Abstract
Cigarette smoking is common despite adverse health effects. Nicotine’s effects on learning may contribute to addiction by enhancing drug-context associations. Effects of nicotine on learning could be direct or could occur by altering systems that modulate cognition. Because thyroid signaling can alter cognition and nicotine/smoking may change thyroid function, nicotine could affect learning through changes in thyroid signaling. These studies investigate the functional contributions of thyroid receptor (TR) subtypes β and α1 to nicotine-enhanced learning and characterize the effects of acute nicotine and learning on thyroid hormone levels. We conducted a high throughput screen of transcription factor activity to identify novel targets that may contribute to the effects of nicotine on learning. Based on these results, which showed that combined nicotine and learning uniquely acted to increase TR activation, we identified TRs as potential targets of nicotine. Further analyses were conducted to determine the individual and combined effects of nicotine and learning on thyroid hormone levels, but no changes were seen. Next, to determine the role of TRβ and TRα1 in the effects of nicotine on learning, mice lacking the TRβ or TRα1 gene and wildtype littermates were administered acute nicotine prior to fear conditioning. Nicotine enhanced contextual fear conditioning in TRα1 knockout mice and wildtypes from both lines but TRβ knockout mice did not show nicotine-enhanced learning. This finding supports involvement of TRβ signaling in the effect of acute nicotine on hippocampus-dependent memory. Acute nicotine enhances learning and these effects may involve processes regulated by the transcription factor TRβ.
Keywords: Acetylcholine, Learning, and Memory, Fear Conditioning, Thyroid, Thyroid Receptor, Gene
1. Introduction
The development of addiction involves neural and synaptic remodeling, events that also occur during normal learning (Kalivas and O’Brien 2008). It has been suggested that drugs of abuse, such as nicotine, may usurp the learning and memory machinery at the neural, cellular, and molecular levels to create maladaptive drug-context and drug-cue associations that drive behavior toward addiction (Gould 2006; Gould and Leach 2013; Hyman 2005; Wolf 2002). Such maladaptive learning may create or strengthen associations between the subjective effects of nicotine and the spatial and discrete cues involved in the drug taking process (i.e., convenience stores, cigarettes, lighters, and packaging) leading to continued drug use (Gould 2010; Gould and Leach 2013). Acute nicotine administration in rodents enhanced performance in a variety of hippocampus-dependent learning and memory tasks (French et al. 2006; Gould and Wehner 1999; Kenney et al. 2012a; Levin and Rose 1991; Socci et al. 1995) including contextual fear conditioning. Identifying the cell signaling processes underlying the enhancement of learning and memory by nicotine not only advances understanding of learning and memory and addiction but may also identify novel targets for the treatment of nicotine addiction and disorders associated with cognitive decline.
While it has been demonstrated that nicotine enhances contextual memory by acting at nicotinic acetylcholine receptors in the hippocampus (Davis et al. 2007), it is unknown if nicotine is also acting on other systems to modulate learning. One potential target is the thyroid hormone receptor (TR) signaling system. Multiple lines of evidence suggest that nicotine and/or cigarette smoking may affect endocrine signaling (For review see Kapoor and Jones 2005; Tweed et al. 2012) including, but not limited to, thyroid signaling. Specifically, there is a substantial literature describing alterations in thyroid function in current and former smokers (Bertelsen and Hegedus 1994; Schlienger et al. 2003; Wiersinga 2013). Smokers generally have higher thyroid hormone levels and lower levels of thyroid stimulating hormone (TSH), which is typically inversely proportional to thyroid hormone level (Christensen et al. 1984; Ericsson and Lindgarde 1991; Fisher et al. 1997; Jorde and Sundsfjord 2006); however, there is also evidence that smoking can decrease thyroid function (Soldin et al. 2009). Behavioral and electrophysiological studies in rodents suggest that chronic nicotine can abolish hypothyroidism-induced neural deficiencies. Specifically, chronic nicotine ameliorated learning deficits in rats with experimentally-induced hypothyroidism (Alzoubi et al. 2006b) and nicotine also reversed hypothyroidism-induced deficits in synaptic plasticity (Alzoubi et al. 2006a; 2007). The hypothyroidism-induced deficit in synaptic plasticity was associated with decreases in the activity of learning-related molecules such as CREB and ERK1/2 (Alzoubi and Alkadhi 2007; Gerges and Alkadhi 2004). Chronic nicotine ameliorated the molecular, electrophysiological, and behavioral/cognitive disruption caused by induced-hypothyroidism and this may represent convergent or compensatory mechanisms of action. The previously mentioned studies reveal an interaction between the effects of nicotine and thyroid function in experimentally compromised animal models (i.e., thyroidectomized animals that had ~50% normal thyroid hormone levels).
In addition to direct effects of smoking on thyroid function, second hand exposure may also alter thyroid function. Maternal and paternal cigarette smoking may have effects on the thyroid function of their children. The evidence suggests that children of smokers have enlarged thyroids and higher levels of thyroglobulin, a protein that is important in the synthesis of the prohormone thyroxine (T4), the secreted form of thyroid hormone (Chanoine et al. 1991). Parental smoking is also associated with higher levels of T4 and lower levels of TSH in infants (Meberg and Marstein 1986). These studies suggest that chronic exposure to nicotine alters thyroid signaling; however, it is not entirely clear from these studies if these effects are due to nicotine, or due to other components of tobacco smoke, such as thiocyanate (Bertelsen and Hegedus 1994; Wiersinga 2013). It is also unclear from human association studies if smoking causes alterations in thyroid hormones or if subjects with alterations in thyroid hormones tend to smoke cigarettes.
The effects of acute nicotine on thyroid function and thyroid hormone receptor (TR) signaling is largely unknown. Animal models suggest that acute nicotine administration did not directly alter thyroid hormone levels (Cam and Bassett 1983; Huffman et al. 1991), but did reduce TSH levels (Andersson et al. 1988), which is usually indicative of higher thyroid hormone levels. Further, levels of brain thyroid hormones may be independent of serum hormone concentrations, and brain TR activity may not correspond perfectly to serum hormone levels. Thus, this study examined if acute nicotine-associated changes in thyroid signaling are involved in nicotine-enhancement of learning, and if disrupted thyroid signaling alters the effects of acute nicotine on learning. Specifically, this study tested: 1) if nicotine and learning uniquely alter hippocampal transcription factor activity (including thyroid receptor activity) that may relate to its effects on hippocampus-dependent learning, 2) if disrupted TR signaling in knockout mice attenuates the effects of nicotine on hippocampus-dependent learning and memory, and 3) if acute nicotine and contextual fear conditioning have an effect on serum thyroid hormone status.
2. Methods
2.1 Subjects
Subjects used for the transcription factor array experiment (N=12) and hormone analysis (N=24) were male C57BL/6J mice (Jackson Laboratories, Bar Harbor ME) 8-12 weeks old at the start of training. Male and female TR mutant mice (TRβ WT and KO (N=141); TRα1 WT and KO (N=141)) aged 8-12 weeks at start of training were used for behavioral studies. Mutant mice, originally generated by Forrest, Wikstrom and colleagues, were purchased from Jackson Laboratories. TRβ mutants contained a mutation that disrupted transcription of the entire TRβ gene (TRβ1 and TRβ2) (Forrest et al. 1996b). The TRα1 mutation was specific for TRα1, such that mutant mice expressed functional TRα2 (Wikstrom et al. 1998). Both colonies were backcrossed to parent strain (C57BL/6) for greater than 15 generations, resulting in mice estimated to be >99.9% genetically identical to C57BL/6 mice (Conner 2002) indicating that they are on a suitable background strain and that results should generalize to the C57BL/6 strain. All mice were maintained in a temperature and humidity controlled vivarium with ad libitum access to standard lab chow and water. Mutant mice were bred, maintained, and tested at Temple University according to NIH guidelines. All procedures were approved by the Temple University Institutional Animal Care and Use Committee.
2.2 Apparatus
Fear conditioning training and testing took place in Plexiglas (26.5 × 20.4 × 20.8 cm) conditioning chambers with stainless steel rod grid floors (2 mm diameter) spaced 1 cm apart as previously described (Kenney et al. 2010). Grid floors were connected to a scrambled shock generator (Med-Associates) that delivered 0.57 mA foot shocks. Conditioning chambers, controlled by LabView software, were housed inside sound attenuating chambers (Med-Associates, St. Albans, VT). Each chamber also contained a house light (4 watt) as well as a ventilation fan that produced a constant white noise (65 dB) and provided air circulation. Cued fear conditioning testing took place in an altered context. Altered context testing occurred in chambers of a different size (20 × 23 × 19 cm) contained within sound attenuating chambers (Med-Associates, St. Albans, VT) located in a different room from conditioning chambers. The altered context chambers differed in construction in that they had aluminum side-walls and a flat plastic floor. Additionally, vanilla extract was added within each of the chambers to further alter the context. All chambers were cleaned with 70% ethanol before and after each training or testing session.
Auditory startle testing occurred in sound attenuating chambers using SR-Lab Equipment (San Diego Instruments, San Diego, CA). Mice were constrained to Plexiglas cylinders (38mm internal diameter) that contained a shock grid with 7 rods. The cylinders rested on a platform containing an accelerometer attached to a PC running SR-Lab software.
2.3 Drug preparation and administration
For all experiments, (−) nicotine hydrogen tartrate (reported as freebase weight) was dissolved in physiological saline (Sigma) and all doses were administered at a dose volume of 10 mL/kg. For phenotyping experiments, acute nicotine (0, 0.09, 0.18, or 0.36 mg/kg) was administered via intraperitoneal injection (IP) to mice 5 minutes prior to the initiation of training and both testing sessions (context and cued). For analysis of serum thyroid hormone levels and the transcription factor array experiment, acute nicotine (0, 0.09, or 0.18 mg/kg) was administered (IP) 5 minutes prior to contextual fear conditioning training or to a home cage control. Nicotine doses are based on a dose found to produce plasma nicotine levels similar to those of human smokers (Davis et al. 2005).
2.4 Fear Conditioning Training and Testing
For each nicotine dose, TRβ and TRα1 wildtype (WT) and knockout (KO) mice were trained and tested in a combined contextual and cued fear conditioning paradigm (Portugal et al. 2012). Fear conditioning is a useful tool to assess multiple forms of memory and can be conducted in only 2 days, facilitating the examination of acute nicotine’s effects on learning. Briefly, mice were placed into conditioning chambers and were allowed to explore for 2 minutes, at which time a conditioned stimulus (CS, white noise, 85 dB) was presented continuously for 30 seconds and co-terminated with an unconditioned stimulus (US, footshock) lasting 2 seconds. After the CS-US pairing, a 2 minute inter-trial-interval elapsed prior to a second CS-US pairing. Mice were returned to their home cages 30 seconds after the second CS-US pairing. 24 hours after conditioning, mice were returned to the training context and assessed for freezing for 5 minutes. Freezing to the training context was used as a measure of hippocampus-dependent contextual memory. At least one hour after contextual testing, mice were placed into the altered context for 6 minutes. During the initial 3 minutes, altered context (pre-CS) freezing was assessed and used as a measure of generalized freezing (Baldi et al. 2004). After the initial 3 minutes, the CS was presented continuously for an additional 3 minutes and freezing to the CS was assessed and used to measure hippocampus-independent memory for the CS-US association (Logue et al. 1997; Phillips and LeDoux 1992).
In addition to the use of an auditory cue CS, an alternate CS modality, a light-CS, was used to assess learning in mice to examine sensory modality specific defects. Light-CS training and cued testing occurred as previously described except that in place of a white noise, the house light was used as the CS. Additionally, light-CS training and testing occurred under low ambient light levels with red filters placed over room lights to increase the salience of the CS. For all experiments, freezing behavior was assessed using a sampling procedure of 1 second out of every 10 seconds by researchers blind to drug and genotype conditions. Freezing was defined as lack of all movement other than respiration (Blanchard and Blanchard 1969). Percent freezing was calculated as the number of times observed freezing divided by the total number of sampled time points expressed as a percent.
2.5 Nuclear Protein Extraction
Mice were administered acute nicotine (0 or 0.09 mg/kg, IP) 5 minutes prior to training in the fear conditioning paradigm as previously described, except that no auditory cue was used as no effect of nicotine was observed in the KO studies. Saline treated homecage animals were used as controls for these experiments. Thirty minutes after training, or an equivalent time after saline homecage treatment, mice were sacrificed by cervical dislocation. Brains were dissected out and bilateral dorsal hippocampi were isolated and immediately flash frozen, as prior work has shown that acute nicotine works in dorsal hippocampus to enhance learning (Kenney et al. 2012b; Raybuck and Gould 2010). Nuclear protein extraction from hippocampal tissue was performed using Panomics Nuclear Extraction Kit (AY2002) according to the manufacturer’s whole tissue instructions. Briefly, hippocampal tissue was homogenized in nuclear extraction buffer and centrifuged at 4° C. The resulting supernatant, which contained the nuclear extract, was collected and used for the transcription factor array experiments. Each treatment group consisted of pooled hippocampi from 4 animals to obtain sufficient nuclear extract.
2.6 Transcription Factor Array
To investigate changes in hippocampal transcription factor activity, Panomics TranSignal protein/DNA Assay Kits (Arrays I, II, III, and V) with spin column separation were used (MA1210, MA1211, MA1212, and MA1214, respectively). Following the manufacturer’s instructions, 10 μg of nuclear extract was incubated with a biotin-labelled DNA probe mixture. Protein bound probes were isolated using the spin columns included with the kit. Following isolation of protein-DNA complexes, eluted and labelled probes were hybridized to TranSignal membranes (for a total of 319 unique consensus binding sites for transcription factor binding) overnight at 42° C. After hybridization, each membrane was transferred from its hybridization bottle to its own container and washed. Following washing, membranes were incubated with Streptavidin-horseradish peroxidase (HRP) conjugate and allowed to incubate. Chemiluminescent imaging was used to detect signal using a Gel Logic 1500 imaging system and accompanying software (Kodak).
2.7 Auditory Startle
Four weeks following fear conditioning and testing, mice were tested in an auditory startle paradigm based on the protocol used by Kazdoba and colleagues (2007). Startle testing consisted of a single session beginning with a 5 minute acclimation period. Following acclimation, 6 trials each of 500 ms bursts of white noise (0, 70, 80, 90,100, 110, 120, and 130 dB) for a total of 48 trials, were presented. Average startle values for each animal were calculated for each dB level. Bursts were presented in a pseudorandom order and variable trial interval ranging from 12 to 20 seconds.
2.8 Competition Enzyme Linked Immunosorbent Assay (ELISA) Analyses
To investigate if acute nicotine significantly altered thyroid hormone levels, serum was collected 30 minutes after acute nicotine (0, or 0.18 mg/kg, IP) treatment for ELISA (Alpha Diagnostics) analyses that evaluated total levels of T4 (Kit #1100) and metabolically active thyroid hormone (tri-iodothyronine,T3, Kit #1700). 30 minutes was chosen based on the transcription factor array experiment that identified thyroid hormone receptor activity in the hippocampus 30 minutes after treatment. Briefly, approximately 500 μL blood was collected from the descending vena cava into microtainer serum collection tubes (BD) with clot activator and gel separator and tubes were immediately inverted 5 times to activate clotting. Blood was allowed to clot for at least 30 minutes before being centrifuged for 5 minutes at 10,000 rpm. Resulting supernatant was collected and stored at −80° C until analysis. ELISAs were run using manufacturer’s recommended protocols. Briefly, 25 or 50 μL (for T4 and T3 assays, respectively) serum were added to 96 well plates previously coated with anti-thyroid hormone antibodies. Then, horseradish peroxidase (HRP) conjugated thyroid hormone solution was added to the wells to competitively bind to their respective antibodies for 60 minutes at room temperature. Wells were then aspirated and washed 3 times prior to the addition of an HRP substrate solution. Plates were then incubated with HRP substrate solution for 15 minutes at room temperature and a blue color developed. Finally, 50 μL stop solution was added and the blue color turned to yellow. Plates were then read on a 96 well plate reader (Bio-Rad) at 450 nM. Absorbance values were inversely proportional to thyroid hormone levels. Using a standard curve, thyroid hormone levels were calculated (interpolated) and graphed. Also, as a more sensitive measure of thyroid hormone status, a T3/T4 ratio was calculated for each animal. Finally, to determine if combined nicotine and fear conditioning selectively altered thyroid hormone signaling, ELISA analyses were repeated with the following groups: saline-treated homecage, nicotine-treated homecage, saline-treated fear conditioning, and nicotine-treated fear conditioning.
2.9 Statistical Analysis
For the transcription factor array experiment, blots of experimental groups (Sal+FC and Nic+FC) were expressed as fold-change over control group (Sal+No FC) and anything over 2-fold or under 0.5-fold was considered significant, as per the manufacturer’s recommended protocol. For fear conditioning experiments, 2×2 ANOVAs were conducted with genotype (WT and KO) and drug treatment (saline or nicotine) as between subjects factors to compare the percentage of time spent freezing between WT mice treated with saline, WT mice treated with nicotine, KO mice treated with saline, and KO mice treated with nicotine for all phases of testing (context, pre-CS, and CS). Prior to analyses, male and female freezing levels were compared across each group to determine if there were sex-based differences in freezing; there were no differences and therefore, males and females were grouped together for statistical analyses. Bonferroni corrected post-hoc analyses were used to determine if nicotine treatment differed from saline treatment in each genotype. A planned comparison of saline versus nicotine treated WT animals was used as a positive control for nicotine’s effects in every study. For light-cued fear conditioning, an unpaired student’s t-test was used to compare WT and KO freezing levels and a paired t-test was used to evaluate the efficacy of light-cued fear conditioning procedure. Auditory startle results were analyzed by a 2×8 mixed model ANOVA with genotype as a between subjects variable and decibel (dB) as a within subjects variable. Bonferroni corrected post hoc tests were used to compare WT to KO at each dB level.
3. Results
3.1 Transcription Factor Array Analyses
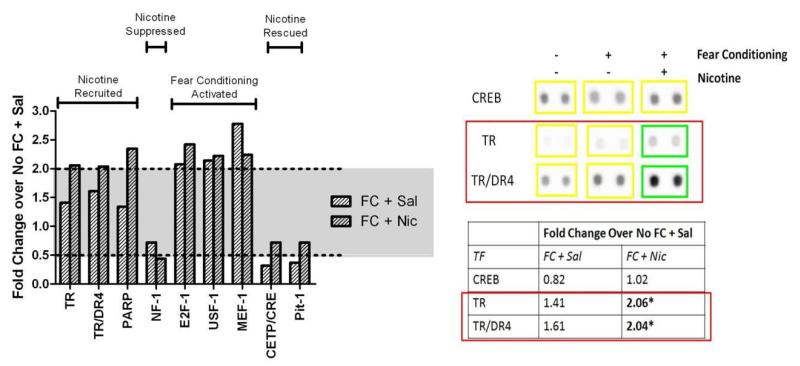
The results of the transcription factor arrays revealed that a select subset of transcription factors (3 out of 319) were activated in the hippocampus 30 minutes after fear conditioning in the presence of nicotine (FC+Nic) that were not activated in animals conditioned in the absence of nicotine (FC+Sal); both groups were compared to saline-treated homecage-control animals (No FC+Sal). In other words, nicotine recruited the activity of these 3 transcription factors that were not normally activated during a hippocampal learning event (Fig. 1). Thyroid receptor (TR), TR direct repeat 4 (TR/DR4), and poly-(ADP-ribose) polymerase (PARP) showed little activity after fear conditioning in the absence of nicotine (1.41-fold, 1.61-fold, and 1.34-fold changes in activity compared to controls (No FC+Sal), respectively), but nicotine recruited their involvement during fear conditioning (2.06-fold, 2.04-fold, and 2.35-fold changes in activity compared to No FC+Sal control group, respectively).
Fig. 1.
The effect of fear conditioning and nicotine on transcription factor activity.
Combined nicotine and fear conditioning treatment significantly increased the activity of TR, TR/DR4, and PARP and decreased the activity of NF-1. Fear conditioning alone significantly increased the activity of E2F-1, USF-1, and MEF-1. Fear conditioning alone significantly decreased and nicotine rescued the activity of CETP/CRE and Pit-1. Significance (above or below dotted line) was set at 2-fold and 0.5-fold change compared to control (Sal+No FC). Representative dots are shown on the right for CREB (no change) and TRs.
Fear conditioning alone (FC+Sal) activated 3 transcription factors. Fear conditioning resulted in a 2.08-fold increase in E2F transcription factor 1 (E2F-1) activity, a 2.14-fold increase in myocyte-specific enhancer-binding nuclear factor 1 (MEF-1) activity, and a 2.78-fold increase in upstream transcription factor 1 (USF-1) activity compared to controls (No FC+Sal). Nicotine treatment (FC+Nic) did not further augment the activity of these transcription factors (2.42-fold, 2.22-fold, and 2.24-fold change in activity for E2F1, MEF-1, and USF-1, respectively). Thus, hippocampus-dependent learning may activate these transcription factors, but nicotine does not contribute to any additional activation. Fear conditioning alone (FC+Sal) resulted in a 0.32-fold change (i.e., a reduction in activity) in cholesteryl ester transfer protein (CETP/CRE) activity and a 0.37-fold change in pituitary specific transcription factor (Pit-1) activity, but the presence of nicotine (FC+Nic) rescued the reduction in activity of both these transcription factors (0.79-fold change in activity for both). Fear conditioning (FC+Sal) did not alter the activity of nuclear factor 1 (NF-1) (0.72-fold change in activity), but the addition of nicotine (FC+Nic) suppressed its activity to 0.44-fold compared to naïve homecage control (No FC+Sal) activity.
3.2 Effects of Acute Nicotine on Contextual and Cued Fear Conditioning in TRβ Mutant Mice
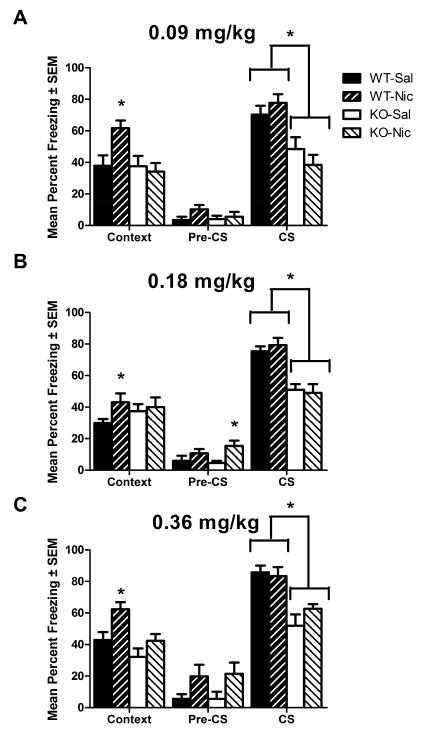
TRβ WT and KO mice administered acute nicotine (0, 0.09, 0.18, or 0.36 mg/kg, IP) were evaluated for nicotine-enhanced fear conditioning (Fig. 2). ANOVA evaluating the effect of acute nicotine (0 or 0.09 mg/kg, IP) on freezing to context revealed a significant effect of genotype on contextual freezing levels: F(1,43)=5.679, p<0.05; no effect of nicotine on contextual freezing levels: p>0.05; and a significant interaction: F(1,43)=5.433, p<0.05 (Fig. 2A). Bonferroni corrected post-hoc tests revealed a significant effect of nicotine on freezing levels in WT mice (p<0.05), but no effect of nicotine on freezing levels in KO mice (p>0.05). ANOVA on contextual freezing levels in TRβ mutant mice treated with nicotine (0 or 0.18 mg/kg, IP) revealed no effect of genotype, nicotine, and no interaction (Fig. 2B). Planned comparison analysis revealed a significant effect of nicotine on freezing levels in WT mice (p<0.05), but not KO mice (p>0.05). ANOVA on contextual freezing levels in TRβ mutant mice treated with nicotine (0 or 0.36 mg/kg, IP) revealed a significant effect of genotype on freezing levels: F(1,23)=10.17, p<0.05; a significant effect of nicotine on freezing levels: F(1,23)=9.544, p<0.05; and no interaction (Fig. 2C). Bonferroni corrected post-hoc tests revealed a significant effect of nicotine on freezing levels in WT mice (p<0.05), but no effect of nicotine in KO mice (p>0.05). Nicotine consistently enhanced hippocampus-dependent memory in WT mice across all doses tested. In contrast, nicotine had no effect on hippocampus-dependent memory in TRβ KO mice.
Fig. 2.
The effect of acute nicotine on contextual and cued fear conditioning in TRβ mutant mice. A) 0 or 0.09 mg/kg, IP. B) 0 or 0.18 mg/kg, IP. C) 0 or 0.36 mg/kg, IP. Nicotine consistently enhanced contextual fear conditioning in WT mice but not KO mice (p<0.05 for all doses). At one dose (0.18 mg/kg), nicotine increased Pre-CS freezing, but effects were not consistent (p<0.05) and did not relate to nicotine’s effects on contextual conditioning. Deletion of TRβ consistently disrupted cued freezing (CS freezing) (p<0.05 for all doses). * indicates p<0.05.
The pre-CS portion of fear conditioning testing can indicate levels of generalized freezing. ANOVAs comparing TRβ mutant mice treated with 3 different doses of nicotine revealed no effect on pre-CS freezing of 0.09 mg/kg nicotine: p>0.05; a significant effect of 0.18 mg/kg nicotine: F(1,63)=7.011, p<0.05; and no effect of 0.36 mg/kg nicotine: p>0.05. For the 0.18 mg/kg nicotine comparison, Bonferroni corrected post hoc tests revealed a significant effect of nicotine in KO mice, but not WT mice. Because WT mice showed nicotine-enhanced contextual learning (Fig. 2), while KOs did not, these data indicate that increased generalized freezing likely did not contribute to the observed effects.
The cued fear conditioning portion of testing examines hippocampus-independent effects of drugs and genes, as cued fear conditioning does not require a functioning hippocampus (Phillips and LeDoux 1992). ANOVA of freezing to an auditory cue in TRβ mutant mice treated with nicotine (0 or 0.09 mg/kg, IP) revealed a significant effect of genotype: F(1,43)=23.71, p<0.05; no significant effect of nicotine; and no interaction. ANOVA of freezing to an auditory cue in TRβ mutant mice treated with nicotine (0 or 0.18 mg/kg, IP) revealed a significant effect of genotype: F(1,63)=33.25, p<0.05; no significant effect of nicotine; and no interaction. Finally, analysis of the effect of nicotine (0 or 0.36 mg/kg, IP) on auditory cue-evoked freezing in TRβ mutant mice revealed a significant effect of genotype: F(1,23)=27.65, p<0.05; no significant effect of nicotine; and no interaction. Mice lacking TRβ showed a significant reduction in the formation of the hippocampus-independent cued memory, and this effect was observed independent of nicotine treatment.
Based on the cued fear conditioning deficit observed in TRβ KO mice, several follow-up analyses were conducted to exclude the possibility that altered TR signaling affects hippocampus-independent learning. Cued fear conditioning is most often conducted with an auditory CS, and the literature suggests that TRβ mutant mice exhibit deafness (Forrest et al. 1996a). Based on the current results, however, TRβ KO mice showed attenuated cued fear conditioning rather than completely abolished performance, indicating that mice were able to at least modestly perceive sound. To ascertain the impact of hearing deficits in TRβ KO mice on cued fear conditioning and to determine their ability to learn to associate the presence of a visual CS and an aversive US, TRβ WT and KO mice were assessed for light-cued fear conditioning (data not shown). Unpaired student’s t-test comparing freezing levels between TRβ WT and KO mice in contextual and cued fear conditioning revealed no differences for either test (p>0.05 for each test). Finally, a paired samples t-test for all subjects comparing Pre-CS “Altered Context” freezing to freezing in the presence of the CS revealed successful cued fear conditioning: t(15)=3.088, p<0.05 and indicated that light-cued fear conditioning was successfully learned by all animals.
3.3 Evaluation of Auditory Processing in TRβ and TRα1 Mutant Mice
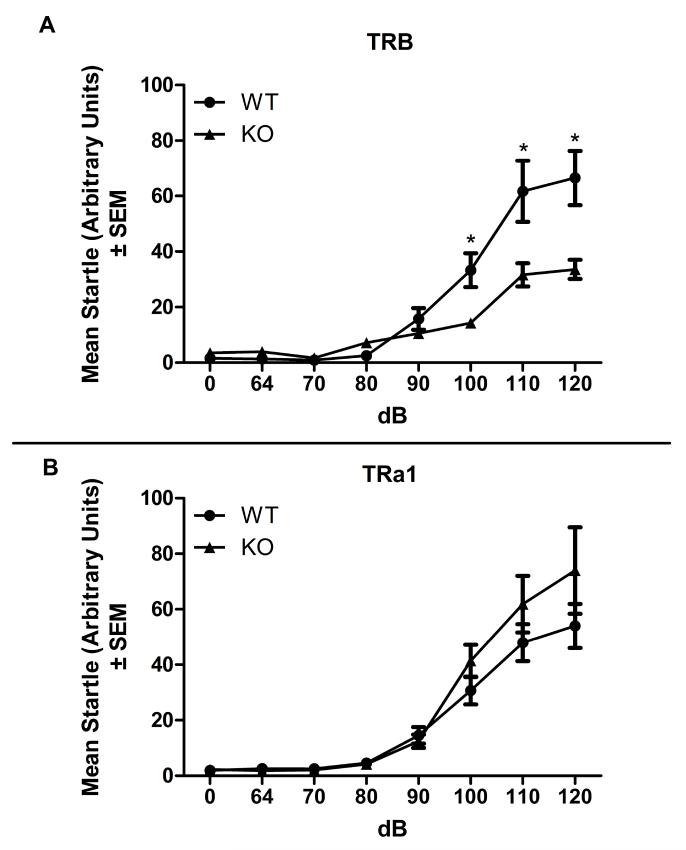
To further examine auditory function in TRβ KO mice, auditory startle response was evaluated in TRα1 and TRβ mutant mice (Fig. 3). A two factor ANOVA with TRβ genotype as a between subjects factor (WT and KO) and dB level (0, 65, 70, 80, 90, 100, 110, and 120) as a within subjects factor (Fig. 3A) revealed a significant effect of genotype: F(1,63)=7.377, p<0.05; a significant effect of dB: F(7,63)=67.22, p<0.05; and a significant interaction: F(7,63)=9.872, p<0.05 on auditory startle reactivity. Bonferroni corrected post-hoc tests revealed a significant deficit in KO auditory startle at 100, 110, and 120 dB compared to WT mice (ps<0.05). In contrast, ANOVA analysis of auditory startle reactivity in TRα1 mice in auditory startle (Fig. 3B) revealed no significant effect of genotype; a significant effect of dB: F(7,105)=60.11, p<0.05; and no interaction. These results confirm an attenuated auditory response system in TRβ KO mice. Conversely, TRα1 mice exhibit normal levels of auditory startle as well as auditory cued fear conditioning.
Fig. 3.
Auditory startle in TR mutant mice.
A) TRβ mutant mice. B) TRα1 mutant mice. Deletion of TRβ produced a modest deficit in auditory startle response at 100, 110, and 120 dB (p<0.05 for each comparison). Deletion of TRα1 had no effect on auditory startle response at any dB level (p>0.05 for all comparisons).
3.4 Effects of Acute Nicotine on Contextual and Cued Fear Conditioning in TRα1 Mutant Mice
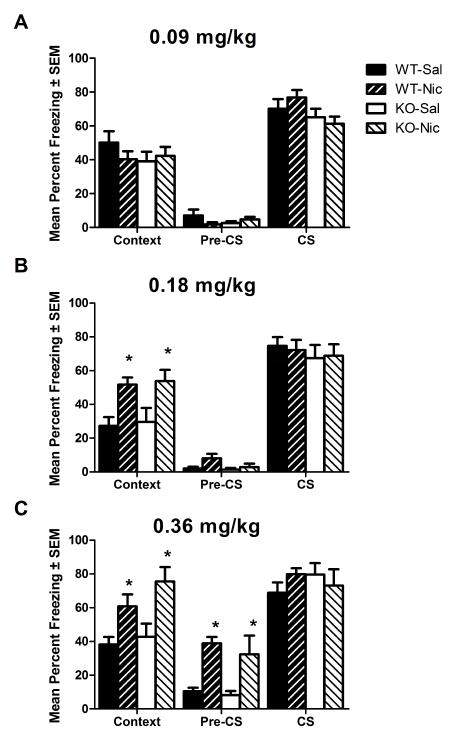
To determine if disruption of TRα1 similarly alters acute nicotine-enhanced hippocampus-dependent memory, TRα1 WT and KO mice administered acute nicotine (0, 0.09, 0.18, or 0.36 mg/kg, IP) were evaluated for nicotine-enhanced fear conditioning (Fig. 4). For TRα1 mutant mice tested in contextual fear conditioning after acute nicotine (0 or 0.09 mg/kg, IP), an ANOVA revealed no significant effect of genotype on contextual freezing levels; no effect of nicotine; and no interaction (Fig. 4A). Planned comparisons revealed no effect of nicotine in WT mice (ps>0.05) on contextual freezing levels. ANOVA of TRα1 mutant mice tested for contextual fear-evoked freezing with a 2-fold higher dose of acute nicotine (0 or 0.18 mg/kg, IP) revealed no effect of genotype; a significant effect of nicotine: F(1,35)=16.45, p<0.05; and no interaction (Fig. 4B). Bonferroni corrected post-hoc tests revealed a significant effect of nicotine on contextual freezing in both WTs and KOs (ps<0.05). Additionally, ANOVA of TRα1 mutant mice tested for contextual fear conditioning after acute nicotine (0 or 0.36 mg/kg, IP) revealed no effect of genotype on contextual freezing levels; a significant effect of nicotine on contextual freezing levels: F(1,26)=16.80, p<0.05; and no interaction (Fig. 4C). Bonferroni corrected post-hoc tests revealed a significant effect of nicotine on contextual freezing levels in KOs and WTs (p<0.05). These results revealed a clear dissociation between the effects of altered TRα1 and TRβ signaling on nicotine-enhanced hippocampus-dependent learning and memory.
Fig. 4. The effect of acute nicotine on contextual and cued fear conditioning in TRα1 mutant mice.
A) 0 or 0.09 mg/kg, IP. B) 0 or 0.18 mg/kg, IP. C) 0 or 0.36 mg/kg, IP. Nicotine had no effect in WTs or KOs at 0.09 mg/kg (p>0.05 for both genotypes). Nicotine enhanced contextual fear conditioning in WTs and KOs at 0.18 and 0.36 mg/kg (p<0.05 for all comparisons). Nicotine increased Pre-CS freezing at the highest dose tested (0.36 mg/kg) (p<0.05). Neither nicotine nor genotype had any effect on CS freezing (p>0.05 for all comparisons).
In contrast, ANOVA analysis of pre-CS freezing in TRα1 mutant mice treated with 3 different doses of nicotine revealed no effect of 0.09 mg/kg nicotine: p>0.05; no effect of 0.18 mg/kg nicotine: p>0.05; and a significant effect of 0.36 mg/kg nicotine: F(1,19)=24.67, p<0.05. Bonferroni corrected post hoc tests revealed significant effects of nicotine in WT and KO mice. Overall, there were no dose-dependent effects of nicotine on pre-CS freezing and the observed effects were not related to context freezing.
ANOVA on auditory cue-evoked freezing in of cued fear conditioning in TRα1 mutant mice revealed no significant effects of genotype; no effects of nicotine; and no interactions for any dose of nicotine tested (0, 0.09, 0.18, or 0.36 mg/kg, IP). These results indicate that, unlike TRβ KO mice, TRα1 KO mice exhibited normal levels of cued fear conditioning, which is evidence of both normal hearing and hippocampus-independent memory.
3.5 Effects of Acute Nicotine and Fear Conditioning on Serum Thyroid Hormone Levels
The effects of acute nicotine treatment on serum levels of thyroid hormones was assessed using ELISAs for T4 and T3 were carried out (Table 1). Serum was collected from C57BL/6J male mice treated with acute nicotine (0 or 0.18 mg/kg, IP). The analysis of thyroid hormone levels revealed no significant effect of nicotine treatment on hormone levels. Student’s t-tests comparing T3 and T4 levels revealed no significant effects of acute nicotine treatment (ps>0.05) on either hormone level. Further, acute nicotine did not affect the T3/T4 ratio (p>0.05), which can be indicative of subtle shifts in hormone secretion and availability. A follow-up analysis tested the effect of combined acute nicotine (0 or 0.18 mg/kg, IP) and contextual fear conditioning training on hormone levels (Table 1), but the ANOVA analysis revealed no effect on T3 levels, T4 levels, or on the T3/T4 ratio (p>0.05 for all comparisons).
Table 1.
Evaluation of the effects of acute nicotine or combined acute nicotine and fear conditioning on thyroid hormone status.
| ELISA Plate |
Treatment Group | T3 (ng/mL) | T4 (ug/dL) | T3/T4 Ratio |
|---|---|---|---|---|
| ELISA 1 | Acute Saline n=8 |
2.004 ± 0.1025 | 3.625 ± 0.1854 | 0.05334 ± 0.00181 |
| ELISA 1 | Acute Nicotine (0.18 mg/kg) n=10 |
1.777 ± 0.0930 | 3.291 ± 0.2289 | 0.05213 ± 0.00165 |
| ELISA 2 | Acute Saline Homecage n=4 |
1.472 ± 0.192 | 4.066 ± 0.168 | 0.0363 ± 0.0049 |
| ELISA 2 | Acute Nicotine (0.18 mg/kg) Homecage n=4 |
1.409 ± 0.092 | 4.095 ± 0.228 | 0.0349 ± 0.0035 |
| ELISA 2 | Acute Saline Fear Conditioned (2 × 0.57 mA) n=4 |
1.627 ± 0.184 | 4.131 ± 0.246 | 0.0392 ± 0.0034 |
| ELISA 2 | Acute Nicotine (0.18 mg/kg) Fear Conditioned (2 × 0.57 mA) n=4 |
1.526 ± 0.064 | 4.282 ± 0.142 | 0.0359 ± 0.0024 |
Table shows the calculated values of T3, T4, and T3/T4 ratios (Mean ± SEM) based on ELISA analyses.
4. Discussion
The present study identifies hippocampal TR signaling, specifically through TRβ, as a potentially novel signaling pathway involved in acute nicotine-enhanced hippocampal learning and memory. In contrast, changes in hippocampal TR signaling through TRα1 did not alter nicotine-enhanced hippocampus-dependent learning. The biochemical studies presented here sought to elucidate the mechanism responsible for nicotine-enhanced contextual fear conditioning. In order to test whether TR signaling was associated with nicotine-enhanced contextual fear conditioning, a series of transcription factor arrays were used to identify potential pathways that could lead to changes in hippocampal gene expression that may underlie the effects of acute nicotine on learning. Strikingly, there were only three transcription factor targets that were uniquely activated by learning in the presence of nicotine (TR, TR-DR4, and PARP), and two of these targets (TR and TR-DR4) were related to thyroid signaling. Specifically, the transcription factor array revealed increased TR activity at thyroid hormone response element half-sites, where one TR binds and dimerizes with other nuclear receptors (i.e. RXR), and direct repeat 4 sequences, where two TRs bind and dimerize to control gene transcription. These biochemical results indicate that learning in the presence of nicotine activates TRs and the behavioral results indicate that altered TRβ signaling disrupts nicotine-enhanced hippocampus-dependent learning and memory. Thus, TRβ signaling could either directly or indirectly modulate the effects of acute nicotine on learning.
Nicotine could directly alter thyroid hormone levels, either in the central nervous system or the periphery, and this could have a direct influence over learning and memory processes. Nicotine’s actions at glial cell-located nAChRs increases deiodinase 2 activity (Gondou et al. 1999), which catalyzes the activation of thyroid hormone by converting it from T4 to T3 (Arrojo et al. 2013; Bianco and Kim 2006; Williams and Bassett 2009). It has been suggested that many of the functions of thyroid hormone in neurons (i.e., activity at TRs) are specifically dependent on deiodinase 2 activity in glial cells (Asteria 1998) and this may represent a mechanism whereby nicotine can drive an increase in hippocampal TR activity. Nicotine-augmented TR signaling could lead to changes in gene expression of plasticity-related genes such as reelin or brain-derived neurotrophic factor (Giordano et al. 1992; Sui et al. 2010), both of which can be controlled by thyroid hormone. The present results identified increased hippocampal TR activity without a corresponding change in peripheral (serum) thyroid hormone levels. These results suggest no effect on thyroid secretion or general effects on metabolism, but instead support the idea that there are more localized effects on TR signaling, including effects in the hippocampus. The present studies not only reveal potential involvement of thyroid hormone signaling (particularly TRβ) in nicotine’s effects on learning, but also demonstrated that learning in the presence of nicotine selectively recruits hippocampal TR activity. Thyroid hormones have been shown to increase both ERK (Lei et al. 2008; Pantos et al. 2007) and PKA (Ghosh et al. 2005; Sarkar et al. 1999) activation, thus providing one potential pathway through which thyroid signaling could contribute to the enhancement of learning by acute nicotine. In support of this, a series of experiments identified these cell signaling cascades as critical for acute nicotine’s effects on learning. Specifically, nicotine-enhanced learning requires both ERK (Raybuck and Gould 2007) and PKA (Gould et al. 2014) activation.
Another possible explanation for the results obtained here is that there is an indirect link between TR signaling and the effects of nicotine on learning and memory; and that developmental alterations in thyroid signaling alter the effects of nicotine on learning. TRs control transcription of genes such as choline acetyltransferase (ChAT) (Quirin-Stricker et al. 1994) and NMDA receptor subunits (Lee et al. 2003). Therefore, constitutive changes in TR signaling (especially during development), such as those that could occur with KO mice, could alter processes involved in the effects of nicotine on learning. For instance, it is possible that the constitutive deletion of TRβ led to altered development of the cholinergic system through effects on ChAT expression, and this could have contributed to the diminished effects of nicotine on learning in the KO mice. In support, disrupted thyroid function during development led to a decrease in ChAT expression in learning and memory-related brain regions including the hippocampus (Sawin et al. 1998). Further, ChAT levels correlated with performance in a contextual fear conditioning paradigm (Woolf et al. 2001) and alterations in this system could attenuate nicotine’s effects on learning and memory. Alternatively, disrupted thyroid signaling may alter NMDA receptor expression patterns, which could affect the interaction between the glutamatergic and cholinergic neurotransmitter systems that are important for hippocampal learning and memory (Andre et al. 2011; Gould and Lewis 2005). In support of this, developmental hypothyroidism leads to an increase in select NMDA receptor subunit expression (NR1) and deficits in spatial learning and synaptic plasticity (Opazo et al. 2008). However, the current results found hippocampus-dependent and hippocampus-independent learning and memory largely intact in TR mutant mice, which suggests any potential subtle alterations in glutamatergic or acetylcholinergic signaling do not produce gross learning and memory impairments but could still potentially disrupt nicotine’s effects on learning. Therefore, while there may be a direct signaling component whereby nicotine increases TRβ signaling to enhance learning, it is also possible that disrupted thyroid signaling during development causes changes to other systems and this interferes with the ability of acute nicotine to enhance learning.
The current study also supports a critical role TRβ signaling plays in the development of normal auditory processing (Forrest et al. 1996a). Previously, using auditory brainstem evoked potentials, it was determined that TRβ KOs were deaf (Forrest et al. 1996a). The current study used a behavioral startle paradigm and observed similar, although not as profound, loss of auditory behavioral responses in TRβ KO mice. Furthermore, despite alterations in auditory processing, our results showed remarkably intact learning and memory systems in TRβ KO mice, such that their learning in drug-free contextual fear conditioning and their delay-cued fear conditioning to a light-CS (data not shown) was indistinguishable from WT littermates. This indicates that genetic disruption of either TR does not overtly affect either hippocampus-dependent or hippocampus-independent learning, whereas decreases in thyroid hormones can disrupt learning (Ge et al. 2012; Gerges et al. 2001).
A surprising finding from the current studies was that 0.09 mg/kg acute nicotine did not enhance contextual freezing in TRα1 WT or KO mice. While this result contrasted with established results with C57BL/6J mice and the effects observed in TRβ WT mice, it was not without precedent. Previously, differences in acute nicotine-enhanced contextual learning were observed between WTs of different nicotinic acetylcholine receptor mutant mouse lines (Lotfipour et al. 2013; Wehner et al. 2004). Further, the embryonic stem cell line used for the creation of many mutant mice (129), including those used here (Forrest et al. 1996b; Wikstrom et al. 1998), comes from an inbred mouse strain that does not show nicotine-enhanced learning at the doses that C57BL/6J mice do (Portugal et al. 2012). Thus, some residual genetic contribution from the 129 strain may play a role in differences between WTs of various mutant mouse lines.
In summary, using behavioral, genetic and pharmacological approaches, the results presented here indicate that combined nicotine and fear conditioning led to an increase in activity of hippocampal TRs (both T3RE half-sites and direct repeat sites) 30 minutes after contextual fear conditioning in the presence of nicotine and that thyroid hormone signaling at TRβ may be important for acute nicotine’s effects on hippocampal learning. In addition, independent of nicotine treatment status, TRβ KO mice exhibited deficits in auditory cue learning that were likely attributable to hearing deficits because visual cue learning was unaffected in these mice. Furthermore, acute nicotine did not affect serum thyroid hormone levels (neither T3 nor T4) 30 minutes after treatment nor did combined nicotine and fear conditioning treatment, although a limited set of conditions were tested, and higher doses of nicotine may significantly alter serum thyroid hormone levels. Taken together, these results point to a novel interaction between the nicotinic acetylcholinergic and thyroid signaling systems. The potential cross-talk between these signaling systems raises the possibility that novel pharmacological targets for the treatment of nicotine addiction may include thyroid signaling molecules.
Acute nicotine recruits unique transcription factor activity during learning.
Thyroid Receptor β is critical for nicotine’s acute effects on learning.
Thyroid Receptor α does not play a role in nicotine’s acute effects on learning.
WT and Thyroid Receptor α KO mice exhibit stronger learning after acute nicotine.
Acknowledgements
This work was funded with grant support from the National Institute on Drug Abuse (T.J.G., DA017949). PTL and JWK were supported by NIH-NIDA Training Grant (DA007237). All experiments comply with the current laws of the United States of America. The authors would also like to thank Drs. Vinay Parikh and Lauren Ellman for critical reading of previous versions of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest The authors declare no conflict of interest.
References
- Alzoubi KH, Aleisa AM, Alkadhi KA. Molecular studies on the protective effect of nicotine in adult-onset hypothyroidism-induced impairment of long-term potentiation. Hippocampus. 2006a;16:861–74. doi: 10.1002/hipo.20217. [DOI] [PubMed] [Google Scholar]
- Alzoubi KH, Aleisa AM, Alkadhi KA. Nicotine prevents disruption of the late phase LTP-related molecular cascade in adult-onset hypothyroidism. Hippocampus. 2007;17:654–64. doi: 10.1002/hipo.20306. [DOI] [PubMed] [Google Scholar]
- Alzoubi KH, Aleisa AM, Gerges NZ, Alkadhi KA. Nicotine reverses adult-onset hypothyroidism-induced impairment of learning and memory: Behavioral and electrophysiological studies. Journal of neuroscience research. 2006b;84:944–53. doi: 10.1002/jnr.21014. [DOI] [PubMed] [Google Scholar]
- Alzoubi KH, Alkadhi KA. A critical role of CREB in the impairment of late-phase LTP by adult onset hypothyroidism. Experimental neurology. 2007;203:63–71. doi: 10.1016/j.expneurol.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Andersson K, Fuxe K, Eneroth P, Harfstrand A, Agnati LF. Involvement of D1 dopamine receptors in the nicotine-induced neuro-endocrine effects and depletion of diencephalic catecholamine stores in the male rat. Neuroendocrinology. 1988;48:188–200. doi: 10.1159/000125007. [DOI] [PubMed] [Google Scholar]
- Andre JM, Leach PT, Gould TJ. Nicotine ameliorates NMDA receptor antagonist-induced deficits in contextual fear conditioning through high-affinity nicotinic acetylcholine receptors in the hippocampus. Neuropharmacology. 2011;60:617–25. doi: 10.1016/j.neuropharm.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrojo EDR, Fonseca TL, Werneck-de-Castro JP, Bianco AC. Role of the type 2 iodothyronine deiodinase (D2) in the control of thyroid hormone signaling. Biochimica et biophysica acta. 2013;1830:3956–64. doi: 10.1016/j.bbagen.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asteria C. Crucial role for type II iodothyronine deiodinase in the metabolic coupling between glial cells and neurons during brain development. European journal of endocrinology / European Federation of Endocrine Societies. 1998;138:370–1. doi: 10.1530/eje.0.1380370. [DOI] [PubMed] [Google Scholar]
- Baldi E, Lorenzini CA, Bucherelli C. Footshock intensity and generalization in contextual and auditory-cued fear conditioning in the rat. Neurobiology of learning and memory. 2004;81:162–6. doi: 10.1016/j.nlm.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Bertelsen JB, Hegedus L. Cigarette smoking and the thyroid. Thyroid. 1994;4:327–31. doi: 10.1089/thy.1994.4.327. [DOI] [PubMed] [Google Scholar]
- Bianco AC, Kim BW. Deiodinases: implications of the local control of thyroid hormone action. The Journal of clinical investigation. 2006;116:2571–9. doi: 10.1172/JCI29812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Crouching as an index of fear. Journal of comparative and physiological psychology. 1969;67:370–5. doi: 10.1037/h0026779. [DOI] [PubMed] [Google Scholar]
- Cam GR, Bassett JR. The effect of acute nicotine administration on plasma levels of the thyroid hormones and corticosterone in the rat. Pharmacology, biochemistry, and behavior. 1983;19:559–61. doi: 10.1016/0091-3057(83)90135-1. [DOI] [PubMed] [Google Scholar]
- Chanoine JP, Toppet V, Bourdoux P, Spehl M, Delange F. Smoking during pregnancy: a significant cause of neonatal thyroid enlargement. British journal of obstetrics and gynaecology. 1991;98:65–8. doi: 10.1111/j.1471-0528.1991.tb10313.x. [DOI] [PubMed] [Google Scholar]
- Christensen SB, Ericsson UB, Janzon L, Tibblin S, Melander A. Influence of cigarette smoking on goiter formation, thyroglobulin, and thyroid hormone levels in women. The Journal of clinical endocrinology and metabolism. 1984;58:615–8. doi: 10.1210/jcem-58-4-615. [DOI] [PubMed] [Google Scholar]
- Conner DA. Mouse colony management. In: Ausubel Frederick M, et al., editors. Current protocols in molecular biology. 2002. Chapter 23: Unit 23 8. [DOI] [PubMed] [Google Scholar]
- Davis JA, James JR, Siegel SJ, Gould TJ. Withdrawal from chronic nicotine administration impairs contextual fear conditioning in C57BL/6 mice. J Neurosci. 2005;25:8708–13. doi: 10.1523/JNEUROSCI.2853-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Kenney JW, Gould TJ. Hippocampal alpha4beta2 nicotinic acetylcholine receptor involvement in the enhancing effect of acute nicotine on contextual fear conditioning. J Neurosci. 2007;27:10870–7. doi: 10.1523/JNEUROSCI.3242-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericsson UB, Lindgarde F. Effects of cigarette smoking on thyroid function and the prevalence of goitre, thyrotoxicosis and autoimmune thyroiditis. Journal of internal medicine. 1991;229:67–71. doi: 10.1111/j.1365-2796.1991.tb00308.x. [DOI] [PubMed] [Google Scholar]
- Fisher CL, Mannino DM, Herman WH, Frumkin H. Cigarette smoking and thyroid hormone levels in males. International journal of epidemiology. 1997;26:972–7. doi: 10.1093/ije/26.5.972. [DOI] [PubMed] [Google Scholar]
- Forrest D, Erway LC, Ng L, Altschuler R, Curran T. Thyroid hormone receptor beta is essential for development of auditory function. Nature genetics. 1996a;13:354–7. doi: 10.1038/ng0796-354. [DOI] [PubMed] [Google Scholar]
- Forrest D, Hanebuth E, Smeyne RJ, Everds N, Stewart CL, Wehner JM, Curran T. Recessive resistance to thyroid hormone in mice lacking thyroid hormone receptor beta: evidence for tissue-specific modulation of receptor function. The EMBO journal. 1996b;15:3006–15. [PMC free article] [PubMed] [Google Scholar]
- French KL, Granholm AC, Moore AB, Nelson ME, Bimonte-Nelson HA. Chronic nicotine improves working and reference memory performance and reduces hippocampal NGF in aged female rats. Behavioural brain research. 2006;169:256–62. doi: 10.1016/j.bbr.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Ge JF, Peng L, Hu CM, Wu TN. Impaired learning and memory performance in a subclinical hypothyroidism rat model induced by hemi-thyroid electrocauterisation. Journal of neuroendocrinology. 2012;24:953–61. doi: 10.1111/j.1365-2826.2012.02297.x. [DOI] [PubMed] [Google Scholar]
- Gerges NZ, Alkadhi KA. Hypothyroidism impairs late LTP in CA1 region but not in dentate gyrus of the intact rat hippocampus: MAPK involvement. Hippocampus. 2004;14:40–5. doi: 10.1002/hipo.10165. [DOI] [PubMed] [Google Scholar]
- Gerges NZ, Stringer JL, Alkadhi KA. Combination of hypothyroidism and stress abolishes early LTP in the CA1 but not dentate gyrus of hippocampus of adult rats. Brain research. 2001;922:250–60. doi: 10.1016/s0006-8993(01)03181-x. [DOI] [PubMed] [Google Scholar]
- Ghosh M, Gharami K, Paul S, Das S. Thyroid hormone-induced morphological differentiation and maturation of astrocytes involves activation of protein kinase A and ERK signalling pathway. The European journal of neuroscience. 2005;22:1609–17. doi: 10.1111/j.1460-9568.2005.04351.x. [DOI] [PubMed] [Google Scholar]
- Giordano T, Pan JB, Casuto D, Watanabe S, Arneric SP. Thyroid hormone regulation of NGF, NT-3 and BDNF RNA in the adult rat brain. Brain research Molecular brain research. 1992;16:239–45. doi: 10.1016/0169-328x(92)90231-y. [DOI] [PubMed] [Google Scholar]
- Gondou A, Toyoda N, Nishikawa M, Yonemoto T, Sakaguchi N, Tokoro T, Inada M. Effect of nicotine on type 2 deiodinase activity in cultured rat glial cells. Endocrine journal. 1999;46:107–12. doi: 10.1507/endocrj.46.107. [DOI] [PubMed] [Google Scholar]
- Gould TJ. Nicotine and hippocampus-dependent learning: implications for addiction. Molecular neurobiology. 2006;34:93–107. doi: 10.1385/MN:34:2:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TJ. Addiction and Cognition. Addiction Science and Clinical Practice. 2010;5:4–14. [PMC free article] [PubMed] [Google Scholar]
- Gould TJ, Leach PT. Cellular, molecular, and genetic substrates underlying the impact of nicotine on learning. Neurobiology of learning and memory. 2013 doi: 10.1016/j.nlm.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TJ, Lewis MC. Coantagonism of glutamate receptors and nicotinic acetylcholinergic receptors disrupts fear conditioning and latent inhibition of fear conditioning. Learning & memory (Cold Spring Harbor, NY. 2005;12:389–98. doi: 10.1101/lm.89105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TJ, Wehner JM. Nicotine enhancement of contextual fear conditioning. Behavioural brain research. 1999;102:31–9. doi: 10.1016/s0166-4328(98)00157-0. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Wilkinson DS, Yildirim E, Poole RL, Leach PT, Simmons SJ. Nicotine shifts the temporal activation of hippocampal protein kinase A and extracellular signal-regulated kinase 1/2 to enhance long-term, but not short-term, hippocampus-dependent memory. Neurobiology of learning and memory. 2014;109:151–9. doi: 10.1016/j.nlm.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman LJ, Michalkiewicz M, Connors JM, Pietrzyk Z, Hedge GA. Muscarinic modulation of the vasodilatory effects of vasoactive intestinal peptide at the rat thyroid gland. Neuroendocrinology. 1991;53:69–74. doi: 10.1159/000125699. [DOI] [PubMed] [Google Scholar]
- Hyman SE. Addiction: a disease of learning and memory. The American journal of psychiatry. 2005;162:1414–22. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- Jorde R, Sundsfjord J. Serum TSH levels in smokers and non-smokers. The 5th Tromso study. Exp Clin Endocrinol Diabetes. 2006;114:343–7. doi: 10.1055/s-2006-924264. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, O’Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2008;33:166–80. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- Kapoor D, Jones TH. Smoking and hormones in health and endocrine disorders. European journal of endocrinology / European Federation of Endocrine Societies. 2005;152:491–9. doi: 10.1530/eje.1.01867. [DOI] [PubMed] [Google Scholar]
- Kazdoba TM, Del Vecchio RA, Hyde LA. Automated evaluation of sensitivity to foot shock in mice: inbred strain differences and pharmacological validation. Behavioural pharmacology. 2007;18:89–102. doi: 10.1097/FBP.0b013e3280ae6c7c. [DOI] [PubMed] [Google Scholar]
- Kenney JW, Adoff MD, Wilkinson DS, Gould TJ. The effects of acute, chronic, and withdrawal from chronic nicotine on novel and spatial object recognition in male C57BL/6J mice. Psychopharmacology. 2012a;217:353–65. doi: 10.1007/s00213-011-2283-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney JW, Florian C, Portugal GS, Abel T, Gould TJ. Involvement of hippocampal jun-N terminal kinase pathway in the enhancement of learning and memory by nicotine. Neuropsychopharmacology. 2010;35:483–92. doi: 10.1038/npp.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney JW, Raybuck JD, Gould TJ. Nicotinic receptors in the dorsal and ventral hippocampus differentially modulate contextual fear conditioning. Hippocampus. 2012b;22:1681–90. doi: 10.1002/hipo.22003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PR, Brady D, Koenig JI. Thyroid hormone regulation of N-methyl-D-aspartic acid receptor subunit mRNA expression in adult brain. Journal of neuroendocrinology. 2003;15:87–92. doi: 10.1046/j.1365-2826.2003.00959.x. [DOI] [PubMed] [Google Scholar]
- Lei J, Mariash CN, Bhargava M, Wattenberg EV, Ingbar DH. T3 increases Na-K-ATPase activity via a MAPK/ERK1/2-dependent pathway in rat adult alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2008;294:L749–54. doi: 10.1152/ajplung.00335.2007. [DOI] [PubMed] [Google Scholar]
- Levin ED, Rose JE. Nicotinic and muscarinic interactions and choice accuracy in the radial-arm maze. Brain research bulletin. 1991;27:125–8. doi: 10.1016/0361-9230(91)90293-s. [DOI] [PubMed] [Google Scholar]
- Logue SF, Paylor R, Wehner JM. Hippocampal lesions cause learning deficits in inbred mice in the Morris water maze and conditioned-fear task. Behavioral neuroscience. 1997;111:104–13. doi: 10.1037//0735-7044.111.1.104. [DOI] [PubMed] [Google Scholar]
- Lotfipour S, Byun JS, Leach P, Fowler CD, Murphy NP, Kenny PJ, Gould TJ, Boulter J. Targeted Deletion of the Mouse alpha2 Nicotinic Acetylcholine Receptor Subunit Gene (Chrna2) Potentiates Nicotine-Modulated Behaviors. J Neurosci. 2013;33:7728–41. doi: 10.1523/JNEUROSCI.4731-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meberg A, Marstein S. Smoking during pregnancy--effects on the fetal thyroid function. Acta paediatrica Scandinavica. 1986;75:762–6. doi: 10.1111/j.1651-2227.1986.tb10287.x. [DOI] [PubMed] [Google Scholar]
- Opazo MC, Gianini A, Pancetti F, Azkcona G, Alarcon L, Lizana R, Noches V, Gonzalez PA, Marassi MP, Mora S, Rosenthal D, Eugenin E, Naranjo D, Bueno SM, Kalergis AM, Riedel CA. Maternal hypothyroxinemia impairs spatial learning and synaptic nature and function in the offspring. Endocrinology. 2008;149:5097–106. doi: 10.1210/en.2008-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantos C, Xinaris C, Mourouzis I, Malliopoulou V, Kardami E, Cokkinos DV. Thyroid hormone changes cardiomyocyte shape and geometry via ERK signaling pathway: potential therapeutic implications in reversing cardiac remodeling? Molecular and cellular biochemistry. 2007;297:65–72. doi: 10.1007/s11010-006-9323-3. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behavioral neuroscience. 1992;106:274–85. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Portugal GS, Wilkinson DS, Kenney JW, Sullivan C, Gould TJ. Strain-dependent effects of acute, chronic, and withdrawal from chronic nicotine on fear conditioning. Behavior genetics. 2012;42:133–50. doi: 10.1007/s10519-011-9489-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirin-Stricker C, Nappey V, Simoni P, Toussaint JL, Schmitt M. Trans-activation by thyroid hormone receptors of the 5′ flanking region of the human ChAT gene. Brain research Molecular brain research. 1994;23:253–65. doi: 10.1016/0169-328x(94)90232-1. [DOI] [PubMed] [Google Scholar]
- Raybuck JD, Gould TJ. Extracellular signal-regulated kinase 1/2 involvement in the enhancement of contextual fear conditioning by nicotine. Behavioral neuroscience. 2007;121:1119–24. doi: 10.1037/0735-7044.121.5.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybuck JD, Gould TJ. The role of nicotinic acetylcholine receptors in the medial prefrontal cortex and hippocampus in trace fear conditioning. Neurobiology of learning and memory. 2010;94:353–63. doi: 10.1016/j.nlm.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Biswas SC, Chatterjee O, Sarkar PK. Protein kinase A linked phosphorylation mediates triiodothyronine induced actin gene expression in developing brain. Brain research Molecular brain research. 1999;67:158–64. doi: 10.1016/s0169-328x(99)00056-x. [DOI] [PubMed] [Google Scholar]
- Sawin S, Brodish P, Carter CS, Stanton ME, Lau C. Development of cholinergic neurons in rat brain regions: dose-dependent effects of propylthiouracil-induced hypothyroidism. Neurotoxicology and teratology. 1998;20:627–35. doi: 10.1016/s0892-0362(98)00020-8. [DOI] [PubMed] [Google Scholar]
- Schlienger JL, Grunenberger F, Vinzio S, Goichot B. Annales d’endocrinologie. 2003;64:309–15. [PubMed] [Google Scholar]
- Socci DJ, Sanberg PR, Arendash GW. Nicotine enhances Morris water maze performance of young and aged rats. Neurobiology of aging. 1995;16:857–60. doi: 10.1016/0197-4580(95)00091-r. [DOI] [PubMed] [Google Scholar]
- Soldin OP, Goughenour BE, Gilbert SZ, Landy HJ, Soldin SJ. Thyroid hormone levels associated with active and passive cigarette smoking. Thyroid. 2009;19:817–23. doi: 10.1089/thy.2009.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui L, Ren WW, Li BM. Administration of thyroid hormone increases reelin and brain-derived neurotrophic factor expression in rat hippocampus in vivo. Brain research. 2010;1313:9–24. doi: 10.1016/j.brainres.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Tweed JO, Hsia SH, Lutfy K, Friedman TC. The endocrine effects of nicotine and cigarette smoke. Trends in endocrinology and metabolism: TEM. 2012;23:334–42. doi: 10.1016/j.tem.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehner JM, Keller JJ, Keller AB, Picciotto MR, Paylor R, Booker TK, Beaudet A, Heinemann SF, Balogh SA. Role of neuronal nicotinic receptors in the effects of nicotine and ethanol on contextual fear conditioning. Neuroscience. 2004;129:11–24. doi: 10.1016/j.neuroscience.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Wiersinga WM. Smoking and thyroid. Clinical endocrinology. 2013;79:145–51. doi: 10.1111/cen.12222. [DOI] [PubMed] [Google Scholar]
- Wikstrom L, Johansson C, Salto C, Barlow C, Campos Barros A, Baas F, Forrest D, Thoren P, Vennstrom B. Abnormal heart rate and body temperature in mice lacking thyroid hormone receptor alpha 1. The EMBO journal. 1998;17:455–61. doi: 10.1093/emboj/17.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GR, Bassett JH. Deiodinases: the balance of thyroid hormone: local control of thyroid hormone action: role of type 2 deiodinase. The Journal of endocrinology. 2009;209:261–72. doi: 10.1530/JOE-10-0448. [DOI] [PubMed] [Google Scholar]
- Wolf ME. Addiction: making the connection between behavioral changes and neuronal plasticity in specific pathways. Molecular interventions. 2002;2:146–57. doi: 10.1124/mi.2.3.146. [DOI] [PubMed] [Google Scholar]
- Woolf NJ, Milov AM, Schweitzer ES, Roghani A. Elevation of nerve growth factor and antisense knockdown of TrkA receptor during contextual memory consolidation. J Neurosci. 2001;21:1047–55. doi: 10.1523/JNEUROSCI.21-03-01047.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]