Abstract
A number of studies have examined the ability of the endogenous cannabinoid anandamide to elicit Δ9 -tetrahydrocannabinol (THC)-like subjective effects, as modeled through the THC discrimination paradigm. In the present study, we compared transgenic mice lacking fatty acid amide hydrolase (FAAH), the enzyme primarily responsible for anandamide catabolism, to wildtype counterparts in a THC discrimination procedure. THC (5.6 mg/kg) served as a discriminative stimulus in both genotypes, with similar THC dose-response curves between groups. Anandamide fully substituted for THC in FAAH knockout, but not wildtype, mice. Conversely, the metabolically stable anandamide analog O-1812 fully substituted in both groups, but was more potent in knockouts. The CB1 receptor antagonist rimonabant dose-dependently attenuated THC generalization in both groups and anandamide substitution in FAAH knockouts. Pharmacological inhibition of monoacylglycerol lipase (MAGL), the primary catabolic enzyme for the endocannabinoid 2-arachidonoylglycerol (2-AG), with JZL184 resulted in full substitution for THC in FAAH knockout mice and nearly full substitution in wildtypes. Quantification of brain endocannabinoid levels revealed expected elevations in anandamide in FAAH knockout mice compared to wildtypes and equipotent dose-dependent elevations in 2-AG following JZL184 administration. Dual inhibition of FAAH and MAGL with JZL195 resulted in roughly equipotent increases in THC-appropriate responding in both groups. While the notable similarity in THC's discriminative stimulus effects across genotype suggests that the increased baseline brain anandamide levels (as seen in FAAH knockout mice) do not alter THC's subjective effects, FAAH knockout mice are more sensitive to the THC-like effects of pharmacologically induced increases in anandamide and MAGL inhibition (e.g., JZL184).
Keywords: Δ9-tetrahydrocannabinol, anandamide, fatty acid amide hydrolase, 2-arachidonoylglycerol, drug discrimination, rimonabant
1. Introduction
The major psychoactive constituent of marijuana, Δ9 -tetrahydrocannabinol (THC; Gaoni and Mechoulam 1964), exerts a range of pharmacological effects via activation of the endogenous cannabinoid (endocannabinoid) system. This system is comprised of two known receptors, cannabinoid type 1 (CB1) and cannabinoid type 2 (CB2), with notable differences in their distribution and function. Centrally located CB1 receptors are involved in THC's psychoactivity (Wiley et al. 1995), as well as its effects on anxiety (Tambaro and Bortolato 2012), cognition (Hart et al. 2001), appetite (Kirkham 2009), and other physiological processes. A number of endogenous ligands have been identified for the endocannabinoid system, including the two best-characterized ligands: N-arachidonoylethanolamine (hereto referred as anandamide) and 2-arachidonoylglycerol (2-AG). These important neuromodulators are synthesized and released on demand (as opposed to being stored in vesicles), and they are short-lived in vivo, with anandamide rapidly degraded predominantly by fatty acid amide hydrolase (FAAH; Cravatt et al. 1996), and 2-AG metabolized primarily by monoacylglycerol lipase (MAGL; Blankman et al. 2007).
Development of pharmacological and genetic tools for probing the functions of the endocannabinoid system has aided understanding of its complex actions. For example, pharmacological inhibitors of FAAH (e.g., URB597, PF3845), MAGL (e.g., JZL184, KML29), and both enzymes (e.g. JZL195, SA57) have been generated and evaluated, as have transgenic mice lacking either of these enzymes (Cravatt et al. 2001; Schlosburg et al. 2010). Of these two knockout strains, mice lacking FAAH have been better characterized and were selected for study in the present set of experiments. FAAH knockout mice possess roughly 15-fold higher levels of anandamide than wildtype counterparts and display a characteristic set of cannabimimetic responses to anandamide, including antinociception, catalepsy, hypomotility, and hypothermia (Cravatt et al. 2001). Further, these mice display a hypoalgesic phenotype (Lichtman et al. 2004), and enhanced cognitive impairment in the Morris water maze following anandamide or JZL184 treatment (Varvel et al. 2006; Wise et al. 2012). FAAH knockout mice have been trained in THC drug discrimination, a pharmacologically selective animal model of marijuana's subjective effects (Balster and Prescott 1992), but that study did not include characterization of the discrimination or direct comparison to THC-trained wildtype mice (Ignatowska-Jankowska et al. 2014). FAAH knockout mice (but not wildtype mice) also have been trained to discriminate anandamide from vehicle in drug discrimination (Walentiny et al. 2011).
The objectives of this study were to characterize the discriminative stimulus effects of THC in FAAH knockout mice compared to wildtype controls, and to determine potential phenotypic differences in responding with cannabinoid agonists and endocannabinoid metabolic enzyme inhibitors. Specifically, dose-response determinations were conducted with THC, anandamide, O-1812 (a metabolically stable anandamide analog; Di Marzo et al. 2001), JZL184 (MAGL inhibitor; Long et al. 2009a), and JZL195 (dual FAAH/MAGL inhibitor; Long et al. 2009b). Challenge tests with the CB1 receptor antagonist rimonabant were conducted when appropriate.
2.0 Material and Methods
2.1. Subjects
Adult male FAAH knockout (-/-; N=16) and wildtype mice (+/+; N=14) on a C57BL/6 background were derived from heterozygous breeding pairs at Virginia Commonwealth University and served as subjects in the THC discrimination study. An additional 23 FAAH knockouts and 24 wildtype male mice were used to quantify anandamide and 2-AG levels following JZL184 administration. Mice were housed individually in a temperature controlled (20-22°C) vivarium on a 12 h light/dark cycle. Mice in the discrimination study were maintained at 85-90% of their free-feeding body weights by restricting postsession feeding and had ad libitum access to water in their home cage. All methods were approved by the Institutional Animal Care and Use Committee at Virginia Commonwealth University and adhered to the “Guide for the Care and Use of Laboratory Animals” (National Research Council 2003).
2.2. Apparatus
Experimental sessions were conducted in sound- and light-attenuated operant conditioning chambers (Med Associates, St. Albans, VT). Each chamber contained two nose-poke apertures on the front panel. A recessed food receptacle connected to a pellet hopper was centered in between the response apertures. Stimulus lights above each aperture were illuminated during experimental sessions. Fans provided ventilation and masking noise. A computer running Med-PC software (Med Associates) was used to control session parameters and record data.
2.3. Drugs
THC and rimonabant obtained from the National Institute on Drug Abuse (Bethesda, MD) and anandamide (Organix Inc., Woburn, MA) were dissolved in a solution of 0.78% Tween-80 (Fischer Scientific, Pittsburgh, PA) and 99.22% saline. JZL184 and JZL195 (provided by Dr. Benjamin Cravatt, The Scripps Research Institute, La Jolla, CA) and O-1812 (Organix Inc.) were dissolved in an ethanol, emulphor-620, (Rhone-Poulenc, Inc., Princeton, NJ) and saline mixture at a ratio of 1:1:18. THC, anandamide, and O-1812 were administered s.c. 30 min prior to the session. JZL184 and JZL195 were administered i.p. 2 h presession. For antagonism tests, rimonabant was administered i.p. 10 min before treatment with the respective agonist/enzyme inhibitor. All drugs were administered at a volume of 10 ml/kg. Doses were administered in ascending order.
2.4. Discrimination Procedure
Mice were prompted to initiate nose poke behavior in an overnight operant session, during which each response on either aperture resulted in delivery of a 14 mg sweetened pellet (Bio-Serv, Frenchtown, NJ). Next, fixed ratio requirements were systematically increased during daily training sessions (15 min) until each subject was required to respond 10 times consecutively (i.e., fixed ratio 10; FR10) to receive a food pellet. Reliable responding on an FR10 schedule was obtained on both apertures before proceeding to discrimination training.
For discrimination training, drug and vehicle apertures (i.e., left or right) were randomly assigned to each subject. During sixteen daily training sessions, subjects were administered 5.6 mg/kg THC or vehicle according to a double alternation sequence of drug delivery (i.e., DDVVDDVV…). Responses during these training sessions were restricted to the condition-appropriate aperture (i.e., were errorless) by inserting a rubber stopper into the inactive aperture.
Following errorless training, the double alternation sequence of drug and vehicle administration continued and subjects were allowed to respond on either aperture. However, only responses on the condition-appropriate aperture resulted in the delivery of reinforcement according to the FR10 schedule. A response on the incorrect aperture reset the counter on the condition-appropriate aperture. During these training sessions, three criteria were used as indices of successful acquisition of the task: 1) first completed ratio on the condition-appropriate aperture, 2) ≥ 80% of total responses made on the condition-appropriate aperture, and 3) response rate ≥ 10 responses/min. Testing began once a subject met all three of these criteria during 7 out of 8 consecutive training sessions.
During testing, session parameters were identical to training sessions, except that responses on either aperture resulted in reinforcement presentation according to an FR10 schedule. Prior to the initiation of each dose-response evaluation, control tests were conducted with the training drug and vehicle to demonstrate stimulus control. Tests were conducted up to twice per week, provided subjects met the three training criteria described above during the most recent training sessions with THC and vehicle. A minimum 72 h washout period was implemented between test sessions for most compounds, and test sessions with JZL184 or JZL195 were conducted at least one week apart due to their longer half-lives.
First, a THC dose-effect determination was conducted in all subjects, followed by a rimonabant challenge against the THC training dose. Next, systemic anandamide and its metabolically stable analog O-1812 were assessed for their ability to produce THC-like discriminative stimulus effects in each genotype. Rimonabant challenge against 10 mg/kg anandamide was assessed in FAAH knockout mice, but not in FAAH wildtype mice. Lastly, we determined whether either MAGL inhibition (JZL184) or dual inhibition of MAGL and FAAH (JZL195) would result in substitution for THC's discriminative stimulus. Rimonabant antagonism of the effects of 40 mg/kg JZL195 was also evaluated.
2.5. Quantification of Brain Concentrations of 2-AG and Anandamide
Adult male FAAH knockout and wildtype mice were injected i.p. with vehicle (1:1:18) or JZL184 (3-30 mg/kg) two hours before decapitation. After decapitation, the cerebellum was harvested and rapidly cooled by immersion in liquid nitrogen. Tissue was stored at -80°C until use. Anandamide and 2-AG were then extracted using a methanol/chloroform extraction (Burston et al. 2008; Hardison et al. 2006). Samples were homogenized on ice in 2 mL chloroform: methanol (2:1, v/v). The internal standards, 1 pmol anandamide-d8 and 2 nmol 2-AG-d8, were added to each sample, calibrator or control. Samples were mixed and centrifuged after the addition of 0.2 mL of a 0.73% sodium chloride solution. The chloroform was collected and evaporated to dryness with nitrogen. The extracts were reconstituted with 100 μL methanol and placed in autosampler vials for analysis. The injection volume was 20 μL and the auto sampler temperature was set at 5°C. The chromatographic separation of anandamide and 2-AG was performed on a Discovery® HS C18 column 15cm × 2.1mm, 3μm (Supelco: Bellefonte, PA) kept at 40°C. The mobile phase was 10% water with 1g/L ammonium acetate and 0.1% formic acid, and 90% methanol with 1g/L ammonium acetate and 0.1% formic acid. The flow rate was 0.3 mL/min. The following transition ions (m/z) were monitored in positive multiple reaction monitoring (MRM) mode: anandamide, 348>62 & 348>91; anandamide-d8, 356>62; 2-AG, 379>287 & 279>269; and 2-AG, 387>296. Total run time was 10 min.
2.6. Data analysis
The percentage of responses made on the THC-paired aperture for each subject was calculated. Full substitution for THC was defined as ≥ 80% THC-appropriate responding. ED50 values (and 95% confidence intervals) were calculated for drugs that fully substituted for THC using least squares linear regression. Response rates were expressed as responses per min. Response rates (for all compounds) and % THC-appropriate responding (for rimonabant and THC combination tests) were analysed using split-plot analysis of variance (ANOVA), with genotype as the between subjects variable and dose as the within subjects variable. Rimonabant antagonism of anandamide in FAAH knockout mice was analysed with repeated measures ANOVA across dose. Brain levels of anandamide and 2-AG after JZL184 administration were analysed via factorial (genotype × dose) between-subjects ANOVA. For all ANOVAs, significant main effects and/or interactions were further evaluated with Tukey's post hoc tests (α=0.05). Discrimination data for subjects that made fewer than 10 responses during a test session for a particular dose were excluded from analysis, but the corresponding response rate data were included.
3. Results
Thirteen FAAH knockout and eleven wildtype mice met training criteria in a mean (± SEM) of 22.6 (± 1.6) and 25.2 (± 2.5) sessions, respectively, with no significant difference in the number of trials required to meet training criteria, t(22)=0.87, p< 0.05. Control tests were conducted with vehicle and with the 5.6 mg/kg THC training dose prior to each dose-effect curve determination (left side of panels A-D in Figure 1 and panels A, B, E and F in Figure 2). In each case, THC produced responding primarily on the THC-associated aperture whereas vehicle engendered responding primarily on the other aperture. While mean response rates during control tests shown in Figure 2 were similar across test and genotype, mean response rates during control tests shown in Figure 1 exhibited more variability. This variability was more noted in control tests conducted across dose-effect curves rather than within the set of dose-effect data for each test compound. The reason for this unusual variability in the control response rate data is unclear; however, given the predominance of responding on the injection-appropriate aperture during control tests for each compound, choice behavior does not appear to have been affected.
Figure 1.
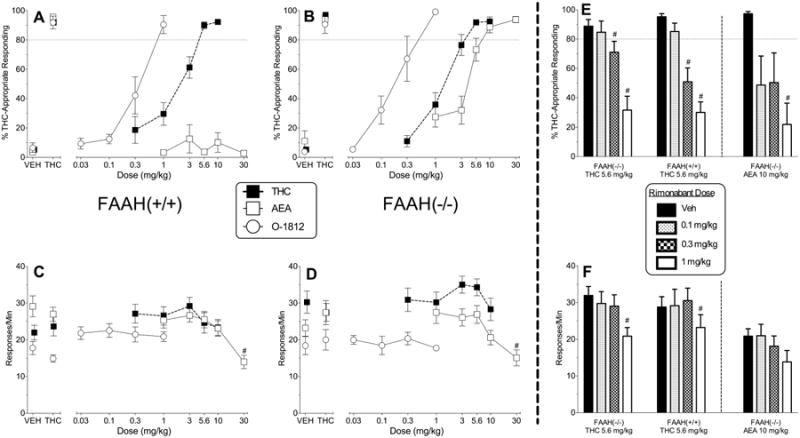
Panels A and B show the effects of THC (filled squares), anandamide (AEA; unfilled squares), and O-1812 (unfilled circles) on % THC-aperture responding in FAAH wild type (+/+; panel A) and knockout (-/-; panel B) mice trained to discriminate 5.6 mg/kg THC vs. vehicle. Response rates for each genotype (responses/min on both apertures) are shown in corresponding bottom panels (panels C and D, respectively). In panels A-D, points above VEH and THC represent the results of control tests with vehicle and THC, respectively, on % THC-aperture responding (panels A and B) and response rates (panels C and D) conducted prior to the dose-effect curve determination for each test compound. The rightmost panels show the effects of 5.6 mg/kg THC in combination with vehicle and with increasing doses of rimonabant (0.1 – 1 mg/kg) on % THC-appropriate responding (panel E) and response rates (panel F) in FAAH knockout and wildtype mice. Also shown in these panels are the effects of 10 mg/kg anandamide in combination with vehicle and with increasing doses of rimonabant (0.1 – 1 mg/kg) on the same measures (right side of panels E and F, respectively). Values represent the mean (±S.E.M.) of data from 13 FAAH knockout mice for THC, THC/rimonabant combination, and anandamide tests and 6 FAAH knockout mice for O-1812 tests. Values represent the mean (±S.E.M.) of data from 9-11 wildtype mice for THC, THC/rimonabant combination, and anandamide tests and 7-8 wildtype mice for O-1812 tests. Number signs (#) designate a significant main effect of dose across genotypes (p< 0.05) and subsequent Tukey post hoc determination of a significant decrease in THC-appropriate responding or response rate compared to mean responding during the vehicle control test.
Figure 2.
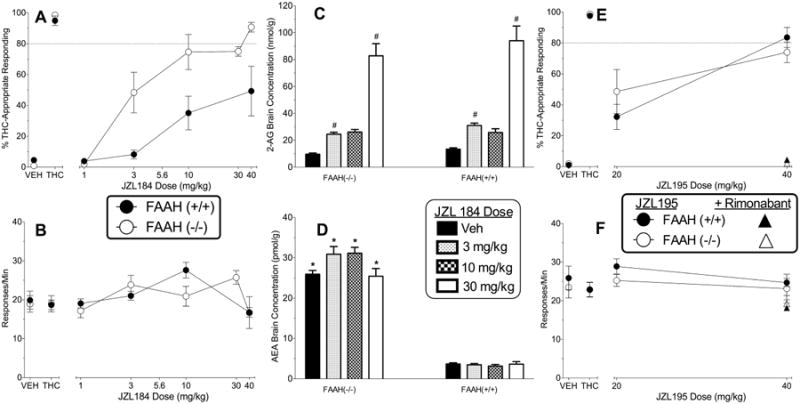
Panel A shows the effects of MAGL inhibitor JZL184 (administered alone) on % THC-aperture responding in FAAH knockout (unfilled circles) and wildtype (filled circles) mice trained to discriminate 5.6 mg/kg THC vs. vehicle. Panel B shows the response rates for each genotype during these tests. The middle panels show concentrations of 2-AG (panel C) and anandamide (panel D) in the brains of FAAH knockout and wildtype mice following a single injection with vehicle or JZL184 (N=5-6 per group). Panels E and F show the effects of the dual MAGL/FAAH inhibitor JZL195 (administered alone) on % THC-appropriate responding and response rates, respectively, in FAAH knockout (unfilled circles) and wildtype (filled circles) mice. Results of combination tests of 40 mg/kg JZL195 and 3 mg/kg rimonabant on % THC-appropriate responding (panel E) and response rates (panel F) in FAAH knockout (unfilled triangles) and wildtype (filled triangles) are also shown. Points above VEH and THC (panels A, B, E, and F) represent the results of control tests with vehicle and THC, respectively, conducted prior to the dose-effect curve determination for each test compound. Values in the drug discrimination graphs represent the mean (±S.E.M.) of data from 6 FAAH knockout mice and 6-7 wildtype mice. Number signs (#) designate a significant main effect of dose across genotype (p< 0.05) and subsequent Tukey post hoc determination of a significant increase in 2-AG concentrations in the brain. Asterisks (*) designate a significant genotype × dose interaction (p< 0.05) and Tukey post hoc determination of a significant increase in anandamide concentrations in the brain of FAAH knockout mice compared to wildtype mice at the same JZL195 dose.
A dose-response assessment with THC revealed full, dose-dependent generalization to the training dose in both wildtype (Figure 1A) and FAAH knockout (Figure 1B) mice. Further, potency for generalization was similar across genotype (Table 1). THC did not decrease response rates (compared to vehicle) in either genotype (Figure 1C and 1D).
Table 1. Cannabinoid potencies in FAAH wildtype and FAAH knockout mice trained to discriminate 5.6 mg/kg THC from vehicle.
| Compound | ED50 (95% confidence limits) | |
|---|---|---|
| FAAH wildtype | FAAH knockout | |
| THC | 1.2 mg/kg (0.9 - 1.5) | 1.2 mg/kg (1.0 – 1.4) |
| Anandamide | No substitution | 4.0 mg/kg (3.3 – 4.9) |
| O-1812 | 0.33 mg/kg (0.24 - 0.43) | 0.17 mg/kg (0.12 – 0.23) |
| JZL184 | ∼ 40 mg/kg | 4.5 mg/kg (2.72 - 7.42) |
| JZL195 | 25.4 mg/kg (21.7 - 29.7) | ∼ 20 mg/kg |
Similar to THC, O-1812 also elicited full, dose-dependent substitution for THC in wildtype (Figure 1A) and FAAH knockout mice (Figure 1B) without significant effect on response rates in either genotype (Figure 1C and 1D). Potency for substitution was approximately 2-fold greater in FAAH knockout (vs. wildtype) mice (Table 1). In addition, O-1812 was more potent than THC in both genotypes (Table 1).
Not unexpectedly, the greatest divergence of results across genotypes was for anandamide. Whereas FAAH knockout mice exhibited full, dose-dependent substitution for THC when dosed with anandamide (Figure 1A), anandamide-treated wildtype mice responded primarily on the vehicle-associated aperture (Figure 1B). In FAAH knockout mice, anandamide was approximately 3 times less potent than THC (Table 1). Despite the dramatic difference in substitution, decreases in response rates were observed in both genotypes with the 30 mg/kg anandamide dose (Figure 1C and 1D) [main effect of dose: F(5,100)=16.11, p< 0.05].
Challenge tests with the CB1 receptor antagonist rimonabant and the training dose (5.6 mg/kg) of THC resulted in dose-dependent decreases in THC-appropriate responding in both genotypes, as compared to injections with rimonabant vehicle and 5.6 mg/kg THC (Figure 1E) [main effect of dose: F(5,100)=45.85, p< 0.05]. Similarly, rimonabant (1 mg/kg) decreased substitution of anandamide (10 mg/kg) for THC in FAAH knockout mice [F(4,16)=7.55, p< 0.05]. Response rates were also reduced in both genotypes at the highest rimonabant dose tested (1 mg/kg; Figure 1F) [main effect of dose: F(5,100)=18.11, p< 0.05]. When administered in combination with vehicle, 1 mg/kg rimonabant significantly reduced response rates and did not substitute for THC (data not shown).
Figure 2 shows the results of substitution tests with the MAGL inhibitor JZL184. In FAAH knockout mice, JZL184 produced dose-dependent increases in THC-appropriate responding, with full substitution at the 40 mg/kg dose (Figure 2A) and an ED50 of 4.5 mg/kg (Table 1). In contrast, JZL184 produced a maximum of approximately 50% responding on the THC-appropriate aperture in the wildtype mice at doses up to 40 mg/kg. Hence, JZL184 was almost 10 times less potent in wildtype mice. These effects occurred in the absence of significant suppressant effects on response rates in either genotype (Figure 2B).
Measurement of endocannabinoid concentrations in the brains of separate mice revealed that doses of 3 and 30 mg/kg JZL184 increased 2-AG concentrations in both genotypes (Figure 2C) [main effect of dose: F(3,39)=80.69, p< 0.05]. Consistent with the absence of FAAH in the knockout mice, anandamide concentrations were significantly higher compared to wildtype mice (Figure 2D) [interaction: F(3,39)=3.89, p< 0.05]. JZL184 did not affect concentrations of anandamide in the brains of either genotype compared to vehicle.
In a previous study (Long et al. 2009b), we demonstrated that the dual FAAH/MAGL inhibitor JZL195 (40 mg/kg) produced full substitution for THC in wildtype mice and that this effect was blocked by rimonabant (Figure 2E). Here, we report that JZL195 also approaches full substitution (approximately 74 % THC-appropriate responding at the 40 mg/kg dose) in FAAH knockout mice, with rimonabant (3 mg/kg) reversal (Figure 2E). Potency was slightly higher in wildtype mice (Table 1). Response rates were not affected by either dose of JZL195. Limited supply of the compound prevented testing of additional doses.
4. Discussion
The present study is one in a series of studies conducted in our lab that were designed, in part, to examine the role of endocannabinoids in THC's discriminative stimulus effects (Ignatowska-Jankowska et al. 2014; Vann et al. 2012; Walentiny et al. 2011; Wiley et al. 2014). In this study, a combination of genetically altered mice (FAAH knockout and widltype) and pharmacological probes (THC, anandamide, O-1812, JZL184, JZL195) were used to manipulate brain levels of anandamide and/or 2-AG. Because FAAH knockout mice lack the primary enzyme that normally metabolizes anandamide, their brain anandamide concentrations substantially exceed those of wildtype mice (present study; Cravatt et al. 2001). Despite this difference, mice in both genotypes learned the THC discrimination task within a similar time frame. Further, analysis of their THC dose-effect curves revealed full dose-dependent substitution with approximately equal potencies. While results with THC in wildtype mice represent a systematic replication of previous experiments in which THC served as an effective discriminative stimulus in C57/BL6 mice (Brents et al. 2013; McMahon et al. 2008; Vann et al. 2009; Wiley et al. 2013), this study is the first to report successful training of THC discrimination in FAAH knockout mice, thus allowing direct comparisons across genotype. As demonstrated previously in wildtype mice (McMahon et al. 2008; Vann et al. 2009), CB1 receptor mediation of THC's discriminative stimulus in both genotypes was indicated by the observed dose-dependent attenuation produced by rimonabant pretreatment. Together, these findings demonstrate that differences in endogenous brain anandamide concentration (e.g., FAAH knockout vs. wildtype mice) do not affect the efficacy or potency of THC as a discriminative stimulus under the present experimental parameters.
Evaluation of the role of anandamide in THC's discriminative stimulus effects was accomplished by substitution tests with anandamide and with O-1812, a metabolically stable anandamide analog. As might be expected, anandamide fully and dose-dependently substituted for THC in mice lacking FAAH, but it failed to substitute in wildtype mice even at a behaviorally active dose that suppressed overall responding. Similarly, most previous studies have shown that anandamide failed to substitute in rats and wildtype mice trained to discriminate THC from vehicle (Järbe et al. 2001; Vann et al. 2009; Wiley et al. 1998) unless it was accompanied by an inhibitor of FAAH (Solinas et al. 2007b; Stewart and McMahon 2011; Vann et al. 2009). Further, injection with a FAAH inhibitor (e.g., PF3845, URB 597) in the absence of co-administration of anandamide did not produce THC-like effects in rodents trained to discriminate THC (Solinas et al. 2007b; Wiley et al. 2014) nor did anandamide alone serve as a discriminative stimulus in rats (Wiley 1999). In contrast, FAAH knockout mice were readily trained to discriminate anandamide from vehicle, with full dose-dependent substitution of anandamide and cross-substitution of THC (Walentiny et al. 2011). Rimonabant reversal of the discriminative stimulus effects of anandamide in anandamide-and THC-trained FAAH knockout mice indicates CB1 receptor mediation of anandamide substitution for itself (Walentiny et al. 2011) and its cross-substitution for THC (present study). Together, these results suggest that anandamide-induced stimulation of CB1 receptors produces THC-like discriminative stimulus effects, but only at brain concentrations much higher than normal physiological levels; i.e., endogenous levels must be enhanced by exogenous administration of anandamide and rapid degradation of this anandamide must be prevented by genetic deletion of FAAH or by co-administration of a FAAH inhibitor.
This hypothesis receives additional support from results of substitution tests with metabolically stable anandamide analogs in animals trained to discriminate THC from vehicle. A variety of these analogs with good affinity for the CB1 receptor have been shown to produce THC-like discriminative stimulus effects in several species (Järbe et al. 2001; Stewart and McMahon 2011; Wiley et al. 1997; Wiley et al. 2004). Further, several of these analogs have been trained as discriminative stimuli, with substitution and cross-substitution with THC as the typically observed pattern (Järbe et al. 2009; Järbe et al. 2001; Wiley et al. 2004; Wiley et al. 2011). Consistent with these previous results, O-1812 fully and dose-dependently substituted for THC in both groups of mice, showing greater potency than anandamide in FAAH knockout mice and compared to THC in mice of both genotypes. Notably, however, O-1812 was roughly twice as potent in FAAH knockout (vs. wildtype) mice, suggesting that increased anandamide present in the brains of FAAH knockout mice may have enhanced sensitivity to the THC-like effects of O-1812. Although rimonabant reversal of O-1812 was not attempted in the present study, previous studies have shown that the cannabinoid discriminative stimulus effects of O-1812 and other anandamide analogs were blocked by rimonabant (Wiley et al. 2004; Wiley et al. 2011).
Following completion of tests with anandamide and O-1812, evaluation of the role of 2-AG in THC's discriminative stimulus effects was initiated. To this end, the MAGL inhibitor JZL184 and the dual FAAH / MAGL inhibitor JZL195 were tested for substitution in THC-discriminating mice of each genotype. In both FAAH knockout and wildtype mice, JZL184 dose dependently increased responding on the THC-associated aperture, an effect that was rimonabant reversible in wildtype mice (Wiley et al. 2014). JZL184 concomitantly elevated brain 2-AG concentrations in both genotypes. While these results seem to suggest that blocking the metabolism of endogenous 2-AG provides sufficient stimulation of CB1 receptors to engender THC-like responding, several findings argue against this hypothesis. First, full substitution occurred only in the FAAH knockout mice, not in the wildtype mice, despite nearly equal increases in JZL184-induced 2-AG concentrations in the brain. Second, other 2-AG inhibitors [N-arachidonyl maleimide (NAM), KML 29] failed to engender substantial responding on the THC lever in wildtype mice trained to discriminate THC from vehicle (Ignatowska-Jankowska et al. 2014; Wiley et al. 2014). Co-administration of NAM with exogenous 2-AG also did not produce substitution for THC (Wiley et al. 2014). Hence, the ability of increased endogenous 2-AG alone to produce THC-like discriminative stimulus effects is still unclear. On the other hand, results from this and other studies suggest increased endogenous brain concentrations of both major endocannabinoids (i.e., 2-AG and anandamide) may be sufficient to elicit THC-like subjective state. For example, the 2-AG metabolic inhibitor JZL184 fully substituted in mice with high brain concentrations of anandamide (i.e., FAAH knockout mice). Further, dual pharmacological inhibition of the metabolism of 2-AG and anandamide (i.e., JZL195) produced full (84%) or nearly full (74%) substitution for THC in wildtype and FAAH knockout mice, respectively, as did coadministration of JZL184 and the FAAH inhibitor URB597 in rats (Wiley et al. 2014). Together, these data suggest that MAGL inhibition alone has the potential to elicit mild to moderate cannabimimetic effects, with a greater propensity to produce THC-like psychoactivity when interfering with the major metabolic processes for anandamide and 2-AG degradation. If its metabolism by FAAH is inhibited, anandamide alone also can produce THC-like discriminative stimulus effects, but only when the brain is flooded by exogenous pharmacological (vs. endogenous physiological) concentrations.
In summary, to the extent that the present results may apply to humans, they suggest that both anandamide and 2-AG may be capable of mimicking the subjective effects of marijuana to varying extents. Consequently, balancing desired therapeutic outcomes with potential abuse-related effects will be of considerable importance to endocannabinoid-based medication development efforts. Based upon the results showing increased sensitivity of FAAH knockout (vs. wildtype) mice to several of the test compounds, any drug development effort will also need to take into account the genetic vulnerability of certain populations to substance abuse. For example, a single nucleotide polymorphism observed in the gene encoding FAAH (385C→A) is correlated with a higher likelihood of street drug use and adverse drug and/or alcohol use (Sipe et al. 2002). While this polymorphism is not associated with alterations in FAAH catabolism (Sipe et al. 2002), it may play a role in withdrawal-related effects in chronic marijuana users (Schacht et al. 2009). Given the observed interplay between the endocannabinoid system and other drugs of abuse (Beardsley et al. 2009; Solinas and Goldberg 2005; Solinas et al. 2007a), alterations in FAAH activity may confer functional changes to an individual's response to marijuana or other drugs of abuse, though this hypothesis will require further rigorous testing.
Highlights.
THC served as a discriminative stimulus in FAAH knockout and wildtype mice.
The THC dose-response curve was similar in both genotypes.
Anandamide substituted for THC in knockout mice only.
MAGL inhibition promoted higher levels of THC-like responding in FAAH knockouts.
Dual inhibition of FAAH and MAGL produced THC-like responding.
Acknowledgments
Research supported by NIH/NIDA Research Grant DA-026449. The authors wish to thank Dr. Aron Lichtman for providing the FAAH knockout and wildtype mice used in this study and Dr. Rehab Abdullah and Mr. Justin Poklis for their technical assistance in endocannabinoid extraction and quantification.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balster RL, Prescott WR. Δ9-Tetrahydrocannabinol discrimination in rats as a model for cannabis intoxication. Neuroscience and Biobehavioral Reviews. 1992;16:55–62. doi: 10.1016/s0149-7634(05)80051-x. [DOI] [PubMed] [Google Scholar]
- Beardsley PM, Thomas BF, McMahon LR. Cannabinoid CB1 receptor antagonists as potential pharmacotherapies for drug abuse disorders. Int Rev Psychiatry. 2009;21:134–42. doi: 10.1080/09540260902782786. [DOI] [PubMed] [Google Scholar]
- Blankman JL, Simon GM, Cravatt BF. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol. 2007;14:1347–56. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brents LK, Zimmerman SM, Saffell AR, Prather PL, Fantegrossi WE. Differential drug-drug interactions of the synthetic cannabinoids JWH-018 and JWH-073: Implications for drug abuse liability and pain therapy. J Pharmacol Exp Ther. 2013;346:350–361. doi: 10.1124/jpet.113.206003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burston JJ, Sim-Selley LJ, Harloe JP, Mahadevan A, Razdan RK, Selley DE, Wiley JL. N-arachidonyl maleimide potentiates the pharmacological and biochemical effects of the endocannabinoid 2-arachidonylglycerol through inhibition of monoacylglycerol lipase. J Pharmacol Exp Ther. 2008;327:546–53. doi: 10.1124/jpet.108.141382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, Lichtman AH. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci U S A. 2001;98:9371–6. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Bisogno T, De Petrocellis L, Brandi I, Jefferson RG, Winckler RL, Davis JB, Dasse O, Mahadevan A, Razdan RK, et al. Highly selective CB(1) cannabinoid receptor ligands and novel CB(1)/VR(1) vanilloid receptor “hybrid” ligands. Biochem Biophys Res Commun. 2001;281:444–451. doi: 10.1006/bbrc.2001.4354. [DOI] [PubMed] [Google Scholar]
- Gaoni Y, Mechoulam R. Isolation, structure, and partial synthesis of an active constituent of hashish. J Amer Chem Soc. 1964;86:1646–1647. [Google Scholar]
- Hardison S, Weintraub ST, Giuffrida A. Quantification of endocannabinoids in rat biological samples by GC/MS: technical and theoretical considerations. Prostaglandins Other Lipid Mediat. 2006;81:106–112. doi: 10.1016/j.prostaglandins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Hart CL, van Gorp W, Haney M, Foltin RW, Fischman MW. Effects of acute smoked marijuana on complex cognitive performance. Neuropsychopharmacology. 2001;25:757–65. doi: 10.1016/S0893-133X(01)00273-1. [DOI] [PubMed] [Google Scholar]
- Ignatowska-Jankowska BM, Ghosh S, Crowe MS, Kinsey SG, Niphakis MJ, Abdullah RA, Tao Q, O'Neal ST, Walentiny DM, Wiley JL, Cravatt BF, Lichtman AH. In vivo characterization of the highly selective monoacylglycerol lipase inhibitor KML29: Antinociceptive activity without cannabimimetic side effects. Br J Pharmacol. 2014;171:1392–1407. doi: 10.1111/bph.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järbe T, Li C, Liu Q, Makriyannis A. Discriminative stimulus functions in rats of AM1346, a high-affinity CB1R selective anandamide analog. Psychopharmacology. 2009;203:229–239. doi: 10.1007/s00213-008-1199-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järbe TU, Lamb RJ, Lin S, Makriyannis A. (R)-methanandamide and Delta 9-THC as discriminative stimuli in rats: tests with the cannabinoid antagonist SR-141716 and the endogenous ligand anandamide. Psychopharmacology (Berl) 2001;156:369–80. doi: 10.1007/s002130100730. [DOI] [PubMed] [Google Scholar]
- Kirkham TC. Cannabinoids and appetite: food craving and food pleasure. Int Rev Psychiatry. 2009;21:163–71. doi: 10.1080/09540260902782810. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Shelton CC, Advani T, Cravatt BF. Mice lacking fatty acid amide hydrolase exhibit a cannabinoid receptor-mediated phenotypic hypoalgesia. Pain. 2004;109:319–27. doi: 10.1016/j.pain.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Long JZ, Li W, Booker L, Burston JJ, Kinsey SG, Schlosburg JE, Pavon FJ, Serrano AM, Selley DE, Parsons LH, Lichtman AH, Cravatt BF. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nature Chem Biol. 2009a;5:37–44. doi: 10.1038/nchembio.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JZ, Nomura DK, Vann RE, Walentiny DM, Booker L, Jin X, Burston JJ, Sim-Selley LJ, Lichtman AH, Wiley JL, Cravatt BF. Dual blockade of FAAH and MAGL identifies behavioral processes regulated by endocannabinoid crosstalk in vivo. Proc Natl Acad Sci U S A. 2009b;106:20270–5. doi: 10.1073/pnas.0909411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon LR, Ginsburg BC, Lamb RJ. Cannabinoid agonists differentially substitute for the discriminative stimulus effects of Delta(9)-tetrahydrocannabinol in C57BL/6J mice. Psychopharmacology (Berl) 2008;198:487–495. doi: 10.1007/s00213-007-0900-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Guidelines for the care and use of mammals in neuroscience and behavioral research. National Academies Press; Washington, D.C: 2003. [PubMed] [Google Scholar]
- Schacht JP, Selling RE, Hutchison KE. Intermediate cannabis dependence phenotypes and the FAAH C385A variant: an exploratory analysis. Psychopharmacology (Berl) 2009;203:511–7. doi: 10.1007/s00213-008-1397-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosburg JE, Blankman JL, Long JZ, Nomura DK, Pan B, Kinsey SG, Nguyen PT, Ramesh D, Booker L, Burston JJ, Thomas EA, Selley DE, Sim-Selley LJ, Liu QS, Lichtman AH, Cravatt BF. Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat Neurosci. 2010;13:1113–9. doi: 10.1038/nn.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipe JC, Chiang K, Gerber AL, Beutler E, Cravatt BF. A missense mutation in human fatty acid amide hydrolase associated with problem drug use. Proc Natl Acad Sci U S A. 2002;99:8394–9. doi: 10.1073/pnas.082235799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Goldberg SR. Motivational effects of cannabinoids and opioids on food reinforcement depend on simultaneous activation of cannabinoid and opioid systems. Neuropsychopharmacology. 2005;30:2035–45. doi: 10.1038/sj.npp.1300720. [DOI] [PubMed] [Google Scholar]
- Solinas M, Scherma M, Tanda G, Wertheim CE, Fratta W, Goldberg SR. Nicotinic facilitation of delta-9-tetrahydrocannabinol (THC) discrimination involves endogenous anandamide. J Pharmacol Exp Ther. 2007a;321:1127–1134. doi: 10.1124/jpet.106.116830. [DOI] [PubMed] [Google Scholar]
- Solinas M, Tanda G, Justinova Z, Wertheim CE, Yasar S, Piomelli D, Vadivel SK, Makriyannis A, Goldberg SR. The endogenous cannabinoid anandamide produces delta-9-tetrahydrocannabinol-like discriminative and neurochemical effects that are enhanced by inhibition of fatty acid amide hydrolase but not by inhibition of anandamide transport. J Pharmacol Exp Ther. 2007b;321:370–80. doi: 10.1124/jpet.106.114124. [DOI] [PubMed] [Google Scholar]
- Stewart JL, McMahon LR. The fatty acid amide hydrolase inhibitor URB 597: interactions with anandamide in rhesus monkeys. Br J Pharmacol. 2011;164:655–66. doi: 10.1111/j.1476-5381.2011.01388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambaro S, Bortolato M. Cannabinoid-related agents in the treatment of anxiety disorders: current knowledge and future perspectives. Recent Pat CNS Drug Discov. 2012;7:25–40. doi: 10.2174/157488912798842269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann RE, Warner JA, Bushell K, Huffman JW, Martin BR, Wiley JL. Discriminative stimulus properties of Delta9-tetrahydrocannabinol (THC) in C57Bl/6J mice. Eur J Pharmacol. 2009;615:102–7. doi: 10.1016/j.ejphar.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann RE, Walentiny DM, Burston JJ, Gamage TF, Wiley JL. Enhancement of the behavioral effects of endogenous and exogenous cannabinoid agonists by phenylmethyl sulfonyl fluoride. Neuropharmacology. 2012;62:1019–1027. doi: 10.1016/j.neuropharm.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varvel SA, Cravatt BF, Engram AE, Lichtman AH. Fatty acid amide hydrolase (-/-) mice exhibit an increased sensitivity to the disruptive effects of anandamide or oleamide in a working memory water maze task. J Pharmacol Exp Ther. 2006;317:251–7. doi: 10.1124/jpet.105.095059. [DOI] [PubMed] [Google Scholar]
- Walentiny DM, Gamage TF, Warner JA, Nguyen TK, Grainger DB, Wiley JL, Vann RE. The endogenous cannabinoid anandamide shares discriminative stimulus effects with (9)-tetrahydrocannabinol in fatty acid amide hydrolase knockout mice. Eur J Pharmacol. 2011;656:63–7. doi: 10.1016/j.ejphar.2011.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL. Cannabis: discrimination of “internal bliss”? Pharmacol Biochem Behav. 1999;64:257–60. doi: 10.1016/s0091-3057(99)00059-3. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Golden KM, Ryan WJ, Balster RL, Razdan RK, Martin BR. Evaluation of cannabimimetic discriminative stimulus effects of anandamide and methylated fluoroanandamide in Rhesus monkeys. Pharmacol Biochem Beh. 1997;58:1139–1143. doi: 10.1016/s0091-3057(97)00327-4. [DOI] [PubMed] [Google Scholar]
- Wiley JL, LaVecchia KL, Karp NE, Kulasegram S, Mahadevan A, Razdan RK, Martin BR. A comparison of the discriminative stimulus effects of Delta(9)-tetrahydrocannabinol and O-1812, a potent and metabolically stable anandamide analog, in rats. Exp Clin Psychopharmacol. 2004;12:173–9. doi: 10.1037/1064-1297.12.3.173. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Lowe JA, Balster RL, Martin BR. Antagonism of the discriminative stimulus effects of delta 9-tetrahydrocannabinol in rats and rhesus monkeys. J Pharmacol Exp Ther. 1995;275:1–6. [PubMed] [Google Scholar]
- Wiley JL, Marusich JA, Lefever TW, Grabenauer M, Moore KN, Thomas BF. Cannabinoids in disguise: Delta-9-Tetrahydrocannabinol-like effects of tetramethylcyclopropyl ketone indoles. Neuropharmacology. 2013;75:145–154. doi: 10.1016/j.neuropharm.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Ryan WJ, Razdan RK, Martin BR. Evaluation of cannabimimetic effects of structural analogs of anandamide in rats. Eur J Pharmacol. 1998;355:113–8. doi: 10.1016/s0014-2999(98)00502-0. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Walentiny DM, Vann RE, Baskfield CY. Dissimilar substitution patterns in mice trained to discriminate Δ9-tetrahydrocannabinol or methanandamide from vehicle. Behav Pharmacol. 2011;22:480–488. doi: 10.1097/FBP.0b013e328348eced. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Walentiny DM, Wright MJ, Jr, Beardsley PM, Burston JJ, Poklis JL, Lichtman AH, Vann RE. Endocannabinoid contribution to Delta(9)-tetrahydrocannabinol discrimination in rodents. Eur J Pharmacol. 2014;737:97–105. doi: 10.1016/j.ejphar.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise LE, Long KA, Abdullah RA, Long JZ, Cravatt BF, Lichtman AH. Dual fatty acid amide hydrolase and monoacylglycerol lipase blockade produces THC-like Morris water maze deficits in mice. ACS Chem Neurosci. 2012;3:369–78. doi: 10.1021/cn200130s. [DOI] [PMC free article] [PubMed] [Google Scholar]


