Abstract
The amygdala is innervated by the cholinergic system and is involved in major depressive disorder (MDD). Evidence suggests a hyper-activate cholinergic system in MDD. Hippocampal Cholinergic Neurostimulating Peptide (HCNP) regulates acetylcholine synthesis. The aim of the present work was to investigate expression levels of HCNP-precursor protein (HCNP-pp) mRNA and other cholinergic-related genes in the postmortem amygdala of MDD patients and matched controls (females: N=16 pairs; males: N=12 pairs), and in the mouse unpredictable chronic mild stress (UCMS) model that induced elevated anxiety-/depressive-like behaviors (females: N=6 pairs; males: N=6 pairs). Results indicate an up-regulation of HCNP-pp mRNA in the amygdala of women with MDD (p<0.0001), but not males, and of UCMS-exposed mice (males and females; p=0.037). HCNP-pp protein levels were investigated in the human female cohort, but no difference was found. There were no differences in gene expression of acetylcholinesterase (AChE), muscarinic (mAChRs) or nicotinic receptors (nAChRs) between MDD subjects and controls or UCMS and control mice, except for an up-regulation of AChE in UCMS-exposed mice (males and females; p=0.044). Exploratory analyses revealed a baseline expression difference of cholinergic signaling-related genes between women and men (p<0.0001). In conclusion, elevated amygdala HCNP-pp expression may contribute to mechanisms of MDD in women, potentially independently from regulating the cholinergic system. The differential expression of genes between women and men could also contribute to the increased vulnerability of females to develop MDD.
Keywords: Hippocampal Cholinergic Neurostimulating Peptide, depression, cholinergic system, postmortem, amygdala, mRNA gene expression
Introduction
Major Depressive Disorder (MDD) is a severe mental disorder that is often chronic and recurrent and that leads to substantial impairments in an individual’s ability to take care of everyday responsibilities. MDD is the leading cause of disability worldwide, as measured by years lost due to disability (WHO, 2008). The World Health Organization ranked MDD as the 3rd leading cause of burden of disease as of 2004, but importantly, projected that MDD would be the number one cause for burden of disease by 2030 (WHO, 2008).
In the 1970s, Janowsky et al. first proposed a possible involvement of the cholinergic system in the etiology of MDD (Janowsky et al., 1974; Janowsky et al., 1972). They hypothesized that a given affective state may represent a balance between central cholinergic and adrenergic neurotransmitter activity in those areas of the brain that regulate affect, with depression being a disease of cholinergic dominance and mania being a disease of adrenergic dominance (Janowsky et al., 1974; Janowsky et al., 1972). This possible mechanism was recently revisited by Mineur and Piccioto (Mineur and Picciotto, 2010). Neurotransmission of the cholinergic system is carried out by acetylcholine (ACh), which is synthesized by cholineacetyltransferase (ChAt) and degraded by acetylcholinesterase (AChE). The main receptors for ACh are the nicotinic (nAChRs) and muscarinic (mAChRs) receptors. Several lines of evidence suggest involvement of the cholinergic system in MDD. Organophosphate poisoning inhibits AChE, resulting in increased ACh, and can cause depressive-like behavior in humans (Gershon and Shaw, 1961). Additionally, a neural nAChR antagonist reduces anxiety-like behavior in mice (Roni and Rahman, 2011) and an α4β2nAChR partial agonist elicits antidepressant properties in the forced swim test in mice (Zhang et al., 2012). Administration of scopolamine (a mAChR antagonist) showed antidepressant properties in unipolar and bipolar patients (Drevets and Furey, 2010; Furey and Drevets, 2006; Furey et al., 2010), and MDD patients on both oral scopolamine and citalopram had better remission rates than with citalopram alone (Khajavi et al., 2012). Many MDD patients exhibit sleep disturbances, including a decrease in rapid eye movement (REM) latency. Interestingly, a cholinergic agonist produced a faster induction of REM sleep only in MDD patients and in subjects at high risk for psychiatric disorders (Palagini et al., 2013). Finally, knockdown of AChE in the hippocampus of adult mice increases anxiety- and depression-like behaviors and susceptibility to social stress, which was prevented by fluoxetine (Mineur et al., 2013). Taken together, these results support the idea that hyper-activation of the cholinergic system may be involved in MDD.
Hippocampal Cholinergic Neurostimulating Peptide (HCNP) is involved in regulating ACh synthesis in a medial septal nucleus culture system (Ojika et al., 1992) by increasing the levels of ChAT in cholinergic neurons (Uematsu et al., 2009). HCNP is an undecapeptide cleaved from the precursor protein (HCNP-pp) (Otsuka and Ojika, 1996). HCNP-pp is also known as phosphatidylethanolamine-binding protein (PEBP) and Raf kinase inhibitor protein (RKIP) (Sedivy, 2011). The release of HCNP from hippocampal culture is specifically mediated by the NMDAR (Ojika et al., 1998). Results suggest that HCNP/HCNP-pp also acts as a key regulator for differentiation of cultured hippocampal progenitor cells (Sagisaka et al., 2010).
At the neural network level, changes in the function of several cortical and subcortical brain regions are thought to underlie the mood regulation deficit in depression (Seminowicz et al., 2004). We previously found differential gene expression in the amygdala of men and women with MDD compared to controls, although with notable sex differences (Guilloux et al., 2012; Sibille et al., 2009). This is in accordance with neuroimaging studies showing reduced volume or grey matter density of the amygdala in female MDD patients compared to control subjects, with no change in male MDD (Hastings et al., 2004; Kong et al., 2013).
The amygdala receives cholinergic input from the Nucleus Basalis of Meynert (Schafer et al., 1998) and expresses both muscarinic and nicotinic receptors (Klein and Yakel, 2006; McDonald and Mascagni, 2010; 2011). However, the cholinergic system in the amygdala has not been studied in detail in MDD subjects. Here, our working hypothesis is that HCNP-pp expression in the amygdala is involved in the pathogenesis of MDD by regulating the cholinergic system through HCNP. The aim of the present work was to investigate gene expression levels of HCNP-pp and genes involved in the cholinergic system in the postmortem amygdala of MDD patients and matched controls, and in a mouse model that elicits increased anxiety-/depressive-like behaviors.
Materials and Methods
Details of all methods are available in the Supplementary Information.
Human postmortem subjects
Brain samples were obtained after consent from next-of-kin during autopsies conducted at the Allegheny County Medical Examiner’s Office (Pittsburgh, PA, USA) using procedures approved by University of Pittsburgh’s Institutional Review Board and Committee for Oversight of Research Involving the Dead. Two cohorts of MDD subjects were examined here (male, n=12 pairs; female, n=16 pairs). Each MDD subject was matched with one control subject for sex and as closely as possible for age (Tables 1 and 2). See cohort details in Supplementary Information and (Guilloux et al., 2012; Sibille et al., 2009). The effects of putative confounds (age, antidepressants, death by suicide, pH, PMI, RNA ratio, RIN) were evaluated. When comparing male MDD subjects versus male controls, or female MDD subjects versus female controls, subject groups did not differ in mean age, postmortem interval (PMI), RNA integrity number (RIN), RNA ratio, or brain pH, as determined by one-way ANOVA (p>0.05). When comparing men and women, pH and RNA ratio were significantly different (p=0.009 and p=0.004, respectively) in MDD patients but they did not differ in RIN. Importantly, RIN is a better indicator of RNA quality than pH (Stan et al., 2006) or RNA ratio (Copois et al., 2007). Subjects did not differ in mean age, antidepressants, death by suicide or PMI. Male and female control subjects did not differ by age, pH, PMI, RNA ratio, or RIN. Significant co-factors were included in the ANCOVA analyses.
Table 1.
Characteristics of male subjects
| Control subjects | Depressed subjects | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||||||
| Case | Mode of death |
Cause of deatha | Age | Race | PMIb | pH | RNA ratio |
RIN | Tobacco | Case | DSM-IV Diagnoses |
MDD subtype |
Mode of death |
Cause of deatha | Age | Race | PMIb | pH | RNA ratio |
RIN | Medicationsc | Tobacco |
| 789 | Accident | Asphyxiation due to hanging |
22 | W | 20.0 | 7 | 2.0 | 7.8 | N | 513 | MDD | Recurrent and familial |
Suicide | Asphyxiation due to Hanging |
24 | W | 13.1 | 6.9 | 1.5 | 7.0 | N | Y |
| 615 | Natural | Ruptured Abdominal Aortic Aneurysm |
62 | W | 7.2 | 6.4 | 1.4 | 7.8 | N | 600 | MDD | Familial | Suicide | Asphyxiation due to Hanging |
63 | W | 9.9 | 6.7 | 1.7 | 7.1 | O | N |
| 551 | Natural | Cardiac Tamponade |
61 | W | 16.4 | 6.6 | 1.3 | 8.3 | U | 613 | MDDe,f | Recurrent and familial |
Suicide | GSW of Head | 59 | W | 15.6 | 7 | 1.5 | 8.7 | O | N |
| 713 | Natural | ASCVD | 58 | W | 37.5 | 7 | 1.6 | 8.4 | Y | 698 | MDD | Recurrent | Suicide | Asphyxiation due to Hanging |
59 | W | 13.0 | 6.8 | 1.5 | 9.0 | D O P | N |
| 1086 | Natural | ASCVD | 51 | W | 24.2 | 6.8 | 1.4 | 8.1 | Y | 863 | MDD | Familial | Natural | ASCVD | 51 | W | 28.3 | 7.2 | 1.5 | 8.4 | N | N |
| 857 | Natural | ASCVD | 48 | W | 16.6 | 6.7 | 2.0 | 8.9 | Y | 868 | MDDd,g | Recurrent and familial |
Accidental | Trauma of Trunk | 47 | W | 10.5 | 6.8 | 1.5 | 8.1 | N | N |
| 1122 | Natural | Cardiac Tamponade |
55 | W | 15.4 | 6.7 | 1.4 | 7.9 | Y | 926 | MDDe | Familial | Natural | Arteriosclerotic and Hypertensive Heart Disease |
56 | W | 19.0 | 7 | 1.4 | 7.3 | D O | Y |
| 852 | Natural | Cardiac Tamponade |
54 | W | 8.0 | 6.8 | 1.8 | 9.1 | Y | 1001 | MDD | Familial | Natural | Arteriosclerotic and Hypertensive Heart Disease |
53 | W | 7.3 | 6.6 | 1.4 | 7.6 | O | Y |
| 1067 | Natural | Hypertensive Heart Disease |
49 | W | 6.0 | 6.6 | 1.4 | 8.2 | N | 1049 | MDD | Familial | Natural | Hypertrophic Cardiomyopathy |
48 | W | 5.4 | 6.6 | 1.5 | 8.4 | D O | N |
| 1031 | Natural | Arteriosclerotic and Hypertensive Cardiovascular Disease |
53 | W | 23.1 | 6.8 | 1.5 | 8.2 | N | 809 | MDDh | Recurrent | Natural | ASCVD | 50 | W | 20.0 | 6.9 | 1.5 | 8.5 | D O | Y |
| 604 | Natural | Hypoplastic Coronary Artery Disease |
39 | W | 19.3 | 7.1 | 2.1 | 8.6 | N | 1060 | MDDi | Familial | Suicide | Hanging | 30 | W | 11.1 | 6.6 | 1.3 | 8.3 | O | N |
| 1047 | Natural | ASCVD | 43 | W | 12.0 | 6.6 | 1.8 | 9.0 | N | 943 | MDDd,g | Familial | Suicide | GSW to Mouth | 56 | W | 15.4 | 6.6 | 1.5 | 8.2 | O | Y |
ASCVD indicates arteriosclerotic cardiovascular disease; ASHCVD indicates arteriosclerotic hypertensive cardiovascular disease.
PMI indicates postmortem interval in hours.
Indicates prescribed medications at time of death (D, Antidepressants; N, No medications; O, Other medication(s); P, Antipsychotic).
Alcohol dependence, current at time of death.
Alcohol abuse, in remission at time of death.
History of psychotic features
Other substance dependence, current at time of death.
In full remission at time of death.
Other substance abuse, current at time of death.
Table 2.
Characteristics of female subjects.
| Control subjects |
Depressed subjects |
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Mode of death |
Cause of deatha | Age | Race | PMIb | pH | RNA ratio |
RIN | Tobacco | Case | DSM-IV Diagnoses |
MDD subtype |
Mode of death |
Cause of deatha | Age | Race | PMIb | pH | RNA ratio |
RIN | Medicationsc | Tobacco |
| 1466 | Accidental | Trauma | 64 | B | 20.0 | 6.7 | 2.0 | 8.8 | N | 803 | MDDg | Recurrent | Accidental | Trauma | 65 | W | 18.0 | 7 | 1.9 | 9.0 | DO | N |
| 1282 | Natural | ASCVD | 39 | W | 24.5 | 6.8 | 1.3 | 7.5 | N | 967 | MDDh | Recurrent | Natural | ASCVD | 40 | W | 22.2 | 6.6 | 1.6 | 7.4 | N | Y |
| 575 | Natural | ASCVD | 55 | B | 11.3 | 6.8 | 1.8 | 9.6 | U | 986 | MDD | Recurrent | Natural | Bronchial asthma | 53 | W | 11.9 | 6.7 | 1.8 | 8.7 | D O | N |
| 1391 | Natural | ASCVD | 51 | W | 7.8 | 6.6 | 1.6 | 7.1 | Y | 1041 | MDDe,i,d | Recurrent | Accidental | Combined drug overdose |
52 | W | 10.3 | 6.5 | 1.5 | 8.4 | B D O P | Y |
| 1034 | Natural | Endocardial fibroelastosis |
23 | W | 8.5 | 6.1 | 2.0 | 7.8 | N | 1157 | MDD | Recurrent | Suicide | Hanging | 26 | W | 13.4 | 6.4 | 1.5 | 7.8 | D | N |
| 567 | Natural | Mitral Valve Prolapse |
46 | W | 15.0 | 6.8 | 2.3 | 8.9 | U | 1190 | MDDh | Recurrent | Suicide | Asphyxiation | 47 | W | 22.3 | 6.6 | 1.6 | 8.0 | N | Y |
| 840 | Natural | ASCVD | 41 | W | 15.4 | 6.8 | 2.0 | 9.1 | Y | 1202 | MDDg | Recurrent | Natural | Pulmonary embolism |
39 | W | 11.2 | 6.4 | 1.8 | 8.0 | D O | Y |
| 546 | Natural | ASCVD | 37 | W | 23.5 | 6.7 | 2.0 | 8.6 | U | 1221 | MDD | Recurrent | Natural | Pulmonary thrombosis |
28 | B | 24.8 | 6.6 | 1.8 | 7.2 | N | N |
| 1092 | Natural | Mitral Valve Prolapse |
40 | B | 16.6 | 6.8 | 1.7 | 8.0 | N | 1249 | MDDf | Recurrent | Accidental | Combined drug overdose |
40 | W | 11.2 | 6.5 | 2.0 | 9.0 | B C D O | Y |
| 1403 | Natural | ASCVD | 45 | W | 12.3 | 6.7 | 1.8 | 8.2 | Y | 1254 | MDD | Recurrent | Suicide | Incised wounds | 39 | W | 12.8 | 6.4 | 1.9 | 9.0 | D | N |
| 1099 | Natural | Cardiomyopathy | 24 | W | 9.1 | 6.5 | 1.9 | 8.6 | Y | 1315 | MDDe | Single episode |
Suicide | Hanging | 28 | W | 12.4 | 7 | 1.5 | 7.9 | N | Y |
| 627 | Natural | COPD | 43 | B | 14.1 | 7.1 | 1.0 | 7.0 | N | 1332 | MDDj,i,g | Recurrent | Natural | ASCVD | 46 | W | 17.5 | 6.7 | 1.6 | 8.9 | B D O | Y |
| 818 | Accidental | Anaphylactic reaction |
67 | W | 24.0 | 7.1 | 1.5 | 8.4 | N | 1356 | MDDg,e | Recurrent | Accidental | Intraperitoneal hemorrhage |
60 | W | 20.6 | 6.1 | 1.8 | 8.5 | D O | N |
| 1081 | Natural | COPD | 57 | W | 14.9 | 6.8 | 1.8 | 9.0 | N | 1360 | MDDi | Single episode |
Suicide | Drowning | 59 | W | 18.1 | 6.4 | 1.4 | 7.6 | D | Y |
| 1196 | Accidental | Asphyxiation | 36 | W | 14.5 | 6.4 | 1.8 | 8.2 | N | 1408 | MDDh | Recurrent | Accidental | Trauma | 37 | W | 15.5 | 6.6 | 1.6 | 7.0 | B D O | N |
| 1355 | Natural | Subarachnoid hemorrhage |
74 | W | 24.9 | 6.6 | 1.9 | 7.0 | N | 10028 | MDDk,l,m,n | Single episode |
Suicide | Gunshot | 72 | W | 23.1 | 6.7 | 1.4 | 7.0 | O | N |
ASCVD indicates arteriosclerotic cardiovascular disease; ASHCVD indicates arteriosclerotic hypertensive cardiovascular disease.
PMI indicates postmortem interval in hours.
Indicates prescribed medications at time of death (B, Benzodiazepines; C, Anticonvulsants; D, Antidepressants; N, No medications; O, Other medication(s); P, Antipsychotic).
History of psychotic features.
Alcohol abuse, current at time of death.
Other substance dependence, in remission at time of death.
In partial remission at time of death.
Alcohol dependence, current at time of death.
Other substance dependence, current at time of death.
Alcohol dependence, in remission at time of death.
Alcohol abuse, in remission at time of death.
Other substance abuse, in remission at time of death.
Other substance abuse, current at time of death.
In full remission at time of death.
Protein purification and Western blotting
Following RNA extraction using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), acetone precipitation of proteins was carried out (Guilloux et al., 2012). Using Western blotting, HCNP-pp signal ratios relative to actin were calculated. To reduce the within- and between-subject measurement variance, samples were processed in matched pairs on the same gel four times, and results were replicated for a total of four different Western blots, with 16 replicates per pair (Curley et al., 2011).
Mouse samples
Amygdala cDNA from a mouse cohort previously described was used (Edgar et al., 2011).
Real-time quantitative polymerase chain reaction (qPCR)
qPCR analyses were performed using specific primers for HCNP-pp, AChE, ChAt, mAChRs (1-4) and nAChRs (α3, α4, α7, and β2) and three internal controls (beta-actin, cyclophilin A, glyceraldehyde-3-phosphate dehydrogenase) on amygdala cDNA samples, as described previously (Sibille et al., 2009). In brief, small PCR products (80–120 basepairs) were amplified in quadruplet on an Opticon real-time PCR machine (BioRad, Waltham, MA, USA). Each qPCR run included one MDD subject and one matched control.
Using a similar qPCR methodology as described above for human samples, qPCR on mouse samples was performed. Each run included one UCMS mouse and one control mouse, matched for sex.
Statistical analysis
Human samples
Diagnosis-related expression differences in gene of interest (GOI) signal were determined by analysis of covariance (ANCOVA) using SPSS (SPSS, Inc., Chicago, IL, USA). Relevant factors showing significant differences by ANOVA were included in the ANCOVA model. The qPCR data were averaged across the four replicates and transformed into expression levels relative to the internal control genes. Variance homogeneity was tested by Levenés test. Sex-related expression differences in GOI signal were determined by analysis of covariance (ANCOVA), using a similar method. Since gene expression in sex-related comparisons did not present variance homogeneity, data were transformed by taking the logarithm (ln) of gene expression values.
Mouse samples
Statistical analysis was performed using SPSS. The qPCR data were averaged across the four replicates and transformed into expression levels relative to the internal control genes. Sex was tested as the main factor in a one-way ANOVA. UCMS and control groups were compared in the ANOVA model with GOI mRNA as the dependent variable and subject group as the main effect.
Both in human and mouse samples, correlation between genes expression was tested by Pearson correlation. p<0.05 was considered statistically significant.
Western blot statistical analysis
A diagnosis-related expression difference in protein relative expression was determined by ANCOVA. The Western blot data were averaged across the sixteen replicates for each subject. Variance homogeneity was tested by Levenés test. To determine relevant covariates, the same approach as for gene expression was used. Covariate factors with significant effects were used in the ANCOVA model with relative protein level as the dependent variable and subject group as the main effect.
Results
HCNP-pp mRNA expression is up-regulated in the amygdala of women with MDD
We investigated mRNA expression of HCNP-pp in postmortem brains of men and women with MDD and in matched control subjects. For scale cofactors (age, pH, RNA ratio, RIN, PMI) and nominal cofactors (sex, tobacco, antidepressants, suicide, and cohort), pH, sex and cohort were significant after Bonferroni-Holm correction and included in the ANCOVA model. We observed a significant increase in HCNP-pp mRNA expression in the combined male/female MDD subject group compared with controls (F=21.794, p<0.0001) (Figure 1a). In view of previously-reported sex differences in MDD-related gene changes in the amygdala and since women are twice as likely to have MDD compared to men (Kessler et al., 2003), we explored the potential contribution of sex to differential HCNP-pp expression in MDD. No scale or nominal cofactors were significant in females. HCNP-pp was significantly increased in the amygdala of women with MDD compared to controls (F=51.316, p<0.0001; Fig 1b). In males, no significant difference was observed in HCNP-pp expression between MDD subjects and controls (age as cofactor; Fig 1c).
Figure 1.
mRNA expression of HCNP-pp in postmortem amygdala of men and women with MDD assessed by qPCR. a. HCNP-pp mRNA expression between MDD and control subjects (F=21,794, p<0.0001), using pH as cofactor. b. HCNP-pp mRNA expression between female MDD and control subjects (F=51.316, p<0.0001). c. HCNP-pp mRNA expression between male MDD and control subjects (non-significant). Error bars represent standard error.
Unchanged HCNP-pp protein level in female amygdala
We next investigated whether the up-regulation of HCNP-pp mRNA in the amygdala of females with MDD was associated with protein changes. Using a Western blot approach in postmortem female amygdala (N=15 pairs), we found no change in HCNP-pp between MDD and controls (F=0.525, p=0.475; cofactors: age and PMI) and no correlation with mRNA expression (Pearson R=0.127, p=0.503).
Absence of expression changes for cholinergic-related genes in the amygdala of MDD subjects
Since HNCP-pp/HCNP affects the production of ACh, we next investigated expression of genes related to the cholinergic system in the same postmortem amygdala samples, including mAChRs (1-4), nAChRs (α3, α4, α7, β2), ChAt, and AChE. The expression levels of ChAt and β2nAChR were too low in these samples, and were thus excluded from further analyses. We found no difference in the expression of these genes between MDD subjects and controls (Figure 2) (cofactors in the ANCOVA model: RNA ratio for AChE, nAChRs α3, α7; age and pH for nAChRs α3, α7). Interestingly, we found a robust main effect of sex on gene expression. Specifically, control females had significantly higher expression levels in compared to control males for AChE, mAChRs (1, 2 and 4), and nAChRs α3, α4 and α7 (cofactors: RNA ratio for nAChRα7; p<0.0001 for all genes examined), except for m3 which showed no differences between control males and females (Figure 3a). This differential expression was also present in MDD subjects. Specifically, AChE (cofactors: RNA ratio), mAChRs (1, 2 and 4, cofactors: pH, RNA ratio) and nAChRs (α3 and α7, cofactors: pH, RNA ratio; α4, cofactors: RNA ratio), showed significantly higher expression levels in female MDD subjects compared to male MDD subjects (p<0.0001 for all genes examined), except for m3 which showed no differences between MDD males and females (Figure 3b). This differential expression between females and males was also observed when analyzing controls and patients together (p<0.0001; data not shown). Cholinergic-related genes were not affected by antidepressant treatment in MDD patients.
Figure 2.
mRNA expression of cholinergic signaling-related genes in postmortem amygdala of controls and MDD subjects assessed by qPCR. mAChRs (1-4), nAChRs (α3, α4, α7), or AChE mRNA expression between MDD and control subjects (non-significant). Error bars represent standard error.
Figure 3.
mRNA expression of cholinergic signaling-related genes in postmortem amygdala of men and women assessed by qPCR. a. mAChRs (1, 2, 4), nAChRs (α3, α4, α7), or AChE mRNA expression between men and women in the control subjects (**, p<0.0001). b. mAChRs (1, 2, 4), nAChRs (α3, α4, α7), or AChE mRNA expression between men and women in the MDD subjects (**, p<0.0001). Error bars represent standard error.
HCNP-pp mRNA expression is up-regulated in the amygdala of mice exposed to chronic stress
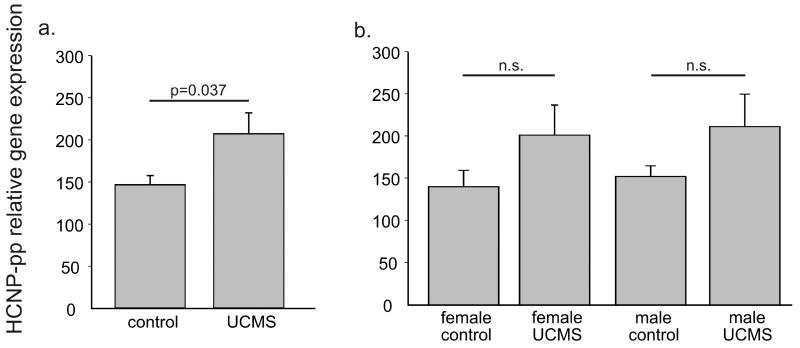
We examined gene expression of HCNP-pp in the amygdala of mice exposed to UCMS and non-stressed controls using samples from a previous study (Edgar et al., 2011). In that study, mice exposed to UCMS for a period of 4 weeks responded with characteristic increases in anxiety-/depressive-like behavior. Analyzing the females and males together (N=12 pairs), we found an up-regulation of HCNP-pp in the amygdala of UCMS mice compared to controls (F=4.912, p=0.037) (Figure 4a). Separated by sex, both groups showed a similar but non-significant increase in HCNP-pp expression (Figure 4b).
Figure 4.
mRNA expression of HCNP-pp in amygdala of mice exposed to UCMS and non-stressed controls assessed by qPCR. a. HCNP-pp mRNA expression between UCMS and control subjects (F=4.912, p=0.037). b. HCNP-pp mRNA expression between female and males UCMS and matched control subjects (n.s. = non-significant). Error bars represent standard error.
AChE expression is up-regulated in the amygdala of UCMS-exposed mice
We examined expression of mAChRs (1-4), nAChRs (α3, α4, α7, β2), ChAt, and AChE in the amygdala of mice exposed to chronic stress. The expression levels of ChAt, α4nAChR, and β2nAChR were too low, and were thus excluded from further analyses. Analyzing the females and males together, we observed an increased expression of AChE in the amygdala of UCMS-exposed mice compared to controls (F=4.559, p=0.044) (Figure 5). No difference in expression of mAChRs or nAChRs expression was found.
Figure 5.
mRNA expression of AChE in amygdala of mice exposed to UCMS and non-stressed controls assessed by qPCR. AChE mRNA expression between UCMS and control subjects (F=4.559, p=0.044). Error bars represent standard error.
We also performed a differential expression analysis in the combined male/female cohort, as performed in the human experiments. No sex differences in gene expression were observed (data not shown).
Gene expression correlation patterns in postmortem amygdala
Given that all cholinergic pathway genes are essential for efficient cholinergic neurotransmission, it is likely that they are expressed in a coordinated fashion. It is thus possible that this co-expression structure may vary in MDD. Therefore, we investigated the degree of relationship between HCNP-pp and cholinergic-related genes expression in the human and mouse samples (Tables 3 and 4, respectively). In humans, we found significant correlations between several cholinergic genes expression both in control and MDD, in both sexes (Table 3). However, the correlation between HCNP-pp and cholinergic-related genes was observed only in men. Specifically, HCNP-pp expression was correlated with AChE and m2 in control and MDD subjects, with m1 and 4 expression in control male subjects and with m3 and nα4 in male MDD subjects. In the mouse amygdala, HCNP-pp expression was correlated with AChE in male and female mice exposed to UCMS and female controls. HCNP-pp expression was also correlated with m1, 2, 4 and nα7 in female UCMS-exposed mice and with m1 in female controls (Table 4).
Table 3.
Gene expression correlation in human postmortem amygdala.
| AChE | m1 | m2 | m3 | m4 | nα3 | nα4 | nα7 | Group | |
|---|---|---|---|---|---|---|---|---|---|
| HCNP-pp | 0.90** | 0.79** | 0.80** | 0.40 | 0.87** | − 0.04 | 0.57 | 0.56 | male control |
| 0.95** | 0.34 | 0.86** | 0.70* | 0.27 | 0.49 | 0.60* | − 0.15 | male MDD | |
| 0.11 | − 0.17 | 0.01 | 0.35 | −0.05 | 0.33 | − 0.15 | − 0.02 | female control | |
| − 0.18 | − 0.23 | − 0.30 | 0.08 | −0.25 | 0.13 | − 0.09 | 0.20 | female MDD | |
|
| |||||||||
| AChE | 0.81** | 0.67* | 0.24 | 0.88** | 0.01 | 0.71** | 0.35 | male control | |
| 0.36 | 0.75** | 0.62* | 0.32 | 0.42 | 0.51 | − 0.24 | male MDD | ||
| 0.64** | 0.60* | 0.55* | 0.62** | 0.21 | 0.50* | 0.03 | female control | ||
| 0.89** | 0.65** | − 0.25 | 0.82** | 0.30 | 0.79** | 0.26 | female MDD | ||
|
| |||||||||
| m1 | 0.79** | 0.51 | 0.90** | − 0.05 | 0.76** | 0.57 | male control | ||
| − 0.04 | 0.46 | 0.96** | − 0.64* | − 0.52 | 0.44 | male MDD | |||
| 0.82** | 0.68** | 0.94** | 0.20 | 0.96** | 0.11 | female control | |||
| 0.69** | − 0.16 | 0.93** | 0.32 | 0.85** | 0.40 | female MDD | |||
|
| |||||||||
| m2 | 0.48 | 0.70* | − 0.11 | 0.58* | 0.50 | male control | |||
| 0.74** | − 0.17 | 0.72** | 0.83** | − 0.32 | male MDD | ||||
| 0.63** | 0.86** | 0.18 | 0.84** | − 0.05 | female control | ||||
| − 0.10 | 0.80** | 0.29 | 0.79** | 0.25 | female MDD | ||||
|
| |||||||||
| m3 | 0.40 | − 0.37 | 0.44 | 0.23 | male control | ||||
| 0.33 | 0.11 | 0.32 | − 0.13 | male MDD | |||||
| 0.71** | 0.40 | 0.72** | 0.05 | female control | |||||
| − 0.08 | 0.42 | − 0.24 | 0.08 | female MDD | |||||
|
| |||||||||
| m4 | 0.03 | 0.74** | 0.61* | male control | |||||
| − 0.68* | − 0.58* | 0.41 | male MDD | ||||||
| 0.09 | 0.93** | − 0.11 | female control | ||||||
| 0.37 | 0.91** | 0.50* | female MDD | ||||||
|
| |||||||||
| nα3 | − 0.21 | − 0.14 | male control | ||||||
| 0.97** | − 0.44 | male MDD | |||||||
| 0.22 | 0.64** | female control | |||||||
| 0.12 | 0.25 | female MDD | |||||||
|
| |||||||||
| nα4 | 0.25 | male control | |||||||
| − 0.47 | male MDD | ||||||||
| 0.10 | female control | ||||||||
| 0.49 | female MDD | ||||||||
p < 0.05 (light grey shading);
p < 0.01 (dark grey shading).
Table 4.
Gene expression correlation in mouse amygdala.
| AChE | m1 | m2 | m3 | m4 | na7 | nb2 | Group | |
|---|---|---|---|---|---|---|---|---|
| HCNP-pp | 0.79 | − 0.02 | 0.01 | 0.73 | 0.10 | − 0.42 | 0.05 | male control |
| 0.93** | 0.79 | − 0.15 | 0.34 | 0.11 | 0.17 | − 0.23 | male UCMS | |
| 0.97** | 0.98** | 0.76 | 0.02 | 0.67 | 0.69 | 0.79 | female control | |
| 0.93** | 0.96** | 0.83* | − 0.03 | 0.93** | 0.88* | 0.79 | female UCMS | |
|
| ||||||||
| AChE | 0.46 | 0.09 | 0.66 | 0.11 | − 0.37 | 0.14 | male control | |
| 0.82* | − 0.01 | 0.51 | 0.10 | − 0.05 | − 0.31 | male UCMS | ||
| 0.95** | 0.90* | 0.08 | 0.78 | 0.82* | 0.89* | female control | ||
| 0.82* | 0.68 | − 0.16 | 0.79 | 0.77 | 0.64 | female UCMS | ||
|
| ||||||||
| m1 | 0.68 | 0.12 | 0.49 | 0.44 | 0.55 | male control | ||
| 0.56 | 0.29 | 0.64 | 0.44 | − 0.14 | male UCMS | |||
| 0.76 | − 0.16 | 0.65 | 0.65 | 0.70 | female control | |||
| 0.86* | 0.11 | 0.98** | 0.93** | 0.88* | female UCMS | |||
|
| ||||||||
| m2 | 0.27 | 0.92* | 0.50 | 0.38 | male control | |||
| − 0.12 | 0.95** | 0.74 | 0.27 | male UCMS | ||||
| 0.16 | 0.86* | 0.92* | 0.82* | female control | ||||
| − 0.02 | 0.92* | 0.94** | 0.75 | female UCMS | ||||
|
| ||||||||
| m3 | 0.29 | − 0.63 | − 0.41 | male control | ||||
| − 0.04 | − 0.54 | − 0.55 | male UCMS | |||||
| 0.30 | 0.40 | 0.47 | female control | |||||
| 0.19 | 0.20 | 0.54 | female UCMS | |||||
|
| ||||||||
| m4 | 0.37 | 0.28 | male control | |||||
| 0.75 | − 0.01 | male UCMS | ||||||
| 0.97** | 0.88* | female control | ||||||
| 0.98** | 0.92** | female UCMS | ||||||
|
| ||||||||
| na7 | 0.84* | male control | ||||||
| 0.27 | male UCMS | |||||||
| 0.94** | female control | |||||||
| 0.91* | female UCMS | |||||||
p < 0.05 (light grey shading);
p < 0.01 (dark grey shading).
Discussion
In the present study, we report an up-regulation of HCNP-pp mRNA in the postmortem amygdala of women with MDD, with no change in men with MDD. Moreover, we found an up-regulation of genes involved in the cholinergic system in women compared to men, but no changes between MDD and control subjects. Also, we report a less robust up-regulation of HCNP-pp mRNA expression and an up-regulation of AChE in the amygdala of mice exposed to UCMS compared to controls. As in humans, no change was observed in genes related to the cholinergic system (except for AChE) between UCMS and control mice. Finally, we report a correlation in the expression of genes related to the cholinergic system in both humans and mice, as expected for an integrated neurotransmitter system. Together, the present results suggest that the differential expression of HCNP-pp observed in female MDD is not correlated with changes in the cholinergic system, at least at the mRNA level and in the amygdala. On the other hand, sex differences in expression of components of the cholinergic system might play a role in the increased susceptibility of women to suffer MDD.
HCNP-pp expression in human postmortem amygdala
The up-regulation of HCNP-pp is in accordance with results showing that an over-expression of HCNP/HCNP-pp in the hippocampus from early life in transgenic mice elicits a depressive-like phenotype in old age (Matsukawa et al., 2010). Also, a reduction of HCNP-pp mRNA was found in the postmortem hippocampus of late Alzheimeŕs disease (AD) patients compared to controls (Maki et al., 2002), but these findings may be related to the overall AD-related reduction in cholinergic neurotransmission (Gil-Bea et al., 2005), rather than to MDD-related mechanisms where a hyper-activation of this system has been proposed.
Despite the observed up-regulation of HCNP-pp in amygdala of women with MDD, we found no changes in protein expression or correlation with mRNA levels, although limitations in detection method may have yielded false negative results (See limitations). This was surprising, although in agreement with previous reports indicating no correlation between HCNP-pp mRNA and protein levels (Tohdoh et al., 1997). Another study by Greenbaum et al. (2003) suggested three reasons for poor correlations between mRNA and protein levels: complicated and varied post-transcriptional mechanisms, protein in vivo half-lives, and/or error and noise in both protein and mRNA experiments (Greenbaum et al., 2003).
HCNP-pp regulation of the cholinergic system in the human amygdala
We found no difference in mRNA expression of AChE, nAChRs, or mAChRs between MDD subjects and matched controls when men and women were analyzed together or separately. Previous studies showed no change of β2nAChRs availability in the amygdala or hippocampus in MDD patients (Saricicek et al., 2012). Also, no difference in α7nAChRs was found between postmortem hippocampus or perirhinal cortex of MDD patients and controls (Thomsen et al., 2011). On the other hand, no association between polymorphisms in m2AChR and MDD was found (Cohen-Woods et al., 2009), although there is opposing evidence (Comings et al., 2002; Wang et al., 2004). These results suggest that the differential expression of HCNP-pp does not affect expression of genes related to the cholinergic system in the amygdala. Interestingly, we found a strong correlation between the expression of HCNP-pp and AChE and ACh receptors in men (both MDD and control), but not in women. Thus, if HCNP-pp increases ACh levels with no increase in AChE or change in the receptors, high levels of ACh in the female brain could increase MDD vulnerability. On the other hand, when analyzing only the cholinergic-related genes, some correlations are present in control but not in MDD and vice versa, both in men and women, indicating that some degree of deregulation is present in the cholinergic system in the amygdala of MDD patients.
Sex difference of cholinergic genes expression in the amygdala
Interestingly, we found a robust up-regulation of genes related to the cholinergic system in women compared to men. We have previously reported differential expression of other genes, related to mitochondrial function for instance, in the amygdala between men and women (Lin et al., 2011), which together suggest that structural differences in amygdala gene expression may contribute to the increased susceptibility of women to suffer from MDD compared to men, and may include a cholinergic component. Indeed, sex differences in cholinergic function were observed, whereas women respond in a greater proportion and magnitude than men to scopolamine treatment for MDD and BD (Furey et al., 2010). Also, female non-smokers have higher availability of β2nAChR in certain brain regions compared to male non-smokers (Cosgrove et al., 2012). Moreover, women respond differently to administration of physostigmine (a reversible cholinesterase inhibitor that elevates ACh levels in the brain) (Rubin et al., 2003; Rubin et al., 1999).
Although the underlying mechanism that explains gender differences remains unclear, there are indications that hormones may have an important role in modulating the cholinergic system. In this sense, primary cultures of rat basal forebrain neurons exposed to physiological concentration of estrogen increased newly synthesized ACh (Pongrac et al., 2004). Also, chronic estradiol replacement significantly enhanced potassium-stimulated acetylcholine release in the hippocampus of ovariectomized rats (Gibbs et al., 2004).
Here, we did not observe differential expression of cholinergic-related genes between male and female mice, suggesting these differences may not be conserved across species, although additional studies are warranted. Studies comparing the expression of cholinergic-related genes in both the central nervous system and periphery in male and female mice are scarce. One study showed increased expression of all mAChRs(1–5) in the frontal cortex in males and of mAChRs 1–4 in the striatum in females, with no difference in other brain regions (including the hippocampus) (Benes et al., 2013). Another study showed differential activity of ChAT between males and females in the hippocampus at 17- and 25-months old but not at 5-months (Frick et al., 2002).
HCNP-pp expression in mouse amygdala
Consistent with the results in MDD subjects, we found an up-regulation of HCNP-pp mRNA expression in the amygdala of mice exposed to UCMS compared to controls, although no sex difference was found. Despite the finding that transgenic mice which over-express HCNP-pp exhibit depressive-like behaviors in old age (Matsukawa et al., 2010), another study reported a reduction of HCNP-pp in the hippocampus of rats exposed to stress (Kim and Kim, 2007). The latter result is in opposition with our observations, which may be explained by differences in methodology and/or brain region studied, namely different stress protocol, outcome measures, and region investigated.
HCNP-pp regulation of the cholinergic system in the mouse amygdala
As in the human cohorts, we did not find differential expression of genes related to the cholinergic system between UCMS-exposed and control mice, except for an up-regulation of AChE in UCMS-exposed mice. The up-regulation of AChE expression in the amygdala of UCMS-exposed mice may be a compensatory effect of increased ACh triggered by HCNP-pp, although further studies are needed to confirm this hypothesis. Also, we found a correlation between HCNP-pp and acetylcholine receptors expression only in females, especially in UCMS-exposed mice, suggesting sex-related differences in mechanisms involved in anxiety-/depressive-like behaviors between humans and mice for the cholinergic system.
Limitations
Some of the limitations of this study are inherent to investigation of heterogeneous cohorts and postmortem brain samples. Large numbers of clinical, demographic, and technical parameters have to be taken into consideration, and results are mostly correlative and cannot provide insight into developmental processes in MDD. The effects of putative confounds (antidepressants, death by suicide, pH, PMI, RNA ratio, RIN) were evaluated, however small samples sizes in parameter-delineated subgroups may have precluded definitive interpretations regarding the potential influence of these factors on the findings. For male MDD subjects, 4/12 had antidepressants present at the time of death and 6/12 died by suicide. For female MDD subjects, 11/16 had antidepressants present at the time of death and 6/16 died by suicide. No difference in HCNP-pp expression or in any other gene examined was found between patients who died by suicide compared to other modes of death or with or without AD (data not shown).
Other variables related to postmortem tissue samples (PMI, pH RNA ratio, RIN) were analyzed. pH of all brains used in this study was ≥6.1, a suggested value in the context of sudden deaths (Lewis, 2002). For RNA ratio, there was no correlation with RIN, which is a better indicator of RNA degradation (Copois et al., 2007). When comparing men and women, pH and RNA ratio were significantly different (p=0.009 and p=0.004, respectively) in MDD patients. Specifically, pH was considered as a cofactor for mAChRs 1, 2 and 4, and nAChRs α3 and α7. RNA ratio was considered as a cofactor for AChE, mAChRs 1, 2 and 4, and nAChRs α3, α7, and α4. Since pH and RNA ratio were not different between control males and females, it is unlikely that the results in MDD subjects were confounded by these parameters. Also RIN values did not differ between MDD females and males, which, as mentioned earlier, is a better indicator of RNA quality than pH (Stan et al., 2006) or RNA ratio (Copois et al., 2007), supporting the significance of the overall findings.
HCNP-pp protein levels were significantly positively correlated with PMI. With one exception, all samples had PMI less than 30 hours, which is acceptable for human studies (Atz et al., 2007). Also, no difference in PMI was found between MDD and control subjects. Moreover, since there is a positive correlation between protein expression and PMI, it does not seem that the protein stability would be affected by this variable.
We measured HCNP-pp protein levels instead of HCNP since there is no commercially available antibody for the latter. Finally, we did not measure ChAt protein levels in the amygdala and only evaluated the cholinergic system in an indirect way by measuring the levels of gene transcript of mAChRs, nAChRs, and AChE.
Summary
In summary, we found an up-regulation of HCNP-pp mRNA in the postmortem amygdala of women with MDD but no change at the protein level. Since HCNP-pp is a precursor protein, changes in processing and post-translational modifications occur and may not have been measured here. Two alternate hypotheses/mechanisms are possible: First, even though we did not find a differential expression of acetylcholine receptors in the amygdala between MDD and control subjects, we cannot rule out a cholinergic deregulation in this structure (since different mechanisms can regulate receptor expression or function). In support, chronic administration of nicotine produced an up-regulation of β2α4nAChRs with no change at the mRNA level (Corringer et al., 2006). The amygdala receives cholinergic input from the Nucleus Basalis of Meynert located in the basal forebrain. ChAt is synthetized in the cytoplasm of the cholinergic neuron and is transported through the axon to the nerve terminals where it synthesizes Ach (Oda, 1999). At the same time, the amygdalar complex projects to the basal forebrain, in particular to the cholinergic neurons of the ventrolateral substantia innominata (Jolkkonen et al., 2002). Thus, it is possible that HCNP-pp, in particular HCNP, exerts its action in a different brain region since ChAt mRNA levels were too low in the amygdala in our cohort, and previous studies showed no detection of ChAt mRNA in the amygdala (Oda, 1999). A possible mechanism is that HCNP travels from the amygdala to the basal forebrain where it can regulate the expression of ChAt mRNA. This hypothesis is in accordance with previous experiments showing that an over-expression of HCNP-pp in the hippocampus increases the levels of ChAt in the septal nucleus (Uematsu et al., 2009), the main cholinergic projection to the hippocampus. However, further experiments are needed to test this hypothesis.
Although regulation the cholinergic system is the more plausible hypothesis for the function of HCNP-pp, considering that a correlation between HCNP-pp and cholinergic-related genes is observed in men, an alternate hypothesis is that the differential expression of HCNP-pp mRNA is affecting the cellular population in the amygdala. In addition to its role in regulating the cholinergic system, HCNP/HCNP-pp is also involved in differentiation of cultured adult rat hippocampal progenitor cells. More specifically, a down-regulation of HCNP-pp was correlated with an up-regulation of GFAP (astrocyte marker) (Sagisaka et al., 2010). There is evidence of decreased GFAP in the amygdala of MDD patients, possibly indicating a reduction of astrocyte density (Altshuler et al., 2010). Also, a reduction in glia was found in MDD patients (Bowley et al., 2002). Taking this in consideration, the up-regulation of HCNP-pp we observed in the amygdala of women with MDD might be preventing progenitor cells from differentiating into astrocytes. Interestingly, reduced volume of the amygdala was reported in female MDD patients compared to control subjects, with no change in male MDD patients (Hastings et al., 2004). However, when analyzing the expression of GFAP in a previous study with the same samples used in our study, no difference between MDD and control postmortem female amygdala was found (Guilloux et al., 2012).
Supplementary Material
Research highlights.
Hippocampal Cholinergic Neurostimulating Peptide (HCNP) is upregulated in depression
HCNP-pp expression is upregulated in the amygdala of female but not men in depression
HCNP-pp expression is upregulated in the amygdala of stressed mice
Findings are independent of changes in cholinergic-related genes
Acknowledgements
This work was supported by National Institute of Mental Health MH084060 (ES), MH086637 (ES). SB was supported by an award jointly sponsored by the Fulbright Program and Bunge y Born Foundation and by a scholarship from CONICET (Consejo Nacional de Investigaciones Científicas y Técnicas). We thank B. French for careful reading of the manuscript.
Role of the funding source. The funding agency had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIMH or the National Institutes of Health.
Footnotes
Conflict of Interest. Marianne Seney in 2014 served as a consultant for NeuroPhage Pharmaceuticals, providing technique-based support.
Contributors. SB and PA designed the study. SB and MS performed the experiments. SB was responsible for the data analysis and wrote the first draft. SB, MS, and ES critically reviewed the manuscript. Each of the authors has reviewed the manuscript and has approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altshuler LL, Abulseoud OA, Foland-Ross L, Bartzokis G, Chang S, Mintz J, et al. Amygdala astrocyte reduction in subjects with major depressive disorder but not bipolar disorder. Bipolar Disord. 2010;12:541–549. doi: 10.1111/j.1399-5618.2010.00838.x. [DOI] [PubMed] [Google Scholar]
- Atz M, Walsh D, Cartagena P, Li J, Evans S, Choudary P, et al. Methodological considerations for gene expression profiling of human brain. J Neurosci Methods. 2007;163:295–309. doi: 10.1016/j.jneumeth.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes J, Mravec B, Kvetnansky R, Myslivecek J. The restructuring of muscarinic receptor subtype gene transcripts in c-fos knock-out mice. Brain Res Bull. 2013;94:30–39. doi: 10.1016/j.brainresbull.2013.01.010. [DOI] [PubMed] [Google Scholar]
- Bowley MP, Drevets WC, Ongur D, Price JL. Low glial numbers in the amygdala in major depressive disorder. Biological Psychiatry. 2002;52:404–412. doi: 10.1016/s0006-3223(02)01404-x. [DOI] [PubMed] [Google Scholar]
- Cohen-Woods S, Gaysina D, Craddock N, Farmer A, Gray J, Gunasinghe C, et al. Depression Case Control (DeCC) Study fails to support involvement of the muscarinic acetylcholine receptor M2 (CHRM2) gene in recurrent major depressive disorder. Human Molecular Genetics. 2009;18:1504–1509. doi: 10.1093/hmg/ddp051. [DOI] [PubMed] [Google Scholar]
- Comings DE, Wu S, Rostamkhani M, McGue M, Iacono WG, MacMurray JP. Association of the muscarinic cholinergic 2 receptor (CHRM2) gene with major depression in women. Am J Med Genet. 2002;114:527–529. doi: 10.1002/ajmg.10406. [DOI] [PubMed] [Google Scholar]
- Copois V, Bibeau F, Bascoul-Mollevi C, Salvetat N, Chalbos P, Bareil C, et al. Impact of RNA degradation on gene expression profiles: assessment of different methods to reliably determine RNA quality. J Biotechnol. 2007;127:549–559. doi: 10.1016/j.jbiotec.2006.07.032. [DOI] [PubMed] [Google Scholar]
- Corringer PJ, Sallette J, Changeux JP. Nicotine enhances intracellular nicotinic receptor maturation: a novel mechanism of neural plasticity? J Physiol Paris. 2006;99:162–171. doi: 10.1016/j.jphysparis.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Esterlis I, McKee SA, Bois F, Seibyl JP, Mazure CM, et al. Sex differences in availability of beta2*-nicotinic acetylcholine receptors in recently abstinent tobacco smokers. Arch Gen Psychiatry. 2012;69:418–427. doi: 10.1001/archgenpsychiatry.2011.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curley AA, Arion D, Volk DW, Asafu-Adjei JK, Sampson AR, Fish KN, et al. Cortical deficits of glutamic acid decarboxylase 67 expression in schizophrenia: clinical, protein, and cell type-specific features. The American Journal of Psychiatry. 2011;168:921–929. doi: 10.1176/appi.ajp.2011.11010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Furey ML. Replication of scopolamine’s antidepressant efficacy in major depressive disorder: a randomized, placebo-controlled clinical trial. Biological Psychiatry. 2010;67:432–438. doi: 10.1016/j.biopsych.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar NM, Touma C, Palme R, Sibille E. Resilient emotionality and molecular compensation in mice lacking the oligodendrocyte-specific gene Cnp1. Translational Psychiatry. 2011;1:e42. doi: 10.1038/tp.2011.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Burlingame LA, Delaney SS, Berger-Sweeney J. Sex differences in neurochemical markers that correlate with behavior in aging mice. Neurobiol Aging. 2002;23:145–158. doi: 10.1016/s0197-4580(01)00237-8. [DOI] [PubMed] [Google Scholar]
- Furey ML, Drevets WC. Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo-controlled clinical trial. Archives of General Psychiatry. 2006;63:1121–1129. doi: 10.1001/archpsyc.63.10.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furey ML, Khanna A, Hoffman EM, Drevets WC. Scopolamine produces larger antidepressant and antianxiety effects in women than in men. Neuropsychopharmacology. 2010;35:2479–2488. doi: 10.1038/npp.2010.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon S, Shaw FH. Psychiatric sequelae of chronic exposure to organophosphorus insecticides. Lancet. 1961;1:1371–1374. doi: 10.1016/s0140-6736(61)92004-9. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Gabor R, Cox T, Johnson DA. Effects of raloxifene and estradiol on hippocampal acetylcholine release and spatial learning in the rat. Psychoneuroendocrinology. 2004;29:741–748. doi: 10.1016/S0306-4530(03)00118-5. [DOI] [PubMed] [Google Scholar]
- Gil-Bea FJ, Garcia-Alloza M, Dominguez J, Marcos B, Ramirez MJ. Evaluation of cholinergic markers in Alzheimer’s disease and in a model of cholinergic deficit. Neurosci Lett. 2005;375:37–41. doi: 10.1016/j.neulet.2004.10.062. [DOI] [PubMed] [Google Scholar]
- Greenbaum D, Colangelo C, Williams K, Gerstein M. Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biol. 2003;4:117. doi: 10.1186/gb-2003-4-9-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilloux JP, Douillard-Guilloux G, Kota R, Wang X, Gardier AM, Martinowich K, et al. Molecular evidence for BDNF- and GABA-related dysfunctions in the amygdala of female subjects with major depression. Molecular Psychiatry. 2012;17:1130–1142. doi: 10.1038/mp.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings RS, Parsey RV, Oquendo MA, Arango V, Mann JJ. Volumetric analysis of the prefrontal cortex, amygdala, and hippocampus in major depression. Neuropsychopharmacology. 2004;29:952–959. doi: 10.1038/sj.npp.1300371. [DOI] [PubMed] [Google Scholar]
- Janowsky DS, el-Yousef MK, Davis JM. Acetylcholine and depression. Psychosom Med. 1974;36:248–257. doi: 10.1097/00006842-197405000-00008. [DOI] [PubMed] [Google Scholar]
- Janowsky DS, el-Yousef MK, Davis JM, Sekerke HJ. A cholinergic-adrenergic hypothesis of mania and depression. Lancet. 1972;2:632–635. doi: 10.1016/s0140-6736(72)93021-8. [DOI] [PubMed] [Google Scholar]
- Jolkkonen E, Miettinen R, Pikkarainen M, Pitkanen A. Projections from the amygdaloid complex to the magnocellular cholinergic basal forebrain in rat. Neuroscience. 2002;111:133–149. doi: 10.1016/s0306-4522(01)00578-4. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) Journal of the American Medical Association. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Khajavi D, Farokhnia M, Modabbernia A, Ashrafi M, Abbasi SH, Tabrizi M, et al. Oral scopolamine augmentation in moderate to severe major depressive disorder: a randomized, double-blind, placebo-controlled study. Journal of Clinical Psychiatry. 2012;73:1428–1433. doi: 10.4088/JCP.12m07706. [DOI] [PubMed] [Google Scholar]
- Kim HG, Kim KL. Decreased hippocampal cholinergic neurostimulating peptide precursor protein associated with stress exposure in rat brain by proteomic analysis. Journal of Neuroscience Research. 2007;85:2898–2908. doi: 10.1002/jnr.21407. [DOI] [PubMed] [Google Scholar]
- Klein RC, Yakel JL. Functional somato-dendritic alpha7-containing nicotinic acetylcholine receptors in the rat basolateral amygdala complex. The Journal of Physiology. 2006;576:865–872. doi: 10.1113/jphysiol.2006.118232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong L, Chen K, Womer F, Jiang W, Luo X, Driesen N, et al. Sex differences of gray matter morphology in cortico-limbic-striatal neural system in major depressive disorder. J Psychiatr Res. 2013;47:733–739. doi: 10.1016/j.jpsychires.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA. The human brain revisited: opportunities and challenges in postmortem studies of psychiatric disorders. Neuropsychopharmacology. 2002;26:143–154. doi: 10.1016/S0893-133X(01)00393-1. [DOI] [PubMed] [Google Scholar]
- Lin LC, Lewis DA, Sibille E. A human-mouse conserved sex bias in amygdala gene expression related to circadian clock and energy metabolism. Mol Brain. 2011;4:18. doi: 10.1186/1756-6606-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki M, Matsukawa N, Yuasa H, Otsuka Y, Yamamoto T, Akatsu H, et al. Decreased expression of hippocampal cholinergic neurostimulating peptide precursor protein mRNA in the hippocampus in Alzheimer disease. Journal of Neuropathology & Experimental Neurology. 2002;61:176–185. doi: 10.1093/jnen/61.2.176. [DOI] [PubMed] [Google Scholar]
- Matsukawa N, Furuya Y, Ogura H, Ojika K. HCNP precursor protein transgenic mice display a depressive-like phenotype in old age. Brain Research. 2010;1349:153–161. doi: 10.1016/j.brainres.2010.06.041. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Neuronal localization of m1 muscarinic receptor immunoreactivity in the rat basolateral amygdala. Brain Structure and Function. 2010;215:37–48. doi: 10.1007/s00429-010-0272-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Neuronal localization of M2 muscarinic receptor immunoreactivity in the rat amygdala. Neuroscience. 2011;196:49–65. doi: 10.1016/j.neuroscience.2011.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Obayemi A, Wigestrand MB, Fote GM, Calarco CA, Li AM, et al. Cholinergic signaling in the hippocampus regulates social stress resilience and anxiety- and depression-like behavior. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:3573–3578. doi: 10.1073/pnas.1219731110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Picciotto MR. Nicotine receptors and depression: revisiting and revising the cholinergic hypothesis. Trends in Pharmacological Sciences. 2010;31:580–586. doi: 10.1016/j.tips.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda Y. Choline acetyltransferase: the structure, distribution and pathologic changes in the central nervous system. Pathology International. 1999;49:921–937. doi: 10.1046/j.1440-1827.1999.00977.x. [DOI] [PubMed] [Google Scholar]
- Ojika K, Kojima S, Ueki Y, Fukushima N, Hayashi K, Yamamoto M. Purification and structural analysis of hippocampal cholinergic neurostimulating peptide. Brain Research. 1992;572:164–171. doi: 10.1016/0006-8993(92)90465-l. [DOI] [PubMed] [Google Scholar]
- Ojika K, Tsugu Y, Mitake S, Otsuka Y, Katada E. NMDA receptor activation enhances the release of a cholinergic differentiation peptide (HCNP) from hippocampal neurons in vitro. Brain research. Developmental brain research. 1998;106:173–180. doi: 10.1016/s0165-3806(98)00014-5. [DOI] [PubMed] [Google Scholar]
- Otsuka Y, Ojika K. Demonstration and characterization of hippocampal cholinergic neurostimulating peptide (HCNP) processing enzyme activity in rat hippocampus. Neurochemical Research. 1996;21:369–376. doi: 10.1007/BF02531654. [DOI] [PubMed] [Google Scholar]
- Palagini L, Baglioni C, Ciapparelli A, Gemignani A, Riemann D. REM sleep dysregulation in depression: state of the art. Sleep Medicine Reviews. 2013;17:377–390. doi: 10.1016/j.smrv.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Pongrac JL, Gibbs RB, Defranco DB. Estrogen-mediated regulation of cholinergic expression in basal forebrain neurons requires extracellular-signal-regulated kinase activity. Neuroscience. 2004;124:809–816. doi: 10.1016/j.neuroscience.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Roni MA, Rahman S. Neuronal nicotinic receptor antagonist reduces anxiety-like behavior in mice. Neuroscience Letters. 2011;504:237–241. doi: 10.1016/j.neulet.2011.09.035. [DOI] [PubMed] [Google Scholar]
- Rubin RT, Abbasi SA, Rhodes ME, Czambel RK. Growth hormone responses to low-dose physostigmine administration: functional sex differences (sexual diergism) between major depressives and matched controls. Psychol Med. 2003;33:655–665. doi: 10.1017/s0033291703007426. [DOI] [PubMed] [Google Scholar]
- Rubin RT, O’Toole SM, Rhodes ME, Sekula LK, Czambel RK. Hypothalamo-pituitary-adrenal cortical responses to low-dose physostigmine and arginine vasopressin administration: sex differences between major depressives and matched control subjects. Psychiatry Res. 1999;89:1–20. doi: 10.1016/s0165-1781(99)00085-2. [DOI] [PubMed] [Google Scholar]
- Sagisaka T, Matsukawa N, Toyoda T, Uematsu N, Kanamori T, Wake H, et al. Directed neural lineage differentiation of adult hippocampal progenitor cells via modulation of hippocampal cholinergic neurostimulating peptide precursor expression. Brain Research. 2010;1327:107–117. doi: 10.1016/j.brainres.2010.02.071. [DOI] [PubMed] [Google Scholar]
- Saricicek A, Esterlis I, Maloney KH, Mineur YS, Ruf BM, Muralidharan A, et al. Persistent beta2*-nicotinic acetylcholinergic receptor dysfunction in major depressive disorder. The American Journal of Psychiatry. 2012;169:851–859. doi: 10.1176/appi.ajp.2012.11101546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer MK, Eiden LE, Weihe E. Cholinergic neurons and terminal fields revealed by immunohistochemistry for the vesicular acetylcholine transporter. II. The peripheral nervous system. Neuroscience. 1998;84:361–376. doi: 10.1016/s0306-4522(97)80196-0. [DOI] [PubMed] [Google Scholar]
- Sedivy JM. Phosphatidylenthanolamine Binding Protein aka Raf Kinase Inhibitor Protein: A Brief History of Its Discovery and the Remarkable Diversity of Biological Functions. For Immunopathol Dis Therap. 2011;2:1–12. doi: 10.1615/ForumImmunDisTher.v2.i1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminowicz DA, Mayberg HS, McIntosh AR, Goldapple K, Kennedy S, Segal Z, et al. Limbic-frontal circuitry in major depression: a path modeling metanalysis. Neuroimage. 2004;22:409–418. doi: 10.1016/j.neuroimage.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Sibille E, Wang Y, Joeyen-Waldorf J, Gaiteri C, Surget A, Oh S, et al. A molecular signature of depression in the amygdala. The American Journal of Psychiatry. 2009;166:1011–1024. doi: 10.1176/appi.ajp.2009.08121760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stan AD, Ghose S, Gao XM, Roberts RC, Lewis-Amezcua K, Hatanpaa KJ, et al. Human postmortem tissue: what quality markers matter? Brain Res. 2006;1123:1–11. doi: 10.1016/j.brainres.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen MS, Weyn A, Mikkelsen JD. Hippocampal alpha7 nicotinic acetylcholine receptor levels in patients with schizophrenia, bipolar disorder, or major depressive disorder. Bipolar Disorders. 2011;13:701–707. doi: 10.1111/j.1399-5618.2011.00961.x. [DOI] [PubMed] [Google Scholar]
- Tohdoh N, Tojo S, Kimura M, Ishii T, Ojika K. Mechanism of expression of the rat HCNP precursor protein gene. Brain Research. Molecular Brain Research. 1997;45:24–32. doi: 10.1016/s0169-328x(96)00125-8. [DOI] [PubMed] [Google Scholar]
- Uematsu N, Matsukawa N, Kanamori T, Arai Y, Sagisaka T, Toyoda T, et al. Overexpression of hippocampal cholinergic neurostimulating peptide in heterozygous transgenic mice increases the amount of ChAT in the medial septal nucleus. Brain Research. 2009;1305:150–157. doi: 10.1016/j.brainres.2009.09.112. [DOI] [PubMed] [Google Scholar]
- Wang JC, Hinrichs AL, Stock H, Budde J, Allen R, Bertelsen S, et al. Evidence of common and specific genetic effects: association of the muscarinic acetylcholine receptor M2 (CHRM2) gene with alcohol dependence and major depressive syndrome. Hum Mol Genet. 2004;13:1903–1911. doi: 10.1093/hmg/ddh194. [DOI] [PubMed] [Google Scholar]
- WHO . The Global Burden of Disease, 2004 Update. 2008. pp. 1–146. [Google Scholar]
- Zhang H, Tuckmantel W, Eaton JB, Yuen PW, Yu LF, Bajjuri KM, et al. Chemistry and behavioral studies identify chiral cyclopropanes as selective alpha4beta2-nicotinic acetylcholine receptor partial agonists exhibiting an antidepressant profile. Journal of Medicinal Chemistry. 2012;55:717–724. doi: 10.1021/jm201157c. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.