Abstract
Background
Because methamphetamine (METH) pharmacokinetics after single iv doses show significant differences between male and female rats, we hypothesized that pharmacokinetic differences in METH disposition could be a contributing factor to the patterns of METH self-administration behaviors in rats.
Methods
For the studies, we used a passive (non-contingent) METH dosing schedule consisting of 27 METH iv bolus injections (0.048 mg/kg) over 2 hrs derived from a previous active (contingent) METH self-administration behavioral study in male rats. After METH dosing of male and female Sprague-Dawley rats (n=5/group), METH and amphetamine serum concentrations were determined by LC-MS/MS. Pharmacokinetic analysis, including predictive mathematical simulations of the data, was then conducted.
Results
Male and female rats achieved relatively stable METH serum concentrations within 20 min, which remained constant from 20–120 min. While not statistically different, METH clearance and volume of distribution values for females were 25% and 33% lower (respectively) than males. Linear regression analysis of predicted METH concentrations from pharmacokinetic simulations versus observed concentrations showed a substantially better correlation with male data than female data (r2 = 0.71 vs. 0.56; slope = 0.95 vs. 0.45, respectively). At 120 min, the time of predicted peak METH serum concentrations, female values were 42% higher than expected, while male values were within 3%.
Conclusions
Unlike METH male pharmacokinetic data, the female data was less predictable during multiple METH administrations and produced overall higher than expected METH concentrations. These findings demonstrate that METH pharmacokinetics could contribute to differences in METH self-administration behaviors in rats.
Keywords: methamphetamine, self-administration, pharmacokinetics, sex differences, rats
1. INTRODUCTION
Studies of (+)-methamphetamine (METH) human abuse patterns have helped delineate the characteristics of METH use (Cho and Melega, 2002; Comer et al., 2001; Hart et al., 2001; McMillan et al., 2004; Melega et al., 2007; Mendelson et al., 1995; Milesi-Hallé et al., 2005). These data suggest METH abuse typically begins with low doses, taken over relatively long intervals of time (Cho and Melega, 2002). Too frequently, this introductory phase is followed by patterns of “binge” use with a quick progression to larger doses and shorter time intervals between doses.
Sex differences are found in a variety of drug abuse models, including psychostimulant self-administration paradigms. For instance, during training female rats acquire heroin and cocaine self-administration behavior in fewer days than males (Lynch and Carroll, 1999) and females display greater locomotor activity following cocaine (Quiñones-Jenab et al., 1999), amphetamine (AMP; Becker, 1999), or METH administration (Milesi-Hallé et al., 2005; 2007; Schindler et al., 2002). In METH self-administration studies, Long-Evans female rats are more vulnerable to acquisition of self-administration behavior and appear more motivated to maintain self-administration than male rats (Roth and Carroll, 2004). Other investigators find that compared to male Wistar rats, females are equally susceptible to METH-induced memory deficits and exhibit higher METH intake and greater relapse to METH-seeking (Reichel et al., 2012). This mirrors aspects of human self-administration studies in which low dose (8–10 mg) AMP administration is a reinforcer in women, but not men (Vansickel et al., 2010).
While neuroendocrine mechanisms could play a role in the different propensity for male and female rats to self-administer METH, pharmacokinetic mechanisms could also be a factor. Sex differences in METH disposition in male and female Sprague-Dawley rats (Milesi-Hallé et al., 2007; 2005) include a faster METH total clearance (CLT) and a greater metabolic conversion of METH to AMP (a pharmacologically active metabolite) in male rats. The female Sprague-Dawley rats also have a lower metabolic clearance of METH, and a greater urinary excretion of unchanged METH (Milesi-Hallé et al., 2005).
The current studies were designed to both determine if the pharmacokinetics of METH could play a significant role in the sexual dimorphism of METH self-administration and if simulations of multiple dosing studies could be used to predict serum METH concentrations. To understand the interaction between the sex differences in METH pharmacokinetics and self-administration in rats, a pharmacokinetic model of passive (non-contingent; Jacobs et al., 2003) METH self-administration was derived from actual patterns of active (contingent) METH self-administration in male Sprague-Dawley rats (McMillan et al., 2004). Using this dosing protocol, the pharmacokinetic profiles of METH and AMP were then characterized in both male and female rats. Simulations of METH concentration versus time profiles using pharmacokinetic modeling software were also performed to evaluate the applicability of pharmacokinetic simulations to accurately predict METH concentrations achieved during a METH multiple-dosing protocol.
2. MATERIALS AND METHODS
2.1 Drugs and Chemicals
(+)-Methamphetamine hydrochloride was obtained from the National Institute on Drug Abuse (Rockville, MD). (±)-Amphetamine–d11 and other chemicals were purchased from Sigma Chemical Company (St. Louis, MO). All other reagents were purchased from Thermo Fisher Scientific Inc. (Waltham, MA), unless otherwise noted. All drug concentrations are expressed as the free base form. METH doses for iv injections were prepared in 0.9% NaCl.
2.2 Development of METH Self-Administration dosing regimen in rats
The METH unit dose, time of dosing, and choice of patterns of METH administration used in the current studies were derived from previous experiments in our laboratory with male Sprague-Dawley rats (McMillan et al., 2004). To aid understanding of the current experimental METH dosing protocol, the key features are as follows. Male Sprague-Dawley rats (n=3) were trained to press levers that delivered food pellets. After responding was established, the schedule was changed to deliver a food pellet after every third response (fixed-ratio [FR3] schedule). After one session under this FR3 schedule, an iv catheter was implanted into each rat’s femoral vein under anesthesia.
Subsequently, METH was made available for self-administration under the FR3 schedule via a syringe pump, mounted over the test chamber and connected to the rat’s catheter through polyethylene tubing. During training sessions, male rats could self-administer METH unit doses of 0.048 mg/kg under the FR3 schedule. The injection volume was approximately 0.07 ml, injected over 1.3-sec; slight volume adjustments were made to correct for body weight differences. Rats were considered to have achieved stable behavior when no significant changes in the rates of responding were observed over four consecutive self-administration sessions. Individual patterns of activity are shown in Figure 1A.
Figure 1.
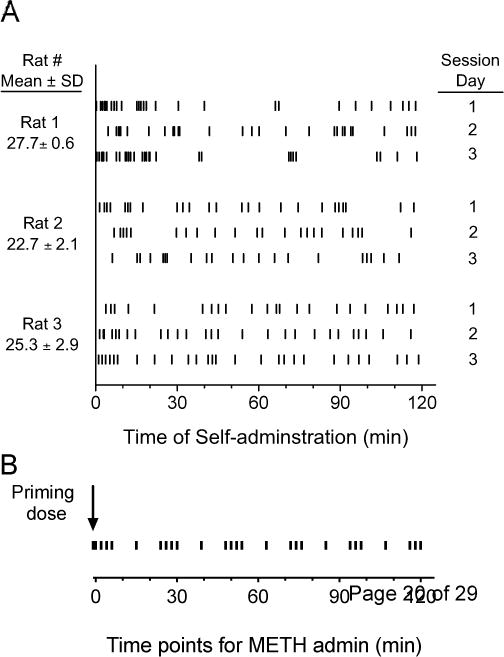
(A) Injections self-administered by rats in 2-hr sessions over three days (McMillan et al., 2004). (B) Drug administration protocol for the current study based on data from male rats that self-administered METH in 2-hr behavioral sessions (see 1A).
2.3 Animals
Male and freely cycling female Sprague-Dawley rats used in these studies were purchased from Hilltop Laboratory Animals Inc. (Scottsdale, PA). Rats were surgically implanted with femoral vein and external jugular vein catheters (Silastic medical-grade tubing, 0.020-in inner diameter and 0.037-in outer diameter; Dow Corning, Midland, MI). Catheters were kept below the skin surface for transport from the vendor and exposed under halothane anesthesia one day before the first experimental procedure. Catheters were kept patent by a daily saline flush (0.2 ml) followed by saline containing 25 U of heparin. Male and female rats were housed in separate cubicles. Each rat was housed in individual cages with a 12-hr light/dark cycle, 22°C environment with free access to water and fed approximately 20 g of pellets daily. This maintained female body weights between 250 to 280 g and male body weights between 270 to 300 g. All studies were conducted in accordance with the Guide for the Care and Use of Laboratory Animals, as adopted and promulgated by the National Institutes of Health. All experiments were performed with the approval of the Animal Care and Use Committee of the University of Arkansas for Medical Sciences.
2.4 Behavioral Habituation and Experimental Apparatus for the Pharmacokinetic Studies
One week prior to experiments, rats were acclimated to metabolism cages (Nalgene Supply, Rochester, NY) and to harnesses (Instech Laboratories, Plymouth Meeting, PA) for six hr/day. The rat’s femoral vein catheter was used for drug administration and the jugular vein catheter was used for blood collection. On the first day of the experiment, the infusion harnesses were connected to a tether that protected two tubing extensions. The drug administration extension (0.023-in inner diameter and 0.038-in outer diameter; Intramedic® Polyethylene Tubing, Becton Dickinson, Parsippany, NJ) was attached to the rat’s femoral vein catheter. The blood withdrawal extension was attached to the rat’s jugular vein catheter. The rat was then placed in the metabolic cage and the tether was attached to a swivel. This allowed free movement in the cage during drug administration and blood sampling. The METH solution (0.2 mg/ml) was dispensed from a 500 μl dead volume syringe (Hamilton Company, Reno, NV) via a polyethylene extension attached to a programmable Harvard infusion pump (Harvard Apparatus, Holliston, MA).
2.5 Drug Administration and Blood Sampling Protocol
Rats in the prototype self-administration studies (McMillan et al., 2004) were shown to promptly achieve METH self-administration behavior. Because the protocol used by McMillan et al. included a METH priming dose before each session, rats in the current studies received one dose of 0.048 mg/kg METH 1 min before the beginning of the self-administration modeling session (Fig. 1). On average, these rats self-administered 26 injections of 0.048 mg/kg METH during the 2-hr sessions (Figure 1A). Including the priming dose, the total number of injections was 27. The average values for their inter-injection intervals and frequency of drug self-administration were also derived from the METH multiple injection protocol. Figure 1B shows the estimated average METH self-administration patterns derived from Figure 1A. METH doses were injected via femoral vein at −1, 0, 2, 4, 6, 15, 24, 26, 28, 30, 39, 48, 50, 52, 54, 63, 72, 74, 76, 85, 94, 96, 98, 107, 116, 118 and 120 min. The volume administered by the pump for each injection was 0.07 ml (for a 280 g rat), as in the original self-administration study. The total METH dose administered over 120 min was 1.3 mg/kg. Blood samples were planned to be collected at 5 min before and 2, 5, 10, 20, 40, 60, 90, 120, 150, 180, 270, 360, 450 and 540 min after drug treatment (for a total of 15 samples). However, not all of these samples could actually be collected. In the end, for nine of the rats we collected only 11–13 samples. The one exception was the first male, from which we collected 17 samples before modifying the protocol. The total blood volume collected during each session did not exceed 1% of the male or female rat total body weight. To limit the possibility of stressing the rats, we collected less blood sample when drug concentrations were expected to be high and more when the concentrations were low. Infusions of sterile saline were also used to replenish fluids. After blood centrifugation, the serum samples were stored at −80°C until analysis.
2.6 Analysis of METH and AMP Samples by Liquid Chromatography Tandem Mass Spectrometry (LC-MS/MS)
The LC-MS/MS method of Hendrickson et al (2004) was used to quantitate METH and AMP serum concentrations, along with quality control standards in rat serum. (±)-Amphetamine-d11 (10 ng/ml solution) was used as an internal standard.
The mobile phase for HPLC consisted of 10 mM ammonium acetate buffer (pH 3.7) with 25% (v/v) acetonitrile and 2.5% (v/v) methanol, with a flow rate of 0.2 ml/min. Injections (25 μL) were made onto a 3 mm Hypersil BDS C18 column (100 × 2.1 mm inner diameter; Thermo Hypersil-Keystone, Bellefonte, PA) at a temperature of 55°C. A Quattro LC Triple Quadrupole Mass Spectrometer (Waters Corporation) fitted with a Z-Spray electrospray interface was used for mass spectrometry analysis in the positive ion mode.
2.7 Pharmacokinetic calculations, statistics, and pharmacokinetic simulations of METH serum concentration-time data
For the model-independent analysis of individual male and female rat concentration-time data, the area under the METH serum concentration-time curve (AUC) from the time of the first METH administration up to 360 min was calculated using the linear trapezoid rule. A linear regression best-fit line to the terminal log-linear serum concentration-time data (after the end dosing at 120 min) was used to obtain the terminal disposition rate constant (λn) and half-life (t1/2) by the equation: t1/2 = 0.693 / λn. The remaining area to time infinity was determined from the predicted concentration on the best-fit line at the time point (tn) of the last measured concentration (Cn): . The sum of these areas was the AUC from time zero to infinity . Total clearance (CLT) was calculated by the equation: and apparent volume of distribution (Vd) was calculated by: Vd = CLT / λn. AMP metabolite t1/2 and were determined by similar calculations. For statistical comparisons of , data were normalized for METH dose.
All statistical analyses of pharmacokinetic parameters were performed with GraphPad 6 (GraphPad Software, Inc, La Jolla, CA). Data are described as the means ± SD. Student’s t tests were used to conduct comparisons between males and females, unless the test for equal variance failed in which case a Mann-Whitney Rank Sum test was conducted on the groups. Harmonic means and pseudo standard deviations of the METH and AMP t1/2 values were calculated as described previously (Lam et al., 1985). A value of p<0.05 was considered to be significant for all analyses.
METH serum concentration-time curves resulting from the 27 dose administration schedule were simulated using the WinNonlin 6.3 pharmacokinetic software (Certara, St. Louis, MO) and were used to make predictions about the METH serum levels during METH self-administration in rats and to optimize the blood sampling protocol. To perform these simulations, a best-fit line was fit to the METH data sets from the Milesi-Hallé (2005) study. Both 1 and 3 mg/kg METH iv doses in male and female rats were analyzed. The best-fit line to each rat concentration-time data set was determined using WinNonlin software with a two-compartment, IV-Bolus macro model and 1/Y2 weighting (Milesi-Hallé et al., 2005). Using this model and the average macroconstants derived from the best-fit lines, we simulated the METH serum concentrations resulting from multiple METH administrations in male and female rats. Average macroconstants for male rats administered 1 mg/kg METH were: A=274 ng/ml, B=38 ng/ml, α=0.05/min, and β=0.01/min. Macroconstants for male rats administered 3 mg/kg METH were: A=741 ng/ml, B=99 ng/ml, α=0.05/min, and β=0.01/min. Macroconstants for female rats administered 1 mg/kg METH were: A=282 ng/ml, B=102 ng/ml, α=0.09/min, and β=0.01/min. Macroconstants for female rats administered 3 mg/kg METH were: A=1753 ng/ml, B=397 ng/ml, α=0.43/min, and β=0.01/min. The predicted METH concentrations at the time of blood draws were compared to the observed values.
Linear regression analysis was then used to fit a best-fit line to pharmacokinetic predicted vs observed concentration data from male and female rats. Confidence intervals (95% level) for these data sets were chosen to represent the variability of the predicted vs. observed METH data. Because of the higher variability of the data than expected, a Grubb’s test was conducted on METH data sets for males and females to determine the possibility of an outlier value in the groups. This analysis showed one animal in the male and female group was an outlier for one of the pharmacokinetic parameters. Because these simulations were intended to be a measure of the best estimate of an average response, we excluded these outliers and used an n=4 per group to make the predicted versus observed comparisons. We calculated the differences between observed and predicted values and analyzed the distribution of differences with a D’Agostino-Pearson normality test.
3. RESULTS
Figure 2 and 3 show the METH and AMP concentration-time profiles for male and female rats (respectively) during the 2-hr dosing sessions. To better understand the fluctuations in METH concentrations induced by the multiple-dosing protocol, the mean METH concentrations (±2 standard deviations) from 20–120 min were plotted on these graphs. These mean concentrations and the mean 120 min METH concentrations at the time of the last injection are listed in Table 1. While the female concentrations were higher on average, the values were not significantly different from males.
Figure 2.
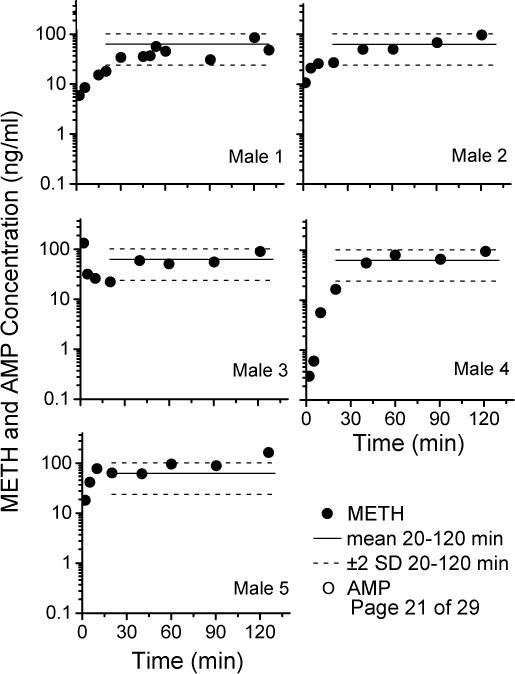
Drug concentration versus time profile for METH (closed circles) and AMP (open circles) for each individual male rat after METH multiple-dosing (0.048 mg/kg METH, 27 doses in 2-hr). The solid line represents the average METH concentrations for all 5 male rats from 20–120 min, and the dotted lines represent ± 2 SD.
Figure 3.
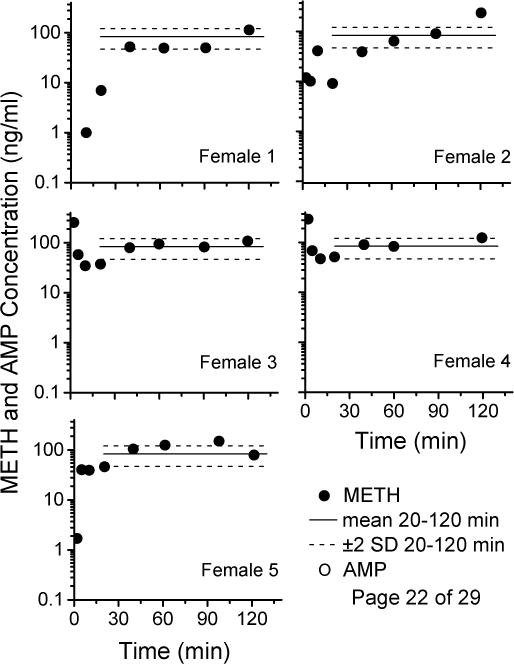
Drug concentration versus time profile for METH (closed circles) and AMP (open circles) for each individual female rat after METH multiple-dosing (0.048 mg/kg METH, 27 doses in 2-hr). The solid line represents the average METH concentrations for all 5 female rats from 20–120 min, and the dotted lines represent ± 2 SD.
Table 1.
Comparison of METH and AMP pharmacokinetic parameters in male and female rats (n=5/group) derived from the individual METH and AMP concentration vs time curves in Figures 2 and 3.
| Parameter | Males | Females |
|---|---|---|
| METH t1/2 (min)a | 55 ± 12 | 55 ± 16 |
| Mean METH conc 20–120 min (ng/ml) | 63 ± 17 | 84 ± 17 |
| METH conc at 120 min (ng/ml) | 106 ± 29 | 139 ± 48 |
| METH (ng.min/ml) | 13767 ± 4405 | 21264 ± 5861 |
| METH (ng.min/ml) | 6617 ± 2160 | 8507 ± 1845 |
| METH (ng.min/ml) | 6611 ± 2313b | 11935 ± 4428 |
| METH Vd (L/kg) | 8 ± 3 | 6 ± 4 |
| METH CLT (ml/min/kg) | 100 ± 25 | 67 ± 25 |
| AMP t1/2 (min)a | 99 ± 19c | 121 ± 9 |
| AMP (ng.min/ml) | 3389 ± 1777c | 3829 ± 792 |
| AMP (ng.min/ml) | 2526 ± 1397c | 3027 ± 625 |
| Molar ratio of AMP/METH | 0.25 ± 0.05c | 0.20 ± 0.03 |
| Molar ratio of AMP/METH | 0.41 ± 0.08c | 0.30 ± 0.08 |
Harmonic mean and pseudo-standard deviation (Lam et al., 1985).
Statistical difference (p<0.05).
One rat excluded (n=4) because of inadequate sampling of AMP terminal elimination concentrations.
Figure 4 shows METH concentrations (open circles) over time in all male (4A) and female rats (4B). The solid line shows the predicted WinNonlin simulated METH concentrations based on male or female baseline pharmacokinetic parameters derived from a 1 mg/kg iv METH dose. This dose was closest to the total 1.3 mg/kg METH dose administered during the current 2 hr dosing study. In Figure 5, the left panels show the correlation between predicted and observed METH concentrations in male (5A) and female (5B) rats. The right panels show the difference between observed and predicted values in male (5C) and female (5D) rats. Based on statistical analysis, the male values were normally distributed while the female values were not.
Figure 4.
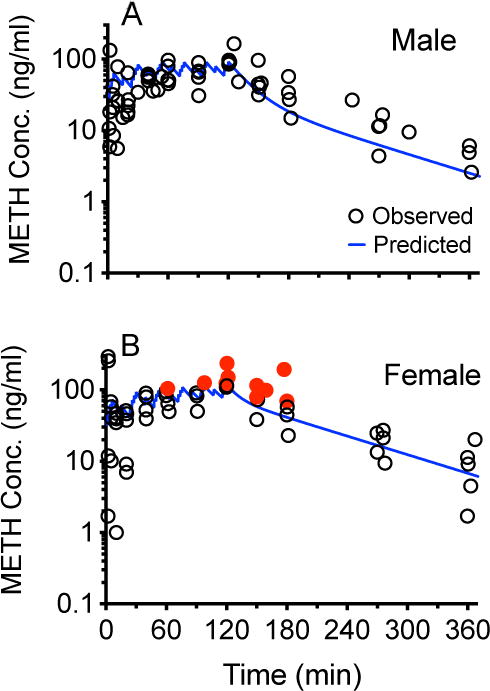
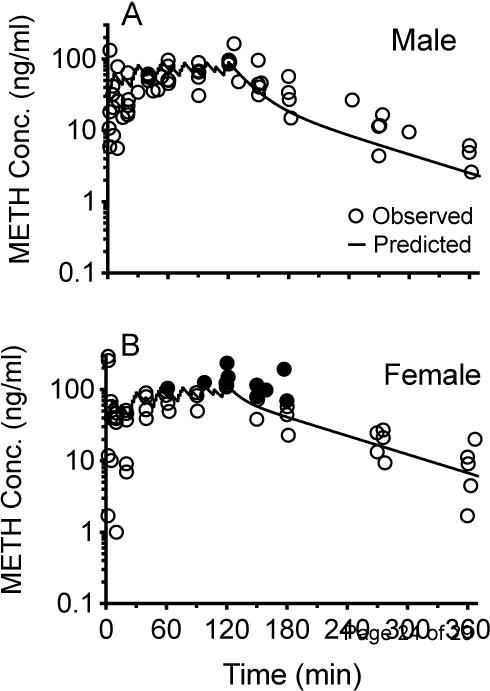
Relationship between predicted (black line) and observed METH serum concentrations (circles) obtained after treating male (A) and female rats (B) with a METH multiple-dosing protocol. In 4B, the red closed circles highlight nine METH concentration values in female rats that were determined (see Figure 5D) to have observed minus predicted values greater than any of the male values found in Figure 5C.
Figure 5.
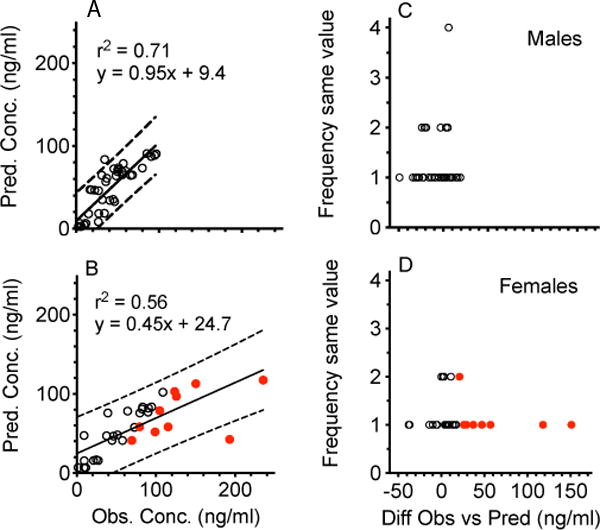
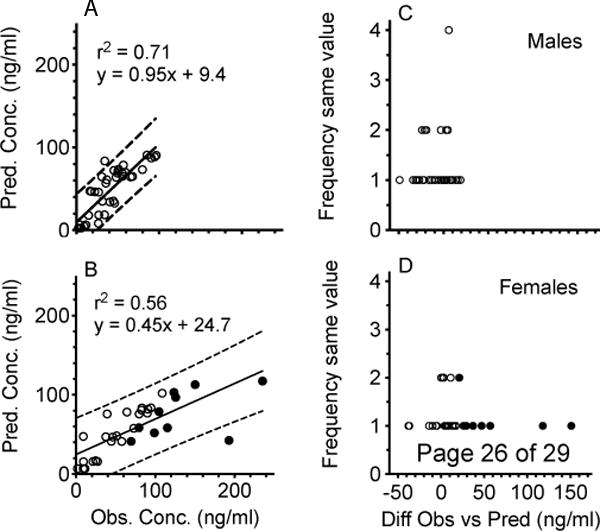
Correlation between predicted and observed METH concentrations in male (5A) and female rats (5B). The black line represents least squares linear regression fits to the male and female data. Dotted lines represent the upper and the lower 95% confidence intervals. The distribution of the frequency of differences in observed and predicted values in male and female rats is shown in figures 5C and 5D, respectively. Based on statistical analysis of these data, the male values in plot 5C were normally distributed while the female values in 5D were not normally distributed (p<0.05). The red closed circles in 5B (and 4B) are used to highlight the observed female concentrations that resulted in large differences in observed minus predicted values in Figure 5D. These ten values appeared to substantially decrease the r2 value and the slope of the best-fit line in Figure 5B.
We also compare the observed METH concentration values with the predicted values resulting from using the pharmacokinetic parameters derived from a 3 mg/kg iv METH dose. The r2 value was 0.7 and 0.68 and the equations for the best-fit line were y=0.85+8.3 and y=0.51+22.4 in male and female rats, respectively. The distribution of difference values in both sexes was similar to the data shown in Figure 5C and 5D, which was based on pharmacokinetic parameters derived from a 1 mg/kg iv METH dose. The male values were also normally distributed while the female values were not.
The METH and AMP pharmacokinetic values from the 27 METH doses in male and female rats are shown in Table 1. CLT, Vd, and t1/2 are also not substantially different from the male and female pharmacokinetic parameters from our previous study of a 1.0 mg/kg iv bolus dose of METH (Milesi-Hallé et al., 2005).
4. DISCUSSION
Previous publications suggest that male and female rats (Long Evans and Wistar) differ in their acquisition, maintenance and reinstatement of METH self-administration (Reichel et al., 2012; Roth and Carroll, 2004). Furthermore, estrogenized ovariectomized female rats self-administered significantly more METH than both male and non-estrogenized ovariectomized female rats (Kucerova et al., 2009). Similarly in human studies, female subjects administered more AMP (a METH-like drug) capsules (8–10 mg) than placebo, while men could not distinguish this low AMP dose from the placebo (Vansickel et al., 2010). Within females, AMP has been reported to cause increased subjective effects in the follicular phase of the menstrual cycle (Terner and de Wit, 2006). These data suggest these differences are affected by sex hormones. However, it is not known if these differences between sexes are mediated by sex-related differences in the central nervous system, pharmacokinetic processes or both.
Results from the current study showed that in both sexes, approximately five METH injections during the first 20 min of the session (equivalent to a cumulative dose between 0.1 and 0.2 mg/kg) allowed most rats to attain relatively stable METH serum concentrations, which then persisted for the remainder of the 120 min session (Figure 2 and 3). The self-administration model used for passive administration of METH in the current study (see Figure 1A) was in agreement with the behavioral results of Munzar et al. (1999). The Munzar studies show most male rats initiate active METH self-administration within five to ten sessions, and quickly attain stable levels of behavioral responding. They also report the rats readily self-administer METH in short intervals in the beginning of the session but later space the frequency of injection in a more uneven fashion. Also, studies by Stefanski et al. (1999) suggest that although male Sprague-Dawley rats alternate irregular phases of METH self-administration with periods of abstinence during a 2-hr session, they are observed to self-administer a burst of injections (5–8) within the first 30 min of the session. These previous behavioral data, along with the pharmacokinetic findings in the current study, suggest that the high frequency of male METH self-administration during the early part of the session results in rapid achievement of relatively stable METH concentrations. From the viewpoint of human clinical pharmacology, this initial self-administration pattern in rats is strikingly similar to the use of loading doses in medical situations that require a more rapid attainment of steady-state levels of a drug.
The current studies also allowed us to investigate the effects of repeated METH injections on METH pharmacokinetic parameters. These effects are of interest because other studies suggest stimulant drugs display different dispositional properties when single and multiple dosing schedules are compared. For example, the metabolism of cocaine in rats differs between acute and self-administered doses (Lau and Sun, 2002). In humans, however, no differences in pharmacokinetics or subjective ratings of METH effects are found when single or multiple dosing METH schedules are compared (Comer et al., 2001). These findings for METH in humans are in agreement with our studies, which showed no substantial differences in pharmacokinetic parameters within a sex after a single 1 mg/kg iv bolus dose (Milesi-Hallé et al., 2005) or after the 27 (0.048 mg/kg) unit doses over two hours used in the current studies.
Another objective for these studies was to determine if METH concentrations during rat self-administration experiments could be predicted from pharmacokinetic simulations. While the observed serum METH concentrations over time appeared to follow the general pattern predicted by the simulations in both male and female rats (Figure 4A and 4B), linear regression analyses of METH predicted vs. actual concentrations for all time points from each sex group (Figure 5A and 5B) showed a higher coefficient of determination in males (r2 = 0.71) than in females (r2 = 0.56). In addition, based on a comparison of the slopes of the best-fit lines (0.95 vs. 0.45) and y-intercept values (9.4 ng/ml vs. 24.7 ng/ml) there was a substantially better correlation of observed vs. predicted values in males than in females (respectively). This greater deviation of the slope from 1.0 and y-intercept from 0 in the female vs male rats appeared to be due to the female METH concentration simulation making predictions that were substantially less than the observed METH concentration values. Specifically, the females had ten concentrations that were greater than any of the male values, and these differences occurred between 60–180 min in the females. These ten values (as concentrations or differences in observed minus predicted values) are shown as red dots in Figures 4B, 5B and 5D.
These unexpected elevations in the observed values between 60–180 min in females could be affected by the stage of the female rat estrous cycle. We did not account for this in our study design since we were relating these data to a prior single dose iv METH study (Milesi-Hallé et al., 2005) involving the use of male and freely cycling female rats. A future study focusing on effects of estrous on METH pharmacokinetics could help to explain these differences. However, in studies of sex difference in METH seeking behavior in Long Evans female rats, estrous cycle stage (as determined from vaginal cytology) did not influence METH-primed reinstatement (Cox et al., 2013).
Because the cumulative METH dose reached 1.3 mg/kg by the end of dosing, it is also possible that the correlation between predicted versus observed METH concentrations were starting to be dose-dependent (and thus showing less predictability). Based on our previous studies, we know male METH pharmacokinetic values are dose-independent after acute iv doses up to at least 3 mg/kg, but female pharmacokinetic values become dose-dependent somewhere between the range of 1 to 3 mg/kg (Milesi-Hallé et al., 2005). Compared to male rat values, the dose-dependency in the female rats is expressed as a slower METH CLT and nonrenal clearance, lower in vivo AMP formation, and greater METH urinary excretion. While not measured, there may also be additional differences in free or Phase II conjugates of 4-hydroxymethamphetamine metabolite(s) (Caldwell et al., 1972).
Although Vd and CLT values were not statistically different (Table 1) for males and females, the CLT and Vd values for females were 25% and 33% lower (respectively) than male values. A lower CLT and lower Vd would both lead to higher serum METH concentrations in the females (and higher AUC values, see Table 1). We think these differences in METH pharmacokinetics, especially the dose-dependent elimination processes in females, could help explain increased behavioral sensitivity of females in METH self-administration studies (i.e., greater METH exposure to female central nervous system). Indeed in our study, the highest serum METH concentrations were predicted to occur in both groups after the final injection at 120 min (Fig 4A and B). In the male rats, the observed concentration values at 120 min were within 3% of predicted values (Fig 4A) while in the female rats the observed values were 42% higher than predicted (Fig 4B). In three of these female rats, the difference was greater than 20 ng/ml. Also in female rats, was significantly increased (Table 1).
In summary, these results showed male and female rats quickly attained (within 20 min) stable METH concentrations and maintained those levels, with relatively low fluctuations during the 120 min dosing session. These studies in Sprague-Dawley rats also provided important evidence that sex-dependent differences in METH pharmacokinetics could contribute to the differences between male and female patterns of self-administration (e.g., Kucerova et al., 2009). In addition, it is possible to accurately predict at least male concentration-time profiles based on knowledge of the experimental conditions and use of pharmacokinetic simulations. The use of METH concentration-time simulations for self-administration experiments could be a powerful tool for future studies since simulated real-time serum concentration data could be correlated with self-administration measurements. This would provide an additional dimension of understanding for the behavioral data without the need to collect blood samples. While this method appears to be less predictive at higher concentrations in female rats, more elaborate modeling (i.e., accounting for the possible nonlinearity or effects of the estrous cycle) might allow better prediction in female rats. Finally, the study of the diversity of effects in the male and female rats offers a broader perspective for discovering the range of susceptibility to adverse effects in humans.
Highlights.
Pharmacokinetic properties produce sex differences in methamphetamine (METH) self-administration
The model included 27 iv bolus METH doses over 120 min based on a previous study
Male and female rats achieved relatively stable METH concentrations within 20 min
Male METH concentrations were accurately predicted from pharmacokinetic simulations
Female METH concentrations were less predictable than male values from 60–180 min
Acknowledgments
The authors thank Bill Hardwick and Sherri Wood for their excellent technical assistance.
Role of Funding Source: This work was supported by NIDA grants R01 DA11560, T32 DA022981 (MDH), a GlaxoSmithKline Graduate Fellowship in Pharmacokinetics (AMH), and the National Center for Advancing Translational Science (ULITR000039). The funding source had no role in the study design; collection, analysis, and interpretation of data; in the writing of this paper; or the decision to submit this paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authorship Contributions
AMH, DEM, and SMO participated in research design. AMH conducted the experiments. AMH, MDH, and SMO performed data analysis. AMH, MDH, DEM, and SMO wrote or contributed to the writing of the manuscript.
Conflict of Interest
SMO has financial interests in and serves as Chief Scientific Officer of InterveXion Therapeutics LLC (Little Rock, AR), a pharmaceutical biotechnology company focused on treating human drug addiction with antibody-based therapies.
Contributor Information
Alessandra Milesi-Hallé, Email: milesihalle@comcast.net.
Michael D. Hambuchen, Email: hambuchenmichaeld@uams.edu.
Donald E. McMillan, Email: demamcmillan@comcast.net.
S. Michael Owens, Email: mowens@uams.edu.
References
- Becker JB. Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacol Biochem Behav. 1999;64:803–812. doi: 10.1016/s0091-3057(99)00168-9. [DOI] [PubMed] [Google Scholar]
- Caldwell J, Dring LG, Williams RT. Metabolism of [14 C] methamphetamine in man, the guinea pig and the rat. Biochem J. 1972;129:11–22. doi: 10.1042/bj1290011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho AK, Melega WP. Patterns of methamphetamine abuse and their consequences. J Addict Dis. 2002;21:21–34. doi: 10.1300/j069v21n01_03. [DOI] [PubMed] [Google Scholar]
- Comer SD, Hart CL, Ward AS, Haney M, Foltin RW, Fischman MW. Effects of repeated oral methamphetamine administration in humans. Psychopharmacology. 2001;155:397–404. doi: 10.1007/s002130100727. [DOI] [PubMed] [Google Scholar]
- Cox BM, Young AB, See RE, Reichel CM. Sex differences in methamphetamine seeking in rats: impact of oxytocin. Psychoneuroendocrinology. 2013;38:2343–2353. doi: 10.1016/j.psyneuen.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CL, Ward AS, Haney M, Foltin RW, Fischman MW. Methamphetamine self-administration by humans. Psychopharmacology. 2001;157:75–81. doi: 10.1007/s002130100738. [DOI] [PubMed] [Google Scholar]
- Hendrickson HP, Milesi-Hallé A, Laurenzana EM, Owens SM. Development of a liquid chromatography-tandem mass spectrometric method for the determination of methamphetamine and amphetamine using small volumes of rat serum. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;806:81–87. doi: 10.1016/j.jchromb.2004.03.038. [DOI] [PubMed] [Google Scholar]
- Jacobs EH, Smit AB, de Vries TJ, Schoffelmeer ANM. Neuroadaptive effects of active versus passive drug administration in addiction research. Trends Pharmacol Sci. 2003;24:566–573. doi: 10.1016/j.tips.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Kucerova J, Vrskova D, Sulcova A. Impact of repeated methamphetamine pretreatment on intravenous self-administration of the drug in males and estrogenized or non-estrogenized ovariectomized female rats. Neuro Endocrinol Lett. 2009;30:663–670. [PubMed] [Google Scholar]
- Lam FC, Hung CT, Perrier DG. Estimation of variance for harmonic mean half-lives. J Pharm Sci. 1985;74:229–231. doi: 10.1002/jps.2600740229. [DOI] [PubMed] [Google Scholar]
- Lau CE, Sun L. The pharmacokinetic determinants of the frequency and pattern of intravenous cocaine self-administration in rats by pharmacokinetic modeling. Drug Metab Dispos. 2002;30:254–261. doi: 10.1124/dmd.30.3.254. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology. 1999;144:77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- McMillan DE, Hardwick WC, Li M, Gunnell MG, Carroll FI, Abraham P, Owens SM. Effects of murine-derived anti-methamphetamine monoclonal antibodies on (+)-methamphetamine self-administration in the rat. J Pharmacol Exp Ther. 2004;309:1248–1255. doi: 10.1124/jpet.103.061762. [DOI] [PubMed] [Google Scholar]
- Melega WP, Cho AK, Harvey D, Laćan G. Methamphetamine blood concentrations in human abusers: application to pharmacokinetic modeling. Synapse. 2007;61:216–220. doi: 10.1002/syn.20365. [DOI] [PubMed] [Google Scholar]
- Mendelson J, Jones RT, Upton R, Jacob P. Methamphetamine and ethanol interactions in humans. Clin Pharmacol Ther. 1995;57:559–568. doi: 10.1016/0009-9236(95)90041-1. [DOI] [PubMed] [Google Scholar]
- Milesi-Hallé A, Hendrickson HP, Laurenzana EM, Gentry WB, Owens SM. Sex-and dose-dependency in the pharmacokinetics and pharmacodynamics of (+)-methamphetamine and its metabolite (+)-amphetamine in rats. Toxicol Appl Pharmacol. 2005;209:203–213. doi: 10.1016/j.taap.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Milesi-Hallé A, McMillan DE, Laurenzana EM, Byrnes-Blake KA, Owens SM. Sex differences in (+)-amphetamine- and (+)-methamphetamine-induced behavioral response in male and female Sprague-Dawley rats. Pharmacol Biochem Behav. 2007;86:140–149. doi: 10.1016/j.pbb.2006.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munzar P, Baumann MH, Shoaib M, Goldberg SR. Effects of dopamine and serotonin-releasing agents on methamphetamine discrimination and self-administration in rats. Psychopharmacology. 1999;141:287–296. doi: 10.1007/s002130050836. [DOI] [PubMed] [Google Scholar]
- Quiñones-Jenab V, Ho A, Schlussman SD, Franck J, Kreek MJ. Estrous cycle differences in cocaine-induced stereotypic and locomotor behaviors in Fischer rats. Behav Brain Res. 1999;101:15–20. doi: 10.1016/s0166-4328(98)00073-4. [DOI] [PubMed] [Google Scholar]
- Reichel CM, Chan CH, Ghee SM, See RE. Sex differences in escalation of methamphetamine self-administration: cognitive and motivational consequences in rats. Psychopharmacology. 2012;223:371–380. doi: 10.1007/s00213-012-2727-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth ME, Carroll ME. Sex differences in the acquisition of IV methamphetamine self-administration and subsequent maintenance under a progressive ratio schedule in rats. Psychopharmacology. 2004;172:443–449. doi: 10.1007/s00213-003-1670-0. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Bross JG, Thorndike EB. Gender differences in the behavioral effects of methamphetamine. Eur J Pharmacol. 2002;442:231–235. doi: 10.1016/s0014-2999(02)01550-9. [DOI] [PubMed] [Google Scholar]
- Stefanski R, Ladenheim B, Lee SH, Cadet JL, Goldberg SR. Neuroadaptations in the dopaminergic system after active self-administration but not after passive administration of methamphetamine. Eur J Pharmacol. 1999;371:123–135. doi: 10.1016/s0014-2999(99)00094-1. [DOI] [PubMed] [Google Scholar]
- Terner JM, de Wit H. Menstrual cycle phase and responses to drugs of abuse in humans. Drug Alcohol Depend. 2006;84:1–13. doi: 10.1016/j.drugalcdep.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Vansickel AR, Stoops WW, Rush CR. Human sex differences in d-amphetamine self-administration. Addiction. 2010;105:727–731. doi: 10.1111/j.1360-0443.2009.02858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


