Abstract
Alcohol and nicotine are often taken together. The mechanisms underlying this frequent co-abuse are not well known. Genetic and pharmacological evidence suggests that the nicotinic acetylcholine receptors (nAChRs) containing the α3 and β4 subunits play a role in alcohol as well as nicotine addiction. AT-1001 is a high affinity α3β4 nAChR partial agonist recently found to block nicotine self-administration and relapse-like behavior in rats. Here, to study the involvement of α3β4 nAChRs in the mechanisms that regulate alcohol abuse we evaluated the effects of AT-1001 on alcohol taking and seeking in Sprague-Dawley rats. AT-1001 reduced operant alcohol self-administration at the highest dose examined (3.0 mg/kg), an effect also observed for food self-administration. A dose of 1.5 mg/kg AT-1001, which had no effect on alcohol or food self-administration, essentially eliminated reinstatement of alcohol seeking induced by yohimbine (0.625 mg/kg) whereas, reinstatement induced by alcohol-associated cues was not altered, nor did AT-1001 induce reinstatement of extinguished self-administration on its own. Finally, AT-1001 showed an anxiolytic activity when measured in the presence or absence of yohimbine stress in the elevated plus maze paradigm. Together, these observations do not support a specific involvement of the α3β4 nAChR in mediating alcohol reward or cue-induced relapse to alcohol seeking but rather indicate that the α3β4 nAChR partial agonism may constitute an attractive approach for treating alcohol use disorders exacerbated by elevated stress response.
1. INTRODUCTION
Alcohol and nicotine are highly co-abused. It has been estimated that 80-90% of alcohol dependent individuals smoke regularly while 60% of smokers binge drink or consume substantial amounts of alcohol (Batel et al., 1995; DiFranza and Guerrera, 1990). Furthermore, while non-dependent drinkers are likely to smoke (Kandel et al., 1997), alcohol-dependent smokers have more severe nicotine dependence, associated with greater difficulties quitting than non-dependent smokers (Romberger and Grant, 2004). This remarkable rate of alcohol and nicotine co-abuse may result from shared genetic factors (Dani and Harris, 2005) and common molecular and cellular targets (Doyon et al., 2013; Hendrickson et al., 2013). For example, both nicotine and alcohol activate the mesocorticolimbic dopaminergic reward circuitry to promote drug use. Nicotine primarily acts via nicotinic acetylcholine receptors (nAChRs), a site where alcohol can also directly or indirectly interact (Cardoso et al., 1999; Le et al., 2000).
nAChRs are pentameric ligand-gated ion channels made up of α and β subunits that form heteromeric or homomeric receptors (Gotti et al., 2006). The α4β2* (*indicates the possibility of additional subunits) and the α7 nAChRs are by far the most prevalent in the central nervous system (Perry et al., 2002; Xiao and Kellar, 2004), and both can be involved in nicotine rewarding properties (Epping-Jordan et al., 1999; Levin et al., 2009; Picciotto, 1998).
However, α3-β4-α5 nAChR gene cluster on chromosome 15 has been associated with an increased likelihood of a smoker to become dependent and smoke a greater number of cigarettes per day (Berrettini et al., 2008; Caporaso et al., 2009; Thorgeirsson et al., 2008). Consistent with the importance of these subunits, inhibition of the α3β4* nAChR subtype with the ibogaine analog 18-methoxycoronaridine (18-MC) was shown to attenuate nicotine self-administration acting in the medial habenula (MHb) and interpeduncular nucleus [IPN, (Glick et al., 2000a; Glick et al., 2011)], two regions particularly rich in these subunits (Sheffield et al., 2000; Wada et al., 1989; Wu et al., 2014). α3β4* nAChRs in the habenulo-interpeduncular (MHb/IPN) system were also found to modulate nicotine withdrawal (Salas et al., 2003; Salas et al., 2009), thus pointing to the α3β4* nAChR in the MHb/IPN pathway as a critical substrate for nicotine dependence.
Human genetic association studies have also linked α3, β4 and α5 subunits with increased risk of alcohol dependence (Joslyn et al., 2008; Wang et al., 2009). In addition, both operant and home cage alcohol self-administration were attenuated following administration of compounds with partial agonist or antagonist activity at α3β4* (Chatterjee et al., 2011; Rezvani et al., 1997) providing evidence for the involvement of the α3 and β4 subunits in alcohol as well as nicotine addiction.
Stressful life events are relevant factors for the initiation, development and maintenance of addiction to drugs of abuse including alcohol. Stress is also a common cause of relapse to drug seeking (Bruijnzeel, 2012; Heilig and Koob, 2007). Recent assumptions point to the habenula as a region critically implicated in behavioral stress responses due to its actions as a major regulator of several neurotransmitter systems, including dopamine, serotonin, norepinephrine, and acetylcholine (Baldwin et al., 2011; Hikosaka, 2010; Velasquez et al., 2014). Thus, due to the high α3β4* nAChR density in the MHb, nicotinic receptors containing these subunits may play a relevant role in stress-induced behaviors associated to drug dependence.
AT-1001 was recently described as a high affinity ligand for the α3β4 nAChR that has partial agonist activity in Ca2+ flux and patch clamp electrophysiological experiments but is a functionally selective antagonist for α3β4* nAChRs through desensitization (Wu et al., 2014; Zaveri et al., 2014). AT-1001 was shown to possess nicotine- but not alcohol-suppressive effects when tested in an operant co-administration paradigm in rats, as it decreased nicotine self-administration at doses that did not affect alcohol self-administration (Cippitelli et al., 2014b; Toll et al., 2012). To better understand if the α3β4* nAChR plays a role in alcohol addiction we examined the effect of AT-1001 on both alcohol-taking and -seeking behaviors, using animal models of alcohol consumption and relapse, respectively. Operant alcohol self-administration and reinstatement experiments were conducted in animals exposed and not exposed to pharmacological stress. In addition, we examined whether treatment with the α3β4* nAChR-selective ligand AT-1001 led to anxiolytic effects under normal (basal) circumstances or during the occurrence of negative affective states associated with pharmacological stress exposure.
2. MATERIAL AND METHODS
2.1 Animals
Male Sprague-Dawley rats (200-225 g at their arrival) were obtained from Charles River (Portage, MI). Rats were housed in a room with a reverse 12-hour light/12-hour dark cycle (lights off at 7:30 AM). All experiments were conducted during the dark phase of the cycle. Animals were acclimated for seven days with water and chow (Teklad Diets, Madison, WI) provided ad libitum and handled for three days before the experiments were conducted. All animal experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Consistent with these guidelines, ongoing statistical testing of data collected was used to minimize the number of animals used, within the constraints of necessary statistical power. All methods used were pre-approved by the Institutional Animal Care and Use Committee at the Torrey Pines Institute for Molecular Studies (Port Saint Lucie, FL).
2.2 Drugs
AT-1001 [N-(2-bromophenyl)-9-methyl-9-azabicyclo [3.3.1] nonan-3-amine] was synthesized at Astraea Therapeutics (Mountain View, CA) by methods described elsewhere (Zaveri et al., 2010). It was suspended in a vehicle containing 2% DMSO, 1% HCl and 97% of 0.5% aqueous hydroxypropyl-cellulose, administered in a 1 ml/kg volume injection, given by a subcutaneous (s.c.) route of administration. Yohimbine hydrochloride and alcohol were purchased from Sigma (St Louis, MO). Alcohol solution (10% v/v) was prepared by diluting 95% alcohol with tap water and made available orally. Yohimbine was dissolved in distilled water and administered intraperitoneally (i.p.) in a 1 ml/kg injection volume. 2ml/kg injection volume was used to improve solubility of yohimbine at 2.5 mg/kg dose.
2.3 Apparatus for operant behavior
Self-administration and reinstatement experiments were conducted in operant conditioning chambers (Med Associates, Inc., St. Albans, VT) enclosed in lit, sound attenuating, ventilated environmental cubicles. Each chamber was equipped with two retractable levers located in the front panel, laterally to a drinking reservoir. When testing food-maintained behavior, drinking reservoir was replaced by a food pellet magazine. The pellet dispenser was positioned behind the front panel of the chambers. Chambers were also equipped with visual stimuli located above the levers (cue lights) and near the top of the chamber on the back panel (house light). Reinforcers were delivered following responses on one (active) lever, while responses on the other lever (inactive) were recorded but did not result in any programmed consequences. Alcohol was connected to the drinking receptacle through a PE-160 tube and delivered by the activation of an infusion pump. A microcomputer controlled the delivery of the reinforcers, presentation of visual stimuli and recording of the behavioral data.
2.4 Intermittent 10% alcohol procedure
One week after arrival, all rats subjected to operant alcohol self-administration and reinstatement experiments were exposed to an intermittent (every other day) alcohol exposure procedure in their home cages as previously described (Cippitelli et al., 2012) where one bottle of 10% (v/v) alcohol solution replaced one bottle of water and was available for the next 24 h. The next day, the 10% alcohol bottle was removed and replaced with a bottle of water. This procedure took place across two weeks for a total of 7 days of alcohol exposure.
2.5.1 Alcohol self-administration
Once the procedure of alcohol exposure in their home cages was terminated, rats were moved to self-administration chambers in which they were trained to self-administer 10% (v/v) alcohol in 30-min daily sessions under a fixed ratio 1 (FR-1) schedule of reinforcement over three weeks (15 sessions). Following a response that delivered alcohol (0.1 ml), a 5-sec time-out (TO) period was in effect, during which the house light was on and responses were recorded but not reinforced. Once a stable baseline was established, animals (N=8) were used to assess the effectiveness of AT-1001 (0.0, 0.75, 1.5, 3.0 mg/kg) using a Latin-square counterbalanced within-subjects design. Test sessions were 4 days apart. Following each test session day, animals were allowed 1 day off, and a new baseline was then established over the following 2 days. Animals received the s.c. injection of drug or vehicle 10 min prior to the self-administration session. Results are described as number of rewards obtained in 30 min. Responses on the inactive lever were also recorded and served as an index of unspecific motor behavior.
2.5.2 Food self-administration
Two weeks following their arrival, one group of rats (N=8) underwent a food restriction procedure in which rats received 16-20 g of chow daily with water freely accessible. After 1 week, rats were trained to self-administer 45-mg food pellets (Test Diet, 5-TUM, Richmond, IN) in 30-min daily sessions on an FR-1 (TO=20 sec) schedule of reinforcement. The TO period was signaled by illumination of a cue light at the top of the active lever for 20 sec and lever pressing during this period did not lead to programmed consequences. Food self-administration was conducted for 7 sessions. Rats were then treated with different doses of AT-1001 (0.0, 0.75, 1.5, 3.0 mg/kg) according to a Latin-square counterbalanced within-subjects design. Drug doses or vehicle were given 10 min before the beginning of the session. Results are described as number of rewards obtained in 30 min. Responses on the inactive lever were also recorded and served as an index of unspecific motor behavior.
2.6 Alcohol self-administration following yohimbine treatment
Rats (N=8) were subjected to the intermittent alcohol exposure in their home cages and then trained to self-administer 10% alcohol as described above. Once reached 15 alcohol self-administration sessions, drug testing was started. Stress exposure consisted of the challenge with the pharmacological stressor yohimbine at doses previously shown to increase alcohol-reinforced lever pressing in rats (Ayanwuyi et al., 2013; Marinelli et al., 2007). AT-1001 (1.5 mg/kg) or vehicle was injected 10 min prior to yohimbine (0.625 mg/kg) treatment or vehicle that was administered 30 min prior to the 30-min self-administration session. Treatments were counterbalanced according to a Latin Square design such that all rats received all treatments. Test sessions were conducted as described above.
2.7.1 Cue-induced reinstatement of alcohol seeking
Following the intermittent alcohol exposure procedure, rats (N=8) carried out daily 30-min sessions of 10% (v/v) alcohol self-administration under an FR-1 schedule of reinforcement for 15 days and were then used to assess cue-induced reinstatement of alcohol seeking. Concurrently with the lever pressing, a 5-s time-out period was in effect. A stimulus predictive of alcohol (orange odor) was also presented immediately after the animals were placed in the operant chambers and immediately before the onset of every conditioning session (Cippitelli et al., 2010b). Alcohol-reinforced responding was then extinguished in daily 30-min sessions that continued for 10 days. In this phase, neither alcohol nor house light and orange cues were available. On the day following the last extinction session (day 1), a 30-min reinstatement session was carried out without any drug treatment. Odor and cue-light, but not alcohol, were presented, and reinstatement response rates (i.e., responses on the previously alcohol-associated lever) were recorded. These response rates were used to assign animals to treatment groups balanced for response rates for the drug treatment experiment that followed. To assess the effects of AT-1001, reinstatement experiments were then conducted every fourth day [on days 4, 7, 10 (Ciccocioppo et al., 2001)]. In a Latin-square counterbalanced order that paralleled that used for the self-administration studies, animals were pretreated with AT-1001 (0.0, 0.75, 1.5 mg/kg, s.c.) 10 min prior the onset of the reinstatement sessions. Responding on the inactive lever was also recorded throughout the experiment.
2.7.2 Yohimbine stress-induced reinstatement of alcohol seeking
This experiment was performed as previously described (Cippitelli et al., 2010a). In brief, after completion of the intermittent alcohol exposure in the home cages, rats (N=7) were allowed daily 30-min self-administration sessions under an FR-1 schedule of reinforcement, for 15 days in order to reach a robust and stable level of alcohol self-administration. Sessions were performed 5 days a week. Once self-administration was established, responses were extinguished over 11 consecutive daily sessions. Extinction sessions were identical to self-administration sessions, except that alcohol was no longer available. After the last extinction session, animals were pre-treated with AT-1001 (0.0, 0.75, or 1.5 mg/kg, s.c.) 10-min prior to administration of yohimbine (0.625 mg/kg, i.p.). Thirty minutes following yohimbine treatment, the reinstatement test was started under the same conditions as extinction sessions. House light was still contingently presented during both extinction and reinstatement phases. Responding on the inactive lever was recorded throughout the experiment to monitor possible nonspecific behavioral effects.
2.7.3 Effect of AT-1001 on reinstatement of alcohol seeking
In a separate control experiment that used new rats (N=8), effects of AT-1001 (0.0, 0.75, or 1.5 mg/kg, s.c.) on response rates following extinction were examined. Procedures for this experiment were identical to those described for yohimbine-induced reinstatement with the exception that no stress was used to elicit reinstatement prior to AT-1001 testing.
2.8 Elevated plus maze (EPM)
To measure anxiety-like responses, the EPM test was used, as previously described (Cippitelli et al., 2014a). In brief, the apparatus was made of black plastic with two open arms (50 × 10 cm) and two closed arms (50 × 10 × 45 cm) connected by a 10 × 10 cm central area. The maze was 50 cm above the floor and placed in a spacious room. Testing was performed under a source of red light. The 5-min test procedure began when the animal was placed in the center of the maze, facing a closed arm. The percent of time spent exploring the open arms and the percent of open arm entries were used as measures of anxiety-like behavior, whereas the number of entries into the closed arms was used as an indicator of general motor activity (Pellow et al., 1985). An entry into an arm was defined as the animal placing all four paws over the line marking that area. The apparatus was cleaned with tap water between each rat performance. To test the effects of AT-1001 on rats exposed and non-exposed to stress, a new group of rats (N=29) were assigned to 4 treatment groups (8 AT-1001/vehicle, 6 vehicle/yohimbine, 6 AT-1001/yohimbine and 9 vehicle/vehicle). AT-1001 (1.5 mg/kg, s.c.) or vehicle was administered 10 min prior to yohimbine (2.5 mg/kg, i.p.) or vehicle which was in turn injected 30 min prior to the EPM.
2.9 Data analysis
Effect of AT-1001 on alcohol and food reinforcement was analyzed by one-way ANOVA with treatment (drug dose) as a within-subject factor. A two-way within-subject ANOVA was used to analyze the effect of AT-1001 on yohimbine-induced increase of alcohol lever pressing. To establish that reinstatement of alcohol seeking was successfully induced, responding during the last EXT session and the respective reinstatement session were separately compared to the vehicle-treated group by one-way within-subject ANOVA. The effect of systemic AT-1001 on reinstatement experiments was analyzed using one-way repeated measures ANOVA with drug dose as a within-subject factor. EPM data were analyzed using a two-way between-subject ANOVA with AT-1001 or vehicle serving as a “pretreatment” factor and yohimbine or vehicle used as a “treatment” factor. The level of significance was set at p<0.05. ANOVAs were followed, where appropriate, by Student Newman-Keuls post hoc tests.
3. RESULTS
3.1 Effect of AT-1001 on operant alcohol self-administration
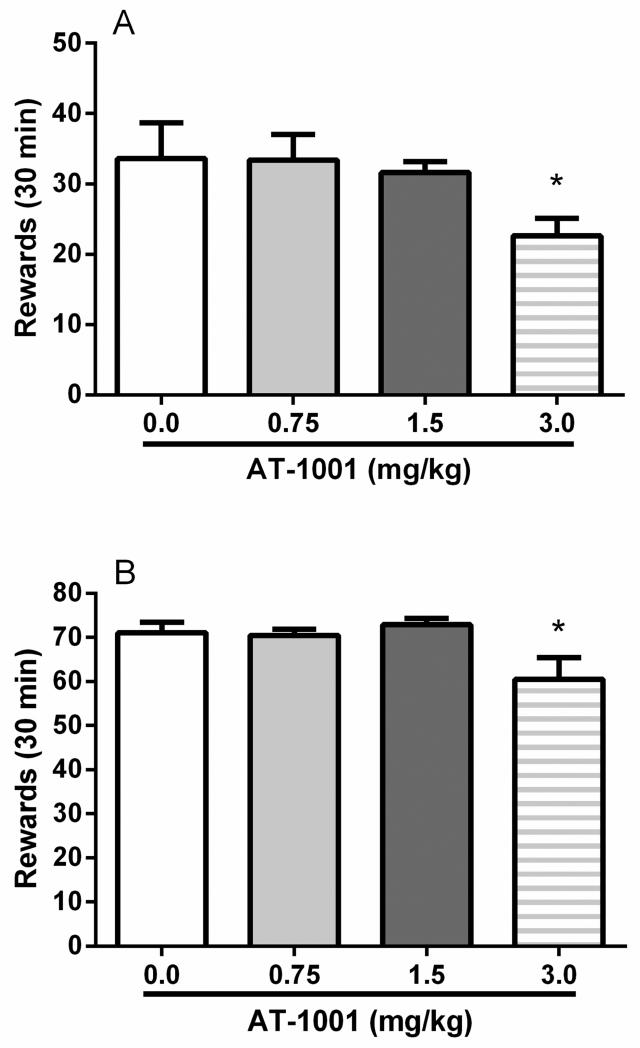
The α3β4 nAChR partial agonist AT-1001 (0.75, 1.5, and 3.0 mg/kg; Figure 1A) attenuated operant responding on the alcohol-reinforced lever (main effect: F(3,21)=3.4; p<0.05). On post hoc comparisons, responding was significantly decreased at 3.0 mg/kg (p=0.05) while lower doses did not differ from vehicle (0.0 mg/kg). Responding on the inactive left lever was not significantly affected by the drug (F(3,21)=0.9; NS).
Figure 1.
Non-selective reduction of operant alcohol self-administration by AT-1001 (0.0, 0.75, 1.5, 3.0 mg/kg, s.c.). The 3.0 mg/kg dose of AT-1001 weakly attenuated (A) 10% (v/v) alcohol (N=8) and (B) food pellet self-administration (N=8) under a FR-1 schedule. Each data point is the mean (±SEM) number of rewards earned in 30 min. *p<0.05, difference from the groups receiving vehicle of AT-1001 (0.0 mg/kg). For detailed statistics, see “Results.”
3.2 Effect of AT-1001 on food self-administration
AT-1001 (0.75, 1.5, and 3.0 mg/kg; Figure 1B) reduced operant responding on the food-reinforced lever (main effect: F(3,21)=4.9; p<0.01). In a similar manner to alcohol self-administration, AT-1001 decreased responding for food at 3.0 mg/kg (p<0.05) while lower doses did not differ from vehicle (0.0 mg/kg). Responding on the inactive left lever was not significantly affected by the drug (F(3,21)=1.2; NS).
3.3 Effect of AT-1001 on yohimbine-induced increase of alcohol self-administration
Overall ANOVA displayed a main effect of yohimbine treatment (F(1,7)=23.1; p<0.01) that was not accompanied by a main effect of AT-1001 (F(1,7)=3.2; NS) or interaction (F(1,7)=1.4; NS). These results indicate that yohimbine (0.625 mg/kg, i.p.) markedly increased alcohol lever pressing (Figure 2). Pre-treatment with AT-1001 failed to significantly block this increased response. Responding on the inactive left lever was not significantly affected by drug treatments [yohimbine: (F(1,7)=0.3; NS); AT-1001: (F(1,7)=0.0; NS); interaction: (F(1,7)=1.2; NS)].
Figure 2.
Pre-treatment with AT-1001 (1.5 mg/kg, s.c.) failed to block the escalation of alcohol self-administration (FR-1) elicited by systemic (i.p.) treatment with yohimbine (YOH) at the dose of 0.625 mg/kg. Results are the mean (±SEM) number of rewards earned in 30 min (N=8). **p<0.01, difference from groups not receiving yohimbine. For detailed statistics, see “Results.”
3.4 Effect of AT-1001 on yohimbine-induced reinstatement of alcohol seeking
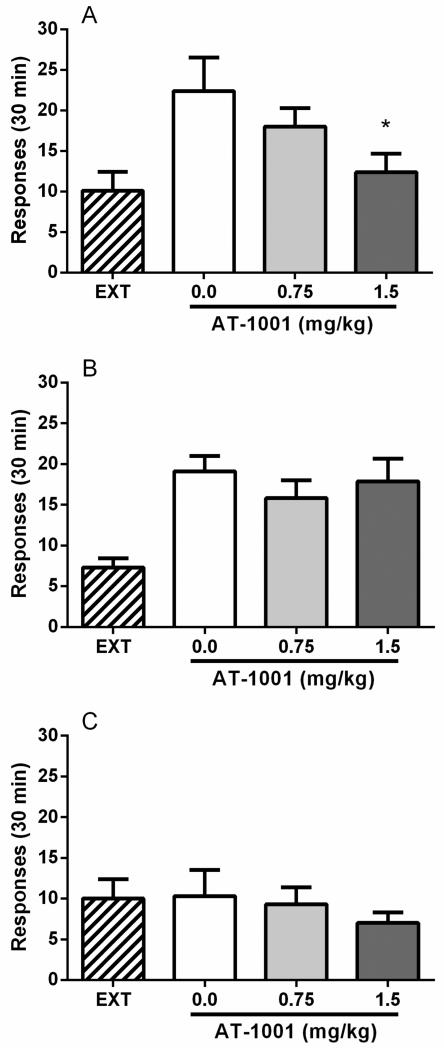
During self-administration prior to extinction training, responses on the alcohol lever on the last alcohol session were 36.9±3.1 (mean ± SEM). During extinction, responding progressively decreased from 28.9±2.8 on the first day to 10.1± 2.3 on the last extinction day. Treatment with yohimbine (0.625 mg/kg, i.p.) induced a robust increase in the number of responses (22.4±4.1) on the alcohol-associated lever compared to the last extinction day (F(1,6)=15.8, p<0.01) whereas inactive lever responding was unaffected (F(1,6)=0.1, NS). Pretreatment with AT-1001 at doses that did not affect alcohol or food baseline responding (0.75 and 1.5 mg/kg) dose-dependently reversed yohimbine-induced responding on the alcohol-associated lever (F(2,12)=4.3, p<0.05). Post hoc analysis showed a statistically significant effect of AT-1001 at the higher dose (P<0.05, Figure 3A). Responding on the inactive lever was not modified by AT-1001 (F(2,12)=0.8, NS).
Figure 3.
Systemic injection of AT-1001 (0.0, 0.75, 1.5 mg/kg, s.c.) (A) blocks yohimbine-induced reinstatement of alcohol seeking but (B) does not affect cue-induced reinstatement. Values represent the mean (±SEM) number of total responses on the alcohol-associated lever in 30 min. There was significant reinstatement induction by yohimbine administration (0.625 mg/kg, i.p.) and cues previously associated with alcohol availability (house light, orange odor), as indicated by responses in rats (N=7 and 8, respectively) exposed to these stimuli in the absence of reward delivery compared with extinction (EXT) responding by the same animals. ##p<0.01 vs. last day of EXT. AT-1001 1.5 mg/kg dose significantly attenuated yohimbine-induced reinstatement while there was not a significant main effect of AT-1001 treatment on cue-induced reinstatement responding. *p<0.05 difference from vehicle (0.0 mg/kg)-treated group. (C) Systemic injection of AT-1001 (0.0, 0.75, 1.5 mg/kg, s.c.) does not elicit reinstatement per se. Values represent the mean (±SEM) number of total responses of (N=8) rats on the alcohol-associated lever in 30 min. For detailed statistics, see “Results”.
3.5 Effect of AT-1001 on cue-induced reinstatement of alcohol seeking
In this group of animals, responses on the alcohol-associated lever on the last alcohol session were 41.9±3.3 (mean ± SEM). During extinction, responding progressively decreased from 27.6±3.0 on the first day to 7.3± 1.1 on the last extinction day. As shown in Figure 3B, following extinction, a robust increase of responding (21.5±2.4) on the alcohol-associated lever pressing was found when previously alcohol-paired stimuli (house light and orange odor) were reintroduced. ANOVA showed a significant difference between responding in the cue-induced reinstatement session and the last one of the extinction phase (F(1,7)=45.9, p<0.001). Inactive lever responses were not changed (F(1,7)=2.3, NS). Pre-treatment with AT-1001 did not affect cue-induced reinstatement (main treatment effect: F(2,14)=1.1, NS). Inactive lever responding was also unaltered (F(2,14)=0.5, NS).
3.6 Effect of AT-1001 on reinstatement of alcohol seeking behavior
As shown in Figure 3C, treatment with AT-1001 in the absence of stimuli that triggered reinstatement (F(1,7)=0.0, NS) did not modify lever pressing on the alcohol-associated lever per se as compared to vehicle (0.0 mg/kg) treatment (F(2,14)=0.9, NS).
3.7 Effect of AT-1001 on anxiety-like behavior
Figure 4A shows the effects of AT-1001 on time spent in the open arms of the EPM following yohimbine or vehicle administration. ANOVA revealed an anxiolytic response of AT-1001 as rats spent a greater amount of time exploring the open arms than animals not receiving AT-1001 (main effect of “pretreatment”: F(1,25)=4.4, p<0.05). In contrast, yohimbine treatment markedly suppressed this variable (main effect of “treatment”: F(1,25)=4.3, p<0.05). There was no “pretreatment × treatment” interaction (F(1,25)=0.1, NS) indicating that experimental anxiety was attenuated by AT-1001 in rats receiving and those not receiving yohimbine stress. The anxiolytic effect of AT-1001 was less reliable when analyzing open arm entries (Figure 4B). Administration of AT-1001 led to a marked but non-significant trend to increased entries onto the open arms [main effect of “pretreatment” (F(1,25)=2.5, NS)] while yohimbine treatment induced anxiety-like behavior (F(1,25)=5.3, p<0.05). There was no “pretreatment × treatment” interaction (F(1,25)=0.9, NS). On analysis of closed arm entries (Figure 4C), AT-1001 administration was ineffective (F(1,25)=1.7, NS) while yohimbine treatment dropped crossings into the closed arms (F(1,25)=12.9, p<0.01) suggesting decreased locomotor behavior for rats treated with 2.5 mg/kg dose of yohimbine. No interaction was observed (F(1,25)=1.1, NS).
Figure 4.
Anxiolytic effect of AT-1001 as assessed in the elevated-plus maze (EPM) test. (A) Rats pretreated with AT-1001 (1.5 mg/kg, s.c.) displayed increased time spent exploring the open arms as compared to rats non-AT-1001 treated exposed or not exposed to yohimbine (YOH, 2.5 mg/kg, i.p.) stress. In contrast, yohimbine increased experimental anxiety as open arm time was decreased. (B) Yohimbine also lowered open arm entries. (C) The elevated anxiety due to yohimbine treatment was accompanied by reduced exploration of the EPM. Values are presented as mean percent (%, ±S.E.M.) of open arm time and entries, and mean (±S.E.M.) number of closed arm entries (N=6-9 per group). *p<0.05 difference from groups pretreated with vehicle (VEH) of AT-1001; #p<0.05, ##p<0.01 difference from groups non-receiving yohimbine. For detailed statistics, see Results.
4.1 DISCUSSION
We found that the extent to which alcohol lever pressing is attenuated by AT-1001 parallels the effect observed while examining operant responding for food. Yohimbine stress-induced increase of operant alcohol self-administration was not significantly reduced but yohimbine-induced reinstatement of alcohol seeking was almost fully blocked by the treatment with AT-1001 at a dose that had no effect on food responding. This effect was behaviorally specific as AT-1001 had no effect on alcohol seeking induced by alcohol-associated cues. AT-1001 also clearly showed anxiolytic properties when tested in an exploratory paradigm of anxiety that may account for this pattern of results.
Due to a lack of subtype-selective ligands, the role of the α3β4 nAChR subtype on drug addiction disorders is poorly understood. For example few studies have investigated the effects of the pharmacological manipulation of the α3β4* nAChR with agonists or antagonists on alcohol consumption. In one of these studies the ibogaine analogue 18-MC, an α3β4* nAChR antagonist selective for α3β4 over α4β2 nAChR (Glick et al., 2000b), was shown to reduce alcohol consumption and preference in alcohol-preferring P rats (Rezvani et al., 1997). In fact, doses of 18-MC that were efficacious in lowering alcohol lever pressing were also found to attenuate self-administration of several other abused drugs such as methamphetamine, morphine, cocaine and nicotine leading to the hypothesis that α3β4* nAChR antagonists may represent a novel approach for treating multiple addictive disorders (Maisonneuve and Glick, 2003). A more recent study described two high affinity partial agonists, CP-601932 and PF-4575180, with some selectivity for α3β4* nAChRs, as anti-alcohol agents able to reduce alcohol self-administration and consumption, indicating that not only the inhibition but also the activation of these nAChR subtypes may attenuate alcohol-related behaviors (Chatterjee et al., 2011). These findings suggest that the effects of nAChR partial agonists and antagonists have promise for the management of alcohol dependence and other alcohol use disorders.
AT-1001 is a high affinity α3β4 nAChR ligand, with about 100-fold binding selectivity versus the α4β2 nAChR (Toll et al., 2012). AT-1001 was recently shown to have low efficacy partial agonist activity at the human α3β4 nAChR transfected into oocytes (Zaveri et al., 2014) and the rat α3β4 nAChR (Cippitelli et al., 2014b). AT-1001 also causes desensitization at the same doses at which it activates the α3β4 nAChRs, resulting in functional antagonism of the receptor (Zaveri et al., 2014). On the other hand, AT-1001 was found to block ACh responses at the α4β2 nAChR at doses only 3-fold higher than at the α3β4 nAChR (Cippitelli et al., 2014b; Zaveri et al., 2014). Emerging results from our laboratory show that AT-1001 reduced nicotine lever pressing at doses that do not alter alcohol responding when tested in an operant co-administration paradigm in which intravenous nicotine and oral alcohol were made simultaneously available (Cippitelli et al., 2014b). In contrast, the α4β2* nAChR partial agonist varenicline, currently the standard medication for smoking cessation, blocked both nicotine and alcohol taking at similar doses in the same paradigm (Cippitelli et al., 2014b). These data suggest that partial activation/functional inhibition at α3β4* receptors would primarily act on decreasing nicotine reinforced behavior, while targeting α4β2* appears to modulate both alcohol and nicotine taking. A study using α4 nAChR knock-out mice, points to a direct involvement of α4 nAChRs in modulating alcohol reward (Hendrickson et al., 2010), supporting this view.
To follow up on these initial findings, the effect of AT-1001 was specifically studied on alcohol self-administration, with alcohol used as the only reinforcer. We found that the 3 mg/kg dose of AT-1001, previously shown to abolish nicotine self-administration (Toll et al., 2012), slightly attenuated responding for alcohol (about 15 % reduction), a response accompanied by a similarly weak reduction of food pellet self-administration when conducted in food-restricted animals. Lower doses were ineffective at reducing either alcohol or food self-administration, but were effective in attenuating the self-administration and reinstatement of nicotine (Cippitelli et al., 2014b; Toll et al., 2012). Therefore, a specific involvement of the α3β4* nAChR in mediating alcohol reward is not supported by our observations, as the effect on alcohol self-administration is marginal as compared to nicotine and non-specific as compared to a natural reward. Additionally, due to the complex AT-1001 in vitro profile, it is possible that doses of AT-1001 as high as 3.0 mg/kg are able to antagonize α4β2* nAChRs, thus leading to a non-specific reduction of alcohol as well as natural rewards. Accordingly, it could be the α4β2* antagonism rather than α3β4* nAChR partial agonism that mediates the anti-alcohol actions described by Chatterjee et al. (2011). However, the selectivity of the anti-alcohol effects (as compared to sucrose consumption) shown in the Chatterjee study represents a discrepancy with findings shown in the present study, which may be explained by the different pharmacological profile of the α3β4* nAChR ligands used.
The habenula, which harbors very high concentrations of α3β4* nAChRs, is a regulator of several neurotransmitter systems, including dopamine, serotonin, norepinephrine, and acetylcholine and is emerging as a region critically important to behavioral responses to stress (Baldwin et al., 2011; Hikosaka, 2010; Velasquez et al., 2014). This rationale prompted us to investigate whether the α3β4* nAChR played a role in altering alcohol self-administration under stressful conditions. The pharmacological stressor yohimbine, an α2 adrenoceptor antagonist that increases noradrenaline cell firing (Aghajanian and VanderMaelen, 1982) has become the most common stress manipulation in alcohol studies, as yohimbine induces anxiety-like responses in both humans (Bremner et al., 1996b) and laboratory animals (Bremner et al., 1996a). This compound induces a stress-like state that leads to increased alcohol self-administration and reinstatement of alcohol seeking (Cippitelli et al., 2010a; Le et al., 2005) which are prevented by the administration of selective corticotropin-releasing factor receptor 1 (CRF1-R) antagonists (Ayanwuyi et al., 2013; Marinelli et al., 2007). We found that a dose of AT-1001, which was ineffective in decreasing alcohol-reinforced lever pressing (1.5 mg/kg), slightly but not significantly attenuated the yohimbine-induced increase in the reinforcing effects of alcohol, indicating that α3β4* nAChRs are probably not crucial to the induction of the excessive alcohol lever pressing due to stress exposure.
In order to further examine the possible role of the α3β4* nAChRs in stress responses associated with alcohol-related behaviors, we determined whether AT-1001 was effective in reducing relapse to alcohol seeking triggered by alcohol-associated cues or pharmacological stress. It has been suggested that cues and stress are additive in inducing alcohol relapse as assessed in the reinstatement paradigm, and their role is mediated by distinct neuropharmacological substrates with cue-induced relapse blocked by the µ-opioid antagonist naltrexone and stress (foot-shock or yohimbine)-induced relapse inhibited by CRF1-R antagonists (Liu and Weiss, 2002; Shaham et al., 2003). Therefore, since mechanisms underlying alcohol seeking can be pharmacologically dissociated we hypothesized that AT-1001 might play a role in neurocircuitry mediating aversive emotional states implicated in stress-induced relapse but not in appetitive mechanisms implicated in cue-induced relapse. In excellent agreement with this hypothesis, our data demonstrate that doses of AT-1001, which were ineffective in reducing baseline alcohol self-administration, blocked yohimbine-induced reinstatement but not cue-induced reinstatement of alcohol seeking. Neither did AT-1001 stimulate or inhibit extinction responding in the absence of other stimuli eliciting relapse-like behavior. These results reveal a behaviorally specific response of the manipulation at α3β4* receptors to suppress stress-induced reinstatement of alcohol seeking without affecting extinction responding or cue-induced reinstatement. This is reminiscent of compounds, such as CRF1-R antagonists and neurokinin 1 receptor (NK1-R) antagonists that target receptors for stress-related neuropeptides (Schank et al., 2011; Schank et al., 2012). This latter similarity is particularly noteworthy because the MHb is enriched in both α3β4* nAChRs (Sheffield et al., 2000) and NK1-Rs (Norris et al., 1993).
Furthermore, MHb projections containing substance P can modulate the activity of the MHb/IPN pathway and are thought to be a source of connectivity between the medial and the lateral portion of the habenula [LHb, (Bianco and Wilson, 2009; Sutherland, 1982)]. In addition, the inactivation of the LHb has been shown to reduce cue-induced reinstatement to cocaine seeking only in the presence of yohimbine, without affecting cue-induced reinstatement by itself. Furthermore, in the same study, the inactivation of the LHb attenuated yohimbine-induced anxiogenic behavior in two different tests of anxiety, the EPM and the defensive burying paradigm, suggesting that the LHb critically regulates anxiogenic states during stressful conditions (Gill et al., 2013). Therefore, one possible mechanism by which the pharmacological manipulation of α3β4* nAChRs by AT-1001 selectively regulates yohimbine-induced reinstatement of alcohol seeking may implicate the recruitment of the substance P/NK1-R system in the unidirectional MHb/LHb connection pathway.
To determine whether the α3β4* nAChR was directly involved in stress mechanisms regardless of the influence of alcohol-mediated responses we examined the effects of AT-1001 in the EPM model of anxiety following yohimbine treatment and under basal (non-stressful) conditions. Under environmental circumstances that elicited robust expression of experimental anxiety, as described by the relatively low basal exploration of the open arms of the control group, yohimbine treatment alone clearly increased the anxiogenic-like response. However, the observed yohimbine-induced reduction in open arm time and entries was also associated with a reduced number of entries into the closed arms, commonly thought to reflect reduced locomotor behavior. We interpret this as evidence of increased expression of inhibitory/passive response to stress that occurs when subjects experience excessive stress (Cippitelli et al., 2014a). The altered locomotor activity of the yohimbine-treated rats, however, does not alter the primary impact of the current results, whereby AT-1001, regardless whether administered prior to yohimbine or vehicle, clearly led to increased open arm time compared to vehicle-yohimbine and vehicle-vehicle treated groups. Due to the ability to act as a functional antagonist at α3β4* nAChRs, the observed anxiolytic activity of AT-1001 is not surprising and is consistent with the anxiolytic effects observed in mice lacking β4 nicotinic receptor subunits (Salas et al., 2003) and in rats administered with 18-MC (Maisonneuve and Glick, 2003), and may account for our observations regarding the inhibition of yohimbine-induced reinstatement of alcohol seeking. In addition, since anxiety is a hallmark of drug withdrawal, the ability of functional antagonism at α3β4* nAChRs to elicit anxiolytic responses may contribute to the key role that these subunits play in mediating withdrawal symptoms of nicotine and other abused drugs such as morphine (Jackson et al., 2013; Muldoon et al., 2014; Salas et al., 2009).
4.2 CONCLUSION
We found that the α3β4* nAChR partial agonist AT-1001 led to a reduction of alcohol self-administration only at doses that also reduced food self-administration. Yohimbine-induced increase of alcohol self-administration was slightly attenuated but not significantly altered by pre-treatment with AT-1001. In contrast, yohimbine- but not cue-induced reinstatement, was reduced by AT-1001 at doses that had no effect on food responding. These results may be due to anxiolytic activity of AT-1001. Altogether the findings reported here suggest the possibility that relapse induced by stressful life events could be inhibited by α3β4* nAChR partial agonists and support a potential utility of these agents to target some aspects of alcohol use disorders, particularly those linked to negative affective states such as anxiety or stress.
α3β4 nAChRs are not specifically involved in alcohol reinforcement processes
α3β4 nAChRs are not involved in cue-induced reinstatement of alcohol seeking
α3β4 nAChRs are implicated in alcohol seeking induced by stress
The α3β4 nAChR partial agonist AT-1001 has anxiolytic activity
α3β4 nAChR represents a target for treatment of stress-related alcohol disorders
Acknowledgments
5. FUNDING
This work was supported by NIH grant 5R01DA023281 to LT and the State of Florida Executive Office of the Governor's Department of Economic Opportunity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. DISCLOSURE
The authors declare no conflict of interest.
REFERENCES
- Aghajanian GK, VanderMaelen CP. alpha 2-adrenoceptor-mediated hyperpolarization of locus coeruleus neurons: intracellular studies in vivo. Science. 1982;215:1394–1396. doi: 10.1126/science.6278591. [DOI] [PubMed] [Google Scholar]
- Ayanwuyi LO, Carvajal F, Lerma-Cabrera JM, Domi E, Bjork K, Ubaldi M, Heilig M, Roberto M, Ciccocioppo R, Cippitelli A. Role of a genetic polymorphism in the corticotropin-releasing factor receptor 1 gene in alcohol drinking and seeking behaviors of marchigian sardinian alcohol-preferring rats. Front Psychiatry. 2013;4:23. doi: 10.3389/fpsyt.2013.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin PR, Alanis R, Salas R. The Role of the Habenula in Nicotine Addiction. J Addict Res Ther. 2011;S1 doi: 10.4172/2155-6105.S1-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batel P, Pessione F, Maitre C, Rueff B. Relationship between alcohol and tobacco dependencies among alcoholics who smoke. Addiction. 1995;90:977–980. doi: 10.1046/j.1360-0443.1995.90797711.x. [DOI] [PubMed] [Google Scholar]
- Berrettini W, Yuan X, Tozzi F, Song K, Francks C, Chilcoat H, Waterworth D, Muglia P, Mooser V. Alpha-5/alpha-3 nicotinic receptor subunit alleles increase risk for heavy smoking. Mol Psychiatry. 2008;13:368–373. doi: 10.1038/sj.mp.4002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco IH, Wilson SW. The habenular nuclei: a conserved asymmetric relay station in the vertebrate brain. Philos Trans R Soc Lond B Biol Sci. 2009;364:1005–1020. doi: 10.1098/rstb.2008.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: I. Preclinical studies. Synapse. 1996a;23:28–38. doi: 10.1002/(SICI)1098-2396(199605)23:1<28::AID-SYN4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: II. Clinical studies. Synapse. 1996b;23:39–51. doi: 10.1002/(SICI)1098-2396(199605)23:1<39::AID-SYN5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW. Tobacco addiction and the dysregulation of brain stress systems. Neurosci Biobehav Rev. 2012;36:1418–1441. doi: 10.1016/j.neubiorev.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso N, Gu F, Chatterjee N, Sheng-Chih J, Yu K, Yeager M, Chen C, Jacobs K, Wheeler W, Landi MT, Ziegler RG, Hunter DJ, Chanock S, Hankinson S, Kraft P, Bergen AW. Genome-wide and candidate gene association study of cigarette smoking behaviors. PLoS One. 2009;4:e4653. doi: 10.1371/journal.pone.0004653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso RA, Brozowski SJ, Chavez-Noriega LE, Harpold M, Valenzuela CF, Harris RA. Effects of ethanol on recombinant human neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1999;289:774–780. [PubMed] [Google Scholar]
- Chatterjee S, Steensland P, Simms JA, Holgate J, Coe JW, Hurst RS, Shaffer CL, Lowe J, Rollema H, Bartlett SE. Partial agonists of the alpha3beta4* neuronal nicotinic acetylcholine receptor reduce ethanol consumption and seeking in rats. Neuropsychopharmacology. 2011;36:603–615. doi: 10.1038/npp.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Angeletti S, Weiss F. Long-lasting resistance to extinction of response reinstatement induced by ethanol-related stimuli: role of genetic ethanol preference. Alcohol Clin Exp Res. 2001;25:1414–1419. doi: 10.1097/00000374-200110000-00002. [DOI] [PubMed] [Google Scholar]
- Cippitelli A, Ayanwuyi LO, Barbier E, Domi E, Lerma-Cabrera JM, Carvajal F, Scuppa G, Li H, Ubaldi M, Heilig M, Roberto M, Ciccocioppo R. Polymorphism in the corticotropin-releasing factor receptor 1 (CRF1-R) gene plays a role in shaping the high anxious phenotype of Marchigian Sardinian alcohol-preferring (msP) rats. Psychopharmacology (Berl) 2014a doi: 10.1007/s00213-014-3743-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cippitelli A, Damadzic R, Hansson AC, Singley E, Sommer WH, Eskay R, Thorsell A, Heilig M. Neuropeptide Y (NPY) suppresses yohimbine-induced reinstatement of alcohol seeking. Psychopharmacology (Berl) 2010a;208:417–426. doi: 10.1007/s00213-009-1741-y. [DOI] [PubMed] [Google Scholar]
- Cippitelli A, Damadzic R, Singley E, Thorsell A, Ciccocioppo R, Eskay RL, Heilig M. Pharmacological blockade of corticotropin-releasing hormone receptor 1 (CRH1R) reduces voluntary consumption of high alcohol concentrations in non-dependent Wistar rats. Pharmacol Biochem Behav. 2012;100:522–529. doi: 10.1016/j.pbb.2011.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cippitelli A, Karlsson C, Shaw JL, Thorsell A, Gehlert DR, Heilig M. Suppression of alcohol self-administration and reinstatement of alcohol seeking by melanin-concentrating hormone receptor 1 (MCH1-R) antagonism in Wistar rats. Psychopharmacology (Berl) 2010b;211:367–375. doi: 10.1007/s00213-010-1891-y. [DOI] [PubMed] [Google Scholar]
- Cippitelli A, Wu J, Gaiolini KA, Mercatelli D, Schoch J, Gorman M, Ramirez A, Ciccocioppo R, Khroyan TV, Yasuda D, Zaveri NT, Pascual C, Xie XS, Toll L. AT-1001: A High Affinity alpha3beta4 nAChR Ligand with Novel Nicotine-Suppressive Pharmacology. Br J Pharmacol. 2014b doi: 10.1111/bph.13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani JA, Harris RA. Nicotine addiction and comorbidity with alcohol abuse and mental illness. Nat Neurosci. 2005;8:1465–1470. doi: 10.1038/nn1580. [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Guerrera MP. Alcoholism and smoking. J Stud Alcohol. 1990;51:130–135. doi: 10.15288/jsa.1990.51.130. [DOI] [PubMed] [Google Scholar]
- Doyon WM, Thomas AM, Ostroumov A, Dong Y, Dani JA. Potential substrates for nicotine and alcohol interactions: a focus on the mesocorticolimbic dopamine system. Biochem Pharmacol. 2013;86:1181–1193. doi: 10.1016/j.bcp.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epping-Jordan MP, Picciotto MR, Changeux JP, Pich EM. Assessment of nicotinic acetylcholine receptor subunit contributions to nicotine self-administration in mutant mice. Psychopharmacology (Berl) 1999;147:25–26. doi: 10.1007/s002130051135. [DOI] [PubMed] [Google Scholar]
- Gill MJ, Ghee SM, Harper SM, See RE. Inactivation of the lateral habenula reduces anxiogenic behavior and cocaine seeking under conditions of heightened stress. Pharmacol Biochem Behav. 2013;111:24–29. doi: 10.1016/j.pbb.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Dickinson HA. 18-MC reduces methamphetamine and nicotine self-administration in rats. Neuroreport. 2000a;11:2013–2015. doi: 10.1097/00001756-200006260-00041. [DOI] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Szumlinski KK. 18-Methoxycoronaridine (18-MC) and ibogaine: comparison of antiaddictive efficacy, toxicity, and mechanisms of action. Ann N Y Acad Sci. 2000b;914:369–386. doi: 10.1111/j.1749-6632.2000.tb05211.x. [DOI] [PubMed] [Google Scholar]
- Glick SD, Sell EM, McCallum SE, Maisonneuve IM. Brain regions mediating alpha3beta4 nicotinic antagonist effects of 18-MC on nicotine self-administration. Eur J Pharmacol. 2011;669:71–75. doi: 10.1016/j.ejphar.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotti C, Zoli M, Clementi F. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol Sci. 2006;27:482–491. doi: 10.1016/j.tips.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson LM, Guildford MJ, Tapper AR. Neuronal nicotinic acetylcholine receptors: common molecular substrates of nicotine and alcohol dependence. Front Psychiatry. 2013;4:29. doi: 10.3389/fpsyt.2013.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson LM, Zhao-Shea R, Pang X, Gardner PD, Tapper AR. Activation of alpha4* nAChRs is necessary and sufficient for varenicline-induced reduction of alcohol consumption. J Neurosci. 2010;30:10169–10176. doi: 10.1523/JNEUROSCI.2601-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O. The habenula: from stress evasion to value-based decision-making. Nat Rev Neurosci. 2010;11:503–513. doi: 10.1038/nrn2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, Sanjakdar SS, Muldoon PP, McIntosh JM, Damaj MI. The alpha3beta4* nicotinic acetylcholine receptor subtype mediates nicotine reward and physical nicotine withdrawal signs independently of the alpha5 subunit in the mouse. Neuropharmacology. 2013;70:228–235. doi: 10.1016/j.neuropharm.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joslyn G, Brush G, Robertson M, Smith TL, Kalmijn J, Schuckit M, White RL. Chromosome 15q25.1 genetic markers associated with level of response to alcohol in humans. Proc Natl Acad Sci U S A. 2008;105:20368–20373. doi: 10.1073/pnas.0810970105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel D, Chen K, Warner LA, Kessler RC, Grant B. Prevalence and demographic correlates of symptoms of last year dependence on alcohol, nicotine, marijuana and cocaine in the U.S. population. Drug Alcohol Depend. 1997;44:11–29. doi: 10.1016/s0376-8716(96)01315-4. [DOI] [PubMed] [Google Scholar]
- Le AD, Corrigall WA, Harding JW, Juzytsch W, Li TK. Involvement of nicotinic receptors in alcohol self-administration. Alcohol Clin Exp Res. 2000;24:155–163. doi: 10.1111/j.1530-0277.2000.tb04585.x. [DOI] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Funk D, Shaham Y. Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology (Berl) 2005;179:366–373. doi: 10.1007/s00213-004-2036-y. [DOI] [PubMed] [Google Scholar]
- Levin ED, Petro A, Rezvani AH, Pollard N, Christopher NC, Strauss M, Avery J, Nicholson J, Rose JE. Nicotinic alpha7- or beta2-containing receptor knockout: effects on radial-arm maze learning and long-term nicotine consumption in mice. Behav Brain Res. 2009;196:207–213. doi: 10.1016/j.bbr.2008.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci. 2002;22:7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonneuve IM, Glick SD. Anti-addictive actions of an iboga alkaloid congener: a novel mechanism for a novel treatment. Pharmacol Biochem Behav. 2003;75:607–618. doi: 10.1016/s0091-3057(03)00119-9. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Funk D, Juzytsch W, Harding S, Rice KC, Shaham Y, Le AD. The CRF1 receptor antagonist antalarmin attenuates yohimbine-induced increases in operant alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology (Berl) 2007;195:345–355. doi: 10.1007/s00213-007-0905-x. [DOI] [PubMed] [Google Scholar]
- Muldoon PP, Jackson KJ, Perez E, Harenza JL, Molas S, Rais B, Anwar H, Zaveri NT, Maldonado R, Maskos U, McIntosh JM, Dierssen M, Miles MF, Chen X, De Biasi M, Damaj MI. The alpha3beta4* nicotinic ACh receptor subtype mediates physical dependence to morphine: mouse and human studies. Br J Pharmacol. 2014;171:3845–3857. doi: 10.1111/bph.12741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris SK, Boden PR, Woodruff GN. Agonists selective for tachykinin NK1 and NK3 receptors excite subpopulations of neurons in the rat medial habenula nucleus in vitro. Eur J Pharmacol. 1993;234:223–228. doi: 10.1016/0014-2999(93)90957-j. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Perry DC, Xiao Y, Nguyen HN, Musachio JL, Davila-Garcia MI, Kellar KJ. Measuring nicotinic receptors with characteristics of alpha4beta2, alpha3beta2 and alpha3beta4 subtypes in rat tissues by autoradiography. J Neurochem. 2002;82:468–481. doi: 10.1046/j.1471-4159.2002.00951.x. [DOI] [PubMed] [Google Scholar]
- Picciotto MR. Common aspects of the action of nicotine and other drugs of abuse. Drug Alcohol Depend. 1998;51:165–172. doi: 10.1016/s0376-8716(98)00074-x. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Overstreet DH, Yang Y, Maisonneuve IM, Bandarage UK, Kuehne ME, Glick SD. Attenuation of alcohol consumption by a novel nontoxic ibogaine analogue (18-methoxycoronaridine) in alcohol-preferring rats. Pharmacol Biochem Behav. 1997;58:615–619. doi: 10.1016/s0091-3057(97)10003-x. [DOI] [PubMed] [Google Scholar]
- Romberger DJ, Grant K. Alcohol consumption and smoking status: the role of smoking cessation. Biomed Pharmacother. 2004;58:77–83. doi: 10.1016/j.biopha.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Salas R, Pieri F, Fung B, Dani JA, De Biasi M. Altered anxiety-related responses in mutant mice lacking the beta4 subunit of the nicotinic receptor. J Neurosci. 2003;23:6255–6263. doi: 10.1523/JNEUROSCI.23-15-06255.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Sturm R, Boulter J, De Biasi M. Nicotinic receptors in the habenulo-interpeduncular system are necessary for nicotine withdrawal in mice. J Neurosci. 2009;29:3014–3018. doi: 10.1523/JNEUROSCI.4934-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schank JR, Pickens CL, Rowe KE, Cheng K, Thorsell A, Rice KC, Shaham Y, Heilig M. Stress-induced reinstatement of alcohol-seeking in rats is selectively suppressed by the neurokinin 1 (NK1) antagonist L822429. Psychopharmacology (Berl) 2011;218:111–119. doi: 10.1007/s00213-011-2201-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schank JR, Ryabinin AE, Giardino WJ, Ciccocioppo R, Heilig M. Stress-related neuropeptides and addictive behaviors: beyond the usual suspects. Neuron. 2012;76:192–208. doi: 10.1016/j.neuron.2012.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Sheffield EB, Quick MW, Lester RA. Nicotinic acetylcholine receptor subunit mRNA expression and channel function in medial habenula neurons. Neuropharmacology. 2000;39:2591–2603. doi: 10.1016/s0028-3908(00)00138-6. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ. The dorsal diencephalic conduction system: a review of the anatomy and functions of the habenular complex. Neurosci Biobehav Rev. 1982;6:1–13. doi: 10.1016/0149-7634(82)90003-3. [DOI] [PubMed] [Google Scholar]
- Thorgeirsson TE, Geller F, Sulem P, Rafnar T, Wiste A, Magnusson KP, Manolescu A, Thorleifsson G, Stefansson H, Ingason A, Stacey SN, Bergthorsson JT, Thorlacius S, Gudmundsson J, Jonsson T, Jakobsdottir M, Saemundsdottir J, Olafsdottir O, Gudmundsson LJ, Bjornsdottir G, Kristjansson K, Skuladottir H, Isaksson HJ, Gudbjartsson T, Jones GT, Mueller T, Gottsater A, Flex A, Aben KK, de Vegt F, Mulders PF, Isla D, Vidal MJ, Asin L, Saez B, Murillo L, Blondal T, Kolbeinsson H, Stefansson JG, Hansdottir I, Runarsdottir V, Pola R, Lindblad B, van Rij AM, Dieplinger B, Haltmayer M, Mayordomo JI, Kiemeney LA, Matthiasson SE, Oskarsson H, Tyrfingsson T, Gudbjartsson DF, Gulcher JR, Jonsson S, Thorsteinsdottir U, Kong A, Stefansson K. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008;452:638–642. doi: 10.1038/nature06846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toll L, Zaveri NT, Polgar WE, Jiang F, Khroyan TV, Zhou W, Xie XS, Stauber GB, Costello MR, Leslie FM. AT-1001: a high affinity and selective alpha3beta4 nicotinic acetylcholine receptor antagonist blocks nicotine self-administration in rats. Neuropsychopharmacology. 2012;37:1367–1376. doi: 10.1038/npp.2011.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasquez KM, Molfese DL, Salas R. The role of the habenula in drug addiction. Front Hum Neurosci. 2014;8:174. doi: 10.3389/fnhum.2014.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada E, Wada K, Boulter J, Deneris E, Heinemann S, Patrick J, Swanson LW. Distribution of alpha 2, alpha 3, alpha 4, and beta 2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J Comp Neurol. 1989;284:314–335. doi: 10.1002/cne.902840212. [DOI] [PubMed] [Google Scholar]
- Wang JC, Grucza R, Cruchaga C, Hinrichs AL, Bertelsen S, Budde JP, Fox L, Goldstein E, Reyes O, Saccone N, Saccone S, Xuei X, Bucholz K, Kuperman S, Nurnberger J, Jr., Rice JP, Schuckit M, Tischfield J, Hesselbrock V, Porjesz B, Edenberg HJ, Bierut LJ, Goate AM. Genetic variation in the CHRNA5 gene affects mRNA levels and is associated with risk for alcohol dependence. Mol Psychiatry. 2009;14:501–510. doi: 10.1038/mp.2008.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Perry DC, Bupp JE, Jiang F, Polgar WE, Toll L, Zaveri NT. [(1)(2)(5)I]AT-1012, a new high affinity radioligand for the alpha3beta4 nicotinic acetylcholine receptors. Neuropharmacology. 2014;77:193–199. doi: 10.1016/j.neuropharm.2013.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Kellar KJ. The comparative pharmacology and up-regulation of rat neuronal nicotinic receptor subtype binding sites stably expressed in transfected mammalian cells. J Pharmacol Exp Ther. 2004;310:98–107. doi: 10.1124/jpet.104.066787. [DOI] [PubMed] [Google Scholar]
- Zaveri N, Jiang F, Olsen C, Polgar W, Toll L. Novel alpha3beta4 nicotinic acetylcholine receptor-selective ligands. Discovery, structure-activity studies, and pharmacological evaluation. J Med Chem. 2010;53:8187–8191. doi: 10.1021/jm1006148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaveri NT, Bertrand S, Yasuda D, Bertrand D. Functional Characterization of AT-1001, an alpha3beta4 Nicotinic Acetylcholine Receptor Ligand, at Human alpha3beta4 and alpha4beta2 nAChR. Nicotine Tob Res. 2014 doi: 10.1093/ntr/ntu170. [DOI] [PMC free article] [PubMed] [Google Scholar]