analyses were performed using R (v2.15.0)50 and the stated statistical test. Survival analyses were performed using event-free survival (EFS) times. 15
Supplementary Material
Supplementary Table 1. Selection of differentially methylated human sequences and their mouse orthologs for investigation. Genes selected met the following criteria: (i) the human and mouse genomes harbored orthologous regions (Supplementary Figure S1A), (ii) methylation changes in human tumors affected multiple CpG sites in the region surrounding the array-associated probe site, and (iii) the average methylation levels at these additional sites were strongly correlated with the array site in human samples (r2 >0.70; Supplementary Figure S1B). The probe sequence used to assay the human CpG site of interest on the Illumina GoldenGate Cancer Panel I methylation array was extended by 50bp proximally and distally using the reference sequences provided by Illumina. This extended sequence was then compared to the mouse assembled genomes using the Blastn algorithm (no filters) (www.ncbi.nlm.nih.gov/Blast.cgi). Orthologous regions of the mouse genome which mapped to the expected gene were identified for further analysis and suitable primers were designed to assess similar sized homologous regions in mouse and human DNA. The positions of the bisulfite sequencing PCR products relative to the transcriptional start site and the number of CpGs analyzed for both are shown. *There is no equivalent mouse transcript to the human transcript used for MEST.
Supplementary Table 2. Primers used for bisulfite and real time PCR. For bisulfite PCR, primers were designed to amplify regions (200-500bp) surrounding the CpG site assessed on the Illumina GoldenGate Cancer Panel I microarray or its orthologous position in the mouse genome. Primers were designed using the program Methprimer (www.urogene.org./methprimer). The annealing temperature for all primers was 55°C, cycling at this temperature was preceded by 5 cycles of ‘touchdown PCR’ from 60°C in increments of −1°C to increase the specificity of the products. The human TRIM29 PCR reactions required the addition of Betaine (Sigma-Aldrich, Gillingham, UK) to the PCR mix (final concentration 1M). For quantitative real time RT-PCR, primers were designed using Primer Express software (Applied Biosystems) to ensure that products overlapped intron/exon boundaries. Dissociation curves confirmed PCR product specificity. Expression was assessed relative to expression of a control gene (28S rRNA or TBP), using the comparative Ct method for relative quantification. Dilution series were carried out to confirm relative expression was consistent over a range of input DNA concentrations.
Supplementary Figure 1. Identification of DNA methylation events associated with MBSHH. A. Clustering of DNA methylation patterns in 230 primary human medulloblastomas identifies four consensus subgroups which are highly significantly related to established SHH and WNT subgroup biomarkers (‘p’ values are shown). The 216 tumors which could be confidently assigned to a subgroup are shown 15. MBSHH, red; Group 3, yellow; WNT, blue; Group 4, green. MBSHH biomarker (SHH mRNA signature / GAB-1/YAP1 IHC) positivity, red. WNT subgroup biomarker (WNT mRNA signature / β-catenin IHC) positivity, blue. Data not available, grey. Magnitudes of the four defining metagenes (V1 to V4; highly expressed metagenes, red; lowly expressed, blue) and subgroup-specific DNA methylation events (unmethylated, green; fully-methylated, red) are shown. B. Principal component analysis loadings plot shows class designations for WNT (blue), MBSHH (red), Group 3 (yellow) and Group 4 (green) tumors and normal cerebella (pink; n=21). Covariance spheroids with a 95% confidence interval have been plotted to demonstrate group memberships.
Supplementary Figure 2. Identification and assessment of DNA methylation alterations in orthologous regions of the mouse and human genomes. A. Examples of regions of homology between mouse and human genomes. Aligned sequences are shown for the regions surrounding TRIM29 (probe P833), CCKAR (probe E79), VAV1 (probe E9) and MEST (probe P4). The probe site interrogated by the Illumina array is boxed (dashed line); other conserved CpG sites are boxed (solid line) and non- conserved CpG sites are underlined. H, human; M, mouse. B. Correlation between the array-assessed β-value and the average methylation score determined by bisulfite sequencing for all CpG sites in the surrounding region (Table S1) for nine genomic regions, in six randomly-selected cerebella and six randomly-selected MBSHH tumors. (RASSF1A; r2, 0.92). C. Methylation levels at individual CpG sites in the assessed Vav1 region in mouse tumors and strain-specific cerebella following analysis by bisulfite sequencing, shown alongside the overall average methylation score for the region. CpG sites are labelled by their position relative to the translational start site of the Vav1 gene. Black circles, ≥80% methylation; three-quarter black circles, ≥60%; half-black circles, ≥40%; quarter-black circles, ≥20% methylation; white circles, <20% methylation.
Supplementary Figure 3. VAV1 hypo-methylation in human medulloblastomas: Regional CpG patterns, molecular and clinical significance. A. Representative examples of bisulfite sequencing on the reverse strand of the region surrounding VAV1 (probe E9). Assayed CpG sites (labelled by their position relative to the translational start site of the Vav1 gene) are underlined and calculated methylated peak height (G-green) relative to total peak height is given for each of the 10 CpG sites analyzed. The methylation score is the average of these values and is shown together with the β value for the CpG site (boxed) assessed on the Illumina array. B. Methylation levels at individual CpG sites in the assessed VAV1 region in representative human tumors (MBSHH (n=25) and non-MBSHH (n=8) tumors) and cerebella (n=7), following analysis by bisulfite sequencing and shown alongside the methylation score. Black circles, ≥80% methylation; three-quarter black circles, ≥60%; half-black circles, ≥40%; quarter-black circles, ≥20% methylation; white circles, <20% methylation. The CpG site assessed by probe E9 is shown, and the cut-off used to discriminate VAV1 hypo-methylated tumors (0.53; Figure 3) is marked. C. Box plots showing methylation levels (β-values) for VAV1 (probe P317) in the MBSHH subgroup (n=50) compared to non- MBSHH (n=166) tumors and cerebella (CB; n=21). The mean cerebellar methylation (-3SD; cut-off marked at 0.30) is shown to distinguish hypo-methylated tumors. Non-MBSHH tumors are shown colored by subgroup (Group 3, yellow; WNT, blue; Group 4, green).
Supplementary Figure 4. VAV1 methylation patterns in multiple pediatric brain tumor types. Box plots show methylation patterns for probes VAV1 E9 (A) and VAV1 P317 (B) for posterior fossa ependymomas (dark green, n=45); supratentorial ependymomas (light green, n=16); pediatric high-grade gliomas (grey, pHGG, n=15) and CNS-PNETs (black, n=30). For comparison, methylation status of non-neoplastic cerebellar (pink, n=21) and cerebral (dark pink, n=16) cohorts are shown (data for the 16 cerebra are previously described 43). Horizontal cut-off lines, corresponding to the mean -3SD cerebellar methylation (probe E9, β=0.53 and probe P317, β=0.30) are shown for comparison with Figures 2A and S3C. ‘p’ values from Kruskal-Wallis tests are shown.
Supplementary movies. Time lapse images documenting the morphology of CGNs following electroporation with equal amounts of vector only (Movie 1); Vav1DN (Movie 2) and Vav1CA (Movie 3).
Acknowledgements
This work was supported by grants from The Brain Tumour Charity (Grants 16/46 (S.C.C. and S.B) and 16/92 (S.C.C., S.B., D.W. and J.L.)), NIH CA-096832, CA-02165-29 (M.F.R.), the American Lebanese Syrian Associated Charities of St. Jude Children’s Research Hospital (M.F.R., D.K.), the Mochida Foundation (D.K.), the Anderson fellowship (D.K.), LoveOliver (S.C.C. and D.W.) and Cancer Research UK (Grant C8464/A13457; S.C.C, S.B. and D.W.). Cell lines D425 Med and MEB-Med8A were gifts from Dr. D. Bigner (Duke University, Durham, USA) and Prof. T. Pietsch (University of Bonn Medical Centre, Bonn, Germany), respectively. Four normal cerebellar DNAs were gifts from Dr. M. Fruhwald (University of Munster, Munster, Germany). Vav1CA cDNA was a gift from Dr. S. Katzav (Hebrew University, Jerusalem, Israel). DAOY and D283 Med cell lines were from the ATCC (Manassas, USA). Approval from the Newcastle and North Tyneside Research Ethics Committee (study reference 07/Q0905/71) was obtained for the collection, storage, and biological study of all material described. Medulloblastomas investigated in this study include samples provided by the UK Children’s Cancer and Leukaemia Group (CCLG) as part of CCLG-approved biological study BS-2007-04.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Pei Y, Moore CE, Wang J, Tewari AK, Eroshkin A, Cho YJ, et al. An animal model of MYC-driven medulloblastoma. Cancer cell. 2012;21:155–67. doi: 10.1016/j.ccr.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taylor MD, Northcott PA, Korshunov A, Remke M, Cho YJ, Clifford SC, et al. Molecular subgroups of medulloblastoma: the current consensus. Acta neuropathologica. 2012;123:465–72. doi: 10.1007/s00401-011-0922-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawauchi D, Robinson G, Uziel T, Gibson P, Rehg J, Gao C, et al. A mouse model of the most aggressive subgroup of human medulloblastoma. Cancer cell. 2012;21:168–80. doi: 10.1016/j.ccr.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gibson P, Tong Y, Robinson G, Thompson MC, Currle DS, Eden C, et al. Subtypes of medulloblastoma have distinct developmental origins. Nature. 2010;468:1095–9. doi: 10.1038/nature09587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pizer BL, Clifford SC. The potential impact of tumour biology on improved clinical practice for medulloblastoma: progress towards biologically driven clinical trials. British journal of neurosurgery. 2009;23:364–75. doi: 10.1080/02688690903121807. [DOI] [PubMed] [Google Scholar]
- 6.Kool M, Jones DT, Jager N, Northcott PA, Pugh TJ, Hovestadt V, et al. Genome Sequencing of SHH Medulloblastoma Predicts Genotype-Related Response to Smoothened Inhibition. Cancer cell. 2014;25:393–405. doi: 10.1016/j.ccr.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ng JM, Curran T. The Hedgehog’s tale: developing strategies for targeting cancer. Nature reviews. 2011;11:493–501. doi: 10.1038/nrc3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rudin CM, Hann CL, Laterra J, Yauch RL, Callahan CA, Fu L, et al. Treatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449. The New England journal of medicine. 2009;361:1173–8. doi: 10.1056/NEJMoa0902903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kimura H, Ng JM, Curran T. Transient inhibition of the Hedgehog pathway in young mice causes permanent defects in bone structure. Cancer cell. 2008;13:249–60. doi: 10.1016/j.ccr.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 10.Yauch RL, Dijkgraaf GJ, Alicke B, Januario T, Ahn CP, Holcomb T, et al. Smoothened mutation confers resistance to a Hedgehog pathway inhibitor in medulloblastoma. Science (New York, NY. 2009;326:572–4. doi: 10.1126/science.1179386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Northcott PA, Jones DT, Kool M, Robinson GW, Gilbertson RJ, Cho YJ, et al. Medulloblastomics: the end of the beginning. Nature reviews. 2012;12:818–34. doi: 10.1038/nrc3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderton JA, Lindsey JC, Lusher ME, Gilbertson RJ, Bailey S, Ellison DW, et al. Global analysis of the medulloblastoma epigenome identifies disease-subgroup-specific inactivation of COL1A2. Neuro Oncol. 2008;10:981–94. doi: 10.1215/15228517-2008-048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Northcott PA, Nakahara Y, Wu X, Feuk L, Ellison DW, Croul S, et al. Multiple recurrent genetic events converge on control of histone lysine methylation in medulloblastoma. Nat Genet. 2009;41:465–72. doi: 10.1038/ng.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hovestadt V, Remke M, Kool M, Pietsch T, Northcott PA, Fischer R, et al. Robust molecular subgrouping and copy-number profiling of medulloblastoma from small amounts of archival tumour material using high-density DNA methylation arrays. Acta neuropathologica. 2013;125:913–6. doi: 10.1007/s00401-013-1126-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwalbe EC, Williamson D, Lindsey JC, Hamilton D, Ryan SL, Megahed H, et al. DNA methylation profiling of medulloblastoma allows robust subclassification and improved outcome prediction using formalin-fixed biopsies. Acta neuropathologica. 2013;125:359–71. doi: 10.1007/s00401-012-1077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uziel T, Zindy F, Xie S, Lee Y, Forget A, Magdaleno S, et al. The tumor suppressors Ink4c and p53 collaborate independently with Patched to suppress medulloblastoma formation. Genes & development. 2005;19:2656–67. doi: 10.1101/gad.1368605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee Y, Kawagoe R, Sasai K, Li Y, Russell HR, Curran T, et al. Loss of suppressor-of-fused function promotes tumorigenesis. Oncogene. 2007;26:6442–7. doi: 10.1038/sj.onc.1210467. [DOI] [PubMed] [Google Scholar]
- 18.Hatton BA, Villavicencio EH, Tsuchiya KD, Pritchard JI, Ditzler S, Pullar B, et al. The Smo/Smo model: hedgehog-induced medulloblastoma with 90% incidence and leptomeningeal spread. Cancer Res. 2008;68:1768–76. doi: 10.1158/0008-5472.CAN-07-5092. [DOI] [PubMed] [Google Scholar]
- 19.Goodrich LV, Milenkovic L, Higgins KM, Scott MP. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science (New York, NY. 1997;277:1109–13. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- 20.Lusher ME, Lindsey JC, Latif F, Pearson AD, Ellison DW, Clifford SC. Biallelic epigenetic inactivation of the RASSF1A tumor suppressor gene in medulloblastoma development. Cancer Res. 2002;62:5906–11. [PubMed] [Google Scholar]
- 21.Taylor RE, Bailey CC, Robinson K, Weston CL, Ellison D, Ironside J, et al. Results of a randomized study of preradiation chemotherapy versus radiotherapy alone for nonmetastatic medulloblastoma: The International Society of Paediatric Oncology/United Kingdom Children’s Cancer Study Group PNET-3 Study. J Clin Oncol. 2003;21:1581–91. doi: 10.1200/JCO.2003.05.116. [DOI] [PubMed] [Google Scholar]
- 22.Northcott PA, Shih DJ, Peacock J, Garzia L, Morrissy AS, Zichner T, et al. Subgroup-specific structural variation across 1,000 medulloblastoma genomes. Nature. 2012;488:49–56. doi: 10.1038/nature11327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abe K, Whitehead IP, O’Bryan JP, Der CJ. Involvement of NH(2)-terminal sequences in the negative regulation of Vav signaling and transforming activity. J Biol Chem. 1999;274:30410–8. doi: 10.1074/jbc.274.43.30410. [DOI] [PubMed] [Google Scholar]
- 24.Fernandez-Zapico ME, Gonzalez-Paz NC, Weiss E, Savoy DN, Molina JR, Fonseca R, et al. Ectopic expression of VAV1 reveals an unexpected role in pancreatic cancer tumorigenesis. Cancer cell. 2005;7:39–49. doi: 10.1016/j.ccr.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 25.Zindy F, Uziel T, Ayrault O, Calabrese C, Valentine M, Rehg JE, et al. Genetic alterations in mouse medulloblastomas and generation of tumors de novo from primary cerebellar granule neuron precursors. Cancer Res. 2007;67:2676–84. doi: 10.1158/0008-5472.CAN-06-3418. [DOI] [PubMed] [Google Scholar]
- 26.Hatten ME, Roussel MF. Development and cancer of the cerebellum. Trends in neurosciences. 2011;34:134–42. doi: 10.1016/j.tins.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Famulski JK, Trivedi N, Howell D, Yang Y, Tong Y, Gilbertson R, et al. Siah regulation of Pard3A controls neuronal cell adhesion during germinal zone exit. Science (New York, NY. 2010;330:1834–8. doi: 10.1126/science.1198480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katzav S, Cleveland JL, Heslop HE, Pulido D. Loss of the amino-terminal helix-loop-helix domain of the vav proto-oncogene activates its transforming potential. Molecular and cellular biology. 1991;11:1912–20. doi: 10.1128/mcb.11.4.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindsey JC, Schwalbe EC, Potluri S, Bailey S, Williamson D, Clifford SC. TERT promoter mutation and aberrant hypermethylation are associated with elevated expression in medulloblastoma and characterise the majority of non-infant SHH subgroup tumours. Acta neuropathologica. 2014;127:307–9. doi: 10.1007/s00401-013-1225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Remke M, Ramaswamy V, Peacock J, Shih DJ, Koelsche C, Northcott PA, et al. TERT promoter mutations are highly recurrent in SHH subgroup medulloblastoma. Acta neuropathologica. 2013;126:917–29. doi: 10.1007/s00401-013-1198-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Denkinger DJ, Borges CR, Butler CL, Cushman AM, Kawahara RS. Genomic organization and regulation of the vav proto-oncogene. Biochimica et biophysica acta. 2000;1491:253–62. doi: 10.1016/s0167-4781(00)00008-7. [DOI] [PubMed] [Google Scholar]
- 32.Katzav S. Flesh and blood: the story of Vav1, a gene that signals in hematopoietic cells but can be transforming in human malignancies. Cancer Lett. 2007;255:241–54. doi: 10.1016/j.canlet.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 33.Wu X, Northcott PA, Dubuc A, Dupuy AJ, Shih DJ, Witt H, et al. Clonal selection drives genetic divergence of metastatic medulloblastoma. Nature. 2012;482:529–33. doi: 10.1038/nature10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wechsler-Reya RJ, Scott MP. Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron. 1999;22:103–14. doi: 10.1016/s0896-6273(00)80682-0. [DOI] [PubMed] [Google Scholar]
- 35.Hahn H, Wojnowski L, Specht K, Kappler R, Calzada-Wack J, Potter D, et al. Patched target Igf2 is indispensable for the formation of medulloblastoma and rhabdomyosarcoma. J Biol Chem. 2000;275:28341–4. doi: 10.1074/jbc.C000352200. [DOI] [PubMed] [Google Scholar]
- 36.Roussel MF, Hatten ME. Cerebellum development and medulloblastoma. Current topics in developmental biology. 2011;94:235–82. doi: 10.1016/B978-0-12-380916-2.00008-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quevedo C, Sauzeau V, Menacho-Marquez M, Castro-Castro A, Bustelo XR. Vav3-deficient mice exhibit a transient delay in cerebellar development. Molecular biology of the cell. 2010;21:1125–39. doi: 10.1091/mbc.E09-04-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fischer KD, Kong YY, Nishina H, Tedford K, Marengere LE, Kozieradzki I, et al. Vav is a regulator of cytoskeletal reorganization mediated by the T-cell receptor. Curr Biol. 1998;8:554–62. doi: 10.1016/s0960-9822(98)70224-6. [DOI] [PubMed] [Google Scholar]
- 39.Sebban S, Farago M, Gashai D, Ilan L, Pikarsky E, Ben-Porath I, et al. Vav1 fine tunes p53 control of apoptosis versus proliferation in breast cancer. PloS one. 2013;8:e54321. doi: 10.1371/journal.pone.0054321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wells CM, Bhavsar PJ, Evans IR, Vigorito E, Turner M, Tybulewicz V, et al. Vav1 and Vav2 play different roles in macrophage migration and cytoskeletal organization. Experimental cell research. 2005;310:303–10. doi: 10.1016/j.yexcr.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 41.Lazer G, Katzav S. Guanine nucleotide exchange factors for RhoGTPases: good therapeutic targets for cancer therapy? Cellular signalling. 2011;23:969–79. doi: 10.1016/j.cellsig.2010.10.022. [DOI] [PubMed] [Google Scholar]
- 42.Oberley MJ, Wang DS, Yang DT. Vav1 in hematologic neoplasms, a mini review. American journal of blood research. 2012;2:1–8. [PMC free article] [PubMed] [Google Scholar]
- 43.Ladd-Acosta C, Pevsner J, Sabunciyan S, Yolken RH, Webster MJ, Dinkins T, et al. DNA methylation signatures within the human brain. American journal of human genetics. 2007;81:1304–15. doi: 10.1086/524110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bibikova M, Lin Z, Zhou L, Chudin E, Garcia EW, Wu B, et al. High-throughput DNA methylation profiling using universal bead arrays. Genome research. 2006;16:383–93. doi: 10.1101/gr.4410706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwalbe EC, Lindsey JC, Straughton D, Hogg TL, Cole M, Megahed H, et al. Rapid diagnosis of medulloblastoma molecular subgroups. Clin Cancer Res. 2011;17:1883–94. doi: 10.1158/1078-0432.CCR-10-2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ellison DW, Kocak M, Dalton J, Megahed H, Lusher ME, Ryan SL, et al. Definition of disease-risk stratification groups in childhood medulloblastoma using combined clinical, pathologic, and molecular variables. J Clin Oncol. 2011;29:1400–7. doi: 10.1200/JCO.2010.30.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Langdon JA, Lamont JM, Scott DK, Dyer S, Prebble E, Bown N, et al. Combined genome-wide allelotyping and copy number analysis identify frequent genetic losses without copy number reduction in medulloblastoma. Genes Chromosomes Cancer. 2006;45:47–60. doi: 10.1002/gcc.20262. [DOI] [PubMed] [Google Scholar]
- 48.Bartolome RA, Molina-Ortiz I, Samaniego R, Sanchez-Mateos P, Bustelo XR, Teixido J. Activation of Vav/Rho GTPase signaling by CXCL12 controls membrane-type matrix metalloproteinase-dependent melanoma cell invasion. Cancer Res. 2006;66:248–58. doi: 10.1158/0008-5472.CAN-05-2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao H, Ayrault O, Zindy F, Kim JH, Roussel MF. Post-transcriptional down-regulation of Atoh1/Math1 by bone morphogenic proteins suppresses medulloblastoma development. Genes & development. 2008;22:722–7. doi: 10.1101/gad.1636408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.RDevelopmentCoreTeam . In R Foundation for Statistical Computing. Vienna: 2011. R: a language and environment for statistical computing. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Selection of differentially methylated human sequences and their mouse orthologs for investigation. Genes selected met the following criteria: (i) the human and mouse genomes harbored orthologous regions (Supplementary Figure S1A), (ii) methylation changes in human tumors affected multiple CpG sites in the region surrounding the array-associated probe site, and (iii) the average methylation levels at these additional sites were strongly correlated with the array site in human samples (r2 >0.70; Supplementary Figure S1B). The probe sequence used to assay the human CpG site of interest on the Illumina GoldenGate Cancer Panel I methylation array was extended by 50bp proximally and distally using the reference sequences provided by Illumina. This extended sequence was then compared to the mouse assembled genomes using the Blastn algorithm (no filters) (www.ncbi.nlm.nih.gov/Blast.cgi). Orthologous regions of the mouse genome which mapped to the expected gene were identified for further analysis and suitable primers were designed to assess similar sized homologous regions in mouse and human DNA. The positions of the bisulfite sequencing PCR products relative to the transcriptional start site and the number of CpGs analyzed for both are shown. *There is no equivalent mouse transcript to the human transcript used for MEST.
Supplementary Table 2. Primers used for bisulfite and real time PCR. For bisulfite PCR, primers were designed to amplify regions (200-500bp) surrounding the CpG site assessed on the Illumina GoldenGate Cancer Panel I microarray or its orthologous position in the mouse genome. Primers were designed using the program Methprimer (www.urogene.org./methprimer). The annealing temperature for all primers was 55°C, cycling at this temperature was preceded by 5 cycles of ‘touchdown PCR’ from 60°C in increments of −1°C to increase the specificity of the products. The human TRIM29 PCR reactions required the addition of Betaine (Sigma-Aldrich, Gillingham, UK) to the PCR mix (final concentration 1M). For quantitative real time RT-PCR, primers were designed using Primer Express software (Applied Biosystems) to ensure that products overlapped intron/exon boundaries. Dissociation curves confirmed PCR product specificity. Expression was assessed relative to expression of a control gene (28S rRNA or TBP), using the comparative Ct method for relative quantification. Dilution series were carried out to confirm relative expression was consistent over a range of input DNA concentrations.
Supplementary Figure 1. Identification of DNA methylation events associated with MBSHH. A. Clustering of DNA methylation patterns in 230 primary human medulloblastomas identifies four consensus subgroups which are highly significantly related to established SHH and WNT subgroup biomarkers (‘p’ values are shown). The 216 tumors which could be confidently assigned to a subgroup are shown 15. MBSHH, red; Group 3, yellow; WNT, blue; Group 4, green. MBSHH biomarker (SHH mRNA signature / GAB-1/YAP1 IHC) positivity, red. WNT subgroup biomarker (WNT mRNA signature / β-catenin IHC) positivity, blue. Data not available, grey. Magnitudes of the four defining metagenes (V1 to V4; highly expressed metagenes, red; lowly expressed, blue) and subgroup-specific DNA methylation events (unmethylated, green; fully-methylated, red) are shown. B. Principal component analysis loadings plot shows class designations for WNT (blue), MBSHH (red), Group 3 (yellow) and Group 4 (green) tumors and normal cerebella (pink; n=21). Covariance spheroids with a 95% confidence interval have been plotted to demonstrate group memberships.
Supplementary Figure 2. Identification and assessment of DNA methylation alterations in orthologous regions of the mouse and human genomes. A. Examples of regions of homology between mouse and human genomes. Aligned sequences are shown for the regions surrounding TRIM29 (probe P833), CCKAR (probe E79), VAV1 (probe E9) and MEST (probe P4). The probe site interrogated by the Illumina array is boxed (dashed line); other conserved CpG sites are boxed (solid line) and non- conserved CpG sites are underlined. H, human; M, mouse. B. Correlation between the array-assessed β-value and the average methylation score determined by bisulfite sequencing for all CpG sites in the surrounding region (Table S1) for nine genomic regions, in six randomly-selected cerebella and six randomly-selected MBSHH tumors. (RASSF1A; r2, 0.92). C. Methylation levels at individual CpG sites in the assessed Vav1 region in mouse tumors and strain-specific cerebella following analysis by bisulfite sequencing, shown alongside the overall average methylation score for the region. CpG sites are labelled by their position relative to the translational start site of the Vav1 gene. Black circles, ≥80% methylation; three-quarter black circles, ≥60%; half-black circles, ≥40%; quarter-black circles, ≥20% methylation; white circles, <20% methylation.
Supplementary Figure 3. VAV1 hypo-methylation in human medulloblastomas: Regional CpG patterns, molecular and clinical significance. A. Representative examples of bisulfite sequencing on the reverse strand of the region surrounding VAV1 (probe E9). Assayed CpG sites (labelled by their position relative to the translational start site of the Vav1 gene) are underlined and calculated methylated peak height (G-green) relative to total peak height is given for each of the 10 CpG sites analyzed. The methylation score is the average of these values and is shown together with the β value for the CpG site (boxed) assessed on the Illumina array. B. Methylation levels at individual CpG sites in the assessed VAV1 region in representative human tumors (MBSHH (n=25) and non-MBSHH (n=8) tumors) and cerebella (n=7), following analysis by bisulfite sequencing and shown alongside the methylation score. Black circles, ≥80% methylation; three-quarter black circles, ≥60%; half-black circles, ≥40%; quarter-black circles, ≥20% methylation; white circles, <20% methylation. The CpG site assessed by probe E9 is shown, and the cut-off used to discriminate VAV1 hypo-methylated tumors (0.53; Figure 3) is marked. C. Box plots showing methylation levels (β-values) for VAV1 (probe P317) in the MBSHH subgroup (n=50) compared to non- MBSHH (n=166) tumors and cerebella (CB; n=21). The mean cerebellar methylation (-3SD; cut-off marked at 0.30) is shown to distinguish hypo-methylated tumors. Non-MBSHH tumors are shown colored by subgroup (Group 3, yellow; WNT, blue; Group 4, green).
Supplementary Figure 4. VAV1 methylation patterns in multiple pediatric brain tumor types. Box plots show methylation patterns for probes VAV1 E9 (A) and VAV1 P317 (B) for posterior fossa ependymomas (dark green, n=45); supratentorial ependymomas (light green, n=16); pediatric high-grade gliomas (grey, pHGG, n=15) and CNS-PNETs (black, n=30). For comparison, methylation status of non-neoplastic cerebellar (pink, n=21) and cerebral (dark pink, n=16) cohorts are shown (data for the 16 cerebra are previously described 43). Horizontal cut-off lines, corresponding to the mean -3SD cerebellar methylation (probe E9, β=0.53 and probe P317, β=0.30) are shown for comparison with Figures 2A and S3C. ‘p’ values from Kruskal-Wallis tests are shown.
Supplementary movies. Time lapse images documenting the morphology of CGNs following electroporation with equal amounts of vector only (Movie 1); Vav1DN (Movie 2) and Vav1CA (Movie 3).