Abstract
Background
Brain perfusion is altered in both alcohol dependence and stimulant dependence. Although most substance users also abuse/depend on alcohol concurrently (polysubstance users; PSU), rigorous perfusion research in PSU is limited. Also, the relationships of perfusion abnormalities with cognition, impulsivity or decision making are not well known.
Methods
Arterial spin labeling MRI and neuropsychological measures assessed perfusion levels and neurocognition in 20 alcohol dependent individuals with comorbid stimulant dependence (PSU), 26 individuals dependent on alcohol only (ALC), and 31 light/non-drinking controls (LD). The patient groups included smokers and non-smokers.
Results
ALC had lower perfusion than LD in subcortical and cortical brain regions including the brain reward/executive oversight system (BREOS). Contrary to our hypothesis, regional perfusion was generally not lower in PSU than ALC. However, smoking PSU had lower perfusion than smoking ALC in several regions, including BREOS. Lower BREOS perfusion related to greater drinking severity in smoking substance users and to greater smoking severity in smoking ALC. Lower regional perfusion in ALC and PSU correlated with worse performance in different cognitive domains; smoking status affected perfusion-cognition relationships in ALC only. Lower BREOS perfusion in both substance using groups related to higher impulsivity.
Conclusion
Although regional perfusion was not decreased in PSU as a group, the combination of cigarette smoking and polysubstance use is strongly related to hypoperfusion in important cortical and subcortical regions. As lower perfusion relates to greater smoking severity, worse cognition and higher impulsivity, smoking cessation is warranted for treatment-seeking PSU and ALC.
Keywords: MRI, Perfusion, Blood Flow, Polysubstance, Alcohol, Smoking
1. INTRODUCTION
Arterial spin labeling magnetic resonance imaging, a non-invasive measurement of regional cerebral blood flow, provides functional information which is tightly coupled with glucose metabolism (Chen et al., 2011; Jueptner and Weiller, 1995). Regional cerebral blood flow (aka, perfusion) and glucose metabolism are intimately linked to brain function (Raichle et al., 1976).
Perfusion is altered in individuals with stimulant dependence and also in those with alcohol dependence. For instance, in cocaine dependence, frontal and temporal brain regions show relatively low perfusion (see review by Nnadi et al., 2005). In alcohol dependent individuals (ALC) at 1–8 weeks of abstinence from alcohol, reduced perfusion is observed in frontal and parietal regions and does not recover with abstinence (Durazzo et al., 2010; Gazdzinski et al., 2006; Mon et al., 2009; Sullivan et al., 2013; see review by Moselhy et al. (2001)). Similarly, glucose metabolism is decreased in frontal regions of ALC and cocaine dependent individuals (Adams et al., 1993; Dao-Castellana et al., 1998; Volkow et al., 1992a, 1992b, 1994; Wang et al., 1993). In alcoholism, both chronic alcohol and tobacco use are associated with altered perfusion: higher alcohol consumption is associated with lower frontal and parietal perfusion (Kuruoglu et al., 1996; Melgaard et al., 1990; Nicolas et al., 1993), and greater cigarette smoking severity is associated with lower frontal and/or parietal perfusion in smoking ALC (sALC) (Gazdzinski et al., 2006; Mon et al., 2009), but see (Sullivan et al., 2013). These reductions in regional blood flow and glucose metabolism are of functional importance as they are associated with relapse to drinking within the first year after treatment (Durazzo et al., 2010a) and poorer neurocognition in cocaine dependence (Browndyke et al., 2004; Goldstein et al., 2004, 2001; review by Hanlon et al., 2013) and in alcohol dependence (Adams et al., 1993; Dao-Castellana et al., 1998; Goldstein et al., 2004; Melgaard et al., 1990; Nicolas et al., 1993; Wang et al., 1993), although with some contradictions (Demir et al., 2002; Gazdzinski et al., 2006; Mon et al., 2009). Furthermore, research has been conducted on measures of self-regulation/inhibitory control (e.g., impulsivity, risk taking, decision making) in alcohol dependence (Fein et al., 2004; Gonzalez et al., 2007; Noel et al., 2007; Tomassini et al., 2012), cocaine dependence (LoBue et al., 2014; Moeller et al., 2001), and in polysubstance dependent alcoholics (De Wilde et al. 2013a,b). However, the relationships of these measures, highly relevant to sustained abstinence and relapse, with regional cerebral perfusion have generally not been studied in substance users.
Furthermore, most substance users today are polysubstance users (PSU), with diagnoses of concurrent dependence on or abuse of more than one substance including alcohol. PSU are currently the largest group needing treatment in the United States (SAMHSA, 2011). However, brain perfusion research in PSU is sparse. Only two reports compared a PSU group (cocaine + alcohol) and a group dependent on cocaine only to controls. Both showed lower frontal and parietal perfusion in PSU abstinent from substances for a few days compared to the cocaine only group, with frontal perfusion deficits recovering after 21 days of abstinence (Gottschalk and Kosten, 2002; Kosten et al., 1998). However, these studies did not report on measures of neurocognition or self-regulation known to be impacted in PSU (De Wilde et al., 2013a,b).
Currently, it is unclear if ALC and PSU differ on magnitude and pattern of regional perfusion – concurrent multiple substance use may impact cerebral blood flow stronger than monosubstance use – and if the relationships of regional perfusion with cognition and self-regulation vary between these groups. Discerning such group differences could inform better treatments targeted at the requirements of different substance dependent populations. Here, we compared primary alcohol dependent individuals (i.e., ALC) to those with alcohol + stimulant dependence (i.e., PSU) and also compared these treatment-seeking groups to light drinking healthy controls (LD). We measured cortical and subcortical perfusion, with focus on regions which are associated with the development and maintenance of addictive disorders, largely localized to specific regions in frontal and mesial temporal lobes, limbic system, and striatum (e.g., Haber and Knutson, 2010; Makris et al., 2008). In this report, these regions are collectively referred to as the brain reward/executive oversight system (BREOS; Durazzo et al., 2012a, 2014b), also termed the extended brain reward system (Durazzo et al., 2010b, 2011). Substance use disorders are associated with long-lasting plastic changes in neuronal and glial tissue of BREOS regions subserving ‘top-down’ inhibitory control/executive functions (Volkow et al., 2011). Compromised integrity of top-down regulatory BREOS regions is related to dysregulation of striatum and limbic regions involved in reward and motivation (George and Koob, 2010) and also to dysfunction in traditional neurocognitive abilities involving executive functions, working memory, processing speed, and visuospatial skills (Gazzaley and D’Esposito, 2007). We hypothesized that: (1) ALC and PSU at 1 month of abstinence have greater perfusion deficits in the BREOS than LD, and that alcohol dependent PSU have greater regional perfusion deficits than ALC after controlling for potentially different alcohol consumption, (2) in both ALC and PSU, greater drinking and smoking severities are associated with lower perfusion, and (3) regional hypoperfusion is associated with poorer neuropsychological performance. In additional analyses, we examined the effects of smoking status on regional perfusion and its dependence on age.
2. MATERIALS AND METHODS
2.1. Participants
All participants provided written informed consent for procedures approved by University of California, San Francisco and San Francisco VA Medical Center. Twenty-six ALC and 20 PSU treatment-seekers were recruited from VA and Kaiser Permanente substance abuse treatment centers. ALC and PSU met DSM-IV criteria for alcohol dependence, and PSU met additional criteria for dependence on at least one psychostimulant (cocaine 100%, methamphetamine 40%) and marijuana use disorder (60%). ALC and PSU were 1 month abstinent from all substances except nicotine, with no group difference in duration of abstinence. Inclusion and exclusion criteria for all groups are described elsewhere (Abe et al., 2012; Durazzo et al., 2007). Briefly, exclusions were neurological and psychiatric disorders which affect neurobiology or neurocognition, but not hepatitis C, type-2 diabetes, hypertension, and unipolar mood disorder, highly prevalent in addiction (Hasin et al., 2007; Stinson et al., 2005). Thirty-one LD (25 nonsmokers, 6 smokers) recruited from the community served as controls. Smokers were allowed to smoke ad libitum before each assessment and during breaks.
2.2. Substance Use and Neurocognitive Assessment
The clinical and neurocognitive assessments are detailed elsewhere (Durazzo et al., 2007). Briefly, ALC and PSU completed the Structured Clinical Interview for DSM-IV Axis I disorders Patient Edition, v2.0 (SCID-I/P; First et al., 1998); LD were administered the screening module. All participants completed the Beck Depression Inventory (BDI; Beck, 1978). Alcohol consumption was estimated with the lifetime drinking history interview (Skinner and Sheu, 1982; Sobell et al., 1988). Nicotine dependence was assessed with the Fagerstrom Tolerance Test for Nicotine Dependence (Fagerstrom et al., 1991). For PSU, lifetime substance use history (other than alcohol) was assessed with an in-house interview (Abe et al., 2012; Mon et al., 2014). In PSU, the average monthly use of cocaine (n=20) and methamphetamine (n=8) noted in parenthesis was 80 ± 123 (24 ± 34) g in the previous year, 68 ± 108 (16 ± 16) g over lifetime, with an average duration of 25 ± 8 (19 ± 5) years. A standard neurocognitive battery was administered and cognitive domains were calculated (see Durazzo et al., 2006, 2012b). The Barratt Impulsivity Scale (BIS-11; Patton et al., 1995) assessed self-reported impulsivity and task-based tests assessed risk taking (Balloon Analogue Risk Task, BART; Lejuez et al., 2002) and decision making (Iowa Gambling Task, IGT; Bechara et al., 1994).
2.3. MR Data Acquisition and Processing
MR imaging was performed on a 4T Bruker MedSpec system with a Siemens Trio console (Siemens, Erlangen, Germany) and an 8-channel transmit-receive head coil. The 3D sagittal T1-weighted sequence (1x1x1 mm3) used a magnetization prepared rapid gradient echo acquisition and the 2D axial T2-weighted sequence (0.9x0.9x3 mm3) a turbo-spin echo. Perfusion-weighted MRIs used a continuous arterial spin labeling (ASL) single-shot echo-planar imaging sequence (Detre et al., 1992), producing sixteen oblique-axial 5-mm-thick slices oriented along the orbital-meatal line (in-plane resolution=5x3.8 mm2, 1.45 mm slice gap, TR/TE=5200/9 ms repetition/echo time, 1590 ms post-labeling delay, 90° flip angle).
All participants were instructed to remain awake with eyes closed during the 7 minute whole-brain ASL perfusion sequence. Structural MRI data were aligned with perfusion data using a fluid-flow warping based distortion correction algorithm, and corrections for partial volume effects controlled for brain atrophy; method details are described elsewhere (Tosun et al., 2010). Regional cerebral blood flow images were corrected for partial volume effects and co-aligned with FreeSurfer v5.1 parcelated labels (Fischl et al., 2002, 2004) to yield subject-specific perfusion averages in pre-defined regions. The inferior 2–3 slices from all ASL datasets were removed due to distortions in inferior slices. FreeSurfer regions with at least 50% gray matter tissue were included in statistical analyses. The perfusion regions were voxel-weighted and combined into distinct regions of interest (ROIs: BREOS, Cortical, Subcortical) and subregions1. The BREOS ROI in this report was comprised of the anterior cingulate cortex (ACC), dorsal prefrontal cortex (dorsal PFC), inferior frontal gyrus (IFG), insula, and orbitofrontal cortex (OFC), while the Cortical ROI was the sum of all cortical regions including BREOS.
2.4. Statistical Analyses
We compared perfusion in ROIs and subregions across 26 ALC, 20 PSU, and 31 LD. Multivariate analyses of variance (MANOVA) assessed for demographic and other descriptive group differences between the 3 groups. Separate analyses of covariance (ANCOVA) examined: (i) perfusion in ROIs and subregions followed by pairwise comparisons testing regional perfusion differences among groups, (ii) main effects and interactions of smoking status (combined ALC+PSU) on perfusion, and (iii) neuropsychological performance between groups. Linear regression examined the associations of perfusion with cognition, impulsivity, and decision making measures. Covariates included age, years of education, body mass index (BMI), American National Adult Reading Test (AMNART) score and drinking variables (substance dependent groups only) since these variables can influence MR and cognitive outcome measures. A modified Bonferroni procedure (Sankoh et al., 1997) adjusted the alpha level (.05) to account for multiplicity by using the number of subregions and their average inter-correlation coefficients; adjusted p-values were: BREOS p=.041, Cortical ROI p=.026, Subcortical ROI p=.028, and composite of ROIs p=.045. Effect sizes for mean differences between groups were calculated with Cohen’s d (Cohen, 1988). Partial correlational analyses used Pearson product moment. All statistical analyses employed SPSS v21.
3. RESULTS
3.1. Demographics
PSU were of similar age than LD, but ALC were older than both PSU and LD. Both ALC and PSU had fewer years of education than LD, with no difference between ALC and PSU (Table 1). ALC and PSU were not different on any drinking severity measure, but PSU began drinking heavily 8 years earlier than ALC. When separated by smoking status (Table 2), sALC consumed more alcoholic drinks per month than nsALC; however, sPSU and nsPSU did not differ on these monthly drinking averages. PSU and ALC had a similar level of nicotine dependence (Fagerstrom Total), but sALC had more lifetime years of smoking and pack-years than sPSU. Nine ALC and 2 PSU had medically controlled hypertension, but perfusion did not significantly differ between those with and without hypertension. Both ALC and PSU reported higher depressive symptoms (BDI) than LD (both p<.03), but BDI did not significantly correlate with regional perfusion, cognition, impulsivity, or risk taking measures.
Table 1.
Participant Demographics
| Variable | ALC | PSU | LD |
|---|---|---|---|
| N (female) | 26 (6) $ | 20 (1) $ | 31 (5) $ |
| Age (years) | 54 ± 10 * | 45 ± 9 ** | 47 ± 11 |
| Education (years) | 14 ± 2 * | 13 ± 1 + | 16 ± 2 |
|
American National Adult Reading Test (AMNART) |
116 ± 8 | 108 ± 8 ** + | 120 ± 6 |
|
Onset Age of Heavy Drinking |
27 ± 12 | 19 ± 4 ** | - |
|
Body Mass Index (BMI) |
25 ± 5 | 28 ± 4 + | 25 ± 3 |
|
Beck Depression Inventory (BDI) |
14 ± 9 * | 10 ± 7 + | 4 ± 4 |
|
Time since last cigarette before MRI (hours) |
1 ± 1 | 1 ± 1 | - |
p<.01 vs. LD;
p<.008 vs. ALC,
p<.03 vs. LD,
p>.05 Chi-square test with Yates correction
ALC Individuals dependent on alcohol only
PSU Alcohol dependent individuals with comorbid stimulant dependence
LD Light/non-drinking controls
Table 2.
Drinking & Smoking Severities in ALC and PSU
| p-value | p-value | ||||
|---|---|---|---|---|---|
| Variable | sALC [nsALC] | sALC vs. nsALC |
sPSU [nsPSU] | sPSU vs. nsPSU |
sALC vs. sPSU |
|
N smoker [nonsmoker] |
13 [13] | - | 13 [7] | - | ns |
| Fagerstrom Total | 4 ± 2 | - | 5 ± 1 | - | ns |
| Cigarette pack-years | 28 ± 17 | - | 9 ± 11 | - | 0.002 |
| Cigarettes per day | 17 ± 7 | - | 12 ± 7 | - | ns |
|
Lifetime Smoking (years) |
31 ± 10 | - | 18 ± 10 | - | 0.005 |
|
Fagerstrom Current Level (years) |
21 ± 14 | - | 15 ± 13 | - | ns |
|
Abstinence Duration (days) |
36 ± 4 [31 ± 11] | ns | 27 ± 10 [33 ± 8] | ns | 0.007 |
|
1 Yr Avg (drinks/month) |
472 ± 219 [222 ± 134] | 0.002 | 226 ± 242 [347 ± 175] | ns | 0.01 |
|
3 Yr Avg (drinks/month) |
448 ± 212 [231 ± 151] | 0.008 | 244 ± 263 [371 ± 308] | ns | 0.04 |
|
8 Yr Avg (drinks/month) |
384 ± 173 [227 ± 150] | 0.02 | 217 ± 186 [321 ± 319] | ns | 0.02 |
|
Total Lifetime Avg (drinks/month) |
257 ± 106 [172 ± 93] | 0.04 | 198 ± 122 [261 ± 223] | ns | ns |
|
Lifetime Drinking (years) |
34 ± 8 [38 ± 16] | ns | 26 ± 12 [33 ± 8] | ns | ns |
|
Total Alcohol Consumed (lifetime_kg) |
1047 ± 572 [1098 ± 614] | ns | 938 ± 684 [1553 ± 1599] | ns | ns |
|
Onset Age of Heavy Drinking |
25 ± 7 [30 ± 15] | ns | 19 ± 5 [19 ± 3] | ns | 0.02 |
|
Duration of Heavy Drinking (years) |
23 ± 6 [25 ± 12] | ns | 17 ± 10 [26 ± 10] | ns | ns |
sALC Smoking individuals dependent on alcohol only
nsALC Non-smoking individuals dependent on alcohol only
sPSU Smoking alcohol dependent individuals with comorbid stimulant dependence
sPSU Non-smoking alcohol dependent individuals with comorbid stimulant dependence
3.2. Regional perfusion by group (PSU, ALC, LD)
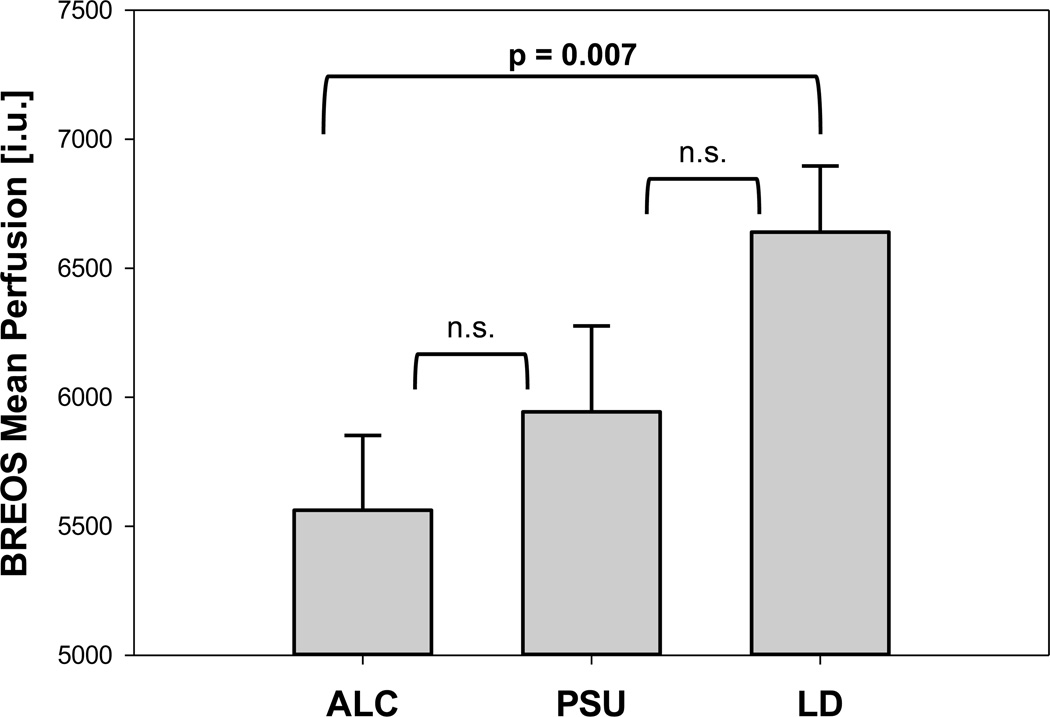
After correction for multiple comparisons, all significant perfusion differences were observed between ALC and LD only. ALC had lower perfusion than LD in the BREOS [F(1,52)=7.94, p<.008] (Fig. 1), Cortical [F(1,47)=9.46, p<.005], and Subcortical ROIs [F(1,51)=7.98, p<.008]. Table 3 lists ROI and subregion perfusion by group2. There was no regional hyperperfusion in PSU or ALC compared with LD. PSU and ALC did not differ significantly on perfusion in any region; however, a distinct pattern of perfusion deficits emerged for ALC and PSU in striatum (4559 ± 1375 and 4761 ± 1407, respectively) and subcortical regions: Whereas ALC had the lowest perfusion in thalamus and caudate, PSU had the lowest perfusion in putamen.
Fig. 1.
Mean BREOS Perfusion by group (estimated mean ± standard error from a group model with BMI as a covariate).
BREOS Brain Reward/Executive Oversight System
ALC Individuals dependent on alcohol only
PSU Alcohol dependent individuals with comorbid stimulant dependence
LD Light/non-drinking controls
Table 3.
Perfusion by group and region (estimated means from a group model comparing perfusion in ALC, PSU, and LD)
| Mean ± Standard Deviation | Effect Size (Cohen’s d) | ||||||
|---|---|---|---|---|---|---|---|
| ROIs & Subregions | ALC | PSU | LD | LD vs. ALC | LD vs. PSU | PSU vs. ALC | |
| BREOS | 5562 ± 1421 | 5943 ± 1455 | 6640 ± 1430 | 0.76 * | 0.48 | 0.27 | |
| Cortical | 5743 ± 1497 | 6130 ± 1535 | 7049 ± 1507 | 0.87 * | 0.61 | 0.26 | |
| Subcortical | 4061 ± 1258 | 4293 ± 1287 | 5017 ± 1269 | 0.76 * | 0.57 * | 0.18 | |
| Cortical subregions | |||||||
| ACC | 5538 ± 1789 | 5975 ± 1828 | 6604 ± 1795 | 0.59 * | 0.35 | 0.24 | |
| Dorsal PFC | 5446 ± 1343 | 6222 ± 1369 | 6464 ± 1353 | 0.75 * | 0.18 | 0.57 | |
| IFG | 5597 ± 1682 | 6045 ± 1716 | 6657 ± 1697 | 0.63 * | 0.36 | 0.26 | |
| OFC | 5546 ± 1574 | 5542 ± 1609 | 6432 ± 1590 | 0.56 * | 0.56 | 0.00 | |
| Pars Opercularis | 5890 ± 1894 | 6381 ± 1938 | 7159 ± 1905 | 0.67 * | 0.41 | 0.26 | |
| Insula | 5591 ± 1728 | 6025 ± 1773 | 6899 ± 1727 | 0.76 * | 0.50 | 0.25 | |
| Hippocampus | 5468 ± 1935 | 4939 ± 1992 | 6514 ± 1945 | 0.54 | 0.80 * | −0.27 # | |
| Pre-Central | 5840 ± 1526 | 6492 ± 1562 | 6923 ± 1535 | 0.71 * | 0.28 | 0.42 | |
| Superior Temporal | 6175 ± 1775 | 6726 ± 1812 | 7326 ± 1786 | 0.65 * | 0.33 | 0.31 | |
| Middle Temporal | 7345 ± 1919 | 7113 ± 1965 | 7909 ± 1921 | 0.29 | 0.41 | −0.12 | |
| Paracentral | 5243 ± 1628 | 5781 ± 1670 | 6652 ± 1638 | 0.86 * | 0.53 | 0.33 | |
| Post-Central | 6081 ± 1439 | 6514 ± 1474 | 7085 ± 1445 | 0.70 * | 0.39 | 0.30 | |
| Inferior Parietal lobule | 6067 ± 1823 | 6469 ± 1866 | 7752 ± 1827 | 0.92 * | 0.70 * | 0.22 | |
| Posterior Cingulate | 6784 ± 2352 | 7168 ± 2407 | 8645 ± 2367 | 0.79 * | 0.62 $ | 0.16 | |
| Isthmus Cingulate | 7300 ± 2358 | 7978 ± 2414 | 8641 ± 2370 | 0.57 * | 0.28 | 0.28 | |
| Precuneus | 6335 ± 1916 | 6915 ± 1960 | 7563 ± 1930 | 0.64 * | 0.33 | 0.30 | |
| Cuneus | 7148 ± 2090 | 7168 ± 2141 | 7880 ± 2093 | 0.35 | 0.34 | 0.01 | |
| Lateral Occipital | 6080 ± 2044 | 6417 ± 2095 | 6509 ± 2039 | 0.21 | 0.04 | 0.16 | |
| Lingual | 7152 ± 2313 | 7414 ± 2373 | 7841 ± 2256 | 0.30 | 0.18 | 0.11 | |
| Subcortical subregions | |||||||
| Accumbens Area | 5398 ± 1368 | 4989 ± 1552 | 5398 ± 1529 | 0.00 | 0.28 | −0.28 # | |
| Caudate | 3843 ± 1363 | 4338 ± 1393 | 5217 ± 1379 | 1.00 * | 0.64 | 0.36 | |
| Putamen | 5069 ± 1562 | 4959 ± 1597 | 6044 ± 1576 | 0.62 * | 0.69 * | −0.07 | |
| Pallidum | 677 ± 358 | 733 ± 366 | 775 ± 361 | 0.27 | 0.12 | 0.16 | |
| Thalamus | 3762 ± 1304 | 4205 ± 1300 | 4901 ± 1260 | 0.89 * | 0.53 | 0.34 | |
significant after correction for multiple comparisons (see Methods)
Pairwise comparisons p-value:
p<.04
PSU<ALC (p = n.s.)
ALC Individuals dependent on alcohol only
PSU Alcohol dependent individuals with comorbid stimulant dependence
LD Light/non-drinking controls
BREOS Brain Reward/Executive Oversight System
ACC Anterior Cingulate Cortex
Dorsal PFC Dorsal Prefrontal Cortex
IFG Inferior Frontal Gyrus
OFC Orbitofrontal Cortex
3.3. Effects of substance and tobacco use severity on regional perfusion
3.3.1. Drinking severity
Drinking severity did not significantly correlate with regional perfusion in either the ALC or PSU group. However, when separated by smoking status, perfusion was negatively related to drinking severity in smokers only. In sALC, greater drinking severity correlated with lower perfusion in BREOS (r>−.65, p<.03) and its subregions (dorsal PFC, OFC, and IFG), and in paracentral and post-central subregions (all r>−.71, p<.003). Similarly, in sPSU, drinking severity was negatively associated with perfusion in BREOS and its subregions (ACC, IFG), in the paracentral region, accumbens area, and hippocampus (all r>−.63, p<.014), as well as in the middle temporal region, and cuneus (all r<−.45, p<.028). Although PSU began drinking heavily at an earlier age than ALC, this was not associated with greater regional perfusion deficits in PSU.
3.3.2. Cocaine consumption severity
In PSU, longer duration of cocaine use and quantity in the previous year and over lifetime (grams/month) did not significantly correlate with lower perfusion in any of the three ROIs (r = −.22 to −.41, p > .08).
3.3.3. Smoking severity
In sALC, more pack-years and lifetime years smoking correlated significantly with lower perfusion in most subregions; in sPSU it did not. In sALC, the strongest dose-response relationships were with perfusion in posterior cingulate, precuneus, lingual, middle and superior temporal regions, cuneus, insula, caudate, and putamen (all r>−.61, p<.005).
3.4. Effects of smoking status on regional perfusion
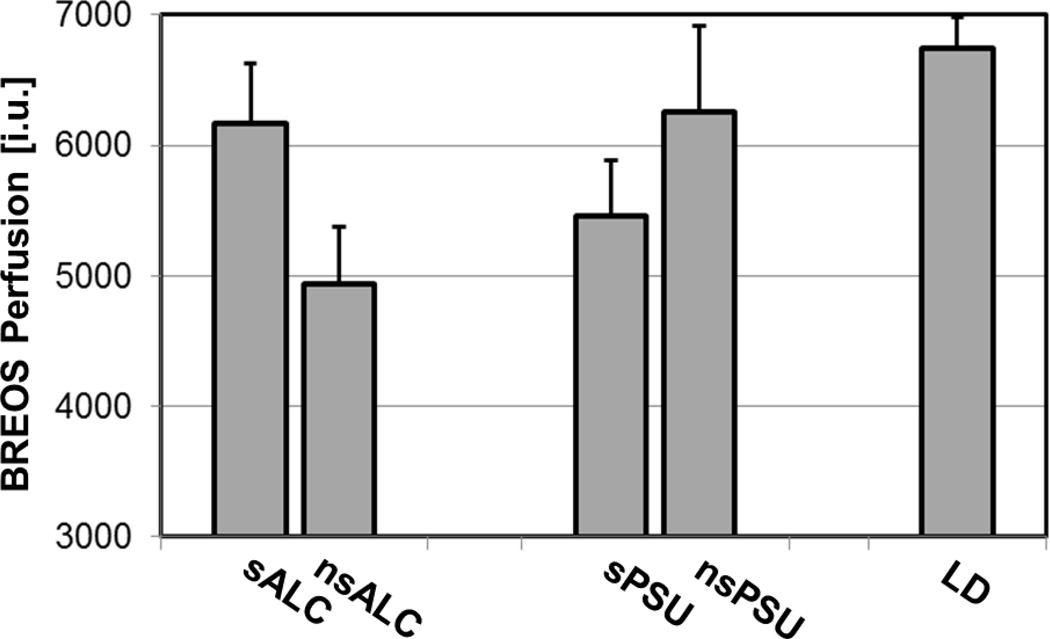
ANCOVAs yielded no main effects for smoking status or interactions between smoking status and substance group (i.e., ALC and PSU) in the BREOS, the Cortical ROI, or their respective subregions. There was a trend to a smoking status x group interaction for the Subcortical ROI [F(1, 34)=3.79, p<.061]: sALC and sPSU had higher perfusion than their nonsmoking counterparts. There were no main effects for smoking status or substance group on perfusion in any ROI or subregion; however, sPSU had lower perfusion than sALC in Cortical and Subcortical ROIs and in BREOS regions (see Fig. 2) such as ACC, OFC, insula, hippocampus, cuneus, pars opercularis, accumbens, and putamen (all p <.05). Of note was a lack of significant perfusion differences between nsALC and nsPSU in any region. Furthermore, despite the absence of main effects for smoking status or age in the combined ALC+PSU group, smoking status x age interactions were observed for the inferior parietal lobule and isthmus cingulate (both p<.04); this indicates a steeper decline in parietal perfusion with increasing age in smoking substance users than in their nonsmoking counterparts.
Fig. 2.
Mean BREOS Perfusion by smoking status (estimated mean ± standard error from a group model with the 4 substance using groups; covariate were 3-year monthly drinking average and body mass index). LD group mean shown only for comparison.
BREOS Brain Reward/Executive Oversight System
sALC Smoking individuals dependent on alcohol only
nsALC Non-smoking individuals dependent on alcohol only
sPSU Smoking alcohol dependent individuals with comorbid stimulant dependence
sPSU Non-smoking alcohol dependent individuals with comorbid stimulant dependence
LD Light/non-drinking controls
3.5. Neuropsychological performance by group (PSU, ALC, LD)
All significant group differences for measures of neurocognition, BIS, BART, and IGT were observed between ALC and LD; PSU had an intermediate performance and did not differ significantly from either group (see Table 4). For example, global cognition was significantly lower in ALC vs. LD (ES=0.98), was not significantly reduced in PSU (ES=0.64), and not different between PSU and ALC (ES=0.32). The performance of PSU vs. ALC was not explained by group differences in drinking or smoking measures.
Table 4.
Cognition, Impulsivity, and Risk Taking by group (estimated means from a group model comparing cognition/impulsivity, risk taking and decision making measures in ALC, PSU, and LD)
| Mean ± Standard Deviation | Effect Size (Cohen’s d) | |||||
|---|---|---|---|---|---|---|
| Cognitive Domains | ALC | PSU | LD |
LD vs. ALC |
LD vs. PSU |
PSU vs. ALC |
| Global Cognition (averaged z-scores of all 11 domains) |
46 ± 6 | 48 ± 6 | 52 ± 6 | 0.98 * | 0.64 | 0.32 |
| Auditory-Verbal Learning | 47 ± 10 | 51 ± 11 | 58 ± 10 | 1.10 * | 0.66 | 0.38 |
| Auditory-Verbal Memory | 45 ± 9 | 49 ± 10 | 55 ± 10 | 1.10 * | 0.65 | 0.41 |
| Executive Functioning | 48 ± 7 | 50 ± 8 | 52 ± 8 | 0.52 | 0.21 | 0.29 |
| Processing Speed | 49 ± 6 | 50 ± 7 | 51 ± 7 | 0.29 | 0.18 | 0.11 |
| Cognitive Efficiency | 48 ± 6 | 50 ± 6 | 51 ± 6 | 0.57 | 0.11 | 0.45 |
| Fine Motor | 46 ± 11 | 44 ± 12 | 47 ± 11 | 0.10 | 0.30 | 0.20 |
| Intelligence | 51 ± 6 | 52 ± 7 | 57 ± 6 | 0.97 * | 0.81 | 0.13 |
| Visuospatial Learning | 40 ± 10 | 40 ± 12 | 44 ± 11 | 0.37 | 0.39 | 0.03 |
| Visuospatial Memory | 41 ± 12 | 41 ± 13 | 46 ± 13 | 0.44 | 0.43 | 0.01 |
| Visuospatial Skills | 46 ± 9 | 51 ± 10 | 52 ± 9 | 0.68 * | 0.14 | 0.51 |
| Working Memory | 49 ± 6 | 50 ± 7 | 53 ± 6 | 0.67 * | 0.58 | 0.08 |
| Impulsivity, Risk Taking & Decision Making | ||||||
| Barratt Impulsivity Scale (BIS-11) Total | 66 ± 11 | 65 ± 12 | 58 ± 12 | 0.77 $ | 0.69 | 0.08 |
| Attentional-Impulsivity | 17 ± 4 | 16 ± 4 | 14 ± 4 | 0.70 * | 0.36 | 0.34 |
| Motor-Impulsivity | 23 ± 4 | 23 ± 4 | 21 ± 5 | 0.37 | 0.37 | 0.01 |
| Nonplanning-Impulsivity | 26 ± 5 | 27 ± 5 | 22 ± 5 | 0.83 $ | 0.94 $ | 0.10 |
| Balloon Analogue Risk Task (BART) | ||||||
| Adjusted Avg Pumps | 31 ± 13 | 32 ± 13 | 25 ± 14 | 0.41 | 0.49 | 0.07 |
| Iowa Gambling Task (IGT) Net Total | 11 ± 28 | 6 ± 30 | 24 ± 31 | 0.42 | 0.62 | 0.19 |
Pairwise comparisons p-value:
p <.05
p <.09
ALC Individuals dependent on alcohol only
PSU Alcohol dependent individuals with comorbid stimulant dependence
LD Light/non-drinking controls
3.5.1. Correlations of perfusion with cognitive and behavioral measures
3.5.1.1. Cognition
Regional perfusion was correlated with cognitive performance in both PSU and ALC, but distinctly different perfusion-cognition patterns emerged between groups (see Table 5). In PSU, the correlations were not affected by smoking status and lower regional perfusion was related to worse cognitive performance. In ALC correlations between perfusion and cognitive domains were different between smokers and nonsmokers. In sALC, higher frontal perfusion correlated with worse cognitive performance, whereas in nsALC, lower perfusion correlated with worse cognitive performance.
Table 5.
BMI-corrected partial correlations (r*) between regional perfusion and cognitive domain measures in PSU and ALC (ALC separated by smoking status).
| Group | Perfusion Subregion | Cognitive Domain | r* | p | ||
|---|---|---|---|---|---|---|
| PSU | Insula | Visuospatial Memory | 0.44 | 0.020 | ||
| Inferior Parietal Lobule | 0.61 | 0.007 | ||||
| Thalamus | 0.57 | 0.007 | ||||
| Insula | Visuospatial Skills | 0.55 | 0.012 | |||
| Pars Opercularis | 0.54 | 0.014 | ||||
| Precuneus | 0.53 | 0.018 | ||||
| Putamen | 0.52 | 0.021 | ||||
| sALC ^ | Paracentral | Visuospatial Skills | −0.74 | 0.003 | ||
| Auditory-Verbal Memory | −0.61 | 0.035 | ||||
| Executive Functioning | −0.62 | 0.029 | ||||
| Inferior Frontal Gyrus (IFG) | Visuospatial Skills | −0.68 | 0.049 | |||
| OFC | Auditory-Verbal Memory | −0.63 | 0.029 | |||
| nsA LC | Hippocampus | Auditory-Verbal Memory | 0.67 | 0.015 | ||
| Inferior Parietal Lobule | 0.64 | 0.012 |
BMI- and age-corrected
BMI Body Mass Index
PSU Alcohol dependent individuals with comorbid stimulant dependence
sALC Smoking individuals dependent on alcohol only
nsALC Non-smoking individuals dependent on alcohol only
3.5.1.2. Impulsivity
BIS scores correlated negatively with perfusion in both ALC and PSU. Correlations were strong in all ROIs of ALC and only moderate in all ROIs of PSU. In ALC, higher attention-impulsivity correlated with lower perfusion in the BREOS, Subcortical ROI, and subregions (ACC, pars opercularis, paracentral, precentral, insula, posterior cingulate, striatum, caudate, and putamen) (all r>−.52, p<.03). In PSU, higher motor-impulsivity related to lower BREOS perfusion (r>−.51, p<.021). Higher nonplanning-impulsivity correlated with lower perfusion in all ROIs of PSU (all r>−.46, p<.032) and with lower perfusion in ACC, OFC, pars opercularis, and accumbens of ALC (all r>−.50, p<.046).
3.5.1.3. Risk Taking and Decision Making
In ALC, lower perfusion in Cortical ROI and IFG were associated with higher Adjusted Average Pumps on the BART (more risk taking) (both r>−.70, p<.009); no such correlations were observed in PSU. Lower pallidum perfusion in PSU correlated with lower IGT scores (worse decision making) (r>.66, p<.005). In contrast, in ALC, lower perfusion in Cortical and Subcortical ROIs and subregions (IFG, thalamus) correlated with higher IGT (all r>−.55, p<.025), independent of differences in drinking severity between sALC and nsALC.
4. DISCUSSION
Our main analyses indicate that (i) regional perfusion in PSU was not significantly lower than in ALC and perfusion was significant lower only in ALC compared with light/non-drinking controls, (ii) greater drinking severity correlated with lower regional perfusion in both sALC and sPSU, (iii) greater smoking severity correlated with lower perfusion in ALC only, (iv) lower perfusion had cognitive ramifications in both groups, but cognition related to regional perfusion differently in PSU and ALC; smoking status affected these relationships differently in PSU and ALC, and (v) lower regional perfusion related to higher impulsivity albeit to different subscales in both ALC and PSU. We found no significant main effects of smoking on regional perfusion. When groups were classified by smoking status; however, sPSU had lower perfusion than sALC in brain regions critical to inhibitory control (ACC, OFC, insula), reward and conditioning (accumbens area, hippocampus, putamen). Finally, smoking substance users showed a steeper decline in parietal perfusion with increasing age than nonsmoking substance users.
Consistent with previous reports, ALC had lower perfusion than LD in the Cortical and Subcortical ROIs including the BREOS, a neural network critical to the development and maintenance of addiction (Durazzo et al., 2010a; Gazdzinski et al., 2006; Mon et al., 2009; Sullivan et al., 2013; Volkow et al., 1992). Contrary to our a priori hypothesis, however, regional perfusion was not lower in PSU as a group than ALC. Furthermore, the regional pattern of subcortical perfusion deficits was distinctly different in ALC (thalamus, caudate) and PSU (putamen); this appears to be a function of smoking status (seen in the trend for a smoking status x substance group interaction) and also of drinking severity (significantly stronger in sALC than nsALC, but similar between sPSU and nsPSU). Since others have not reported on subcortical perfusion or volume differences between ALC and PSU, our results warrant further study. The observed ROI perfusion levels in PSU were in-between those of LD and ALC despite similar drinking severities in ALC (216 drinks/month) and PSU (221 drinks/month) over lifetime, suggesting different effects of abused substances on perfusion or premorbid effects. In an acute cocaine + alcohol administration study, interactions between cocaine and alcohol were shown to heighten the vasoactive effects of cocaine (McCance-Katz et al., 1998); such interactions may contribute to the relatively higher perfusion seen chronically in our PSU. Here, relatively high perfusion in PSU may be explained, at least partially, by an unstable vascular system or the occurrence of gliosis in damaged tissue (Kosten et al., 1998; Silverman et al., 1997) as speculated for cocaine dependence (Gottschalk and Kosten, 2002).
Greater drinking severity was related specifically to lower perfusion in BREOS, only observed in smoking substance abusers, and not related to parietal or subcortical perfusion. This dose-response relationship is consistent with the majority of perfusion studies in ALC (Gazdzinski et al., 2006; Kuruoglu et al., 1996; Melgaard et al., 1990; Nicolas et al., 1993) but not all (Mon et al., 2009), and congruent with the apparently greater vulnerability of anterior frontal regions to chronic alcohol consumption. In sALC but not in sPSU, greater smoking severity related to lower perfusion in parietal, temporal and subcortical regions, suggesting susceptibility of select regions to cigarette smoking effects (Durazzo et al., 2014a) and consistent with healthy (elderly) smokers showing greater rates of atrophy in select regions than nonsmokers (Durazzo et al., 2012a).
Previous reports did not evaluate correlations between perfusion and cognition in PSU, although perfusion-cognition associations in cocaine dependence have been documented (reviewed by Hanlon et al., 2013). We found that regional perfusion correlated with distinctly different cognitive domains in ALC and PSU, highlighting the clinical relevance of specific perfusion-cognition patterns in substance dependence. Furthermore, perfusion-cognition relationships differed in sALC and nsALC in a way that suggests that smoking has a detrimental impact on both perfusion and cognition in ALC; this interpretation supports our previous report of worse cognitive performance in sALC than nsALC (Durazzo et al., 2013).
Higher attention-impulsivity in ALC than LD and generally elevated impulsivity in both ALC and PSU compared with LD is consistent with the literature in substance users (see review Stanford et al., 2009 and references therein); to the best of our knowledge, the association between impulsivity and brain perfusion level in PSU has not been explored previously. Lower BREOS perfusion related to ALC’s inability to focus on a task (attention-impulsivity) and to PSU’s tendency to respond fast without thinking (motor-impulsivity), while more widespread cortical and subcortical perfusion deficits in PSU were associated with their lack of foresight (non-planning impulsivity). In addition, poorer decision making performance (lower IGT score) in PSU correlated with lower pallidum perfusion; this is different from “pure” cocaine dependent individuals in whom worse IGT performance related to hypoperfusion in frontal cortex (Adinoff et al., 2003; Bolla et al., 2003; Tucker et al., 2004). By contrast, in ALC, poorer decision making was associated with higher perfusion in Cortical and Subcortical ROIs. Lower perfusion in substance users, which is associated with impulsivity and decision making measures in this study, may be related to lower volumes of frontal gray matter observed in individuals with cocaine, alcohol, and polysubstance abuse versus controls (Fein et al., 2002; O’Neill et al., 2001; Tanabe et al., 2009), since poorer decision making has also been associated with smaller frontal brain volumes in PSU (Tanabe et al., 2009). Therefore, the differences between ALC and PSU in their relationships of impulsivity and decision making to regional perfusion, together with reported correlations between low IGT performance and relapse in PSU (De Wilde et al., 2013b), highlight the importance of considering the concept of self-control when designing/implementing behavioral treatments specific to ALC and to PSU, in addition to treating the perfusion deficits that may potentially underlie these constructs.
Interestingly, sPSU showed lower perfusion than sALC in Cortical and Subcortical ROIs and in multiple BREOS subregions, despite greater pack-years or lifetime years smoking in sALC. This suggests that the combination of smoking and polysubstance use, perhaps exerting aggregate adverse effects on brain vascular integrity, is particularly damaging to cortical and subcortical perfusion, which in turn related to worse visuospatial test performance. It remains to be seen if the potential resolution of perfusion deficits in abstinent PSU also relate to improvements in visuospatial skills, as we observed for recovery of parietal cortex volume in abstinent ALC (Mon et al., 2014).
Limitations of this cross-sectional study were that alcohol and cocaine consumption measures were based on self-report. We could not assess smoking effects in LD (too few smokers) or gender effects across groups (too few women) and as they were conducted in a relatively small cohort that precluded the inclusion of multiple covariates in the model simultaneously. Although we controlled statistically for age differences between groups, the differences in age was a limitation. Further, unbalanced membership in nsPSU vs sPSU may have affected the results of the smoking analyses. We also cannot rule out the possible contributions of premorbid and additional environmental factors to the neurobiological group differences as they were not assessed in this research. Furthermore, the lack of a significant smoking main effect on regional perfusion should not be interpreted to reflect a lack of smoking effects on the brain vasculature/microvasculature, as our subgroup analyses clearly show. Blood flow velocities (Gdovinova 2001; Terborg et al., 2002) or capillary transit times are all important for healthy tissue perfusion (e.g., Østergaard et al., 2013), but they were not measured in this study.
Although 1-month-abstinent PSU as a group did not have greater perfusion deficits than primary ALC in any region investigated, sPSU had lower perfusion than sALC in cortical and subcortical brain regions critical to addictive behavior. This suggests that the combination of smoking and polysubstance use impairs cerebral perfusion in these regions. Significantly reduced frontal perfusion in smoking PSU, combined with (non-significantly) poorer cognition and higher impulsivity, may hamper the ability of PSU to maintain long-term abstinence. Since lower perfusion is cognitively relevant and has been related to relapse in ALC, smoking cessation is highly warranted for treatment-seeking PSU and ALC. Future longitudinal studies of change in recovering substance users may strengthen the case for a direct relationship between perfusion and neuropsychological deficits. Perhaps a dual treatment approach which aims to increase perfusion pharmacologically while employing cognitive-behavioral treatment to enhance self-control, decision making, and coping strategies may prove efficacious in real-world substance abuse treatment. Finally, changes in perfusion, cognition and self-regulation with abstinence, under consideration of the effects of cigarette smoking, should be explored as potential biomarkers of relapse risk and sustained abstinence.
Supplementary Material
Highlights.
-
➢
We compared polysubstance dependent to alcohol dependent individuals on perfusion
-
➢
Perfusion in polysubstance users was between that of the alcohol and control groups
-
➢
Perfusion was significantly lower only in the alcohol group compared with controls
-
➢
Greater drinking and smoking severity was associated with lower perfusion
-
➢
Lower perfusion had cognitive ramifications and related to higher impulsivity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
REFERENCES
- Abe C, Mon A, Durazzo TC, Pennington DL, Schmidt TP, Meyerhoff DJ. Polysubstance and alcohol dependence: Unique abnormalities of magnetic resonance-derived brain metabolite levels. Drug Alcohol Depend. 2012;130:30–37. doi: 10.1016/j.drugalcdep.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams KM, Gilman S, Koeppe RA, Kluin KJ, Brunberg JA, Dede D, Berent S, Kroll PD. Neuropsychological deficits are correlated with frontal hypometabolism in positron emission tomography studies of older alcoholic patients. Alcohol. Clin. Exp. Res. 1993;17:205–210. doi: 10.1111/j.1530-0277.1993.tb00750.x. [DOI] [PubMed] [Google Scholar]
- Adinoff B, Devous MD, Cooper DB, Best SE, Chandler P, Harris T, Cervin CA, Cullum CM. Resting regional cerebral blood flow and gambling task performance in cocaine-dependent subjects and healthy comparison subjects. Am. J. Psychiatry. 2003;160:1892–1894. doi: 10.1176/appi.ajp.160.10.1892. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Beck AT. Depression Inventory. Philadelphia: Center for Cognitive Therapy; 1978. [Google Scholar]
- Bjork JM, Hommer DW, Grant SJ, Danube C. Impulsivity in abstinent alcohol-dependent patients: relation to control subjects and type 1-/type 2-like traits. Alcohol. 2004;34:133–150. doi: 10.1016/j.alcohol.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, London ED, Kiehl KA, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, Funderburk FR, Ernst M. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. Neuroimage. 2003;19:1085–1094. doi: 10.1016/s1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browndyke JN, Tucker KA, Woods SP, Beauvals J, Cohen RA, Gottschalk PC, Kosten TR. Examining the effect of cerebral perfusion abnormality magnitude on cognitive performance in recently abstinent chronic cocaine abusers. J. Neuroimaging. 2004;14:162–169. [PubMed] [Google Scholar]
- Chen Y, Wolk DA, Reddin JS, Korczykowski M, Martinez PM, Musiek ES, Newberg AB, Julin P, Arnold SE, Greenberg JH, Detre JA. Voxel-level comparison of arterial spin-labeled perfusion MRI and FDG-PET in Alzheimer disease. Neurology. 2011;77:1977–1985. doi: 10.1212/WNL.0b013e31823a0ef7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis For The Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Dao-Castellana MH, Samson Y, Legault F, Martinot JL, Aubin HJ, Crouzel C, Feldman L, Barrucand D, Rancurel G, Feline A, Syrota A. Frontal dysfunction in neurologically normal chronic alcoholic subjects: metabolic and neuropsychological findings. Psychol. Med. 1998;28:1039–1048. doi: 10.1017/s0033291798006849. [DOI] [PubMed] [Google Scholar]
- De Wilde B, Bechara A, Sabbe B, Hulstijn W, Dom G. Risky decision-making but not delay discounting improves during inpatient treatment of polysubstance dependent alcoholics. Front. Psychiatry. 2013a;4:91. doi: 10.3389/fpsyt.2013.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wilde B, Verdejo-Garcia A, Sabbe B, Hulstijn W, Dom G. Affective decision-making is predictive of three-month relapse in polysubstance-dependent alcoholics. Eur. Addict. Res. 2013b;19:21–28. doi: 10.1159/000339290. [DOI] [PubMed] [Google Scholar]
- Demir B, Ulug B, Lay Ergun E, Erbas B. Regional cerebral blood flow and neuropsychological functioning in early and late onset alcoholism. Psychiatry Res. 2002;115:115–125. doi: 10.1016/s0925-4927(02)00071-9. [DOI] [PubMed] [Google Scholar]
- Durazzo T, Gazdzinski S, Mon A, Meyerhoff D. Cortical perfusion in alcohol-dependent individuals during short-term abstinence: relationships to resumption of hazardous drinking after treatment. Alcohol. 2010a;44:201–210. doi: 10.1016/j.alcohol.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo T, Insel PS, Weiner MW The Alzheimer’s Disease Neuroimaging Initiative. Greater regional brain atrophy rate in healthy elders with a history of cigarette smoking. Alzheimer’s Dement. 2012a;8:513–519. doi: 10.1016/j.jalz.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Mattsson N, Weiner MW Alzheimer’s Disease Neuroimaging Initiative. Smoking and increased Alzheimer’s disease risk: a review of potential mechanisms. Alzheimer’s Dement. 2014a;10(3) Suppl:S122–S145. doi: 10.1016/j.jalz.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ, Nixon SJ. A comprehensive assessment of neurocognition in middle-aged chronic cigarette smokers. Drug Alcohol Depend. 2012b;122:105–111. doi: 10.1016/j.drugalcdep.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Mon A, Pennington D, Abé C, Gazdzinski S, Meyerhoff DJ. Interactive effects of chronic cigarette smoking and age on brain volumes in controls and alcohol-dependent individuals in early abstinence. Addict. Biol. 2014;19:132–143. doi: 10.1111/j.1369-1600.2012.00492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Pathak V, Gazdzinski S, Mon A, Meyerhoff DJ. Metabolite levels in the brain reward pathway discriminate those who remain abstinent from those who resume hazardous alcohol consumption after treatment for alcohol dependence. J. Stud. Alcohol Drugs. 2010b;71:278–89. doi: 10.15288/jsad.2010.71.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Pennington DL, Schmidt TP, Mon A, Abe C, Meyerhoff DJ. Neurocognition in 1-month-abstinent treatment-seeking alcohol-dependent individuals: interactive effects of age and chronic cigarette smoking. Alcohol. Clin. Exp. Res. 2013;37:1794–1803. doi: 10.1111/acer.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Rothlind JC, Gazdzinski S, Banys P, Meyerhoff DJ. A comparison of neurocognitive function in nonsmoking and chronically smoking short-term abstinent alcoholics. Alcohol. 2006;39:1–11. doi: 10.1016/j.alcohol.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Rothlind JC, Gazdzinski S, Banys P, Meyerhoff DJ. Chronic smoking is associated with differential neurocognitive recovery in abstinent alcoholic patients: a preliminary investigation. Alcohol. Clin. Exp. Res. 2007;31:1114–1127. doi: 10.1111/j.1530-0277.2007.00398.x. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Tosun D, Buckley S, Gazdzinski S, Mon A, Fryer SL, Meyerhoff DJ. Cortical thickness, surface area, and volume of the brain reward system in alcohol dependence: relationships to relapse and extended abstinence. Alcohol. Clin. Exp. Res. 2011;35:1187–1200. doi: 10.1111/j.1530-0277.2011.01452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerstrom KO, Heatherton TF, Kozlowski LT. Nicotine addiction and its assessment. Ear Nose Throat J. 1991;69:763–765. [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Meyerhoff DJ. Prefrontal cortical volume reduction associated with frontal cortex function deficit in 6-week abstinent crack-cocaine dependent men. Drug Alcohol Depend. 2002;68:87–93. doi: 10.1016/s0376-8716(02)00110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Klein L, Finn P. Impairment on a simulated gambling task in long-term abstinent alcoholics. Alcohol. Clin. Exp. Res. 2004;28:1487–1491. doi: 10.1097/01.alc.0000141642.39065.9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders - Patient Edition (SCID-I/P, Version 2.0, 8/98 revision) New York, NY: Biometrics Research Department; 1998. [Google Scholar]
- Gazzaley A, D’Esposito M. Unifying prefrontal cortex function: executive control, neural networks, and top-down modulation. In: Miller BL, editor. The Human Frontal Lobes: Functions and Disorders. New York: The Guilford Press; 2007. pp. 187–206. [Google Scholar]
- Gazdzinski S, Durazzo T, Jahng GH, Ezekiel F, Banys P, Meyerhoff D. Effects of chronic alcohol dependence and chronic cigarette smoking on cerebral perfusion: a preliminary magnetic resonance study. Alcohol. Clin. Exp. Res. 2006;30:947–958. doi: 10.1111/j.1530-0277.2006.00108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gdovinova Z. Blood flow velocity in the middle cerebral artery in heavy alcohol drinkers. Alcohol Alcohol. 2001;36:346–348. doi: 10.1093/alcalc/36.4.346. [DOI] [PubMed] [Google Scholar]
- George O, Koob GF. Individual differences in prefrontal cortex function and the transition from drug use to drug dependence. Neurosci. Biobehav. Rev. 2010;35:232–247. doi: 10.1016/j.neubiorev.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Leskovjan AC, Hoff AL, Hitzemann R, Bashan F, Khalsa SS, Wang GJ, Fowler JS, Volkow ND. Severity of neuropsychological impairment in cocaine and alcohol addiction: association with metabolism in the prefrontal cortex. Neuropsychologia. 2004;42:1447–1458. doi: 10.1016/j.neuropsychologia.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND, Wang GJ, Fowler JS, Rajaram S. Addiction changes orbitofrontal gyrus function: involvement in response inhibition. Neuroreport. 2001;12:2595–2599. doi: 10.1097/00001756-200108080-00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R, Bechara A, Martin EM. Executive functions among individuals with methamphetamine or alcohol as drugs of choice: preliminary observations. J. Clin. Exp. Neuropsychol. 2007;29:155–159. doi: 10.1080/13803390600582446. [DOI] [PubMed] [Google Scholar]
- Gottschalk P, Kosten T. Cerebral perfusion defects in combined cocaine and alcohol dependence. Drug Alcohol Depend. 2002;68:95–104. doi: 10.1016/s0376-8716(02)00109-6. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Beveridge TJ, Porrino LJ. Recovering from cocaine: insights from clinical and preclinical investigations. Neurosci. Biobehav. Rev. 2013;37:2037–2046. doi: 10.1016/j.neubiorev.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch. Gen. Psychiatry. 2007;64:830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Jueptner M, Weiller C. Review: does measurement of regional cerebral blood flow reflect synaptic activity? Implications for PET and fMRI. Neuroimage. 1995;2:148–156. doi: 10.1006/nimg.1995.1017. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Cheeves C, Palumbo J, Seibyl JP, Price LH, Woods SW. Regional cerebral blood flow during acute and chronic abstinence from combined cocaine-alcohol abuse. Drug Alcohol Depend. 1998;50:187–195. doi: 10.1016/s0376-8716(98)00038-6. [DOI] [PubMed] [Google Scholar]
- Kuruoglu AC, Arikan Z, Vural G, Karatas M, Arac M, Isik E. Single photon emission computerised tomography in chronic alcoholism: Antisocial personality disorder may be associated with decreased frontal perfusion. Br. J. Psychiatry. 1996;169:348–354. doi: 10.1192/bjp.169.3.348. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, Strong DR, Brown RA. Evaluation of a behavioral measure of risk taking: the Balloon Analogue Risk Task (BART) J. Exp. Psychol. Appl. 2002;8:75–84. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- LoBue C, Cullum CM, Braud J, Walker R, Winhusen T, Suderajan P, Adinoff B. Optimal neurocognitive, personality and behavioral measures for assessing impulsivity in cocaine dependence. Am. J. Drug Alcohol Abuse. 2014;40:455–462. doi: 10.3109/00952990.2014.939752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey S, Paulus M. Are there volumetric brain differences associated with the use of cocaine and amphetamine-type stimulants? Neurosci. Biobehav. Rev. 2013;37:300–316. doi: 10.1016/j.neubiorev.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy DN, Caviness VS, Marinkovic K, Breiter HC, Gasic GP, Harris GJ. Decreased volume of the brain reward system in alcoholism. Biol. Psychiatry. 2008;64:192–202. doi: 10.1016/j.biopsych.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCance-Katz EF, Kosten TR, Jatlow P. Concurrent use of cocaine and alcohol is more potent and potentially more toxic than use of either alone--a multiple-dose study. Biol. Psychiatry. 1998;44:250–259. doi: 10.1016/s0006-3223(97)00426-5. [DOI] [PubMed] [Google Scholar]
- Melgaard B, Henriksen L, Ahlgren P, Danielsen UT, Sorensen H, Paulson OB. Regional cerebral blood flow in chronic alcoholics measured by single photon emission computerized tomography. Acta. Neurol. Scand. 1990;82:87–93. doi: 10.1111/j.1600-0404.1990.tb01594.x. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Dougherty DM, Barratt ES, Schmitz JM, Swann AC, Grabowski J. The impact of impulsivity on cocaine use and retention in treatment. J. Subst. Abuse Treat. 2001;21:193–198. doi: 10.1016/s0740-5472(01)00202-1. [DOI] [PubMed] [Google Scholar]
- Mon A, Durazzo TC, Gazdzinski S, Meyerhoff DJ. The impact of chronic cigarette smoking on recovery from cortical gray matter perfusion deficits in alcohol dependence: longitudinal arterial spin labeling MRI. Alcohol. Clin. Exp. Res. 2009;33:1314–1321. doi: 10.1111/j.1530-0277.2009.00960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mon A, Durazzo TC, Gazdzinski S, Abe C, Pennington DL, Schmidt TP, Meyerhoff DJ. Structural brain differences in alcohol-dependent individuals with and without comorbid substance dependence. Drug Alcohol Depend. 2014;144:170–177. doi: 10.1016/j.drugalcdep.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moselhy HF, Georgiou G, Kahn A. Frontal lobe changes in alcoholism: a review of the literature. Alcohol Alcohol. 2001;36:357–368. doi: 10.1093/alcalc/36.5.357. [DOI] [PubMed] [Google Scholar]
- Nicolas JM, Catafau AM, Estruch R, Lomena FJ, Salamero M, Herranz R, Monforte R, Cardenal C, Urbano-Marquez A. Regional cerebral blood flow-SPECT in chronic alcoholism: relation to neuropsychological testing. J. Nucl. Med. 1993;34:1452–1459. [PubMed] [Google Scholar]
- Nixon SJ, Paul R, Phillips M. Cognitive efficiency in alcoholics and polysubstance abusers. Alcohol. Clin. Exp. Res. 1998;22:1414–1420. doi: 10.1111/j.1530-0277.1998.tb03929.x. [DOI] [PubMed] [Google Scholar]
- Nnadi CU, Mimiko OA, McCurtis HL, Cadet JL. Neuropsychiatric effects of cocaine use disorders. J. Natl. Med. Assoc. 2005;97:1504–1515. [PMC free article] [PubMed] [Google Scholar]
- Noel X, Bechara A, Dan B, Hanak C, Verbanck P. Response inhibition deficit is involved in poor decision making under risk in nonamnesic individuals with alcoholism. Neuropsychology. 2007;21:778–786. doi: 10.1037/0894-4105.21.6.778. [DOI] [PubMed] [Google Scholar]
- O’Neill J, Cardenas VA, Meyerhoff DJ. Separate and interactive effects of cocaine and alcohol dependence on brain structures and metabolites: quantitative MRI and proton MR spectroscopic imaging. Addict. Biol. 2001;6:347–361. doi: 10.1080/13556210020077073. [DOI] [PubMed] [Google Scholar]
- Østergaard L, Jespersen SN, Mouridsen K, Mikkelsen IK, Jonsdottir KY, Tietze A, et al. The role of the cerebral capillaries in acute ischemic stroke: the extended penumbra model. J. Cereb. Blood Flow Metab. 2013;33:635–648. doi: 10.1038/jcbfm.2013.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J. Clin. Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Grubb RL, Jr, Gado MH, Eichling JO, Ter-Pogossian MM. Correlation between regional cerebral blood flow and oxidative metabolism. In vivo studies in man. Arch. Neurol. 1976;33:523–526. doi: 10.1001/archneur.1976.00500080001001. [DOI] [PubMed] [Google Scholar]
- SAMHSA. Results From The 2010 National Survey On Drug Use And Health: Summary Of National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration, NSDUH Series H-41, HHS Publication No.(SMA) 11-4658; 2011. [Google Scholar]
- Sankoh AJ, Huque MF, Dubey SD. Some comments on frequently used multiple endpoint adjustment methods in clinical trials. Stat. Med. 1997;16:2529–2542. doi: 10.1002/(sici)1097-0258(19971130)16:22<2529::aid-sim692>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Silverman DG, Kosten TR, Jatlow PI, Gutter V, Fleming J, O’Connor TZ, Byck R. Decreased digital flow persists after the abatement of cocaine-induced hemodynamic stimulation. Anesth. Analg. 1997;84:46–50. doi: 10.1097/00000539-199701000-00008. [DOI] [PubMed] [Google Scholar]
- Skinner HA, Sheu WJ. Reliability of alcohol use indices. The Lifetime Drinking History and the MAST. J. Stud. Alcohol. 1982;43:1157–1170. doi: 10.15288/jsa.1982.43.1157. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, Riley DM, Schuller R, Pavan DS, Cancilla A, Klajner F, Leo GI. The reliability of alcohol abusers’ self-reports of drinking and life events that occurred in the distant past. J. Stud. Alcohol. 1988;49:225–232. doi: 10.15288/jsa.1988.49.225. [DOI] [PubMed] [Google Scholar]
- Stanford MS, Mathias CW, Dougherty DM, Lake SL, Anderson NE, Patton JH. Fifty years of the Barratt Impulsiveness Scale: an update and review. Pers. Individ. Dif. 2009;47:385–395. [Google Scholar]
- Stinson FS, Grant BF, Dawson DA, Ruan WJ, Huang B, Saha T. Comorbidity between DSM-IV alcohol and specific drug use disorders in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend. 2005;80:105–116. doi: 10.1016/j.drugalcdep.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Muller-Oehring E, Pitel AL, Chanraud S, Shankaranarayanan A, Alsop DC, Rohlfing T, Pfefferbaum A. A selective insular perfusion deficit contributes to compromised salience network connectivity in recovering alcoholic men. Biol. Psychiatry. 2013;74:547–555. doi: 10.1016/j.biopsych.2013.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe J, Tregellas JR, Dalwani M, Thompson L, Owens E, Crowley T, Banich M. Medial orbitofrontal cortex gray matter is reduced in abstinent substance-dependent individuals. Biol. Psychiatry. 2009;65:160–164. doi: 10.1016/j.biopsych.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terborg C, Bramer S, Weiller C, Rother J. Short-term effect of cigarette smoking on CO(2)-induced vasomotor reactivity in man: a study with near-infrared spectroscopy and tanscranial Doppler sonography. J. Neurol. Sci. 2002;205:15–20. doi: 10.1016/s0022-510x(02)00308-8. [DOI] [PubMed] [Google Scholar]
- Tomassini A, Struglia F, Spaziani D, Pacifico R, Stratta P, Rossi A. Decision making, impulsivity, and personality traits in alcohol-dependent subjects. Am. J. Addict. 2012;21:263–267. doi: 10.1111/j.1521-0391.2012.00225.x. [DOI] [PubMed] [Google Scholar]
- Tosun D, Mojabi P, Weiner MW, Schuff N. Joint analysis of structural and perfusion MRI for cognitive assessment and classification of Alzheimer’s disease and normal aging. Neuroimage. 2010;52:186–197. doi: 10.1016/j.neuroimage.2010.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker KA, Potenza MN, Beauvais JE, Browndyke JN, Gottschalk PC, Kosten TR. Perfusion abnormalities and decision making in cocaine dependence. Biol. Psychiatry. 2004;56:527–530. doi: 10.1016/j.biopsych.2004.06.031. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Hitzemann R, Wang GJ, Fowler JS, Burr G, Pascani K, Dewey SL, Wolf AP. Decreased brain metabolism in neurologically intact healthy alcoholics. Am. J. Psychiatry. 1992a;149:1016–1022. doi: 10.1176/ajp.149.8.1016. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Hitzemann R, Wang GJ, Fowler JS, Wolf AP, Dewey SL, Handlesman L. Long-term frontal brain metabolic changes in cocaine abusers. Synapse. 1992b;11:184–190. doi: 10.1002/syn.890110303. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D. Addiction circuitry in the human brain. Annu. Rev. Pharmacol. Toxicol. 2011;52:321–336. doi: 10.1146/annurev-pharmtox-010611-134625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Hitzemann R, Fowler JS, Overall JE, Burr G, Wolf AP. Recovery of brain glucose metabolism in detoxified alcoholics. Am. J. Psychiatry. 1994;151:178–183. doi: 10.1176/ajp.151.2.178. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Roque CT, Cestaro VL, Hitzemann RJ, Cantos EL, Levy AV, Dhawan AP. Functional importance of ventricular enlargement and cortical atrophy in healthy subjects and alcoholics as assessed with PET, MR imaging, and neuropsychologic testing. Radiology. 1993;186:59–65. doi: 10.1148/radiology.186.1.8416587. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.