Abstract
Changes in tissue composition and cellular architecture have been associated with neurological disease, and these in turn can affect biomechanical properties. Natural biological factors such as aging and an individual’s sex also affect underlying tissue biomechanics in different brain regions. Understanding the normal changes is necessary before determining the efficacy of stiffness imaging for neurological disease diagnosis and therapy monitoring. The objective of this study was to evaluate global and regional changes in brain stiffness as a function of age and sex, using improved MRE acquisition and processing that has been shown to provide median stiffness values that are typically reproducible to within 1% in global measurements and within 2% for regional measurements. Furthermore, this is the first study to report the effects of age and sex over the entire cerebrum volume and over the full frontal, occipital, parietal, temporal, deep gray matter/white matter (insula, deep gray nuclei and white matter tracts), and cerebellum volumes. In 45 volunteers, we observed a significant linear correlation between age and brain stiffness in the cerebrum (P<.0001), frontal lobes (P<.0001), occipital lobes (P=.0005), parietal lobes (P=.0002), and the temporal lobes (P<.0001) of the brain. No significant linear correlation between brain stiffness and age was observed in the cerebellum (P=.74), and the sensory-motor regions (P=.32) of the brain, and a weak linear trend was observed in the deep gray matter/white matter (P=.075). A multiple linear regression model predicted an annual decline of 0.011±0.002 kPa in cerebrum stiffness with a theoretical median age value (76 years old) of 2.56±0.08 kPa. Sexual dimorphism was observed in the temporal (P=.03) and occipital (P=.001) lobes of the brain, but no significant difference was observed in any of the other brain regions (P>.20 for all other regions). The model predicted female occipital and temporal lobes to be 0.23 kPa and 0.09 kPa stiffer than males of the same age, respectively. This study confirms that as the brain ages, there is softening; however, the changes are dependent on region. In addition, stiffness effects due to sex exist in the occipital and temporal lobes.
Keywords: aging, brain MRE, brain stiffness, elastography, gender bias, sexual dimorphism
1. INTRODUCTION
Changes in tissue composition and cellular architecture have been associated with neurological disease. For instance, Alzheimer’s disease presents pathologically with neuritic plaque development, intracellular neurofibrillary tangles, and neurodegeneration (McKhann et al., 2011). The hallmarks of multiple sclerosis (MS) are focal demyelination, inflammation, scar formation and axonal degeneration (Pittock and Lucchinetti, 2007). Also, brain tumors can present with a variety of histological features such as nuclear atypia, increased proliferation, microvascular proliferation and necrosis (Huse and Holland, 2010). These changes in tissue architecture influence the underlying biomechanical properties of brain tissue. As a result, there is an active effort to non-invasively and accurately measure the mechanical properties of the brain in vivo.
Several groups have investigated the role of magnetic resonance elastography (MRE), a quantitative stiffness imaging technique capable of measuring the biomechanical properties of tissues in vivo, for the diagnosis of neurological diseases. With this technique, significant changes in tissue biomechanics have been reported in brain tumor (Xu et al., 2007), normal pressure hydrocephalus (Streitberger et al., 2011), multiple sclerosis (Streitberger et al., 2012; Wuerfel et al., 2010), and Alzheimer’s disease (Murphy et al., 2011). In addition to measuring biomechanical changes observed in the brain due to disease, it is also important to understand how natural biological factors such as age and sex affect the underlying tissue biomechanics in different brain regions. Understanding these changes is necessary before determining the efficacy of stiffness imaging for neurological disease diagnosis and therapy monitoring, especially for the purposes of understanding diffuse and slowly progressing diseases.
Currently, stiffness imaging of the brain is in its early development, with few publications reporting on the influence of age and sex on stiffness. Although the current literature consistently demonstrates that stiffness decreases with age, the dependence of stiffness on sex is still somewhat controversial. In 2009, Sack et al (Sack et al., 2009) reported that the cerebrum of women were estimated to be 180 Pa stiffer than age-matched men, but in 2011 this same group reported no difference between cerebrum stiffness of men and women (Sack et al., 2011). Several neurological conditions such as autism, attention deficit/hyperactivity disorder, conduct disorder, specific language impairment, Tourette syndrome, dyslexia, depression, anxiety disorder, and anorexia nervosa have all been shown to exhibit sexual dimorphism (Bao and Swaab, 2010; Baron-Cohen et al., 2011; Ruigrok et al., 2014). Therefore, it is important to establish whether or not the tissue mechanics of healthy human control groups exhibit sexual dimorphisms, especially if brain MRE is going to be used to investigate neurological conditions in the future.
Some evidence also exists suggesting that the biomechanical properties of the brain are non-uniform throughout the entire brain volume. For instance, Zhang et al (Zhang et al., 2011) reported that cerebellum stiffness was found to be significantly lower (P<.001) than cerebrum stiffness across a cohort of 8 healthy subjects (22–43 years old). More recent publications have reported on a few brain regions (Guo et al., 2013; Johnson et al., 2013); however these measurements were limited to small regions inside the brain and not the entire brain volume. In 2013, Guo et al (Guo et al., 2013) performed 3-dimensional MRE on 23 healthy volunteers between the ages of 22 to 72. The authors only reported on four specific brain regions (the head of the caudate nucleus, thalamus, corpus callosum genu, and white matter), and stated that regional differences between brain regions did exist. However, the authors were primarily focused on developing a high resolution MRE technique that looked at the viscoelastic properties of brain tissue, and did not focus on the effects of age or sex inside or outside of these regions. In the same year, Johnson et al (Johnson et al., 2013) demonstrated that the corpus callosum and corona radiata were significantly stiffer than other white matter within the brain in healthy volunteers (24–53 in age). Once again, these authors focused on specific regions within the brain and did not look at changes with respect to sex and age.
The objective of this study was to measure global and regional changes in brain stiffness in healthy volunteers as a function of age and sex, in an age cohort that is commonly affected by neurological disorders. An improved MRE acquisition and processing method was used, that has previously been shown to provide median stiffness values that are typically reproducible within 1%, in global measurements, and within 2% for regional measurements (Murphy et al., 2013). Furthermore, this is the first study to report the effects of age and sex over the entire cerebrum volume and over the full frontal, occipital, parietal, temporal, deep gray matter/white matter (insula, deep gray nuclei and white matter tracts) and cerebellum volumes.
2. MATERIALS AND METHODS
2.1. Study Population
This study was approved by our institutional review board and the subjects were imaged after obtaining written informed consent. A total of 45 healthy (22 males, 23 female) amyloid-PET negative, cognitively normal subjects in the age range of 56–89 years (mean=74, median=76) were included in the study (Table 1). The subjects were recruited from a longitudinal study of aging (Roberts et al., 2008) in which they had already undergone Pittsburgh compound B positron emission tomography (PiB PET) imaging to determine they did not have an abnormal amyloid burden (Jack et al., 2010). This population was part of a well characterized group of subjects known to be cognitively normal without degenerative diseases and offer a reliable measure of healthy volunteers.
Table 1.
Study Population Demographic
| Age Range (years) |
n (M) | Mean Age±SD | Mean Age Males±SD |
Mean Age Females±SD |
|---|---|---|---|---|
| 56 | 2 (1) | 56.0±0.0 | 56.0±0.0 | 56.0±0.0 |
| 60–69 | 13 (6) | 64.0±3.5 | 64.2±2.6 | 63.9±2.6 |
| 70–79 | 16 (7) | 75.6±2.2 | 75.4±2.1 | 75.8±2.4 |
| 80–89 | 14 (7) | 84.4±3.0 | 84.9±2.9 | 83.7±3.3 |
| Total | 45 | |||
2.2. MR Elastography Imaging
Image acquisition included a modified spin-echo echo planar imaging sequence to acquire MRE data with the following imaging parameters: 60-Hz vibration frequency; TR/TE=3600/62 ms; FOV=24 cm; 72×72 image matrix reconstructed to 80×80; 48 contiguous 3-mm-thick axial slices; one 18.2-ms motion-encoding gradient on each side of the refocusing RF pulse; x, y, and z motion encoding directions; and 8 phase offsets spaced evenly over one period of 60-Hz motion. Approximately 10 minutes (6.5 minutes with vibrations on, 3 minutes with no vibrations) of imaging time was needed for the MRE portion of the exam. A 3D IR-SPGR T1 weighted image (sagittal orientation; frequency encoding in the superior-inferior direction; TR/TE = 6.3/2.8 ms; flip angle = 11 degrees; TI = 400 ms; FOV = 27 cm; 256×256 acquisition matrix; BW = ±31.25 kHz; 1.75× ASSET acceleration in the anterior-posterior direction; and 200 1.2-mm slices), was acquired for brain mask and brain atlas co-registration purposes. The total examination time, including all imaging and patient positioning, was approximately 30 minutes.
MRE post processing was performed utilizing a previously described pipeline (Murphy et al., 2013) and, for a given brain region, can be summarized in 3 steps: 1) calculating the first temporal harmonic of the curl of the displacement images; 2) smoothing the result with a quartic smoothing kernel; and 3) calculating the shear stiffness (defined as the product of wave speed squared and density, where density is assumed to be that of water) using direct inversion of the Helmholtz wave equation. These steps are performed with adaptive filtering and derivative calculations that avoid using data outside a given region. Furthermore, the inversion algorithm uses an iterative approach to correct stiffness values depending on the ratio of the amplitude of the curled displacement image over the standard deviation of the curled no motion data (SNR) for a sliding 3×3×3 window, excluding pixels outside the region of interest mask. The median stiffness of each region is then calculated after further erosion of the region by one pixel.
To define the regions, a lobar atlas in a standard template space was warped to the subject’s T1 weighted image using a unified segmentation algorithm implemented in SPM5 (Ashburner and Friston, 2005). The T1 weighted image was then registered to the magnitude data from the MRE exam along with the segmentation images and the warped atlas. Finally, these images were re-sliced to calculate the GM, WM and CSF content in MRE space. The brain and regional masks were generated by marking any voxel where GM content plus WM content was greater than CSF content. The pipeline thus masks out voxels with significant contributions from cerebral spinal fluid (>50%), minimizes partial volume and edge effects, attempts to correct areas of low SNR and low wave amplitude, and has previously been shown to have a coefficient of variation of less than 1% for global brain stiffness and less than 2% for the lobes of the brain and the cerebellum (Murphy et al., 2013).
2.3. Image Processing and Data Analysis
The median stiffness in the cerebrum, frontal lobes, occipital lobes, parietal lobes, temporal lobes, sensory motor, deep gray matter/white matter (insula, deep gray nuclei and white matter tracts), and the cerebellum were calculated for each volunteer. Initially, median stiffness for each region of the brain was fitted independently to a multiple linear regression model, using statistical analysis software (JMP® Pro 9.0.1, SAS Institute, Inc., Cary, NC) with sex, age, and sex-age interaction as the independent model parameters. A significant linear relationship was not observed for the sex-age interaction (additive effect) for any region within the brain. Therefore, a non-additive effect model was then fitted with sex and age as the only independent predictors. A p-value was reported for the overall model for each region of the brain, as calculated from the f-ratio of an analysis of variance (ANOVA) statistic. A p-value for each term within each model was also calculated for both sex and age predictor terms.
3. RESULTS
All of the multiple linear regression model results are summarized in Table 2. A significant linear correlation between age and brain stiffness was observed in the cerebrum (P<.0001), frontal lobes (P<.0001), occipital lobes (P=.0005), parietal lobes (P=.0002), and the temporal lobes (P<.0001) of the brain. No significant linear correlation between brain stiffness and age was observed in the cerebellum (P=.74) and the sensory motor regions (P=.32) of the brain, and a weak trend was observed in the deep gray matter/white matter (P=.075). The multiple linear regression models predicted an annual decline of 0.011±0.002 kPa in cerebrum stiffness with a theoretical stiffness of 2.56±0.08 kPa for the median volunteer age of 76 years old.
Table 2.
Parameter estimates of multiple linear regression models for all 45 healthy cognitively normal volunteers.
| Brain Region | Age Slope (kPa/Year) (p-value) |
Sex Bias (kPa)* (p-value) |
Predicted stiffness at age 76 (kPa) |
Overall F-test p-value |
R2 |
|---|---|---|---|---|---|
| Cerebrum | −0.011±0.002 (<.0001) |
−0.01 ±0.01 (p=.5178) |
2.56 ±0.08 | <0.0001 | 0.5453 |
| Frontal Lobes | −0.012±0.002 (<.0001) |
0.01 ±0.02 (p=.7373) |
2.6 ±0.1 | <0.0001 | 0.4308 |
| Occipital Lobes | −0.013±0.004 (.0005) |
−0.11 ±0.03 (p=.0010) |
2.8 ±0.2 | <0.0001 | 0.4134 |
| Parietal Lobes | −0.011±0.003 (.0002) |
−0.02 ±0.03 (p=.5442) |
2.6 ±0.2 | 0.0008 | 0.2881 |
| Temporal Lobes | −0.014±0.002 (<.0001) |
−0.04 ±0.02 (p=.0326) |
2.7 ±0.1 | <0.0001 | 0.5380 |
| Deep GM/WM | −0.006±0.003 (.075) |
−0.02 ±0.03 (p=.48) |
3.0 ±0.3 | 0.1423 | 0.0887 |
| Cerebellum | −0.001±0.003 (.740) |
−0.02 ±0.03 (p=.38) |
2.2 ±0.2 | 0.6276 | 0.0219 |
| Sensory-motor | −0.005±0.005 (.330) |
0.04 ±0.04 (p=.34) |
2.8 ±0.3 | 0.4210 | 0.0403 |
In the linear regression model stiffness = (zero intercept) + (sex bias)(sex) + (age slope)(age), where sex = +1 for males and −1 for females. Therefore, the sex difference in the occipital lobe = (−0.11)(−1)−(−.11)(1) = 0.22 kPa.
The median stiffness and the mean median stiffness ± the standard deviation over the entire volunteer cohort for each brain region has been summarized in table 3.
Table 3.
Stiffness measurements over entire volunteer cohort.
| Brain Region | Mean Median Stiffness ± Standard Deviation (kPa) |
Median Stiffness (kPa) |
|---|---|---|
| Cerebrum | 2.6 ± 0.1 | 2.6 |
| Frontal Lobes | 2.7 ± 0.2 | 2.7 |
| Occipital Lobes | 2.8 ± 0.3 | 2.8 |
| Parietal Lobes | 2.6 ± 0.2 | 2.6 |
| Temporal Lobes | 2.8 ± 0.2 | 2.8 |
| Deep GM/WM | 3.0 ± 0.2 | 3.0 |
| Cerebellum | 2.2 ± 0.2 | 2.2 |
| Sensory-motor | 2.8 ± 0.3 | 2.8 |
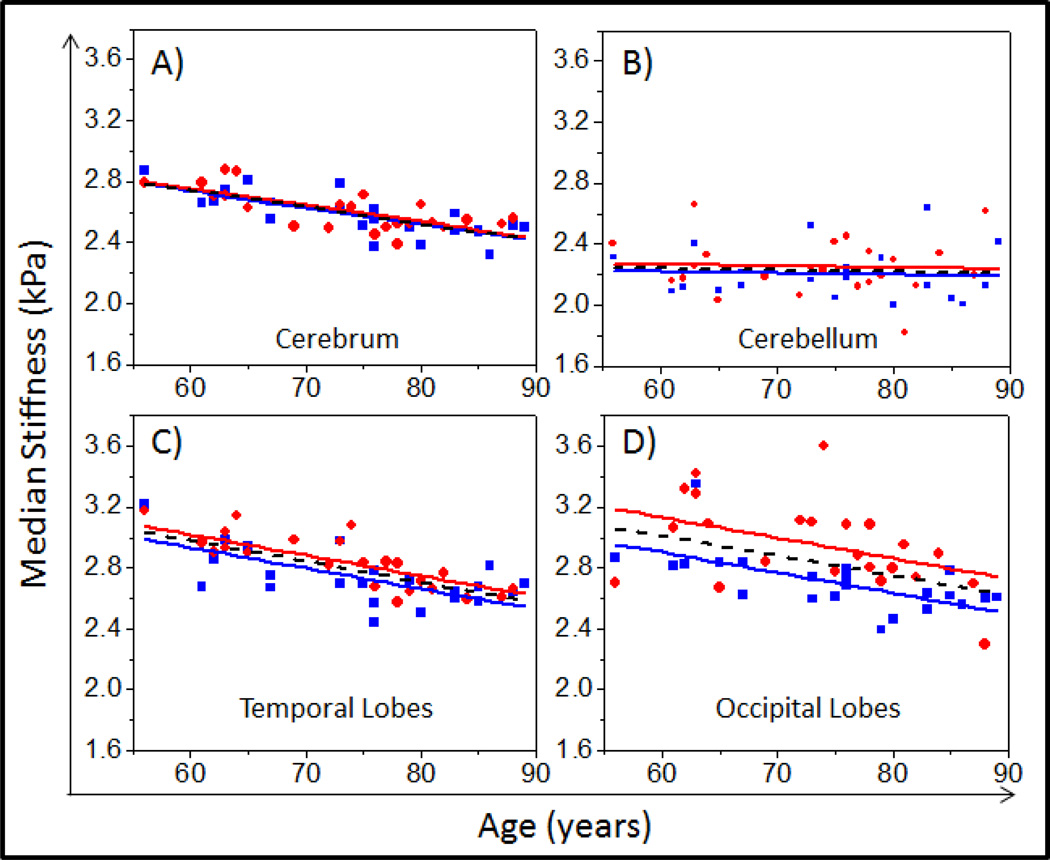
A plot of stiffness as a function of age and sex is shown for the cerebrum (Figure 1A), cerebellum (Figure 1B), temporal lobes (Figure 1C) and occipital lobes (Figure 1D) for all volunteers. Figure 1A illustrates the similar relationship between brain stiffness and age for both men and women over the entire cerebrum volume. Figure 1B demonstrates the relatively constant stiffness of the cerebellum with regard to age and sex.
Figure 1.
Plot of median brain stiffness (kPa) versus age in male (blue squares) and female (red circles) populations in the A) cerebrum, B) cerebellum, C) temporal lobes, and D) occipital lobes. Trend lines given by the multiple linear regression model are plotted for males (blue solid line) and females (red solid line). A stiffness versus age-only linear regression line has also been plotted (dashed black line) for comparison purposes.
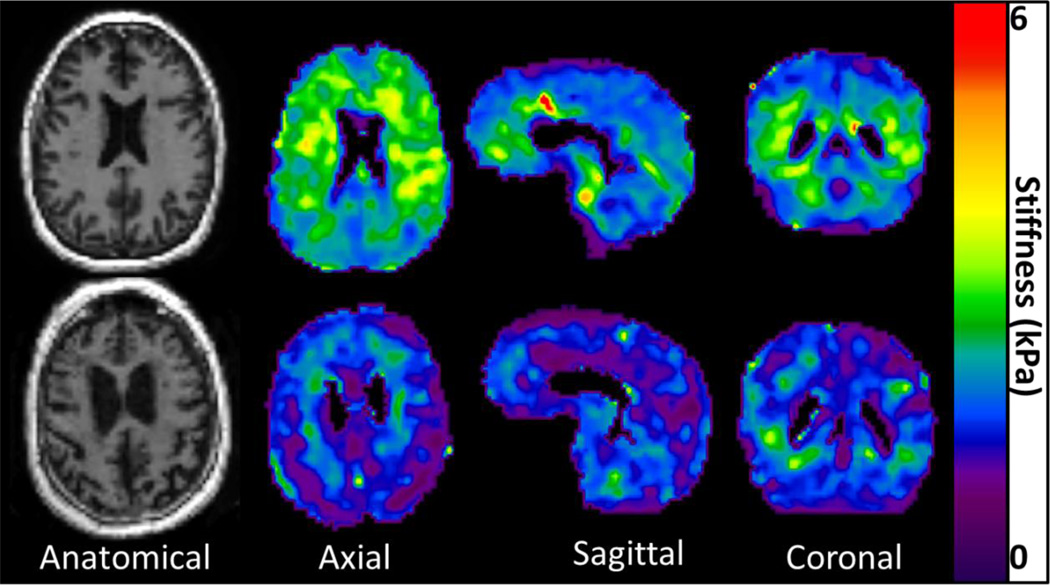
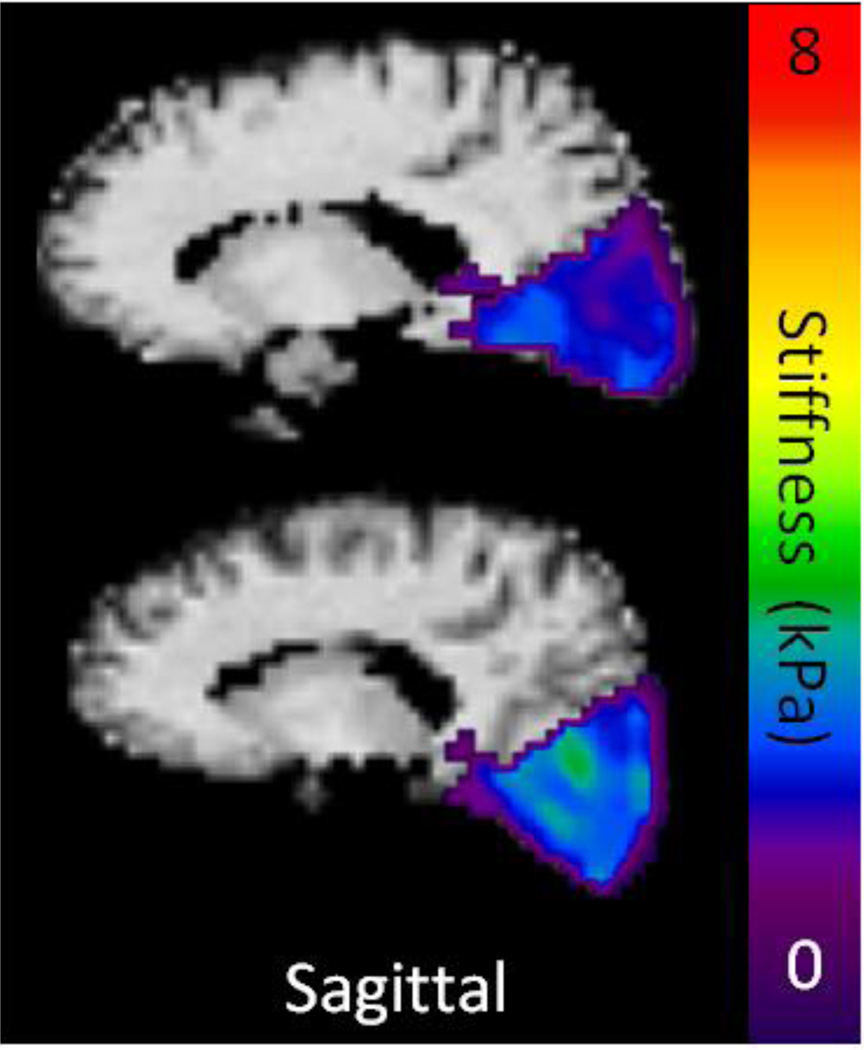
A sex effect was observed in the temporal (P=.03) (Figure 1C) and occipital (P=.001) (Figure 1D) lobes of the brain, but no significant difference was observed in any of the other brain regions (P>.2 for all other regions). The linear regression model predicted female occipital and temporal lobes to be 0.228 kPa and 0.085 kPa stiffer than males of the same age, respectively. Axial, sagittal, and coronal stiffness maps of a 56 year old male are compared with that of an 89 year old male in Figure 2, demonstrating the apparent softening of the brain with age. It should be noted that regions affected by brain atrophy and increased CSF appear to be much softer than the surrounding tissue. These regions are excluded in the processing of our stiffness measurements by eliminating pixels with CSF content greater than 50% and eroding pixels at regional boundaries. Nevertheless, it is clear that even tissues distant from atrophy affected regions have reduced stiffness in the older volunteer. Sagittal stiffness maps of the occipital lobes of an age matched (73 year old) male and female volunteer are shown in Figure 3 to illustrate the softer male occipital lobe in normal volunteers.
Figure 2.
An example of the overall decrease in cerebrum stiffness observed with aging. Axial anatomical images and elastograms of a 56 (top row) year old and an 89 (bottom row) year old cognitively normal males.
Figure 3.
Example elastograms overlaid on the corresponding T1-weighted images of 73 year old age matched male (top row) and female (bottom row) volunteers. The overall stiffness of the occipital lobes appears to be higher in the female volunteer.
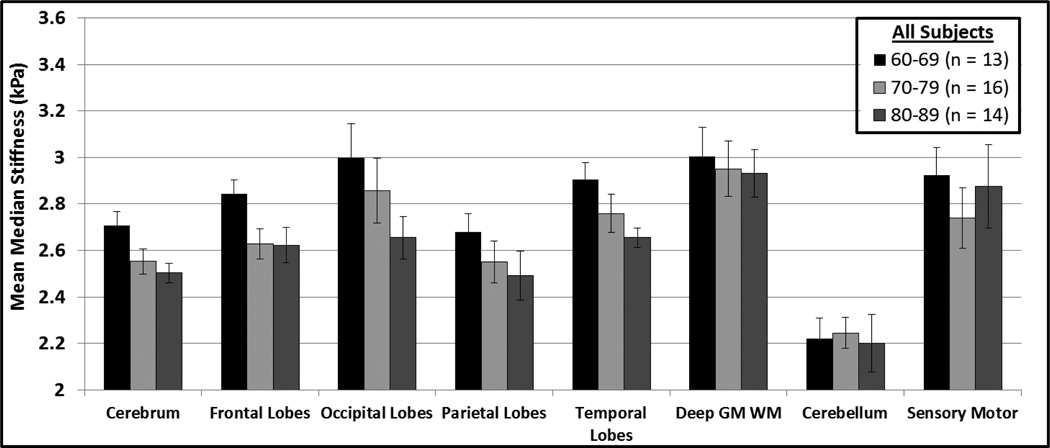
For visual purposes only the mean median stiffness per decade is shown in Figure 4 for the entire volunteer cohort. Figure 4 illustrates a decrease in stiffness with age in the cerebrum, frontal lobes, occipital lobes, parietal lobes, and temporal lobes, as predicted by the linear regression model.
Figure 4.
Mean median stiffness per decade for all volunteers in the 60–89 year age range. The error bars represent the 95% confidence intervals of the means.
4. DISCUSSION
This work has demonstrated that the stiffness of some regions of the brain have a significant linear correlation with age and sex, while others do not. Specifically, stiffness of the cerebrum is found to decrease with age by approximately −0.011±0.002 kPa/year. This value is comparable to the 0.0075 kPa/year decline in global brain storage modulus previously reported by Sack et al. (Sack et al., 2009) at a vibrational frequency of 62.5 Hz. However, those measurements were based on three transverse slices within the cerebrum at the level of the ventricles and not the entire cerebrum volume (Sack et al., 2009). Stiffness measurements were independent of age for deep gray/white matter, cerebellum, and sensory motor regions of the brain. The cerebellum stiffness (2.23±0.18 kPa) was found to be significantly lower (P<.001) than the cerebrum stiffness across all subjects, which agrees with findings reported by Zhang et al (Zhang et al., 2011) in younger subjects (22–43 years old).
Some previous studies have reported an association with sex and MRE, while others reported no association (Sack et al., 2009; Sack et al., 2011). We found only the occipital and temporal lobe stiffness to be dependent on sex. Specifically, the linear regression model predicted female occipital and temporal lobes to be 0.23 kPa and 0.09 kPa stiffer than males of the same age, respectively. This finding suggests that region selection could explain the discrepancies in the sex effect apparent in the literature. We note that our processing tries to carefully remove the influence of cerebrospinal fluid and edge effects from our stiffness measurements, which may help isolate tissue-specific characteristics.
In this study, the rate of change in stiffness in the temporal (12±2 Pa/year) and occipital lobes (13±4 Pa/year) were equivalent, within error, and the temporal lobe did not demonstrate the approximate 2 fold increase in slope as previously reported by Sack et al in 2011(Sack et al., 2011). However, the rate of change in stiffness in the occipital lobes between the two studies did overlap, within error. It is difficult to comment on the discrepancy observed in the temporal lobes, since Sack et al. did not include error bars or uncertainties in their measurements, but these variations could have been due to different age cohorts used in the two studies. Also, in this study the entire volunteer cohort was screened for abnormal amyloid plaque burden, which may have reduced contamination in the volunteer cohort.
On the other hand, our study did not use a viscoelastic model or multiple frequencies to calculate more than one stiffness coefficient, which may contribute to the observed differences. In future studies, it may be beneficial to use stiffness inversion algorithms that try to account for the anisotropy of the fibers in the brain (Qin et al., 2013; Romano et al., 2014; Romano et al., 2012). Furthermore, these algorithms in combination with multi-directional vibrational excitation could help improve the sensitivity and accuracy of the technique in regions of high anisotropy, such as the corpus callosum.
The mechanism behind the sexual dimorphism observed in this study is unknown, although the observation is not unique to brain stiffness. Previous groups have reported morphological and chemical differences, both globally and in specific brain regions, between men and women (Goldstein et al., 2001; Ruigrok et al., 2014). In general, brain size scales roughly with height and therefore men have larger brain volumes (Filipek et al., 1994; Gur et al., 1999; Witte et al., 2010), while women have a higher grey matter to white matter ratio (Allen et al., 2003; Sowell et al., 2007). In certain cases, current stiffness reconstruction algorithms tend to underestimate the stiffness of smaller objects, which may introduce a bias. Nevertheless, it is encouraging that in this study, we found that women, who have been shown to have statistically significant smaller brain volumes, demonstrated a higher stiffness in certain regions. This suggests that these findings may be even more significant than what the p-values report, if such a bias does exist in the data. Global cerebral blood flow has also been shown to be higher in women. Although differences exist, it is unclear exactly how or if these factors affect brain stiffness. It is interesting to note, however, that Witelson et al (Witelson et al., 1995), in a histology study, have shown that the density of neurons in the posterior temporal cortex was 11% higher in women than in men, which may be a potential contributing factor to the stiffer temporal lobes we observed in women. Also, other groups have reported that women have increased gray matter composition in the temporal associated cortices (Good et al., 2001; Sowell et al., 2007), as well as the lingual gyri (located in the occipital lobes) (Goldstein et al., 2001), further supporting the same regional sexual dimorphisms observed in this study. It should be noted that as a result of our post-processing methodology to reduce the artifactual tissue softening resulting from CSF in our stiffness measurements, the contribution of gray matter in the stiffness measurements is limited.
The mechanism behind the decrease in stiffness as a function of aging is also not completely understood. There is some evidence to support the idea that global gray matter volume decreases linearly with age (Allen et al., 2005; Good et al., 2001). This may suggest that gray matter composition could potentially contribute to the underlying mechanism for maintaining brain stiffness, which could not only explain the aging trend, but also the sexual dimorphism observations. Age-dependent studies conducted in animal models have shown that as the brain develops from adolescence to adulthood the brain tissue stiffens (Finan et al., 2012). However, this study only looked at two groups, (post-natal day 17 or 18 and fully mature females) and the authors did not report the exact age at full term. This makes it difficult to predict what the exact age difference was between the two groups. Another study, looking at 42 age controlled rats ranging from 13 days to 90 days demonstrated that immature rat brains were significantly stiffer than mature rat brains (Gefen et al., 2003). This further supports our findings in healthy volunteers. Future studies investigating the correlation between stiffness and tissue composition will be important in order to completely understanding the mechanisms influencing brain stiffness with respect to age and sex.
5. CONCLUSIONS
In conclusion, our study confirms that in an older population, as the brain ages, there is softening; however, this is not true for all regions of the brain. In addition, stiffness effects due to sex exist in the occipital and temporal lobes. Although the mechanisms behind these changes are not fully understood, it is important that they are recognized, especially when comparing different control and patient cohorts.
Highlights.
Changes in brain stiffness as a function of age and sex were investigated, in vivo.
This study observed that in this older population, as the brain ages, there is softening.
Brain stiffness changes with respect to age are dependent on brain region.
Stiffness effects due to sex exist in the occipital and temporal lobes.
Age and sex need to be considered when designing future brain MRE studies.
Acknowledgements
This research was funded by grant EB001981 (R.L.E.) and grant AG16574-14PILOT2 (J.H.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Allen JS, Bruss J, Brown CK, Damasio H. Normal neuroanatomical variation due to age: The major lobes and a parcellation of the temporal region. Neurobiol Aging. 2005;26:1245–1260. doi: 10.1016/j.neurobiolaging.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Allen JS, Damasio H, Grabowski TJ, Bruss J, Zhang W. Sexual dimorphism and asymmetries in the gray-white composition of the human cerebrum. Neuroimage. 2003;18:880–894. doi: 10.1016/s1053-8119(03)00034-x. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Bao AM, Swaab DF. Sex differences in the brain, behavior, and neuropsychiatric disorders. Neuroscientist. 2010;16:550–565. doi: 10.1177/1073858410377005. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Lombardo MV, Auyeung B, Ashwin E, Chakrabarti B, Knickmeyer R. Why are autism spectrum conditions more prevalent in males? PLoS Biol. 2011;9:e1001081. doi: 10.1371/journal.pbio.1001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipek PA, Richelme C, Kennedy DN, Caviness VS., Jr The young adult human brain: an MRI-based morphometric analysis. Cereb Cortex. 1994;4:344–360. doi: 10.1093/cercor/4.4.344. [DOI] [PubMed] [Google Scholar]
- Finan JD, Elkin BS, Pearson EM, Kalbian IL, Morrison B., 3rd Viscoelastic properties of the rat brain in the sagittal plane: effects of anatomical structure and age. Ann Biomed Eng. 2012;40:70–78. doi: 10.1007/s10439-011-0394-2. [DOI] [PubMed] [Google Scholar]
- Gefen A, Gefen N, Zhu Q, Raghupathi R, Margulies SS. Age-dependent changes in material properties of the brain and braincase of the rat. J Neurotrauma. 2003;20:1163–1177. doi: 10.1089/089771503770802853. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS, Faraone SV, Tsuang MT. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb Cortex. 2001;11:490–497. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RNA, Friston KJ, Frackowiak RSJ. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Guo J, Hirsch S, Fehlner A, Papazoglou S, Scheel M, Braun J, Sack I. Towards an elastographic atlas of brain anatomy. PLoS One. 2013;8:e71807. doi: 10.1371/journal.pone.0071807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Turetsky BI, Matsui M, Yan M, Bilker W, Hughett P, Gur RE. Sex differences in brain gray and white matter in healthy young adults: correlations with cognitive performance. Journal of Neuroscience. 1999;19:4065–4072. doi: 10.1523/JNEUROSCI.19-10-04065.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huse JT, Holland EC. Targeting brain cancer: advances in the molecular pathology of malignant glioma and medulloblastoma. Nat Rev Cancer. 2010;10:319–331. doi: 10.1038/nrc2818. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Wiste HJ, Vemuri P, Weigand SD, Senjem ML, Zeng G, Bernstein MA, Gunter JL, Pankratz VS, Aisen PS, Weiner MW, Petersen RC, Shaw LM, Trojanowski JQ, Knopman DS. Brain beta-amyloid measures and magnetic resonance imaging atrophy both predict time-to-progression from mild cognitive impairment to Alzheimer's disease. Brain : a journal of neurology. 2010;133:3336–3348. doi: 10.1093/brain/awq277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CL, McGarry MD, Gharibans AA, Weaver JB, Paulsen KD, Wang H, Olivero WC, Sutton BP, Georgiadis JG. Local mechanical properties of white matter structures in the human brain. Neuroimage. 2013;79:145–152. doi: 10.1016/j.neuroimage.2013.04.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MC, Huston J, 3rd, Jack CR, Jr, Glaser KJ, Manduca A, Felmlee JP, Ehman RL. Decreased brain stiffness in Alzheimer's disease determined by magnetic resonance elastography. J Magn Reson Imaging. 2011;34:494–498. doi: 10.1002/jmri.22707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MC, Huston J, 3rd, Jack CR, Jr, Glaser KJ, Senjem ML, Chen J, Manduca A, Felmlee JP, Ehman RL. Measuring the characteristic topography of brain stiffness with magnetic resonance elastography. PLoS One. 2013;8:e81668. doi: 10.1371/journal.pone.0081668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittock SJ, Lucchinetti CF. The pathology of MS: new insights and potential clinical applications. Neurologist. 2007;13:45–56. doi: 10.1097/01.nrl.0000253065.31662.37. [DOI] [PubMed] [Google Scholar]
- Qin EC, Sinkus R, Geng GQ, Cheng S, Green M, Rae CD, Bilston LE. Combining MR elastography and diffusion tensor imaging for the assessment of anisotropic mechanical properties: A phantom study. Journal of Magnetic Resonance Imaging. 2013;37:217–226. doi: 10.1002/jmri.23797. [DOI] [PubMed] [Google Scholar]
- Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF, Ivnik RJ, Tangalos EG, Petersen RC, Rocca WA. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30:58–69. doi: 10.1159/000115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano A, Guo J, Prokscha T, Meyer T, Hirsch S, Braun J, Sack I, Scheel M. In vivo waveguide elastography: Effects of neurodegeneration in patients with amyotrophic lateral sclerosis. Magn Reson Med. 2014;72:1755–1761. doi: 10.1002/mrm.25067. [DOI] [PubMed] [Google Scholar]
- Romano A, Scheel M, Hirsch S, Braun J, Sack I. In vivo waveguide elastography of white matter tracts in the human brain. Magn Reson Med. 2012;68:1410–1422. doi: 10.1002/mrm.24141. [DOI] [PubMed] [Google Scholar]
- Ruigrok AN, Salimi-Khorshidi G, Lai MC, Baron-Cohen S, Lombardo MV, Tait RJ, Suckling J. A meta-analysis of sex differences in human brain structure. Neurosci Biobehav Rev. 2014;39C:34–50. doi: 10.1016/j.neubiorev.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack I, Beierbach B, Wuerfel J, Klatt D, Hamhaber U, Papazoglou S, Martus P, Braun J. The impact of aging and gender on brain viscoelasticity. Neuroimage. 2009;46:652–657. doi: 10.1016/j.neuroimage.2009.02.040. [DOI] [PubMed] [Google Scholar]
- Sack I, Streitberger KJ, Krefting D, Paul F, Braun J. The influence of physiological aging and atrophy on brain viscoelastic properties in humans. PLoS One. 2011;6:e23451. doi: 10.1371/journal.pone.0023451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Kan E, Woods RP, Yoshii J, Bansal R, Xu D, Zhu H, Thompson PM, Toga AW. Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cereb Cortex. 2007;17:1550–1560. doi: 10.1093/cercor/bhl066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streitberger KJ, Sack I, Krefting D, Pfuller C, Braun J, Paul F, Wuerfel J. Brain viscoelasticity alteration in chronic-progressive multiple sclerosis. PLoS One. 2012;7:e29888. doi: 10.1371/journal.pone.0029888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streitberger KJ, Wiener E, Hoffmann J, Freimann FB, Klatt D, Braun J, Lin K, McLaughlin J, Sprung C, Klingebiel R, Sack I. In vivo viscoelastic properties of the brain in normal pressure hydrocephalus. NMR Biomed. 2011;24:385–392. doi: 10.1002/nbm.1602. [DOI] [PubMed] [Google Scholar]
- Witelson SF, Glezer II, Kigar DL. Women have greater density of neurons in posterior temporal cortex. Journal of Neuroscience. 1995;15:3418–3428. doi: 10.1523/JNEUROSCI.15-05-03418.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte AV, Savli M, Holik A, Kasper S, Lanzenberger R. Regional sex differences in grey matter volume are associated with sex hormones in the young adult human brain. Neuroimage. 2010;49:1205–1212. doi: 10.1016/j.neuroimage.2009.09.046. [DOI] [PubMed] [Google Scholar]
- Wuerfel J, Paul F, Beierbach B, Hamhaber U, Klatt D, Papazoglou S, Zipp F, Martus P, Braun J, Sack I. MR-elastography reveals degradation of tissue integrity in multiple sclerosis. Neuroimage. 2010;49:2520–2525. doi: 10.1016/j.neuroimage.2009.06.018. [DOI] [PubMed] [Google Scholar]
- Xu L, Lin Y, Han JC, Xi ZN, Shen H, Gao PY. Magnetic resonance elastography of brain tumors: preliminary results. Acta Radiol. 2007;48:327–330. doi: 10.1080/02841850701199967. [DOI] [PubMed] [Google Scholar]
- Zhang J, Green MA, Sinkus R, Bilston LE. Viscoelastic properties of human cerebellum using magnetic resonance elastography. J Biomech. 2011;44:1909–1913. doi: 10.1016/j.jbiomech.2011.04.034. [DOI] [PubMed] [Google Scholar]