Abstract
Background
A substantial number of planned clinical trials for sickle cell disease (SCD) have terminated early due to insufficient patient enrollment.
Purpose
To describe attitudes towards clinical trials among a sample of adults with SCD and identify patient-level factors associated with these attitudes.
Methods
Our data came from a sample [N = 291] of primarily adults with SCD participating in the Improving Patient Outcomes with Respect and Trust (IMPORT) study, which is a federally-funded observational study of SCD patient experiences in seeking health care. Attitudes towards clinical trials were assessed using items from the Perceptions of Participation in Clinical Research instrument. Patient factors examined as potential correlates of clinical trial attitudes were demographics, disease severity, engagement in self-care, trust, healthcare experience ratings, and prior history of participation in clinical trials. Multiple regression analyses were used to identify patient-level correlates of clinical trial attitudes.
Results
Our sample of SCD patients expressed overwhelmingly favorable attitudes about clinical trials, with 77% to 92% of our sample expressing agreement with a series of positive statements about clinical trials in general. Demographics, engagement in self-care, healthcare experience ratings, and prior trial participation each explained significant portions of the variability in clinical trial attitudes.
Limitations
The generalizability of our results to the entire SCD population may be of concern as the study participants were all receiving care at comprehensive sickle cell centers and already participating in clinical research.
Conclusions
Our results suggest that, in principle, adults with SCD enrolled in an observational study express very positive general attitudes about clinical trial participation and that specific factors attached to particular clinical trial opportunities may play a greater role in a SCD patient’s decision to participate than a general unwillingness to participate.
Keywords: Sickle Cell Disease, Attitudes, Patient Recruitment, Clinical Trials
Introduction
Clinical trials are necessary to develop new treatments to improve the quality of life. One community in particular need of new treatment modalities from the clinical research enterprise is sickle cell disease (SCD).1 Sickle cell disease is a serious genetic disorder of the blood that causes severe pain, progressive end-organ damage, and early mortality. One of the most common genetic disorders found across the world, SCD is also the most common genetic disorder affecting African-Americans in the U.S., and it is the most common inherited condition identified by newborn screening programs in this country, having a birth prevalence between 1 per every 2500 and 1 per every 2000 births.2, 3 Hydroxyurea is the only disease-modifying drug therapy available to treat SCD. Research to identify additional disease-modifying therapeutics would increase the treatment options available for SCD patients and are sorely needed.1
Advocates in the SCD community have traditionally bemoaned a seeming lack of priority and funding directed at SCD research progress.4, 5 When clinical trials for SCD have been planned and started, many have terminated early because of insufficient enrollment.6 A recently-closed trial surveyed its investigators about potentially modifiable challenges to recruitment and implementation, finding that features of the protocol design and staffing at study sites, eligibility criteria, and competing research protocols were significant barriers contributing to under-enrollment and early trial termination.6 Data available on Clinicaltrials.gov demonstrates that 56% percent of SCD studies that are terminated before study completion are done so because of slow rates of recruitment (19 out of 34 studies).7
Very little is known about the factors that affect SCD patient participation in clinical research. The few studies addressing this topic have focused on the factors that affect the decisions of children/adolescents with SCD or their parents.8–13 Yet a large body of literature exists that examines the determinants of African-American participation in research generally. This research has found an important role for the attitudes that individuals have about clinical research. Negative attitudes about clinical trial participation, such as the belief that one will be treated like a “guinea pig” if taking part in a clinical trial, are found in numerous studies to serve as barriers to research enrollment, while positive attitudes, such as a belief in receiving personal benefit from study participation, has been identified as a facilitator of African-American research participation.14–18
We examined the responses of adults with SCD to a survey of attitudes toward the potential benefits of clinical trials. Our specific aims were to describe the attitudes of adults with SCD toward clinical trials and to identify factors associated with these attitudes.
Methods
Study Design, Subjects and Setting
Our study was conducted using data from the Improving Patient Outcomes with Respect and Trust (IMPORT) study. Funded by the National Heart, Lung and Blood Institute, the IMPORT study is an observational cohort of SCD patient experiences with healthcare at two academic medical centers. The Institutional Review Boards of both centers approved the research protocols for the study.
Persons eligible to participate in the IMPORT study were: 1) age 15 years or older, 2) possess any of the following sickle hemoglobinopathies: HbSS, HbSC, Hb S/B-thalassemia or HbSS/a-thalassemia, 3) reported no plans to move in the next three years, and 4) expressed willingness to adhere to study procedures. Research assistants recruited eligible patients from waiting rooms of adult and pediatric SCD clinics at the two study sites, and all study subjects gave written informed consent or assent if less than 18 years old.
Data Collection Procedures
At the time of study enrollment, participants completed a comprehensive baseline questionnaire administered by an audio computer-assisted self-interview (ACASI) system. On average, each ACASI interview took approximately 45 minutes to complete and patients were paid $50 for their time. We collected data on their perceptions of the previous quality of their healthcare experiences, basic demographic information, health status, clinical complications, and psychosocial attitudes. The specific measures collected and used for the current analysis are as follows:
Dependent Variable: Attitudes Towards Clinical Trials
We modified and used the “Perceptions of Participation in Clinical Research” (PPCR) instrument described and adapted by Kennedy and Burnett.19, 20 Specifically, we used 4 items from the “Participation Benefits” sub-scale of the PPCR. Using a 5-point likert response set (strongly disagree to strongly agree), the “Participation Benefits” sub-scale assesses respondent agreement with the following statements: 1) Clinical trials are a necessary way to learn about treatments; 2) It is important for people to take part in clinical trials; 3) Participation in a clinical trial can help me and my family; and 4) Participation in a clinical trial can help future generations. We present the results of responses to these items in two different ways: We first dichotomized the items into “agreement” (a response of agree or strongly agree) vs. “lack of agreement” (a response of unsure, disagree, or strongly disagree) on each item. For our primary statistical analyses, we constructed an overall scale score by taking the mean of the 4 items (total score ranging from 1 to 5, with higher scores indicating more positive attitudes about the benefits of clinical trials). This scale demonstrated good internal consistency in our sample with a Cronbach’s alpha of 0.813.
Independent Variables: Potential Correlates of Clinical Trial Attitudes
We examined 6 broad categories of independent variables as potential correlates of SCD patient clinical trial attitudes: Demographics (patient age, sex, and education), Disease severity (general health status, the presence/absence of daily chronic pain, and annual emergency department and inpatient utilization), Patient engagement in self-care (the Sickle Cell Self-Efficacy Scale,21 and the Sickle Cell Self-Care instrument)22, Healthcare experiential factors (the quality of previous communication with healthcare providers23, the extent to which providers previously treated the respondent with respect or partnership during clinical encounters, and whether the patient perceived race or disease-based discrimination from their providers)24, 25, Patient trust (in medical professionals and in the healthcare system)26, 27, and the patient’s prior history with clinical trials (assessed by asking a single dichotomous question from the PPCR’s history with clinical trials subsection that read, “Have you ever participated in a clinical trial for sickle cell disease?” (yes/no)).19, 20
Statistical Analyses
We used basic descriptive statistics to describe our sample’s history with clinical trials and to explore their item-level attitudes toward clinical trials. Bivariate associations between the potential patient-level correlates and their aggregated clinical trial attitude scores were assessed using t-tests, one-way ANOVA, or Pearson’s correlations as appropriate. We used hierarchical multiple regression analyses using nested models to identify variables, or categories of variables, that could explain significant portions of the variability observed in clinical trial attitude scores. We specified the order of entry of variables for our regression model following Cohen’s recommended method.28 Patient demographic factors were entered into the model first (model 1), followed by disease severity factors (model 2), healthcare experience ratings (model 3), patient trust (model 4), attitudes toward engagement in self-care (model 5), and finally history with clinical trials (model 6). Each model sought to investigate the added explanatory power attributed to a particular block of variables after accounting for the effects of all previously entered variables.
A p-value of <0.05 was the threshold used to determine statistical significance in both the bivariate and multivariable analyses. All analyses were conducted using the Stata 12.1 statistical software package.29
Results
Patient Demographics
Sample characteristics are provided in Table 1. Two-hundred ninety one (291) people participated in the IMPORT study and provided enough data for inclusion in our analytic sample. The majority of participants (54%) are female with a mean age of 34.5. Nearly two thirds (65%) of the participants reported a high school or less level of education. Thirty-eight percent of the participants perceived their health status as being poor or fair, while 54% of the participants reported daily chronic pain. A little over half of the respondents reported prior participation in a clinical trial.
Table 1.
IMPORT Sample Demographic Characteristics (n = 291)
| Age (mean, SD) | 34.5(12.5) | |
|---|---|---|
| Sex (n, Col %) | ||
| Male | 134 | 46.0% |
| Female | 157 | 54.0% |
| Total | 291 | |
| Education (n, Col %) | ||
| High School or less | 185 | 64.9% |
| Some College | 45 | 15.8% |
| College Grad & more | 55 | 19.3% |
| Total | 285 | |
| Perceived Health Status (n, Col %) | ||
| Poor/Fair | 111 | 38.4% |
| Good | 123 | 42.6% |
| V.Good/Excellent | 55 | 19.0% |
| Total | 289 | |
| Daily Chronic Pain (n, Col %) | ||
| No | 132 | 45.7% |
| Yes | 157 | 54.3% |
| Total | 289 | |
| ED Utilization in past 12months (n, Col %) | ||
| None | 47 | 16.3% |
| 1 to 2 | 93 | 32.2% |
| 3 to 5 | 78 | 27.0% |
| 6 to 10 | 42 | 14.5% |
| More than 10 | 29 | 10.0% |
| Total | 289 | |
| Hospital Utilization in past 12months (n, Col %) | ||
| None | 68 | 23.5% |
| 1 to 2 | 108 | 37.4% |
| 3 to 5 | 71 | 24.6% |
| 6 to 10 | 18 | 6.2% |
| More than 10 | 24 | 8.3% |
| Total | 289 | |
| Study Site (n, Col %) | ||
| Howard | 124 | 42.6% |
| JHU | 167 | 57.4% |
| Total | 291 | |
| Have you previously participated in a clinical trial for sickle cell disease? | ||
| No | 136 | 47.6% |
| Yes | 150 | 52.4% |
| Total | 286 | 100.0% |
Clinical Trial Attitudes
Respondent attitudes toward the potential benefits of clinical trials were overwhelmingly positive (Figure 1). The results of our bivariate examinations for relationships between patient-level factors of various types and patient aggregated clinical trial attitude scores are found in Tables 2 and 3. A number of factors were found to be significantly associated with clinical trial attitudes. More education, better perceived health status, and previous clinical trial participation were all associated with more positive attitudes about clinical trials (Table 2). As shown in Table 3, better patient engagement in the form of greater utilization of recommended self-care behaviors was weakly, yet significantly, associated with more positive attitudes (r = 0.26, p < 0.01), as was greater perceived partnership with healthcare providers during healthcare encounters over the prior year (r = 0.16, p = 0.01).
Figure 1.
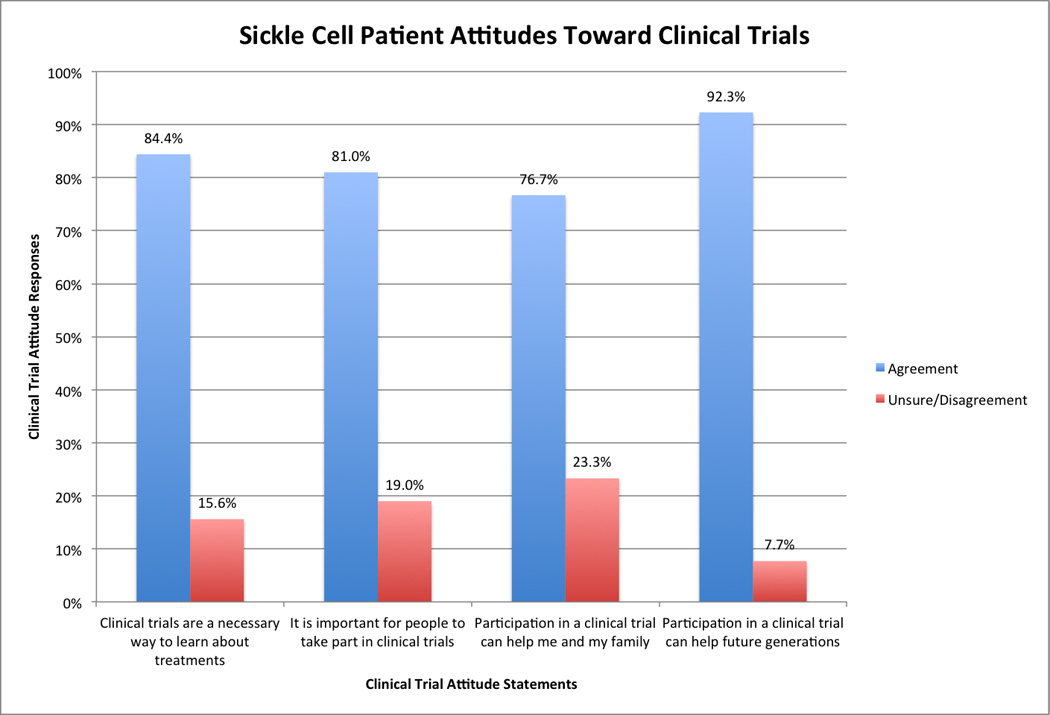
Sickle cell patient attitudes toward clinical trials.
Table 2.
Bivariate Associations between Patient Demographics, Clinical Trial History, and Clinical Trial Attitudes
| Clinical Trial History | Mean (SD) Clinical Trial Attitude Score (higher scores = more positive attitudes toward trials) |
P-value |
|---|---|---|
| Have you previously participated in a clinical trial for sickle cell disease? | 0.001* | |
| No | 4.03 (0.65) | |
| Yes | 4.27 (0.54) | |
| Patient Demographic Characteristics | ||
| Patient's Sex | 0.36 | |
| Male | 4.12 (0.61) | |
| Female | 4.19 (0.60) | |
| Education | 0.01* | |
| High School or less | 4.09 (0.64) | |
| Some College | 4.23 (0.46) | |
| College Grad & more | 4.37 (0.54) | |
| Perceived Health Status | 0.0003* | |
| Poor/Fair | 4.03 (0.62) | |
| Good | 4.15 (0.61) | |
| V.Good/Excellent | 4.44 (0.47) | |
| Daily Chronic Pain | 0.88 | |
| No | 4.17 (0.61) | |
| Yes | 4.15 (0.61) | |
| ED Utilization in the past 12months | 0.648 | |
| None | 4.21 (0.57) | |
| 1 to 2 | 4.17 (0.57) | |
| 3 to 5 | 4.15 (0.63) | |
| 6 to 10 | 4.23 (0.69) | |
| More than 10 | 4.02 (0.54) | |
| Hospital Utilization in the past 12months | 0.388 | |
| None | 4.26 (0.59) | |
| 1 to 2 | 4.17 (0.59) | |
| 3 to 5 | 4.10 (0.64) | |
| 6 to 10 | 4.24 (0.66) | |
| More than 10 | 4.02 (0.57) | |
| Study Site | 0.55 | |
| Howard | 4.19 (0.61) | |
| JHU | 4.14 (0.60) |
Table 3.
Pearson Correlations between Patient Characteristics, Healthcare Experience Ratings, and Psychosocial Attitudes with Clinical Trial Attitudes
| Clinical Trial Attitudes | |||
|---|---|---|---|
| rho | p-value | N | |
| Demographics | |||
| 1) Age | 0.11 | 0.07 | 280 |
| Patient Engagement in Self Care | |||
| 2) Sickle Cell Disease Self-Efficacy | 0.11 | 0.07 | 271 |
| 3) Sickle Cell Self-Care Score | 0.26 | 0.00* | 275 |
| Healthcare Provider Experience Ratings | |||
| 4) Interpersonal Trust | 0.05 | 0.37 | 272 |
| 5) Institutional Distrust | −0.02 | 0.75 | 273 |
| 6) Provider Communication Rating | 0.05 | 0.41 | 278 |
| 7) Provider Partnership Rating | 0.16 | 0.01* | 279 |
| 8) Provider Respectfulness Rating | −0.01 | 0.90 | 277 |
| 9) Perceived Race-based Discrimination | −0.00 | 0.94 | 274 |
| 10) Perceived Disease-based Discrimination | 0.09 | 0.13 | 277 |
Explaining Variability in Clinical Trial Attitudes: Hierarchical Multiple Regression Results
The results of our hierarchical multiple regression analyses using nested models are shown in Table 4. Considered as a group, patient demographic characteristics were found to explain 4.8% (p = 0.007) of the variability in clinical trial attitudes. Interestingly, measures of disease severity did not significantly explain any of the variability in clinical trial attitudes after adjustment for patient demographic characteristics. Adding measures of healthcare experience ratings to the model accounted for another 4.1% (p = 0.054) of the variability in clinical trial attitudes even after adjustment for patient demographics and disease severity measures. Measures of patient trust were added to the analysis next, but did not significantly account for any of the variability in clinical trial attitudes beyond that accounted for by demographics and healthcare experiences (p = 0.619). A patient’s level of engagement in healthcare significantly accounted for 3.8% (p = 0.006) of the variance in clinical trial attitudes even after adjustment for patient demographics, disease severity, healthcare experience ratings, and measures of patient trust. Previous participation in clinical trials, added to the analysis at the end and by itself, significantly accounted for 1.6% of the variability in clinical trial attitudes (p = 0.036). In total, the various patient-level factors analyzed accounted for 20% of the variability in clinical trial attitudes overall.
Table 4.
Hierarchical Regression Analysis for Clinical Trial Attitudes (n = 251)
| Models | df† | F† | ΔR2 † | p-value |
|---|---|---|---|---|
| 1. Demographics | 3 | 4.11 | .048 | .007 |
| 2. Disease Severity | 11 | 1.33 | .055 | .211 |
| 3. Healthcare Experience Ratings | 5 | 2.21 | .041 | .054 |
| 4. Patient Trust | 2 | 0.48 | .004 | .619 |
| 5. Patient Engagement in Self-Care | 2 | 5.28 | .038 | .006 |
| 6. Prior Trial Participation | 1 | 4.46 | .016 | .036 |
| Total R2 | 0.20 | |||
df = Model degrees of freedom; F = F-statistic for the model; Δ R2 = proportion of variability in the dependent variable attributed to the model
Model 1 - Demographics: Patient Age, Education
Model 2 – Model 1 + Disease Severity (Perceived health status, ED utilization, Inpatient Utilization, Daily Chronic Pain)
Model 3 – Model 2 + Healthcare Experience Ratings (Provider Communication Ratings, Provider Partnership Ratings, Perceived racial/ethnic discrimination, perceived disease-based discrimination)
Model 4 – Model 3 + Patient Trust (Trust in Medical Professionals, Distrust in Medical Institutions)
Model 5 – Model 4 + Patient Engagement in Self-Care (SCD Self-Care Score, SCD Self-Efficacy)
Model 6 – Model 5 + Prior Trial Participation (Previous Participation in a clinical trial [yes vs. no])
Discussion
The attitudes that African-Americans have toward clinical trials are known determinants of African-American patient willingness to enroll in studies, with positive attitudes and a belief in the benefits of clinical trials serving as important facilitators of trial participation.15–18 We found among our cohort of patients with SCD substantially positive attitudes toward clinical trials. Specifically, our cohort expressed generally high levels of endorsement of the potential benefits of clinical trials.
The patients in our sample generally agreed that clinical trials are necessary to learn about treatments, that it is important for people to take part in clinical trials, and that clinical trials can potentially help the patient, their family, or future generations. This is suggestive of a high level of belief in the potential benefits of clinical trials among SCD patients, and a low level of general negativity about clinical trials among this patient population. Numerous studies among African-American respondents generally, as well as among African-American patient populations with chronic diseases, have found that attitudes and perceptions about clinical trials, the potential benefits of clinical trials, and the identity of the individuals and communities perceived to benefit from clinical trials, are major determinants of African-American willingness to participate in clinical research.15–18 Negative attitudes, such as believing that one will be treated as a guinea pig if taking part in a clinical trial, or believing that the African-American community is not likely to share in the benefits that result from clinical research, have been identified as important barriers to African-American willingness to participate in research.16, 18 Conversely, the belief that the individual potential research participant, family, friends, or the African-American community in general are likely to benefit from the research has been identified as an important facilitator of African-American research participation.15, 16, 18 The level of belief in the potential benefits of clinical trial participation observed in the current study suggests one important facilitator of clinical trial participation that the SCD community may already possess in high levels, and it may be targeted and used in conjunction with efforts to improve actual rates of clinical trial enrollment for this population.
A number of patient-level factors were found to correlate with clinical trial attitudes. Patient demographics, particularly education, were found to significantly explain some portion of the variability in clinical trial attitudes. This is consistent with other studies that have found greater education to be a facilitator of research participation among African-Americans generally, as well as African-Americans with cancer.16, 19 Educating SCD patients about clinical trials, their need, and how they work, may serve a role in helping patients feel more positive about the clinical trial enterprise, thus facilitating their participation in research opportunities.
Patient beliefs about the quality of their interpersonal experiences in seeking care were found to correlate with clinical trial attitudes. African-American patient experiences of disrespectful or inequitable treatment in healthcare settings have been shown to have a negative effect on African-American patient willingness to participate in clinical research.14 In academic medical settings where clinicians are heavily involved in both patient care and clinical research, the quality of the patient’s healthcare experiences will almost inevitably have some effect on how patients view clinical trials if the same clinicians who provide their care are also the ones engaged with the clinical trial enterprise. Thus, efforts to improve the interpersonal quality of care for pain that is delivered to patients with SCD, which is currently a major problem in the care of this patient population, and is an important goal of health policy efforts, may also help the research enterprise.
Somewhat surprisingly, patient trust in medical professionals or in healthcare institutions was not found to be independently associated with clinical trial attitudes. While almost universally hypothesized to have a major impact on African-American patient willingness to participate in research, empirical studies of trust have actually been mixed with regards to it being found to be a significant, independent predictor of African-American patient willingness to participate in research or actual enrollment in research studies.16 The fact that trust was entered into the statistical model after the effect of prior patient experiences with healthcare had already been accounted for may explain this finding as patient experiences are known to be strong predictors of patient trust.30–32 This finding might also suggest that trust, to the extent that it plays a role in decisions to participate in research, may exert its greatest impact for SCD patients when considering a specific research opportunity. Studies that examine SCD patient trust in medical researchers in addition to healthcare providers are much needed to help further evaluate these relationships.
The fact that patient engagement in self-care behaviors independently accounted for a portion of the variability in clinical trial attitudes is a particularly interesting finding. Patients who more frequently engaged in recommended self-care behaviors exhibited more positive attitudes about clinical trials. This suggests that participating in clinical trials may be an attempt to express some level of agency in fighting one’s disease. This attitude may be extremely important for an individual in the context of a disease that introduces a great deal of uncertainty and a great sense of “lacking control” in the lives of those affected by it.
Prior participation in clinical trials was found to be associated with more positive attitudes toward clinical trials. This same finding has been made for a variety of different patient populations, including among parents of children with SCD.9 This suggests that participating in a clinical trial can lead to a sense of familiarity that contributes to positive feelings about clinical trials going forward. Once they have participated, these patients may be more likely to participate again, suggesting that registries of SCD patients who have participated in clinical trials may serve as a useful resource for investigators.
Some important limitations of our study must be considered. Our data on clinical trial attitudes were collected from a group of SCD patients already enrolled and participating in an observational research study of SCD patient experiences in care. Although the parent study is not a clinical trial, this may be a self-selected group of patients who are already predisposed to having more positive attitudes. Nevertheless, we observed a very high response rate among the patients we approached for recruitment into our larger study. Further, the 291 participants enrolled in the IMPORT study comprise approximately 38% of the approximately 757 patients actively followed at our two study sites. These two facts considered together provide further support for our main finding and provide evidence in favor of the likely representativeness of our study participants when compared to SCD patients receiving care at other comprehensive care centers across the country. Therefore, while our respondents may not be representative of the wider SCD patient community overall, they are likely representative of those SCD patients across the country who have the most exposure to potential research opportunities given their affiliation with comprehensive care centers and academic medical centers. Because the attitudes we observed in this study were on average very high, our results may be subject to a ceiling effect that limits our ability to identify additional patient-level factors that may impact patient attitudes towards clinical trials. Even with this potential effect, though, we were still able to identify a number of factors with small, but statistically significant and important, effects on clinical trial attitudes. Finally, our outcome in this study was patient attitudes toward clinical trials in general. While our results shed light on SCD patient attitudes towards the clinical research enterprise, additional research must be conducted to explore the factors that affect SCD patient decision-making when considering actual and specific opportunities to participate in therapeutic clinical trials in the “real-world”.
Conclusions
Our results provide important insights into SCD patient attitudes towards clinical trials. We found that SCD patients have a high level of belief in the potential benefits of clinical trials in general, which is a known facilitator of research participation among African-American patient populations in general. There is an apparent disconnect, then, between the high level of endorsement of the potential benefits of clinical trials among SCD patients observed in our study, and the difficulties found in recruiting sufficient numbers of SCD patients for clinical trials that is observed in the SCD research community. This suggests to us that biomedical researchers need to pay close attention to the specific characteristics of particular clinical trial opportunities that may facilitate, or serve as barriers to, SCD patient participation. For example, there may be aspects to the ways that researchers tend to approach SCD patients with research opportunities that can be modified to improve patient participation. The inclusion/exclusion criteria of many SCD research opportunities may be too stringent given the goals of the study and the distribution of certain characteristics in the patient population. The design of SCD research studies must take into account the goals of the study, the characteristics of the population, and the fact that SCD is a rare condition in North America and Europe. Novel approaches may be needed in order to take these important contextual factors into account while attempting to maximize the number of SCD patients who could potentially take part in the research. Similarly, systems or resources that reduce the logistical burdens of trial participation, and that make trial participation more convenient for patients, need to be explored and implemented as a routine component of any SCD research trial.
The inadequate level of patient participation in clinical trials for SCD continues to be a major barrier to the progress of biomedical research for this patient population. The attitudes of the patient community toward clinical trials are known to serve as a major determinant of their participation in studies. We observed a great degree of positive attitudes about clinical trials generally from our SCD cohort, which should facilitate a high level of SCD patient enrollment into clinical studies. Our results suggest, then, that those who fund, design, and conduct SCD research need to turn the focus of their attention to other factors that may impact SCD patient participation in specific clinical trial opportunities.
Acknowledgements
The authors thank all members and participants of the IMPORT study for their contributions. The full list of IMPORT investigators includes the authors, as well as the following individuals – Johns Hopkins Investigators: C. Patrick Carroll, MD; Tanita Woodson, Jordan Wilks, Benjamin Ajiboye, Nina Shah. Howard University Investigators: Abiodun Akintilo; Margaret Fadojutimi-Akinsiku; Patricia Oneal, MD; Adriana Medina; Nouraie, Seyed- Mehdi; John Kwagyan; Kemi Owoyemi; and Ronke Ajala.
Study data were collected and managed using REDCap electronic data capture tools hosted at Johns Hopkins University. REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies, providing: 1) an intuitive interface for validated data entry; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for importing data from external sources.
Funding
This study was funded by a grant from the National, Heart, Lung and Blood Institute (NHLBI) (#1R01HL088511-01). Dr. Haywood’s effort was funded by a Career Development Award from the NHLBI (#1K01HL108832-01). Dr. Lanzkron’s effort was funded by a Career Development Award from the NHLBI (#K23HL083089). The funders played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Grant Support:
This study was funded by a grant from the National, Heart, Lung and Blood Institute [#1R01HL088511-01]. The efforts of Dr. Haywood and Dr. Lanzkron were funded by Career Development Awards from the National, Heart, Lung, and Blood Institute [Haywood: #1K01HL108832-01; Lanzkron: #K23HL083089]. The funders played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest Statement
The authors declare that there is no conflict of interest.
Contributor Information
Sophie Lanzkron, Email: slanzkr@jhmi.edu.
Marie Diener-West, Email: mdiener@jhsph.edu.
Jennifer Haythornthwaite, Email: jhaytho1@jhmi.edu.
John J. Strouse, Email: jstrous1@jhmi.edu.
Shawn Bediako, Email: bediako@umbc.edu.
Gladys Onojobi, Email: g_onojobi@Howard.edu.
Mary Catherine Beach, Email: mcbeach@jhmi.edu.
References
- 1.Hoots WK, Shurin SB. Future directions of sickle cell disease research: the NIH perspective. Pediatr Blood Cancer. 2012;59:353–357. doi: 10.1002/pbc.24180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaye CI, Committee on Genetics. Accurso F, et al. Newborn screening fact sheets. Pediatrics. 2006;118:e934–e963. doi: 10.1542/peds.2006-1783. [DOI] [PubMed] [Google Scholar]
- 3.Weatherall D. The inherited disorders of haemoglobin: an increasingly neglected global health burden. Indian J Med Res. 2011;134:493–497. [PMC free article] [PubMed] [Google Scholar]
- 4.Scott RB. Health care priority and sickle cell anemia. JAMA. 1970;214:731–734. [PubMed] [Google Scholar]
- 5.Smith LA, Oyeku SO, Homer C, et al. Sickle cell disease: a question of equity and quality. Pediatrics. 2006;117:1763–1770. doi: 10.1542/peds.2005-1611. [DOI] [PubMed] [Google Scholar]
- 6.Peters-Lawrence MH, Bell MC, Hsu LL, et al. Clinical trial implementation and recruitment: lessons learned from the early closure of a randomized clinical trial. Contemp Clin Trials. 2012;33:291–297. doi: 10.1016/j.cct.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.U.S. National Institutes of Health. ClinicalTrials.gov. [July 7, 2013]; http://clinicaltrials.gov.
- 8.Barakat LP, Patterson CA, Mondestin V, et al. Initial development of a questionnaire evaluating perceived benefits and barriers to pediatric clinical trials participation. Contemp Clin Trials. 2013;34:218–226. doi: 10.1016/j.cct.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Liem RI, Cole AH, Pelligra SA, et al. Parental attitudes toward research participation in pediatric sickle cell disease. Pediatr Blood Cancer. 2010;55:129–133. doi: 10.1002/pbc.22450. [DOI] [PubMed] [Google Scholar]
- 10.Jones FC, Broome ME. Focus groups with African American adolescents: enhancing recruitment and retention in intervention studies. J Pediatr Nurs. 2001;16:88–96. doi: 10.1053/jpdn.2001.23151. [DOI] [PubMed] [Google Scholar]
- 11.Wynn L, Miller S, Faughnan L, et al. Recruitment of infants with sickle cell anemia to a Phase III trial: data from the BABY HUG study. Contemp Clin Trials. 2010;31:558–563. doi: 10.1016/j.cct.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nield-Anderson L, Dixon JK, Lee K. Random assignment and patient choice in a study of alternative pain relief for sickle cell disease. West J Nurs Res. 1999;21:266–274. doi: 10.1177/01939459922043767. [DOI] [PubMed] [Google Scholar]
- 13.Omondi NA, Ferguson SE, Majhail NS, et al. Barriers to hematopoietic cell transplantation clinical trial participation of african american and black youth with sickle cell disease and their parents. J Pediatr Hematol Oncol. 2013;35:289–298. doi: 10.1097/MPH.0b013e31828d5e6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shavers-Hornaday VL, Lynch CF, Burmeister LF, et al. Why are African Americans under-represented in medical research studies? Impediments to participation. Ethn Health. 1997;2:31–45. doi: 10.1080/13557858.1997.9961813. [DOI] [PubMed] [Google Scholar]
- 15.Schmotzer GL. Barriers and facilitators to participation of minorities in clinical trials. Ethn Dis. 2012;22:226–230. [PubMed] [Google Scholar]
- 16.Rivers D, August EM, Sehovic I, et al. A systematic review of the factors influencing African Americans' participation in cancer clinical trials. Contemp Clin Trials. 2013;35:13–32. doi: 10.1016/j.cct.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 17.Rivera-Goba MV, Dominguez DC, Stoll P, et al. Exploring decision-making of HIV-infected Hispanics and African Americans participating in clinical trials. J Assoc Nurses AIDS Care. 2011;22:295–306. doi: 10.1016/j.jana.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corbie-Smith G, Thomas SB, Williams MV, et al. Attitudes and beliefs of African Americans toward participation in medical research. J Gen Intern Med. 1999;14:537–546. doi: 10.1046/j.1525-1497.1999.07048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kennedy BM, Burnett MF. Clinical research trials: a comparison of African Americans who have and have not participated. J Cult Divers. 2002;9:95–101. [PubMed] [Google Scholar]
- 20.Kennedy BM, Burnett MF. Clinical research trials: factors that influence and hinder participation. J Cult Divers. 2007;14:141–147. [PubMed] [Google Scholar]
- 21.Edwards R, Telfair J, Cecil H, et al. Reliability and validity of a self-efficacy instrument specific to sickle cell disease. Behav Res Ther. 2000;38:951–963. doi: 10.1016/s0005-7967(99)00140-0. [DOI] [PubMed] [Google Scholar]
- 22.Lenoci JM, Telfair J, Cecil H, et al. Self-care in adults with sickle cell disease. West J Nurs Res. 2002;24:228–245. doi: 10.1177/01939450222045879. [DOI] [PubMed] [Google Scholar]
- 23.Hays RD, Shaul JA, Williams VS, et al. Psychometric properties of the CAHPS 1.0 survey measures. Consumer Assessment of Health Plans Study. Med Care. 1999;37:MS22–MS31. doi: 10.1097/00005650-199903001-00003. [DOI] [PubMed] [Google Scholar]
- 24.Stewart AL, Napoles-Springer A, Perez-Stable EJ. Interpersonal processes of care in diverse populations. Milbank Q. 1999;77:305–339. 274. doi: 10.1111/1468-0009.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stewart AL, Napoles-Springer AM, Gregorich SE, et al. Interpersonal processes of care survey: patient-reported measures for diverse groups. Health Serv Res. 2007;42:1235–1256. doi: 10.1111/j.1475-6773.2006.00637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dugan E, Trachtenberg F, Hall MA. Development of abbreviated measures to assess patient trust in a physician, a health insurer, and the medical profession. BMC Health Serv Res. 2005;5:64. doi: 10.1186/1472-6963-5-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rose A, Peters N, Shea JA, et al. Development and testing of the health care system distrust scale. J Gen Intern Med. 2004;19:57–63. doi: 10.1111/j.1525-1497.2004.21146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen J, Cohen P. Applied multiple regression/correlation analysis for the behavioral sciences. 2 ed. Hillsdale, N.J.: Hillsdale, N.J.: 1983. p. 545. [Google Scholar]
- 29.StataCorp. Stata Statistical Software: Release 12. 2011 [Google Scholar]
- 30.Haywood C, Jr, Lanzkron S, Ratanawongsa N, et al. The association of provider communication with trust among adults with sickle cell disease. J Gen Intern Med. 2010;25:543–548. doi: 10.1007/s11606-009-1247-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haywood C, Jr, Lanzkron S, Ratanawongsa N, et al. Hospital self-discharge among adults with sickle-cell disease (SCD): associations with trust and interpersonal experiences with care. J Hosp Med. 2010;5:289–294. doi: 10.1002/jhm.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elander J, Beach MC, Haywood C. Respect, trust, and the management of sickle cell disease pain in hospital: comparative analysis of concern-raising behaviors, preliminary model, and agenda for international collaborative research to inform practice. Ethn Health. 2011;16:405–421. doi: 10.1080/13557858.2011.555520. [DOI] [PMC free article] [PubMed] [Google Scholar]


