African Americans disproportionally experience uncontrolled asthma1. Because asthma control is now the focus of asthma management,2 publicly available questionnaires like the Asthma Control Test™ (ACT)3 and childhood Asthma Control Test (ACTc)4 represent the most accessible, standardized, non-physician methods of assessing control and are typically considered cost-effective and easily implemented asthma care strategies for primary care offices. Both the ACT (≥12 years old) and ACTc (<12 years old) query asthma symptoms over the past four weeks and use a cutpoint of ≤ 19 for not well-controlled asthma3–4. Because neither questionnaire has been validated in African American children5–10, we sought to determine whether the ACT and the ACTc are appropriate screening tools for asthma control in this population.
This retrospective study was approved by the University of North Carolina Institutional Review Board. From a list maintained by an asthma educator for all pediatric asthma patients seen either at the pulmonology or allergy/immunology clinics, we reviewed the medical record and included data from all children who met the following inclusion criteria: self-identified African-American, ages 5–18, and had at least 2 visits for which ACT/c and spirometry were both performed between January 1, 2011 and November 1, 2013. Fifty-seven children were identified. The primary author reviewed charts from each visit and determined physician-assessment of asthma control by either 1) provider’s written explicit determination of control or, if this were not available, 2) reviewing medication management strategies (classifying continuation of current management or de-escalation of therapy as “well-controlled” and the escalation of therapy as “not well-controlled”). Although investigators were not blinded to spirometry and ACT/c scores, these values were, in most cases, collected by a second investigator independent of chart review for asthma control.
By this method, we collected a total of 130 observations/visits with 84 visits in 41 children ≥12 years old (45 well-controlled visits and 39 not well-controlled visits by MD assessment) and 46 visits in 22 children < 12 years old (24 well-controlled visits and 22 not well-controlled visits by MD assessment). The overall average number of visits per each child in this study was 2.3. All visits of a child were included in the analyses, and multilevel analysis was used to to account for the correlation of the values within a child across visits. Wilcoxon rank sum test was used to compare the ACT medians across categories of physician assessment of asthma control (controlled versus not controlled). We determined the agreement of the ACT score at the standard cutpoint of >19 with physician assessment of control using measures of sensitivity, specificity, and Cohen’s kappa. A receiver-operating curve was used to determine the best ACT cutpoint for assessment of control in this population. A logistic regression model was used with ‘control by physician’ as the dependent variable and ‘ACT score’ as the independent variable. This model included as potential confounders FEV1 and FEV1/FVC (dichotomized at 80%) and age of the child (age <12 or ≥12) as a potential moderator of the effect of ACT score. These models also included a random intercept effect for child in order to account for the correlation among the repeated measures in a child.
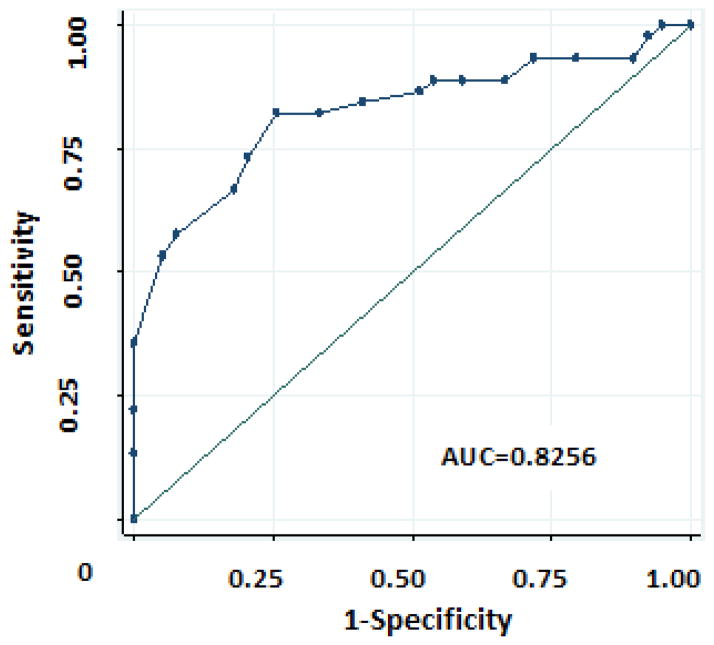
Adolescents ages 12–18 years with controlled asthma by physician assessment had higher ACT scores than those without controlled asthma (p<0.0001). An ACT score of >19 was strongly associated with physician-assessment of control in this group (Fisher’s exact <0.001). However, this cutpoint provided only a sensitivity of 67% and a specificity of 82% in determining physician assessment of asthma control in this minority group. Upon further investigation, we were able to identify that a cutpoint of 18 demonstrated the highest area under the curve (AUC=0.8256) in this group (Figure 1) with a sensitivity of 74% and a specificity of 67%. Spirometry was important in predicting physician assessment of control, especially FEV1/FVC ≥80% (p=0.03). However, ACT score did not agree with spirometry-measured control defined by FEV1 >80% (kappa=0.20, p=0.0317).
Figure 1.
Receiver operating curve showing ACT scores compared to MD control assessment in children ≥12yo.
When reviewing the entire data set of children ages 5–18 years old, logistic regression demonstrated that higher ACT scores are associated with increased odds of one’s asthma being classified as controlled by MD assessment (p=0.0001). However, older age (being ≥12 compared to <12) significantly affects this relationship (p=0.028), and, after adjusting for this effect, ACT score was no longer found to be a significant predictor of physician-assessed control (p=0.642). This age cut-off distinguished those children who self-administer the ACT (≥12 years) from the caregiver-administered cACT (<12 years old). In children <12 years old, we saw no difference in ACTc scores between controlled or not well-controlled asthmatics as determined by physician assessment (p=0.10). Using a standard cutpoint of >19 for asthma control using the ACTc was not associated with physician assessment of control (Fisher’s exact=0.3883) and did not agree with spirometry-measured control defined by FEV1>80% (kappa=0.34, p=0.0095). Additionally, a cutpoint of >19 only had a sensitivity of 50% and a specificity of 64% in these younger children.
In this study, we demonstrate that the ACTc as currently used may not accurately predict asthma control in African American children < 12-years-old, showing limited functionality in this group with low sensitivity and specificity for mirroring the gold standard of physician assessment of asthma control. Surprisingly, this points to a clear discrepancy between caregiver and provider perception of asthma control in children < 12 years old. While the ACTc queries both child and caregiver, greater weight is given to those caregiver-answered questions. Hence, failure of a caregiver to recognize not well-controlled asthma has implications for impacting asthma treatment plans and may also contribute to the increased risks of morbidity and mortality associated with uncontrolled asthma in this population.
Although the ACT had better performance in children ≥12-years-old (who do fill out their own questionnaires), we also found that sensitivity for determining asthma control could be increased by changing the ACT cutpoint to 18 rather than >19 (with a previously reported sensitivity of 71.3% and specificity of 70.8% in a primarily Caucasian population6).
Limitations to our study include its retrospective nature, small sample size, unknown impact of socioeconomic status or the nature of the visit (scheduled versus urgent care), and possible investigator bias (non-blinding to ACT/c and spirometry results). Overall, our results suggest that current use of the ACT and ACTc may require adjustment to better correlate with physician assessment of asthma control in African American children. Additional prospective studies are required to further explore these findings and inform alternate clinical practice standards.
Clinical Implications.
The Asthma Control Test™ (ACT) and childhood ACT are standardized, non-physician methods to assess asthma control but were validated in primarily Caucasian populations. Overall, our results suggest that current use of the ACT and ACTc may require adjustment to better correlate with physician assessment of asthma control in African American children.
Acknowledgments
Sources of Funding: The North Carolina Children’s Promise; MLH is supported by NIEHS K23-ES021745 and the AAAAI ARTrust Gail G. Shapiro Award.
To NC Children’s Promise Grant for funding support for statistical assistance. To the North Carolina Translational and Clinical Sciences (NC TraCS) Institute for providing statistical support.
Abbreviations
- ACT
Asthma Control Test
- ACTc
childhood Asthma Control Test
- FEV1
Forced Expiratory Volume in one second
Footnotes
Author Contributions:
a. Conception and design of study – Todoric, Hernandez
b. Data generation – Todoric, Alarcon, Vadlamudi
c. Analysis and interpretation of data – Todoric, Bangdiwala, Hernandez
d. Preparation or critical review of manuscript – Todoric, Bangdiwala, Vadlamudi, Alarcon, Hernandez
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Krista Todoric, Email: ktodorich@gmail.com.
Shrikant Bangdiwala, Email: kant@unc.edu.
Anusha Vadlamudi, Email: avadlam@gmail.com.
Lisa Alarcon, Email: lisa.Alarcon@unchealth.unc.edu.
Michelle Hernandez, Email: michelle_hernandez@med.unc.edu.
References
- 1.United States Department of Health and Human Services. Office of Minority Health. [Accessed June 17, 2014];Asthma and African Americans. 2012 Aug; Available at http://minorityhealth.hhs.gov/templates/content.aspx?ID=6170.
- 2.US Department of Health and Human Resources: National Institutes of Health. The National Heart Lung and Blood Institute and the National Asthma Education and Prevention Program. . Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Released August 28, 2007. [Google Scholar]
- 3.Asthma Control Test (ACT) [Accessed June 19, 2014];Quality Metric 2014. 2014 Available at http://www.qualitymetric.com/WhatWeDo/DiseasespecificHealthSurveys/AsthmaControlTestACT/tabid/190/Default.aspx.
- 4.Childhood Asthma Control Test. [Accessed June 19, 2014];GlaxoSmithKline 2005. Available at http://www.michaelaye.com/actchild.pdf.
- 5.Cloutier MM, Schatz M, Castro M, et al. Asthma outcomes: composite scores of asthma control. J Allergy Clin Immunol. Mar;129(3 Suppl):S24–33. doi: 10.1016/j.jaci.2011.12.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schatz M, Sorkness CA, Li JT, et al. Asthma Control Test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol. 2006;117(3):549–56. doi: 10.1016/j.jaci.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 7.Thomas M, Kay S, Pike J, et al. The Asthma Control Test (ACT) as a predictor of GINA guideline-defined asthma control: analysis of a multinational cross-sectional survey. Prim Care Respir J Mar. 2009;18(1):41–9. doi: 10.4104/pcrj.2009.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu AH, Zeiger RS, Sorkness CA, et al. Development and cross-sectional validation of the Childhood Asthma Control Test. J Allergy Clin Immunol. 2007;119:817–25. doi: 10.1016/j.jaci.2006.12.662. [DOI] [PubMed] [Google Scholar]
- 9.Liu AH, Zeiger RS, Sorkness CA, et al. The Childhood Asthma Control Test: retrospective determination and clinical validation of a cut point to identify children with very poorly controlled asthma. J Allergy Clin Immunol. 2010;126(2):267–73. doi: 10.1016/j.jaci.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 10.Nathan RA, Sorkness CA, Kosinski M, et al. Development of the asthma control test: A survey for assessing asthma control. J Allergy Clin Immunol. 2004;(113):59–65. doi: 10.1016/j.jaci.2003.09.008. [DOI] [PubMed] [Google Scholar]