Abstract
BACKGROUND
Increasing evidence suggests that chronic ethanol exposure decreases dopamine (DA) neurotransmission in the nucleus accumbens (NAc), contributing to a hypodopaminergic state during withdrawal. However, few studies have investigated adaptations in presynaptic DA terminals after chronic intermittent ethanol (CIE) exposure. In monkeys and rats, chronic ethanol exposure paradigms have been shown to increase DA uptake and D2 autoreceptor sensitivity.
METHODS
The current study examined the effects of ethanol on DA terminals in CIE exposed mice during two time-points after the cessation of CIE exposure. DA release and uptake were measured using fast scan cyclic voltammetry in NAc core slices from C57BL/6J mice, 0 and 72 hours following three weekly cycles (4 days of 16 hrs ethanol vapor/8 hrs room air/day + 3 days withdrawal) of CIE vapor exposure.
RESULTS
Current results showed that DA release was reduced, uptake rates were increased, and inhibitory D2-type autoreceptor activity was augmented following CIE exposure in mice.
CONCLUSIONS
Overall, these CIE-induced adaptations in the accumbal DA system reduce DA signaling and therefore reveal several potential mechanisms contributing to a functional hypodopaminergic state during alcohol withdrawal.
Keywords: Chronic ethanol, Dopamine, Nucleus accumbens, Mouse
1. INTRODUCTION
In the nucleus accumbens (NAc), acute administration of ethanol elevates dopamine (DA) levels (Yim et al., 1998, 2000; Tang et al., 2003), an effect that has been implicated in its rewarding properties (Wise, 2004). As with many drugs of abuse, chronic or repeated exposure to ethanol produces compensatory neuroadaptations that oppose acute drug effects, normalizing function during intoxication, but resulting in below-normal function in the absence of the drug (Koob, 2003). Following chronic ethanol exposure, the mesolimbic DA system is fundamentally hypoactive. For example, dopaminergic neurons in the VTA of ethanol-dependent rats exhibit reduced tonic and burst firing rates (Diana et al., 1993; Bailey et al., 2001; Shen, 2003; Shen et al., 2007) during withdrawal. Animals exposed to chronic ethanol also have decreased expression of tyrosine hydroxylase (TH) coupled with augmented levels of DA transporter (DAT) in the striatum (Rothblat et al, 2001; Healey et al 2008), consistent with an attenuation in DA signaling. Together, these observations suggest that there are multiple functional changes in DA regulation that reduce signaling during ethanol withdrawal. However, to date, there have been no studies examining the changes in DA uptake in the NAc of a mouse model of ethanol dependence.
In addition to DATs, presynaptic DA signaling is also regulated by D2 autoreceptors which inhibit DA release (Phillips et al., 2002) and synthesis (Wolf and Roth, 1990) while facilitating DA uptake (Wu et al., 2002; Garris et al., 2003). Previous studies have revealed sensitization of D2 autoreceptors following chronic exposure to ethanol. For example, chronic ethanol intake in monkeys results in supersensitivity of release-regulating D2 autoreceptors (Budygin et al., 2003). In rats, chronic oral ethanol intake has been shown to increase total (pre- and post-synaptic) D2 receptor density in the NAc (Kim et al., 1997; Djouma and Lawrence, 2002; Sari et al., 2006). However, a previous study from our lab showed that 10 days of intermittent ethanol exposure (CIE) in rats did not affect functional D2 autoreceptor sensitivity (Budygin et al., 2007). In mice, sensitizing regimens of intraperitoneal ethanol injections have been shown to increase the function of D2 receptors in the NAc (Souza-Formigoni et al., 1999; Abrahao et al., 2012). Although there is evidence for supersensitivity of pre- and post-synaptic D2 receptors following chronic ethanol exposure, the goal of the current study, was to determine the changes specifically in presynaptic D2 autoreceptor function following repeated CIE exposure in mice.
A CIE vapor exposure paradigm for mice was developed by Becker and colleagues as a means to quantify behavioral and biochemical changes associated with chronic ethanol exposure (Becker and Lopez, 2004; Lopez and Becker 2005, Griffin et al, 2009). For example, in C57 mice, this CIE exposure/withdrawal paradigm produces: (1) behavioral sensitization to ethanol (Zapata et al., 2006), (2) handling-induced seizures during withdrawal (Becker and Hale, 1993; Metten et al., 2010), (3) increased ethanol drinking (Becker and Lopez, 2004; Lopez and Becker, 2005; Griffin et al., 2009) and (4) blunted DA responses to ethanol during withdrawal (Zapata and Shippenberg, 2006; Zapata et al., 2006). Over the past decade, the ethanol field has used C57 mice as a model of dependence to study the neurobiological and behavioral effects of chronic exposure to ethanol due to their propensity to voluntarily consume large amounts of ethanol (Yoneyama et al., 2008). However, while the behavioral effects of CIE are well documented, studies investigating the neurobiological underpinnings of these behavioral manifestations are limited, especially in the NAc (Zapata and Shippenberg, 2006; Zapata et al., 2006; Jeanes et al., 2011; Griffin et al., 2014).
In the present study, ex vivo fast-scan cyclic voltammetry was used to examine the effects of repeated CIE exposure on presynaptic DA release and uptake in the core of the NAc, as well as changes in the functional sensitivity of D2 autoreceptors, at two different times after the cessation of CIE exposure (0 and 72 hour time-points). The primary goal of these experiments was to identify CIE-induced changes in presynaptic DA signaling that may underlie reduced DA system function in the NAc during withdrawal in C57 mice.
2. MATERIALS AND METHODS
2.1 Animals
Male C57BL/6 mice (8 –12 weeks; Jackson Laboratories, Bar Harbor, ME) were used for all experiments. Animals were housed in groups of three to four per cage with food and water ad libitum (12-hr light-dark cycle). Experimental protocols adhered to National Institutes of Health Animal Care guidelines and were approved by the Wake Forest University Institutional Animal Care and Use Committee.
2.2 CIE and Withdrawal
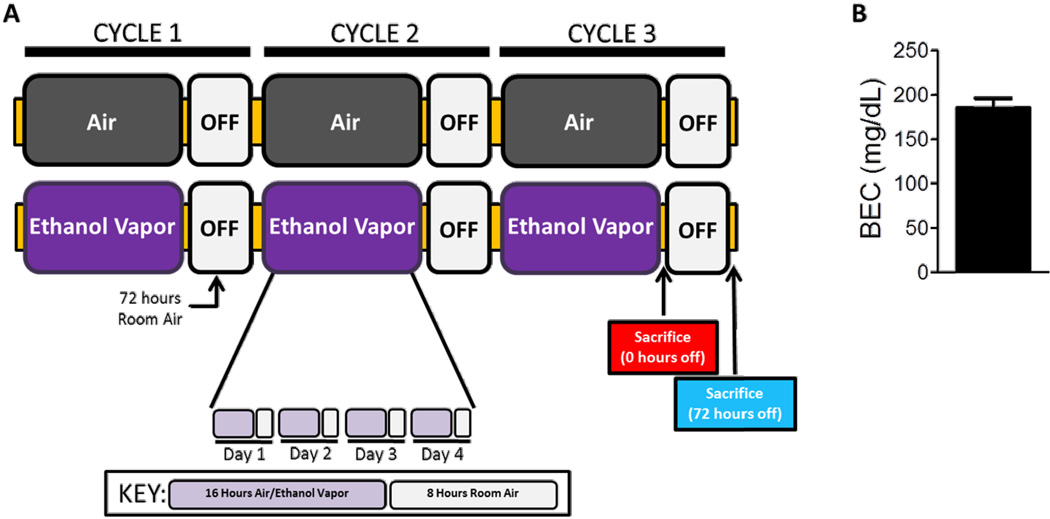
The general design of the repeated ethanol exposure and withdrawal paradigm was adapted from Becker and colleagues (Becker, 1994; Becker et al, 1997) with the following modifications. Mice were assigned to either control or ethanol exposure groups. The ethanol group underwent 16 hours of continuous ethanol vapor exposure followed by 8 hours off in room air each day for four days, followed by three days of abstinence (1 cycle; Fig 1A). This procedure was repeated three times for a total of 3 cycles. A loading dose of 1.0 g/kg ethanol (20% w/v, CIE group) or saline (Air group) and the alcohol dehydrogenase inhibitor, pyrazole (85 mg/kg) in 0.9% saline was administered i.p. prior to entering the ethanol vapor or air inhalation chamber. Ethanol vapor was delivered to the ethanol inhalation chamber by volatilizing 190 proof ethanol and mixing the ethanol vapor with fresh air at a rate of 10 L/min. The concentration of ethanol in the chamber was monitored at the completion of each cycle by taking a 2 mL air sample through a port in the chamber door.
Figure 1.
Chronic intermittent ethanol (CIE) exposure paradigm and blood ethanol concentrations (BECs). (A) CIE experimental procedure. Mice were exposed to four “cycles” of CIE, each consisting of ethanol vapor or room air inhalation for 16h/day with 8 hours of abstinence for four days, followed by 72 hours of room air exposure. Cycles were repeated three times. Mice were sacrificed either immediately after cessation of third cycle (0 hour time-point) or at 72 hours after cessation of the third cycle (72 hour time-point). (B) Blood ethanol concentrations (BEC) were evaluated to ensure that physiologically and behaviorally relevant BECs were achieved and maintained throughout the experiment. Mean BEC were 185.1 ±11.3 mg/dl.
2.3 Blood Ethanol Concentrations
Blood ethanol concentrations (BEC) were measured at the end of each cycle to ensure physiologically and behaviorally relevant BECs (Griffin et al., 2009). Blood samples were collected from the mice using the submandibular vein punch immediately after removal from the ethanol vapor chamber. Blood samples (5 µL) were mixed with 0.38 N trichloroacetic acid (10 µL) and centrifuged at 12,000 g. The supernatant was used in a spectrophotometric enzymatic assay (229-29; Sekisui Diagnostics LLC, Lexington, MA). The mean BEC for the mice exposed to three cycles of the ethanol vapor was 185.1 ± 11.3 mg/dl (mean ± SEM; Fig. 1B).
2.4 Tissue preparation
Immediately upon completion of the vapor exposure (or following 72 hours of abstinence), mice were sacrificed by decapitation and brains were rapidly removed and transferred into ice-cold, pre-oxygenated (95% O2/5% CO2) artificial cerebral spinal fluid (aCSF) consisting of (in mM): NaCl (126), KCl (2.5), NaH2PO4 (1.2), CaCl2 (2.4), MgCl2 (1.2), NaHCO3 (25), glucose (11), L-ascorbic acid (0.4) and pH was adjusted to 7.4. The brain was sectioned into 400 µm-thick coronal slices containing the striatum with a vibrating tissue slicer (Leica VT1000S, Vashaw Scientific, Norcross, GA) and transferred to a submersion recording chamber perfused at 1 ml/min at 32 °C with oxygenated aCSF.
2.5 Fast Scan Cyclic Voltammetry
Following an equilibration period (30-min), a carbon fiber microelectrode (approximately 150 µM length, 7 µM radius) and a bipolar stimulating electrode (Plastics One, Roanoke, VA) were placed in close proximity to each other (approximately 100 µM apart) into the NAc core. DA was evoked by a single, rectangular, electrical pulse (350 µA, 4 ms) applied every 5 min. Changes in current were monitored every 100 ms using fast-scan cyclic voltammetry (Kennedy et al., 1992) by applying a triangular waveform (−0.4 to +1.2 to −0.4 V vs Ag/AgCl, 400 V/s). One slice was used per animal per experiment. Once the extracellular DA response was stable (variation between peak height data did not exceed 10%), quinpirole, a D2/D3 autoreceptor agonist, was added cumulatively to the bath (0.1–1.0µM). Immediately following the completion of each experiment, recording electrodes were calibrated by recording their response (in current; nA) to 3 µM DA in aCSF using a flow-injection system. Baseline measures were made using averaged data from all slices, with statistical outliers removed. For autoreceptor studies, only data with signals that allowed modeling through the entire cumulative dose curve were included.
To determine kinetic parameters, evoked levels of DA were modeled using Michaelis–Menten kinetics, as a balance between release and uptake (Wightman et al., 1988). Michaelis–Menten modeling of baseline DA signals provides parameters that describe the amount of DA released following stimulation (i.e., the peak height of the signal) and the maximal rate of DA uptake (Vmax). For baseline modeling, we followed standard voltammetric modeling procedures by setting the apparent Km value to 160 nM for each animal based on well-established research on the affinity of DA for the DAT (Wu et al., 2001), whereas baseline Vmax values were allowed to vary as the maximal rate of DA uptake. All voltammetry data were collected and modeled using Demon Voltammetry and Analysis Software (Yorgason et al., 2011).
2.6 Chemicals and Drugs
Components of the artificial cerebrospinal fluid and neurotransmitter standards were of the highest quality obtainable from Sigma-Aldrich (St. Louis, Missouri). Quinpirole, a D2/D3 autoreceptor agonist, was obtained from Sigma-Aldrich (St. Louis, Missouri).
2.7 Statistics
All statistical analyses were conducted using GraphPad Prism 5 (GraphPad Software, La Jolla, CA). Baseline DA release and uptake measures were compared using a one-way ANOVA followed by Dunnett’s post-hoc analysis. Autoreceptor sensitivity was assessed using two-way repeated measures ANOVA with Bonferroni’s post hoc test. The EC50 of quinpirole was quantified using the log[agonist] vs. response calculation for each treatment group. A one-way ANOVA was used to statically test the change in potency of quinpirole following CIE exposure. The significance level was set at p<0.05.
3. RESULTS
3.1 CIE exposure reduces electrically stimulated DA release in the NAc
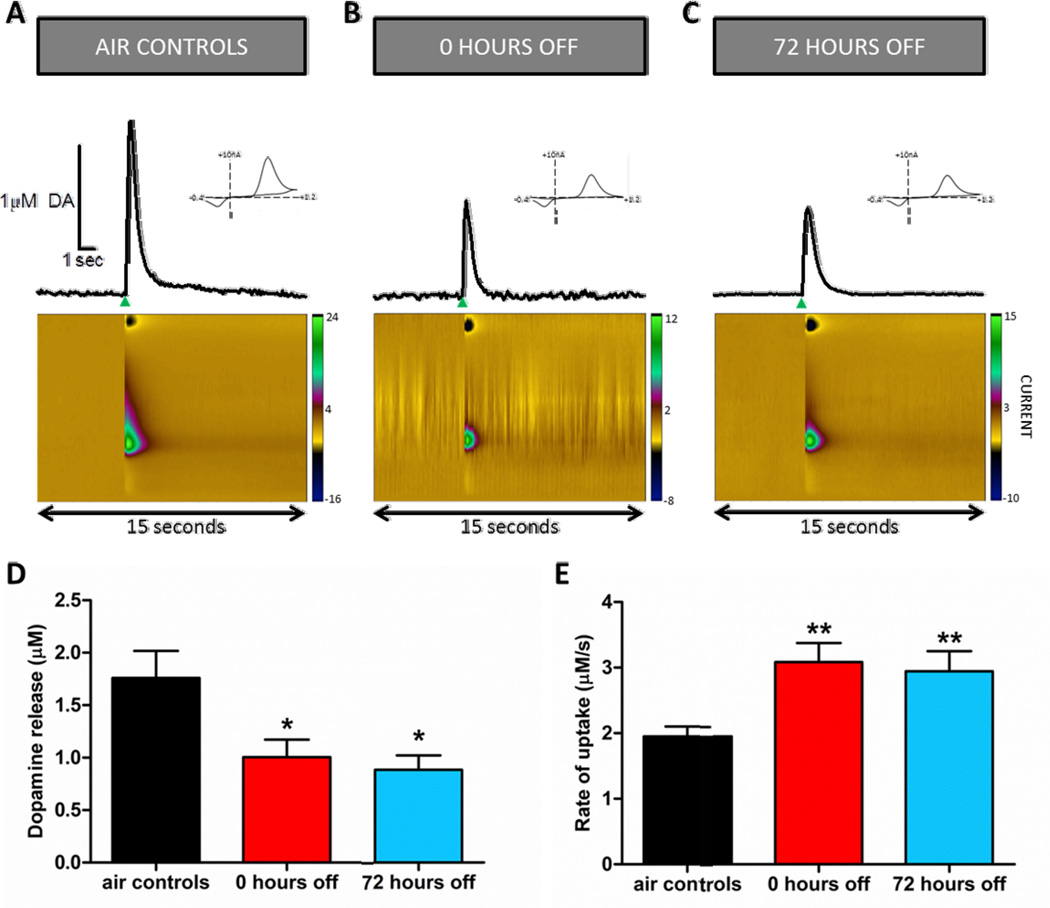
Chronic ethanol exposure alters electrically stimulated DA release in the NAc compared to control animals (Fig. 2). Electrically stimulated DA release traces show a decrease in DA release, shown by the decreased peak of the representative DA trace, during the 0 and 72 hour abstinence time points compared to control animals (Fig. 2A–C). A one-way ANOVA revealed a significant difference in DA release ([DA]/pulse; Fig. 2D; F(2,16)=5.32, p<0.05). Dunnett’s multiple comparison post-hoc analysis showed that DA release measured at the 0 (p<0.05) and 72 (p<0.05) hour time-points following CIE exposure was significantly decreased compared to release in control animals. There was no significant difference between the 0 and 72 hour time-points.
Figure 2.
Chronic intermittent ethanol (CIE) exposure drives decreased dopamine (DA) system signaling. Representative voltammetric curves illustrating electrically stimulated DA release (A) control mice (air exposed) and mice treated with CIE exposure at the (B) 0 hour and (C) 72 hour time-points. The green arrow represents the electrical stimulus. Representative traces of electrically stimulated DA release and uptake in control and ethanol exposed animals measured by voltammetry in NAc brain slices shows faster uptake and reduced release after ethanol exposure. The single pulse stimulation occurs at the time of the upward inflection of the curve, when DA is released. False color plots demonstrate changes in current over time and in response to electrically evoked DA release (X-axis: time, Y-axis: command voltage (scanned from −0.4 to +1.2V), Z-axis: current). Cyclic voltammogram (insets), demonstrating the peak oxidation (0.6V) and reduction (−0.2V) voltage indicate that the evoked signal was DA. (D) Chronic ethanol (0 hour time-point, red bar, n=7; 72 hour time-point, blue bar, n=5) significantly reduced electrically stimulated DA release ([DA]/pulse) as compared to control animals (black bar, n=7). (E) Chronic ethanol significantly increases the maximal rate of DA uptake (Vmax; 0 hour time-point, red bar, n=12; 72 hour time-point, blue bar, n=12) as compared to control animals (black bar, n=22). **p<0.01; *p<0.05.
3.2 Maximal rate of uptake at the DAT increased after CIE
The maximal rate of DA uptake (Vmax) was measured using ex vivo voltammetry. Chronic ethanol exposure resulted in a significant increase (F(2,43)=8.32, p<0.001) in Vmax (Fig. 2E). Post-hoc analysis showed increased uptake at both the 0 (p<0.05) and 72 (p<0.05) hour time points as compared to control animals. There was no significant difference in Vmax between the 0 and 72 hour time-points.
3.3 Increased D2 autoreceptor function immediately after CIE exposure
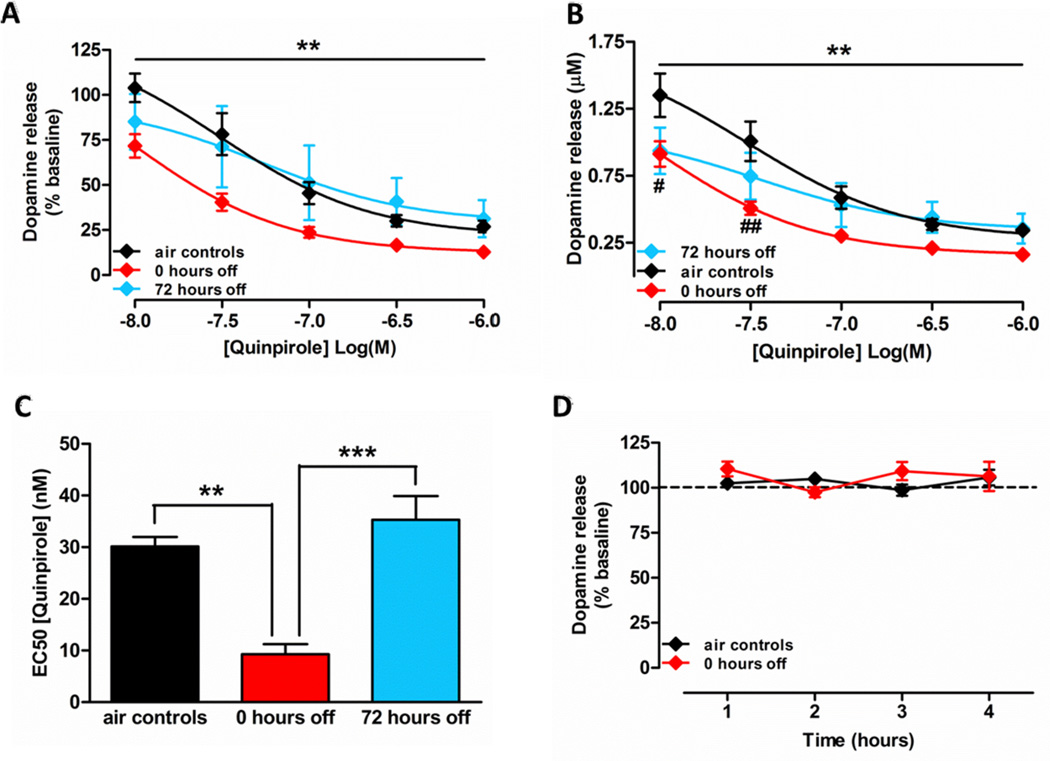
In order to examine the effects of CIE exposure on D2 receptors, quinpirole, a D2/D3 autoreceptor agonist was bath applied to NAc slices. DA release was significantly attenuated following the application of quinpirole. An overall interaction between concentration of quinpirole and chronic ethanol or air treatment was observed across the three treatment groups as measured by percent change from baseline DA release (Fig. 3A; F(8,40)=3.18, p<0.01). DA release was decreased in a concentration-dependent manner across all three treatment groups (F(4,10)=130.7, p<0.001).
Figure 3.
Chronic ethanol increases D2 autoreceptor sensitivity. (A) Percent change in quinpirole-mediated DA release. A two-way repeated measures ANOVA comparing the control (black symbols; n=4), 0 hour abstinent (red symbols; n=5), and 72 hour abstinent (blue symbols; n=4) animals revealed a significant interaction of concentration and treatment. (B) Representation of quinpirole-mediated absolute DA release. DA release was concentration-dependently reduced by autoreceptor activation in all groups. However, post hoc analysis revealed a significant supersensitivity in response in 0 hour time-point animals as compared to controls. D2 autoreceptor activity measured after 72 hours of abstinence showed no significant difference when compared to controls or the 0 hour time-point animals. (C) Potency of quinpirole increased transiently after CIE exposure. A significant increase in potency was observed at the 0 hour time-point. (D) Stimulated DA release was not altered over a period of four hours in NAc slices of air exposed control animals and CIE exposed animals at the 0 hour time-point (n=4 in both groups). ***p<0.001; **p<0.01; *p<0.05.
An increased sensitivity of terminal-release regulating DA autoreceptors in ethanol exposed animals at the 0 hour time-point was observed. This supersensitive response was not present following 72 hours of withdrawal. A two-way repeated measures ANOVA revealed a significant interaction of concentration and treatment, (Fig. 3B; F(8,40)=3.36, p<0.01); with a significant concentration-dependent decrease in DA release across the three groups (F(4,10)=112.1, p<0.001). Post-hoc pairwise comparisons (Bonferroni) showed significant differences at the 0.01 (p<0.05) and 0.03 (p<0.01) µM concentrations of quinpirole in accumbal brain slices from ethanol exposed animals at the 0 hour time-point.
A one-way ANOVA revealed a significant effect of CIE exposure on the EC50 of quinpirole (Fig. 3C; F(2,10)=23.48, p<0.001). Specifically, the concentration at which the effect of quinpirole was 50% of its maximum decreased immediately after the cessation of CIE exposure (0 hour time-point), indicating greater autoreceptor sensitivity to inhibition. However, 72 hours after the cessation of CIE exposure, the EC50 of quinpirole returned to the EC50 observed in air exposed control animals. Thus, a significant reduction in EC50 concentration was observed in a comparison between the 0 hour time-point and air controls (p<0.01), and the 0 hour and 72 hour time-points (p<0.001).
In order to verify that the quinpirole-induced decrease in DA release was a true effect of the drug and not a non-specific decrease in release over several hours in the slice chamber, DA release was measured in NAc slices of air exposed control animals and CIE exposed animals at the 0 hour time-point for four hours (Fig. 3D). No significant effect of time was found between the two groups (p>0.05). Because no significant difference was observed between air exposed control animals and CIE exposed animals after 72 hours in the quinpirole experiments, control data were not collected for this group.
4. DISCUSSION
CIE vapor exposure increases voluntary ethanol consumption in C57 mice (Becker and Lopez, 2004; Lopez and Becker, 2005; Griffin et al., 2009); however, the neurobiological underpinnings of these changes are not well understood. Previous literature suggests that increased ethanol intake in dependent animals may be related to altered DA neurotransmission (Budygin et al 2003; Carroll et al, 2006). Therefore, we examined CIE-induced changes in DA signaling in the NAc core using ex vivo fast scan cyclic voltammetry. The current data showed that CIE exposure resulted in decreased accumbal DA system function characterized by attenuated DA release, augmented DA uptake, and increased D2 autoreceptor sensitivity.
4.1 CIE-mediated changes in DA transmission
CIE exposure resulted in decreased presynaptic DA signaling. Specifically, electrically stimulated DA release was decreased at both the 0 and 72 hour time-points after CIE exposure. These results are consistent with previous literature showing decreased tonic and phasic activity of DA neurons in the VTA of ethanol-dependent animals (Diana et al., 1993; Bailey et al., 2001; Shen, 2003; Shen et al., 2007). The DA release measured in the current study using voltammetry is stimulated, phasic release, which is involved in regulating motivated behaviors (Ostlund et al., 2014).
DA uptake was increased at both the 0 and 72 hour time-points after CIE exposure in the current study. One of the major regulators of extracellular DA levels is DA uptake through DATs. Slice voltammetry studies have shown that chronic exposure to ethanol increases DA uptake in the NAc in monkeys and rats, potentially as a compensatory mechanism to reduce chronically elevated DA levels. An enhancement of DA uptake was observed in monkeys exposed to a long-term (18 months) voluntary ethanol consumption paradigm (Budygin et al., 2003). Similarly, a 10 day intermittent ethanol vapor exposure in rats resulted in an augmentation of DA uptake rates (Budygin et al, 2007). Additonally, chronic ethanol has been shown to increase synaptosomal DA uptake rates in rats (Carroll et al., 2006) and repeated ethanol administration has been observed to increase DAT binding sites in mice (Itzhak and Martin, 1999), potentially increasing DAT function. Thus, it appears that chronic ethanol reliably produces withdrawal-associated increases in DA uptake.
4.2 CIE-induced supersensitivity of D2 autoreceptors
Chronic ethanol exposure has been shown to increase the responsiveness of release-regulating D2 autoreceptors in monkeys (Budygin et al., 2003) and mice (Perra et al., 2011), but not in rats (Budgyin et al., 2007; Sari et al., 2006, but see Kim et al., 1997). In rats, DA autoreceptor responses were either unchanged (Budgyin et al., 2007; Sari et al., 2006) or total D2 receptor expression levels were increased (Kim et al., 2007) following chronic ethanol exposure. Data presented here suggest that autoreceptor sensitivity is transiently increased at the 0 hour time-point in mice, with functionality returning to control levels following 72 hours of ethanol withdrawal. The transient nature of this change, species, and differences in CIE exposure protocols used may account for the lack of consistency in the literature.
Pre-synaptic D2 autoreceptors are involved in the regulation of DA synthesis and release via a negative feedback mechanism (Wolf and Roth, 1990; Missale et al., 1998; Phillips et al., 2002). D2 autoreceptors are coupled to Gi/o and inhibit adenylyl cyclase and calcium channels (Ford, 2014). Furthermore, these autoreceptors inhibit vesicular DA release through hyperpolarization of terminals by activating G-protein-activated inwardly rectifying potassium channels (Beaulieu and Gainetdinov, 2011; Ford, 2014). In the current study, CIE exposure-induced D2 autoreceptor supersensitivity could, at least in part, explain the decrease in electrically stimulated DA release. While it is plausible that post-synaptic D2 receptors may influence DA release, an ex vivo voltammetry study shows that, unlike in wild-type mice, DA release is unaffected by quinpirole in D2 autoreceptor knockout mice (Bello et al., 2011). Thus the quinpirole-induced augmented decrease in DA release observed at the 0 hour time-point in the current study is likely due to D2 autoreceptor supersensitivity.
D2 autoreceptors are primarily involved in the inhibition of DA release, however, these autoreceptors can facilitate DA uptake via an increase in rate of uptake per transporter (Cass and Gerhardt, 1994; Wu et al., 2002; Phillips et al., 2003). In accumbal slices, a single pulse electrical stimulation does not release enough DA to alter DA uptake via a D2-mediated mechanism; higher frequency stimulation is necessary to induce D2-mediated changes in DA uptake (Benoit-Marand et al., 2001; Phillips et al., 2003; Benoit-Marand et al., 2011). However, the current data show that CIE exposure results in an enhanced sensitivity of D2 autoreceptors. Therefore, it is possible that CIE exposure amplifies the effects of D2 receptors on DA uptake and thus single pulse stimulations in the NAc of CIE exposed animals may result in activation of D2 autoreceptors, potentially contributing to the observed augmentation of uptake rates specifically during the 0 hour time-point. While the decreased release and increased uptake observed at the 0 hour time-point may be explained by D2 supersensitivity, at the 72 hour time-point these changes may be due to a different mechanism entirely, or may be a persistent result driven by transient increases in D2 receptor sensitivity. Thus, the supersensitivity of D2 autoreceptors following ethanol administration may, in part, explain the attenuated DA release and enhanced DA uptake observed in the current study.
4.3 DA transmission and ethanol reward processing
Based on work from several laboratories, DA neurotransmission is known to regulate motivational states, thus affecting ethanol intake. Specifically, the state of hypodopaminergia has been linked to low motivational states and anhedonia (Trevisan et al., 1998; Melis et al., 2005; Koob and Volkow, 2010). Moreover, ethanol-preferring rats have lower DA innervation (measured by TH immunostaining) in the NAc than non-preferring rats (Casu et al., 2002a, 2002b) and therefore a lower content of DA (McBride et al., 1991, 1995), suggesting that a low-DA state may drive high ethanol intake. Additionally, mice and rats exposed chronically to ethanol vapor drink more ethanol compared to animals exposed to air (Gilpin et al., 2008; Lopez and Becker, 2005; Griffin et al., 2009). Based on the alterations in DA signaling observed in the current study, it is possible that reduced DA transmission following ethanol dependence or CIE exposure drives the augmented ethanol drinking in dependent animals in order to alleviate the symptoms produced by hypodopaminergia during withdrawal. Taken together, neurobiological data from the current and previous studies, and post-CIE exposure behavioral data suggest that DA in the NAc contributes to a negative affective state during withdrawal, and acute ethanol-induced increases in DA levels may alleviate that state.
4.4 Conclusion
Despite decades of research, the neurochemical underpinnings of alcohol use disorders remain unclear. Because the striatal DA system is an important site of action for many drugs of abuse (Calipari and Jones, 2014; Calipari et al., 2014), including ethanol (Yim et al., 1998), we examined changes in presynaptic DA kinetics in a mouse model of dependence (Becker and Hale, 1993). In summary, our data show that CIE produces a pattern of presynaptic DA signaling changes, including decreased DA release, increased DA uptake, and D2 autoreceptor supersensitivity, resulting in functional hypodopaminergia during withdrawal. This hypodopaminergic state may drive withdrawal symptoms, such as anhedonia, that lead to relapse drinking behaviors in rodent models and potentially in humans.
HIGHLIGHTS.
The following are the most important findings of the current study:
Chronic intermittent ethanol (CIE) exposure results in decreased dopamine release in the nucleus accumbens of mice. This decrease in stimulated dopamine release was observed at the 0 hours off time point (immediately after cessation of CIE exposure) and at the 72 hours off time point (after 72 hours of withdrawal).
CIE exposure increased dopamine uptake rate at the dopamine terminals at the 0 and 72 hours off time points.
The current data showed that potency of quinpirole increased during the 0 hours off time point. However no significant difference between quinpirole potency was found between the air-exposed control animals and CIE exposed animals at the 72 hours off time point. These data suggest that D2 receptors were supersensitive transiently after CIE exposure.
Acknowledgments
Role of funding source:
This work was funded by NIH grants U01 AA014091, P01 AA021099 (SRJ), T32 AA007565-21 (ANK, KNH, JHR), and F31 DA035558 (JHR). None of the funding sources had any involvement in study design, collection, analysis and interpretation of the data, in writing of the manuscript, and decision to submit for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors:
ANK, JHR, KNH, and SRJ were responsible for the study concept and design. JHR, KNH, and JKK conducted all the experiments. ANK, JHR, KNH, and JKK analyzed these data. ANK drafted the manuscript. JHR and SRJ provided critical revision of the manuscript for important intellectual content. All authors critically reviewed content and approved final version for publication.
Conflict of interest:
No conflict to declare.
REFERENCES
- Abrahao KP, Quadros IM, Andrade AL, Souza-Formigoni ML. Accumbal dopamine D2 receptor function is associated with individual variability in ethanol behavioral sensitization. Neuropharmacology. 2012;62:882–889. doi: 10.1016/j.neuropharm.2011.09.017. [DOI] [PubMed] [Google Scholar]
- Bailey CP, O’Callaghan MJ, Croft AP, Manley SJ, Little HJ. Alteration in mesolimbic dopamine function during the abstinence period following chronic ethanol consumption. Neuropharmacology. 2001;41:989–999. doi: 10.1016/s0028-3908(01)00146-0. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol. Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- Becker HC. Positive relationship between the number of prior ethanol withdrawal episodes and the severity of subsequence withdrawal seizures. Psychopharmacology. 1994;116:26–32. doi: 10.1007/BF02244867. [DOI] [PubMed] [Google Scholar]
- Becker HC, Hale RL. Repeated episodes of ethanol withdrawal potentiate the severity of subsequent withdrawal seizures: an animal model of alcohol withdrawal "kindling". Alcohol. Clin. Exp. Res. 1993;17:94–98. doi: 10.1111/j.1530-0277.1993.tb00731.x. [DOI] [PubMed] [Google Scholar]
- Becker HC, Diaz-Granados JL, Weathersby RT. Repeated ethanol withdrawal experience increases the servity and duration of subsequent withdrawal seizures in mice. Alcohol. 1997;14:319–326. doi: 10.1016/s0741-8329(97)87949-9. [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol. Clin. Exp. Res. 2004;28:1829–1838. doi: 10.1097/01.alc.0000149977.95306.3a. [DOI] [PubMed] [Google Scholar]
- Bello EP, Mateo Y, Gelman DM, Noaín D, Shin JH, Low MJ, Alvarez VA, Lovinger DM, Rubinstein M. Cocaine supersensitivity and enhanced motivation for reward in mice lacking dopamine D2 autoreceptors. Nat. Neurosci. 2011;14:1033–1038. doi: 10.1038/nn.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit-Marand M, Borrelli E, Gonon F. Inhibition of dopamine release via presynaptic D2 receptors: time course and functional characteristics in vivo. J. Neurosci. 2001;21:9134–9141. doi: 10.1523/JNEUROSCI.21-23-09134.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit-Marand M, Ballion B, Borrelli E, Boraud T, Gonon F. Inhibition of dopamine uptake by D2 antagonists: an in vivo study. J. Neurochem. 2011;116:449–458. doi: 10.1111/j.1471-4159.2010.07125.x. [DOI] [PubMed] [Google Scholar]
- Blanchard BA, Steindorf S, Wang S, Glick SD. Sex differences in ethanol-induced dopamine release in nucleus accumbens and in ethanol consumption in rats. Alcohol. Clin. Exp. Res. 1993;17:968–973. doi: 10.1111/j.1530-0277.1993.tb05650.x. [DOI] [PubMed] [Google Scholar]
- Brodie MS, Appel SB. Dopaminergic neurons in the ventral tegmental area of C57BL/6J and DBA/2J mice differ in sensitivity to ethanol excitation. Alcohol. Clin. Exp. Res. 2000;24:1120–1124. [PubMed] [Google Scholar]
- Budygin EA, John CE, Mateo Y, Daunais JB, Friedman DP, Grant KA, Jones SR. Chronic ethanol exposure alters presynaptic dopamine function in the striatum of monkeys: a preliminary study. Synapse. 2003;50:266–268. doi: 10.1002/syn.10269. [DOI] [PubMed] [Google Scholar]
- Budygin EA, Oleson EB, Mathews TA, Lack AK, Diaz MR, McCool BA, Jones SR. Effects of chronic alcohol exposure on dopamine uptake in rat nucleus accumbens and caudate putamen. Psychopharmacology. 2007;193:495–501. doi: 10.1007/s00213-007-0812-1. [DOI] [PubMed] [Google Scholar]
- Calipari ES, Jones SR. Sensitized nucleus accumbens dopamine terminal responses to methylphenidate and dopamine transporter releasers after intermittent-access selfadministration. Neuropharmacology. 2014;82:1–10. doi: 10.1016/j.neuropharm.2014.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Ferris MJ, Melchior JR, Bermejo K, Salahpour A, Roberts DC, Jones SR. Methylphenidate and cocaine self-administration produce distinct dopamine terminal alterations. Addict. Biol. 2014;19:145–155. doi: 10.1111/j.1369-1600.2012.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll MR, Rodd ZA, Murphy JM, Simon JR. Chronic ethanol consumption increases dopamine uptake in the nucleus accumbens of high alcohol drinking rats. Alcohol. 2006;40:103–109. doi: 10.1016/j.alcohol.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cass WA, Gerhardt GA. Direct in vivo evidence that D2 dopamine receptors can modulate dopamine uptake. Neurosci. Lett. 1994;176:259–263. doi: 10.1016/0304-3940(94)90096-5. [DOI] [PubMed] [Google Scholar]
- Casu MA, Colombo G, Gessa GL, Pani L. Reduced TH-immunoreactive fibers in the limbic system of Sardinian alcohol-preferring rats. Brain Res. 2002a;924:242–251. doi: 10.1016/s0006-8993(01)03296-6. [DOI] [PubMed] [Google Scholar]
- Casu MA, Dinucci D, Colombo G, Gessa GL, Pani L. Reduced DAT- and DBH-immunostaining in the limbic system of Sardinian alcohol-preferring rats. Brain Res. 2002b;948:192–202. doi: 10.1016/s0006-8993(02)03220-1. [DOI] [PubMed] [Google Scholar]
- Diana M, Brodie M, Muntoni A, Puddu MC, Pillolla G, Steffensen S, Spiga S, Little HJ. Enduring effects of chronic ethanol in the CNS: basis for alcoholism. Alcohol. Clin. Exp. Res. 2003;27:354–361. doi: 10.1097/01.ALC.0000057121.36127.19. [DOI] [PubMed] [Google Scholar]
- Diana M, Rossetti ZL, Gessa G. Rewarding and aversive effects of ethanol: interplay of GABA, glutamate and dopamine. Alcohol Alcohol. Suppl. 1993;2:315–319. [PubMed] [Google Scholar]
- Djouma E, Lawrence AJ. The effect of chronic ethanol consumption and withdrawal on mu-opioid and dopamine D(1) and D(2) receptor density in Fawn-Hooded rat brain. J. Pharmacol. Exp. Ther. 2002;302:551–559. doi: 10.1124/jpet.102.035915. [DOI] [PubMed] [Google Scholar]
- Ford CP. The role of D2-autoreceptors in regulating dopamine neuron activity and transmission. Neuroscience. 2014 doi: 10.1016/j.neuroscience.2014.01.025. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garris PA, Budygin EA, Phillips PE, Venton BJ, Robinson DL, Bergstrom BP, Rebec GV, Wightman RM. A role for presynaptic mechanisms in the actions of nomifensine and haloperidol. Neuroscience. 2003;118:819–829. doi: 10.1016/s0306-4522(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Lumeng L, Koob GF. Dependence-induced alcohol drinking by alcohol-preferring (P) rats and outbred Wistar rats. Alcohol. Clin. Exp. Res. 2008;32:688–696. doi: 10.1111/j.1530-0277.2008.00678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Lopez MF, Becker HC. Intensity and duration of chronic ethanol exposure is critical for subsequent escalation of voluntary ethanol drinking in mice. Alcohol. Clin. Exp. Res. 2009;33:1893–1900. doi: 10.1111/j.1530-0277.2009.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Haun HL, Hazelbaker CL, Ramachandra VS, Becker HC. Increased extracellular glutamate in the nucleus accumbens promotes excessive ethanol drinking in ethanol dependent mice. Neuropsychopharmacology. 2014;39:707–717. doi: 10.1038/npp.2013.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healey JC, Winder DG, Kash TL. Chronic ethanol exposure leads to divergent control of dopaminergic synapses in distinct target regions. Alcohol. 2008;42:179–190. doi: 10.1016/j.alcohol.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzhak Y, Martin JL. Effects of cocaine, nicotine, dizocipline and alcohol on mice locomotor activity: cocaine-alcohol cross-sensitization involves upregulation of striatal dopamine transporter binding sites. Brain Res. 1999;818:204–211. doi: 10.1016/s0006-8993(98)01260-8. [DOI] [PubMed] [Google Scholar]
- Jeanes ZM, Buske TR, Morrisett RA. In vivo chronic intermittent ethanol exposure reverses the polarity of synaptic plasticity in the nucleus accumbens shell. J. Pharmacol. Exp. Ther. 2011;336:155–164. doi: 10.1124/jpet.110.171009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerlhag E, Landgren S, Egecioglu E, Dickson SL, Engel JA. The alcohol-induced locomotor stimulation and accumbal dopamine release is suppressed in ghrelin knockout mice. Alcohol. 2011;45:341–347. doi: 10.1016/j.alcohol.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Kennedy RT, Jones SR, Wightman RM. Dynamic observation of dopamine autoreceptor effects in rat striatal slices. J. Neurochem. 1992;59:449–455. doi: 10.1111/j.1471-4159.1992.tb09391.x. [DOI] [PubMed] [Google Scholar]
- Kim MO, Lee YK, Choi WS, Kim JH, Hwang SK, Lee BJ, Kang SG. Prolonged ethanol intake increases D2 dopamine receptor expression in the rat brain. Mol. Cells. 1997;7:682–687. [PubMed] [Google Scholar]
- Koob GF. Alcoholism: allostasis and beyond. Alcohol. Clin. Exp. Res. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Becker HC. Effect of patter and number of chronic ethanol exposure on subsequent voluntary ethanol intake in C57BL/6J mice. Psychopharmacology. 2005;181:688–696. doi: 10.1007/s00213-005-0026-3. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Murphy JM, Gatto GJ, Levy AD, Lumeng L, Li TK. Serotonin and dopamine systems regulating alcohol intake. Alcohol Alcohol. Suppl. 1991;1:411–416. [PubMed] [Google Scholar]
- McBride WJ, Bodart B, Lumeng L, Li TK. Association between low contents of dopamine and serotonin in the nucleus accumbens and high alcohol preference. Alcohol. Clin. Exp. Res. 1995;19:1420–1422. doi: 10.1111/j.1530-0277.1995.tb01001.x. [DOI] [PubMed] [Google Scholar]
- Melis M, Spiga S, Diana M. The dopamine hypothesis of drug addiction: hypodopaminergic state. Int. Rev. Neurobiol. 2005;63:101–154. doi: 10.1016/S0074-7742(05)63005-X. [DOI] [PubMed] [Google Scholar]
- Metten P, Sorensen ML, Cameron AJ, Chia-Hua Y, Crabbe JC. Withdrawal severity after chronic intermittent ethanol in inbred mouse strains. Alcohol. Clin. Exp. Res. 2010;34:1552–1564. doi: 10.1111/j.1530-0277.2010.01240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol. Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Ostlund SB, LeBlanc KH, Kosheleff AR, Wassum KM, Maidment NT. Phasic mesolimbic dopamine signaling encodes the facilitation of incentive motivation produced by repeated cocaine exposure. Neuropsychopharmacology. 2014;39:2441–2449. doi: 10.1038/npp.2014.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perra S, Clements MA, Bernier BE, Morikawa H. In vivo ethanol experience increases D(2) autoinhibition in the ventral tegmental area. Neuropsychpharmacology. 2011;36:993–1002. doi: 10.1038/npp.2010.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips PE, Hancock PJ, Stamford JA. Time window of autoreceptor-mediated inhibition of limbic and striatal dopamine release. Synapse. 2002;44:15–22. doi: 10.1002/syn.10049. [DOI] [PubMed] [Google Scholar]
- Phillips PE, Johns JM, Lubin DA, Budygin EA, Gainetdinov RR, Lieberman JA, Wightman RM. Presynaptic dopaminergic function is largely unaltered in mesolimbic and mesostriatal terminals of adult rats that were prenatally exposed to cocaine. Brain Res. 2003;961:63–72. doi: 10.1016/s0006-8993(02)03840-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JH, Calipari ES, Mathews TA, Jones SR. Greater ethanol-induced locomotor activation in DBA/2J versus C57BL/6J mice is not predicted by presynaptic striatal dopamine dynamics. PLoS One. 2013;8:e83852. doi: 10.1371/journal.pone.0083852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothblat DS, Rubin E, Schneider JS. Effects of chronic alcohol ingestion on the mesostriatal dopamine system in the rat. Neurosci. Lett. 2001;300:63–66. doi: 10.1016/s0304-3940(01)01548-8. [DOI] [PubMed] [Google Scholar]
- Sari Y, Bell RL, Zhou FC. Effects of chronic alcohol and repeated deprivations on dopamine D1 and D2 receptor levels in the extended amygdala of inbred alcohol-preferring rats. Alcohol. Clin. Exp. Res. 2006;30:46–56. doi: 10.1111/j.1530-0277.2006.00010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen RY. Ethanol withdrawal reduced the number of spontaneously active ventral tegmental area dopamine neurons in conscious animals. J. Pharamcol. Exp. Ther. 2003;307:566–572. doi: 10.1124/jpet.103.053371. [DOI] [PubMed] [Google Scholar]
- Shen RY, Choong KC, Thompson AC. Long-term reduction in ventral tegmental are dopamine neuron population activity following repeated stimulant or ethanol treatment. Biol. Psychiatry. 2007;61:93–100. doi: 10.1016/j.biopsych.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Souza-Formigoni ML, De Lucca EM, Hipólide DC, Enns SC, Oliveira MG, Nobrega JN. Sensitization to ethanol's stimulant effect is associated with region-specific increases in brain D2 receptor binding. Psychopharmacology. 1999;146:262–267. doi: 10.1007/s002130051115. [DOI] [PubMed] [Google Scholar]
- Tang A, George MA, Randall JA, Gonzales RA. Ethanol increases extracellular dopamine concentration in the ventral striatum in C57BL/6 mice. Alcohol. Clin. Exp. Res. 2003;27:1083–1089. doi: 10.1097/01.ALC.0000075825.14331.65. [DOI] [PubMed] [Google Scholar]
- Trevisan LA, Boutros N, Petrakis IL, Krystal JH. Complications of alcohol withdrawal: pathophysiological insights. Alcohol Health Res. World. 1998;22:61–66. [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J. Pharmacol. Exp. Ther. 1993;267:250–258. [PubMed] [Google Scholar]
- Weiss F, Parsons LH, Schulteis G, Hyytia P, Lorang MT, Bloom FE, Koob GF. Ethanol self-administration restores withdrawal-associated deficiencies in accumbal dopamine and 5-hydroxytryptamine release in dependent rats. J. Neurosci. 1996;16:3474–3485. doi: 10.1523/JNEUROSCI.16-10-03474.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman RM, Amatore C, Engstrom RC, Hale PD, Kristensen EW, Kuhr WG, May LJ. Real-time characterization of dopamine overflow and uptake in the rat striatum. Neuroscience. 1988;25:513–523. doi: 10.1016/0306-4522(88)90255-2. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat. Rev. Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Roth RH. Autoreceptor regulation of dopamine synthesis. Ann. N. Y. Acad. Sci. 1990;604:323–343. doi: 10.1111/j.1749-6632.1990.tb32003.x. [DOI] [PubMed] [Google Scholar]
- Wu Q, Reith ME, Wightman RM, Kawagoe KT, Garris PA. Determination of release and uptake parameters from electrically evoked dopamine dynamics measured by real-time voltammetry. J. Neurosci. Methods. 2001;112:119–133. doi: 10.1016/s0165-0270(01)00459-9. [DOI] [PubMed] [Google Scholar]
- Wu Q, Reith ME, Walker QD, Kuhn CM, Carroll FI, Garris PA. Concurrent autoreceptor-mediated control of dopamine release and uptake during neurotransmission: an in vivo voltammetric study. J. Neurosci. 2002;22:6272–6281. doi: 10.1523/JNEUROSCI.22-14-06272.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim HJ, Schallert T, Randall PK, Gonzales RA. Comparison of local and systemic ethanol effects on extracellular dopamine concentration in rat nucleus accumbens by microdialysis. Alcohol. Clin. Exp. Res. 1998;22:367–374. [PubMed] [Google Scholar]
- Yim HJ, Robinson DL, White ML, Jaworski JN, Randall PK, Lancaster FE, Gonzales RA. Dissociation between the time course of ethanol and extracellular dopamine concentrations in the nucleus accumbens after a single intraperitoneal injection. Alcohol. Clin. Exp. Res. 2000;24:781–788. [PubMed] [Google Scholar]
- Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol. 2008;42:149–160. doi: 10.1016/j.alcohol.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorgason JT, Espana RA, Jones SR. Demon voltammetry and analysis software: analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. J. Neurosci. Methods. 2011;202:158–164. doi: 10.1016/j.jneumeth.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata A, Shippenberg TS. Endogenous kappa opioid receptor systems modulate the responsiveness of mesoaccumbal dopamine neurons to ethanol. Alcohol. Clin. Exp. Res. 2006;30:592–597. doi: 10.1111/j.1530-0277.2006.00069.x. [DOI] [PubMed] [Google Scholar]
- Zapata A, Gonzales RA, Shippenberg TS. Repeated ethanol intoxication induces behavioral sensitization in the absence of a sensitized accumbens dopamine response in C57BL/6J and DBA/2J mice. Neuropsychopharmacology. 2006;31:396–405. doi: 10.1038/sj.npp.1300833. [DOI] [PMC free article] [PubMed] [Google Scholar]