Abstract
Interest in nanoneuromedicine has grown rapidly due to the immediate need for improved biomarkers and therapies for psychiatric, developmental, traumatic, inflammatory, infectious and degenerative nervous system disorders. These, in whole or in part, are a significant societal burden due to growth in numbers of affected people and in disease severity. Lost productivity of the patient and his or her caregiver, and the emotional and financial burden cannot be overstated. The need for improved health care, treatment and diagnostics are immediate. A means to such an end is nanotechnology. Indeed, recent developments of health-care enabling nanotechnologies and nanomedicines range from biomarker discovery including neuroimaging to therapeutic applications for degenerative, inflammatory and infectious disorders of the nervous system. This review focuses on the current and future potential of the field to positively affect clinical outcomes.
Keywords: nanoneuromedicine, diagnostics, neurodegenerative disorders, nanotechnology, drug development
1. Introduction
The field of nanoneuromedicine offers real opportunities to harness unique therapeutic approaches to address diseases of the nervous system where often few options exist. Because of the enormous potential of the field, it was chosen as the theme for the 2014 meeting of the American Society for Nanomedicine.1 In addition to improved therapies, newer, safer and more sensitive-specific imaging modalities as well as improved diagnostics for disease detection are immediately needed.
Nervous system disorders, due to infection, trauma or degenerative disorders, represent a significant societal burden with parallel broad unmet needs. In many and sometimes most cases, current treatments are simply inadequate to affect disease progression or even ameliorate symptoms and signs of brain injury or degeneration. Significant challenges abound and are associated with the transport of therapeutic or imaging contrast agents across the blood-brain barrier (BBB) into the nervous system and retain the ability to achieve targeted delivery to appropriate brain or spinal cord subregions.2 Nanomedicines can facilitate solutions to such problems. This and related enabling technologies, can increase drug-drug interactions, facilitate disease ameliorating immunomodulation, enable pathogen clearance and improve nervous system delivery of biologically active molecules. Included are multifunctional therapeutic, imaging and diagnostic devices currently referred to as theranostics.3 However, limitations for improved drug delivery to the nervous system are not trivial, including the potential for secondary toxicities. Thus, any new formulation must balance a drug therapeutic index. This highlights a quite diverse and multifaceted field of research in biomarker discovery, bioimaging and theranostics. If successful, therapies to address neurodegenerative, immune and infectious diseases of the nervous system could be realized and more options would be available for human use.
2. Biomarker Discovery, Bioimaging and Theranostics
The abilities to diagnose and monitor neurological diseases have seen considerable growth in the recent decades. Nonetheless, in understanding the mechanisms and pathology of neurodegenerative diseases, the development of strategies to detect neurological diseases at early stages and prior to the emergence of overt symptoms is still a challenge for scientists and physicians in the field. In this context, nanotechnology-based techniques have gained tremendous interest as a tool in the efforts to improve the effectiveness of the imaging of central nervous system (CNS) functions and disease states as well as to advance neurosurgical practice. Most notably is bioimaging. Magnetic resonance imaging (MRI) has emerged as the most important tool in the diagnosis of brain disorders. Positron emission tomography (PET) imaging is not far behind and has already allowed improved understanding of the time course of a range of nervous system disorders including for the pathophysiology of Alzheimer's disease (AD). This has been seen through the application of radiolabeled amyloid ligands.4-6
Nanoparticles containing iron, gadolinium and manganese were studied extensively as contrast agents. Among them superparamagnetic iron oxide (SPIO) nanoparticles have garnered interest due to their large surface area, magnetic properties and low toxicity. Biocompatible SPIO nanoparticles consist of a crystalline iron oxide core (in the form of magnetite, Fe3O4, or maghemite, γFe2O3) encased in polymer or a coated monomer (Figure 1, upper panel).7, 8 The particles can be classified according to their size in several categories: particles with a mean diameter of 50 to 180 nm, referred to as standard SPIOs (e.g. ferumoxides coated with dextran); ultra-small SPIO (USPIOs) nanoparticles with a diameter of 10 to 50 nm; and very-small SPIO (VSPIOs) nanoparticles less than 10 nm in diameter.9 The nature of the surface coatings determine the physical and biologic properties such as the overall size, surface charge, coating density, toxicity and degradability. These affect the fate of SPIO in body fluids and cells10. The nonspecific uptake of SPIO nanoparticles by the reticuloendothelial system (RES) has found clinical application for imaging liver tumors11, 12 and lymph nodes.13 Ferumoxytol, the USPIO nanoparticles coated with polyglucose sorbitol carboxymethyl ether approved for intravenous iron replacement therapy in patients with chronic kidney disease,14 was recently investigated as an MR contrast for brain tumors.15, 16 Unlike gadolinium-based agents, contrast enhancement of brain malignancies with ferumoxytol requires intracellular uptake by mononuclear phagocytes (MP; perivascular macrophages and microglia) and reactive astrocytes with maximal signal enhancement at 24-48 hours after injection.9 The extended USPIO residence time is believed to promote their uptake by circulating cells. This suggests that USPIOs, combined with perfusion-weighted imaging can accurately gauge tumor progression.
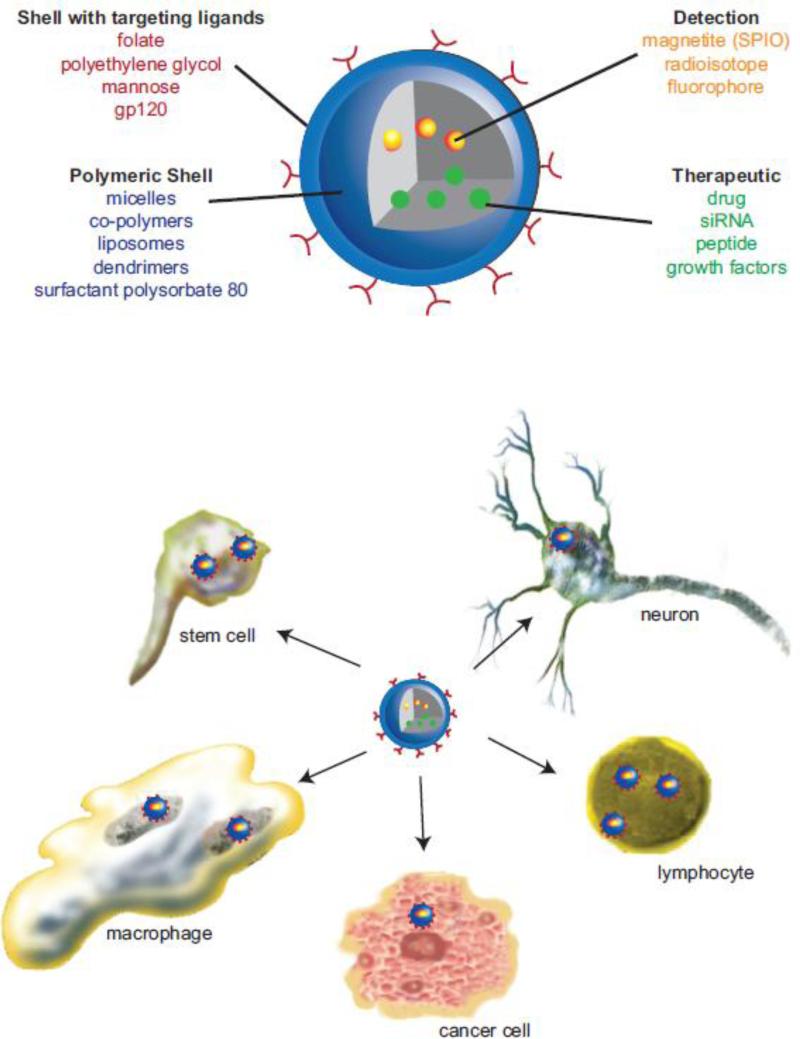
Figure 1. Polymer composition strategies for nanomedicines.
A range of nanomedicines has been developed for drug delivery. These include particle platforms that have versatile and tunable composites for large surface to volume ratios, an optimal surface charge, hydrophobicity, and controllable particle shape and size. Descriptions are made for cargos and surface targeting modifications. The availability of enhanced imaging modalities has facilitated bioimaging and theranostics applications. These are illustrated and represent the development and use of polymer drug conjugates, dendrimers, micelles, liposomes, solid lipid nanoparticles and polymeric nanoparticles (upper panel). The abilities of these nanoformulations to cross the blood-brain barrier and target specific neural and glial cells underpin their therapeutic activity in disease and drug storage capacities (lower panel).
Since MP are present in a range of intracranial pathologies from glial tumors to many inflammatory disorders, ferumoxytol and other USPIO may be useful for imaging diseases. Labeling of circulating monocytes by systemic administration of USPIO nanoparticles were applied to spatiotemporal profiles of MP infiltration in stroke models.17, 18 Studies demonstrated delayed influx of blood-borne monocytes in affected brain regions. The potential of using ferumoxtran-10 (USPIO coated with dextran) for imaging ischemic lesions in patients suffering from stroke was evaluated.19 Contrast enhancement was observed primarily within the infarcted brain region attributed to the USPIO nanoparticle-labeled macrophage brain infiltration. The latter was supported by a combination of gadolinium-enhanced and USPIO nanoparticle-enhanced MRI.17, 18 Similar observations were reported by Beckmann et al.20 in studies of cerebral amyloid angiopathy in amyloid precursor protein mouse AD models. Systemic administration of SPIO improved the MRI detection of microvascular lesions in the brains of the mice, and also led to the labeling of additional microvascular alteration sites. For AD, it was suggested that monocytes take up SPIO nanoparticles in the circulation then penetrate the brain after attraction by chemokines produced by amyloid beta (Aβ)-stimulated glia. This is true in inflammatory diseases of the nervous system. Indeed, macrophage activity can be visualized with USPIO nanoparticles using MRI tests in patients with relapsing-remitting multiple sclerosis.21 Alternatively to labeling circulating monocyte-macrophages, visualization of activity may be achieved with isolated cells loaded with SPIO nanoparticles through in vitro incubation prior to systemic administration. Such a strategy has been applied in stroke models to depict inflammatory cell biodistribution.22
Multifunctional modifications of SPIO nanoparticles with specific ligands such as antibodies, peptides, aptamers and other targeting molecules offer the ability to monitor SPIO nanoparticle accumulation at the disease site (Figure 1, lower panel). This results in enhanced contrast and improved diagnostics. For example, SPIO nanoparticles conjugated with chlorotoxin (a neurotoxin known to target glioma) show increased uptake in glioma cells and are being developed to improve imaging of brain tumors.23, 24 Polyethylene glycol (PEG)-coated USPIO nanoparticles chemically coupled with Aβ1-42 peptide have provided the opportunities for simultaneous targeting and imaging of amyloid plaques in AD transgenic mice. This is seen following intravenous injection without the need to co-inject an agent to transiently open the BBB.25 The amyloid plaques detected by longitudinal bioimaging were confirmed with matched histological sections. Such systems are very useful for early diagnosis and also for direct measurements of anti-amyloid therapies.
In sites of inflammation in stroke, multiple sclerosis, and HIV-dementia, circulating blood leukocytes are the first to migrate across activated endothelium. In particular, vascular cell adhesion molecule-1 (VCAM-1) plays an important role in leukocyte recruitment to the brain.26-28 Thus, targeting contrast agents to adhesion molecules in inflamed, activated cerebral endothelium is a potent strategy for early diagnosis. The feasibility of VCAM-1 visualization in acute brain inflammation was demonstrated with VCAM-1 antibody conjugated to microparticles of iron oxide (VCAM-MPIO).29, 30 In this case, the application of micron-size of MPIO allowed delivery of a high iron payload to the targeted sites of disease. In addition, due to their size, micron-size SPIO (MSPIO) particles are less susceptible than USPIO to extravasation or non-specific uptake by endothelial cells, and therefore retain specificity for molecular targets. VCAM-1-targeted MRI revealed that pre-symptomatic lesions could be quantified in an experimental autoimmune encephalomyelitis (EAE) of multiple sclerosis when found undetectable by gadolinium-enhanced MRI.31 An alternative to VCAM-1 targeting is the direct detection of neuroinflammation by targeting E- and P-selectins. This demonstrates the fact that intercellular adhesion molecules are up-regulated as part of host response to injury.32 Van Kasteren et al. designed glyco-USPIO decorated with a biomarker ligand sialyl LewisX which showed excellent targeting to activated endothelium and allowed pre-symptomatic in vivo brain imaging of brain diseases in several clinically relevant animal models.33 Similarly, USPIO nanoparticles coated with a short heptapeptide (IELLQAR) that target selectin binding sites34 were successfully used for mapping E-selectin expression following traumatic brain injury.35
Nanoparticles based on biodegradable poly(n-butyl cyanoacrylate) (PBCA) coated with the surfactant polysorbate 80 were investigated as carriers for drug delivery to the brain. The ability of these nanoparticles to bypass the BBB has been attributed to polysorbate-80 mediated affinity for apolipoproteins B and E and the subsequent transcytosis through low-density lipoprotein receptors present on brain endothelial cells.36, 37 This mechanism was utilized to deliver BBB-impermeable molecular imaging probes into the brain for visualization of amyloid plaques.38 Further, MRI of wild type mouse brain revealed contrast enhancement of brain parenchyma after intravenous administration of PBCA nanoparticles loaded with gadobutrol, a gadolinium-based contrast agent routinely used in humans for imaging anatomical lesions. Similarly, PBCA nanoparticles were utilized for the brain delivery of radiolabeled amyloid-affinity chelator, 125I-clioquinol, a derivative of quinoline imaging probes.39 Nanoparticulate encapsulation of 125I-clioquinol into PBCA nanoparticles resulted in significantly greater brain uptake, enhanced retention of the drug and labeling of amyloid deposits in AD transgenic mice. These data collectively indicate the future potential of nanocarrier-mediated delivery of molecular imaging probes to improve diagnostic specificity.
The use of nanotechnology-based approaches for cell therapy and tissue engineering has shown promise in brain and spinal cord injury. Stem cells have been shown to selectively target injured brain and spinal cord tissue and improve functional recovery (Figure 2).40, 41 The ex vivo loading of cells with magnetic nanoparticles allowed the in vivo tracking and monitoring of grafted cells in the host organism with MRI after transplantation. Successful in vivo detection and migration monitoring of SPIO-labeled cells was demonstrated in numerous preclinical studies related to implantation of hematopoietic, mesenchymal or neuronal cells in the CNS.40, 42-44 Clinical trials based on this approach involved tracking autologous neural stem cells by MRI in traumatic head injury and bone marrow stem cells in chronic spinal cord injury and were shown to be safe and effective.45, 46 Despite the encouraging results of initial trials, cell tracking using SPIO labels is limited by dilution of contrast agent during cellular proliferation, possible transfer of label from dying cells to surrounding endogenous cells (e.g. macrophages or microglia), and inability to discriminate between live and dead labeled cells. Thus, interpretation of signal changes during long-term MRI cell tracking might be difficult and requires caution.47 In addition, the clinical MRI agents Feridex® (Endorem) and Resovist® are no longer commercially available. Feridex was discontinued by AMAG Pharma in 2008, while Resovist was approved for the European market in 2001, but production was abandoned in 2009; thus new SPIO suitable for clinical applications will have to be developed. Li et al, reviews the approaches for the development of MR contrast agents suitable for cell labeling.48
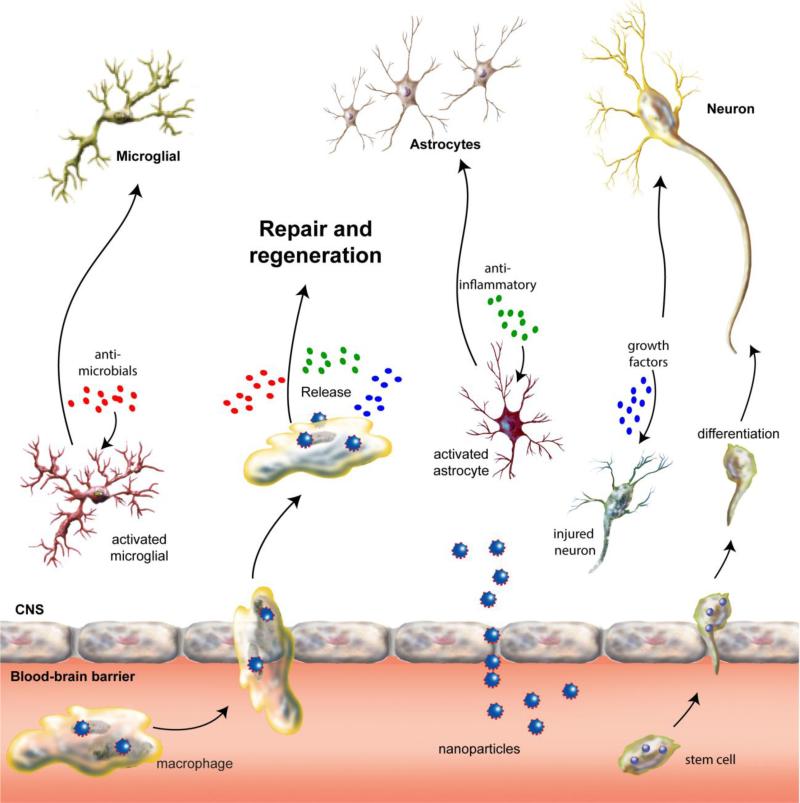
Figure 2. Pathogenesis and nanomedicine treatment of neuroinfectious diseases.
Disease in the CNS is caused, in largest measure, by genetic, degenerative, immune and infectious events. This results in neuronal injury or death, astroglial and microglial activation, or infection with consequent secretion of inflammatory neurotoxic mediators. Nanomedicines can directly cross the blood-brain barrier, affect physiological response barrier function or be carried within circulating immunocytes (monocyte/macrophages and lymphocytes) and stem cells. Once inside the brain, they release their cargo and affect ongoing disease processes leading to clearance of microbial infections, neuronal repair and/or anti-inflammatory responses leading to restoration of glial homeostasis.
Semiconductor fluorescent quantum dots (QDs), nanoscale-sized particles, are used extensively for visualization and tracking of living cells. Manipulations of the core material and size allow synthesis of a wide array of QDs emitting at various wavelengths, including the near-infrared region, which is optimal for deep-tissue imaging.49 The long-term stability and brightness of QDs as well as the possibility for attachment of different bioactive molecules to their outer shells make them perfect candidates for in vitro and in vivo targeting and imaging. For example, diffusion dynamics of glycine receptors in living spinal neurons were analyzed using single-QD tracking.50 In this study, the fluorescence and electron microscopy images were acquired with the same probes, which provided both the temporal dynamics and high-resolution localization of the diffusing receptors in the neuronal membrane. Wang et al. demonstrated the feasibility and specificity of using antibody-labeled QDs for rapidly visualizing epidermal growth factor receptor expression in human brain tumor cells and in surgical frozen section slides of glioma tissue.51 Recently, Feng et al. developed QDs conjugated with an anti-Aβ antibody to track the state of Aβ accumulation in vivo in a mouse model of AD.52 While QD-based optical imaging represents a valuable tool to address cellular and molecular questions of interest, one of the remaining issues with QD probes is in vivo toxicity. Modification of the surface of QDs by PEG or other polymers significantly improved their biocompatibility; however, the long-term fate of polymer-coated QDs in living organisms is not fully understood. Safety concerns need to be addressed before applications of QDs can be translated into human clinical use.
Recently, the arsenal of nanoparticle-based technologies has been further expanded by the design of multifunctional constructs combining diagnostic and therapeutic functions within the same nanocarrier (Figure 1, upper panel). These “theranostic” platforms enable a noninvasive assessment of the pharmacokinetics, tissue biodistribution and accumulation of drugs at the target site (Figure 1, lower panel). Such an approach can be used to optimize the drug delivery systems and treatment regimens in order to achieve maximal therapeutic efficacy and minimize drug-induced side effects. By doing so, theranostic nanoparticles might also contribute to the development of “personalized” treatment options. Numerous exciting examples were developed in recent years, especially for the treatment of cancer.53, 54 Reddy and colleagues have developed multifunctional polyacrylamide-based nanoparticles consisting of a surface-localized tumor vasculature targeting F3 peptide, an encapsulated photosensitizer (Photofrin) and an iron oxide imaging agent. Serial MRI was used for determination of pharmacokinetics and distribution of nanoparticles within the tumor. A combination treatment of F3-targeted nanoparticles followed by photodynamic therapy in glioma-bearing rats showed a significant improvement in survival rate in treated animals that were found tumor-free at the end of the study.55 In another study, researchers demonstrated that dendrimer-grafted gadoliniumfunctionalized nanographene oxide nanoparticles carrying epirubicin and miRNA can be detected by MRI to identify the tumor area and quantify the concentration of therapeutics within the tumor in a mouse glioma model.56 The capacity of theranostic agents to delineate the peri-infarct region and achieve a therapeutic effect in brains of ischemic injured animals was also demonstrated.57 These investigators prepared stealth immunoliposomes carrying the drug citicoline and a contrast agent, a gadolinium-labeled lipid. HSP72 protein, an inducible form of HSP70 that translocates to the cellular membrane under stress conditions such as ischemia, was selected to specifically target the peri-infarct tissue.58 Using MRI, they found that after intravenous administration, about 80% of anti-HSP72 liposomes were located on the periphery of the ischemic lesion, and animals treated with citicoline encapsulated in these liposomes presented significantly smaller lesion volumes compared to controls. These findings demonstrate that targeted theranostic nanoparticles represent an interesting platform for noninvasive monitoring of the effectiveness of the therapy. Although the data of theranostic approaches being used to target areas located inside the brain parenchyma are currently limited, these examples clearly demonstrate the potential of nanotheranostics to bring much-needed treatments for neurological diseases.
Biomarkers are molecules that indicate the biological status of a disease59 and, therefore, can provide invaluable information for clinical diagnosis such as monitoring response to treatment, as well as, aid in the development and evaluation of novel therapies. Sensitive and accurate detection of biomarkers in human body fluids could offer essential input to early diagnosis for neurological diseases. In the past decade, various nanomaterials (gold (Au) nanoparticles, QDs, SPIO, carbon nanotubes and nanowires) have been extensively studied to improve the sensitivity and specificity of biomarker detection.60-63 For example, a bio-barcode amplification assay based on a sandwich process involving oligonucleotide-modified Au nanoparticle and magnetic microparticles, both functionalized with antibodies against a specific antigen, was utilized for ultrasensitive detection of soluble amyloid-β-derived diffusible ligands (ADDL) in cerebral spinal fluid (CSF) at clinically relevant concentrations.64 Elevated concentrations of ADDLs were detected in the CSF of AD patients compared with CSF from non-demented controls. Another sandwich assay was developed for fast detection of Alzheimer's tau protein using a combination of hybrid magnetic nanoparticles functionalized with monoclonal anti-tau antibodies and polyclonal anti-tau immobilized Au nanoparticles as the recognition and surface-enhanced Raman scattering component, respectively.65 Ultrasensitive immunosensors for detection of Aβ peptides based on surface plasmon resonance66 or scanning tunneling microscopy-based electrical detection67 utilized specific monoclonal antibody fragments immobilized on the surface of Au nanoparticles as recognition elements. Yang et al. synthesized and characterized SPIO coated with antibodies against Aβ-40 or Aβ-42 and employed them as an immunoassay platform.68 In combination with immunomagnetic reduction technology, these biofunctionalized SPIO targeted Aβs with high specificity and exhibited ultralow detection limits (~ 10 pg/mL). Furthermore, levels of Aβ-40 or Aβ-42 peptides detected in blood plasma samples from normal and AD patients correlated with clinical diagnosis. Aβ screening methodology based on the electrochemical sensing of saccharide–protein interactions has also been reported.69 The densely packed sialic acid areas for recognition of Aβ were arranged on the surface of Au nanoparticles electrodeposited on a screen-printed carbon strip. The intrinsic oxidation signal of tyrosine residues from captured Aβ peptides was detected and monitored using differential pulse voltammetry. Neely et al. demonstrated that monoclonal anti-tau antibody-coated Au nanoparticles were used for detection of CSF tau by employing a two-photon Rayleigh scattering assay.70 The plasmon absorbance of the Au nanoparticles also was exploited in the design of a colorimetric assay for neurotransmitters involved in PD pathology.71
The exceptional optical properties of QDs also make them useful as signal amplification agents in biomarker detection.72 Recently, core-shell CdSe/ZnS QDs were used in an assay designed to detect apolipoprotein E (ApoE) as a potential biomarker for AD.73 The QDs proved to be highly effective reporters and exhibited up to a 7-fold enhancement in limit of detection compared to a conventional enzyme-linked immunosorbent assay targeting ApoE. This allowed assaying very small volumes (1 μL) of human serum with high sensitivity and acceptable precision and accuracy. Further fine-tuning of microarrays for use with QDs will facilitate improved biosensing and diagnostics.
Other nanoparticle-based technologies have also been investigated for biomarker detection. An et al. exploited Au-doped titanium oxide nanotube arrays to develop a photoelectrochemical immunosensor for α-synuclein detection.74 A parallel approach was proposed based on a dual signal amplification using G4-polyamidoamine dendrimer-encapsulated Au nanoparticles and enhanced Au nanoparticle labels.75 The designed immunosensor displayed an excellent analytical performance with a detection limit of 14.6 pg/mL for α-synuclein. Recently, vertically aligned ZnO nanowire arrays were fabricated on 3D graphene foam and used to selectively detect uric acid and dopamine by a differential pulse voltammetry method at a detection limit of 1 nM.75 This method was further used to show the feasibility of using uric acid as a biomarker in the serum of PD patients. Apart from these examples, there are a number of other studies that use nanomaterials for developing sensing mechanisms that will allow detecting and measuring the concentrations of pathogenic markers in biological samples at clinically relevant concentrations. Although the majority of the reported data are related to the proof-of-concept studies, the current findings strongly suggest that nanotechnology has a real potential to contribute to early detection, diagnoses and treatments of neurodegenerative diseases.
3. Nanomedicines for Infectious Diseases
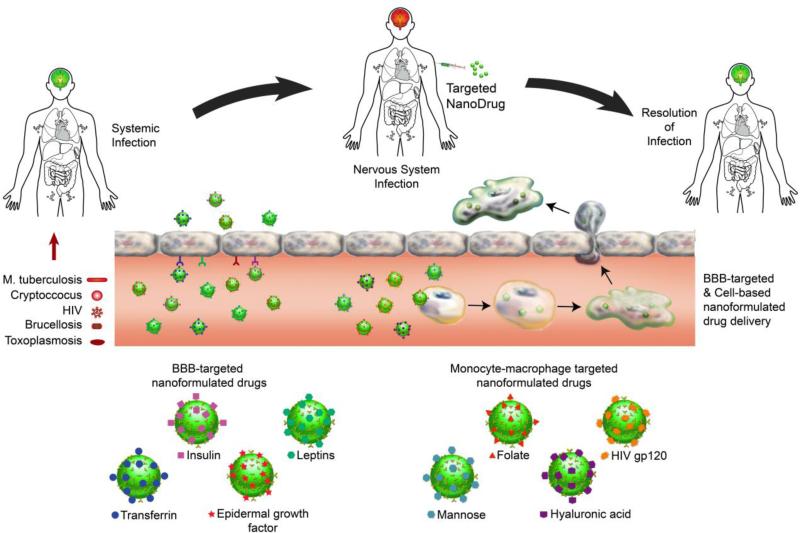
Nanoneuromedicines are being developed to increase drug penetration into sites of active microbial infection while limiting systemic toxicities. Longer acting medicines would also improve regimen adherence. Thus, to facilitate drug therapeutic efficacy by improving pharmacokinetics and disease region-specific nervous system drug biodistribution as well as immune-directed microbial clearance best defines the field of infectious disease-linked nanoneuromedicine. The overarching goal is to actively target, and then eliminate sites of persistent infection, inflammation or degeneration.76-78 In recent years, a number of nanomedicines were developed for the treatment, detection and prevention of infectious diseases.79 The platform has focused on liposomes, polymeric nanoparticles, dendrimers, micelles and SLNs to improve water-solubility of poorly-water-soluble drugs and subsequently enhance drug stability to sites of infection. Specific drug targeting to endothelial cell receptors and use of cell-based carriage of nanomedicines serves to facilitate CNS delivery (Figure 3).
Figure 3. Targeted nanoformulated drug delivery for infectious diseases of the nervous system.
Nanoformulated antimicrobial drugs can be targeted to brain endothelial cell receptors such as insulin, leptin, transferrin and epidermal growth factor receptors to promote transfer across the blood-brain barrier (BBB). They can also be targeted to monocyte-macrophage receptors such as folate, CD4, mannose and CD44 receptors to promote cell uptake for macrophage-based drug delivery across the BBB. The nanoformulated antimicrobial agent that is decorated with the appropriate ligand for the targeted cellular receptor can be administered systemically with the insurance it will either find a BBB-target cell or an appropriate carrier cell such as MPs that support transport across the BBB. Once inside the brain the drug cargo can be released from free nanoparticles or macrophages to facilitate resolution of microbial infection.
The CNS infections where nanomedicines are currently being developed include bacterial meningitis, rabies, malaria and HIV. Research is active in models of human disease and in translational research. For example, treatment strategies for Staphylococcus aureus and Cryptococcus neoformans meningitis in rabbits were successful with self-assembled cationic antimicrobial peptides of cholesterol-conjugate G3R6TAT.80, 81 The manufactured particles easily crossed the BBB and were shown to be equally effective as vancomycin and amphotericin B in attenuating meningeal infections and their sequelae without affecting liver function or causing imbalances in blood electrolytes. Both are known complications in treating bacterial and fungal disease. Other studies showed that delivery of vancomycin into a drug-resistant S. aureus strain using a folic acid-conjugated chitosan nanocarrier improved delivery of the medicine,82 highlighting the notion that nanoparticle delivery could positively affect treatment outcomes for multidrug resistance in bacteria. Benefits were seen with such an approach in reducing oxidative stress that follows S. aureus infections. Indeed, diminished lipid peroxidation, protein oxidation, nitrite generation, DNA damage and glutathione were seen with the emergence of antioxidant enzymes.
Adjunctive therapies are often used in combination with antimicrobials to reduce the inflammatory events that contribute to CNS damage and long-term impairments associated with CNS infectious diseases;83-85 however, the high incidence of adverse side effects with corticosteroids limits their use.83 In a recent study, nano-sterically stabilized liposomal formulations of the glucocorticoid β-methasone hemisuccinate were used in conjunction with artemisone to enhance the efficacy of the antiplasmodial in an experimental mouse model of cerebral malaria with no glucocorticoid-related side effects.86 Of importance, the liposomal formulation of the glucocorticoid resulted in accumulation of the drug in the brains of infected mice, but not healthy mice. The use of nanoparticles for delivery of anti-inflammatory agents has also been described for Escherichia coli-induced meningitis87 and demonstrated that a water-soluble malonic acid derivative of carboxyfullerene could reduce CNS levels of TNFα and IL-1β and inhibit neutrophil infiltration across the BBB. In other reports, nanoparticle systems comprised of dendrimers with multiple reactive surface groups have also demonstrated anti-prion activity in part through alteration of the conformation of misfolded prion proteins (reviewed by McCarthy et al.88), and maltotriose modified poly (propyleneimine) dendrimers have been shown to be capable of crossing the BBB.89
Ultimately, the best treatment for controlling CNS infectious disease is vaccination. Nanoparticles have been developed for improving the immunogenicity and efficacy of vaccines against several CNS infectious diseases. A dendrimer-DNA complex (dendriplex) using a plasmid vaccine construct of the rabies virus glycoprotein gene was complexed with a novel poly(ether imine) (PETIM) dendrimer and used to immunize mice that were subsequently challenged with a standard rabies virus strain.90 These mice demonstrated 4-fold improved viral titers 14 days after immunization compared to mice immunized with the unformulated plasmid viral construct. In addition, all mice receiving the dendriplex vaccine compared with 60% of the mice receiving the unformulated vaccine survived viral challenge. In another study, Knuschke et al. used functionalized triple-shell calcium phosphate (CaP) nanoparticles as carriers for toll-like receptor 9 ligand CpG and antigenic peptides to induce a robust immune response and protection from Friend virus-induced splenomegaly and reduction of viral load.91 This system provided a proof of concept for the development of nanoparticle-based vaccines for retroviral infection.91
Nanoparticle-based detection systems are being developed to provide early and sensitive means for diagnosis of infectious disease. Early diagnosis is critically important for effective treatment of CNS infections. Reddy and coworkers recently described the use of Au nanoparticles to enhance detection of meningococcal antigen by an acoustic wave immunosensor method.92 By binding the cell surface outer membrane protein 85 (OMP85) of N. meningitides to Au nanoparticles and interacting these complexes with antibodies immobilized on a PVDF-coated quartz crystal microbalance, detection of as little as 312 ng/ml OMP85 in blood or CSF could be readily observed.
Various nanoparticle-based approaches have also been described for treatment of HIV infection in the CNS. These notably include drug polymer conjugates, dendrimers, micelles, liposomes, SLNs, nanosuspensions, polymeric nanoparticles and cell-mediated nanoparticle delivery.93 Of note, improved CNS bioavailability of efavirenz and an increase in the relative exposure index for the drug was described using intranasal administration of efavirenz-loaded Pluronic® block copolymer (poly(ethylene oxide)-poly(propylene oxide) polymeric) micelles.94 Pluronic® block copolymers can inhibit efflux transporters (P-glycoprotein and multidrug resistance-associated protein) on brain microvascular endothelial cells, thus facilitating delivery of drug across the BBB.95 Liposomal formulations are used to improve pharmacokinetics of both hydrophobic and hydrophilic drugs. Jin and coworkers demonstrated that liposomal formulations of a zidovudine prodrug (AZT-myristate) provided a 2-fold increase of drug in the brain compared to an equivalent dose of free AZT.96 Liposomal formulations targeted to transferrin and insulin receptors on endothelial cells are also being explored for enhanced delivery of drugs, including antiretrovirals, to the brain.97, 98 In other studies, Saiyed and coworkers demonstrated improved penetration of magnetic azidothymidine 5’-triphosphate liposomal nanoformulations upon application of an external magnetic field.99 Cationic nanogel formulations of nucleoside reverse transcriptase inhibitors (NRTIs) decorated with the peptide binding (AP) brain-specific ApoE receptor exhibited improved CNS antiviral activity compared to non-formulated NRTIs and with low neurotoxicity.100 SLNs are also being investigated for improved antiretroviral drug pharmacokinetics and CNS delivery. Kuo and Su demonstrated that stavudine, delavirdine and saquinavir encapsulated in SLNs more readily passed across an artificial BBB compared to unencapsulated drugs.101 In other in vitro studies, Kuo and Ko used the insulin-like peptidomimetic monoclonal antibody, 83-14 MAb, as a targeting moiety to improve penetration of saquinavir SLNs across an artificial BBB.102 While these in vitro results are promising, supportive in vivo studies are needed to demonstrate the utility of targeted SLNs for improved CNS antiretroviral therapy (ART) delivery.
To extend circulation longevity of nanomedicines that are targeted for specific diseases such as cancer, many drug delivery systems have been designed to evade the immune system, thus improving delivery of drug to the desired target while reducing untoward immune reactivity.76 Over the last decade the strategy of targeting nanoparticles to MP, lymphocytes and stem cells to use them as Trojan horses for delivery of anti-infective medicines has been explored to facilitate drug delivery for a variety of infectious and neurodegenerative diseases.77, 103 Our own laboratories have developed the concept of MP delivery of nanoART to extend circulating drug levels and target sites of HIV replication including the CNS.104-106
Targeted cell-based delivery is also being developed as a means of carrying drug nanoparticles across biologic barriers, such as the BBB. The phagocytic and chemotactic capabilities of MP can be harnessed by targeted systems to deliver drugs to CNS disease sites and to other protected sites such as lymphoid tissue.77, 103 Proof of concept was demonstrated for delivery of nanoformulated catalase (nanozymes) to the CNS and for delivery of nanoART to localized CNS HIV-1 infection in mouse models of Parkinson's disease (PD) and HIV encephalitis (HIVE), respectively.105, 107 CNS targeting of bone marrow macrophages (BMM) loaded ex vivo with indinavir nanoparticles was determined in an HIVE mouse model. BMM loaded with indinavir nanoparticles were administered intravenously to mice and provided indinavir release up to 14 days. Of significance, indinavir was present in infected brain regions where there was significant inhibition of HIV replication.105 Intracellular transfer of nanoART from MDM to brain microvascular endothelial cells was confirmed in vitro by Kanmogne et al., and this transfer could be enhanced by addition of folate on the nanoparticle surface as a targeting ligand.108 The results demonstrated that nanoART could transfer NP through cell-to-cell contacts, and thus facilitate the penetration of nanoART across the BBB. By targeting drugs to MP, delivery can be achieved to sites of disease or infection that are normally inaccessible to free drug in circulation.
Of importance, however, an effective cell-based delivery system is dependent on the normal function of the cell carrier. Thus, for macrophages, their normal functions of phagocytosis, migration, and release of immune-modulating cytokines and chemokines should be maintained in nanoparticle-loaded cells. Martinez-Skinner et al. used dynamic global proteomic changes in macrophages loaded with nanoART to identify changes linked to immune cell migration and chemotaxis, cytokine and chemokine production, lipid metabolism, free radical scavenging, and cell differentiation.109 Protein changes were substantiated by functional assays that indicated nanoART uptake induced a macrophage activation phenotype that is primed for further nanoART uptake, storage and cell migration, which would thus enhance the capacity of the cell to deliver drug to the site of disease.109
For cell-based drug delivery, the carrier cell must deliver sufficient amounts of therapeutically active drug to the site of infection and disease. For this to occur, once inside the cell, the drug must be localized in stable, non-degrading subcellular compartments that facilitate release at the target site.110 Cationic nanoparticles decrease acidification of lysosomal compartments, and thus are generally less likely to be degraded than are anionic particles.103 In addition, trafficking of nanoparticles to non-degrading endosomal subcellular compartments can also reduce lysosomal degradation. Our studies on macrophage delivery of nanoART, demonstrated that ritonavir nanoART prepared with P188, DSPE-mPEG2000, and DOTAP as surfactants were taken up by macrophages via clathrin-mediated endocytosis and trafficked to recycling (Rab 11+ and Rab 14+) endosomal compartments.111 Therapeutically active nanoparticles were released intact at the cell surface. Of particular importance, active targeting of the nanoformulated antimicrobials to specific cell compartments not only enhanced cell storage, but also allowed the drug to be directed to the cell compartments where the infectious agents replicate.111 Such an effect was recently demonstrated for nanoART; wherein, atazanavir nanoART were co-localized in the same macrophage endosomal compartments utilized for HIV-1 replication.112 Thus, not only can nanoART be carried intact by macrophages to sites of disease; but for microbial and viral infections that target macrophages, the drug may also be delivered to the site of microbial and viral replication.
Nanotechnological approaches can impact not only therapy, but also imaging, diagnostics and theranostics to enable early disease diagnosis coupled with therapeutics as well as morphological and/or functional imaging. Such approaches allow for investigation of the disease progression or recovery associated with different therapeutic approaches such as nanoparticle or stem-cell based strategies.
4. Nanomedicine and neurodegenerative diseases
Neurodegenerative diseases, such as PD, AD, and amyotrophic lateral sclerosis (ALS), represent a wide range of devastating progressive conditions associated with the deterioration or loss of neurons in specific locations of the CNS. A major challenge in the treatment of neurodegenerative diseases, including PD is the restricted access of drug molecules across the BBB. In this regard, nanotechnology-based drug delivery strategies hold great potential in the management and treatment of these diseases. In this section, we will discuss the current applications of nano-based drug-delivery systems for the treatment of neurodegenerative disorders with particular emphasis on PD.
As the second most common neurodegenerative disorder, PD affects over a million Americans with an annual cost of several billion dollars. The disease is characterized by the loss of dopaminergic neurons in the substantia nigra pars compacta of the mid-brain with a drastic decrease in striatal dopamine and its metabolites. Another important pathological hallmark of PD is the production of intraneuronal proteinaceous cytoplasmic inclusions called Lewy bodies, the primary structural component of which is α-synuclein. Cardinal motor signs of PD include resting tremor, rigidity, bradykinesia and postural instability. It has been increasingly recognized that the non-motor symptoms, such as sleep disturbances, depression, cognitive impairment, anosmia, constipation and autonomic dysfunctions precede the classic motor symptoms by several years.113 Currently, the gold standard of treatment for PD remains the oral administration of dopamine agonists such as levodopa. Although levodopa provides the greatest benefit for motor symptoms, it is unable to stop or compensate for the continual loss of dopamine neurons. Furthermore, the effectiveness of levodopa fades rapidly; and its long-term use often results in serious motor fluctuations. Thus, more effective treatments for PD patients are urgently needed.
In recent years, nanotechnology has been employed in an effort to enhance the efficacy of PD therapy. Major advantages of using nanosystems as drug delivery agents include specific delivery for targeted action in the CNS, effectively overcoming barriers to CNS, and improving the bioavailability and therapeutic efficacy of anti-parkinsonian agents. One specific example of nanotechnology in advanced experimental treatment of PD is the brain-targeted delivery of dopamine. Using an intracranial nano-enabled scaffold device implantable in the parenchyma of the frontal lobe of the brain, Pillay and colleagues showed that the inclusion of dopamine-loaded cellulose acetate phthalate NPs into a binary cross-linked alginate scaffold facilitated local dopamine delivery in a rat model.114 Recently, systemic delivery of dopamine has been developed. Trapani et al. found that dopamine-loaded chitosan NPs were less cytotoxic than free dopamine in vitro. In vivo brain microdialysis experiments in rats demonstrated that intraperitoneal administration of the dopamine-loaded chitosan NPs effectively increased striatal dopamine levels.115, 116 Unfortunately, these studies did not explore the efficacy of administering dopamine-loaded NPs in modulating motor activity and brain biochemical changes in an animal model of PD. In a very recent study, Rashed et al. attempted to use polyvinylpyrrolidone-poly (acrylic acid) (PVP/PAA) nanogels synthesized by γ-radiation-induced template polymerization to systemically deliver dopamine to the brain.117 Intraperitoneal administration of the dopamine-loaded PVP/PAA nanogels improved striatal dopamine levels and catalepsy scores in reserpine-treated rats. Significant increases in their long-term survival and restoration of their normal activity were also found in the reserpine-treated rats following subchronic administration of dopamine-loaded PVP/PAA nanogels. Additional animal experiments performed in a rotenone PD rat model demonstrated that dopamine-loaded PVP/PAA nanogels improved the mitochondrial dysfunction induced by rotenone. Nonetheless, these disease-modifying effects of nanogel-based delivery remain preliminary and need further confirmation.
Nanodelivery of dopaminergic agonists like levodopa, apomorphine, ropinirole and bromocriptine is being pursued also because of the potential to improve brain uptake and reduce side effects associated with these compounds. Levodopa methyl ester, a highly soluble pro-drug that is hydrolysable by plasma esterases, was encapsulated with benserazide in poly (lactic-co-glycolic acid) (PLGA) NPs. This method of administering levodopa successfully abolished levodopa-induced dyskinesia in rats.118 More recently, intranasal delivery of levodopa NPs has been explored.119 Levodopa encapsulated in chitosan NPs was incorporated in a thermo-reversible gel prepared using Pluronic PF127, and then delivered via intranasal route, which increased drug levels in the brain.119 In another study, Md et al. developed a system for nose-to-brain delivery of bromocriptine-loaded chitosan NPs.120 Intranasal administration of bromocriptine-loaded chitosan NPs effectively increased brain uptake of bromocriptine and prevented haloperidol-induced catalepsy and akinesia in a mouse PD model. An oil-based nanocarrier system (nanoemulsion gel) for ropinirole transdermal delivery has shown efficacy in the 6-OHDA-lesioned rat model.121 While these studies have shown some promise in improving the bioavailability of dopaminergic agonists, more pre-clinical validations are needed before being applied in clinical settings.
Other targeting molecules include antioxidants,122, 123 peptides,124 and neurotrophic factors. The neurotrophic factor nerve growth factor (NGF) was absorbed on PBCA NPs coated with polysorbate 80 to enhance its pharmacological efficacy in the brain.125 Intravenous administration of the NP-bound NGF prevented amnesia and improved memory in the acute scopolamine-induced amnesia rat model. This formulation also demonstrated significant protection against MPTP-induced motor symptoms. Even combinatorial delivery of several neurotrophic factors has been recently investigated, yielding promising outcomes.126, 127 Lectin-functionalized, polyethylene glycol–block-poly-(d,l)-lactic-co-glycolic acid NPs loaded with haloperidol and further functionalized with Solanum tuberosum lectin (STL) achieved higher drug concentrations in the striatum when administered intranasally than when delivered by intraperitoneal injection.128 The study also found a significantly higher percentage of STL-functionalized NPs present in the striatum and olfactory bulb relative to non-functionalized NPs. To cite another example, the macromolecular drug urocortin peptide, when encapsulated in odorranalectin-conjugated PEG-PLGA NPs, was able to reduce dopaminergic neurodegeneration and subsequent behavioral deficits in hemi-Parkinsonian rats.129 Recently, Mito-apocynin, a derivative of apocynin, which is a known NADPH oxidase inhibitor, has been shown to attenuate behavioral deficits in LRRK2 transgenic mouse model of PD.130 Ongoing studies are focused on encapsulating mito-apocynin with polyanhydride nanoparticles to prolong the brain bioavailability of the drug.
Despite the relatively early stages of their development, overall these nanodelivery systems continue to represent a promising new direction for PD therapy. Furthermore, most current studies were performed in rodent models; and thus, their therapeutic potential to treat various neurodegenerative diseases has yet to be evaluated in pre-clinical animal models before eventual clinical testing. Oxidative stress and neuroinflammation have been established as major pathophysiological mechanisms of many neurological diseases including PD, but also for AD and ALS. Inflammation caused by trauma, infection or even through the process of aging generates reactive oxygenated species and reactive nitrogen species resulting in the activation of microglia. While microglia help to contain the basal inflammation at quiescent stage, they end up, during excessive activation, secreting more pro-inflammatory cytokines including TNF-α. Both neurons as well as glial cells produce antioxidants.131 However, the persistence of free radicals beyond the pool of available antioxidants leads to neurodegeneration and subsequent neurological symptoms. Currently, efforts are being made to develop and transport naturally occurring and synthetically made antioxidants as well as neuroprotective drugs to the brain.
A major drawback of using such antioxidants alone is their failure to cross the BBB as well as the inability to target deeper areas of the brain such as the hippocampus, midbrain and brain stem. Hence larger and repeated doses of the drug are needed to elicit a protective response. Drugs encapsulated in nanoparticles, on the other hand, can effectively cross the BBB owing to their small size and in some cases, their chemistry.132, 133 In addition, surface functionalization of nanoparticles with a ligand whose membrane receptors are present on specific neurons can help target these nanoparticles to these neurons. For example, PLGA-coated curcumin NPs functionalized with Tet-1 peptide were able to eliminate amyloid aggregates.134 The Tet-1 functionalization allowed better uptake of NPs in GI-1 glioma cells as evidenced by flow cytometry analysis; however, these results were not validated by in vivo studies. Melanocortin-loaded polysorbate 80-coated NPs reduced lipid peroxidation while increasing antioxidant reactivity in various regions of the brain.135 In another experiment, intravenous or oral administration of dalargin-adsorbed NPs provided analgesic effects as assessed by the hot-plate test in mice. The same drug, when delivered without NPs was unable to provide pain-relief, as the drug could not cross the BBB.136 However, if the nanoformulation is administered via the circulatory system, they acquire opsonins on their surface and can be phagocytized.137 One way to circumvent this problem is to deliver nanoparticles via intra-nasal injections. The particles then reach the CNS via the olfactory or trigeminal tracts. For example, nasal injection of nimodipine (calcium channel blocker)-encapsulated methoxy poly(ethylene glycol)-poly(lactic acid) (MPEG-PLA) NPs lead to 1.6- to 3.3-fold higher drug concentrations in the brain than nasal administration of the drug solution alone.138
Nanomaterials can also be therapeutically used to modulate detrimental immune responses in the CNS. Multiple sclerosis (MS) is an immune-mediated inflammatory disease of the CNS, characterized by destruction of the protective myelin sheath that insulates neurons. Recent evidence has highlighted the possibility of using nanotechnology in MS patients as a potential new tool to deliver drugs with immunosuppressive activity. In a study of EAE, which is the most commonly used experimental model for MS, Kizelsztein et al. demonstrated that encapsulation of tempamine, a stable radical with antioxidant and proapoptotic activities, in nanoliposomes shows efficacy in inhibiting EAE in mice.139 Later, Yeste and colleagues used Au NPs to deliver a tolerogenic compound in combination with oligodendrocyte antigen to dendritic cells to induce antigen-specific regulatory T cells (Tregs). Au NPs loaded with an aryl hydrocarbon receptor ligand and a T-cell epitope from myelin oligodendrocyte glycoprotein (MOG35-55) promoted the generation of Tregs by dendritic cells in vitro. When injected intraperitoneally to EAE mice, these NPs effectively increased the Treg population and suppressed disease development.140 Using the relapsing-remitting EAE (R-EAE) in a murine Th1/17-mediated model of EAE, Hunter et al. developed a biodegradable PLG NP as a myelin antigen carrier and showed its efficacy in generating robust tolerance and preventing disease development.141
Dendrimers are repetitively branched molecules with nanometer-scale dimensions. Owing to their intrinsic ability to localize to activated microglia and astrocytes, dendrimers can be used against immune diseases by delivering immunosuppressive drugs to target tissues. For example, polyamidoamine (PAMAM) dendrimers have been reported by Wang et al. to deliver the antioxidant and anti-inflammatory agent N-acetyl cysteine (NAC) to the brain.142 In vitro experiments indicated that conjugating NAC with a PAMAM dendrimer increased its antioxidant and anti-inflammatory properties relative to free NAC. Subsequently, another study from the same group demonstrated in an animal model that intravenously administered dendrimer-NAC conjugates localized in the inflammation sites associated with cerebral palsy, leading to reduced neuroinflammation and improved cerebral palsy symptoms.143 Similarly, the dendrimer-based strategy achieved sustained suppression of inflammation in a model of retinal degeneration.144 More recently, in a spinal cord injury study, Cerqueira et al. showed that methylprednisolone-loaded carboxymethylchitosan dendrimer NPs are internalized by microglia, astrocytes and oligodendrocytes, modulating the release of growth factors while limiting the titer of pro-inflammatory molecules.145 Moreover, local administration of these dendrimer NPs to the spinal cord of Wistar rats following lateral hemi-section lesions improved their locomotor outcomes. In recent years, much attention has been focused on neurological disorders (chronic traumatic encephalopathies, CTE) that develop following single or repeated head injuries. Repetitive mild traumatic brain injury can lead to diffuse axonal injury as well as persistent neuroinflammation. This persistent neuroinflammation and associated neurodegeneration can lead to the development of chronic neurodegenerative diseases. The use of nanoformulations as a potential drug delivery platform to the CNS could prove critical in treating CTE.146-148 Indeed, Ruozi et al. have shown that cerebrolysin-loaded poly-lactide-co-glycolide NPs reduced brain edema and possibly limited the degree of BBB permeability typically seen after concussive head injury.149
5. Nanotoxicology
Man-made nanoparticles provide new opportunities for the creation of new consumer products and the manufacture of new materials for therapeutic and imaging applications as discussed here. Likewise, their potential benefit to human health has increased exponentially in recent years, but realizing this potential requires that any adverse effects to human health be minimized and characterized.
Nanotoxicology is a new branch of toxicology that addresses the adverse human and environmental health effects associated with nanoparticles.150-156 The main source of nanotoxicity comes from environmental and occupational exposure to nanoparticles derived from metals such as copper, magnesium, sodium, potassium, calcium and iron. A second, more recent source of nanotoxicity stems from specialized nanoparticles serving as novel platforms for the target-specific delivery of therapeutics. Although their benefits tend to outweigh their ill effects, nanotoxic and immunogenic aspects of nanoparticles can no longer be overlooked. Upon passive entry into the cell, the nanoparticles have direct access to the cytoplasm and subcellular organelles. Depending on their intracellular localization, nanoparticles can induce oxidative stress, inflammation, DNA damage, cardiovascular effects and coagulation.157 Nanoparticles have been shown to enter the brain primarily by inhalation, specifically by crossing into the brain through the olfactory nerves.158 Besides the CNS, nanoparticles also enter the GI-tract, circulatory system, liver, kidney, spleen and lymphatic systems. Diseases such as asthma, bronchitis, emphysema, lung cancer, neurodegenerative diseases, Crohn's disease, colon cancer, arteriosclerosis, blood clots, arrhythmia and heart diseases, systemic lupus erythematosus, scleroderma, and rheumatoid arthritis as well as liver and spleen diseases are all associated with nanoparticle toxicity.158-160
Metal nanoparticles are particularly toxic to the CNS. Adverse neurological effects due to occupational exposure of non-particulate manganese (Mn), aluminium (Al) and iron are relatively well known. For example, chronic exposure to Al and iron have been linked to both PD and AD.161 Mn and neurotoxicity are now clearly linked in humans.162, 163 In the condition known as manganism, occupational exposure to Mn in miners and welders results in psychiatric and motor disturbances, with symptoms resembling those of idiopathic PD and that contribute to its etiopathogenesis. Currently, Mn nanomaterials are being pursued in metallurgic and chemical sectors;164 and therefore, neurotoxicological research on emerging Mn nanoparticle technologies are urgently needed.164
Our own recent work characterizing the neurotoxicological effects of Mn nanoparticles on dopaminergic neuronal cells suggests that environmental exposure to certain metallic nanoparticles may cause serious health problems in humans.165 Thus, a systematic characterization of potential adverse effects of nanomaterials will ultimately help formulate benign nanoformulations for human applications.
6. Conclusions and Outlook
Nanomedicine offers exciting possibilities to overcome the significant challenges associated with diagnosis, imaging and therapies to address the malfunction of the nervous system. This is a broad area of research with enormous potential and current efforts represent just the tip of the iceberg.
Nanoscale systems are extremely promising for safe, effective, targeted/site-specific, and sustained delivery of anti-inflammatory and immunomodulatory agents, growth factors and other bioactive molecules to treat neurodegenerative disorders and infectious diseases. Nanomedicine enables therapeutics and imaging agents to effectively cross the blood brain barrier. In this context, immunoprotective approaches harnessing the immune system through nanotechnology to address neurodegenerative disorders and traumatic brain injury are very novel and can provide a new paradigm for the treatment of such conditions.
While therapies represent an important aspect of nanoneuromedicine, diagnostics as well as imaging can benefit enormously from recent developments in this new field as well. Delivery of therapeutics as well as imaging and contrast agents need to overcome some similar challenges such as being able to traverse the BBB. Advances in delivery of therapeutics can lead to better diagnostics, better delivery of imaging agents and development of new theranostics as well. Much of the work in this area so far has been conducted in various animal models; and showing efficacy in clinical studies, while addressing any potential nanotoxicological issues, is the next important step in moving this field forward.
Acknowledgement
We thank Robin Taylor for outstanding editorial and graphic support.
Funding: Funding for this work (Grant account no. W81XWH-11-1-0700) was provided by the US Army Medical Research and Materiel Command. University of Nebraska Medical Center research support includes individual donations from Dr. Carol Swarts and Frances and Louie Blumkin, the Vice Chancellor's office of Research, University of Nebraska Medical Center, and ViiV Healthcare. National Institutes of Health grants to researchers at Iowa State University and the University of Nebraska Medical Center: P01 MH064570, R01 MH104147, P01 DA028555, R01 NS036126, P01 NS031492, 2R01 NS034239, P30 MH062261, R01 AG043540, P01 NS43985, (HEG); R01 NS070190 (RLM); P20GM103480 (TB), R01 NS 074443R01 NS039958, R01 ES010586 and R01 ES019267 (AGK) are gratefully acknowledged.
Abbreviations
- Aβ
amyloid beta
- AD
Alzheimer's disease
- ADDL
amyloid-β-derived diffusible ligands
- ALS
amyotrophic lateral sclerosis
- ApoE
apolipoprotein E
- ART
antiretroviral therapy
- BBB
blood-brain barrier
- CNS
central nervous system
- CSF
cerebral spinal fluid
- CTE
chronic traumatic encephalopathies
- EAE
experimental autoimmune encephalomyelitis
- MOG
myelin oligodendrocyte glycoprotein
- MP
mononuclear phagocytes
- MPIO
microparticles of iron oxide
- MRI
magnetic resonance imaging
- MS
multiple sclerosis
- NAC
N-acetyl cysteine
- NGF
nerve growth factor
- NRTI
nucleoside reverse transcriptase inhibitors
- PAMAM
polyamidoamine
- PBCA
poly(n-butyl cyanoacrylate)
- PD
Parkinson's disease
- PEG
polyethylene glycol
- PET
positron emission tomography
- QD
quantum dots
- RES
reticuloendothelial system
- SLNs
solid lipid nanoparticles
- SPIO
superparamagnetic iron oxide
- STL
Solanum tuberosum lectin
- Tregs
regulatory T cells
- USPIO
ultra-small superparamagnetic iron oxide
- VCAM
vascular cell adhesion molecule-1
- VSPIO
very-small superparamagnetic iron oxide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Singh D, McMillan JM, Kabanov AV, Sokolsky-Papkov M, Gendelman HE. Bench-to-bedside translation of magnetic nanoparticles. Nanomedicine (Lond) 2014;9:501–16. doi: 10.2217/nnm.14.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alam MI, Beg S, Samad A, Baboota S, Kohli K, Ali J, et al. Strategy for effective brain drug delivery. Eur J Pharm Sci. 2010;40:385–403. doi: 10.1016/j.ejps.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Wang H, Chen X. Applications for site-directed molecular imaging agents coupled with drug delivery potential. Expert Opin Drug Deliv. 2009;6:745–68. doi: 10.1517/17425240902889751. [DOI] [PubMed] [Google Scholar]
- 4.Jack CR, Jr., Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, et al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–16. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ni R, Gillberg PG, Bergfors A, Marutle A, Nordberg A. Amyloid tracers detect multiple binding sites in Alzheimer's disease brain tissue. Brain. 2013;136:2217–27. doi: 10.1093/brain/awt142. [DOI] [PubMed] [Google Scholar]
- 6.Nordberg A, Rinne JO, Kadir A, Langstrom B. The use of PET in Alzheimer disease. Nat Rev Neurol. 2010;6:78–87. doi: 10.1038/nrneurol.2009.217. [DOI] [PubMed] [Google Scholar]
- 7.Laurent S, Forge D, Port M, Roch A, Robic C, Vander Elst L, et al. Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem Rev. 2008;108:2064–110. doi: 10.1021/cr068445e. [DOI] [PubMed] [Google Scholar]
- 8.Wagner S, Schnorr J, Pilgrimm H, Hamm B, Taupitz M. Monomer-coated very small superparamagnetic iron oxide particles as contrast medium for magnetic resonance imaging: preclinical in vivo characterization. Invest Radiol. 2002;37:167–77. doi: 10.1097/00004424-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Weinstein JS, Varallyay CG, Dosa E, Gahramanov S, Hamilton B, Rooney WD, et al. Superparamagnetic iron oxide nanoparticles: diagnostic magnetic resonance imaging and potential therapeutic applications in neurooncology and central nervous system inflammatory pathologies, a review. J Cereb Blood Flow Metab. 2010;30:15–35. doi: 10.1038/jcbfm.2009.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie J, Liu G, Eden HS, Ai H, Chen X. Surface-engineered magnetic nanoparticle platforms for cancer imaging and therapy. Acc Chem Res. 2011;44:883–92. doi: 10.1021/ar200044b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stark DD, Weissleder R, Elizondo G, Hahn PF, Saini S, Todd LE, et al. Superparamagnetic iron oxide: clinical application as a contrast agent for MR imaging of the liver. Radiology. 1988;168:297–301. doi: 10.1148/radiology.168.2.3393649. [DOI] [PubMed] [Google Scholar]
- 12.Ros PR, Freeny PC, Harms SE, Seltzer SE, Davis PL, Chan TW, et al. Hepatic MR imaging with ferumoxides: a multicenter clinical trial of the safety and efficacy in the detection of focal hepatic lesions. Radiology. 1995;196:481–8. doi: 10.1148/radiology.196.2.7617864. [DOI] [PubMed] [Google Scholar]
- 13.Weissleder R, Elizondo G, Wittenberg J, Lee AS, Josephson L, Brady TJ. Ultrasmall superparamagnetic iron oxide: an intravenous contrast agent for assessing lymph nodes with MR imaging. Radiology. 1990;175:494–8. doi: 10.1148/radiology.175.2.2326475. [DOI] [PubMed] [Google Scholar]
- 14.Lu M, Cohen MH, Rieves D, Pazdur R. FDA report: Ferumoxytol for intravenous iron therapy in adult patients with chronic kidney disease. Am J Hematol. 2010;85:315–9. doi: 10.1002/ajh.21656. [DOI] [PubMed] [Google Scholar]
- 15.Neuwelt EA, Varallyay CG, Manninger S, Solymosi D, Haluska M, Hunt MA, et al. The potential of ferumoxytol nanoparticle magnetic resonance imaging, perfusion, and angiography in central nervous system malignancy: a pilot study. Neurosurgery. 2007;60:601–11. doi: 10.1227/01.NEU.0000255350.71700.37. [DOI] [PubMed] [Google Scholar]
- 16.Gahramanov S, Raslan AM, Muldoon LL, Hamilton BE, Rooney WD, Varallyay CG, et al. Potential for differentiation of pseudoprogression from true tumor progression with dynamic susceptibility-weighted contrast-enhanced magnetic resonance imaging using ferumoxytol vs. gadoteridol: a pilot study. Int J Radiat Oncol Biol Phys. 2011;79:514–23. doi: 10.1016/j.ijrobp.2009.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kleinschnitz C, Bendszus M, Frank M, Solymosi L, Toyka KV, Stoll G. In vivo monitoring of macrophage infiltration in experimental ischemic brain lesions by magnetic resonance imaging. J Cereb Blood Flow Metab. 2003;23:1356–61. doi: 10.1097/01.WCB.0000090505.76664.DB. [DOI] [PubMed] [Google Scholar]
- 18.Wiart M, Davoust N, Pialat JB, Desestret V, Moucharrafie S, Cho TH, et al. MRI monitoring of neuroinflammation in mouse focal ischemia. Stroke. 2007;38:131–7. doi: 10.1161/01.STR.0000252159.05702.00. [DOI] [PubMed] [Google Scholar]
- 19.Saleh A, Schroeter M, Ringelstein A, Hartung HP, Siebler M, Modder U, et al. Iron oxide particle-enhanced MRI suggests variability of brain inflammation at early stages after ischemic stroke. Stroke. 2007;38:2733–7. doi: 10.1161/STROKEAHA.107.481788. [DOI] [PubMed] [Google Scholar]
- 20.Beckmann N, Gerard C, Abramowski D, Cannet C, Staufenbiel M. Noninvasive magnetic resonance imaging detection of cerebral amyloid angiopathy-related microvascular alterations using superparamagnetic iron oxide particles in APP transgenic mouse models of Alzheimer's disease: application to passive Abeta immunotherapy. J Neurosci. 2011;31:1023–31. doi: 10.1523/JNEUROSCI.4936-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dousset V, Brochet B, Deloire MS, Lagoarde L, Barroso B, Caille JM, et al. MR imaging of relapsing multiple sclerosis patients using ultra-small-particle iron oxide and compared with gadolinium. AJNR Am J Neuroradiol. 2006;27:1000–5. [PMC free article] [PubMed] [Google Scholar]
- 22.Oude Engberink RD, Blezer EL, Hoff EI, van der Pol SM, van der Toorn A, Dijkhuizen RM, et al. MRI of monocyte infiltration in an animal model of neuroinflammation using SPIO-labeled monocytes or free USPIO. J Cereb Blood Flow Metab. 2008;28:841–51. doi: 10.1038/sj.jcbfm.9600580. [DOI] [PubMed] [Google Scholar]
- 23.Lyons SA, O'Neal J, Sontheimer H. Chlorotoxin, a scorpion-derived peptide, specifically binds to gliomas and tumors of neuroectodermal origin. Glia. 2002;39:162–73. doi: 10.1002/glia.10083. [DOI] [PubMed] [Google Scholar]
- 24.Veiseh O, Sun C, Gunn J, Kohler N, Gabikian P, Lee D, et al. Optical and MRI multifunctional nanoprobe for targeting gliomas. Nano Lett. 2005;5:1003–8. doi: 10.1021/nl0502569. [DOI] [PubMed] [Google Scholar]
- 25.Wadghiri YZ, Li J, Wang J, Hoang DM, Sun Y, Xu H, et al. Detection of amyloid plaques targeted by bifunctional USPIO in Alzheimer's disease transgenic mice using magnetic resonance microimaging. PLoS One. 2013;8:e57097. doi: 10.1371/journal.pone.0057097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elices MJ, Osborn L, Takada Y, Crouse C, Luhowskyj S, Hemler ME, et al. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990;60:577–84. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- 27.Cannella B, Raine CS. The adhesion molecule and cytokine profile of multiple sclerosis lesions. Ann Neurol. 1995;37:424–35. doi: 10.1002/ana.410370404. [DOI] [PubMed] [Google Scholar]
- 28.Polman CH, O'Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]
- 29.McAteer MA, Sibson NR, von Zur Muhlen C, Schneider JE, Lowe AS, Warrick N, et al. In vivo magnetic resonance imaging of acute brain inflammation using microparticles of iron oxide. Nat Med. 2007;13:1253–8. doi: 10.1038/nm1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoyte LC, Brooks KJ, Nagel S, Akhtar A, Chen R, Mardiguian S, et al. Molecular magnetic resonance imaging of acute vascular cell adhesion molecule-1 expression in a mouse model of cerebral ischemia. J Cereb Blood Flow Metab. 2010;30:1178–87. doi: 10.1038/jcbfm.2009.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Serres S, Mardiguian S, Campbell SJ, McAteer MA, Akhtar A, Krapitchev A, et al. VCAM-1-targeted magnetic resonance imaging reveals subclinical disease in a mouse model of multiple sclerosis. FASEB J. 2011;25:4415–22. doi: 10.1096/fj.11-183772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.von Andrian UH, Mackay CR. T-cell function and migration. Two sides of the same coin. N Engl J Med. 2000;343:1020–34. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- 33.van Kasteren SI, Campbell SJ, Serres S, Anthony DC, Sibson NR, Davis BG. Glyconanoparticles allow pre-symptomatic in vivo imaging of brain disease. Proc Natl Acad Sci U S A. 2009;106:18–23. doi: 10.1073/pnas.0806787106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fukuda MN, Ohyama C, Lowitz K, Matsuo O, Pasqualini R, Ruoslahti E, et al. A peptide mimic of E-selectin ligand inhibits sialyl Lewis X-dependent lung colonization of tumor cells. Cancer Res. 2000;60:450–6. [PubMed] [Google Scholar]
- 35.Chapon C, Franconi F, Lacoeuille F, Hindre F, Saulnier P, Benoit JP, et al. Imaging E-selectin expression following traumatic brain injury in the rat using a targeted USPIO contrast agent. MAGMA. 2009;22:167–74. doi: 10.1007/s10334-008-0161-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blasi P, Giovagnoli S, Schoubben A, Ricci M, Rossi C. Solid lipid nanoparticles for targeted brain drug delivery. Adv Drug Deliv Rev. 2007;59:454–77. doi: 10.1016/j.addr.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 37.Hasadsri L, Kreuter J, Hattori H, Iwasaki T, George JM. Functional protein delivery into neurons using polymeric nanoparticles. J Biol Chem. 2009;284:6972–81. doi: 10.1074/jbc.M805956200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koffie RM, Farrar CT, Saidi LJ, William CM, Hyman BT, Spires-Jones TL. Nanoparticles enhance brain delivery of blood-brain barrier-impermeable probes for in vivo optical and magnetic resonance imaging. Proc Natl Acad Sci U S A. 2011;108:18837–42. doi: 10.1073/pnas.1111405108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roney CA, Arora V, Kulkarni PV, Antich PP, Bonte FJ. Nanoparticulate radiolabelled quinolines detect amyloid plaques in mouse models of Alzheimer's disease. Int J Alzheimers Dis. 2009;2009:481031. doi: 10.4061/2009/481031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sykova E, Jendelova P. In vivo tracking of stem cells in brain and spinal cord injury. Prog Brain Res. 2007;161:367–83. doi: 10.1016/S0079-6123(06)61026-1. [DOI] [PubMed] [Google Scholar]
- 41.Kubinova S, Sykova E. Nanotechnology for treatment of stroke and spinal cord injury. Nanomedicine (Lond) 2010;5:99–108. doi: 10.2217/nnm.09.93. [DOI] [PubMed] [Google Scholar]
- 42.Borlongan CV. Recent preclinical evidence advancing cell therapy for Alzheimer's disease. Exp Neurol. 2012;237:142–6. doi: 10.1016/j.expneurol.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song M, Mohamad O, Gu X, Wei L, Yu SP. Restoration of intracortical and thalamocortical circuits after transplantation of bone marrow mesenchymal stem cells into the ischemic brain of mice. Cell Transplant. 2013;22:2001–15. doi: 10.3727/096368912X657909. [DOI] [PubMed] [Google Scholar]
- 44.Ugoya SO, Tu J. Bench to bedside of neural stem cell in traumatic brain injury. Stem Cells Int. 2012;2012:141624. doi: 10.1155/2012/141624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bulte JW. In vivo MRI cell tracking: clinical studies. AJR Am J Roentgenol. 2009;193:314–25. doi: 10.2214/AJR.09.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bulte JW, Walczak P, Gleich B, Weizenecker J, Markov DE, Aerts HC, et al. MPI Cell Tracking: What Can We Learn from MRI? Proc Soc Photo Opt Instrum Eng. 2011;7965:79650z. doi: 10.1117/12.879844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berman SC, Galpoththawela C, Gilad AA, Bulte JW, Walczak P. Long-term MR cell tracking of neural stem cells grafted in immunocompetent versus immunodeficient mice reveals distinct differences in contrast between live and dead cells. Magn Reson Med. 2011;65:564–74. doi: 10.1002/mrm.22613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li L, Jiang W, Luo K, Song H, Lan F, Wu Y, et al. Superparamagnetic iron oxide nanoparticles as MRI contrast agents for non-invasive stem cell labeling and tracking. Theranostics. 2013;3:595–615. doi: 10.7150/thno.5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, et al. Quantum dots for live cells, in vivo imaging, and diagnostics. Science. 2005;307:538–44. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dahan M, Levi S, Luccardini C, Rostaing P, Riveau B, Triller A. Diffusion dynamics of glycine receptors revealed by single-quantum dot tracking. Science. 2003;302:442–5. doi: 10.1126/science.1088525. [DOI] [PubMed] [Google Scholar]
- 51.Wang J, Yong WH, Sun Y, Vernier PT, Koeffler HP, Gundersen MA, et al. Receptor-targeted quantum dots: fluorescent probes for brain tumor diagnosis. J Biomed Opt. 2007;12:044021. doi: 10.1117/1.2764463. [DOI] [PubMed] [Google Scholar]
- 52.Feng L, Long HY, Liu RK, Sun DN, Liu C, Long LL, et al. A quantum dot probe conjugated with abeta antibody for molecular imaging of Alzheimer's disease in a mouse model. Cell Mol Neurobiol. 2013;33:759–65. doi: 10.1007/s10571-013-9943-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lammers T, Aime S, Hennink WE, Storm G, Kiessling F. Theranostic nanomedicine. Acc Chem Res. 2011;44:1029–38. doi: 10.1021/ar200019c. [DOI] [PubMed] [Google Scholar]
- 54.Mura S, Couvreur P. Nanotheranostics for personalized medicine. Adv Drug Deliv Rev. 2012;64:1394–416. doi: 10.1016/j.addr.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 55.Reddy GR, Bhojani MS, McConville P, Moody J, Moffat BA, Hall DE, et al. Vascular targeted nanoparticles for imaging and treatment of brain tumors. Clin Cancer Res. 2006;12:6677–86. doi: 10.1158/1078-0432.CCR-06-0946. [DOI] [PubMed] [Google Scholar]
- 56.Yang HW, Huang CY, Lin CW, Liu HL, Huang CW, Liao SS, et al. Gadolinium-functionalized nanographene oxide for combined drug and microRNA delivery and magnetic resonance imaging. Biomaterials. 2014;35:6534–42. doi: 10.1016/j.biomaterials.2014.04.057. [DOI] [PubMed] [Google Scholar]
- 57.Agulla J, Brea D, Argibay B, Novo M, Campos F, Sobrino T, et al. Quick adjustment of imaging tracer payload, for in vivo applications of theranostic nanostructures in the brain. Nanomedicine. 2014;10:851–8. doi: 10.1016/j.nano.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 58.Agulla J, Brea D, Campos F, Sobrino T, Argibay B, Al-Soufi W, et al. In vivo theranostics at the peri-infarct region in cerebral ischemia. Theranostics. 2013;4:90–105. doi: 10.7150/thno.7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.De Gruttola VG, Clax P, DeMets DL, Downing GJ, Ellenberg SS, Friedman L, et al. Considerations in the evaluation of surrogate endpoints in clinical trials. summary of a National Institutes of Health workshop. Control Clin Trials. 2001;22:485–502. doi: 10.1016/s0197-2456(01)00153-2. [DOI] [PubMed] [Google Scholar]
- 60.Zhang X, Guo Q, Cui D. Recent advances in nanotechnology applied to biosensors. Sensors (Basel) 2009;9:1033–53. doi: 10.3390/s90201033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Choi YE, Kwak JW, Park JW. Nanotechnology for early cancer detection. Sensors (Basel) 2010;10:428–55. doi: 10.3390/s100100428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang Y, Guo Y, Xianyu Y, Chen W, Zhao Y, Jiang X. Nanomaterials for ultrasensitive protein detection. Adv Mater. 2013;25:3802–19. doi: 10.1002/adma.201301334. [DOI] [PubMed] [Google Scholar]
- 63.Shao H, Yoon TJ, Liong M, Weissleder R, Lee H. Magnetic nanoparticles for biomedical NMR-based diagnostics. Beilstein J Nanotechnol. 2010;1:142–54. doi: 10.3762/bjnano.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Georganopoulou DG, Chang L, Nam JM, Thaxton CS, Mufson EJ, Klein WL, et al. Nanoparticle-based detection in cerebral spinal fluid of a soluble pathogenic biomarker for Alzheimer's disease. Proc Natl Acad Sci U S A. 2005;102:2273–6. doi: 10.1073/pnas.0409336102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zengin A, Tamer U, Caykara T. A SERS-based sandwich assay for ultrasensitive and selective detection of Alzheimer's tau protein. Biomacromolecules. 2013;14:3001–9. doi: 10.1021/bm400968x. [DOI] [PubMed] [Google Scholar]
- 66.Lee JH, Kang DY, Lee T, Kim SU, Oh BK, Choil JW. Signal enhancement of surface plasmon resonance based immunosensor using gold nanoparticle-antibody complex for beta-amyloid (1-40) detection. J Nanosci Nanotechnol. 2009;9:7155–60. doi: 10.1166/jnn.2009.1613. [DOI] [PubMed] [Google Scholar]
- 67.Kang DY, Lee JH, Oh BK, Choi JW. Ultra-sensitive immunosensor for beta-amyloid (1-42) using scanning tunneling microscopy-based electrical detection. Biosens Bioelectron. 2009;24:1431–6. doi: 10.1016/j.bios.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 68.Yang CC, Yang SY, Chieh JJ, Horng HE, Hong CY, Yang HC, et al. Biofunctionalized magnetic nanoparticles for specifically detecting biomarkers of Alzheimer's disease in vitro. ACS Chem Neurosci. 2011;2:500–5. doi: 10.1021/cn200028j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chikae M, Fukuda T, Kerman K, Idegami K, Miura Y, Tamiya E. Amyloid-beta detection with saccharide immobilized gold nanoparticle on carbon electrode. Bioelectrochemistry. 2008;74:118–23. doi: 10.1016/j.bioelechem.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 70.Neely A, Perry C, Varisli B, Singh AK, Arbneshi T, Senapati D, et al. Ultrasensitive and highly selective detection of Alzheimer's disease biomarker using two-photon Rayleigh scattering properties of gold nanoparticle. ACS Nano. 2009;3:2834–40. doi: 10.1021/nn900813b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Baron R, Zayats M, Willner I. Dopamine-, L-DOPA-, adrenaline-, and noradrenaline-induced growth of Au nanoparticles: assays for the detection of neurotransmitters and of tyrosinase activity. Anal Chem. 2005;77:1566–71. doi: 10.1021/ac048691v. [DOI] [PubMed] [Google Scholar]
- 72.Xing Y, Chaudry Q, Shen C, Kong KY, Zhau HE, Chung LW, et al. Bioconjugated quantum dots for multiplexed and quantitative immunohistochemistry. Nat Protoc. 2007;2:1152–65. doi: 10.1038/nprot.2007.107. [DOI] [PubMed] [Google Scholar]
- 73.Morales-Narvaez E, Monton H, Fomicheva A, Merkoci A. Signal enhancement in antibody microarrays using quantum dots nanocrystals: application to potential Alzheimer's disease biomarker screening. Anal Chem. 2012;84:6821–7. doi: 10.1021/ac301369e. [DOI] [PubMed] [Google Scholar]
- 74.An Y, Tang L, Jiang X, Chen H, Yang M, Jin L, et al. A photoelectrochemical immunosensor based on Au-doped TiO2 nanotube arrays for the detection of alpha-synuclein. Chemistry. 2010;16:14439–46. doi: 10.1002/chem.201001654. [DOI] [PubMed] [Google Scholar]
- 75.Yue HY, Huang S, Chang J, Heo C, Yao F, Adhikari S, et al. ZnO nanowire arrays on 3D hierachical graphene foam: biomarker detection of Parkinson's disease. ACS Nano. 2014;8:1639–46. doi: 10.1021/nn405961p. [DOI] [PubMed] [Google Scholar]
- 76.Wagner V, Dullaart A, Bock AK, Zweck A. The emerging nanomedicine landscape. Nature biotechnology. 2006;24:1211–7. doi: 10.1038/nbt1006-1211. [DOI] [PubMed] [Google Scholar]
- 77.McMillan J, Batrakova E, Gendelman HE. Cell delivery of therapeutic nanoparticles. Prog Mol Biol Transl Sci. 2011;104:563–601. doi: 10.1016/B978-0-12-416020-0.00014-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim BY, Rutka JT, Chan WC. Nanomedicine. N Engl J Med. 2010;363:2434–43. doi: 10.1056/NEJMra0912273. [DOI] [PubMed] [Google Scholar]
- 79.Blecher K, Nasir A, Friedman A. The growing role of nanotechnology in combating infectious disease. Virulence. 2011;2:395–401. doi: 10.4161/viru.2.5.17035. [DOI] [PubMed] [Google Scholar]
- 80.Liu L, Xu K, Wang H, Tan PK, Fan W, Venkatraman SS, et al. Self-assembled cationic peptide nanoparticles as an efficient antimicrobial agent. Nat Nanotechnol. 2009;4:457–63. doi: 10.1038/nnano.2009.153. [DOI] [PubMed] [Google Scholar]
- 81.Wang H, Xu K, Liu L, Tan JP, Chen Y, Li Y, et al. The efficacy of self-assembled cationic antimicrobial peptide nanoparticles against Cryptococcus neoformans for the treatment of meningitis. Biomaterials. 2010;31:2874–81. doi: 10.1016/j.biomaterials.2009.12.042. [DOI] [PubMed] [Google Scholar]
- 82.Chakraborty SP, Sahu SK, Pramanik P, Roy S. In vitro antimicrobial activity of nanoconjugated vancomycin against drug resistant Staphylococcus aureus. Int J Pharm. 2012;436:659–76. doi: 10.1016/j.ijpharm.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 83.Borchorst S, Moller K. The role of dexamethasone in the treatment of bacterial meningitis - a systematic review. Acta Anaesthesiol Scand. 2012;56:1210–21. doi: 10.1111/j.1399-6576.2012.02698.x. [DOI] [PubMed] [Google Scholar]
- 84.de Vries HE, Kuiper J, de Boer AG, Van Berkel TJ, Breimer DD. The blood-brain barrier in neuroinflammatory diseases. Pharmacol Rev. 1997;49:143–55. [PubMed] [Google Scholar]
- 85.van de Beek D, Brouwer MC, Thwaites GE, Tunkel AR. Advances in treatment of bacterial meningitis. Lancet. 2012;380:1693–702. doi: 10.1016/S0140-6736(12)61186-6. [DOI] [PubMed] [Google Scholar]
- 86.Waknine-Grinberg JH, Even-Chen S, Avichzer J, Turjeman K, Bentura-Marciano A, Haynes RK, et al. Glucocorticosteroids in nano-sterically stabilized liposomes are efficacious for elimination of the acute symptoms of experimental cerebral malaria. PLoS One. 2013;8:e72722. doi: 10.1371/journal.pone.0072722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tsao N, Kanakamma PP, Luh TY, Chou CK, Lei HY. Inhibition of Escherichia coli-induced meningitis by carboxyfullerence. Antimicrob Agents Chemother. 1999;43:2273–7. doi: 10.1128/aac.43.9.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McCarthy JM, Appelhans D, Tatzelt J, Rogers MS. Nanomedicine for prion disease treatment: new insights into the role of dendrimers. Prion. 2013;7:198–202. doi: 10.4161/pri.24431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Janaszewska A, Ziemba B, Ciepluch K, Appelhans D, Voit B, Klajnert B, et al. The biodistribution of maltotriose modified poly(propylene imine) (PPI) dendrimers conjugated with fluorescein-proofs of crossing blood-brain-barrier. New Journal of Chemistry. 2012;36:350–353. [Google Scholar]
- 90.Ullas PT, Madhusudana SN, Desai A, Sagar BK, Jayamurugan G, Rajesh YB, et al. Enhancement of immunogenicity and efficacy of a plasmid DNA rabies vaccine by nanoformulation with a fourth-generation amine-terminated poly(ether imine) dendrimer. Int J Nanomedicine. 2014;9:627–34. doi: 10.2147/IJN.S53415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Knuschke T, Bayer W, Rotan O, Sokolova V, Wadwa M, Kirschning CJ, et al. Prophylactic and therapeutic vaccination with a nanoparticle-based peptide vaccine induces efficient protective immunity during acute and chronic retroviral infection. Nanomedicine. 2014 doi: 10.1016/j.nano.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 92.Reddy SB, Mainwaring DE, Kobaisi MA, Zeephongsekul P, Fecondo JV. Acoustic wave immunosensing of a meningococcal antigen using gold nanoparticle-enhanced mass sensitivity. Biosens Bioelectron. 2012;31:382–7. doi: 10.1016/j.bios.2011.10.051. [DOI] [PubMed] [Google Scholar]
- 93.Edagwa BJ, Zhou T, McMillan JM, Liu XM, Gendelman HE. Development of HIV Reservoir Targeted Long Acting Nanoformulated Antiretroviral Therapies. Curr Med Chem. 2014;21:4186–98. doi: 10.2174/0929867321666140826114135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chiappetta DA, Hocht C, Opezzo JA, Sosnik A. Intranasal administration of antiretroviral-loaded micelles for anatomical targeting to the brain in HIV. Nanomedicine (Lond) 2013;8:223–37. doi: 10.2217/nnm.12.104. [DOI] [PubMed] [Google Scholar]
- 95.Batrakova E, Lee S, Li S, Venne A, Alakhov V, Kabanov A. Fundamental relationships between the composition of pluronic block copolymers and their hypersensitization effect in MDR cancer cells. Pharm Res. 1999;16:1373–9. doi: 10.1023/a:1018942823676. [DOI] [PubMed] [Google Scholar]
- 96.Jin SX, Bi DZ, Wang J, Wang YZ, Hu HG, Deng YH. Pharmacokinetics and tissue distribution of zidovudine in rats following intravenous administration of zidovudine myristate loaded liposomes. Pharmazie. 2005;60:840–3. [PubMed] [Google Scholar]
- 97.Chen H, Qin Y, Zhang Q, Jiang W, Tang L, Liu J, et al. Lactoferrin modified doxorubicin-loaded procationic liposomes for the treatment of gliomas. Eur J Pharm Sci. 2011;44:164–73. doi: 10.1016/j.ejps.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 98.Soni V, Kohli DV, Jain SK. Transferrin-conjugated liposomal system for improved delivery of 5-fluorouracil to brain. J Drug Target. 2008;16:73–8. doi: 10.1080/10611860701725381. [DOI] [PubMed] [Google Scholar]
- 99.Saiyed ZM, Gandhi NH, Nair MP. Magnetic nanoformulation of azidothymidine 5'-triphosphate for targeted delivery across the blood-brain barrier. Int J Nanomedicine. 2010;5:157–66. doi: 10.2147/ijn.s8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gerson T, Makarov E, Senanayake TH, Gorantla S, Poluektova LY, Vinogradov SV. Nano-NRTIs demonstrate low neurotoxicity and high antiviral activity against HIV infection in the brain. Nanomedicine. 2014;10:177–85. doi: 10.1016/j.nano.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kuo YC, Su FL. Transport of stavudine, delavirdine, and saquinavir across the blood-brain barrier by polybutylcyanoacrylate, methylmethacrylate-sulfopropylmethacrylate, and solid lipid nanoparticles. Int J Pharm. 2007;340:143–52. doi: 10.1016/j.ijpharm.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 102.Kuo YC, Ko HF. Targeting delivery of saquinavir to the brain using 83-14 monoclonal antibody-grafted solid lipid nanoparticles. Biomaterials. 2013;34:4818–30. doi: 10.1016/j.biomaterials.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 103.Batrakova EV, Gendelman HE, Kabanov AV. Cell-mediated drug delivery. Expert Opin Drug Deliv. 2011;8:415–33. doi: 10.1517/17425247.2011.559457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dou H, Destache CJ, Morehead JR, Mosley RL, Boska MD, Kingsley J, et al. Development of a macrophage-based nanoparticle platform for antiretroviral drug delivery. Blood. 2006;108:2827–35. doi: 10.1182/blood-2006-03-012534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dou H, Grotepas CB, McMillan JM, Destache CJ, Chaubal M, Werling J, et al. Macrophage delivery of nanoformulated antiretroviral drug to the brain in a murine model of neuroAIDS. J Immunol. 2009;183:661–9. doi: 10.4049/jimmunol.0900274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nowacek AS, McMillan J, Miller R, Anderson A, Rabinow B, Gendelman HE. Nanoformulated antiretroviral drug combinations extend drug release and antiretroviral responses in HIV-1-infected macrophages: implications for neuroAIDS therapeutics. J Neuroimmune Pharmacol. 2010;5:592–601. doi: 10.1007/s11481-010-9198-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Batrakova EV, Li S, Reynolds AD, Mosley RL, Bronich TK, Kabanov AV, et al. A macrophage-nanozyme delivery system for Parkinson's disease. Bioconjug Chem. 2007;18:1498–506. doi: 10.1021/bc700184b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kanmogne GD, Singh S, Roy U, Liu X, McMillan J, Gorantla S, et al. Mononuclear phagocyte intercellular crosstalk facilitates transmission of cell-targeted nanoformulated antiretroviral drugs to human brain endothelial cells. Int J Nanomedicine. 2012;7:2373–88. doi: 10.2147/IJN.S29454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Martinez-Skinner AL, Veerubhotla RS, Liu H, Xiong H, Yu F, McMillan JM, et al. Functional proteome of macrophage carried nanoformulated antiretroviral therapy demonstrates enhanced particle carrying capacity. J Proteome Res. 2013;12:2282–94. doi: 10.1021/pr400185w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Duncan R, Richardson SC. Endocytosis and intracellular trafficking as gateways for nanomedicine delivery: opportunities and challenges. Mol Pharm. 2012;9:2380–402. doi: 10.1021/mp300293n. [DOI] [PubMed] [Google Scholar]
- 111.Kadiu I, Gendelman HE. Macrophage bridging conduit trafficking of HIV-1 through the endoplasmic reticulum and Golgi network. J Proteome Res. 2011;10:3225–38. doi: 10.1021/pr200262q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Guo D, Zhang G, Wysocki TA, Wysocki BJ, Gelbard HA, Liu XM, et al. Endosomal Trafficking of Nanoformulated Antiretroviral Therapy Facilitates Drug Particle Carriage and HIV Clearance. J Virol. 2014;88:9504–13. doi: 10.1128/JVI.01557-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chaudhuri KR, Healy DG, Schapira AH, E. National Institute for Clinical Non-motor symptoms of Parkinson's disease: diagnosis and management. Lancet Neurol. 2006;5:235–45. doi: 10.1016/S1474-4422(06)70373-8. [DOI] [PubMed] [Google Scholar]
- 114.Pillay S, Pillay V, Choonara YE, Naidoo D, Khan RA, du Toit LC, et al. Design, biometric simulation and optimization of a nano-enabled scaffold device for enhanced delivery of dopamine to the brain. Int J Pharm. 2009;382:277–90. doi: 10.1016/j.ijpharm.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 115.Trapani A, De Giglio E, Cafagna D, Denora N, Agrimi G, Cassano T, et al. Characterization and evaluation of chitosan nanoparticles for dopamine brain delivery. Int J Pharm. 2011;419:296–307. doi: 10.1016/j.ijpharm.2011.07.036. [DOI] [PubMed] [Google Scholar]
- 116.De Giglio E, Trapani A, Cafagna D, Sabbatini L, Cometa S. Dopamine-loaded chitosan nanoparticles: formulation and analytical characterization. Anal Bioanal Chem. 2011;400:1997–2002. doi: 10.1007/s00216-011-4962-y. [DOI] [PubMed] [Google Scholar]
- 117.Rashed ER, Abd El-Rehim HA, El-Ghazaly MA. Potential efficacy of dopamine loaded-PVP/PAA nanogel in experimental models of Parkinsonism: Possible disease modifying activity. J Biomed Mater Res A. 2014 doi: 10.1002/jbm.a.35312. [DOI] [PubMed] [Google Scholar]
- 118.Yang X, Zheng R, Cai Y, Liao M, Yuan W, Liu Z. Controlled-release levodopa methyl ester/benserazide-loaded nanoparticles ameliorate levodopa-induced dyskinesia in rats. Int J Nanomedicine. 2012;7:2077–86. doi: 10.2147/IJN.S30463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sharma S, Lohan S, Murthy RS. Formulation and characterization of intranasal mucoadhesive nanoparticulates and thermo-reversible gel of levodopa for brain delivery. Drug Dev Ind Pharm. 2014;40:869–78. doi: 10.3109/03639045.2013.789051. [DOI] [PubMed] [Google Scholar]
- 120.Md S, Khan RA, Mustafa G, Chuttani K, Baboota S, Sahni JK, et al. Bromocriptine loaded chitosan nanoparticles intended for direct nose to brain delivery: Pharmacodynamic, Pharmacokinetic and Scintigraphy study in mice model. Eur J Pharm Sci. 2013;48:393–405. doi: 10.1016/j.ejps.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 121.Azeem A, Talegaonkar S, Negi LM, Ahmad FJ, Khar RK, Iqbal Z. Oil based nanocarrier system for transdermal delivery of ropinirole: a mechanistic, pharmacokinetic and biochemical investigation. Int J Pharm. 2012;422:436–44. doi: 10.1016/j.ijpharm.2011.10.039. [DOI] [PubMed] [Google Scholar]
- 122.Carroll RT, Bhatia D, Geldenhuys W, Bhatia R, Miladore N, Bishayee A, et al. Brain-targeted delivery of Tempol-loaded nanoparticles for neurological disorders. J Drug Target. 2010;18:665–74. doi: 10.3109/10611861003639796. [DOI] [PubMed] [Google Scholar]
- 123.Brynskikh AM, Zhao Y, Mosley RL, Li S, Boska MD, Klyachko NL, et al. Macrophage delivery of therapeutic nanozymes in a murine model of Parkinson's disease. Nanomedicine (Lond) 2010;5:379–96. doi: 10.2217/nnm.10.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Herve P, Wijdenes J, Bergerat JP, Bordigoni P, Milpied N, Cahn JY, et al. Treatment of corticosteroid resistant acute graft-versus-host disease by in vivo administration of anti-interleukin-2 receptor monoclonal antibody (B-B10). Blood. 1990;75:1017–23. [PubMed] [Google Scholar]
- 125.Kurakhmaeva KB, Djindjikhashvili IA, Petrov VE, Balabanyan VU, Voronina TA, Trofimov SS, et al. Brain targeting of nerve growth factor using poly(butyl cyanoacrylate) nanoparticles. J Drug Target. 2009;17:564–74. doi: 10.1080/10611860903112842. [DOI] [PubMed] [Google Scholar]
- 126.Herran E, Ruiz-Ortega JA, Aristieta A, Igartua M, Requejo C, Lafuente JV, et al. In vivo administration of VEGF- and GDNF-releasing biodegradable polymeric microspheres in a severe lesion model of Parkinson's disease. Eur J Pharm Biopharm. 2013;85:1183–90. doi: 10.1016/j.ejpb.2013.03.034. [DOI] [PubMed] [Google Scholar]
- 127.Lampe KJ, Kern DS, Mahoney MJ, Bjugstad KB. The administration of BDNF and GDNF to the brain via PLGA microparticles patterned within a degradable PEG-based hydrogel: Protein distribution and the glial response. J Biomed Mater Res A. 2011;96:595–607. doi: 10.1002/jbm.a.33011. [DOI] [PubMed] [Google Scholar]
- 128.Piazza J, Hoare T, Molinaro L, Terpstra K, Bhandari J, Selvaganapathy PR, et al. Haloperidol-loaded intranasally administered lectin functionalized poly(ethylene glycol)-block-poly(D,L)-lactic-co-glycolic acid (PEG-PLGA) nanoparticles for the treatment of schizophrenia. Eur J Pharm Biopharm. 2014;87:30–9. doi: 10.1016/j.ejpb.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 129.Wen Z, Yan Z, Hu K, Pang Z, Cheng X, Guo L, et al. Odorranalectin-conjugated nanoparticles: preparation, brain delivery and pharmacodynamic study on Parkinson's disease following intranasal administration. J Control Release. 2011;151:131–8. doi: 10.1016/j.jconrel.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 130.Dranka BP, Gifford A, McAllister D, Zielonka J, Joseph J, O'Hara CL, et al. A novel mitochondrially-targeted apocynin derivative prevents hyposmia and loss of motor function in the leucine-rich repeat kinase 2 (LRRK2) transgenic mouse model of Parkinson's disease. Neurosci Lett. 2014;583C:159–164. doi: 10.1016/j.neulet.2014.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jang BC, Paik JH, Kim SP, Shin DH, Song DK, Park JG, et al. Catalase induced expression of inflammatory mediators via activation of NF-kappaB, PI3K/AKT, p70S6K, and JNKs in BV2 microglia. Cell Signal. 2005;17:625–33. doi: 10.1016/j.cellsig.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 132.Schubert D, Dargusch R, Raitano J, Chan SW. Cerium and yttrium oxide nanoparticles are neuroprotective. Biochem Biophys Res Commun. 2006;342:86–91. doi: 10.1016/j.bbrc.2006.01.129. [DOI] [PubMed] [Google Scholar]
- 133.Gao H, Yang Z, Zhang S, Cao S, Shen S, Pang Z, et al. Ligand modified nanoparticles increases cell uptake, alters endocytosis and elevates glioma distribution and internalization. Sci Rep. 2013;3:2534. doi: 10.1038/srep02534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mathew A, Fukuda T, Nagaoka Y, Hasumura T, Morimoto H, Yoshida Y, et al. Curcumin loaded-PLGA nanoparticles conjugated with Tet-1 peptide for potential use in Alzheimer's disease. PLoS One. 2012;7:e32616. doi: 10.1371/journal.pone.0032616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schaffazick SR, Siqueira IR, Badejo AS, Jornada DS, Pohlmann AR, Netto CA, et al. Incorporation in polymeric nanocapsules improves the antioxidant effect of melatonin against lipid peroxidation in mice brain and liver. Eur J Pharm Biopharm. 2008;69:64–71. doi: 10.1016/j.ejpb.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 136.Schroeder U, Sommerfeld P, Sabel BA. Efficacy of oral dalargin-loaded nanoparticle delivery across the blood-brain barrier. Peptides. 1998;19:777–80. doi: 10.1016/s0196-9781(97)00474-9. [DOI] [PubMed] [Google Scholar]
- 137.Owens DE, 3rd, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm. 2006;307:93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 138.Zhang QZ, Zha LS, Zhang Y, Jiang WM, Lu W, Shi ZQ, et al. The brain targeting efficiency following nasally applied MPEG-PLA nanoparticles in rats. J Drug Target. 2006;14:281–90. doi: 10.1080/10611860600721051. [DOI] [PubMed] [Google Scholar]
- 139.Kizelsztein P, Ovadia H, Garbuzenko O, Sigal A, Barenholz Y. Pegylated nanoliposomes remote-loaded with the antioxidant tempamine ameliorate experimental autoimmune encephalomyelitis. J Neuroimmunol. 2009;213:20–5. doi: 10.1016/j.jneuroim.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 140.Yeste A, Nadeau M, Burns EJ, Weiner HL, Quintana FJ. Nanoparticle-mediated codelivery of myelin antigen and a tolerogenic small molecule suppresses experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2012;109:11270–5. doi: 10.1073/pnas.1120611109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hunter Z, McCarthy DP, Yap WT, Harp CT, Getts DR, Shea LD, et al. A biodegradable nanoparticle platform for the induction of antigen-specific immune tolerance for treatment of autoimmune disease. ACS Nano. 2014;8:2148–60. doi: 10.1021/nn405033r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang B, Navath RS, Romero R, Kannan S, Kannan R. Anti-inflammatory and anti-oxidant activity of anionic dendrimer-N-acetyl cysteine conjugates in activated microglial cells. Int J Pharm. 2009;377:159–68. doi: 10.1016/j.ijpharm.2009.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kannan S, Dai H, Navath RS, Balakrishnan B, Jyoti A, Janisse J, et al. Dendrimer-based postnatal therapy for neuroinflammation and cerebral palsy in a rabbit model. Sci Transl Med. 2012;4:130ra46. doi: 10.1126/scitranslmed.3003162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Iezzi R, Guru BR, Glybina IV, Mishra MK, Kennedy A, Kannan RM. Dendrimer-based targeted intravitreal therapy for sustained attenuation of neuroinflammation in retinal degeneration. Biomaterials. 2012;33:979–88. doi: 10.1016/j.biomaterials.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 145.Cerqueira SR, Oliveira JM, Silva NA, Leite-Almeida H, Ribeiro-Samy S, Almeida A, et al. Microglia response and in vivo therapeutic potential of methylprednisolone-loaded dendrimer nanoparticles in spinal cord injury. Small. 2013;9:738–49. doi: 10.1002/smll.201201888. [DOI] [PubMed] [Google Scholar]
- 146.Lin Y, Pan Y, Shi Y, Huang X, Jia N, Jiang JY. Delivery of large molecules via poly(butyl cyanoacrylate) nanoparticles into the injured rat brain. Nanotechnology. 2012;23:165101. doi: 10.1088/0957-4484/23/16/165101. [DOI] [PubMed] [Google Scholar]
- 147.Das M, Wang C, Bedi R, Mohapatra SS, Mohapatra S. Magnetic micelles for DNA delivery to rat brains after mild traumatic brain injury. Nanomedicine. 2014;10:1539–48. doi: 10.1016/j.nano.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Samuel EL, Duong MT, Bitner BR, Marcano DC, Tour JM, Kent TA. Hydrophilic carbon clusters as therapeutic, high-capacity antioxidants. Trends Biotechnol. 2014;32:501–505. doi: 10.1016/j.tibtech.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ruozi B, Belletti D, Forni F, Sharma A, Muresanu D, Mossler H, et al. Poly (D,L-lactide-co-glycolide) Nanoparticles Loaded with Cerebrolysin Display Neuroprotective Activity in a Rat Model of Concussive Head Injury. CNS Neurol Disord Drug Targets. 2014 doi: 10.2174/1871527313666140806145540. [DOI] [PubMed] [Google Scholar]
- 150.Balshaw DM, Philbert M, Suk WA. Research strategies for safety evaluation of nanomaterials, Part III: nanoscale technologies for assessing risk and improving public health. Toxicol Sci. 2005;88:298–306. doi: 10.1093/toxsci/kfi312. [DOI] [PubMed] [Google Scholar]
- 151.Borm P, Klaessig FC, Landry TD, Moudgil B, Pauluhn J, Thomas K, et al. Research strategies for safety evaluation of nanomaterials, part V: role of dissolution in biological fate and effects of nanoscale particles. Toxicol Sci. 2006;90:23–32. doi: 10.1093/toxsci/kfj084. [DOI] [PubMed] [Google Scholar]
- 152.Holsapple MP, Farland WH, Landry TD, Monteiro-Riviere NA, Carter JM, Walker NJ, et al. Research strategies for safety evaluation of nanomaterials, part II: toxicological and safety evaluation of nanomaterials, current challenges and data needs. Toxicol Sci. 2005;88:12–7. doi: 10.1093/toxsci/kfi293. [DOI] [PubMed] [Google Scholar]
- 153.Thomas K, Sayre P. Research strategies for safety evaluation of nanomaterials, Part I: evaluating the human health implications of exposure to nanoscale materials. Toxicol Sci. 2005;87:316–21. doi: 10.1093/toxsci/kfi270. [DOI] [PubMed] [Google Scholar]
- 154.Thomas T, Thomas K, Sadrieh N, Savage N, Adair P, Bronaugh R. Research strategies for safety evaluation of nanomaterials, part VII: evaluating consumer exposure to nanoscale materials. Toxicol Sci. 2006;91:14–9. doi: 10.1093/toxsci/kfj129. [DOI] [PubMed] [Google Scholar]
- 155.Tsuji JS, Maynard AD, Howard PC, James JT, Lam CW, Warheit DB, et al. Research strategies for safety evaluation of nanomaterials, part IV: risk assessment of nanoparticles. Toxicol Sci. 2006;89:42–50. doi: 10.1093/toxsci/kfi339. [DOI] [PubMed] [Google Scholar]
- 156.Warheit DB, Borm PJ, Hennes C, Lademann J. Testing strategies to establish the safety of nanomaterials: conclusions of an ECETOC workshop. Inhal Toxicol. 2007;19:631–43. doi: 10.1080/08958370701353080. [DOI] [PubMed] [Google Scholar]
- 157.Hoet PH, Bruske-Hohlfeld I, Salata OV. Nanoparticles - known and unknown health risks. J Nanobiotechnology. 2004;2:12. doi: 10.1186/1477-3155-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.De Jong WH, Borm PJ. Drug delivery and nanoparticles:applications and hazards. Int J Nanomedicine. 2008;3:133–49. doi: 10.2147/ijn.s596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bonner JC. Nanoparticles as a potential cause of pleural and interstitial lung disease. Proc Am Thorac Soc. 2010;7:138–41. doi: 10.1513/pats.200907-061RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Becker H, Herzberg F, Schulte A, Kolossa-Gehring M. The carcinogenic potential of nanomaterials, their release from products and options for regulating them. Int J Hyg Environ Health. 2011;214:231–8. doi: 10.1016/j.ijheh.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 161.Cannon JR, Greenamyre JT. The role of environmental exposures in neurodegeneration and neurodegenerative diseases. Toxicol Sci. 2011;124:225–50. doi: 10.1093/toxsci/kfr239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Aschner M, Lukey B, Tremblay A. The Manganese Health Research Program (MHRP): status report and future research needs and directions. Neurotoxicology. 2006;27:733–6. doi: 10.1016/j.neuro.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 163.Guilarte TR. Manganese and Parkinson's disease: a critical review and new findings. Environ Health Perspect. 2010;118:1071–80. doi: 10.1289/ehp.0901748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Han MJ, Ozaki T, Yu J. Electronic structure and magnetic properties of small manganese oxide clusters. J Chem Phys. 2005;123:34306. doi: 10.1063/1.1953387. [DOI] [PubMed] [Google Scholar]
- 165.Afeseh Ngwa H, Kanthasamy A, Gu Y, Fang N, Anantharam V, Kanthasamy AG. Manganese nanoparticle activates mitochondrial dependent apoptotic signaling and autophagy in dopaminergic neuronal cells. Toxicol Appl Pharmacol. 2011;256:227–40. doi: 10.1016/j.taap.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]