Abstract
Enhancement of tonic inhibition mediated by extrasynaptic α5-subunit containing GABAA receptors (GABAARs) has been proposed as the mechanism by which a variety of anesthetics, including the general anesthetic etomidate, impair learning and memory. Since α5 subunits preferentially partner with β3 subunits, we tested the hypothesis that etomidate acts through β3-subunit containing GABAARs to enhance tonic inhibition, block LTP, and impair memory. We measured the effects of etomidate in wild type mice and in mice carrying a point mutation in the GABAAR β3-subunit (β3-N265M) that renders these receptors insensitive to etomidate. Etomidate enhanced tonic inhibition in CA1 pyramidal cells of the hippocampus in wild type but not in mutant mice, demonstrating that tonic inhibition is mediated by β3-subunit containing GABAARs. However, despite its inability to enhance tonic inhibition, etomidate did block LTP in brain slices from mutant mice as well as in those from wild type mice. Etomidate also impaired fear conditioning to context, with no differences between genotypes. In studies of recombinant receptors expressed in HEK293 cells, α5β1γ2L GABAARs were insensitive to amnestic concentrations of etomidate (1 [.proportional]M and below), whereas α5β2γ2L and α5β3γ2L GABAARs were enhanced. We conclude that etomidate enhances tonic inhibition in pyramidal cells through its action on α5β3-containing GABAA receptors, but blocks LTP and impairs learning by other means - most likely by modulating α5β2-containing GABAA receptors. The critical anesthetic targets underlying amnesia might include other forms of inhibition imposed on pyramidal neurons (e.g. slow phasic inhibition), or inhibitory processes on non-pyramidal cells (e.g. interneurons).
Keywords: Hippocampus, Tonic inhibition, Etomidate, LTP, Learning, GABA receptors
1. Introduction
The ability of general anesthetics to cause sedation, amnesia, and immobility has been a subject of interest and intense study for many years. There is now an emerging recognition that these different anesthetic end-points may reflect different anesthetic actions at the molecular, cellular and network levels (Rudolph and Antkowiak, 2004). The present study addresses the mechanism by which the general anesthetic etomidate impairs learning and memory.
GABAA receptors (GABAARs) are heteropentameric ligand-gated anion channels responsible for the majority of inhibitory synaptic transmission in the brain. Functional GABAA receptors most commonly incorporate two α-subunits, two β-subunits, and one γ-subunit (Olsen and Sieghart, 2008). GABAARs are considered to be important targets of a variety of agents, including etomidate (Jones et al., 1992; Jones and Harrison, 1993; Uchida et al., 1995). Because of its favorable hemodynamic profile, etomidate is used in the clinical setting to induce anesthesia in patients at risk for cardiovascular compromise, and in other select circumstances such as electroconvulsive therapy (Forman, 2011). It has also become an important experimental drug because its receptor sensitivity can be controlled by genetic manipulation: the discovery that receptors that incorporate β1-subunits (together with α1-subunits) are markedly less sensitive to etomidate compared to those that incorporate β2- or β3-subunits (Sanna et al., 1997) led to the identification of a single amino acid residue in the pore-forming transmembrane domain that controls etomidate sensitivity (Belelli et al., 1997). Mice carrying single point mutations in these subunits (β2-N265S or β3-N265M) were found to resist etomidate's action in studies of sedation and immobility (Jurd et al., 2003; Reynolds et al., 2003). The impact of these mutations on etomidate-induced amnesia was not tested.
It was shown previously that genetic and pharmacologic manipulations that reduce or eliminate inhibitory current carried by GABAAR α5-subunits (α5-KO) are resistant to etomidate's suppression of long term potentiation (LTP) in vitro and learning and memory in vivo (Cheng et al., 2006). Because the majority of α5-GABAARs are located extrasynaptically on pyramidal cells, where they mediate a persistent conductance termed “tonic inhibition” (Caraiscos et al., 2004), and tonic inhibition is strongly enhanced by amnestic drugs, it was proposed that the effect of etomidate on synaptic plasticity is due to its enhancement of tonic inhibition (Cheng et al., 2006; Orser, 2007; Martin et al., 2009). Since α5-subunits preferentially partner with β3-subunits (Luddens et al., 1994; Sur et al., 1998), we hypothesized that mice carrying the N265M mutation in the β3-subunit would similarly resist etomidate's enhancement of tonic inhibition and suppression of LTP in vitro and learning in vivo.
We found that tonic inhibition in the β3-N265M mice was indeed insensitive to etomidate, showing that this form of inhibition is mediated by β3-subunit containing GABAARs. Surprisingly, although etomidate did not enhance tonic inhibition in these mice, it did suppress synaptic plasticity in vitro and learning in vivo. Studies of expressed recombinant receptors showed that GABAARs incorporating β1-subunits together with α5-subunits were insensitive to amnestic concentrations of etomidate, mirroring previous results from studies of α1-containing GABAARs (Sanna et al., 1997). We conclude that tonic inhibition in CA1 pyramidal cells is mediated by α5β3-subunit containing GABAARs, but that this form of inhibition does not play a key role in anesthetic suppression of synaptic plasticity in these neurons. Rather, etomidate appears to control LTP of Shaffer collateral synapses, and fear conditioning to context, by modulating α5β2-GABAARs.
2. Materials and Methods
All experiments were performed in accordance with the National Institutes of Health guide the Guide for the Care and use of Laboratory Animals (NIH Publications No. 8023, revised 1978) and were approved by the University of Wisconsin Institutional Animal Care and Use Committee, Madison, Wisconsin, or by the University of California Institutional Animal Care and Use Committee, San Francisco, California. All efforts were made to minimize animals suffering and reduce the number of animals used.
2.1 Mice
The male offspring of heterozygous breeding pairs homozygous for an asparagine-tomethionine point mutation at position 265 of the GABAA receptor β3 subunit (β3-N265M), and homozygous wild-type controls, were used for this study. The strain background of the β3-N265M mice was 129X1/SvJ. Mice were genotyped using DNA template from tail tips, amplified by PCR using the specific primers: RJM-8 (5′-GTT CAG CTT CCA TTC TCA CTG-3′) and RJM-24 (5′-GCT ATG GCT TTC TGG TGG AG-3′). Animals were housed in the animal care facility under 12-h cycles of light and dark and had continuous access to standard mouse chow and water.
2.2 Brain slice preparation
LTP
Hippocampal brain slices were prepared from mice aged 42-77 days (57±9, n=31). Before decapitation mice were anesthetized with 2.5% isoflurane (Novaplus, Hospira, Inc., Lake Forest, IL), then the brain was removed, blocked by removing the cerebellum and olfactory cortex, glued to a microtome slice tray with cyanoacrylate glue (Krazy Glue Instant, Westerville, OH), and placed for slicing in ice-cold cutting artificial cerebrospinal fluid (“cutting aCSF”) containing (in mM) 127 NaCl, 1.9 KCl, 2.7 MgSO4x7H2O, 0.9 CaCl2x2H2O, 26 NaHCO3, 1.2 KH2PO4, 1 ascorbic acid, 15 glucose, bubbled with 95% O2-5% CO2 (“carbogen”). Coronal slices 500 [.proportional]m thick were cut using a vibratome (Leica VT 100S, Leica Microsystems Nussloch GmbH, Nussloch, Germany). A portion of the slice including the hippocampus was trimmed with a scalpel to ensure proper fit within a custom-manufactured microfluidic recording chamber (Blake et al., 2010). Brain slices recovered in a holding chamber filled with carbogenated recording aCSF containing (in mM) 127 NaCl, 1.9 KCl, 26 NaHCO3, 1.2 KH2PO4, 1.4 MgSO4x7H2O, 2.2 CaCl2x2H2O, 15 glucose, 1 ascorbic acid for at least 60 min at room temperature (20 - 22 °C). This same solution was used for recording (“recording aCSF”).
Tonic and synaptic currents
Hippocampal brain slices were prepared from mice aged 40-50 days (44±1, n=7). Before decapitation mice were anesthetized with 2.5-3% isoflurane then the brain was removed and placed in ice-cold N-methyl-D-glucamine (NMDG)-based cutting solution containing (in mM): 2.5 KCl, 1.25 Na2HPO4, 25 NaHCO3, 10 MgSO4x7H2O, 0.5 CaCl2x2H2O, 25 glucose, 110 NMDG, 2.5 sodium ascorbate, bubbled with carbogen (pH adjusted with 6N HCl to 7.3, 300-310 mOsm) (Ting and Feng, 2011). Horizontal slices 350 μm thick were cut with oscillating blade microtome 7000 smz2 vibratome (Campden Instruments, Loughborough, England). Thereafter slices recovered while submerged in warmed (35 °C), carbogenated cutting solution which was next slowly exchanged (at a rate of 5 ml/min) with warmed (35 °C), carbogenated recording aCSF contain ing (in mM): 130 NaCl, 2.5 KCl, 1.25 Na2HPO4, 25 NaHCO3, 2 MgSO4x7H2O, 2 CaCl2x2H2O, 10 glucose, 2.5 sodium ascorbate (pH 7.3, 300-310 mOsm). The exchange process was completed in 30 minutes. The slices were maintained at room temperature until they were transferred to the recording chamber.
2.3 Cell culture and recombinant receptor expression
Human embryonic kidney cells (HEK-293T, American Type Culture Collection, Manassas, VA) were cultured in minimum essential medium with L-glutamine and Earle's salts (Life Technologies, Carlsbad, CA) supplemented with 10% fetal bovine serum (Thermo Scientific, Waltham, MA) and penicillin-streptomycin (Sigma-Aldrich, St. Louis, MO) in at 37°C under a 5% CO2 atmosphere. Cells were initially plated onto 60 mm dishes and co-transfected 24 hours later with pUNIV vectors (Venkatachalan et al., 2007) containing appropriate rat GABAAR subunits (α5,β1,β2,β3,γ2L) or eGFP cDNAs using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). The ratio of α vs. β vs. γ subunits was 1:1:3, and a total of 0.6 µg of cDNA was used for transfections. After 24 hours cells were re-plated onto 12 mm glass coverslips, and electrophysiological studies were conducted 48-72 hours post-transfection.
2.4 Data acquisition
LTP
Brain slices were transferred to a microfluidic recording chamber (Blake et al., 2010) perfused with recording aCSF at a flow rate of 2.5 ml/min. The bath temperature was maintained at 30 ± 0.5 °C using an in-line temperat ure controller (Warner Instruments Corp., Hamden, CT). A 16-channel linear recording electrode (50 μm separating recording sites; NeuroNexus Technologies, Ann Arbor, MI) was inserted orthogonal to the hippocampal layers, in the middle of CA1, at a depth (along the rostral - caudal axis) of 200 μm beneath the surface of the tissue. Field excitatory postsynaptic potentials (fEPSPs) were electrically evoked by a tungsten stereotrode stimulating electrode (0.5 M , World Precision Instruments, Sarasota, FL) placed in stratum radiatum for activation of the Schaffer collateral/commissural path (SC). Recorded signals were amplified 1000x, band-pass filtered between 1-3000 Hz (model LYNX-8 amplifiers, Neuralynx Inc., Tucson, AZ), digitized at 10 kHz using an analog-to-digital converter (Digidata 1440A, Molecular Devices, Sunnyvale, CA), and acquired using pClamp software (Version 10.2, Molecular Devices). Stimuli of 0.1 ms duration were delivered using a constant current stimulus isolator (model A365D, World Precision Instruments, Sarasota, FL). SC axons were stimulated at 0.03 Hz, using stimulus intensity (“baseline”) adjusted to evoke responses below half-maximal fEPSP amplitude. Baseline stimulus amplitude was typically between 30 −90 μA. LTP protocols consisted of a 30 min stable baseline recording period in which evoked fEPSP slope changed by less than 10%, followed by a theta burst stimulus (TBS), and then an additional 60 min recording period. The LTP-inducing TBS (“40x5Hz”) consisted of 10 bursts delivered every 200 ms (i.e. 5 Hz inter-burst interval) with each burst consisting of 4 pulses separated by 10 ms (i.e. 100 Hz inter-stimulus interval). The stimulus intensity during the burst was adjusted to evoke half-maximal population spike amplitude, judged by measuring the amplitude of the downward-going negative voltage peak in the pyramidal channel (see below) online during recordings.
Tonic and synaptic currents
Brain slices were transferred to a submersion-style recording chamber perfused at a flow rate of 2.5-3 ml/min with recording aCSF containing kynurenic acid (KA, 3 mM). The bath temperature was maintained at 30 ± 0.5 °C using an in-line temperature controller (Warner Instruments Corp.). Pyramidal cells in the CA1 region were visualized with a 40x water immersion objective and IR-DIC video camera installed on BX50WI microscope (Olympus America Inc., Center Valley, PA). Patch clamp recordings were obtained using a MultiClamp 700B amplifier (Molecular Devices), low pass filtered at 4 kHz, digitized at 10 kHz using an analog-to-digital converter (Digidata 1322A, Molecular Devices), and acquired using pClamp software (Version 10.3, Molecular Devices). Borosilicate glass pipettes (O.D. 1.5 mm x I.D. 0.86 mm, Sutter Instruments, Novato, CA) were pulled to tip diameters of μ1 μm using a horizontal puller (P-97, Sutter Instruments) then filled with an intracellular solution containing (in mM) 90 CsCl, 30 KCl, 5 NaCl, 10 NaHEPES, 5 EGTA, 4 Mg2ATP 2, 0.4 Na3GTP, 10 Na2phosphocreatine, 4 QX-314, pH adjusted with 1M CsOH to 7.3 (290±5 mOsm). The resistances of filled pipettes were 5-7 MΩ. Whole-cell, voltage-clamp recordings were performed at a -60 mV holding potential. Cell capacitances and membrane resistances were measured using the software membrane test algorithm. Series resistances were not compensated but were monitored, and recordings were discontinued if resistance increased more than 25%. Uncompensated series resistances were 10-20 MΩ.
Recombinant receptors
Recordings were performed at room temperature (21°C) on the stage of an inverted microscope equipped with DIC optics and epifluorescence (Leica DM IRB, Leica Microsystems, Wetzlar GmbH, Germany). The recording chamber was perfused with recording solution containing (in mM): 145 NaCl, 5 KCl, 10 HEPES, and 1.8 CaCl2, pH adjusted with 10 M NaOH to 7.4 (305 mOsm). Transfected cells were identified by expression of eGFP. Outside-out excised patch recordings were made using borosilicate glass pipettes filled with (in mM): 130 KCl, 10 HEPES, 5 EGTA, 1 MgCl2, and 5 Mg2ATP, pH adjusted with 10 M KOH to 7.4 (310 mOsm). Patches were held at −40 mV. Currents were low-pass-filtered at 2 kHz with a four-pole Bessel filter, and data were collected at 20 kHz using an Axopatch 200A amplifier (Axon Instruments, Foster City, CA), a Digidata 1200 (Axon Instruments), and Clampex 10.4 (Axon Instruments). Solutions were applied using a custom gravity-fed multibarreled device fabricated with polyimide tubes with OD 0.0142”/ ID 0.0122” (Cole-Parmer, Vernon Hills, IL), and attached to a stepper motor-based microscope translation stage (ITK Dr. Kassen GmbH, Lahnau, Germany). Open tip exchange rates of 20 ms (10-90% rise time) were achieved. GABA was dissolved fresh in bath solution. Etomidate was prepared as stock (50 mM in DMSO), stored at −20 °C, and diluted in recording solution the day of an experiment.
2.5 Behavioral testing
Experiments were conducted at the University of California, San Francisco. Three to 10 male mice, between 60 to 80 days old, of each genotype (7 on average per group) were assessed at 0, 7.5, 9.38, 10.3, 11.25 and 15 mg/kg etomidate. These doses were chosen based on a previous study indicating that etomidate's ED50 for conditional freezing for a similar fear conditioning protocol in 129/SvJ x C57BL/6J mice was 11 mg/kg (Benkwitz et al., 2007). A 35% propylene glycol solution (Sigma, St. Louis, MO) was used for all vehicle injections. The intraperitoneal injection of etomidate was given 30 minutes before training, and all mice were injected at 7.7 ml/kg body weight.
Thirty minutes after the injection, groups of four mice at a time were transported from their home cage and transferred to fear conditioning chambers (27 cm L×24.5 cm W×20 cm H) constructed of clear acrylic. The chamber floor was made of 31 stainless steel bars (3 mm in diameter, spaced 7 mm center to center) and was connected to a shock delivery system (San Diego Instruments, San Diego, CA). Before and after each session, the chambers walls were cleaned with 5% Pine Scented Disinfectant (Midland, Inc., Sweetwater, TN). Pine solution was placed in a dish underneath each chamber as well. Room lights were left on and white noise (65 db) was played in the background. The odor, tactile, auditory and visual stimuli of the chamber comprised the training “context”.
After a 3-min baseline period, mice received 6 shocks (1 mA, 2 s), separated by 1 min. They were removed from the chamber 30 s after the last shock. Mice were tested for fear to the training context the following day by placement back in their training chamber in the absence of shock for a period of 8 min. The context tested was identical to the training context.
Freezing, the absence of all movement except that necessary for respiration, is an innate defensive response in rodents and is a reliable measure of learned fear (Fanselow, 1980). A camera installed in an experimental room transmitted a video to the monitor placed in adjoining room where animals were scored online by the observer. The observer scoring the behavior was blinded to the genotypes of the mice. Each animal's behavior was scored every 8 s during the test. If the animal showed freezing, it was given a score of “1” for that observation; if the animal showed movement, it was given a score of “0” for that observation. A percentage was calculated by dividing the number of freezing observations a mouse had by the total number possible during the observation period. This number represented the animal's freezing score.
To derive ED50 values for etomidate suppression of fear conditioning, data were fit by a logistic dose response curve: y = A2 + (A1-A2)/(1 + (x/x0)^p), where A1 (max value) was fixed at 1 and A2 (min value) was fixed at 0, and ED50=x0.
2.6 Data analysis
Electrophysiological data were analyzed using Clampfit 10.2 and 10.3 (Molecular Devices), Origin 9.0 (Microcal Software Inc., Northampton, MA), GraphPad Prism 6.05 (GraphPad Software Inc., La Jolla, CA), custom-written R programming language scripts (R Foundation for Statistical Computing, Vienna, Austria), and Mini Analysis Program 6 (Synaptosoft Inc., Decatur, GA).
LTP
The recording electrode site used for LTP analysis was the site with the largest amplitude fEPSP in response to the baseline stimulus (“dendritic channel”). Field EPSP slopes were analyzed using Clampfit (10.2, Molecular Devices) to determine the maximum rate of rise at the dendritic channel. The electrode site closest to the pyramidal cell layer (“pyramidal channel”), used for population spike analysis, was chosen by examining the evoked waveform in response to a stimulus that was suprathreshold for population spike generation. Slices without population spikes of at least 0.5 mV prior to TBS, or that showed unstable responses before or after TBS, were excluded from analysis. The coefficient of variation of the pre-tetanus baseline for the slices that met these inclusion criteria was 0.09. LTP was calculated as the percentage change of the fEPSP slope collected 10 min pre-TBS and the last 10 min (51-60 min) post-TBS of the LTP experiment.
Tonic inhibition
The level of tonic inhibition present in CA1 pyramidal cells was measured by adding the non-competitive GABAA receptor antagonist picrotoxin (PTX, 200 μM) to the aCSF superfusate during whole cell recording. The amount of tonic current was calculated as the difference in the baseline current before and after the addition of PTX. The mean current values were obtained from Gaussian fits to all point amplitude histograms. Histograms (1 pA bin-width) were constructed using 1 min of data before and 20 sec of data after the PTX effect had stabilized, which required μ2 min. The histogram generated from data before PTX application had skewed distribution towards larger negative value. To ensure that sIPSCs were not included into the measurement of tonic inhibition, the Gaussian fit was applied to the unskewed (outward current) portion of the distribution. The resulting parameters were used to simulate a symmetric Gaussian curve. The difference between peak values of two simulated Gaussians was used as the measure of the baseline tonic current.
Synaptic currents
To detect sIPSCs, the search protocol threshold was set at 3 times the root mean square (RMS) noise level, which typically was 3-6 pA. For each cell, the averaged frequency and amplitude characteristics of sIPSCs were computed by the software. For each cell, at least 40 sIPSCs were averaged, normalized, and characterized by their 10-90% rise, rates of fast (τ1) and slow (τ2) components, and weighted (τw) decay times, and fraction of the fast component of deactivation (A1) (Table 1). The sIPSCs used for averaging were selected based on the presence of a stable baseline level and the lack of spontaneous events during the deactivation phase. These events were aligned at the time of half-maximal amplitude of the rising phase. The decay phases of averaged fast sIPSCs were fitted to bi-exponential functions using a Simplex fitting algorithm Mini Analysis Program 6 (Synaptosoft Inc.). Weighted decay time constant was calculated using the formula: τw= ((A1/(A1+A2)*τ1)+((A2/(A1+A2)*τ2).
Table 1.
Characteristics of spontaneous inhibitory postsynaptic currents recorded from CA1 pyramidal cells in WT and β3-N265M mice, in the absence (Ctrl) and presence of 1 μM etomidate (Etom). RT - 10-90% rise time, τ1- fast component of deactivation, τ2– slow component of deactivation, A1- fractional contribution of fast component, τw- weighted time constant of deactivation.
| GABAA,fast | Frequency (s−1) | 10-90% RT (ms) | Amplitude (pA) | τ1 (ms) | τ2 (ms) | A1 (%) | τw (ms) | |
|---|---|---|---|---|---|---|---|---|
| WT | Ctrl | 21.1±2.7 | 0.61±0.03 | -37.8±2.4 | 7.0±0.6 | 13.7±2.3 | 73±6 | 8.5±0.6 |
| Etom | 14.9±2.0 | 0.59±0.04 | -34.1±3.6 ** | 4.1±0.4 | 21.7±3.1 ** | 38±3 *** | 14.9±2.0 | |
| P3-N265M | Ctrl | 19.3±1.7 | 0.60±0.04 | -39.6±3.6 | 4.9±0.4 * | 15.2±2.7 | 67±7 | 7.7±0.5 |
| Etom | 17.2±1.5 | 0.60±0.04 | -34.6±2.5 | 4.3±0.6 | 17.6±2.0 | 55±5 | 9.8±0.2 | |
p<0.05
p<0.01
p<0.001, n=7 cells for each condition.
Recombinant receptors
For each excised-patch, 1-5 responses were averaged. Peak current amplitude was identified visually and the mean value of the surrounding 200 data points (10 ms) was measured. GABA concentration-response data were fit using GraphPad Prism software to the sigmoidal dose-response equation Y=Bottom + (Top-Bottom)/(1+10^((LogEC50-X)*HillSlope)), where Y is the peak response to a given GABA concentration, Top is the maximal amplitude of current, X is GABA concentration, and Bottom was constrained to 0. EC10-GABA was calculated using the equation logEC50=logECF - (1 / HillSlope)*log(F/(100-F)), where F was set to 10. The calculated EC10-GABA derived from these fits was used in experiments to evaluate modulation by etomidate. The percent potentiation of GABA-evoked responses by etomidate was expressed as ((Amplitude Drug) / ((Amplitude Control + Amplitude Drug) / 2) - 1)*100.
2.10 Statistics
Data are presented as mean ± SEM, with n specifying the number of mice, slices or recorded cells. Unless noted otherwise, statistical comparisons were performed using two-way ANOVA, with Tukey's post-hoc test for pairwise comparisons and p-values adjusted for multiple comparisons. For experiments assessing tonic inhibitory current under control conditions, a one-sample t-test was used to test the null hypothesis that the shift in baseline current upon PTX application was equal to zero. For experiments on expressed recombinant receptors, a one-sample t-test was used to test the null hypothesis that potentiation of the GABA-evoked response was equal to zero. For all experiments, the critical value for statistical significance was set at p < 0.05.
Variance in the ratio between independent estimates was calculated by the formula
where δA/B is the standard deviation of the ratio, A is the mean of A, B is the mean of B, δA is the standard deviation of B, and δB is the standard deviation of B.
2.11 Chemicals
For experiments in vitro, etomidate ((R)-1-(1-phenylethyl)-1H-imidazole-5-carboxylic acid ethyl ester) was purchased from Tocris (Bristol, UK) as a powder that was dissolved in DMSO and kept in aliquots at 50 mM concentration. For each experiment, an aliquot was thawed and diluted appropriately in recording aCSF. The final concentration of DMSO did not exceed 0.1%, which in previous studies was found to have no effect on GABAARs (Harney et al., 2003). Effects of etomidate were quantified in slices that had been pre-incubated in etomidate for at least 1 hour (Benkwitz et al., 2007). For behavioral experiments in vivo, etomidate was purchased pre-dissolved in a 35% propylene glycol solution at a concentration of 2 mg etomidate/ml solution (Hospira, Inc; Lake Forest, IL). All salts were obtained from Sigma-Aldrich, and kynurenic acid from Abcam Biochemicals®, NaHEPES from ChemCruz™ Biochemicals, and CaCl2x2H2O from Fisher Scientific.
3. Results
3.1 Etomidate enhances tonic inhibition in WT but not β3-N265M mice
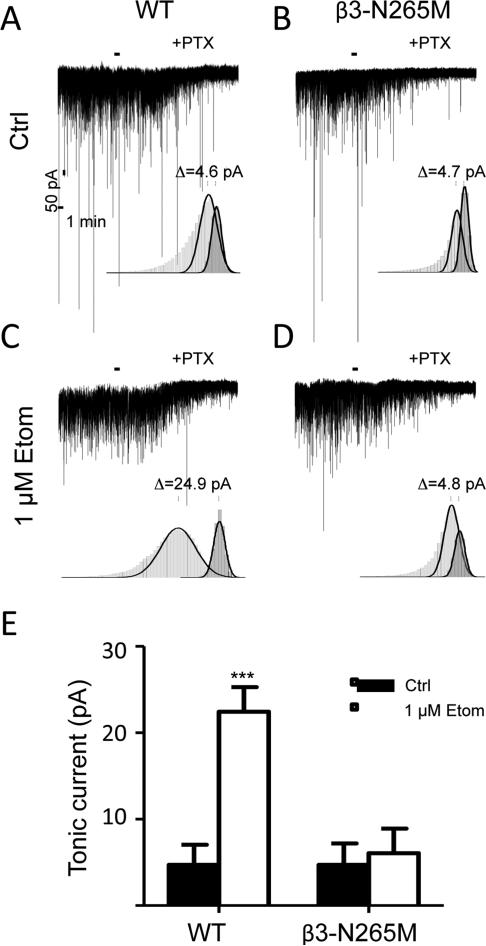
To test whether tonic inhibition is mediated by GABAA receptors that contain β3-subunits, we measured and compared the amplitudes of tonic currents in wild type and β3-N265M mice, under control conditions and in the presence of 1 μM etomidate (Fig.1). Under control conditions, the amplitude of tonic current (measured as the change in the baseline current upon addition of picrotoxin) approached, but did not reach, statistical significance (WT 4.7±2.3 pA, n=7, p=0.09; β3-N265M 4.7±2.5 pA, n=7, p=0.1; one-sample t-test). These values did not differ between genotypes (p=0.99). Etomidate increased the amplitude of tonic current in WT mice (22.45±2.8 pA; p=0.005 vs. WT control) but not in β3-N265M mice (6.05±2.8 pA; p=0.98 vs. mutant control).
Fig.1. Tonic inhibition is mediated by β3-subunit containing GABAA receptors.
(A and C) Representative traces containing spontaneous inhibitory currents recorded in WT mice in the absence (Ctrl) and presence of etomidate (1 μM Etom). (B and D) Representative traces containing spontaneous inhibitory currents recorded in β3N265M mice in the absence (Ctrl) and presence of etomidate (1 μM Etom). Insets show frequency histograms (light and dark grey) with overlaid Gaussian fits (black). The peaks of the fits were used as a measure of the amplitude of tonic currents before and after application of the non-competitive GABAARs channel-blocker, picrotoxin (PTX, 200 μM). (E) Summary bar plot of average amplitudes of tonic inhibition measured in the two genotypes under different conditions, ***p<0.001, n=7 cells for each condition.
We conclude from these results that tonic inhibition in CA1 pyramidal neurons arises mostly or entirely from receptors that incorporate β3-subunits.
3.2. Etomidate modulates fast phasic inhibition in WT but not β3-N265M mice
Spontaneous inhibitory postsynaptic currents (sIPSCs) were evident in our whole cell recordings before PTX was applied. We characterized and compared the properties of these “GABAA,fast” sIPSCs in WT and β3-N265M mice, in the absence and presence of 1 μM etomidate (Table 1).
Under control conditions, the only characteristic of sIPSCs that differed between genotypes was the fast component of decay (τ1, p=0.033; for all others p>0.8). Etomidate increased the weighted decay time constant (τw) in WT mice (175±26%, p=0.005), but not in β3-N265M mice (127±8%, p=0.7225). This change was driven by an increase in the relative proportion of the slow component of decay (A2, p=0.0005), a reduction in the time constant of the fast component of decay (τ1, p=0.0025), and a trend toward an increase in the time constant of the slow component (τ2, p=0.15). None of these effects were observed in mutant mice, indicating that GABAA,fast IPSCs are mediated largely by β3-containing GABAARs. There was also a trend toward reduced sIPSC frequency produced by etomidate in WT (p= 0.16) but not in mutant mice (p=0.88). This finding suggests that the cells that give rise to fast spontaneous IPSCs are themselves inhibited via β3-GABAARs.
3.3 Etomidate blocks LTP in WT and β3-N265M mice
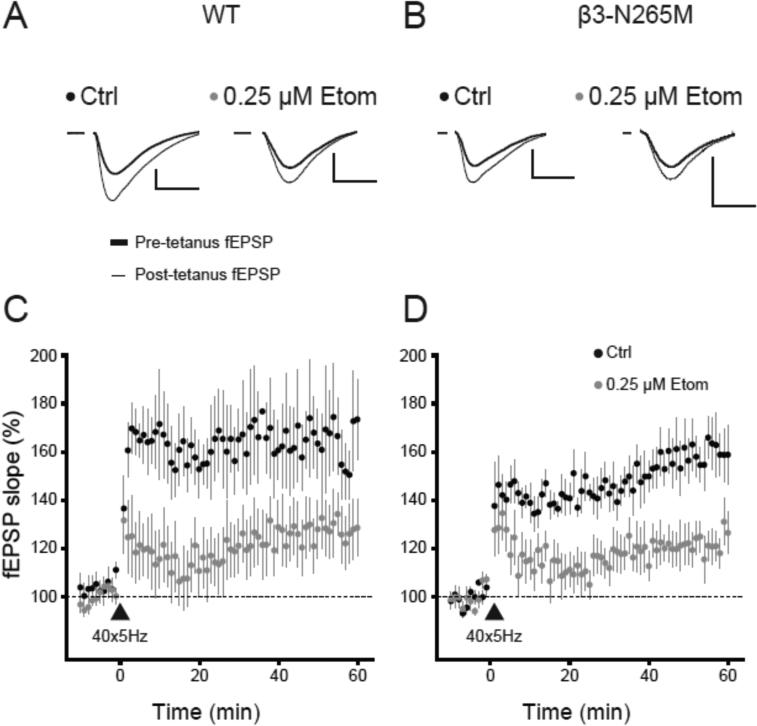
It has been proposed that enhancement of tonic inhibition in CA1 pyramidal cells by etomidate accounts for its ability to block synaptic plasticity in vitro and learning and memory in vivo (Cheng et al., 2006). Thus, we hypothesized that etomidate would fail to block LTP or impair memory in β3-N265M mice, since it did not potentiate tonic inhibition. To test this hypothesis, we induced LTP of the fEPSP using TBS in slices from WT and β3-N265M mice, under control conditions and in the presence of 0.25 μM etomidate (Fig.2).
Fig.2. Effect of etomidate on long-term potentiation does not depend on β3-subunit containing GABAA receptors.
(A and B) Representative traces showing field excitatory post-synaptic potentials (fEPSPs) recorded from stratum radiatum of the CA1 region in slices from WT (A) and β3-N265M mice (B) in the absence (Ctrl) and presence of etomidate (0.25 μM Etom). Traces are the averages of fEPSPs acquired 10 min pre-TBS (thick line) and the last 10 min (51-60 min) post-TBS (thin line). Scale bars indicate 0.5 mV and 10 ms. (C and D) Summary time series of the fEPSPs slope changes following 40x5Hz TBS (triangle) in WT (C) and β3-N265M mice (D) in the absence (black circles, Ctrl) and presence of etomidate (grey circles, 0.25 μM Etom), n=7-8 slices per group.
Theta burst stimulation induced LTP under drug-free conditions in both genotypes (WT 165±17%, n=7; β3-N265M 160±10%, n=7). Etomidate reduced the amplitude of LTP (WT 129±11%, n=8; β3-N265M 122±7%, n=7), by an amount that did not differ between genotypes (genotype F(1,25)=0.4, p=0.5; drug F(1,25)=10.24, p<0.01; interaction F(1,25)=0.01, p=0.9; twoway ANOVA).
From these results we conclude that, contrary to our expectation, etomidate is able to block synaptic plasticity in vitro in β3-N265M mice as well as in WT mice. The dissociation between the effect of etomidate on tonic inhibition and on LTP suggests that enhanced tonic inhibition is not the mechanism by which etomidate blocks TBS-induced synaptic plasticity.
3.4 Etomidate impairs fear conditioning to context in WT and β3-N265M mice
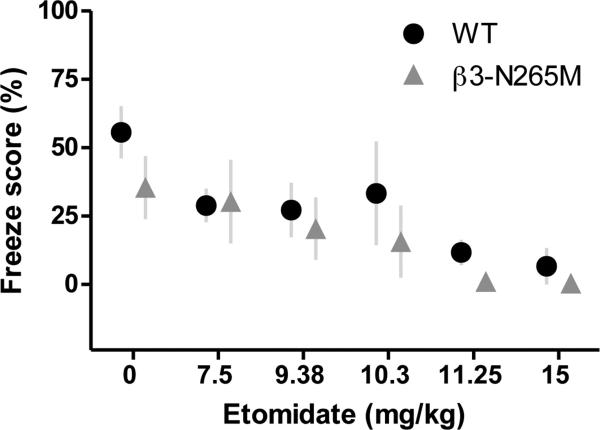
To test whether etomidate impairs learning and memory in vivo by modulating GABAA receptors that incorporate β3-subunits, we compared effects of different doses of etomidate on fear conditioning to context in WT and β3-N265M mice (Fig.3).
Fig.3. Effect of etomidate on contextual fear conditioning does not depend on β3-subunit containing GABAA receptors.
Learning was assessed as % time spent freezing when re-exposed to context in WT (black circles) and β3-N265M (grey triangles) mice. Increasing doses of etomidate reduced freezing scores equally in both genotypes, n= 3-10 mice per group, 7 mice on average.
Under drug-free conditions, freezing scores did not differ in WT vs. β3-N265M mice (55.7±9.5%, n=10 vs. 35±11.5%, n=8; p=0.861). Increasing doses of etomidate (7.5-15 mg/kg) reduced freezing scores in both genotypes (Fig. 3), with an ED50 dose of 8.2±1.1 mg/kg, 95% confidence interval [CI] 4.5-9.6 mg/kg in WT, and 8.8±0.8 mg/kg, 95% confidence interval [CI] 6.6-10.7 mg/kg in β3-N265M mice.
From these results we conclude that etomidate is able to block learning and memory in vivo in β3-N265M mice as in WT mice, thus mirroring our findings above for TBS-induced LTP. Taken together, our findings indicate that neither tonic inhibition nor β3-GABAARs contribute critically to the amnestic effect of etomidate.
3.5 Amnestic concentrations of etomidate do not modulate α5β1γ2L GABAA receptors
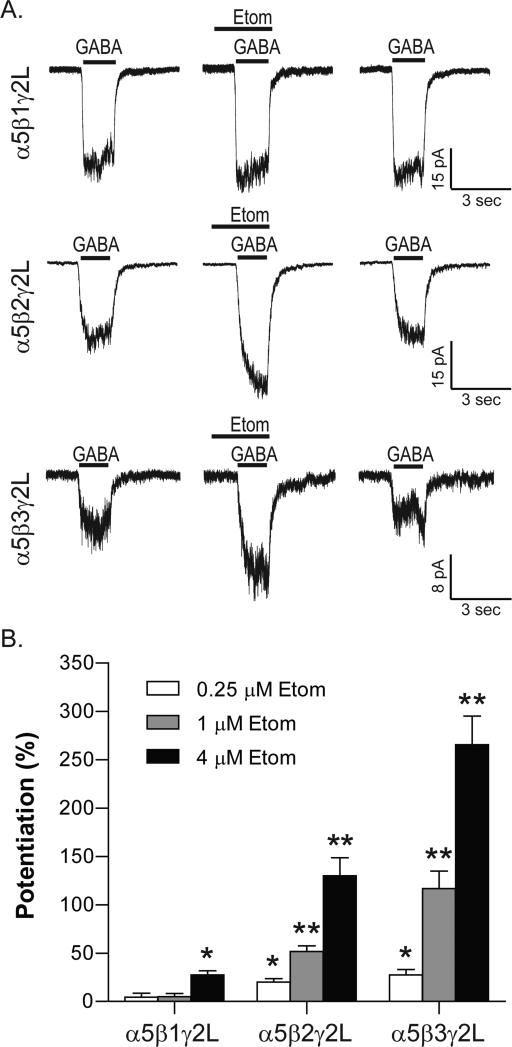
Previous studies of recombinant α1βxγ2S receptors expressed in Xenopus oocytes showed that etomidate modulates receptors that incorporate β2- or β3-subunits, but not β1-subunits (Sanna et al., 1997). This result would seem to indicate that the target of etomidate in β3-N265M mice must be receptors that incorporate β2-subunits. However, a recent report that 3 μM etomidate potentiates GABA-evoked responses of α5β1γ2-GABAARs to similar extent as α5β3γ2-GABAARs (Janssen et al., 2009) caused us to re-examine the selectivity of etomidate for β2 and β3 versus β1-subunits when expressed in combination with α5 subunits.
A summary of results is presented graphically in Fig. 4. At a concentration of 0.25 μM, which corresponds to ED50-amnesia (Benkwitz et al., 2007), etomidate potentiated responses of α5β2γ2L− and α5β3γ2L-GABAARs, but not α5β1γ2L-GABAARs. Similarly, 1 μM etomidate potentiated α5β2γ2L− and α5β3γ2L-GABAARs, but not α5β1γ2L-GABAARs. Only in the presence of 4 μM etomidate, which corresponds to 16 times the ED50-amnesia concentration, were α5β1γ2L-GABAARs potentiated (27±14%, p=0.0002, one-sample t-test). However, the amount of potentiation was significantly smaller than for α5β2γ2L− and α5β3γ2L-GABAARs (130±74.5%, p<0.0001 and 266±102%, p<0.0001, respectively).
Fig.4. α5β1γ2 GABAARs resist potentiation by etomidate.
(A) Responses of recombinant receptors in excised outside-out patches in the absence and presence of 1 μM etomidate. Receptors were activated by EC10 concentrations of GABA, which were 16 μM for α5β1γ2L, 6.2 μM for α5β2γ2L, and 1.3 μM for α5β3γ2L. (B) Summary bar plot of average potentiating effect of 0.25 μM, 1 μM and 4 μM etomidate on GABA-evoked responses. *p<0.001, **p<0.0001, n=11-19 patches for each condition.
From these results we conclude that amnestic concentrations of etomidate do selectively target β2- and β3-GABAARs. Therefore, effects of etomidate on LTP and learning in vivo arose most likely from modulation of α5β2-GABAARs.
4. Discussion
The data presented here support the hypothesis that α5β3-GABAARs underlie tonic inhibition in hippocampal CA1 pyramidal cells. However, they also show that impairment of learning in vivo and LTP in vitro by etomidate is independent of β3-subunit containing GABAARs. This dissociation between enhanced tonic inhibition and impaired plasticity and memory challenges the notion that tonic inhibition mediates the amnestic effect of etomidate, as derived from studies of α5-KO mice (Cheng et al., 2006; Martin et al., 2009).
To arrive at these conclusions we used mice carrying a point mutation in the β3-subunit of the GABAAR that renders these receptors insensitive to etomidate (Jurd et al., 2003). This model allowed us to dissect the roles of different β-subunits in etomidate enhancement of tonic inhibition and suppression of learning and memory. Our results are consistent with previous reports that α5-GABAARs mediate tonic inhibition in CA1 pyramidal neurons (Caraiscos et al., 2004), and that α5-GABAARs are important for learning and memory (Collinson et al., 2002; Crestani et al., 2002). However our data indicate that α5-subunits do so in partnership with β2-subunits. One caveat is that we have not measured α5-subunit expression directly, so it is possible that associated changes in these subunits may have occurred in these mice. However, substantial changes in α5 subunit expression seems unlikely, since the knock-in mutation does not alter expression of the β3 subunit itself, nor of any of the other subunits that have been measured (α1, α2, α3, β2/3, γ2) (Jurd et al., 2003). Moreover, reduction in the expression of α5-GABAARs would itself be expected to prevent etomidate from impairing LTP (Cheng et al., 2006), which did not occur in these mice (Fig. 3).
The finding that etomidate achieves its amnestic effects through selective modulation of α5β2-containing GABAARs is unexpected. Previous investigators showed that α5 subunits are essential for etomidate-induced amnesia (Cheng et al., 2006), that α5 subunits preferentially co-assemble with β3 subunits (Luddens et al., 1994; Sur et al., 1998), that properties of recombinant α5β3γ2 receptors are similar to those of native receptors in CA1 pyramidal cells, which are enriched in α5 and β3 subunits (Burgard et al., 1996; Sur et al., 1998; Caraiscos et al., 2004), and that α5 and β3 subunits are co-depleted in mice lacking either the β3 or α5 subunits (Olsen and Homanics, 2000). Nevertheless, there is evidence that native α5β2 receptors do exist, though they constitute a minority of α5-containing receptors (Ju et al., 2009).
Our findings support behavioral results obtained in forebrain specific β3-KO mice showing that β3-GABAARs do not mediate etomidate-induced amnesia (Rau et al., 2011). Previous experiments in β3-N265M mice similarly showed that propofol-induced amnesia is independent of β3-GABAARs (Zeller et al., 2007). Experiments with isoflurane produced conflicting results: studies in β3-N265M knock-in mice indicated no contribution of β3 subunits (Liao et al., 2005) but studies in forebrain-specific knockout suggested a partial contribution (Rau et al., 2011). Because of the lower likelihood for compensatory changes in receptor expression in knock-in vs. knock-out mice, the present results strengthen the evidence that etomidate acts through receptors that lack β3-subunits.
At doses that impair memory, etomidate also causes sedation, as indicated by a decrease in exploratory activity, (Benkwitz et al., 2007). Since sedation has been attributed to modulation of β2-containing GABAARs (Jurd et al., 2003; Reynolds et al., 2003), this effect may well have contributed to the β2-GABAAR-mediated learning impairment that we observed in vivo. However, this consideration does not apply to experiments in vitro. Therefore, the finding that etomidate suppresses LTP in β3-N265M mice implicates β2-GABAARs in etomidate-induced amnesia specifically, rather than indirectly by causing sedation.
Since β3-GABAAR-mediated tonic inhibition appears not to be the means by which etomidate impairs synaptic plasticity and learning, what inhibitory processes might be involved? Our analysis of spontaneous GABAA,fast IPSCs showed that they are mediated primarily by β3-GABAARs. Also, fast inhibitory currents are relatively insensitive to amnestic concentrations of etomidate (Dai et al., 2009). Taken together, these findings indicate that GABAA,fast inhibition is unlikely to contribute substantially. By contrast, GABAA,slow, an inhibitory current that overlaps anatomically with excitatory receptors on the dendrites (Pearce, 1993), was shown previously to engage α5-GABAARs (Zarnowska et al., 2009), and to be particularly sensitive to amnestic concentrations of etomidate (Dai et al., 2009). This form of inhibition thus appears to be well suited to anesthetic control of synaptic plasticity and memory.
It is also possible that etomidate controls synaptic plasticity and memory by targeting interneurons. GABAA,slow synapses have been observed in interneurons (Banks et al., 2000), and activation of synaptic α5-GABAARs significantly reduces the excitability of oriens lacunosum-moleculare (OLM) interneurons (Salesse et al., 2011). In addition, OLM interneurons have been shown to impact mnemonic processes in the hippocampus by inhibiting other interneurons located in stratum radiatum of the hippocampus (Leao et al., 2012). Thus, etomidate may target non-β3-subunit containing GABAARs on interneurons to impair learning and memory and synaptic plasticity through “disinhibition” (Freund and Gulyas, 1997; Pi et al., 2013; Wolff et al., 2014). Experiments that restrict changes in receptor expression or anesthetic sensitivity to specific classes of neurons (Lee and Maguire, 2013) will be useful for testing these possibilities.
Highlights.
β3-containing GABAA receptors mediate tonic inhibition in CA1 pyramidal neurons
Etomidate suppresses long term potentiation independently of β3-GABAA receptors
Etomidate impairs fear conditioning to context independently of β3-GABAA receptors
Enhanced tonic inhibition does not underlie memory impairment in this animal model
Acknowledgement
This research was supported by the NIH grants: GM047818, GM101497, GM086448. The authors thank Mark Perkins for expert technical assistance and Dr. Sijian Wang for statistical consultation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Banks MI, White JA, Pearce RA. Interactions between distinct GABA(A) circuits in hippocampus. Neuron. 2000;25:449–457. doi: 10.1016/s0896-6273(00)80907-1. [DOI] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ, Peters JA, Wafford K, Whiting PJ. The interaction of the general anesthetic etomidate with the gamma-aminobutyric acid type A receptor is influenced by a single amino acid. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:11031–11036. doi: 10.1073/pnas.94.20.11031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benkwitz C, Liao M, Laster MJ, Sonner JM, Eger EI, 2nd, Pearce RA. Determination of the EC50 amnesic concentration of etomidate and its diffusion profile in brain tissue: implications for in vitro studies. Anesthesiology. 2007;106:114–123. doi: 10.1097/00000542-200701000-00020. [DOI] [PubMed] [Google Scholar]
- Blake AJ, Rodgers FC, Bassuener A, Hippensteel JA, Pearce TM, Pearce TR, Zarnowska ED, Pearce RA, Williams JC. A microfluidic brain slice perfusion chamber for multisite recording using penetrating electrodes. Journal of Neuroscience Methods. 2010;189:5–13. doi: 10.1016/j.jneumeth.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgard EC, Tietz EI, Neelands TR, Macdonald RL. Properties of recombinant gamma-aminobutyric acid A receptor isoforms containing the alpha 5 subunit subtype. Molecular Pharmacology. 1996;50:119–127. [PubMed] [Google Scholar]
- Caraiscos VB, Elliott EM, You-Ten KE, Cheng VY, Belelli D, Newell JG, Jackson MF, Lambert JJ, Rosahl TW, Wafford KA, MacDonald JF, Orser BA. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by α5 subunit-containing γ-aminobutyric acid type A receptors. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:3662–3667. doi: 10.1073/pnas.0307231101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng VY, Martin LJ, Elliott EM, Kim JH, Mount HTJ, Taverna FA, Roder JC, MacDonald JF, Bhambri A, Collinson N, Wafford KA, Orser BA. α5GABAA Receptors Mediate the Amnestic But Not Sedative-Hypnotic Effects of the General Anesthetic Etomidate. The Journal of Neuroscience. 2006;26:3713–3720. doi: 10.1523/JNEUROSCI.5024-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinson N, Kuenzi FM, Jarolimek W, Maubach KA, Cothliff R, Sur C, Smith A, Otu FM, Howell O, Atack JR, McKernan RM, Seabrook GR, Dawson GR, Whiting PJ, Rosahl TW. Enhanced learning and memory and altered GABAergic synaptic transmission in mice lacking the alpha 5 subunit of the GABAA receptor. J Neurosci. 2002;22:5572–5580. doi: 10.1523/JNEUROSCI.22-13-05572.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani F, Keist R, Fritschy JM, Benke D, Vogt K, Prut L, Bluthmann H, Mohler H, Rudolph U. Trace fear conditioning involves hippocampal alpha5 GABA(A) receptors. Proc Natl Acad Sci U S A. 2002;99:8980–8985. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai S, Perouansky M, Pearce RA. Amnestic concentrations of etomidate modulate GABAA,slow synaptic inhibition in hippocampus. Anesthesiology. 2009;111:766–773. doi: 10.1097/ALN.0b013e3181b4392d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS. Conditioned and unconditional components of post-shock freezing. Pavlov J Biol Sci. 1980;15:177–182. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- Forman SA. Clinical and molecular pharmacology of etomidate. Anesthesiology. 2011;114:695–707. doi: 10.1097/ALN.0b013e3181ff72b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Gulyas AI. Inhibitory control of GABAergic interneurons in the hippocampus. Canadian Journal of Physiology and Pharmacology. 1997;75:479–487. [PubMed] [Google Scholar]
- Harney SC, Frenguelli BG, Lambert JJ. Phosphorylation influences neurosteroid modulation of synaptic GABAA receptors in rat CA1 and dentate gyrus neurones. Neuropharmacology. 2003;45:873–883. doi: 10.1016/s0028-3908(03)00251-x. [DOI] [PubMed] [Google Scholar]
- Janssen MJ, Ade KK, Fu Z, Vicini S. Dopamine modulation of GABA tonic conductance in striatal output neurons. J Neurosci. 2009;29:5116–5126. doi: 10.1523/JNEUROSCI.4737-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MV, Harrison NL. Effects of volatile anesthetics on the kinetics of inhibitory postsynaptic currents in cultured rat hippocampal neurons. J Neurophysiol. 1993;70:1339–1349. doi: 10.1152/jn.1993.70.4.1339. [DOI] [PubMed] [Google Scholar]
- Jones MV, Brooks PA, Harrison NL. Enhancement of gamma-aminobutyric acid-activated Cl-currents in cultured rat hippocampal neurones by three volatile anaesthetics. J Physiol. 1992;449:279–293. doi: 10.1113/jphysiol.1992.sp019086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju YH, Guzzo A, Chiu MW, Taylor P, Moran MF, Gurd JW, MacDonald JF, Orser BA. Distinct properties of murine alpha 5 gamma-aminobutyric acid type a receptors revealed by biochemical fractionation and mass spectroscopy. J Neurosci Res. 2009;87:1737–1747. doi: 10.1002/jnr.21991. [DOI] [PubMed] [Google Scholar]
- Jurd R, Arras M, Lambert S, Drexler B, Siegwart R, Crestani F, Zaugg M, Vogt K, Ledermann B, Antkowiak B. General anesthetic actions in vivo strongly attenuated by a point mutation in the GABAA receptor beta3 subunit. FASEB J. 2003;17:250–252. doi: 10.1096/fj.02-0611fje. [DOI] [PubMed] [Google Scholar]
- Leao RN, Mikulovic S, Leao KE, Munguba H, Gezelius H, Enjin A, Patra K, Eriksson A, Loew LM, Tort ABL, Kullander K. OLM interneurons differentially modulate CA3 and entorhinal inputs to hippocampal CA1 neurons. Nat Neurosci. 2012;15:1524–1530. doi: 10.1038/nn.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee V, Maguire J. Impact of inhibitory constraint of interneurons on neuronal excitability. J Neurophysiol. 2013;110:2520–2535. doi: 10.1152/jn.00047.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao M, Sonner JM, Jurd R, Rudolph U, Borghese CM, Harris RA, Laster MJ, Eger EI., 2nd Beta3-containing gamma-aminobutyric acidA receptors are not major targets for the amnesic and immobilizing actions of isoflurane. Anesthesia and Analgesia. 2005;101:412–418. doi: 10.1213/01.ANE.0000154196.86587.35. [DOI] [PubMed] [Google Scholar]
- Luddens H, Seeburg PH, Korpi ER. Impact of beta and gamma variants on ligand-binding properties of gamma-aminobutyric acid type A receptors. Mol Pharmacol. 1994;45:810–814. [PubMed] [Google Scholar]
- Martin LJ, Oh GH, Orser BA. Etomidate targets alpha5 gamma-aminobutyric acid subtype A receptors to regulate synaptic plasticity and memory blockade. Anesthesiology. 2009;111:1025–1035. doi: 10.1097/ALN.0b013e3181bbc961. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Homanics GE. Function of GABAA receptors:insights from mutant and knockout mice. Lippincot, Williams & Willkins; Philadelphia: 2000. [Google Scholar]
- Olsen RW, Sieghart W. International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol Rev. 2008;60:243–260. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orser BA. Lifting the fog around anesthesia. Sci Am. 2007;296:54–61. doi: 10.1038/scientificamerican0607-54. [DOI] [PubMed] [Google Scholar]
- Pearce RA. Physiological evidence for two distinct GABAA responses in rat hippocampus. Neuron. 1993;10:189–200. doi: 10.1016/0896-6273(93)90310-n. [DOI] [PubMed] [Google Scholar]
- Pi HJ, Hangya B, Kvitsiani D, Sanders JI, Huang ZJ, Kepecs A. Cortical interneurons that specialize in disinhibitory control. Nature. 2013;503:521–524. doi: 10.1038/nature12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau V, Oh I, Liao M, Bodarky C, Fanselow MS, Homanics GE, Sonner JM, Eger EI., 2nd Gamma-aminobutyric acid type A receptor beta3 subunit forebrain-specific knockout mice are resistant to the amnestic effect of isoflurane. Anesthesia and Analgesia. 2011;113:500–504. doi: 10.1213/ANE.0b013e3182273aff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds DS, Rosahl TW, Cirone J, O'Meara GF, Haythornthwaite A, Newman RJ, Myers J, Sur C, Howell O, Rutter AR, Atack J, Macaulay AJ, Hadingham KL, Hutson PH, Belelli D, Lambert JJ, Dawson GR, McKernan R, Whiting PJ, Wafford KA. Sedation and Anesthesia Mediated by Distinct GABAA Receptor Isoforms. The Journal of Neuroscience. 2003;23:8608–8617. doi: 10.1523/JNEUROSCI.23-24-08608.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph U, Antkowiak B. Molecular and neuronal substrates for general anaesthetics. Nat Rev Neurosci. 2004;5:709–720. doi: 10.1038/nrn1496. [DOI] [PubMed] [Google Scholar]
- Salesse C, Mueller CL, Chamberland S, Topolnik L. Age-dependent remodelling of inhibitory synapses onto hippocampal CA1 oriens-lacunosum moleculare interneurons. The Journal of Physiology. 2011;589:4885–4901. doi: 10.1113/jphysiol.2011.215244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna E, Murgia A, Casula A, Biggio G. Differential subunit dependence of the actions of the general anesthetics alphaxalone and etomidate at gamma-aminobutyric acid type A receptors expressed in Xenopus laevis oocytes. Molecular Pharmacology. 1997;51:484–490. [PubMed] [Google Scholar]
- Sur C, Quirk K, Dewar D, Atack J, McKernan R. Rat and Human Hippocampal α5 Subunit-Containing γ-Aminobutyric AcidA Receptors Have α5β3γ2 Pharmacological Characteristics. Molecular Pharmacology. 1998;54:928–933. doi: 10.1124/mol.54.5.928. [DOI] [PubMed] [Google Scholar]
- Ting JT, Feng G. Improved methods for acute brain slice preparation from adult and aging brain. Society for Neuroscience Annual Meeting Abstracts Book. 2011;520.29 [Google Scholar]
- Uchida I, Kamatchi G, Burt D, Yang J. Etomidate potentiation of GABAA receptor gated current depends on the subunit composition. Neurosci Lett. 1995;185:203–206. doi: 10.1016/0304-3940(95)11263-v. [DOI] [PubMed] [Google Scholar]
- Wolff SB, Grundemann J, Tovote P, Krabbe S, Jacobson GA, Muller C, Herry C, Ehrlich I, Friedrich RW, Letzkus JJ, Luthi A. Amygdala interneuron subtypes control fear learning through disinhibition. Nature. 2014;509:453–458. doi: 10.1038/nature13258. [DOI] [PubMed] [Google Scholar]
- Zarnowska ED, Keist R, Rudolph U, Pearce RA. GABAA receptor α5 subunits contribute to GABAA, slow synaptic inhibition in mouse hippocampus. Journal of Neurophysiology. 2009;101:1179–1191. doi: 10.1152/jn.91203.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller A, Arras M, Jurd R, Rudolph U. Mapping the contribution of beta3-containing GABAA receptors to volatile and intravenous general anesthetic actions. BMC Pharmacol. 2007;7:2. doi: 10.1186/1471-2210-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]