Abstract
Canines suffer from and serve as strong translational animals models for many immunological disorders and infectious diseases. Routine vaccination has been a mainstay of protecting dogs through the stimulation of robust antibody responses and expansion of memory T cell populations. Commercially available reagents and described techniques are limited for identifying and characterizing canine T cell subsets and evaluating T cell-specific effector function. To define reagents for delineating naïve versus activated T cells and identify antigen-specific T cells, we tested anti-human and anti-bovine T-cell specific cell surface marker reagents for cross-reactivity with canine peripheral blood mononuclear cells (PBMCs. Both CD4+ and CD8+ T cells from healthy canine donors showed reactivity to CCL19-Ig, a CCR7 ligand, and coexpression with CD62L. An in vitro stimulation with concanavalin A validated downregulation of CCR7 and CD62L expression on stimulated healthy control PBMCs, consistent with an activated T cell phenotype. Anti-IFNγ antibodies identified antigen-specific IFNγ-producing CD4+ and CD8+ T cells upon in vitro vaccine antigen PBMC stimulation. PBMC isolation within 24 hours of sample collection allowed for efficient cell recovery and accurate T cell effector function characterization. These data provide a reagent and techniques platform via flow cytometry for identifying canine T cell subsets and characterizing circulating antigen-specific canine T cells for potential use in diagnostic and field settings.
Keywords: dog, T cell, CCR7, CD62L, flow cytometry, vaccine
2. Introduction
Domestication and tractability have allowed do gs to serve as research subjects for canine-specific diseases as well as models for human disorders. In particular, dogs serve as robust translational models in cardiovascular (Hohnloser et al., 2009), neoplastic (Khanna et al., 2006; Klopfleisch et al., 2010), immunological (Creevy et al., 2003; Marsella and Girolomoni, 2009), neurological (Awano et al., 2009; Selkoe et al., 1987), and genetic (Wilbe et al., 2010) research studies. Canines are also susceptible to and serve as models of zoonotic diseases such as leishmaniasis and American trypanosomiasis and hence used to evaluate anti-parasitic chemotherapeutic regimens (Guedes et al., 2002). Routine vaccination in canines allows an opportunity to assess the development of an appropriate immunological response to foreign antigens. Techniques and commercially available reagents are scarce for studying the canine immune system, especially as compared to those available for humans. As basic research pursues translational applications in animals more physiologically similar to humans, and veterinary medicine strives for more individualized patient therapies, an increasing need exists for identifying, characterizing, and monitoring the canine immune response.
The First International Canine Leukocyte Antigen Workshop (CLAW) was a significant step in identifying canine homologs of human CD antigens that delineated leukocyte populations by monoclonal antibodies (Cobbold and Metcalfe, 1994). Clusters of antibodies collected from several sources identified canine equivalents of CD4, CD8, and Thy1.1 antigens from peripheral blood. Additional antibodies reactive to canine leukocyte antigens including CD45R (Aguiar et al., 2005) CD45RA (Caniatti et al., 1996), CD11/CD18 (Danilenko et al., 1992a; Moore et al., 1990), and CD62L (Crockett-Torabi and Fantone, 1997) and to platelet and erythrocyte antigens (Schuberth et al., 2007) have been described separately from the CLAW workshop. Testing of monoclonal antibodies specific for cytokines in other species have also identified IL-4-, IL-8-, and IFN-γ-producing canine PBMCs and expanded the repertoire of canine specific reagents (Pedersen et al., 2002). However, despite these advances, delineating and characterizing naïve, activated, and memory T cell subsets in canines has remained limited.
The aim of this project was to identify and validate immunological reagents for characterizing canine T cells through phenotypic and effector function evaluation-based assays. Detection of the canine cross-reactive CCL19-hIg, a ligand for CCR7, identified naïve and antigen-experienced but not recently activated canine T cells. CCR7 cell surface expression was consistent with CD62L, an L-selectin expressed by naïve and central memory T cells during homing to secondary lymphoid organs. Decreases in CCR7 and CD62L expression following antigen stimulation or mitogen activation correlated with upregulation of the activation marker, CTL2.58, and delineated activated T cells. IFNγ-production following PBMC whole vaccine stimulation defined antigen-specific T cell effector function. Extended time between blood collection and PBMC isolation of up to twenty-four hours revealed no significant loss in identifying vaccine-specific IFNγ-producing T cells. These data provide a reagent platform for identifying and characterizing canine T cell populations and assessing antigen-specific effector function.
3. Materials and Methods
3.1. Animals and isolation of mononuclear cells
Approximately 40–50mls of blood from four clinically healthy adult (>3 years of age) mixed breed dogs were drawn into heparinized tubes (Vacutainer, Becton-Dickinson, Franklin Lakes, NJ, USA) by venipuncture. Isolation of peripheral blood mononuclear cells (PBMCs) occurred immediately following collection or as otherwise indicated and as previously described for human subjects (Albareda et al., 2009). PBMCs were washed in Hank’s buffered balance salt solution (Mediatech Inc., Manassas, VA, USA) and resuspended in RPMI-1640 (Mediatech Inc.) completed with 50uM 2-β-mercaptoethanol, 2mM L-glutamine, 25µg/mL gentamicin, 200U/mL penicillin (Mediatech Inc), 2µg/mL streptomycin (Mediatech Inc), 1mM sodium pyruvate, and 10% heat-inactivated (30min, 56°C) and aggregate-removed (800gx30min) fetal calf serum (HI-FCS) (HyClone Laboratories, ThermoScientific, Logan, UT, USA). Resuspended cells were frozen in media containing 10% dimethyl sulfoxide (Acros Organics, Fair Lawn, NJ, USA) in liquid nitrogen for long-term storage. Prior to use, PBMCs were recovered, thawed at 37°C, washed and resuspended in complete RPMI-1640 + 10% HI-FCS. These purification, storage, and recovery procedures consistently yielded >95% viability, as determined by microscopic examination of trypan blue dye exclusion. All animal use protocols were approved by the University of Georgia Institutional Animal Care and Use Committee.
3.2. PBMC antibody reactivity
For testing canine T cells antibody reactivity, a minimum of 2×105 PBMCs, were stained at 1:50 antibody dilution in PBS containing 1% BSA and 0.05% sodium azide (PAB; both from Sigma-Aldrich, St. Louis, MO, USA) at 4°C for 45 min. Antibodies tested were those defined in Table 1. Cells were fixed in 2% formaldehyde prior to flow cytometric collection.
Table 1.
Reagent panel used to study canine peripheral mononuclear cells.
| Antigen | Antibody | Host species |
Target species |
Source (citation) | Reagent specificity | Dog PBMC reactivity |
Reactivity in this study |
Discriminatory |
|---|---|---|---|---|---|---|---|---|
| Leukocyte cell lineages | ||||||||
| CD3 | CA17.2A12 | mouse | dog | AbD Serotec (Byrne et al., 2000) | T cells | (Byrne et al., 2000) | + | n.d. |
| CD11a | CA11.4D3 | mouse | dog | AbD Serotec (Cobbold and Metcalfe, 1994; Danilenko et al., 1992b) | integrin, all leukocytes | (Cobbold and Metcalfe, 1994) | − | no |
| CD11c | BU15 | mouse | human | AbD Serotec | dendritic cells, monocytes, macrophages, neutrophils | (Trowald-Wigh et al., 1993) | + | n.d. |
| Lymphocyte subsets | ||||||||
| B cell | LSM11.425 | mouse | dog | BD Pharminogen (Cobbold and Metcalfe, 1994; Gebhard and Carter, 1992; Ruslander et al., 1997) | B cells | (Cobbold and Metcalfe, 1994) | + | yes |
| Pan T cell | LSM8.358 | mouse | dog | BD Pharminogen (Cobbold and Metcalfe, 1994) | T cells | (Cobbold and Metcalfe, 1994) | + | yes |
| CD4 | YKIX302.9 | rat | dog | AbD Serotec (Cobbold and Metcalfe, 1994) | CD4+ T cells | (Cobbold and Metcalfe, 1994) | + | yes |
| CD4 | RPA-T4 | mouse | human | BioLegend (Schlossman et al., 1995) | CD4+ T cells | − | ||
| CD8 | YCATE55.9 | rat | dog | AbD Serotec (Cobbold and Metcalfe, 1994) | CD8+ T cells | (Cobbold and Metcalfe, 1994) | + | yes |
| CD8a | RPA-T8 | mouse | human | BioLegend (Schlossman et al., 1995) | CD8+ T cells | − | ||
| T cell-specific antigens | ||||||||
| T cell activation | CTL2.58 | mouse | dog | BD Pharminogen (Ruslander et al., 1997) | activated T cells | + | yes | |
| CD25 | 2A3 | mouse | human | BD Biosciences (Urdal et al., 1984) | activated & regulatory T cells, activated B cells | − | ||
| CD27 | M-T271 | mouse | human | BD Pharminogen (Bigler et al., 1988) | tumor necrosis factor R’; T cell costimulatory molecule | − | ||
| CD28 | CD28.2 | mouse | human | BD Pharminogen (Verwilghen et al., 1993) | T cell costimulatory molecule, required for T cell activation | − | ||
| CD44 | IM7 | rat | mouse | AbD Serotec (McKallip et al., 2002) | activated effector-memory T cells | (Cobbold and Metcalfe, 1994) | + | no |
| CD45RA | HI100 | mouse | human | BioLegend (Iannello et al., 2010) | naïve T cells | − | ||
| CD45RA | CA4.ID3 | mouse | dog | Abd Serotec (Caniatti et al., 1996; Cobbold and Metcalfe, 1994) | naïve T cells | (Moreno et al., 1999) | + | no |
| CD45RO | UCHL1 | mouse | human | Beckman Coulter | memory T cells | (Galkowska et al., 1996) | + | no |
| CD62L | FMC46 | mouse | human | AbD Serotec (Pilarski et al., 1991) | lymphocyte homing, naïve & memory T cells | (Schuberth et al., 2007) | + | yes |
| CD62L | DREG-56 | mouse | human | BD Pharminogen (Kishimoto et al., 1990) | lymphocyte homing, naïve, & memory T cells | − | ||
| CD127 | HIL-7R-M2 | mouse | human | BD Pharminogen (Goodwin et al., 1990) | IL-7 R’ subunit | − | ||
| CD197 (CCR7) | 3D12 | rat | human | BD Pharminogen (Sallusto et al., 1999) | secondary lymphoid organ entry R’ | − | ||
| CCL19-hIg | ELC | human | (Hargreaves et al., 2001) | chemokine ligand for CCR7 on T cells for secondary lymphoid organ migration | + | yes | ||
| Other antigens, receptors, and cytokines | ||||||||
| CD85j | GHI/75 | mouse | human | BD Pharminogen (Colonna et al., 1997) | leukocyte R’ providing inhibitory signals to APCs | − | ||
| HLA-ABC | 09HHLA01E | Chemicon Australia (Murakawa et al., 2000) | human leukocyte antigen MHCI | + | n.d. | |||
| CX3CR1 | rabbit | human | Torrey Pines Biolab | chemokine R’ expressed by activated T cells (TH1), natural killer, epithelial & endothelial cells | + | n.d. | ||
| Interferon-γ | CC302 | mouse | bovine | AbD Serotec (Fellman et al., 2011; Pedersen et al., 2002) | natural killer & T cell effector cytokine | (Fellman et al., 2011; Pedersen et al., 2002) | + | yes |
Abbreviations: MHCI, major histocompatibility complex I; R’, receptor; APC, antigen presenting cell.
3.3. T cell stimulation and proliferation assessment assays
A total of 4×105 PBMCs were stimulated in a 96-well flat-bottom tissue culture plate (Costar, Corning, NY, USA) at 37°C in the presence of media, 15µg/mL anti-canine CD3 (AbD Serotec, Raleigh, NC, USA), or 0.25µg/mL concanavalin A (Sigma-Aldrich) for the indicated days. For assessment of proliferation, PBMCs were washed twice with PBS, incubated with 5µM CFSE (Molecular Probes, Eugene, OR), quenched with FCS, and plated. Two days post-stimulation, centrifuged and washed cells were incubated with or without CCL19-hIg (ELC; (Hargreaves et al., 2001)) supernatant at 4°C for 45 min. Antibodies used were anti-CD8-Pacific Blue, anti-CD62L-PE (AbD Serotec), anti-human IgG-AF488 (Molecular Probes), and a cocktail containing anti-dog Pan T cell-APC, anti-B cell-PE, and anti-dog T cell Activation marker-FITC (Dog Activated T Lymphocyte Cocktail, BD Pharmingen, BD Biosciences, San Jose, CA). 7-amino-actinomycin D (7AAD; BD Pharmingen) was included for live/dead cell discrimination. Cells were stained in PAB for 45 min at 4°C, washed, and fixed in 2% formaldehyde.
3.4 Intracellular cytokine staining
For assaying IFNγ levels, 4×105 PBMCs were stimulated for 5hr in the presence of 2ng/mL phorbol 12-myristate 13-acetate (PMA), 4µg/mL Ca2+ ionomycin (Sigma-Aldrich), and brefeldin A (BD GolgiPlug; BD Biosciences) (Pedersen et al., 2002). For polyclonal activation, PBMCs were plated with 15µg/mL anti-CD3 (AbD Serotec) or diluted whole vaccine antigens, incubated overnight at 37°C and brefeldin A added 5hr prior to end of incubation. Canine vaccines were IMRAB 3TF (Rabies; Merial, Athens, GA, USA), Duramune 5 (Canine distemper-Adenovirus Type 2-Parainfluenza-Parvovirus (DAPP); Fort Dodge Animal Health, Fort Dodge, IA, USA), and Leptovax 4 (Leptospirosis bacterial extract; Fort Dodge Animal Health) vaccines. Cells were stained with anti-CD8-Pacific Blue and anti-CD4-FITC (AbD Serotec) followed by intracellular staining with anti-bovine anti-IFN-γ AF647 (AbD Serotec) according to the BD Cytofix/Cytoperm kit (BD Biosciences).
3.5 Flow Cytometry and Analysis
A minimum of 250,000 events were collected for each sample on a CyAn ADP using Summit, version 4.3 (Beckman Coulter, Fullerton, CA, USA). Where appropriate, fluorescence minus one controls were used to establish gating measures. FlowJo flow cytometry analysis software, version 9 (Tree Star, Ashland, OR, USA) was used for analyses.
4. Results
As CD4+ and CD8+ T cells play critical roles in various infections, our primary efforts focused on identifying T cells and discriminatory surface markers for naïve, activated, and memory T cells subsets. A reagent panel used for testing of cross-reactivity with canine PBMCs included antibodies purchased commercially or kindly provided by other investigators (Table 1). A reagent was judged as positive if reactive with >2% of PBMCs. Utilizing this strategy, fifteen of twenty-six reagents were cross-reactive with canine PBMCs as denoted in Table 1, with eleven of twelve (91.7%) previously referenced reactive antibodies confirmed in this study. If a reagent distinguished distinct cell populations individually or following incubation in a T cell stimulation protocol, the reagent was found to be discriminatory, as noted in Table 1.
For antibodies recognizing putative cell surface markers on T cells, we repeated staining to validate marker expression on CD4+ and CD8+ T cells. Canine T cell reactivity was found with CCL19-Ig (ELC), a fusion protein serving as a chemokine ligand of CCR7 (Hargreaves et al., 2001) and previously described to have reactivity with naïve and central memory ovine T cells (Debes et al., 2005). As circulating T cells traffic from the blood and home to lymph nodes, naïve and central memory T cells express CCR7 and other adhesion molecules, like L-selectin (CD62L) and LFA-1, to tether and arrest to endothelium for transmigration across high endothelial venules. To investigate if circulating canine CD4+ and CD8+ T cells express both CCR7 and L-selectin (CD62L), PBMCs were stained with anti-CD4, anti-CD8, CCL19-Ig, and anti-CD62L. In clinically healthy canine donors, CD4+ and CD8+ T cells expressed measureable levels of CCR7 (Fig 1A). Unlike CCR7 expression in human CD8+ T cells (Campbell et al., 2001), CCR7 expression by canine CD8+ T cells varied greatly between individual dogs. In comparison to humans and sheep where >85% of CD4+ T cells express CCR7 (Campbell et al., 2001; Debes et al., 2005), approximately 40–50% of canine CD4+ T cells from healthy donors expressed CCR7. The observed difference is likely explained by the decreased ability of CCL19-hIg to delineate distinct peaks associated with CCR7+ and CCR7− canine CD4+ T cells populations (Fig 1A) versus a difference in species or health status of the hosts. Exploration of alternative secondary fluorophore combinations yielded similar CCR7+CD4+ T cell percentages (not shown). Both canine CD4+ and CD8+ T cells also showed reactivity to the lymph node homing marker CD62L (Fig 1B) and this expression agreed with that of CCR7 (Fig 1C), as the predominance of CD4+CD62Lhi T cells expressed CCR7, results consistent with that observed in human peripheral blood (Campbell et al., 2001). Taken together, the cell surface markers of CCR7 and CD62L identified canine CD4+ and CD8+ T cell populations consistent with the phenotype of naïve or central memory T cells.
Figure 1. Co-expression of CCR7 and CD62L on canine CD8+ and CD4+ T cells.
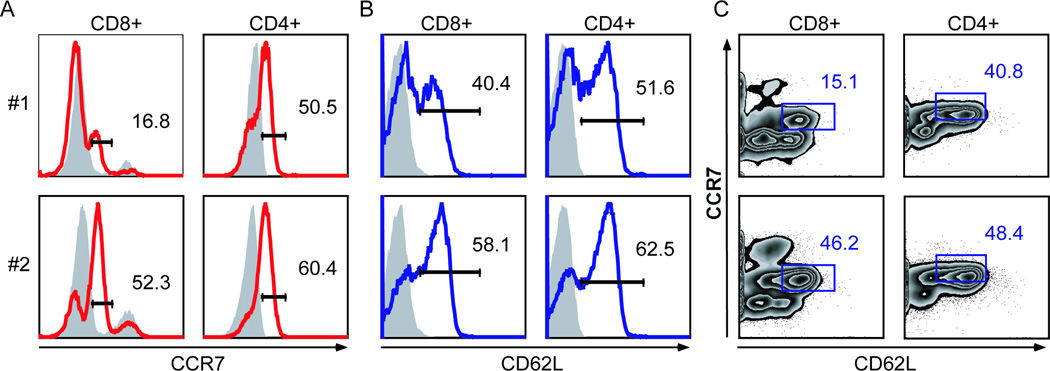
Canine PBMCs from two clinically healthy dogs (#1 and #2) were incubated for 1 hour at 37°C and stained with or without CCL19-hIg (ELC), a CCR7 ligand. Cells were stained with anti-CD8, anti-CD4, anti-CD62L, anti-human secondary antibody, and 7AAD. Percentages of CD8+7AAD− and CD4+7AAD− T cells expressing CCR7 (A) and CD62L (B). Shaded lines are fluorescence-minus-one (FMO) controls for the indicated marker. C, Percentages of CD8+7AAD− or CD4+7AAD− T cells co-expressing CCR7 and CD62L. Gating strategies are determined by FMO controls, especially noting the highest CCR7 peak present in experimental and FMO control samples is due to nonspecific secondary antibody binding.
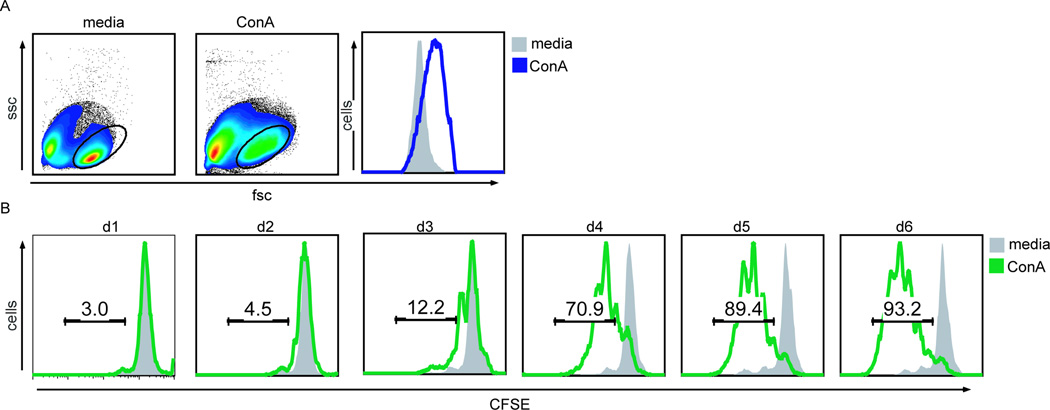
To evaluate if CCR7 a nd other cross-reactive antibodies identified in Table 3.1 were discriminatory for naïve versus activated T cells, a method and assay for T cell activation was used. For this purpose, CFSE-labeled canine PBMCs were incubated in media alone or with the T cell mitogen concanavalin A (ConA), and stimulation and proliferation was evaluated by changes in cell size and CFSE dilution. A comparison of forward versus side scatter revealed PBMC stimulated with ConA were on average volumetrically larger than unstimulated cells, consistent with cellular activation (Fig 2A). CFSE dilution indicated cell division of ConA-stimulated CD8+ T cells as early as three days post stimulation (Fig 2B).
Figure 2. Measuring stimulation and proliferation using CFSE time-series data.
A, Forward scatter (fsc) versus side scatter plots (ssc) of CFSE-labeled PBMCs incubated with media or concavalin A (ConA) for 4 days. Gated cells are displayed in the fsc histogram, comparing ConA-stimulation (blue line) to media alone (grey). B, CFSE-labeled PBMCs were harvested on the indicated days and stained with 7AAD and anti-CD8 antibodies. Histograms represent percentages of CD8+7AAD− T cells with CFSE dilution following incubation with media (grey shaded) versus ConA (green line).
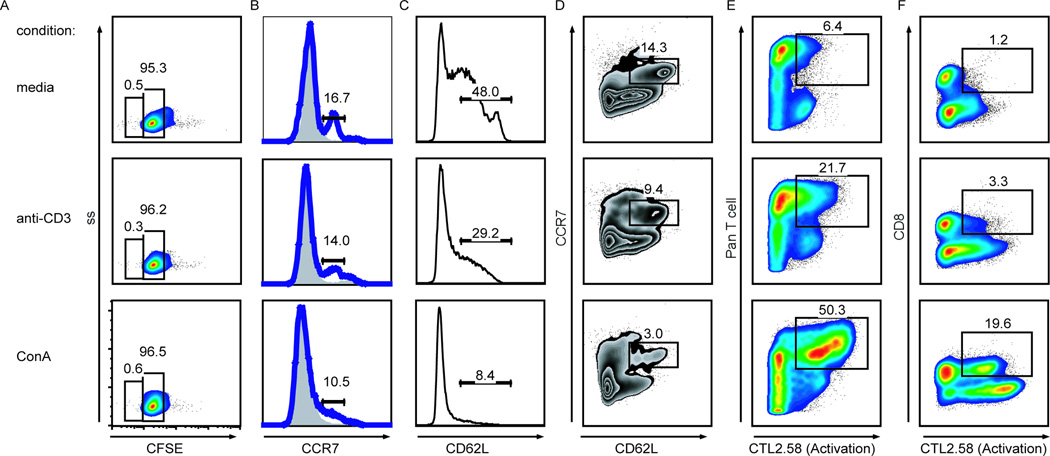
As we proposed the CCR7 and CD62L reagents identified naïve and central memory canine T cells, we hypothesized that following T cell activation canine T cells would decrease expression of CCR7 and CD62L. To address this hypothesis, canine PBMCs were cultured in the presence of anti-CD3, ConA, or media alone. Even before detectable proliferation by CFSE dilution two days post stimulation (Fig 3A), CD8+ T cells expressed less CCR7 (Fig 3B) and CD62L (Fig 3C) when activated. Expression levels of these two markers were consistent with the strength of stimulus, as ConAstimulated CD8+ T cells expressed less CCR7 and CD62L than cells stimulated with anti-CD3. Co-expression of both CCR7 and CD62L also decreased in stimulated cells, with ConA-stimulated cells showing greater decreases than cells incubated with anti-CD3 (Fig 3D). Down-regulation of CCR7 and CD62L cell surface expression further correlated with increased expression of the activation marker (CTL2.58+) on stimulated T cells (Fig 3E) and CD8+ T cells (Fig 3F). These experiments identified markers for defining activated CTL2.58+CD8+ T cells and naïve or central memory-like CCR7+CD62LhiCD8+ T cells in dogs. Testing of other canine T cell reactive reagents listed in Table 1, including CD45RA, CD45RO, and CD11a, in media versus stimulatory conditions did not alter antibody T cell reactivity in our stimulation assay or were not detectable using secondary antibody reagents and therefore were not investigated further. These results are noted as “not discriminatory” in Table 1.
Figure 3. CCR7, CD62L, and CTL2.58 expression of canine PBMCs in stimulation conditions.
Canine PBMCs incubated two days with media, anti-CD3, or ConA, harvested, and stained with various antibody combinations. A, Proliferation of CFSE-labeled CD8+ T cells. B–D, PBMCs incubated with (blue line) and without (grey shaded) CCL19-hIg and stained with anti-human secondary antibody and anti-CD62L. Histograms of CCR7 (B) and CD62L (C) expression of CD8+ T cells. D, Percentages of CD8+ T cells co-expressing CCR7 and CD62L. E–F, PBMCs stained with the dog activated T lymphocyte antibody cocktail, 7AAD, and anti-CD8. Percentages of stimulated PanT+ (E) and CD8+ (F) T cells reactive to the CTL2.58 antibody, an activation marker.
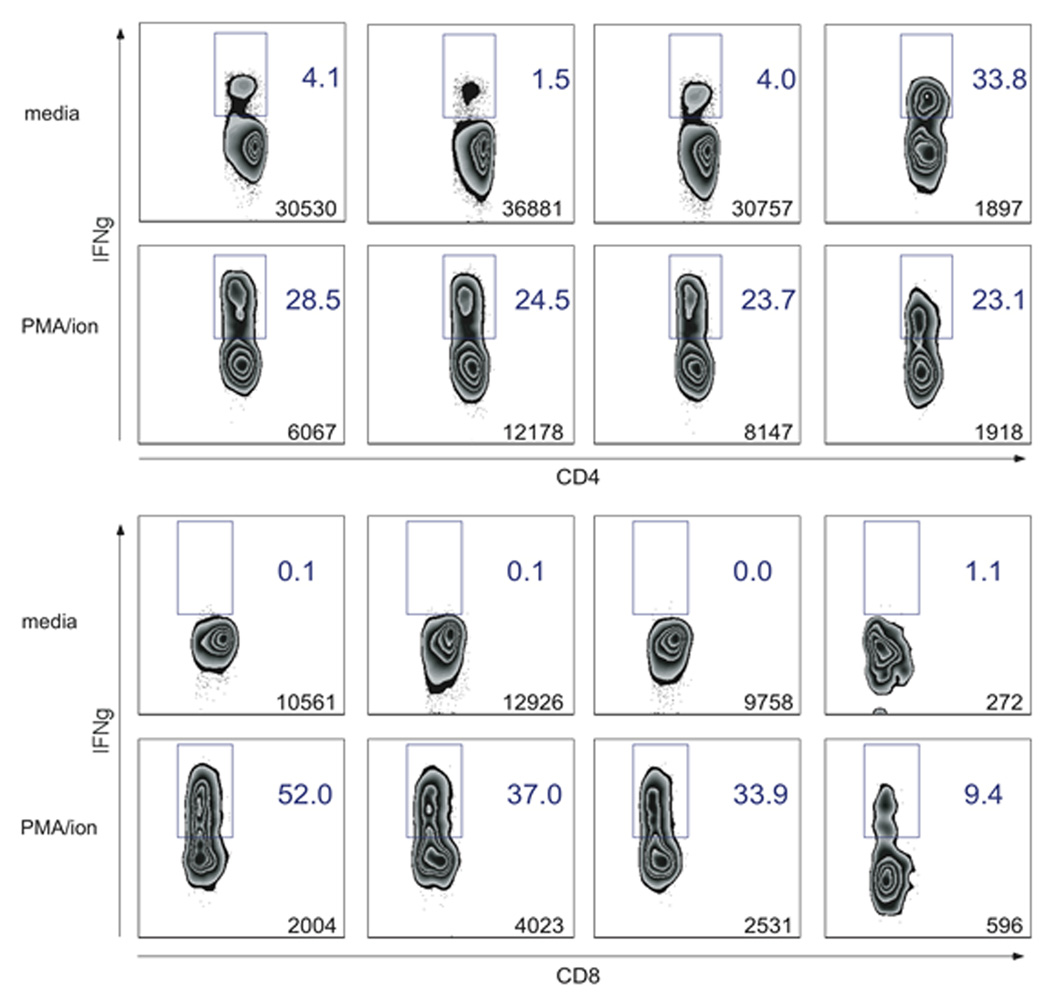
Cognate antigen recognition through the TCR results in effector cytokine production, and thereby provides a method for evaluating and delineating antigen-specific T cells. To assay the production of the effector cytokine IFNγ by canine T cells in response to previously seen antigens, PBMCs were isolated from three healthy dogs vaccinated three weeks prior with IMRAB 3TF (Rabies), Duramune 5 (Canine distemper-Adenovirus Type 2-Parainfluenza-Parvovirus (DAPP)), and Leptovax 4 (Leptospirosis bacterial extract) vaccines. Following incubation of cells with media or vaccine antigen overnight, intracellular IFNγ staining was performed to identify vaccine-specific CD4+ and CD8+ T cells. CD4+ T cells from three of three dogs produced IFNγ in response to rabies vaccine, whereas only one of three had IFNγ-producing cells when stimulated with DAPP vaccine antigens (Fig 4A). CD8+ T cells from two of three dogs produced IFNγ in response to rabies vaccine, but all appeared to not be significantly different from media controls when stimulated with the DAPP vaccine (Fig 4B). Despite differences in vaccine sources and stimulus concentrations, the low levels of detectable of IFNγ-producing T cells following canine distemper vaccine (DAPP) stimulation were consistent with previously published ELISAs of stimulated PBMC culture supernatants (Valli et al., 2010). Both CD4+ and CD8+ T cell subsets failed to produce robust IFNγ in response to the Leptovax 4 vaccine, suggesting this vaccine failed to elicit a strong T cell specific immune response three weeks post administration, consistent with the predominant humoral response elicited by the bacterial antigens of the leptospiral vaccine (Adler and Faine, 1977). These results provide a flow cytometric method for identifying antigen-specific CD4+ and CD8+ T cells and evaluating IFNγ production for individual canine T cells.
Figure 4. IFNγ production by canine CD4+ and CD8+ T cells upon stimulation with vaccine antigens.
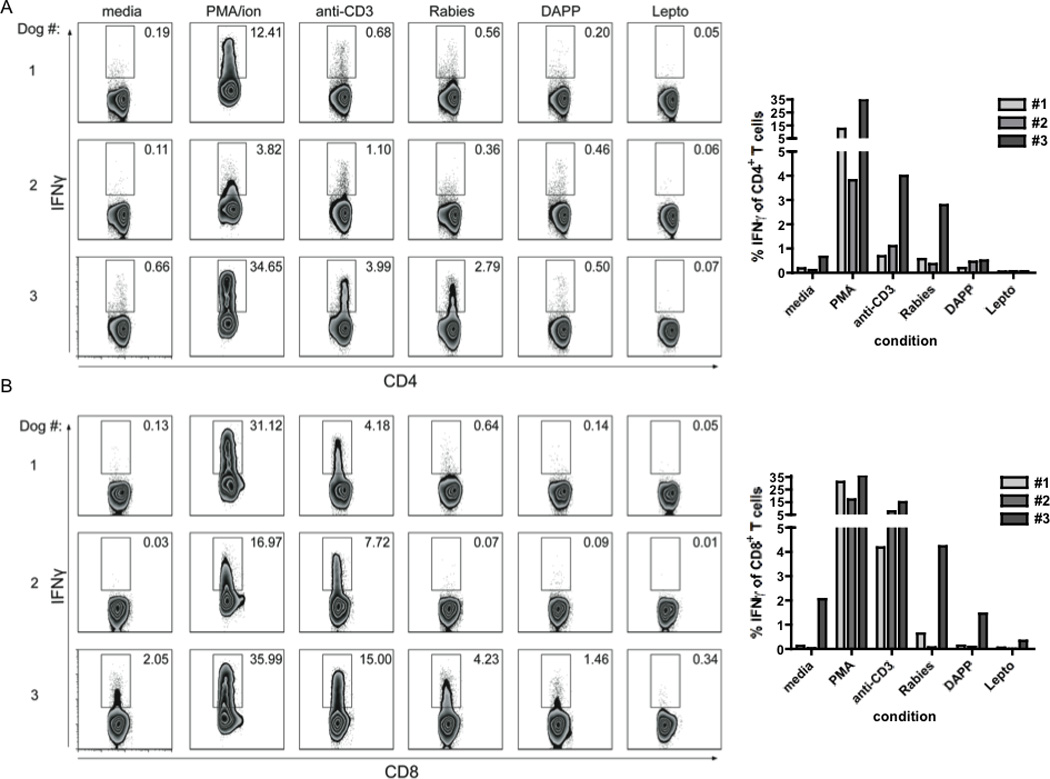
PBMCs collected from three dogs three weeks post vaccination with IMRAB 3TF (Rabies), Duramune 5 (DAPP), and Leptovax 4 (Lepto) were incubated with media, PMA/Ca2+ ionomycin, and vaccines. GolgiPlug was added 5hr prior to end of incubation. Cells were stained with anti-CD8, anti-CD4, and anti-bovine IFNγ. A–B, Numbers indicate percentages of CD4+ (A) and CD8+ (B) T cells producing cytokine in each condition.
In fieldwork settings, circumstances may arise where PBMC isolation will occur hours following sample collection, as is the case in field sampling. We asked how this timing would affect sample quality and ability to identify antigen-specific and cytokine-producing T cells. To address this question, we determined recovery and effector function of T cells isolated at various times post-blood collection. Incubation of cells with media or PMA/Ca2+ ionomycin followed by intracellular cytokine staining revealed no significant loss in quantities or percentages of IFNγ-producing T cells isolated up to 24 hours post collection (Fig 5). In PBMCs purified 48 hours following venipuncture, a five-fold reduction in recovery of both CD4+ and CD8+ T cell as compared to the 24 hours post collection time point was observed. Additionally, unstimulated CD4+ T cells produced significantly higher levels of IFNγ. These data provide an optimal method for PBMC isolation for application to fieldwork settings and for accurate evaluation of T cell effector function.
Figure 5. Viability and effector function of canine CD4+ and CD8+ T cells measured over a 48hr period.
Canine blood was collected, incubated on ice, and PBMCs isolated at the indicated time points. PBMCs were incubated at 37°C with PMA/ionomycin or media alone in the presence of GolgiPlug for 5 hours. Cells were stained with anti-CD8 and anti-CD4, permeabilized, and stained for IFNγ. Numbers indicate percentage (gated) and count (bottom right) of CD8+ (A) and CD4+ (B) T cells producing IFNγ at each isolation time point.
5. Discussion and Conclusion
T cells play a central role in the initiation and regulation of the immune response. T cells modulate cell surface expression of ligands and receptors to execute effector function and communicate with the environment. T-cell receptor complex signaling and CD28 co-stimulation results in naïve T cells downregulating lymph node homing receptors (CD62L and CCR7) while upregulating expression of cell adhesion molecules (CD44), T-cell receptor signaling (CD45RO), and early activation (CD69) antigens. Reliable expression of these cell surface molecules following cognate antigen encounter affords T cell monitoring during disease processes. Diagnosis and progression of human T cell lymphoproliferative disorders have been examine through flow cytometic immunophenotyping. Similar individualized diagnostics are being pursued for veterinary patients, but the paucity of literature describing the detection and characterization of antigen-driven T cell responses limits reaching this goal.
To address the growing need of canine immune system characterization for individualized diagnoses and toward furthering dogs as suitable translational research models, we aimed to identify and validate canine-specific reagents for discriminating naïve versus activated T cells. Novel canine T cell reactivity of CCL19-Ig defined CCR7 expression in CD4+ and CD8+ T cells of healthy donors. CCR7 co-expression with CD62L and decreased expression following stimulation validated these markers for defining naïve or central memory CD8+ T cells. The activation marker CTL2.58 further delineated activated T cells. Combining these reagents into a single panel would allow for identification of naïve or central memory T cells as CCR7+CD62LhiCTL2.58− and activated T cells as CCR7−CD62LloCTL2.58+. To our knowledge, these results are the first to describe and validate these markers for identifying naïve and activated canine T cell subsets.
With cognate antigen recognition stimulating cytokine-mediated T cell effector function, identification of IFNγ-producing T cells is critical for evaluating appropriate T cell responses. Monitoring antigen-specific T cell responses induction is a critical component for evaluating efficacy of T cell-mediated vaccines, especially in the era of canine oral malignant melanoma DNA vaccines (Grosenbaugh et al., 2011) where cytotoxic T cells would be proposed to mediate protection. Here, we described a method for defining vaccine-specific CD4+ and CD8+ T cells through intracellular cytokine staining following rabies, canine distemper-adenovirus-parainfluenza-parvovirus, and leptospirosis bacterial extract vaccination. Determining induction, kinetics, and effector function of these antigen-specific T cells are ideal for evaluating appropriate and protective vaccine responses and defining goals for future vaccines, where T cells are primary mediators of immune control for the targeted disease.
In instances where immediate PMBC isolation may be unavailable due to geographical location, evaluating appropriate storage conditions, subsequent cell isolation, and cytokine production following extended times post blood sampling is critical. For example, immunophenotyping of lymphoma of a dog living in a rural area or research studies involving characterization of T cell responses of dogs living in an international locale would likely require significant time between blood collection and PBMC isolation and storage. Data herein illustrates these canine subjects can be examined and immune responses characterized, expanding the translational application of this study beyond laboratory settings. These techniques and reagents are powerful in scenarios where human vaccination efforts would be unethical or inaccessible and dogs serve as models of natural infection and appropriate targets for control efforts, such as American trypanosomiasis (Padilla et al., 2010).
In conclusion, limited resources and techniques for phenotyping and characterizing canine T cells have restricted canine adaptive immune system studies. The tools defined here provide a platform for defining T cell subsets and identifying circulating antigen-specific T cells. The impact of this work for improving canine health and facilitating the translational applications of dogs for human disease modeling is promising. As individualized T cell immunophenotyping becomes more accessible for human patients, application to veterinary medicine and animal patients is encouraging.
Acknowledgements
We greatly acknowledge Lisa Reynolds of the University of Georgia College of Veterinary Medicine and Julie Nelson of the Center for Tropical and Emerging Global Diseases Flow Cytometry Facility at the University of Georgia, and members of the Tarleton Research Group for helpful discussion. ANH was supported in part by an NIH training grant T32 AI-060546 to the CTEGD. This work was supported by NIH Grant R01 AI089952 awarded to RLT.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare they have no competing interests.
References
- Adler B, Faine S. Host immunological mechanisms in the resistance of mice to leptospiral infections. Infection and Immunity. 1977;17:67–72. doi: 10.1128/iai.17.1.67-72.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguiar PHP, Barrouin-Melo SM, Franke CR, dos Santos RRB, Silva TMC, Mengel JO, dos-Santos WLC, Pontes-de-Carvalho L. A monoclonal antibody against a canine CD45 homologue: Analysis of tissue distribution, biochemical properties and in vitro immunological activity. Vet J. 2005;173:158–166. doi: 10.1016/j.tvjl.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Albareda MC, Olivera GC, Laucella SA, Alvarez MG, Fernandez ER, Lococo B, Viotti R, Tarleton RL, Postan M. Chronic human infection with Trypanosoma cruzi drives CD4+ T cells to immune senescence. J Immunol. 2009;183:4103–4108. doi: 10.4049/jimmunol.0900852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awano T, Johnson GS, Wade CM, Katz ML, Johnson GC, Taylor JF, Perloski M, Biagi T, Baranowska I, Long S, March PA, Olby NJ, Shelton GD, Khan S, O'Brien DP, Lindblad-Toh K, Coates JR. Genome-wide association analysis reveals a SOD1 mutation in canine degenerative myelopathy that resembles amyotrophic lateral sclerosis. P Natl Acad Sci USA. 2009;106:2794–2799. doi: 10.1073/pnas.0812297106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigler RD, Bushkin Y, Chiorazzi N. S152 (CD27). A modulating disulfide-linked T cell activation antigen. Journal of immunology. 1988;141:21–28. [PubMed] [Google Scholar]
- Byrne KM, Kim HW, Chew BP, Reinhart GA, Hayek MG. A standardized gating technique for the generation of flow cytometry data for normal canine and normal feline blood lymphocytes. Vet Immunol Immunop. 2000;73:167–182. doi: 10.1016/s0165-2427(99)00163-4. [DOI] [PubMed] [Google Scholar]
- Campbell JJ, Murphy KE, Kunkel EJ, Brightling CE, Soler D, Shen Z, Boisvert J, Greenberg HB, Vierra MA, Goodman SB, Genovese MC, Wardlaw AJ, Butcher EC, Wu L. CCR7 expression and memory T cell diversity in humans. Journal of immunology. 2001;166:877–884. doi: 10.4049/jimmunol.166.2.877. [DOI] [PubMed] [Google Scholar]
- Caniatti M, Roccabianca P, Scanziani E, Paltrinieri S, Moore PF. Canine Lymphoma: Immunocytochemical Analysis of Fine-needle Aspiration Biopsy. Vet Pathol. 1996;33:204–212. doi: 10.1177/030098589603300210. [DOI] [PubMed] [Google Scholar]
- Cobbold S, Metcalfe S. Monoclonal antibodies that define canine homologues of human CD antigens: summary of the First International Canine Leukocyte Antigen Workshop (CLAW) Tissue Antigens. 1994;43:137–154. doi: 10.1111/j.1399-0039.1994.tb02315.x. [DOI] [PubMed] [Google Scholar]
- Colonna M, Navarro F, Bellon T, Llano M, Garcia P, Samaridis J, Angman L, Cella M, Lopez-Botet M. A common inhibitory receptor for major histocompatibility complex class I molecules on human lymphoid and myelomonocytic cells. The Journal of experimental medicine. 1997;186:1809–1818. doi: 10.1084/jem.186.11.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creevy KE, Bauer TR, Tuschong LM, Embree LJ, Colenda L, Cogan K, Starost MF, Haskins ME, Hickstein DD. Canine leukocyte adhesion deficiency colony for investigation of novel hematopoietic therapies. Vet Immunol Immunop. 2003;94:11–22. doi: 10.1016/s0165-2427(03)00057-6. [DOI] [PubMed] [Google Scholar]
- Crockett-Torabi E, Fantone JC. L-selectin stimulation of canine neutrophil initiates calcium signal secondary to tyrosine kinase activation. Am J Physiol-Heart C. 1997;272:H1302–H1308. doi: 10.1152/ajpheart.1997.272.3.H1302. [DOI] [PubMed] [Google Scholar]
- Danilenko DM, Moore PF, Rossitto PV. Canine Leukocyte Cell-Adhesion Molecules (Leucams) - Characterization of the Cd11/Cd18 Family. Tissue Antigens. 1992a;40:13–21. doi: 10.1111/j.1399-0039.1992.tb01952.x. [DOI] [PubMed] [Google Scholar]
- Danilenko DM, Moor e PF, Rossitto PV. Canine leukocyte cell adhesion molecules (LeuCAMs): characterization of the CD11/CD18 family. Tissue Antigens. 1992b;40:13–21. doi: 10.1111/j.1399-0039.1992.tb01952.x. [DOI] [PubMed] [Google Scholar]
- Debes GF, Arnold CN, Young AJ, Krautwald S, Lipp M, Hay JB, Butcher EC. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nature Immunology. 2005;6:889–894. doi: 10.1038/ni1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellman CL, Stokes JV, Archer TM, Pinchuk LM, Lunsford KV, Mackin AJ. Cyclosporine A affects the in vitro expression of T cell activation-related molecules and cytokines in dogs. Vet Immunol Immunop. 2011;140:175–180. doi: 10.1016/j.vetimm.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Galkowska H, Waldemar LO, Wojewodzka U. Reactivity of antibodies directed against human antigens with surface markers on canine leukocytes. Vet Immunol Immunop. 1996;53:329–334. doi: 10.1016/S0165-2427(96)05604-8. [DOI] [PubMed] [Google Scholar]
- Gebhard DH, Carter PB. Identification of canine T-lymphocyte subsets with monoclonal antibodies. Vet Immunol Immunop. 1992;33:187–199. doi: 10.1016/0165-2427(92)90181-o. [DOI] [PubMed] [Google Scholar]
- Goodwin RG, Friend D, Ziegler SF, Jerzy R, Falk BA, Gimpel S, Cosman D, Dower SK, March CJ, Namen AE, et al. Cloning of the human and murine interleukin-7 receptors: demonstration of a soluble form and homology to a new receptor superfamily. Cell. 1990;60:941–951. doi: 10.1016/0092-8674(90)90342-c. [DOI] [PubMed] [Google Scholar]
- Grosenbaugh DA, Leard AT, Bergman PJ, Klein MK, Meleo K, Susaneck S, Hess PR, Jankowski MK, Jones PD, Leibman NF, Johnson MH, Kurzman ID, Wolchok JD. Safety and efficacy of a xenogeneic DNA vaccine encoding for human tyrosinase as adjunctive treatment for oral malignant melanoma in dogs following surgical excision of the primary tumor. American journal of veterinary research. 2011;72:1631–1638. doi: 10.2460/ajvr.72.12.1631. [DOI] [PubMed] [Google Scholar]
- Guedes PMD, Veloso VM, Tafuri WL, Galvao LMD, Carneiro CM, de Lana M, Chiari E, Soares KA, Bahia MT. The dog as model for chemotherapy of the Chagas' disease. Acta Trop. 2002;84:9–17. doi: 10.1016/s0001-706x(02)00139-0. [DOI] [PubMed] [Google Scholar]
- Hargreaves DC, Hyman PL, Lu TT, Ngo VN, Bidgol A, Suzuki G, Zou YR, Littman DR, Cyster JG. A coordinated change in chemokine responsiveness guides plasma cell movements. Journal of Experimental Medicine. 2001;194:45–56. doi: 10.1084/jem.194.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohnloser SH, Crijns HJ, van Eickels M, Gaudin C, Page RL, Torp-Pedersen C, Connolly SJ. Effect of dronedarone on cardiovascular events in atrial fibrillation. The New England journal of medicine. 2009;360:668–678. doi: 10.1056/NEJMoa0803778. [DOI] [PubMed] [Google Scholar]
- Iannello A, Boulassel MR, Samarani S, Debbeche O, Tremblay C, Toma E, Routy JP, Ahmad A. Dynamics and consequences of IL-21 production in HIV-infected individuals: a longitudinal and cross-sectional study. Journal of immunology. 2010;184:114–126. doi: 10.4049/jimmunol.0901967. [DOI] [PubMed] [Google Scholar]
- Khanna C, Lindblad-Toh K, Vail D, London C, Bergman P, Barber L, Breen M, Kitchell B, McNeil E, Modiano JF, Niemi S, Comstock KE, Ostrander E, Westmoreland S, Withrow S. The dog as a cancer model. Nature Biotechnology. 2006;24:1065–1066. doi: 10.1038/nbt0906-1065b. [DOI] [PubMed] [Google Scholar]
- Kishimoto TK, Jutila MA, Butcher EC. Identification of a human peripheral lymph node homing receptor: a rapidly down-regulated adhesion molecule. P Natl Acad Sci USA. 1990;87:2244–2248. doi: 10.1073/pnas.87.6.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopfleisch R, Lenze D, Hummel M, Gruber AD. Metastatic canine mammary carcinomas can be identified by a gene expression profile that partly overlaps with human breast cancer profiles. Bmc Cancer. 2010;10 doi: 10.1186/1471-2407-10-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsella R, Girolomoni G. Canine models of atopic dermatitis: a useful tool with untapped potential. The Journal of investigative dermatology. 2009;129:2351–2357. doi: 10.1038/jid.2009.98. [DOI] [PubMed] [Google Scholar]
- McKallip RJ, Do Y, Fisher MT, Robertson JL, Nagarkatti PS, Nagarkatti M. Role of CD44 in activation-induced cell death: CD44-deficient mice exhibit enhanced T cell response to conventional and superantigens. International immunology. 2002;14:1015–1026. doi: 10.1093/intimm/dxf068. [DOI] [PubMed] [Google Scholar]
- Moore PF, Rossitto PV, Danilenko DM. Canine Leukocyte Integrins - Characterization of a Cd18 Homolog. Tissue Antigens. 1990;36:211–220. doi: 10.1111/j.1399-0039.1990.tb01831.x. [DOI] [PubMed] [Google Scholar]
- Moreno J, Nieto J, Chamizo C, Gonzalez F, Blanco F, Barker DC, Alva J. The immune response and PBMC subsets in canine visceral leishmaniasis before, and after, chemotherapy. Vet Immunol Immunop. 1999;71:181–195. doi: 10.1016/s0165-2427(99)00096-3. [DOI] [PubMed] [Google Scholar]
- Murakawa T, Nakajima J, Ono M, Murakami A, Suematsu Y, Takamoto S. Allogenicity of cryopreserved human fibroblasts: cryopreservation does not downregulate the allogenicity of fibroblasts making up the matrices of allografts. J Thorac Cardiovasc Surg. 2000;120:712–719. doi: 10.1067/mtc.2000.109238. [DOI] [PubMed] [Google Scholar]
- Padilla AM, Brandan CMP, Basombrio MA. Vaccine Development for Chagas Disease. In: Telleria J, Tibayrenc M, editors. American Trypanosomiasis Chagas Disease. New York: Elsevier; 2010. pp. 827–841. [Google Scholar]
- Pedersen LG, Castelruiz Y, Jacobsen S, Aasted B. Identification of monoclonal antibodies that cross-react with cytokines from different animal species. Vet Immunol Immunop. 2002;88:111–122. doi: 10.1016/s0165-2427(02)00139-3. [DOI] [PubMed] [Google Scholar]
- Pilarski LM, Turley EA, Shaw AR, Gallatin WM, Laderoute MP, Gillitzer R, Beckman IG, Zola H. FMC46, a cell protrusion-associated leukocyte adhesion molecule-1 epitope on human lymphocytes and thymocytes. Journal of immunology. 1991;147:136–143. [PubMed] [Google Scholar]
- Ruslander DA, Gebhard DH, Tompkins MB, Grindem CB, Page RL. Immunophenotypic characterization of canine lymphoproliferative disorders. In Vivo. 1997;11:169–172. [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- Schlossman SF, Boumsell L, Gilks W. Leukocyte Typing V: White Cell Differentiation Antigens. New York: Oxford University Press; 1995. [Google Scholar]
- Schuberth H, Kucinskiene G, Chu R, Faldyna M. Reactivity of cross-reacting monoclonal antibodies with canine leukocytes, platelets and erythrocytes. Vet Immunol Immunop. 2007;119:47–55. doi: 10.1016/j.vetimm.2007.06.013. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ, Bell DS, Podlisny MB, Price DL, Cork LC. Conservation of brain amyloid proteins in aged mammals and humans with Alzheimer's disease. Science. 1987;235:873–877. doi: 10.1126/science.3544219. [DOI] [PubMed] [Google Scholar]
- Trowald-Wigh G, Johannisson A, Hakansson L. Canine neutrophil adhesion proteins and Fc-receptors in healthy dogs and dogs with adhesion protein deficiency, as studied by flow cytometry. Vet Immunol Immunop. 1993;38:297–310. doi: 10.1016/0165-2427(93)90089-m. [DOI] [PubMed] [Google Scholar]
- Urdal DL, March CJ, Gillis S, Larsen A, Dower SK. Purification and chemical characterization of the receptor for interleukin 2 from activated human T lymphocytes and from a human T-cell lymphoma cell line. P Natl Acad Sci USA. 1984;81:6481–6485. doi: 10.1073/pnas.81.20.6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valli JL, Williamson A, Sharif S, Rice J, Shewen PE. In vitro cytokine responses of peripheral blood mononuclear cells from healthy dogs to distemper virus, Malassezia and Toxocara. Vet Immunol Immunop. 2010;134:218–229. doi: 10.1016/j.vetimm.2009.09.023. [DOI] [PubMed] [Google Scholar]
- Verwilghen J, Vandenberghe P, Wallays G, de Boer M, Anthony N, Panayi GS, Ceuppens JL. Simultaneous ligation of CD5 and CD28 on resting T lymphocytes induces T cell activation in the absence of T cell receptor/CD3 occupancy. Journal of immunology. 1993;150:835–846. [PubMed] [Google Scholar]
- Wilbe M, Jokinen P, Truve K, Seppala EH, Karlsson EK, Biagi T, Hughes A, Bannasch D, Andersson G, Hansson-Hamlin H, Lohi H, Lindblad-Toh K. Genome-wide association mapping identifies multiple loci for a canine SLE-related disease complex. Nat Genet. 2010;42:250–254. doi: 10.1038/ng.525. [DOI] [PubMed] [Google Scholar]