Abstract
Objective
The primary aim of The Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-be (nuMoM2b) is to determine maternal characteristics, including genetic, physiological response to pregnancy, and environmental factors that predict adverse pregnancy outcomes (APOs).
Methods
Nulliparous women in the first trimester of pregnancy were recruited into an observational cohort study. Participants were seen at three study visits during pregnancy and again at delivery. We collected data from in-clinic interviews, take-home surveys, clinical measurements, ultrasound studies, and chart abstractions. Maternal biospecimens (serum, plasma, urine and cervico-vaginal fluid) at antepartum study visits and delivery specimens (placenta, umbilical cord, cord blood) were collected, processed, and stored. The primary outcome of the study was defined as pregnancy ending prior to 37+0 weeks gestation. Key study hypotheses involve APOs of spontaneous preterm birth, preeclampsia, and fetal growth restriction.
Results
10,037 women were recruited to the study. Basic characteristics of the cohort at screening are reported in this Methods paper.
Conclusion
The nuMoM2b cohort study methods and procedures presented can help investigators when planning future projects.
Keywords: Pregnancy, nulliparous, adverse pregnancy outcomes prediction, methods, cohort study, psychosocial
Introduction
Adverse pregnancy outcomes such as preterm birth represent a significant problem in maternal-child health. One in nine pregnancies results in a preterm birth each year in the United States (11.55% in 2012).1 Despite the recent small decline in preterm birth rates due to the decline in late preterm births, preterm birth is still more common in the U.S. than in many developing countries.1–3 The rate of early preterm birth, < 32 weeks of gestation, has not changed since 1990, accounting for 2% of all births but 54% of infant mortality in the United States. A major challenge in efforts to address this problem is the current limitation in our understanding of underlying causes and pathophysiologic pathways. An adverse outcome, such as preterm birth, in a prior pregnancy is typically the best predictor of adverse pregnancy outcome recurrence risk.4 As such, clinicians frequently rely on prior obstetrical history to guide decisions during prenatal care. Women who will give birth to their first child (nulliparas) comprise about 40% of deliveries in the United States.1 This large proportion of women lack previous pregnancy information to guide risk assessment and, as such, adverse outcomes in these first pregnancies are particularly difficult to predict and prevent. Although previously published studies regarding prediction of adverse pregnancy outcomes have incorporated some clinical and biological variables, the derived models they produced have been limited by their inability to accurately predict the adverse outcomes.5–10 In an effort to address this gap in knowledge, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) established the Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-be (nuMoM2b) in 2009 to study nulliparous women.
Adverse pregnancy outcomes such as preterm birth, preeclampsia, and fetal growth restriction result in the majority of perinatal morbidities and mortality.11–13 Many attempts have been made to identify women destined to develop preeclampsia prior to the onset of disease. The most significant risk factors are either nulliparity with 5% developing preeclampsia, or previous history of preeclampsia with a recurrence rate of 20%. Low dose aspirin is an intervention that has been shown to be effective in decreasing the recurrence risk of preeclampsia in women with previous preterm preeclampsia; however, this is a small subgroup of women.14 Low dose aspirin has not been shown to be effective in preventing preeclampsia in nulliparas who constitute the majority of women affected by preeclampsia.15 Therefore, the ability to enhance prediction in nulliparas in which low dose aspirin may be of benefit would be important. Although there is currently no effective prophylactic or therapeutic intervention other than delivery, early prediction of fetal growth restriction is of clinical importance as it would increase sonographic surveillance for at risk fetuses as well as interventions such as antenatal surveillance if fetal growth restriction were detected. Because placental dysfunction underlies hypertensive disorders which are associated with an increased risk of fetal growth restriction it logically follows that the same biomarkers or ultrasound findings examined in preeclampsia may be useful for prediction of fetal growth restriction.10, 16, 17 The identification of women at increased risk for preterm birth has been traditionally directed towards various epidemiologic, clinical, and environmental risk factors. The most significant of these risk factors include history of prior spontaneous preterm birth, elevated mid-gestation cervico-vaginal fetal fibronectin level, and shortened mid-gestation cervical length.4 While effective approaches to prevent preterm birth are established based on history and/or mid-trimester observation, such as progesterone supplementation and cerclage, there are limited studies of identifying first trimester markers for nulliparous women at increased risk for preterm birth for which available effective interventions have been identified.
The underlying mechanisms of preterm birth, preeclampsia, and fetal growth restriction are interrelated, and were therefore evaluated as part of this study. The overarching goals of the study are to 1) determine maternal characteristics, including genetic, physiological responses to pregnancy, and environmental factors that predict adverse pregnancy outcomes; 2) identify features of placental development, structure, and function that are associated with adverse pregnancy outcomes; and 3) characterize genetic, growth, and developmental parameters of the fetus that are associated with adverse pregnancy outcomes. The study includes examination of a broad array of maternal and paternal socio-demographic factors; maternal nutritional, behavioral, and psychosocial assessments; clinical and sonographic measures; and collection of specimens to allow genomic, proteomic, and other biomarker analyses. These data will be used to inform an understanding of risk factors for, mechanisms related to, and causes of adverse pregnancy outcomes and to examine the predictive utility of these factors.
The purpose of this report is to provide a detailed description of the study design, methods, and preliminary demographic characteristics of the cohort.
Materials and Methods
Participants and recruitment
Nulliparous women with singleton pregnancies were recruited from hospitals affiliated with eight clinical centers: Case Western University; Columbia University; Indiana University; University of Pittsburgh; Northwestern University; University of California at Irvine; University of Pennsylvania; and University of Utah. The Data Coordinating and Analysis Center is RTI International. The Appendix lists the individual hospitals involved in recruitment of the cohort. Each site’s local governing Institutional Review Board(s) approved the nuMoM2b protocol and procedures.
Pregnant women attending affiliated obstetric clinics who planned to deliver their baby at one of the clinical site hospitals were screened for eligibility in the first trimester of pregnancy. Women were recruited if they had a viable singleton gestation, were between 6 weeks + 0 days gestation and 13 weeks + 6 days gestation (based on a documented ultrasound crown-rump length measurement by a certified nuMoM2b sonographer-see Table 1 for detailed dating criteria utilized), and had no prior pregnancy lasting 20 weeks or greater based on self-report. Study staff confirmed gestational age of any prior deliveries or pregnancy loss through a review of medical records if available. In addition, recollection of a birth weight for a prior pregnancy of 360 grams or greater was considered evidence of a pregnancy lasting at least 20 weeks. Exclusion criteria were: maternal age less than13 years; history of 3 or more spontaneous abortions; likely fatal fetal malformation evident before enrollment; known fetal aneuploidy; assisted reproduction with a donor oocyte; multifetal reduction; participation in an intervention study anticipated to influence maternal or fetal outcomes; prior enrollment in nuMoM2b; plan to terminate the pregnancy; or inability to provide consent. Trained study personnel screened for eligibility, explained the study, and administered informed consent. The study was explained in the participant’s native language (including Spanish and Vietnamese). Participants in the nuMoM2b study also were offered participation in Network-wide substudies of sleep disordered breathing, sleep actigraphy, and fetal adrenal gland ultrasound. These nested cohort studies will be reported separately.
Table 1.
Determination of the project estimated date of delivery (EDD)/gestational age
| Circumstance of pregnancy dating before visit | Final project EDD based on | |
|---|---|---|
|
| ||
| Unsure LMP | CRL measured (cm) and put into Hadlock formula35: | |
| Project EDD = Project ultrasound date + (280 – ultrasound estimated gestational age in days | ||
|
| ||
| “Sure” LMP | CRL measured confirming LMP or changing the EDD. Project EDD = LMP +28 days if confirmed. If not, Project EDD = ultrasound EDD Cutoffs utilized: |
|
| EGA at 1st ultrasound by LMP | Project US agreement with LMP | |
|
| ||
| <70 weeks | ±3 days | |
|
| ||
| 70 to 75 weeks | ±4 days | |
|
| ||
| 80 to 106 weeks | ±5 days | |
|
| ||
| 110 to 126 weeks | ±6 days | |
|
| ||
| 130 weeks or more | ±7 days | |
|
| ||
| Pregnancy conceived using artificial insemination | Project EDD = Date of insemination + 266 days | |
|
| ||
| Pregnancy conceived by in-vitro fertilization | For a “3-day” fresh/frozen embryo replacement date:
|
|
For a “5-day” fresh/frozen replacement date:
| ||
LMP= last normal menstrual period; CRL = crown rump length measured by 1st trimester ultrasound; EDD = estimated due date; US = ultrasound
Study standardization and design
A common protocol and manual of operations were utilized for all aspects of the study at all sites. Obstetric and newborn care was delivered according to local practices and standards at the individual clinical site hospitals. Trained and credentialed study personnel performed all study procedures. Responsibility for general study procedure training rested with the site Principal Investigator and Study Coordinator. Formally certified sonographers performed ultrasound studies. A local certification was required for crown-rump length and fetal biometry measurements that included images on at least 3 women showing proper measurements as defined locally by a Network site expert. Measurements of cervical length (CL) and uterine artery Doppler (UAD) velocimetry were centrally certified by national experts. (Appendix B)
Study visits, information collected, specimens, and procedures
Study visits were not part of clinical care. Consequently, research data were not made available to the clinical care provider except for results of sonographic examination for predefined criteria (see below). Study visit timing was chosen to be spread across gestation, to occur prior to the time of most preterm births, and generally to correlate with times in prenatal care when women see caregivers: first trimester, early-mid second trimester, and late second-early third trimester. The timing to correspond to regular clinical care visits was chosen to facilitate ultimate translation into clinical care if predictive markers were to be established. Study visits generally lasted 1–2 hours. Study adherence was maximized by compensation of participants for their time and effort. The exact amount and types of compensation (e.g., car seats) were decided by each site. Table 2 provides an overview of interview data collection and clinical measurements, Table 3 provides a list of standardized questionnaire instruments, and Table 4 provides a summary of biospecimens collected, by study visit. Visit 1 occurred between 6+0 and 13+6 weeks gestation. Blood pressure, height, weight, and waist, hip and neck circumference were measured. Detailed interviews were performed to collect demographic characteristics, medical history, physical activity documentation, experience with nausea and vomiting of pregnancy, medication and supplement use, alcohol, tobacco, and drug use, nutritional intake, perceived stress, reactions to racism, symptoms of depression, motivation for and intendedness of pregnancy, pregnancy knowledge, and relationship with a partner/father of the baby. In addition, questionnaires regarding sleep, trait anxiety, perceived social support, and difficulties in pregnancy were taken home after Visit 1 to be completed at home and returned. Blood and urine samples were obtained. Separate cervical and vaginal fluid samples were obtained by speculum exam for fetal fibronectin and stored for other future assays such as cytokine and microbiologic analyses. The participant self-collected additional samples of vaginal fluid for fetal fibronectin assays and for storage
Table 2.
Question Domains and Clinical Evaluations by Study Visit
| Question Domains, Samples, and Clinical Evaluations | Study Visit*
|
Delivery | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
|
| ||||
| Demographic characteristics | ||||
| • Demographics of Mother/Changes | X | X | X | |
| • Background of Mother’s Parents | X | |||
| • Demographics of Father and Background of Father’s Parents (if appropriate) | X | |||
|
| ||||
| Standard instruments (see Table 3 and text for individual timing) | X | X | X | |
|
| ||||
| Medical history | ||||
| • Mother | X | X | X | X |
| • Mother’s family | X | X | X | |
| Medications and Supplements | X | X | X | X |
| Substance use- Alcohol, Tobacco, Drugs | X | X | X | X |
|
| ||||
| Other Psychological factors | ||||
| • Reactions to race | X | |||
| • Pregnancy intendedness | X | |||
| • Difficulties in pregnancy | X | X | ||
| • Relationship with father of baby | X | X | X | |
|
| ||||
| Signs/Symptoms preceding delivery hospitalization | X | |||
|
| ||||
| Participant assessment of route and reasons for delivery | X | |||
|
| ||||
| Blood pressure** and weight | X | X | X | |
|
| ||||
| Height; waist, hip, and neck circumference | X | |||
|
| ||||
| Ultrasound for crown-rump length | X | |||
|
| ||||
| Ultrasound for fetal biometry | X | X | ||
|
| ||||
| Ultrasound for cervical length | X | X | ||
|
| ||||
| Ultrasound for uterine artery Doppler measurement | X | X | ||
Study visits 1, 2, and 3 were during the following gestational age intervals, respectively: 6 week 0 days to 13 weeks 6 days; 16 weeks 0 days to 21 weeks 6 days; and 22 weeks 0 days to 29 weeks 6 days.
If a blood pressure reading >140/90 was obtained the participant was to rest for 10 minutes and have it repeated in the standard way. The 2nd blood pressure was then reported. No notification was required if the 2nd value was elevated as all participants were receiving routine prenatal care in which blood pressure was recorded.
Table 3.
Standard Instruments by Domain and Study Visit When Administered
| Domain | Instrument | Study Visit* | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Health Literacy | Rapid Estimate of Adult Literacy in Medicine Short Form (REALM-SF)36 | X | ||
| Nutrition | Modified Block 2005 Food Frequency Questionnaire37 | X | ||
| Physical Activity | Recent Physical Activity Log38 | X | X | X |
| Psychosocial | Experiences of Discrimination (EOD)39 | X | ||
| Multidimensional Scale of Perceived Social Support (MSPSS)40 | X | |||
| Pregnancy Experience Scale, Brief Version (PES-Brief)41 | X | |||
| Connor-Davidson Resilience Scale (CD-RISC)42 | X | |||
| Sleep | Women’s Health Initiative Insomnia Rating Scale43 | X | X | |
| Epworth Sleepiness Scale44 | X | X | ||
| Restless Legs Syndrome Diagnostic Criteria45 | X | X | ||
| Berlin Questionnaire for Sleep Apnea46 | X | X | ||
| Stress/Mental Health | State-Trait Anxiety Inventory- Trait Subscale (STAI-T)47 | X | ||
| Edinburgh Postnatal Depression Scale (EPDS)48 | X | X | ||
| Perceived Stress Scale (PSS-10)49 | X | X | ||
| Other | Pregnancy-Unique Quantification of Emesis and Nausea (PUQE)50 | X | X | X |
Study visits 1, 2, and 3 were during the following gestational age intervals, respectively: 6 week 0 days to 13 weeks 6 days; 16 weeks 0 days to 21 weeks 6 days; and 22 weeks 0 days to 29 weeks 6 days.
Table 4.
Biospecimen Collection by Study Visit
| Biospecimen | Study Visit* | Delivery | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Urine (20 mL), processed to supernatant and cell pellet | X | X | X | |
| Blood (4–8 mL), processed to plasma | X | X | X | X |
| Blood (4–8 mL), processed to serum | X | X | X | X |
| Blood (4–8 mL), whole, for DNA | X | |||
| Cervico-vaginal fluid from cervix, by speculum (2 swabs) | X | |||
| Cervico-vaginal fluid from posterior fornix, by speculum (4 swabs) | X | |||
| Cervico-vaginal fluid, self-collected (3 swabs) | X | X | X | |
| Cord blood (4–8 mL), whole, for DNA | X | |||
| Placenta (0.25–0.50 cm3), for DNA | X | |||
| Umbilical cord segment (2 cm segment), for DNA | X | |||
| Neonatal saliva** (1–2 mL), for DNA | X | |||
| Placenta (2 cm3), formalin fixed, for histology | X | |||
| Fetal membranes (2 cm2), formalin fixed, for histology | X | |||
| Umbilical cord segment (2 cm segment), formalin fixed, for histology | X | |||
| Samples processed within 30 minutes of delivery (optional): | ||||
| Cord blood (4–8 mL), processed to plasma and flash frozen | X | |||
| Placenta (0.25–0.50 cm3), flash frozen, for DNA | X | |||
| Placenta (0.25–0.50 cm3) processed with RNAlater®, for RNA expression | X | |||
| Placenta (0.25–0.50 cm3), flash frozen, for protein and metabolites | X | |||
| Placenta (0.25–0.50 cm3), flash frozen, for epigenetic studies | X | |||
| Fetal membranes (2 cm2), processed with RNAlater®, for RNA expression | X | |||
| Fetal membranes (2 cm2), flash frozen, for protein and metabolites | X | |||
| Fetal membranes (2 cm2), flash frozen, for epigenetics | X | |||
Study visits 1, 2, and 3 were during the following gestational age intervals, respectively: 6 week 0 days to 13 weeks 6 days; 16 weeks 0 days to 21 weeks 6 days; and 22 weeks 0 days to 29 weeks 6 days.
Collected for pregnancies with a clinical diagnosis at delivery of preterm birth (<37 weeks’ GA), pregnancy-associated hypertension, or fetal growth restriction, if the cord blood sample and the placenta sample for fetal DNA were not collected.
Study visit 2 occurred between 16+0 and 21+6 weeks gestation and at least 4 weeks after Visit 1 had occurred. Clinical measurements of blood pressure and weight were repeated and interviews were conducted to gather information on health literacy, perception of experiences with racism, additional demographic characteristics, current physical activity, further details on family history and updates on exposures to alcohol, drugs, and tobacco, and current habits. Updates to record participants’ experience with emesis and nausea during pregnancy, medications, and relationships with a partner/father of the baby were obtained. A questionnaire on resilience was given to the participant to complete at home. Blood and urine were obtained, as were self-collected vaginal fluid samples for fetal fibronectin assay and storage. Sonographic assessment of fetal biometry, cervical length and uterine artery Doppler measurements were performed.
Study visit 3 occurred between 22+0 and 29+6 weeks gestation and at least 4 weeks after Visit 2. Clinical measures were repeated as were additional interview questionnaires. A survey of sleep patterns and habits, pregnancy experience, and difficulties in pregnancy were completed by the participant. Blood, urine, and self-collected vaginal fluid samples were obtained as at study visit 2. Similarly, sonographic assessment of fetal biometry and cervical length were repeated, as was uterine artery Doppler measurement if the study at the second study visit had demonstrated a diastolic notch.
If the participant had a clinical amniocentesis or chorionic villus sample collection, a separate informed consent was administered to collect any excess sample from those procedures to add to the specimen bank. In addition, if a pregnancy loss occurred prior to 20+0 weeks, research personnel collected products of conception if possible.
Criteria for informing the patient’s caregiver of study findings included: identification of a major fetal structural malformation, hydrops, fetal demise, estimated fetal weight <5th percentile (at 2nd or 3rd ultrasound visit), oligohydramnios (a maximal vertical pocket <2 cm or amniotic fluid index <5 cm), a cervical length <15mm prior to 28 weeks’ gestation, fetal bradycardia or tachycardia, or placenta previa or vasa previa found at the 3rd study visit. Given the study was designed as an observational cohort in which we did not want to increase overtreatment based on information that only would have been obtained at a study visit, but also wanted to optimize the chance that women with potentially pathologic findings could have their health care modified appropriately, we chose to inform caregivers of information obtained only when it was determined, a priori, that the knowledge was most clearly necessary to provide adequate clinical care (e.g., the discovery of an intrauterine fetal demise, estimated fetal weight <5th percentile, cervical length <15mm). This guided our consensus selection of the trigger parameters noted above.
At the time of delivery, an interview was conducted to update medical history, medication and supplement use, alcohol, tobacco, and drug exposures, perceived reasons for labor admission and mode of delivery, and to collect intended method of infant feeding. Maternal blood samples were drawn after admission but not later than one hour after delivery. We allowed for the collection of maternal blood just after delivery for women who were admitted and delivered before maternal blood could be collected. This decision was made to optimize the chance that maternal blood could be obtained in the peripartum period, even if a woman delivered so quickly that maternal blood could not be obtained prior to birth. After delivery of the placenta, an umbilical cord blood sample was obtained for DNA, and the placenta, membranes, and umbilical cord were refrigerated until collection of samples. Placenta, membrane and cord samples were taken for DNA and histology. Attempts were made to obtain these samples within 30 minutes of placental delivery, however collection up to 72 hours after delivery was considered acceptable. When collection and processing within 30 minutes was possible, additional placenta and membrane samples for RNA expression, protein and metabolites, and epigenetics, as well as additional cord blood for plasma, were collected if possible (Table 4). These samples were flash frozen in liquid nitrogen with the exception of the samples for RNA expression which were processed using RNAlater®. Appendix C describes details of delivery sample processing. Finally, a neonatal saliva sample for DNA was collected for women with certain APOs if the cord blood sample and the placenta sample for fetal DNA had not been collected.
Samples were all stored locally in −80°C freezers after processing. Formalin fixed samples were stored at room temperature. Full processing details are available upon request from the Data Coordinating and Analysis Center. Data were entered into a secure web-based data capture system. Multiple data quality checks were performed to ensure high fidelity information in the database. Specimens for assay of fetal fibronectin were shipped to Hologic, Inc., in Sunnyvale, CA. All other specimens were shipped to and stored at a biorepository at Fisher BioServices, Inc. in Rockville, MD.
At least 30 days after delivery, a trained, certified chart abstractor assessed all participants’ medical records to record final birth outcomes and any readmissions to the hospital. Certification for chart abstraction was done locally at the Network sites based on study guidelines. The general topics abstracted were: medical history; prenatal care and medication use; prenatal laboratory and diagnostic studies; maternal and neonatal delivery hospitalization medications, diagnoses, and outcomes; and any other maternal hospitalizations.
Standard survey instruments
Standardized and validated survey instruments were chosen in consultation with experts in the field (Table 3). Instruments were given preference if they had been utilized and/or validated in prior pregnancy populations or cohorts. Attempts were made to choose one instrument to represent a domain of interest theorized to be associated with adverse pregnancy outcomes. Accordingly, we chose surveys to measure perceived stress (the Perceived Stress Scale), the amount of social support and personal resiliency that might buffer external stress (the Multi-Dimensional Scale of Social Support and the Connor-Davidson Resiliency scale), symptoms of depression (the Edinburg Postnatal Depression survey) and anxiety (the trait subscale of the State-Trait Anxiety Inventory). Pregnancy-specific feelings were assessed with the Pregnancy Experiences survey and perceptions of discrimination with the Krieger Racism survey. To adequately analyze the association of sleep on adverse pregnancy outcomes, we utilized 4 instruments concerned with different sleep domains: The Berlin Questionnaire to assess for the possibility of sleep apnea, the Women’s Health Initiative Insomnia Rating Scale to ascertain insomnia symptoms, the Epworth Sleepiness Scale to document daytime sleepiness, and the Restless Leg Syndrome Diagnostic Criteria survey. Lastly, health literacy was assessed with the short version of the REALM instrument.
Outcomes and measures
The primary outcome of the study was defined as pregnancy ending due to any cause prior to 37+0 weeks gestation. Key study hypotheses involve the outcomes of spontaneous preterm birth, preeclampsia, and fetal growth restriction.
Preterm birth was defined as delivery of a liveborn or stillborn infant for any cause between 20+0 and 36+6 weeks gestation. Spontaneous preterm birth was defined as delivery occurring subsequent to spontaneous onset of preterm labor or premature rupture of the membranes (PROM) or fetal membrane prolapse. Preterm labor was defined as spontaneous uterine contractions (more than 6 per hour documented by tocodynamometry or maternal history), with the onset before membrane rupture that leads to delivery; documented cervical change of at least 1cm dilation or effacement during the admission; or dilation >2cm or effacement >80% on admission for contractions. PROM was defined as spontaneous rupture of the membranes before the onset of contractions, regardless of subsequent labor augmentation or cesarean delivery. Rupture of the membranes had to be documented by: visible leaking of amniotic fluid from the cervix; presence of vaginal indigo carmine after intra-amniotic instillation; or the presence of any two of the following-- pooling of fluid in the vaginal vault, positive nitrazine test, positive ferning of dried vaginal fluid observed microscopically, or a positive commercially-available biochemical test for PROM. Fetal membrane prolapse was defined by spontaneous descent of the fetal membranes to or past the external cervical os in the absence of uterine contractions (more than 6 contractions per hour documented by tocodynamometry or by maternal history), PROM, maternal fever or uterine tenderness, chorioamnionitis (clinical or amniocentesis diagnosis), or abruptio placentae, regardless of the placement of a cervical suture (cerclage). Women who delivered an infant preterm who did not meet any of those criteria were categorized as spontaneous preterm birth otherwise unspecified.
Indicated preterm birth was defined as delivery following induction or cesarean delivery between 20+0 and 36+6 weeks gestation. The indication for the delivery was recorded as either: pregnancy associated hypertension (including preeclampsia); fetal growth restriction; placental abruption; placenta previa; chorioamnionitis; abnormal fetal testing; fetal conditions including congenital anomalies; maternal medical conditions; “other” indicated preterm birth; or “no documented indication.”
Abortion (spontaneous pregnancy loss less than 20 weeks) was defined as the delivery of a liveborn or fetus experiencing fetal death for any cause before 20+0 weeks. These were classified as “early” if delivery occurred before 14+0 weeks and “late” if it occurred after 14+0 weeks. A term birth was defined as delivery of a liveborn or stillborn infant for any cause at or after 37+0 weeks.
Other maternal outcomes obtained were: hypertension (mild, severe); chronic hypertension; baseline and preeclamptic proteinuria (mild, severe); preeclampsia (also eclampsia, HELLP syndrome, mild and severe preeclampsia); superimposed preeclampsia; gestational hypertension; abruptio placentae; placenta previa; clinical chorioamnionitis; labor induction; labor augmentation; cesarean delivery (scheduled, unplanned, non-urgent, urgent, reason indicated); diabetes mellitus and gestational diabetes categorized by White’s classification; and other outcomes such as deep venous thrombosis, sepsis, acute respiratory distress syndrome, amniotic fluid abnormalities, cerebral vascular accident, severe anemia (hematocrit = 20% and treated with transfusion), massive transfusion (=6 units of packed red cells), ICU admission, surgery required other than for delivery of the infant, cesarean hysterectomy, and death. Quality control checks (via re-abstraction) were performed for primary outcomes by the site principal investigator on a random selection of charts with and without APOs.
Fetus-newborn outcomes included: embryonic death; fetal death before 20+0 weeks gestation; stillbirth or live birth at or after 20+0 weeks gestation; anomalies/malformations; fetal growth restriction; respiratory distress syndrome; ventilator support; continuous positive airway pressure; transient tachypnea of the newborn; oxygen therapy; confirmed neonatal sepsis (early onset, late onset); suspected neonatal sepsis (early onset, late onset); necrotizing enterocolitis (stage 1, 2, or 3); intraventricular hemorrhage (grade I, II, III, or IV); chronic lung disease/bronchopulmonary dysplasia; newborn seizures; retinopathy of prematurity; and infant discharge status.
Statistical considerations
NuMoM2b was designed as a prospective cohort study. The target recruitment was 10,000 nulliparous women with singleton pregnancies enrolled in the first trimester. It was estimated that this would yield 1100 preterm births (PTBs) <37 weeks, 330 preterm births <34 weeks, 700 cases of preeclampsia, 300 cases of fetal growth restriction using a strict definition of <3rd percentile, and 70 cases of stillbirth. Power estimates were computed for detection of a range of odds ratios with a 4:1 ratio of controls to cases, which is suitable for nested cohorts and conservative for the full cohort. The power was calculated for Pearson chi-square tests with a 2-sided a=0.05, assuming 1100 cases (e.g., all PTBs), 770 cases (e.g., spontaneous PTBs), 550 cases (e.g., Caucasian PTBs), 220 cases (e.g., PTBs in another racial group), and 100 cases (i.e., some other small subset of APOs) and 5% and 20% exposure among the controls. With 1100 cases there would be >80% power to detect a modest effect (odds ratio >1.25) of a common exposure (>20%); for a rare exposure (5%), the power would be>80% for an odds ratio as low as 1.5. For 100 cases, power would remain >80% for odds ratios of approximately 2.0 and 3.0 with 20% and 5% exposed among the controls, respectively.
Standard parametric/nonparametric and asymptotic/exact methods for descriptive and inferential analysis will be employed for continuous, binary, ordinal and polytomous variables to describe characteristics of the cohort and nested subcohorts, controls, and cases, and to estimate effects and draw inferences regarding the associations of exposures and predictors with PTB and other APOs, with suitable stratification and modeling adjustments for confounding and assessments of effect modification.
With regard to prediction, we will assess the models based on predictive accuracy (calibration and discrimination). To assess calibration we will examine calibration plots and perform Hosmer-Lemeshow tests. To assess discrimination we will examine risk distributions among cases and controls and corresponding true and false positive rates for various cutoffs through the use of integrated predictiveness and classification curves18, in addition to the traditional receiver operating characteristic curve analysis for the area under the curve.
Results
At the conclusion of recruitment, which had proceeded from October 2010 through September 2013, 10,037 eligible women were consented and enrolled to the nuMoM2b study. The demographic characteristics of the cohort are presented in Table 5. The mean age of women at entry into the study was 27.0 years (standard deviation 5.7 years). This is similar to the 2012 United States mean age at first birth of 25.8 years.1 The gestational age at screening for a majority of the women (61.9%) was 12 weeks or more. Sixty percent (59.7%) of the women were non-Hispanic white, 14.2% were non-Hispanic black, 16.9% were Hispanic, 4.0% were Asian, and 5.1% were classified as other on race/ethnicity. This distribution is close to the general population distribution in the United States in 2010 (13.6% black, 16.3% Hispanic, 5.6% Asian, 7.0% other; http://factfinder2.census.gov).
Table 5.
Baseline Characteristics for Enrolled Women
| Characteristic (N=10,037)* | Statistic |
|---|---|
|
| |
| Maternal age, in years | |
| Mean (standard deviation) | 26.90 (5.7) |
| Median (interquartile range) | 27.0 (22, 31) |
| Category: n (%) | |
| 13–21 | 2128 (21.2) |
| 22–35 | 7228 (72.1) |
| >35 | 670 (6.7) |
| Gravidity: n (%) | |
| 1 | 7437 (74.2) |
| 2 | 1913 (19.1) |
| 3+ | 676 (6.7) |
| Maternal race/ethnicity: n (%) | |
| Non-Hispanic White | 5988 (59.7) |
| Non-Hispanic Black | 1420 (14.2) |
| Hispanic | 1699 (16.9) |
| Asian | 406 (4.0) |
| Other | 514 (5.1) |
| Ever Smoked: n (%) | 4187 (41.8) |
| Smoked during 3 months prior to pregnancy: n (%) | 1782 (17.8) |
| Among smokers during 3 months prior to pregnancy, cigarettes per day: n (%) | |
| <20 cigarettes per day | 1514 (85.4) |
| 20–40 cigarettes per day | 254 (14.3) |
| >40 cigarettes per day | 4 (0.2) |
| Estimated gestational age at screening: n (%) | |
| < 8 weeks 0 days | 150 (1.5) |
| 8 weeks 0 days to 9 weeks 6 days | 1044 (10.4) |
| 10 weeks 0 days to 11 weeks 6 days | 2697 (26.9) |
| 12 weeks 0 days to 13 weeks 6 days | 6136 (61.2) |
| Education status attained: n (%) | |
| less than high school | 815 (8.1) |
| completed high school or GED | 1170 (11.7) |
| some college | 1947 (19.4) |
| associate or technical degree | 1005 (10.0) |
| completed college | 2772 (27.7) |
| degree work beyond college | 2308 (23.0) |
| Method of paying for healthcare: n (%)** | |
| government insurance | 2854 (28.7) |
| military insurance | 65 (0.7) |
| commercial health insurance | 6776 (68.1) |
| personal household income | 1720 (17.3) |
| other | 132 (1.3) |
| Income and size of household relative to Federal poverty level: n (%) | |
| >200% | 5662 (69.7) |
| 100–200% | 1171 (14.4) |
| <100% | 1296 (15.9) |
10,027 of the 10,037 women have data at Visit 1 that is reported above. Sample size varies by characteristic due to missingness.
Percentages will not add up to 100% as participants were allowed to select multiple methods.
Discussion
As a large cohort following nulliparous women starting in first trimester, nuMoM2b will allow for improved understanding preterm birth, preeclampsia, and growth restriction. Multiple predictive algorithms using patient characteristics and biomarkers have been tested as predictive for adverse pregnancy outcomes such as preterm birth, preeclampsia, and growth restriction.7–10, 19–26 Many times these studies have focused on a small number of predictors or have collected information retrospectively. For instance, in a summary of biomarkers to predict preterm birth, of 30 novel biomarkers studied, only 3 had a positive likelihood ratio greater than 10 and these were only evaluated in one small study.7 In general, none of the biomarkers evaluated in the review were considered to be clinically useful and the authors called for large, prospective studies to better evaluate potential predictive biomarkers.7 While a newer approach using Bayesian models combining clinical, demographic, and genetic markers has shown more promise, this was not performed in a U.S. population and the only biomarker incorporated into the model was fetal fibronectin.8 Similarly, predictive models combining maternal factors, biophysical, and biochemical markers to predict preeclampsia have had detection rates as low as 61%.5, 27 The Preterm Prediction Study in the United States found the combination of positive fetal fibronectin and a short cervix, while not highly prevalent, conveyed a 64.7% risk of spontaneous preterm birth in nulliparous women.26 An international cohort of nulliparous women found that combining maternal characteristics and ultrasound measures may provide a high negative predictive value but only detected 37–61% of women who developed preeclampsia.5 One study screened for pregnancy hypertension using maternal history, uterine artery pulsatility index (PI), mean arterial pressure (MAP), and PAPP-A at 11 to 13 weeks of gestation in a cohort 7,797 women.28 Utilizing a screening algorithm combining both maternal factors, history of preeclampsia, ultrasound measures, and biomarkers in blood, it was promising that 90% of cases of early preeclampsia were identified with only a false-positive rate of 5%. However, of 476 pregnancies that screened positive for early preeclampsia, only 32 developed the disease, giving a positive predictive value of 7%.28 Overall, only 21.2% of the pregnancies classified as screen-positive would develop hypertension during pregnancy.28 The recent report from the American College of Obstetricians and Gynecologists Task Force on Hypertension in Pregnancy noted that current evidence suggests that a combination of biomarkers may provide the best predictive accuracy but that, as of 2012, there was not a reliable test.29 The report called for further investigation in the area. Similar predictive models for fetal growth restriction have not been as robust as hoped.6, 10, 24, 30, 31
The prospective and data-intense nature of nuMoM2b provides the opportunity not only for a wealth of demographic, psychosocial, and biomarker data to be included in predictive models but also for genomic, proteomic, and other “-omic” technologies to be assessed.17, 27, 32–34 For these reasons, nuMoM2b presents a special opportunity to understand the complex interactions of gene-environment in the prediction of preterm birth and other adverse perinatal outcomes such as preeclampsia, fetal growth restriction, and stillbirth. Because of the cohort’s geographically diverse population that approximates racial/ethnic diversity of pregnant nulliparas within the United States, these findings are likely to be generalizable.
We expect that results from nuMoM2b, as well as other large international cohort studies of nulliparous women starting early in pregnancy, will allow for identification of women at the highest risk of adverse pregnancy outcome. We expect to achieve this by improved understanding of the heterogeneous phenotypes of preeclampsia, preterm birth, fetal growth restriction, and stillbirth. The nuMoM2b goals inform multiple pre-specified hypotheses relating demographic, clinical, genetic, biomarker, nutritional, and psychosocial aspects in pregnant women to key APOs in pregnancy. The far-reaching and contemporary hypotheses will provide insights into both normal pregnancy outcomes and adverse outcome development in nulliparous women, and perhaps multiparous women as well. By combining detailed demographic and medical information, clinical parameters, ultrasound measurements, genetics, biomarker measurements in biological fluids, and psychosocial and behavioral measures in both pre-specified and exploratory analyses, nuMoM2b results may be used to identify pregnant women at risk for adverse pregnancy outcomes. Through early identification of women at the highest risk, interventions may then be studied to prevent APO occurrence in the first pregnancy. In addition, the potential to identify novel therapeutic and diagnostic targets based upon the nuMoM2b findings may facilitate more individualized prenatal care. The nuMoM2b cohort is positioned to provide valuable insights into nulliparous pregnancies.
Acknowledgments
Support acknowledgement: This study is supported by grant funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD): U10 HD063036, RTI International; U10 HD063072, Case Western Reserve University; U10 HD063047, Columbia University; U10 HD063037, Indiana University; U10 HD063041, University of Pittsburgh; U10 HD063020, Northwestern University; U10 HD063046, University of California Irvine; U10 HD063048, University of Pennsylvania; and U10 HD063053, University of Utah. In addition, support was provided by respective Clinical and Translational Science Institutes to Indiana University (UL1TR001108) and University of California Irvine (UL1TR000153).
Appendix A. nuMoM2b Clinical Sites and Hospitals
-
Case Western Reserve University / Ohio State University
MetroHealth Medical Center
Ohio State University Wexner Medical Center
-
Columbia University / Christiana Care
Columbia University Medical Center
The Allen Hospital
Christiana Hospital
-
Indiana University
IU Health University Hospital
IU Health Methodist Hospital
IU Health North Hospital
IU Health West Hospital
Wishard Memorial Hospital/ Eskenazi Health
-
University of Pittsburgh
Magee Women’s Hospital
Western Pennsylvania Hospital
-
Northwestern University
Northwestern Memorial Hospital
-
University of California at Irvine
UC Irvine Health
Fountain Valley Regional Hospital
Memorial Care Center for Women
Miller Children’s Hospital
-
University of Pennsylvania
Hospital of the University of Pennsylvania
Pennsylvania Hospital
-
University of Utah
University of Utah Hospital
LDS Hospital
McKay Dee Hospital
Utah Valley Regional Medical Center
Intermountain Medical Center
Appendix 1. Procedures and Certification for nuMoM2b Ultrasounds
This text is taken from the nuMoM2b Manual of Operations and Ultrasound Certification Protocol.
Measurements and Procedures Taken at Study Visits
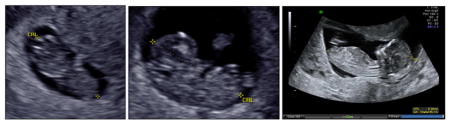
Crown Rump Length
CRL is to be measured on study patients prior to 136.
Transabdominal or transvaginal ultrasound may be used.
Prior to performing CRL measurements, presence of single intrauterine pregnancy with normal fetal cardiac activity should be confirmed.
Magnification and resolution should be such that the margins of the fetus are clearly visible and within the frame, and such that caliper placement can be evaluated.
The CRL is measured in the long axis view either in the midsagittal plane with the fetus in neutral position or in the coronal plane.
The CRL should be measured as the distance between the extreme points of the fetal head and rump
Three technically acceptable measurements should be obtained, and averaged to determine the CRL.
-
Sample images demonstrating measurement of CRL:
Biometry and Amniotic Fluid Measurement
-
Fetal biometric measurements are to be obtained on study patients twice during the pregnancy
Transabdominal or transvaginal ultrasound may be used to obtain biometric measurements
Prior to performing biometric measurements, presence of single intrauterine pregnancy with normal fetal cardiac activity should be confirmed.
Once viability is confirmed, fetal biometric measurements should be obtained as outlined below.
The structure of interest should be magnified such that it occupies at least 50% of the ultrasound image.
Magnification and resolution should be such that the margins of the structure of interest are clearly visible and within the frame, and such that caliper placement can be evaluated.
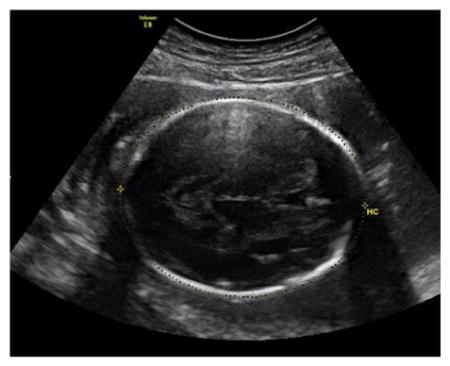
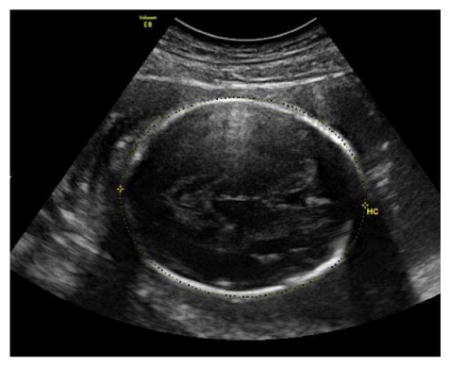
Head Circumference (HC)
The HC ismeasured in an axial image of the fetal head at the level of the thalami and cavum septi pellucidi, and can be measured from the same images used for the BPD.
The thalami and cavum septi pellucidi should be visible in the image.
The atria of the lateral ventricles and cerebellum should not be visible.
Measurement of the HC should be taken around the outer perimeter of the calvarium using the ellipse or manual-trace function.
Three technically acceptable measurements should be obtained, and averaged to determine the HC.
-
Sample image demonstrating measurement of HC:
Biparietal Diameter (BPD)
The BPD is measured in an axial image of the fetal head at the level of the thalami and cavum septi pellucidi, and can be measured from the same images used for the HC.
The thalami and cavum septi pellucidi should be visible in the image.
The atria of the lateral ventricles and cerebellum should not be visible.
The BPD should be measured from the OUTER edge of the proximal calvarium to the INNER edge of the distal calvarium (i.e., “leading edge” to “leading edge”) taking care to ensure that the measurement is made perpendicular to the falx and that the maximum diameter is measured.
Three technically acceptable measurements should be obtained, and averaged to determine the BPD.
-
Sample image demonstrating measurement of BPD:
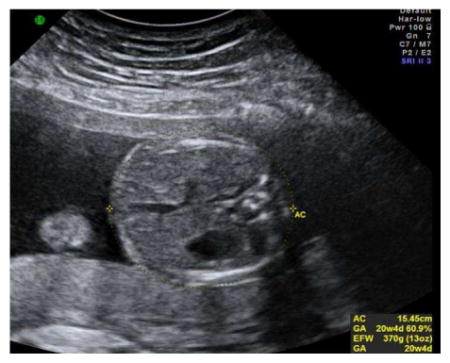
Abdominal Circumference (AC)
The abdominal circumference should be measured on a true transverse plane of the fetal abdomen at the level of the stomach and the junction of the umbilical vein with the portal sinus.
The AC is measured around the outer perimeter of the fetal skin line using the ellipse or manual-trace function.
Three technically acceptable measurements should be obtained, and averaged to determine the AC.
-
Sample image demonstrating measurement of AC:
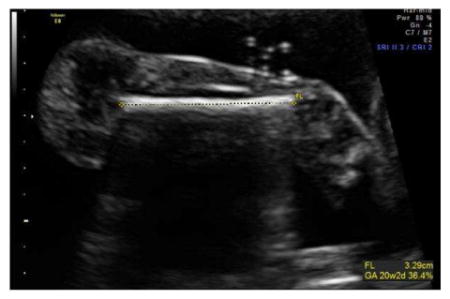
Femur Length (FL)
An image should be obtained demonstrating the long axis of the femur making sure the ultrasound beam is perpendicular to the diaphysis.
FDL is measured as the distance between the midpoints of each end of the calcified femoral diaphysis.
Three technically acceptable measurements should be obtained, and averaged to determine the FDL.
-
Sample image demonstrating measurement of FL:
Amniotic Fluid Index (AFI)
Amniotic fluid volume is to be quantified once during pregnancy at a gestational age between 220 – 296 weeks
Transabdominal ultrasound should be used
The depth of amniotic fluid is to be measured in each of the four quadrants of the maternal abdomen (left lower, left upper, right upper, right lower)
To determine the amniotic fluid index, the deepest vertical pocket of amniotic fluid in EACH of the FOUR quadrants should be measured and summed
When measuring a pocket of amniotic fluid, calipers should be placed PERPENDICULAR to the probe thereby measuring the longest depth of a pocket of fluid
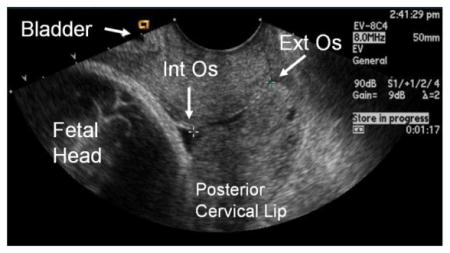
Cervical Length
-
Cervical length is to be assessed on study patients twice during the pregnancy
-
A 5 to 7 MHz endovaginal probe should be used with the endovaginal setting (not OB or abd).
The equipment should be appropriately cleaned w/ soap and water, and soaked according to local protocol.
-
The examination should be performed with an empty maternal bladder, and the patient should be asked to void just before the exam.
If bladder is noted to be large, the exam should be stopped and the patient should be asked to void again.
Insert endovaginal probe into the patient’s vagina under direct real-time vision.
The probe should be held and moved with the sonographers fingers rather than the hand, arm, shoulder.
The following landmarks should be identified in sequence: amniotic fluid and fetus → maternal bladder → internal os → cervical canal→ external os
The probe should be rotated to see best long axis view of the cervical canal.
-
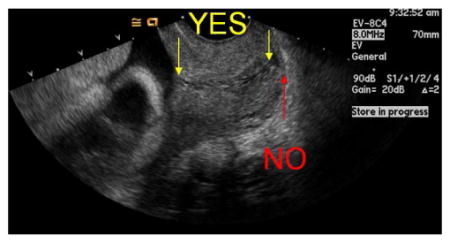
Pressure on the probe should be relaxed until the image begins to blur, then pressure should be reapplied to obtain an optimal image with the following criteria:
The width of the anterior and posterior portion of the cervix must be same.
The entire canal should be well seen, with the internal and external os visible.
-
The image should be magnified such that the canal fills 60 – 75% of screen.
-
Cervical length should then be measured as the distance of closed cervix between the internal and external cervical os.
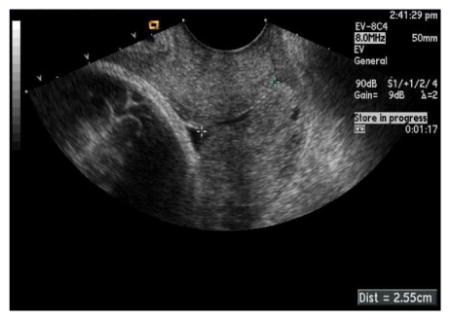
-
Calipers should be placed where then the anterior and posterior walls of the canal touch, and not necessarily at the outer-most edge.
-
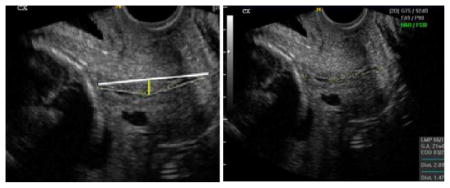
If the distance between the straight line between the internal and external os and the midcanal is > 2 mm, the cervical length should be measured in 2 or more segments.
-
-
Cervical length should be measured repeatedly until the difference between the measurements is < 10%
The shortest best measurement should be recorded (not the average)
-
After the shortest best measurement is obtained, transabdominal fundal and then suprapubic pressure should be applied to assess for the presence of a funnel
Remember to reduce probe pressure while fundal and suprapubic pressure applied
-
If a funnel is noted, the image should be frozen and measurements obtained of the length of the funnel and the length of the closed cervix
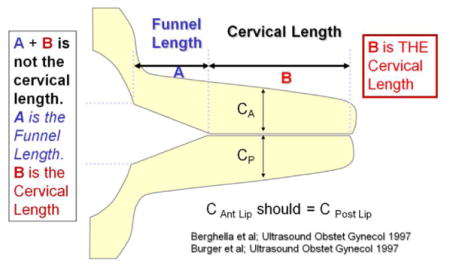
Repeated measurements should be taken until three good quality measurements are obtained that vary by < 10%
Uterine Artery Doppler
Uterine artery Doppler assessments are to be performed on all study patients between 160 – 216 weeks of gestation
-
In those patients noted to have a diastolic notch, systolic notch, or reversed early or late diastolic flow of the uterine artery waveform between 160 and 216 weeks will have repeat Doppler assessment of the uterine artery between 220 and 296 weeks
At least 4 weeks (28 days) should have elapsed between measurement of cervical length at Visit 2 and Visit 3
-
Either transabdominal or transvaginal ultrasound may be used to perform uterine artery Doppler velocimetry. Transabdominal ultrasound, however, should be used primarily, and the transvaginal approach should only be used if transabdominal imaging is deemed to be inadequate (as commonly found in obese patients).
TRANSABDOMINAL APPROACH: After identifying the midline of the cervix in the sagittal plane, the transducer is moved laterally into the iliac fossa until the vascular plexus becomes visible.
TRANSVAGINAL APPROACH: Using color Doppler, the uterine artery can be identified at the level of the internal os as it enters the uterus.
Using color Doppler, identify either the main right or left uterine artery just above the external iliac artery.
-
Either the right or left uterine artery should be sampled with pulsed Doppler, just distal to its crossing of the external iliac artery.
Normal uterine artery flow patterns have a rapid systolic upstroke and slow decay in diastole, with the diastolic velocity pattern increasing throughout gestation.
The heart rate in the uterine artery should be equal to the maternal pulse.
The sweep speed of the Doppler display should be set between 75–100 mm/sec to yield a display of approximately 3–4 maternal waveforms per image (depending on the maternal heart rate).
Optimize the angle of insonation maintaining an angle as close to zero degrees as possible, and preferably less than 30 degrees; use angle correction if needed.
A series of uniform Doppler waveforms should be obtained that fills the entire screen; at a minimum, three consecutive uterine artery waveforms should be obtained that are uniform in appearance with well-defined envelopes.
-
Sample image of pulsed Doppler assessment of uterine artery:
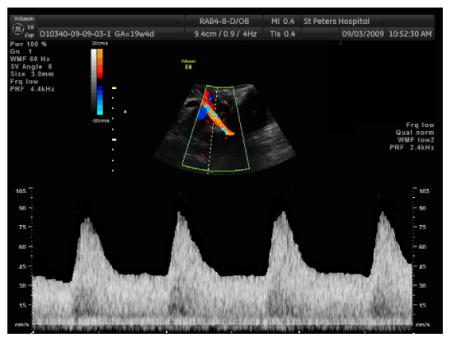
-
Waveform analysis:
For each case, the following measurements and qualitative assessments should be made of the best image that demonstrates a series of ≥ 3 uniform waveforms
Document angle of insonation.
-
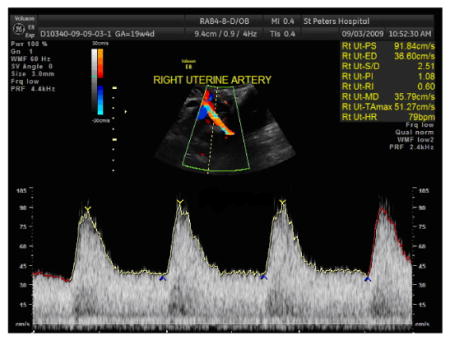
Using the auto- or manual-trace function, measure the heart rate (HR), peak systolic velocity (PSV), systolic/diastolic ratio (S/D), resistance index (RI), and pulsatility index (PI).
If the screen is completely filled with uniform waveforms with well-defined envelopes, then the waveform can be measured using the automated technique.
If difficulty is encountered in obtaining the Doppler waveforms but at least 3 uniform waveforms are obtained, then the most ideal waveform should be manually traced.
-
Sample auto-tracing of uterine artery Doppler waveform:
-
Sample manual tracing of uterine artery Doppler waveform:
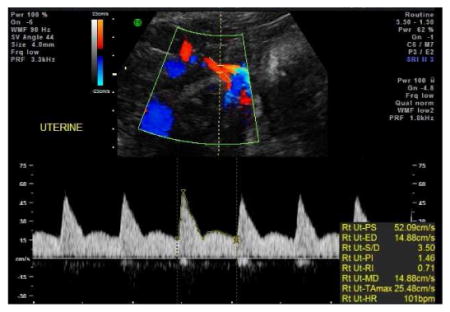
-
Clear the tracing performed above, and then measure the systolic acceleration time
The systolic acceleration time is the distance on the x-axis from the start of systole to the peak of systole; this should be measured by placing a caliper at the beginning of systole and at the peak systolic velocity and reporting the distance between in milliseconds.
-
Determine the presence or absence of an early-diastolic notch
A notch is present when there is a clear upswing in the waveform at the beginning of diastole.
Any perceptible difference between the peak velocity at mid-diastole in comparison to the velocity at the early-diastolic trough should be considered as presence of an early-diastolic notch.
-
Sample uterine artery Doppler waveform WITHOUT early diastolic notch:
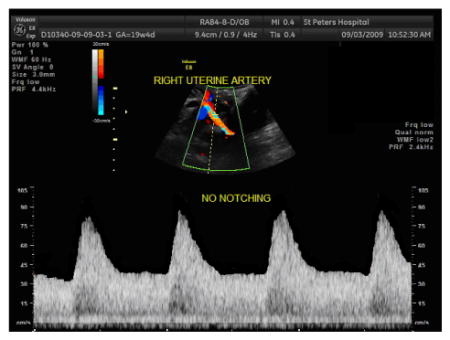
-
Sample uterine artery Doppler waveform WITH early diastolic notch:
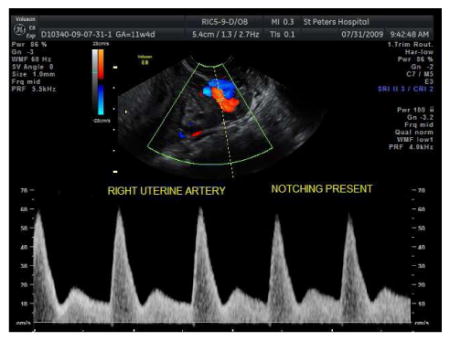
-
If there is a perceptible early-diastolic notch, clear the prior calipers and measure the depth of the early-diastolic notch
The depth is the difference between the peak diastolic velocity at mid-diastole and the lowest velocity in early–diastole.
-
Determine the presence or absence of a systolic notch
A systolic notch is a momentary decrease in the rate of decline in the maximal flow velocity during the decelerative or down stroke phase of the systolic wave.
-
Sample image demonstrating a systolic notch:
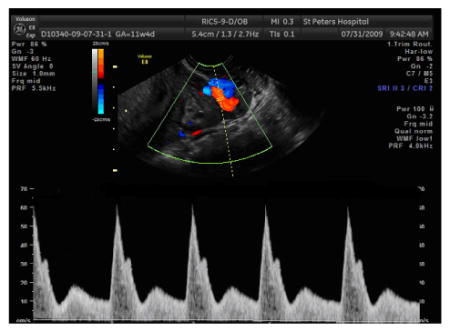
Determine the direction of flow in early- and late- diastole, and classify it as forward, absent or reversed.
Describe the shape of the peak of the systolic portion of the uterine Doppler waveform as “peaked” or “rounded”
Repeat this procedure on contralateral uterine artery
Certification of Sonographers to Obtain Images
To ensure accuracy of ultrasound data, all scans used to derive research data (with the exception of fetal anatomy) – including research ultrasounds and clinical ultrasounds from which data are abstracted – are performed by certified sonographers. Registered Diagnostic Medical Sonographers (RDMS-certified) who have passed the specialty examination in obstetrics and gynecology may become credentialed by submitting appropriate images for review. Likewise, OB/Gyn generalists, MFM attending physicians and MFM fellows who regularly perform obstetrical ultrasound as part of their clinical practice may become credentialed by submitting appropriate images for review. OB/Gyn residents may become credentialed to collect fetal biometry and cervical length measurements under the direct supervision of a credentialed attending or fellow by submitting appropriate images for review.
Fetal Biometry and Amniotic Fluid Measurement Certification
Certification Based on Other Studies
Sonographers and physicians currently certified through the NICHD National Standard for Normal Fetal Growth Study (NICHD/NFGS) to perform fetal biometry measurements (crown rump length [CRL], biparietal diameter [BPD], head circumference [HC], abdominal circumference [AC] and femur diaphysis length [FDL]) and amniotic fluid measurements (amniotic fluid index [AFI]) will be considered certified for these measures on patients enrolled in nuMoM2b once the nuMoM2b Registration ID of the person seeking certification, the name of the study under which certification was obtained (NICHD/NFGS), date of certification, name of the certifier, and expiration date for certification, if any, are submitted to the Data Coordinating and Analysis Center (DCAC) via the study website.
Sonographers and Physicians Not Previously Certified
Sonographers / physicians who are NOT certified through the NFGS must be locally certified for fetal biometry measurements (CRL, BPD, HC, AC and FDL) and amniotic fluid measurements for nuMoM2b. Registered Diagnostic Medical Sonographers (RDMS-certified) who have passed the specialty examination in obstetrics and gynecology may become credentialed by submitting appropriate images and related data for local review. Likewise, OB/Gyn generalists, MFM attendings, and MFM fellows who regularly perform obstetrical ultrasound as part of their clinical practice may become credentialed by submitting this information for review. OB/Gyn residents may become credentialed to collect these measurements under the direct supervision of a credentialed attending or fellow and by submitting appropriate materials for review. The local credentialing process is described below.
The site PI selects one or more physicians who either have completed an MFM fellowship or regularly perform obstetrical ultrasounds as a significant part of his/her practice to serve as local certifiers who review images for local credentialing. Each local certifier must view a 9-minute Webinar training session that reviews the fetal biometry measurements expectations for nuMoM2b. The Webinar and training slides are posted on the Documents/Training Slides/Webinars page of the project website. The training of local certifiers through the Webinar should be documented on the Log of Fetal Biometry Certifiers. This log is maintained in training files by the site coordinator. The site coordinator also provides the names of trained local certifiers to the DCAC so that their names can be added to the nuMoM2b website. Local certifiers may perform fetal biometry and amniotic fluid measurements for nuMoM2b without submitting images for review. However, coordinators must enter appropriate information on the project website indicating that they are self-approved for CRL, BPD, HC, AC, FDL, and AFI.
-
The sonographer or physician seeking certification submits images for each of the structures for which credentialing is sought – CRL, BPD, HC, AC, FDL, and AFI – to the site study coordinator or designee.
One image from each of three different patients should be submitted for each of the biometric measurements. If desired, the same images can be used for both BPD and HC. The three images for a given structure (e.g., HC) should be collected from three individual patients, but images of different structures (e.g., HC and AC) may come from the same patient.
For the amniotic fluid index, four images – one of each quadrant – should be submitted for each of three patients.
The study site coordinator documents receipt of the images from each sonographer or physician seeking certification and submits the set of images and a NuMoM2b Fetal Biometry Credentialing Evaluation Form to a local certifier.
The local certifier reviews the submitted images, records the results of the review on the form, and returns the results to the site coordinator.
-
THREE passing images for each biometric measurement for which credentialing is sought are required to be certified to perform those biometry measurements on study patients. To “pass” for a given image, the image quality, acquisition plane, and caliper placement must all be “acceptable.”
If a sonographer or physician does not receive passing scores for each of the three images for a given type of measurement, the individual is given their results and asked to submit three images for certification for any measures that were not passed. Images that passed certification the first time may be resubmitted as part of this set. If desired, the credentialer can provide guidance on how to adjust technique in order to improve the images chosen for submission.
-
THREE sets of 4 passing images demonstrating accurate measurement of the AFI are required to be certified to perform amniotic fluid measurements on study patients. For an image of a given quadrant to “pass”, the image quality and caliper placement must both be “acceptable”. For an AFI to “pass” for a given patient, all four images (one of each quadrant) must “pass”.
If a sonographer or physician does not receive passing scores for each of the three AFIs submitted, the individual is given their results and asked to submit three additional AFIs. AFIs that passed certification the first time may be resubmitted as part of this set. If desired, the credentialer can provide guidance on how to adjust technique in order to improve the images chosen for submission.
The coordinator keeps track of the certification process. As credentialing for the various measurements is granted, the coordinator enters the nuMoM2b Registration ID of the person to be certified, the date of credentialing, the measures for which the individual has been credentialed, the name of the person who reviewed the images for credentialing purposes, and the expiration date of certification (if any) on the nuMoM2b website.
Level 1 Anatomy Scan Certification
Any sonographer or physician who routinely performs obstetric ultrasound will be considered certified to perform Level 1 anatomy scans on study patients. If the sonographer provides RDMS-certification dates on their Ultrasound Certification Registration form, then the site coordinator may enter the nuMoM2b Registration ID number of that individual, the date of RDMS Certification, and the date RDMS certification expires (if known) on the nuMoM2b website. However, this step is no longer required as part of the nuMoM2b certification process.
Cervical Length Measurement Certification
Certification Based on Other Studies
Sonographers and physicians who were centrally certified to obtain cervical length measurements for the NICHD Maternal-Fetal Medicine Units Network’s (MFMU) Randomized Trial of 17P for Prevention of Preterm Birth in Nulliparous Women with a Short Cervix (SCAN), for the NICHD Vaginal Ultrasound Cerclage Trial (VUCT), or the Effect of Vaginal Progesterone Administration in the Prevention of Preterm Birth in Women with a Short Cervix Trial (also known as Vaginal Progesterone Bioadhesive Gel [Prochieve] Extending Gestation, a New Therapy for Short Cervix Trial [PREGNANT trial]) may be considered certified for these measures for patients enrolled in nuMoM2b. Coordinators enter these credentials on the study website, providing the nuMoM2b Registration ID number, the name of the study under which certification was obtained (SCAN, VUCT, PREGNANT), the date of certification, name of the certifier, and expiration date for certification (if any).
Sonographers and Physicians Not Previously Certified
All personnel performing cervical length measurements who were not certified for one of the above studies must undergo a central certification process through nuMoM2b. Registered Diagnostic Medical Sonographers (RDMS-certified) who have passed the specialty examination in obstetrics and gynecology, OB/Gyn generalists, MFM attendings and MFM fellows who regularly perform obstetrical ultrasound as part of their clinical practice may become credentialed by submitting the materials outlined below for central review. OB/Gyn residents may become credentialed to collect cervical length measurements under the direct supervision of a credentialed attending or fellow by submitting the materials described below for review.
The sonographers or physicians who are NOT certified through one of the above studies should first review the nuMoM2b Cervical Length Measurement Training slides provided by the expert central reviewer. These slides are available on the Documents/Training Slides/Webinars page of the nuMoM2b website.
A sonographer or physician seeking central certification submits three images of cervical length measurements from each of five patients (15 in total) to the site coordinator using the criteria in the training slides to select images. The scans should be accompanied by the NuMoM2b Cervical Length Measurement Credentialing Form.
The expert central reviewer reviews the submitted images via the website and records the results of the review on the electronic NuMoM2b Cervical Length Measurement Credentialing Form. The DCAC provides the results to the coordinator via the study website.
To become certified to perform cervical sonography for nuMoM2b, FOUR of the five sets of images must be acceptable. Sonographers / physicians who do not pass will be asked to submit five new sets of images that meet the criteria in the teaching set. All who perform cervical sonography are welcome to contact the central certifier. NuMoM2b staff can contact him regarding any questions about certification ratings.
Uterine Artery Doppler Certification
Certification
Sonographers and physicians must be centrally certified to perform uterine artery Doppler velocimetry specifically for nuMoM2b. Registered Diagnostic Medical Sonographers (RDMS-certified) who have passed the specialty examination in obstetrics and gynecology, OB/GYN generalists, MFM attendings, and MFM fellows who regularly perform Doppler studies as part of their clinical practice may become credentialed by submitting the materials outlined below for review.
A 15-minute webinar covering standards for the uterine artery Doppler ultrasound is recorded and available for future reference. The Webinar and training slides are posted on the Documents/Training Slides/Webinars page of the project website.
The sonographer or physician seeking central certification submits required images of uterine artery waveforms from three different patients to the site coordinator along with a completed copy of the Uterine Artery Doppler Credentialing Submission Form.
-
For each of the three cases, the submitted images will be evaluated for the following:
Quality of the waveform obtained;
Accurate reporting of the angle of insonation, and use of angle correction;
Accurate measurement (using either auto- or manual-trace) and reporting of the heart rate (HR), peak systolic velocity (PSV), systolic/diastolic ratio (S/D), resistance index (RI), and pulsatility index (PI);
Accurate measurement of systolic acceleration time;
Accurate assessment of the presence or absence of an early-diastolic notch, and if present, accurate measurement of the depth of the early-diastolic notch;
Accurate assessment of the presence or absence of a systolic notch;
Accurate description of the direction of flow in early- and late-diastole as forward, absent or reversed; and
Description of the shape of the peak of the systolic portion of waveform as either peaked or rounded.
All submitted images and results are reviewed centrally via the website by one of the expert central reviewers. The results of the review are recorded on the NuMoM2b Uterine Artery Doppler Certification Evaluation Form. In order to be credentialed to perform uterine artery Doppler scans for nuMoM2b, providers must receive passing scores for all parameters of all three cases. The DCAC provides the results of the review to the coordinator via the study website.
The sonographer or physician who fails certification should seek remedial training from a sonographer or physician who does uterine artery Doppler studies at the site before any further efforts are taken at certification. After this remedial training, the individual may submit three different cases demonstrating uterine artery Doppler waveforms as described above. The new set will again be reviewed by a central reviewer who will record results of the review on the website.
Appendix 2. Collection and Processing of nuMoM2b Cord Blood, Placenta, Membranes, and Umbilical Cord Specimens
This text is taken from Chapter 12 of the nuMoM2b Manual of Operations.
Cord blood, placenta, membranes, and umbilical cord. Cord blood, placenta, membranes, and umbilical cord specimens are collected under either “ideal” or “standard” collection procedures. The ideal is to collect and process the specimens within 30 minutes of delivery of the placenta, with flash freezing in liquid nitrogen for most samples. Under “standard” collection, cord blood is promptly collected and refrigerated along with the whole placenta, membranes, and umbilical cord for later harvesting and processing of a reduced set of samples within 72 hours of delivery. “Ideal” collections were performed by sites with the infrastructure to perform these procedures on a voluntary basis.
Delivery – IDEAL COLLECTION of Cord Blood, Placenta, Membrane, and Umbilical Cord (Voluntary Convenience Sample – Can Process Delivery Samples Immediately)
Under “ideal” collection, cord blood, placenta, membrane, and umbilical cord specimens should be collected and processed within 30 minutes of delivery of the placenta. Also, processing of placenta and membrane specimens collected for RNA under the “ideal” conditions is performed using RNAlater® rather than flash freezing.
Cord Blood (ideal collection) – DNA and Plasma for Protein/Metabolites
Cord blood should be collected as soon as possible after delivery prior to the coagulation of blood in the placenta and cord. It should be obtained by venipuncture of the cord after cleaning the cord with alcohol. It should not be obtained by allowing cord blood to “drip” into tubes. A purple top (EDTA) tube should be collected for DNA (4–8 mL/tube). A second purple top (EDTA) tube should be collected for plasma (4–8 mL/tube). The tubes will be collected by research personnel within 30 minutes of delivery. If there is only a small amount of cord blood, fill the purple top tube for DNA first.
Processing Cord Blood Samples (ideal collection)
DNA/EDTA 10 mL Tube
Affix provided barcode label to a purple top (EDTA) tube (BD Vacutainer 16 × 100 mm tube, Fisher catalog #367863). Affix the matching barcode label to the appropriate specimen tracking form.
Invert tube 10 X.
Place in a −20°C freezer at nurse’s convenience, but no later than 8 hours. Make sure to invert the tube again just before placing in the freezer so the blood is completely mixed.
Store in −20°C freezer until ready to ship.
Ship on dry ice.
Plasma/EDTA 10 mL Tube
Invert EDTA purple top tube 10 X.
Affix a study ID label to purple top EDTA tube (BD Vacutainer 16 × 100 mm tube, Fisher catalog #366643).
Affix provided barcode specimen labels to 2–4 cryovials and set aside 2–4 screw caps with white caps. Use additional labels and cryovials if more than four aliquots are obtained. Affix the matching barcode labels to the appropriate specimen tracking form.
Spin tube at 1500 g for 10 minutes in a refrigerated centrifuge at 4°C immediately after blood is obtained.
If the plasma is hemolyzed, mark the appropriate checkbox on the specimen tracking form.
Using a sterile pipette, transfer a 0.5 mL aliquot of plasma in up to four 2 mL cryovials (Fisherbrand Externally Threaded Cryogenic Vials, Fisher catalog #10-500-26). Close with white caps.
Immediately after the centrifugation and separation process (10 min. max) flash freeze cryovials in liquid nitrogen.
Store in −70°C (or −80°C) freezer.
Ship on dry ice.
Placenta, Membranes, and Umbilical Cord (ideal collection)
The placenta, the attached placental membranes, and the umbilical cord should be immediately put in a placenta bucket after delivery and stored on ice (or in a refrigerator) until they are processed for samples. The placenta should be processed within a maximum of 30 minutes after delivery.
Specimens to be collected include:
frozen samples of placenta, membranes, and umbilical cord, used variously for RNA, protein/metabolites, DNA/epigenetics, and fetal DNA; and
formalin-fixed ambient temperature samples of placenta, membranes, and umbilical cord for histology.
The formalin-fixed ambient temperature samples of placenta, membranes, and cord from each delivery will be stored in one, two, or three 15mL conical tubes along with liquid medium. They will be stored in any order and combination – the smallest number of tubes should be used that can hold all three specimen types with enough room for the liquid medium.
Placental Weight
Before any samples are taken from the placenta, it needs to be weighed.
Cut the umbilical cord off at the insertion site. Make sure to mark the ends of the cord to show which end is the placenta end and which end is the fetal end. Consult with your pathology department regarding their preferred method of marking the cord. Mark the placenta end at least 4 cm from the end, to allow for the removal of the umbilical cord samples without disturbing the mark.
The placenta may be rinsed in normal saline to remove blood clots before weighing.
Leaving the fetal membranes attached, place the placenta on a scale to obtain the weight.
Record the weight on the specimen tracking form.
Once the placenta is weighed, you can proceed to obtain the needed samples of placenta, fetal membranes and umbilical cord segments.
Placental Samples (ideal collection) – RNA, Protein/Metabolites, DNA/Epigenetics, Fetal DNA, and Histology
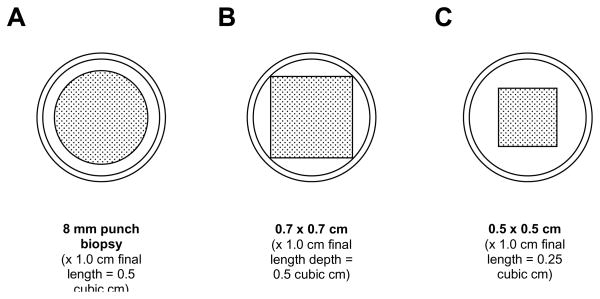
Placental samples are taken with the fetal side of the placenta facing up. Samples to be frozen are taken using a punch biopsy or a scalpel (instructions are described for both in the paragraphs below). Placental samples for histology are taken using a scalpel. For a punch biopsy the cut on the fetal surface is circular and the sample is described according to the diameter of the circle and the depth into the placenta (toward the maternal side); for collection with a scalpel, the cut on the fetal surface is square and the sample is described by the dimensions of the square and the depth of the sample.
A total of five placental samples will be collected, as follows:
four 0.5 cubic cm samples, to be frozen. Each will be collected to a depth of 1.3 cm, and then 0.3 cm (3 mm), including the membranes, will be trimmed from the top (i.e., the fetal side) of the sample, resulting in a final length of 1 cm. Each may be obtained with a 0.8 cm diameter circular surface dimension using an 8 mm punch biopsy, or with a 0.7 × 0.7 cm square surface dimension by cutting with a scalpel. The target volume is 0.5 cubic cm, but a sample as small as 0.25 cubic cm (square surface with 0.5 × 0.5 cm dimensions, X 1 cm final length) is acceptable. Each sample needs to be small enough to be surrounded by liquid medium (where used) in the specimen container (2 mL cryovial, for which the internal diameter is 1 cm). Dimensions should not be larger than 0.8 cm in diameter or 0.7 × 0.7 cm, otherwise the sample may press up against the inner wall of the specimen container. See Figure 1.
one 1 cm × 1 cm × full thickness (about 2 cm in term placentas) sample (for histology), to be fixed in formalin. This will be about 2 cubic cm in term placentas.
Figure 1.
Cross-sections of placental specimens relative to the 1 cm inner diameter of a 2 mL cryovial. Specimens should not press against the inner wall of the cryovial; where liquid medium is used, specimens should be surrounded by the medium. These requirements are satisfied by an 8 mm diameter punch biopsy (panel A) or a scalpel cut specimen with cross-sectional dimensions between 0.7 × 0.7 cm (panel B) and 0.5 × 0.5 cm (panel C). Obtained with a depth of 1 cm, these correspond to a specimen volume of 0.5 cubic cm (panel A) or between 0.5 cubic cm (panel B) and 0.25 cubic cm (panel C).
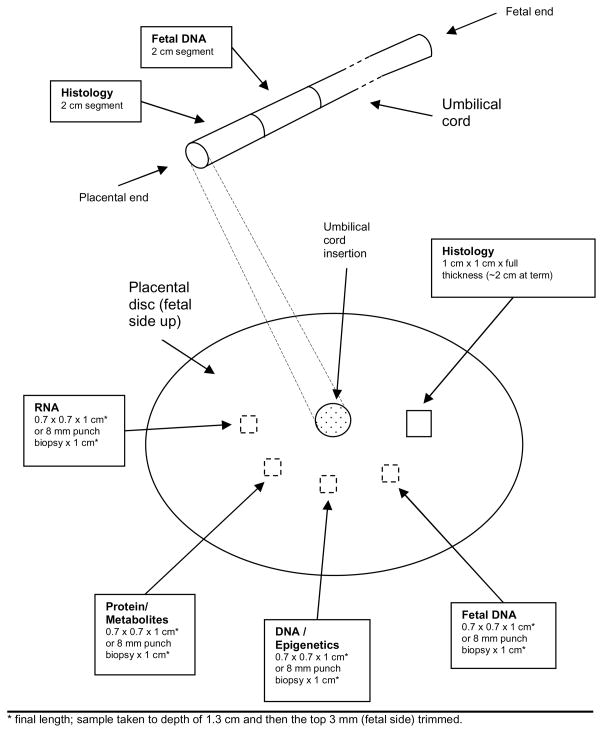
These should be obtained from areas without gross pathology. The samples should be obtained in a semi-circular fashion around the area of cord insertion, as shown in Figure 2. For marginal umbilical cord insertions, obtain the samples in a semi-circular fashion around the center of the placental disc.
Figure 2.
Locations for collection of placenta and umbilical cord samples. Shown are the locations for the ideal delivery collection. (For the standard delivery collection, ignore the locations for specimens for RNA, protein/metabolites, and DNA/epigenetics, which are obtained only under ideal collection.) Locations for the placenta samples other than for histology are shown with dashed lines, because the sample is to be taken to a depth of 1.3 cm and then 3 mm from the top (i.e., the fetal side) is to be trimmed, resulting in a sample from the interior of the placental thickness, with final length of 1 cm.
Processing Placental Samples (ideal collection)
Dispense 20 cc of cold 1X PBS into a 4 ounce tissue container; this will be used for washing the samples. [A separate 4 ounce tissue container will be used for the formalin fixation of the histology samples.]
RNA (This is a priority to process first. Process this sample (through to placement in the refrigerator (step 4) before proceeding to the next sample)
After obtaining the sample, cut the sample into four pieces.
Wash each of the four small pieces by dipping (2×) in cold 1X PBS (about 20 cc) and remove excess liquid by wicking the sample on sterile gauze. Use forceps to wash and manipulate the tissue samples.
Place all four small samples into a 2 mL cryovial (Fisherbrand Externally Threaded Cryogenic Vials, Fisher catalog #10-500-26) with clear cap containing 1mL of RNAlater®. Tube should be labeled with appropriate barcode prior to use; affix the matching label to the appropriate tracking form.
Place cryovial in refrigerator (4°C) for 48 hour s.
After 48 hours, pipette off remaining RNAlater® and discard.
Place and store cryovial in −70°C (or −80°C) freezer.
Ship on dry ice.
Protein/Metabolites
Wash 0.7 × 0.7 × 1 cm sample by dipping (2×) in cold 1X PBS (about 20 cc) and remove excess liquid by wicking the sample on sterile gauze. Use forceps to wash and manipulate the tissue sample.
Place sample into 2 mL cryovial (Fisherbrand Externally Threaded Cryogenic Vials, Fisher catalog #10-500-26) with green cap containing 1 mL 1X DPBS. Tube should be labeled with appropriate barcode prior to use; affix the matching label to the appropriate tracking form.
Make sure the tissue is completely submerged in and surrounded by the 1X DPBS. Otherwise, if the sample presses against the walls of the tube, trim the sample.
Flash freeze in liquid nitrogen within 10 minutes after processing is complete.
Store in −70°C (or −80°C) freezer.
Ship on dry ice.
DNA/Epigenetics
Wash 0.7 × 0.7 × 1 cm sample by dipping (2×) in cold 1X PBS (about 20 cc) and remove excess liquid by wicking the sample on sterile gauze. Use forceps to wash and manipulate the tissue sample.
Place sample in 2 mL cryovial (Fisherbrand Externally Threaded Cryogenic Vials, Fisher catalog #10-500-26) with clear cap with no fluid or buffer. Tube should be labeled with appropriate barcode prior to use; affix the matching label to the appropriate tracking form.
Flash freeze in liquid nitrogen within 10 minutes after processing is complete.
Store in −70°C (or −80°C) freezer.
Ship on dry ice.
Fetal DNA
Wash 0.7 × 0.7 × 1 cm sample by dipping (2×) in cold 1X PBS (about 20 cc) and remove excess liquid by wicking the sample on sterile gauze. Use forceps to wash and manipulate the tissue sample.
Place sample in a 2 mL cryovial (Fisherbrand Externally Threaded Cryogenic Vials, Fisher catalog #10-500-26) with blue cap containing 1 mL of 10 mM Tris-Hcl, 0.1 mM EDTA. Tube should be labeled with appropriate barcode prior to use; affix the matching label to the appropriate tracking form.
Make sure the tissue is completely submerged in and surrounded by the fluid medium. Otherwise, if the sample presses against the walls of the tube, trim the sample.
Flash freeze in liquid nitrogen within 10 minutes after processing is complete.
Store in −70°C (or −80°C) freezer.
Ship on dry ice.
Histology
Wash 1 × 1 × 2 (full thickness) cm sample by dipping (2×) in cold 1X PBS (about 20 cc) and remove excess liquid by wicking the sample on sterile gauze. Use forceps to wash and manipulate the tissue sample.
Place the sample in a separate, empty 4 ounce tissue container and cover with formalin (membrane and umbilical cord histology samples will also be added, see below) and leave the container at room temperature until the next day.
See below for further processing and storage after 24 hours (max 72 hours) of formalin fixation.
Fetal Membranes (ideal collection) – RNA, Protein/Metabolites, DNA/Epigenetics, and Histology
The fetal membranes (which are attached to the placenta and collected and temporarily stored on ice or refrigerator as described above) should be processed within a maximum of 30 minutes after delivery. Three samples (2 × 1 cm) will be collected from the site of rupture. The samples will be washed in 1X PBS. Another 2 × 1 cm sample should be collected at site of rupture for histology, and will be processed and stored together with the placenta and umbilical cord histology samples.
Processing Fetal Membrane Samples (ideal collection)
RNA (This is a priority to process first. Process this sample (through to placement in the refrigerator (step 4) before proceeding to the next sample)
Wash sample by dipping (2×) in cold 1X PBS (about 20 cc) and remove excess liquid by wicking the samples on sterile gauze. Use forceps to wash and manipulate the tissue samples.
Place sample into a 2 mL cryovial (Fisherbrand Externally Threaded Cryogenic Vials, Fisher catalog #10-500-26) with clear cap containing 1mL of RNAlater®. Tube should be labeled with appropriate barcode prior to use; affix the matching label to the appropriate tracking form.
Make sure the entire sample is immersed in the RNAlater®.
Place cryovial in refrigerator (4°C) for 48 hour s.
After 48 hours, pipette off remaining RNAlater® and discard.
Store in −70°C (or −80°C) freezer.
Ship on dry ice.
Proteins/Metabolites
Wash sample by dipping (2×) in cold 1X PBS (about 20 cc) and remove excess liquid by wicking the samples on sterile gauze. Use forceps to wash and manipulate the tissue samples.
Place sample into 2 mL cryovial (Fisherbrand Externally Threaded Cryogenic Vials, Fisher catalog #10-500-26) with green cap containing 1 mL 1X DPBS. Tube should be labeled with appropriate barcode prior to use; affix the matching label to the appropriate tracking form.
Make sure the tissues are completely submerged in the 1X DPBS.
Flash freeze in liquid nitrogen within 10 minutes after processing is complete.
Store in −70°C (or −80°C) freezer.
Ship on dry ice.
DNA/Epigenetics
Wash sample by dipping (2×) in cold 1X PBS (about 20 cc) and remove excess liquid by wicking the samples on sterile gauze. Use forceps to wash and manipulate the tissue samples.
Place sample in 2 mL cryovial (Fisherbrand Externally Threaded Cryogenic Vials, Fisher catalog #10-500-26) with clear cap with no fluid or buffer. Tube should be labeled with appropriate barcode prior to use; affix the matching label to the appropriate tracking form.
Flash freeze in liquid nitrogen within 10 minutes after processing is complete.
Store in −70°C (or −80°C) freezer.
Ship on dry ice.
Histology
Wash sample from near the site of rupture sample by dipping (2×) in cold 1X PBS (about 20 cc) and remove excess liquid by wicking the sample on sterile gauze. Use forceps to wash and manipulate the tissue sample.
Place membranes in same 4 ounce tissue container with formalin as the placenta (and cord segment, see below) and leave it at room temperature until the next day.
See below for further processing and storage after 24 hours (max 72 hours) of formalin fixation.
Umbilical Cord (ideal collection) – Fetal DNA and Histology
The umbilical cord should be processed within a maximum of 30 minutes after delivery. Cut the first 2 cm segment at the placental end of the removed umbilical cord for histology. Cut the next 2 cm segment for fetal DNA. The histology sample will be processed and stored together with the placenta and membrane histology samples.
Processing Umbilical Cord Samples (ideal collection)
Histology – 2 cm segment (first segment from the placental end of the removed umbilical cord)
Wash sample by dipping (2×) in cold 1X PBS (about 20 cc) and remove excess liquid by wicking the sample on sterile gauze. Use forceps to wash and manipulate the tissue sample.
Place umbilical cord segment in same 4 ounce tissue container with formalin as the placenta and membranes; close the container and leave it at room temperature until the next day.
See below for further processing and storage after 24 hours (max 72 hours) of formalin fixation.
Fetal DNA – 2 cm segment (second segment from the placental end of the removed umbilical cord)
Wash sample by dipping (2×) in cold 1X PBS (about 20 cc) and remove excess liquid by wicking the sample on sterile gauze. Use forceps to wash and manipulate the tissue sample.
Place segment in a 15 mL cryovial (Thermo Scientific Nalgene cryogenic vial, Fisherbrand catalog #5005-0015) and cover completely with 10 mM Tris-Hcl, 0.1 mM EDTA. Tube should be labeled with appropriate barcode prior to use; affix the matching label to the appropriate tracking form.
If the diameter of the cord exceeds that of the 15 mL cryovial, trim lengthwise to fit.
Place and store in −70°C (or −80°C) freezer.
Ship on dry ice.
Further processing and storage of 24-hour formalin-fixed placenta, membrane, and umbilical cord samples (ideal collection)
After about 24 hours (max 72 hours), rinse each formalin-fixed sample (placenta, membrane, cord) 1× with 20 cc cold 1X PBS and remove excess liquid by wicking the sample on sterile gauze. Use forceps to wash and manipulate the tissue sample.
Place samples in as many as three 15 mL BD Falcon conical tubes (Fisher catalog #1495970C). Place the samples in the tubes in any order and combination – use the smallest number of tubes that can hold all three specimen types with enough room for the liquid medium (Step 3).
Cover the samples in each tube with 1X PBS.
Tubes will have been labeled with appropriate barcodes prior to use, and the matching labels affixed to the appropriate tracking form.
Store at room temperature.
Ship at room temperature.
Note: If the diameter of the umbilical cord exceeds that of the conical tube (1.5 cm), quarter the cord lengthwise and trim the length to less than 2 cm so that the four pieces of umbilical cord can fit end to end in the tube along with the samples of placenta and membrane.
Delivery – STANDARD COLLECTION of Cord Blood, Placenta, Membrane, and Umbilical Cord (Cannot Process Delivery Samples Immediately)
Under “standard” collection, cord blood is promptly collected and refrigerated along with the whole placenta, membranes, and umbilical cord for later harvesting and processing of a reduced set of samples within 72 hours of delivery.
Cord Blood (standard collection) – DNA
Cord blood should be collected as soon as possible after delivery prior to the coagulation of blood in the placenta and cord. It should be obtained by venipuncture of the cord after cleaning the cord with alcohol. If staff is unable to obtain cord blood by venipuncture, the blood can drip into the tube. A purple top (EDTA) tube should be collected for DNA (4–8 mL). The tube will be collected by labor and delivery personnel and placed in a refrigerator. (A piece of placenta and a segment of umbilical cord will also be obtained for a fetal DNA source, see below.)
Processing Cord Blood Samples (standard collection)
DNA/EDTA 10 mL Tube
Affix provided barcode specimen label to purple top tube (BD Vacutainer 16 × 100 mm tube, Fisher catalog #366643). Affix the matching barcode label to the appropriate specimen tracking form.
Invert tube 10 X and place purple top tube in a refrigerator as soon as possible.
May leave in refrigerator for up to 72 hours.
Once tube is picked up from Labor & Delivery, place in a −20°C freezer. Make sure to invert the tube again just before placing in the freezer so the blood is completely mixed.
Store in −20°C freezer until ready to ship.
Ship on dry ice.
Placenta, Membranes, and Umbilical Cord (standard collection)
The placenta, the attached placental membranes, and the umbilical cord should be immediately put in a placenta bucket after delivery and stored in a refrigerator until they are processed for samples. All processing for frozen samples and all processing of histology samples (up to the immersion in formalin) should be completed within 72 hours after delivery.
Processing steps are described below. In cases where the placenta (and membranes and umbilical cord) is ordered to go to pathology, research staff can take the samples they need before it is sent to pathology if they are available to do so. If the placenta is sent to pathology before research staff can obtain the samples needed for the study, staff should contact the pathology department and make arrangements to pick up the placenta once they are finished with their analyses. All research samples can be obtained once the pathology department is finished with the placenta, even if it has been longer than 72 hours. Procedures for identifying the placenta to the pathology department as a NuMoM2b placenta, for retaining the placenta, cord, and membranes in refrigeration after pathologic examination, and for obtaining the pathologist’s measured placenta weight should be established in advance with the pathology department.
Specimens to be collected include:
frozen samples of placenta and umbilical cord, for fetal DNA
formalin-fixed ambient temperature samples of placenta, membranes, and umbilical cord for histology.
The formalin-fixed ambient temperature samples of placenta, membranes, and cord from each delivery will be stored in one, two, or three 15mL conical tubes along with liquid medium. They will be stored in any order and combination – the smallest number of tubes should be used that can hold all three specimen types with enough room for the liquid medium.
Placental Weight
Before any samples are taken from the placenta, it needs to be weighed.
Cut the umbilical cord off at the insertion site. Make sure to mark the ends of the cord to show which end is the placenta end and which end is the fetal end. Consult with your pathology department regarding their preferred method of marking the cord. Mark the placenta end at least 4 cm from the end, to allow for the removal of the umbilical cord samples without disturbing the mark.
The placenta may be rinsed in normal saline to remove blood clots before weighing.
Leaving the fetal membranes attached, place the placenta on a scale to obtain the weight.
Record the weight on the specimen tracking form.
Once the placenta is weighed, you can proceed to obtain the needed sample of placenta, fetal membranes and umbilical cord segments.
Placental Samples (standard collection) – Fetal DNA and Histology
Placental samples are taken with the fetal side of the placenta facing up. Samples to be frozen are taken using a punch biopsy or a scalpel (instructions are described for both in the paragraphs below). Placental samples for histology are taken using a scalpel. For a punch biopsy the cut on the fetal surface is circular and the sample is described according to the diameter of the circle and the depth into the placenta (toward the maternal side); for collection with a scalpel, the cut on the fetal surface is square and the sample is described by the dimensions of the square and the depth of the sample.
Two placental samples will be collected, as follows:
one 0.5 cubic cm sample, to be frozen. This sample will be collected to a depth of 1.3 cm, and then 0.3 cm (3 mm), including the membranes, will be trimmed from the top (i.e., the fetal side) of the sample, resulting in a final length of 1 cm. The sample may be obtained with a 0.8 cm diameter circular surface dimension using an 8 mm punch biopsy, or with a 0.7 × 0.7 cm square surface dimension by cutting with a scalpel. The target volume is 0.5 cubic cm, but a sample as small as 0.25 cubic cm (square surface with 0.5 × 0.5 cm dimensions, X 1 cm final length) is acceptable. The sample needs to be small enough to be surrounded by the liquid medium in the specimen container (2 mL cryovial, for which the internal diameter is 1 cm). Dimensions should not be larger than 0.8 cm in diameter or 0.7 × 0.7 cm, otherwise the sample may press up against the inner wall of the specimen container. See Figure 1 (above).
one 1 cm × 1 cm × full thickness (about 2 cm in term placentas) sample (for histology), to be fixed in formalin. This will be about 2 cubic cm in term placentas.
These should be obtained from areas without gross pathology. These should be collected from locations as shown in Figure 2 (above).
Processing Placental Samples (standard collection)
Dispense 20 cc of cold 1X PBS into a 4 ounce tissue container; this will be used for washing the samples. [A separate 4 ounce tissue container will be used for the formalin fixation of the histology samples.]
Fetal DNA
Wash 0.7 × 0.7 × 1 cm sample by dipping (2×) in cold 1X PBS (about 20 cc) and remove excess liquid by wicking the sample on sterile gauze. Use forceps to wash and manipulate the tissue sample.
Place sample in a 2 mL cryovial (Fisherbrand Externally Threaded Cryogenic Vials, Fisher catalog #10-500-26) with blue cap containing 1 mL of 10 mM Tris-Hcl, 0.1 mM EDTA. Tube should be labeled with appropriate barcode prior to use; affix the matching label to the appropriate tracking form.
Make sure the tissue is completely submerged in and surrounded by the fluid medium. Otherwise, if the sample presses against the walls of the tube, trim the sample.
Place and store in −70°C (or −80°C) freezer imme diately after processing.
Ship on dry ice
Histology
Wash 1 × 1 × 2 (full thickness) cm sample by dipping (2×) in cold 1X PBS (about 20 cc) and remove excess liquid by wicking the sample on sterile gauze. Use forceps to wash and manipulate the tissue sample.
Place the sample in a separate, empty 4 ounce tissue container and cover with formalin (membrane and umbilical cord histology samples will also be added, see below) and leave the container at room temperature until the next day.
See below for further processing and storage after 24 hours (max 72 hours) of formalin fixation.
Fetal Membranes (standard collection) – Histology
The fetal membranes (which are attached to the placenta and collected and temporarily stored in the refrigerator as described above) should be processed within a maximum of 72 hours after delivery. A 2 × 1 cm sample should be collected at site of rupture for histology, and will be processed and stored together with the placenta and umbilical cord histology samples.
Processing Fetal Membrane Sample (standard collection)
Histology
Wash sample by dipping (2×) in cold 1X PBS (about 20 cc) and remove excess liquid by wicking the sample on sterile gauze. Use forceps to wash and manipulate the tissue sample.
Place membranes in same 4 ounce tissue container with formalin as the placenta (and cord segment, see below) and leave it at room temperature until the next day.
See below for further processing and storage after 24 hours (max 72 hours) of formalin fixation.
Umbilical Cord (standard collection) – Fetal DNA and Histology
The umbilical cord should be processed within a maximum of 72 hours after delivery. Cut the first 2 cm segment at the placental end of the removed umbilical cord for histology. Cut the next 2 cm segment for fetal DNA. The histology sample will be processed and stored together with the placenta and membrane histology samples.
Processing Umbilical Cord Samples (standard collection)
Histology – 2 cm segment (first segment from the placental end of the removed umbilical cord)
Wash sample by dipping (2×) in cold 1X PBS (about 20 cc) and remove excess liquid by wicking the sample on sterile gauze. Use forceps to wash and manipulate the tissue sample.
Place umbilical cord segment in same 4 ounce tissue container with formalin as the placenta and membranes; close the container and leave it at room temperature until the next day.
See below for further processing and storage after 24 hours (max 72 hours) of formalin fixation.
Fetal DNA – 2 cm segment (second segment from the placental end of the removed umbilical cord)
Wash sample by dipping (2×) in cold 1X PBS (about 20 cc) and remove excess liquid by wicking the sample on sterile gauze. Use forceps to wash and manipulate the tissue sample.
Place segment in a 15 mL cryovial (Thermo Scientific Nalgene cryogenic vial, Fisherbrand catalog #5005-0015) and cover completely with 10 mM Tris-Hcl, 0.1 mM EDTA. Tube should be labeled with appropriate barcode prior to use; affix the matching label to the appropriate tracking form.
If the diameter of the cord exceeds that of the 15 mL cryovial, trim lengthwise to fit.
Place and store in −70°C (or −80°C) freezer.
Ship on dry ice.
Further processing and storage of 24-hour formalin-fixed placenta, membrane, and umbilical cord samples (standard collection)
After about 24 hours (max 72 hours), rinse each formalin-fixed sample (placenta, membrane, cord) 1× with 20 cc cold 1X PBS and remove excess liquid by wicking the sample on sterile gauze. Use forceps to wash and manipulate the tissue sample.
Place samples in as many as three 15 mL BD Falcon conical tubes (Fisher catalog #1495970C). Place the samples in the tubes in any order and combination – use the smallest number of tubes that can hold all three specimen types with enough room for the liquid medium (Step 3). See note below if the diameter of the umbilical cord exceeds that of the conical tube.
Cover the samples in each tube with 1X PBS.
Tubes will have been labeled with appropriate barcodes prior to use, and the matching labels affixed to the appropriate tracking form.
Store at room temperature.
Ship at room temperature.
Note: If the diameter of the umbilical cord exceeds that of the conical tube (1.5 cm), quarter the cord lengthwise and trim the length to less than 2 cm so that the four pieces of umbilical cord can fit end to end in the tube along with the samples of placenta and membrane.
Footnotes
Reprints not available from authors.
The authors report no conflict of interest related to this project.
Trial Registry: NCT01322529
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Martin JA, Hamilton BE, Osterman M, Curtin SC, Mathews TJ. Nat Vital Stat Rep. Vol. 62. Hyattsville, MD: National Center for Vital Statistics; 2013. Births: Final data for 2012. [PubMed] [Google Scholar]
- 2.Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–72. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 3.Chang HH, Larson J, Blencowe H, et al. Preventing preterm births: analysis of trends and potential reductions with interventions in 39 countries with very high human development index. Lancet. 2013;381:223–34. doi: 10.1016/S0140-6736(12)61856-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Practice bulletin no. 130: prediction and prevention of preterm birth. Obstet Gynecol. 2012;120:964–73. doi: 10.1097/AOG.0b013e3182723b1b. [DOI] [PubMed] [Google Scholar]
- 5.North RA, McCowan LM, Dekker GA, et al. Clinical risk prediction for pre-eclampsia in nulliparous women: development of model in international prospective cohort. Bmj. 2011;342:d1875. doi: 10.1136/bmj.d1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seed PT, Chappell LC, Black MA, et al. Prediction of preeclampsia and delivery of small for gestational age babies based on a combination of clinical risk factors in high-risk women. Hypertens Pregnancy. 2011;30:58–73. doi: 10.3109/10641955.2010.486460. [DOI] [PubMed] [Google Scholar]
- 7.Conde-Agudelo A, Papageorghiou AT, Kennedy SH, Villar J. Novel biomarkers for the prediction of the spontaneous preterm birth phenotype: a systematic review and meta-analysis. BJOG: An International Journal of Obstetrics & Gynaecology. 2011;118:1042–54. doi: 10.1111/j.1471-0528.2011.02923.x. [DOI] [PubMed] [Google Scholar]
- 8.Lee KA, Chang MH, Park M-H, et al. A model for prediction of spontaneous preterm birth in asymptomatic women. Journal of Women’s Health. 2011;20:1825–31. doi: 10.1089/jwh.2011.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Myatt L, Clifton RG, Roberts JM, et al. First-trimester prediction of preeclampsia in nulliparous women at low risk. Obstetrics & Gynecology. 2012;119:1234–42. doi: 10.1097/AOG.0b013e3182571669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhong Y, Tuuli M, Odibo AO. First-trimester assessment of placenta function and the prediction of preeclampsia and intrauterine growth restriction. Prenatal Diagnosis. 2010;30:293–308. doi: 10.1002/pd.2475. [DOI] [PubMed] [Google Scholar]
- 11.MacDorman MF, Kirmeyer S, Wilson EC. Fetal and Perinatal Mortality, United States, 2006. Nat Vital Stat Rep. 2012;60:1–23. [PubMed] [Google Scholar]
- 12.Leviton A, Paneth N, Reuss ML, et al. Maternal infection, fetal inflammatory response, and brain damage in very low birth weight infants. Developmental Epidemiology Network Investigators Pediatr Res. 1999;46:566–75. doi: 10.1203/00006450-199911000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Dammann O, Leviton A, Gappa M, Dammann CE. Lung and brain damage in preterm newborns, and their association with gestational age, prematurity subgroup, infection/inflammation and long term outcome. Bjog. 2005;112 (Suppl 1):4–9. doi: 10.1111/j.1471-0528.2005.00576.x. [DOI] [PubMed] [Google Scholar]
- 14.Duley L, Henderson-Smart DJ, Meher S, King JF. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2007:CD004659. doi: 10.1002/14651858.CD004659.pub2. [DOI] [PubMed] [Google Scholar]
- 15.Subtil D, Goeusse P, Puech F, et al. Aspirin (100 mg) used for prevention of pre-eclampsia in nulliparous women: the Essai Regional Aspirine Mere-Enfant study (Part 1) BJOG: An International Journal of Obstetrics & Gynaecology. 2003;110:475–84. doi: 10.1046/j.1471-0528.2003.02096.x. [DOI] [PubMed] [Google Scholar]
- 16.Crispi F, Dominguez C, Llurba E, Martin-Gallan P, Cabero L, Gratacos E. Placental angiogenic growth factors and uterine artery Doppler findings for characterization of different subsets in preeclampsia and in isolated intrauterine growth restriction. American Journal of Obstetrics & Gynecology. 2006;195:201–7. doi: 10.1016/j.ajog.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 17.Odibo AO, Zhong Y, Longtine M, et al. First-trimester serum analytes, biophysical tests and the association with pathological morphometry in the placenta of pregnancies with preeclampsia and fetal growth restriction. Placenta. 2011;32:333–8. doi: 10.1016/j.placenta.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pepe MS, Feng Z, Huang Y, et al. Integrating the predictiveness of a marker with its performance as a classifier. Am J Epidemiol. 2008;167:362–8. doi: 10.1093/aje/kwm305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bastek JA, Sammel MD, Srinivas SK, et al. Clinical prediction rules for preterm birth in patients presenting with preterm labor. Obstetrics & Gynecology. 2012;119:1119–28. doi: 10.1097/AOG.0b013e31825503e5. [DOI] [PubMed] [Google Scholar]
- 20.Goetzinger KR, Cahill AG, Kemna J, Odibo L, Macones GA, Odibo AO. First-trimester prediction of preterm birth using ADAM12, PAPP-A, uterine artery Doppler, and maternal characteristics. Prenatal Diagnosis. 2012;32:1002–7. doi: 10.1002/pd.3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bahado-Singh RO, Akolekar R, Mandal R, et al. Metabolomics and first-trimester prediction of early-onset preeclampsia. Journal of Maternal-Fetal & Neonatal Medicine. 2012;25:1840–7. doi: 10.3109/14767058.2012.680254. [DOI] [PubMed] [Google Scholar]
- 22.Parra-Cordero M, Rodrigo R, Barja P, et al. Prediction of early and late pre-eclampsia from maternal characteristics, uterine artery Doppler and markers of vasculogenesis during first trimester of pregnancy. Ultrasound Obstet Gynecol. 2013;41:538–44. doi: 10.1002/uog.12264. [DOI] [PubMed] [Google Scholar]
- 23.Kwon H-S, Kim Y-H, Park Y-W. Uterine artery Doppler velocimetry and maternal weight gain by the mid-second trimester for prediction of fetal growth restriction. Acta Obstetricia et Gynecologica Scandinavica. 2008;87:1291–5. doi: 10.1080/00016340802478133. [DOI] [PubMed] [Google Scholar]
- 24.Melchiorre K, Leslie K, Prefumo F, Bhide A, Thilaganathan B. First-trimester uterine artery Doppler indices in the prediction of small-for-gestational age pregnancy and intrauterine growth restriction. Ultrasound Obstet Gynecol. 2009;33:524–9. doi: 10.1002/uog.6368. [DOI] [PubMed] [Google Scholar]
- 25.Onalan R, Onalan G, Gunenc Z, Karabulut E. Combining 2nd-trimester maternal serum homocysteine levels and uterine artery Doppler for prediction of preeclampsia and isolated intrauterine growth restriction. Gynecologic & Obstetric Investigation. 2006;61:142–8. doi: 10.1159/000090432. [DOI] [PubMed] [Google Scholar]
- 26.Goldenberg RL, Iams JD, Mercer BM, et al. The preterm prediction study: the value of new vs standard risk factors in predicting early and all spontaneous preterm births. NICHD MFMU Network Am J Public Health. 1998;88:233–8. doi: 10.2105/ajph.88.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akolekar R, Syngelaki A, Sarquis R, Zvanca M, Nicolaides KH. Prediction of early, intermediate and late pre-eclampsia from maternal factors, biophysical and biochemical markers at 11–13 weeks. Prenat Diagn. 2011;31:66–74. doi: 10.1002/pd.2660. [DOI] [PubMed] [Google Scholar]
- 28.Poon LC, Kametas NA, Maiz N, Akolekar R, Nicolaides KH. First-trimester prediction of hypertensive disorders in pregnancy. Hypertension. 2009;53:812–8. doi: 10.1161/HYPERTENSIONAHA.108.127977. [DOI] [PubMed] [Google Scholar]
- 29.Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122:1122–31. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 30.Martin AM, Bindra R, Curcio P, Cicero S, Nicolaides KH. Screening for pre-eclampsia and fetal growth restriction by uterine artery Doppler at 11–14 weeks of gestation. Ultrasound Obstet Gynecol. 2001;18:583–6. doi: 10.1046/j.0960-7692.2001.00594.x. [DOI] [PubMed] [Google Scholar]
- 31.Papageorghiou AT, Yu CK, Bindra R, Pandis G, Nicolaides KH. Multicenter screening for pre-eclampsia and fetal growth restriction by transvaginal uterine artery Doppler at 23 weeks of gestation. Ultrasound Obstet Gynecol. 2001;18:441–9. doi: 10.1046/j.0960-7692.2001.00572.x. [DOI] [PubMed] [Google Scholar]
- 32.Davey MA, Watson L, Rayner JA, Rowlands S. Risk scoring systems for predicting preterm birth with the aim of reducing associated adverse outcomes. Cochrane Database Syst Rev. 2011:CD004902. doi: 10.1002/14651858.CD004902.pub4. [DOI] [PubMed] [Google Scholar]
- 33.Honest H, Forbes CA, Duree KH, et al. Screening to prevent spontaneous preterm birth: systematic reviews of accuracy and effectiveness literature with economic modelling. Health Technol Assess. 2009;13:1–627. doi: 10.3310/hta13430. [DOI] [PubMed] [Google Scholar]
- 34.Esplin MS, Merrell K, Goldenberg R, et al. Proteomic identification of serum peptides predicting subsequent spontaneous preterm birth. Am J Obstet Gynecol. 2011;204:391, e1–8. doi: 10.1016/j.ajog.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hadlock FP, Shah YP, Kanon DJ, Lindsey JV. Fetal crown-rump length: reevaluation of relation to menstrual age (5–18 weeks) with high-resolution real-time US. Radiology. 1992;182:501–5. doi: 10.1148/radiology.182.2.1732970. [DOI] [PubMed] [Google Scholar]
- 36.Arozullah AM, Yarnold PR, Bennett CL, et al. Development and validation of a short-form, rapid estimate of adult literacy in medicine. Med Care. 2007;45:1026–33. doi: 10.1097/MLR.0b013e3180616c1b. [DOI] [PubMed] [Google Scholar]
- 37.Block G, Woods M, Potosky A, Clifford C. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol. 1990;43:1327–35. doi: 10.1016/0895-4356(90)90099-b. [DOI] [PubMed] [Google Scholar]
- 38.Hibbard JH, Mahoney ER, Stockard J, Tusler M. Development and testing of a short form of the patient activation measure. Health Serv Res. 2005;40:1918–30. doi: 10.1111/j.1475-6773.2005.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krieger N, Smith K, Naishadham D, Hartman C, Barbeau EM. Experiences of discrimination: validity and reliability of a self-report measure for population health research on racism and health. Soc Sci Med. 2005;61:1576–96. doi: 10.1016/j.socscimed.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 40.Zimet GD, Powell SS, Farley GK, Werkman S, Berkoff KA. Psychometric characteristics of the Multidimensional Scale of Perceived Social Support. J Pers Assess. 1990;55:610–7. doi: 10.1080/00223891.1990.9674095. [DOI] [PubMed] [Google Scholar]
- 41.DiPietro JA, Christensen AL, Costigan KA. The pregnancy experience scale-brief version. J Psychosom Obstet Gynaecol. 2008;29:262–7. doi: 10.1080/01674820802546220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Connor KM, Davidson JR. Development of a new resilience scale: the Connor-Davidson Resilience Scale (CD-RISC) Depress Anxiety. 2003;18:76–82. doi: 10.1002/da.10113. [DOI] [PubMed] [Google Scholar]
- 43.Levine DW, Kaplan RM, Kripke DF, Bowen DJ, Naughton MJ, Shumaker SA. Factor structure and measurement invariance of the Women’s Health Initiative Insomnia Rating Scale. Psychol Assess. 2003;15:123–36. doi: 10.1037/1040-3590.15.2.123. [DOI] [PubMed] [Google Scholar]
- 44.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15:376–81. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- 45.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisi J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–19. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 46.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–91. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 47.Spielberger CD. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; Number of pages. [Google Scholar]
- 48.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–6. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 49.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–96. [PubMed] [Google Scholar]
- 50.Koren G, Boskovic R, Hard M, Maltepe C, Navioz Y, Einarson A. Motherisk-PUQE (pregnancy-unique quantification of emesis and nausea) scoring system for nausea and vomiting of pregnancy. Am J Obstet Gynecol. 2002;186:S228–31. doi: 10.1067/mob.2002.123054. [DOI] [PubMed] [Google Scholar]