Abstract
Disuse osteopenia and bone loss have been extensively reported in long duration space mission and long term bed rest. The pathology of the bone loss is similar to osteoporosis but highly confined to weight bearing bones. The current anabolic and/or anti-resorptive drugs have systemic effects and are costly over extended time, with concerns of long term fracture risk. This study use Low Intensity Pulsed Ultrasound (LIPUS) as a non-invasive acoustic force and anabolic stimulus to countermeasure disuse induced bone loss. Four-month old C57BL/6 mice were randomized to five groups, 1) age-matched (AM), 2) non-suspended sham (NS), 3) nonsuspended –LIPUS (NU), 4) suspended sham (SS), and 5) suspended-LIPUS (SU) groups. After four weeks of suspension, µCT analyses showed significant decreases in trabecular bone volume fraction (BV/TV) (−36%, p<0.005), bone tissue mineral density (TMD) (−3%, p<0.05), trabecular thickness (Tb.Th) (−12.5%, p<0.005), and increase in bone surface/bone volume (+BS/BV) (+16%, p<0.005), relative to age-matched (AM). Application of LIPUS for 20 min/day for 5 days/week, significantly increased TMD (+3%, p<0.05), Tb.Th (+6%, p<0.05), and decreased BS/BV (−10%, p<0.005), relative to suspension alone (SS) mice. Histomorphometry analyses showed a breakdown of bone microstructure under disuse conditions consist with µCT results. In comparison to SS mice, LIPUS treated bone showed increased structural integrity with increased bone formation rates at metaphysical endosteal and trabecular surfaces (+0.104±0.07 vs 0.031±0.30 µm3/µm2/d) relative to SS. Four-point bending mechanical tests of disused SS femurs showed reduced elastic modulus (−53%, p<0.05), yield (−33%, p<0.05) and ultimate strength (−45%, p<0.05) at the femoral diaphysis relative to AM bone. LIPUS stimulation mitigated the adverse effects of disuse on bone elastic modulus (+42%, p<0.05), yield strength (+29%, p<0.05), and ultimate strength (+39%, p<0.05) relative to SS femurs. LIPUS provides the essential mechanical stimulus to retain bone morphological and mechanical integrity in disuse conditions. This study demonstrates LIPUS potential as regional therapeutic agent to countermeasure disuse induced bone loss while maintaining bone's integrity.
Keywords: low intensity pulsed ultrasound, disuse osteopenia, osteoporosis, nonunion fracture, LIPUS, acoustic radiation force, countermeasure, microgravity
Introduction
Disuse osteoporosis due to long duration space travel and/or extended bed rest is a regional phenomenon, mostly concentrated at load bearing sites [1]. Lower limbs are subjected to a high degree of mechanical stress during daily activities that result in functional gravitational forces, ground reaction forces, and dynamic muscular contractions. Lack of mechanical loading due to long-term bed rest, brain/spinal cord injury, paralysis, and long-term exposure to microgravity functional loading can severely compromise bone microstructure and bone mineral density leading to fractures [2, 3]. Lack of mechanical loading resulted from disuse reduces bone formation and increase bone resorption on periosteal, endosteal cortical regions, and trabecular surfaces [4]. Long-term disuse can lead to structural changes in long bones that result in the formation of rounder shaped long bones rather than the typical triangular shape, reducing moment of inertia and strength [5].
The distal most regions of bones show the highest catabolic activity during disuse and the highest anabolic response to mechanical stimulation. Long-term bed rest patients experienced the highest rates of bone loss in bones farthest from the heart while bone formation increased in the skull due to increased interstitial pressure [6]. In a cross-section study of spinal-cord injury patients, Kiratli et al. found 27%, 25%, and 43% reductions in BMD at the femoral neck, mid-shaft, and distal femur, respectively, compared to normal controls [7]. Similar trends have been observed in astronauts, with mean BMD decreases of 5.4% in trabecular bone seen after 6 months of spaceflight [8].
Recently several anti-resorptive/anti-catabolic and anabolic agents have been studied as treatments for osteoporosis. Anti-resorptive agents, such as bisphosphonates [9, 10] and RANKL inhibitors [10], reduce osteoclast differentiation, maturation, and activity, leading to increased bone strength and delayed remodeling. Anabolic agents such as PTH [11] and anti-sclerostin antibodies [12], increase bone formation rates, bone mass, and mechanical strength by enhancing osteoblast activity. Both anti-resorptive and anabolic agents have shown encouraging results in treating osteoporosis in clinical (e.g., bisphosphonates, PTH, strontium ranelate, anti-sclerostin antibody) [13, 14]. These anti-resorptive and/or anabolic therapies have aimed in the systematic effects, while disuse osteoporosis is a more localized pathology. Therefore it is possible that these therapies can lead to undesired effects in healthy bones. Astronauts are made to exercise for 2.5hrs per day to retain their BMD in long space flights but that may not be sufficient for extended space exploration to Mars. Further excessive exercise can lead to bone loss [15].
Disuse osteoporosis is caused by lack of mechanical stimulation; numerous studies have evaluated the use of induced mechanical stimulation as a countermeasure for disuse osteoporosis. Several different modes of mechanical stimulation have been evaluated, namely: cyclic strain [16–19], vibrations [20–22], and Low Intensity Pulsed Ultrasound (LIPUS) [23–27]. Cyclic strain has shown encouraging results in vitro but in vivo applications are limited by skeletal structure. Mechanical vibrations have shown promising results both in vitro and in vivo but there are questions regarding systemic effects of vibrations in whole body vibrations. LIPUS stimulation creates acoustic vibrations that generate localized shear stress on cell membranes and has been shown to induce anabolic responses in osteoblasts [28]. Moreover, LIPUS can be readily applied in vivo and studies and have shown it to have anabolic effects on fresh fractures [29], delayed unions [30, 31], non-unions [30, 31], and osteoporosis [25] both in animal models [29, 30, 32] and clinical studies [33, 34]. LIPUS provides a non-invasive and targeted treatment for specific regions of interest. Furthermore, because the FDA has approved LIPUS for non-union fractures regulatory approval for its use in treating disuse osteoporosis will likely present less of a challenge than other modes of mechanical stimulation.
The objective of this study was to investigate the effects of LIPUS incused acoustic radiation force on the femora and tibiae of hind limb suspended mice using high resolution µCT, dynamic histomorphometry, and mechanical testing. It is hypothesized that daily short duration localized exposure to LIPUS will provide sufficient mechanical stimulation to counteract the disuse induced bone loss in the functional disuse osteopenia of these mice.
Methods and Materials
Animal experiment
The animal protocol was approved by the Stony Brook University IACUC. Twelve-week old black B6/C57J mice were randomized into 5 groups (n=15 per group): Age-matched (AM), Sham non-suspended (NS), Non-suspended + LIPUS (NU), Sham suspended (SS), and Suspended + LIPUS (SU). LIPUS groups were treated with 1 kHz, 20% duty cycle, 30 mw/cm2 pulsed ultrasound exposure for 20 min/day for 5 days per week over 4 weeks with an ultrasound stimulator (Sonicator 740®, Mettle Electronics, Anaheim, CA). To ensure optimal transduction of ultrasound, mice left limb was shaved, acoustic gel was applied as decoupling agent and transducer was placed such that cover both left tibia and femur. For the duration of treatment, the animals were anesthetized with isoflurane. LIPUS was applied to the left femur and tibia in LIPUS treated animals and right legs were used as contralateral untreated controls. Sham groups were treated in same manner except that an inactive ultrasound transducer was utilized. Suspended mice were tail suspended for a period of 4 weeks. The age-matched group was not exposed to suspension or treatment. Tailed suspension studies are considered to be high stress studies (classified as Category C study by IACUC).Animal were under constant observation of veterinary staff for signs for distress associated signs such as weight loss, hair loss, mucus around eye and nose and lack of activity. Our lab has extensive experience in rodent tail suspension studies, to reduce distress levels during the study mice were made accustomed to tail suspension with 2 weeks compliance in which mice were suspended for few hours each day. Furthermore, any mice showing more than 15% weight loss were taken out of study promptly. During study period, animals were observed for signs of stress twice a day and animal weights were measured daily for the duration of the study. In cases of weight loss animals were given flavored treats and to reduce dehydration, animals were given subcutaneous injections of saline. If any animals exhibited more than 20% weight loss, infection or severe distress, veterinary staff was notified and treatment recommendations were followed. All the procedures were guided in accordance with IACUC approved procedures. After 4 weeks the animals were euthanized using CO2 and all four limbs were harvested and stored at −80°C until analysis.
Micro computed tomography
The proximal regions of the right and left tibiae were scanned at 12 micron resolution using µCT (µCT40, SCANCO Medical USA, PA). To be consistent in analyses, a 400 slice region of the bones distal to the end of epiphysis was acquired and the microstructure was evaluated for a region beginning 20 slices below the epiphysis and continuing for 80 slices (app 960 µm). To evaluate the region of interest, contour lines were manually drawn around the trabecular bone every 10 slices and contour lines were morphed to fit the intervening slices.
The regions of interest were then evaluated for: trabecular bone fraction (%, BV/TV), Tissue mineral density (mgHA/cc), Trabecular number (1/mm, Tb.N), Trabecular Thickness (mm, Tb.Th), Trabecular separation (mm, Tb.Sp), and Bone surface/Bone volume (mm2/mm3, BS/BV) to determine the catabolic effects of microgravity on bone microstructure and efficacy of LIPUS treatment in preventing these effects.
Cortical bone was analyzed from the left and right tibiae at the mid-diaphysis, also at 12 micron resolution. Measurements included: cortical bone thickness (Cort.Th), cortical tissue mineral density (Cort. TMD), endosteal surface (Endo S), periosteal surface (Peri S), and bone area (BA).
Dynamic Histomorphometry
Mice were injected with Alizarin red and Calcein. Calcein (15mg/Kg) was injected at weeks 1 and 3 and alizarin red (15mg/Kg) was injected at weeks 2 and 4. Mice were sacrificed after 4 weeks and tibias were stored at −80C. Proximal tibiae were scanned using µCT and samples were prepared for histomorphometry using poly-methyl methacrylate resin (PMMA) embedding. Briefly, the bones were serially dehydrated using 70%, 90%, and 100% isopropyl alcohol, cleaned with petroleum ether and infiltrated with PMMA in three phases. In the first infiltration, the bones were incubated in 85% methyl methacrylate + 15% N-butyl phthalate, the second infiltration was done with 85% methyl methacrylate + 15 % N-butyl phthalate +1g/100ml benzyl peroxide and in third infiltration, the bones were incubated in 85% methyl methacrylate + 14 % N-butyl phthalate +2g/100ml benzoyl peroxide. At the completion of infiltration the samples were then embedded in 85% methyl methacrylate + 14 % N-butyl phthalate +2g/100ml Benzoyl Peroxide solution at 37°C. The tibias were polished and 5µm thick coronal sections were cut using a microtome (Leica Bannockburn, IL). Osteomeasure software (Osteometrics, Decatur, GA, USA) was used to trace calcein and alizarin red labels in proximal tibia. Areas below the growth plates were contoured manually (metaphysis). The areas of evaluation were consistent with the regions scanned by µCT. Regions of interest were assessed in cortical bone, trabecular bone, voids, and double and single labels. Samples were analyzed for mineral deposition rate (Mar, µm/day), defined as the distance between double labels divided by the interval of injections (14 days), double labels from alizarin and calcein were labeled separately and Mar values were averaged to determine average mineral deposition rate over 14 days. Mineralizing surface was obtained by adding the ratio of double label surface to 50% of the ratio of single label surface (dLS/BS + (0.5*sLS/BS). Mineralizing surface was calculated separately for alizarin red and calcein and average mineralizing area has been reported. Average bone formation rates were calculated by averaging alizarin red and calcein for BFR/BS (µm3/µm2/day) values. Data from dynamic histomorphometry using Osteomeaure was also used to calculated Bone volume / Tissue volume (BV/TV), bone surface/ bone volume (BS/BV), Trabecular Thickness (Tb.Th), and Trabecular separation (Tb.Sp).
Mechanical Testing
Four-point bending was performed to determine the stiffness and strength of left femora from the AM, NS, NU, SS, and SU groups. Four-point bending was selected over three point bending to minimize shear stresses. An MTS machine (585 Mini Bionix II) was used along with a 100 N force transducer (SMT1-100N, Interface). All samples were thawed slowly to room temperature. The femurs were loaded in the four-point bending jig such the mid-diaphysis of each femur was positioned in the middle of the supports (Figure 1), the posterior sides were facing upwards, and the load was applied on the posterior – anterior axis. The loading conditions were controlled by Multi-Purpose TestWare software (MTS). The piston was lowered with preload of 1.5 N and then subjected to a ramp load at a rate of 0.1mm/s until complete failure occurred. Force and strain data were recorded and exported to Microsoft Excel for analyses. Cortical beam theory was used to estimate longitudinal strain distribution at the mid-shaft region. The Force/Strain curves, along with the mid-diaphyseal µCT scans, were used to calculate Young’s modulus, stiffness, and strength using the equations listed below [35].
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
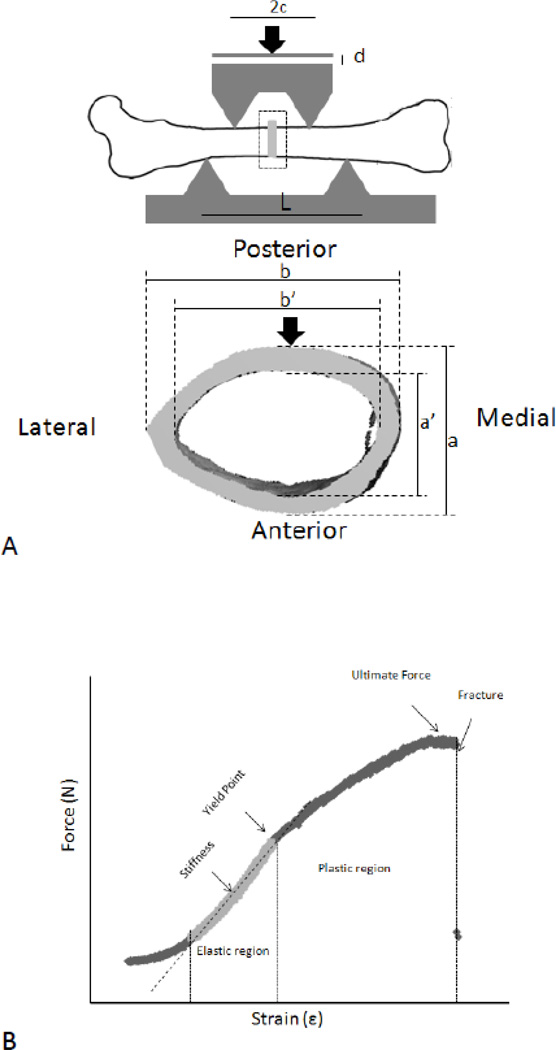
Figure 1.
a): Four-Point bending setup, the load was applied on the posterior – anterior axis. b): Typical force-strain curve generated by four-point bending. Stiffness was calculated from the tangent at the elastic region. Yield and ultimate strength were calculated using the yield point and ultimate force measurements from force-strain curve. The distance between two supports is set approximately at 7 mm; and the gap size between two loaders is set at 3 mm.
Moment of inertia (I) was calculated using equation 1 where a, b, a’, and b’ were measured using µCT images (Figure 1A). Based on previous published works [36, 37] mid-shaft of cortical bone of femur was assumed to be a hollowed structure with uniform diameter along mid-diaphysis. Elastic modulus (E, GPa) was calculated using stiffness (S, N/mm); force over strain data obtained from four-point bending; “L,” defined as the distance between the bottom supports (mm); and “c,” as the distance between the upper loaders and the midpoint of mid-diaphysis, in mm. Stiffness was measured as the slope of the plastic region from the force vs. strain graphs (Figure 1B). Elastic modulus was confirmed using equation 3, 4, and 5. Stress (σ, MPa) and strain (ε) were calculated by taking into account the applied force (F, N), displacement of piston (d, mm), a, c, I, and L.
Statistics
The GraphPad Prism 3.0 software was used to run statistical analyses. All of the data was presented in means ± standard deviation. One-way ANOVA with Newman Keulspost hoc was used to calculate significance within the different groups and time points; student t-test was used within contralateral controls. A p-value of <0.05 was considered to be significant.
Results
Micro CT Shows Anabolic effects of LIPUS in trabecular and cortical bone
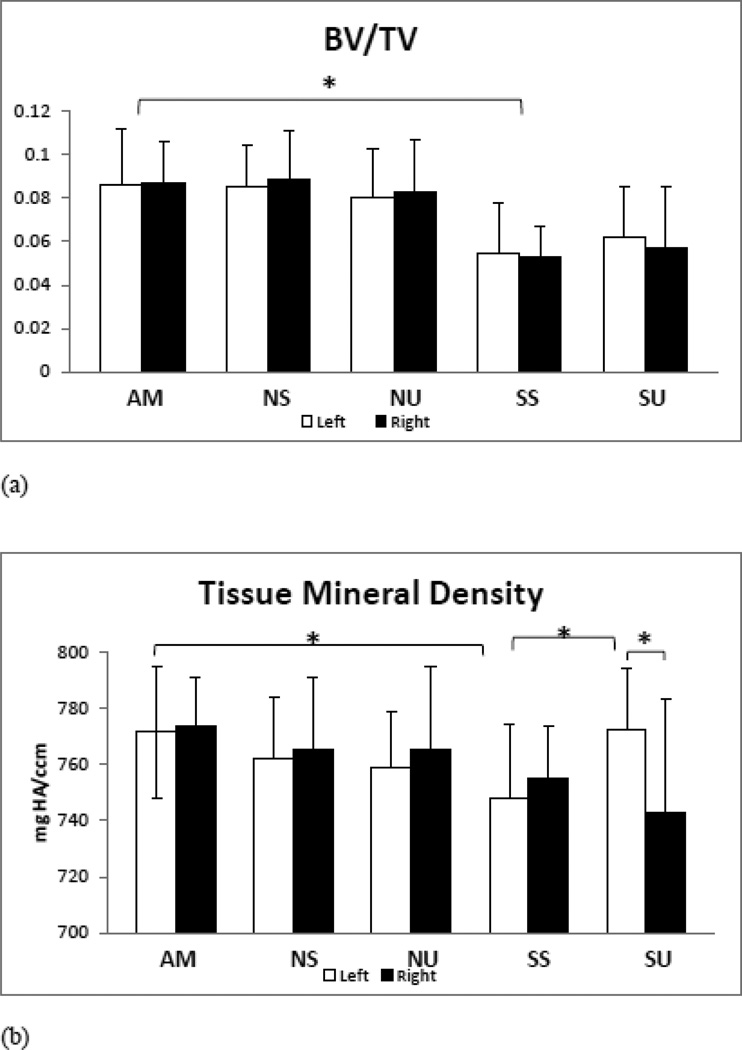
The µCT analyses of the proximal tibiae from AM mice showed dense and intact trabecular struts with no apparent differences when compared to either of the non-suspended groups (NS,NU) (Figure 2). In contrast, trabecular struts in suspended mice (SS) appear to be thinning and breaking down. LIPUS treated tibiae (SU, NU) demonstrate more intact and denser trabecular struts relative to untreated suspended mice (SS). Quantification of µCT parameters showed that SS mice had less −36% trabecular BV/TV (p<0.001, Figure 3a), −3% in bone tissue mineral density (TMD) (p<0.05, Figure 3b), −4% in Tb.N (p<0.05, Figure 3c), −12.5% in Tb.Th (p<0.005, Figure 3d), along with a +16%greater BS/BV, in comparison to AM mice (Table 1). Application of LIPUS increased trabecular TMD by +3% (p<0.05), Tb.Th by +6% (p<0.05), and lowered BS/BV by −10% (p<0.05). BV/TV, Tb.N, and Conn. Den showed positive trends but no significant changes were seen following exposure to LIPUS, when compared to SS mice. Tibiae from NS and NU mice didn’t show any differences with AM controls, suggesting that LIPUS has no adverse effect on healthy bone while showing anabolic effects in unloaded tibia. LIPUS effects were localized to treated left tibia as no significant differences were observed in non-treated NU and SU mice.
Figure 2.
Representative Proximal tibiae longitudinal and cross sectional images showing intact trabecular struts in AM, NS and NU mice. SS mice show disintegrated trabecular struts in proximal tibiae, exposure to LIPUS (SU) show improved trabecular struts structure.
Figure 3.
Series of bar graphs showing the results of µCT assessment of Trabecular Bone, showing significant changes in unloaded mice tibiae. LIPUS treatment show anabolic effects on trabecular bone by significantly increasing TMD, Tb.Th and reducing BS/BV. NS and NU tibiae didn’t show any difference with AM controls, implying that LIPUS has no adverse effect on healthy bone, while showing anabolic effects in unloaded tibiae.
a) BV/TV: −36 % bone volume fraction reduction was calculated in SS mice. LIPUS doesn’t increase bone volume fraction in suspended mice (SU). LIPUS exposure in non-suspended mice showed no difference.
b) TMD: Suspension (SS) significantly lowers bone mineral density in trabecular bone (app −2.5%), LIPUS treatment enhances bone mineral density in SU to nearly the level seen in age-matched animals and non-treated contralateral right tibia.
c) Tb.Th: SS mice showed significantly low trabecular thickness; SU mice showed significant increase.
d) Tb.N: Trabecular number was significantly low in HLS animals; LIPUS expose doesn’t seem to recover trabecular number.
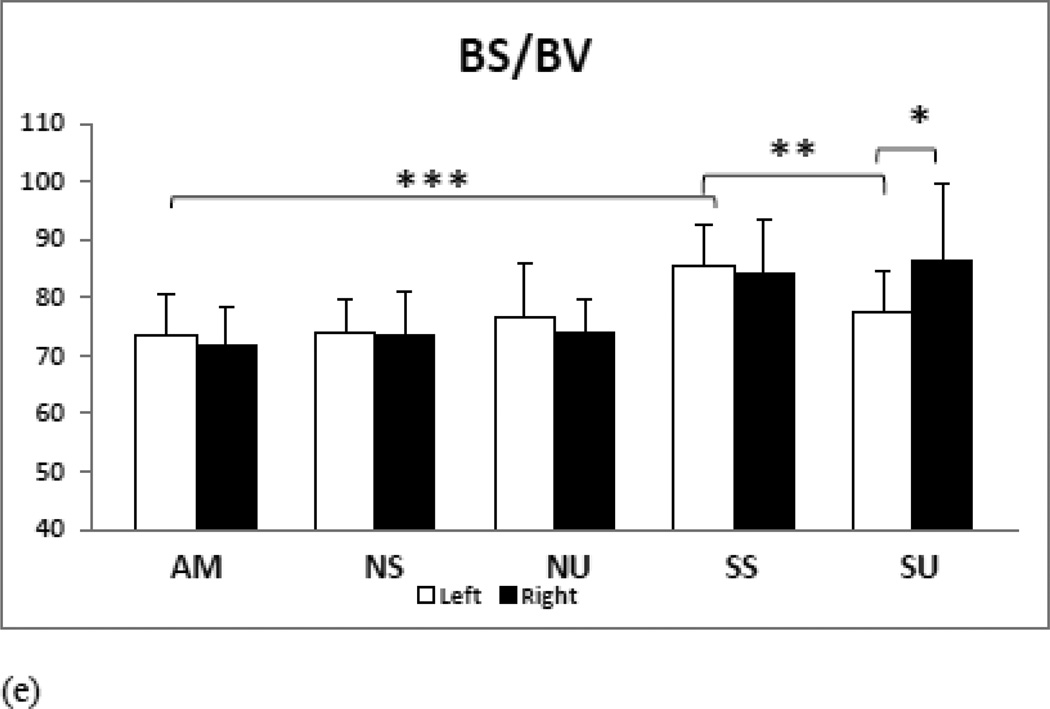
e) BS/BV: SS mice show osteopenic tendencies in bone surface area/volume ratio as it increased significantly and SU mice showed significant decrease in bone surface/volume ratio. Contralateral left tibia shows significant decrease BS/BV relative to non-treated right tibia.
*P < 0.05, **P<0.01, ***P<0.001
Table 1.
MicroCT analyses of trabecular bone at metaphysis of proximal tibia. This table effects of LIPUS on trabecular bone in suspended and non-suspended mice proximal tibia. BV/TV (Bone Volume/ Tissue Volume), TMD (Tissue mineral Density), Tb.Th (Trabecular Thickness), Conn. Den (connectivity Density), Tb.N (trabecular Number) and BS/BV (Bone Surface/Bone volume). Grey shaded rows are for treated tibia (left) and clear one are for non-treated (right) tibia.
| AM | NS | NU | SS | SU | |
|---|---|---|---|---|---|
| BV/TV | 0.086187±0.025 | 0.08515±0.019 | 0.08009±0.025 | 0.05475±0.023*** | 0.06176±0.024 |
| 0.08657±0.028 | 0.08862±0.023 | 0.08292±0.024 | 0.05245±0.014*** | 0.05686±0.089 | |
| TMD (mmHg/cc) | 771.343±23.60 | 762.037±21.77 | 758.847±19.99 | 747.862±26.49* | 772.502±21.50† |
| 773.603±17.07 | 765.202±25.60 | 765.352±29.6 | 754.623±18.72 | 742.396±40.58‡ | |
| Tb.Th (mm) | 0.03894±0.0026 | 0.03792±0.0031 | 0.03635±0.003 | 0.03407±0.0031** | 0.03633±0.004† |
| 0.03753±0.0028 | 0.03782±0.0036 | 0.03772±0.0028 | 0,03322±0.0014* | 0.03293±0.0042‡ | |
| Conn. Dent | 87.4428±48.26 | 83.7609±32.74 | 81.8453±47.15 | 41.4259±31.03 | 45.7749±41.11 |
| 79.756±48.31 | 92.7583±40.83 | 80.6469±45.09 | 36.3662±24.87 | 44.9833±40.68 | |
| Tb.N | 4.86308±0.49 | 4.91501±0.41 | 4.64126±0.47 | 4.18122±0.51** | 4.25086±0.55 |
| 4.82827±0.49 | 4.93701±0.45 | 4.88611±0.39 | 4.20087±0.46* | 3.94239±0.83 | |
| BS/BV | 73.5287±7.00 | 73.9497±5.66 | 76.4512±9.22 | 85.4138±6.97*** | 77.6242±6.96†† |
| 71.7236±6.66 | 73.5559±7.53 | 74.0938±5.50 | 84.309±8.97** | 86.4818±13.03‡ | |
| SMI | 3.0604±0.292 | 3.043646±0.315 | 3.032743±0.235 | 3.298327±0.375 | 3.359457±0.502 |
| 3.098021±0.394 | 3.031267±0.225 | 3.037623±0.302 | 3.377218±0.308 | 3.3386±0.525 |
P<0.05,
P<0.01,
P<0.001 relative to AM.
P < 0.05,
P< 0.01 relative to SS
P<0.05,
P<0.01 relative to left tibia (contra-lateral control)
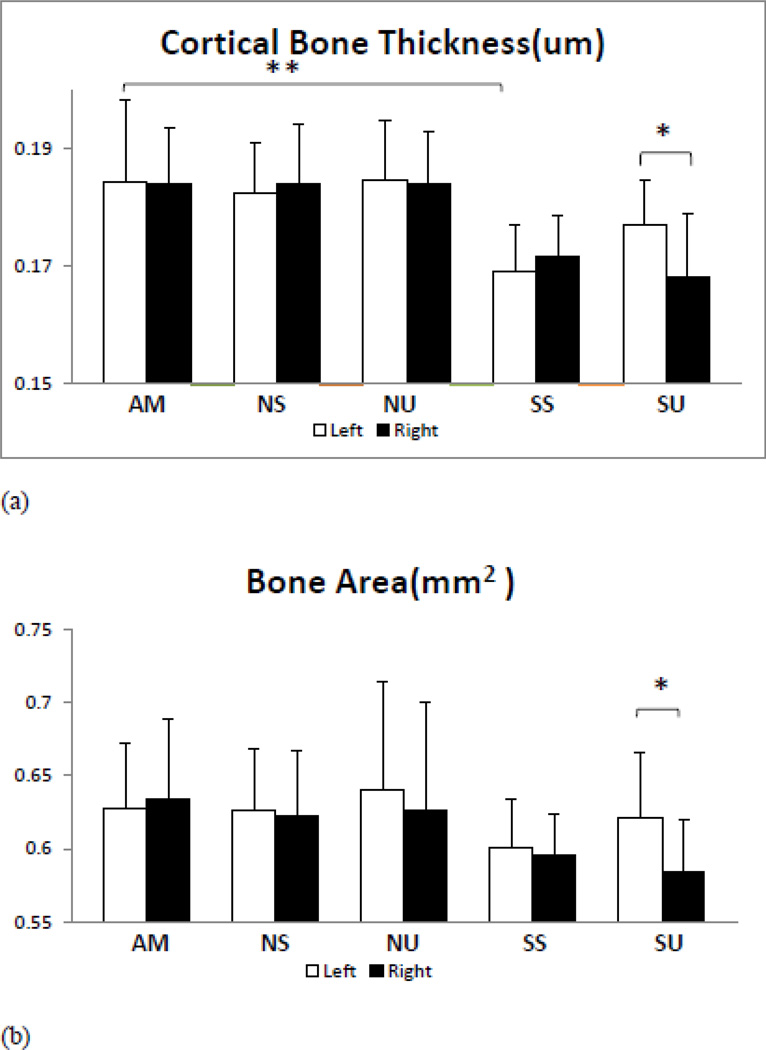
SS group had lower Cort.Th relative to AM mice (−8%, (p< 0.01, Figure 4a), while the other parameters didn’t show any significant changes between AM and SS groups. However, there were trends towards decreased Cort, TMD, Inertia, MoI, Peri S, and BA (Table 2, Figure 4b) but no significant difference. Exposure to LIPUS increased Cort.Th (p<0.05), Peri S (p<0.05), and BA (p<0.05) in SU animals, when compared to contralateral controls (left tibia vs. right tibia), Table 2. Differences between the left tibia of SS and SU were not significant but positive trends were apparent. Trabecular bone was more responsive to unloading and LIPUS treatment than cortical bone. It is speculated that a longer duration of study will show significant difference for cortical bone parameters.
Figure 4.
Cortical bone analyses show lesser effect on cortical bone in SS mice tibia but adverse trend is apparent in inertia, Cort. TMD, MoI, BA and Peri S. LIPUS exposure show significant increase in Cort.Th, BA and Peri S when compared to contralateral controls (left vs. right tibia). No significant differences were observed in NS and NU tibia when compared to AM.
a) Cort.Th: A significant decrease in SS tibia cortical thickness, LIPUS increases Cort.Th significantly relative to untreated tibia of SS animals. SU/SS comparison show positive trend in SU tibia but difference is not significant.
b) BA: Bone Area show similar trends to Peri S, with decreased BA in SS tibia and significant increase SU-L relative to SU-R.
*P < 0.05, **P<0.01, ***P<0.001
Table 2.
MicroCT analyses of cortical bone at mid-diaphysis of tibia. This table effects of LIPUS on cortical bone in suspended and non-suspended mice proximal tibia. Cort.Th (Cortical Thickness), TMD (Tissue mineral Density), Endo.S (Endosteal Surface), Peri. S (Periosteum Surface) and pMOI (polar Moment of Inertia). Grey shaded rows are for treated tibia (left) and clear one are for non-treated (right) tibia.
| Index | AM | NS | NU | SS | SU |
|---|---|---|---|---|---|
| Cort. Th (mm) | 0.184293±0.008 | 0.182508±0.008 | 0.184818±0.0099 | 0.16905±0.008** | 0.176936±0.008 |
| 0.184117±0.010 | 0.183936±0.010 | 0.1842±0.0087 | 0.171663±0.007 | 0.168238±0.010‡ | |
| TMD (mmHg/cc) | 1065.14056±23.97 | 1062.00229±22.30 | 1059.52109±17.48 | 1053.97±16.116 | 1050.53±16.23 |
| 1007.65±21.31 | 1065.93114±20.38 | 1057.894109±18.86 | 1048.327±18.558 | 1051.641±16.64 | |
| Endo. S (mm2) | 2.841675±0.26 | 2.77561667±0.20 | 2.912536364±0.27 | 2.86255±0.28 | 2.9799±0.244 |
| 2.741357±0.27 | 2.8249±0.36 | 2.773427273±0.19 | 2.8973±0.24 | 2.89912±0.21 | |
| Peri. S (mm2) | 4.4008±0.54 | 4.275±0.40 | 4.469244±0.48 | 4.1849±0.47 | 4.4698±0.39 |
| 4.3609±0.36 | 4.3043±0.29 | 4.3569±0.44 | 4.1527±0.37 | 4.1412±0.32‡ | |
| Bone Area (mm2) | 0.6281±0.04 | 0.627±0.04 | 0.640847273±0.07 | 0.60057±0.033 | 0.6214±0.04 |
| 0.6341±0.05 | 0.6231±0.04 | 0.626433636±0.05 | 0.59561±0.0289 | 0.5847±0.035‡ | |
| pMOI(mm4) | 0.2163±0.02 | 0.212±0.04 | 0.2167011±0.029 | 0.19122±0.035 | 0.2059±0.037 |
| 0.2148±0.05 | 0.2112±0.04 | 0.21377±0.05 | 0.19384±0.034 | 0.20286±0.014 |
P<0.05,
P<0.01,
P<0.001 relative to AM
P < 0.05,
P< 0.01 relative to SS
P<0.05,
P<0.01 relative to left tibia (contra-lateral control)
Dynamic Histomorphometry
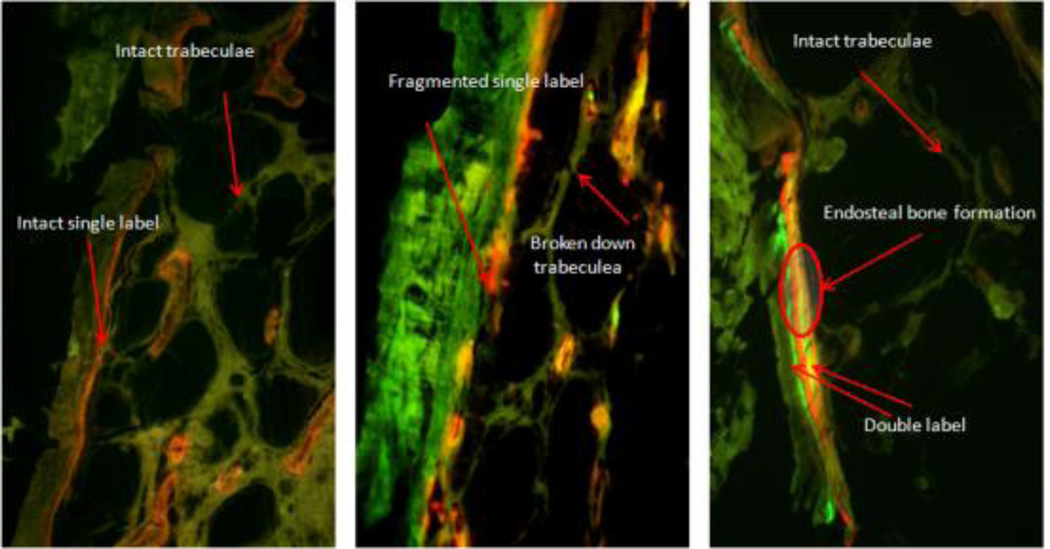
Histomorphometric analyses were only performed on samples from the AM, SS and SU groups as no significant difference were observed in non-suspended groups (NS and NU) in the µCT data. AM mice showed compact cortical bone with intact trabecular struts, along with strong single labeling most prevalent in endosteal and trabecular sites (Figure 5, AM). In contrast, SS mice showed visibly broken and disoriented trabeculi. Fluorochrome labeling was dispersed with sporadic single labels mostly confined to trabecular bone and endosteal surfaces (Figure 5, SS). LIPUS treated tibiae retained their normal bone microstructure and visible double and single labels present on the endosteal surface and trabeculi (Figure 5, SU).
Figure 5.
Mice were injected with calcein (weeks 1 and 3) and alizarin red (weeks 2 and 4). Analyses were performed on proximal tibia metaphyseal sections. AM mice showed intact microstructure along with seamless single labeling, SS mice showed broken down microstructure with little or no labeling, and SU mice showed improved microstructure and with enhanced double labeling along endosteal and trabecular surfaces.
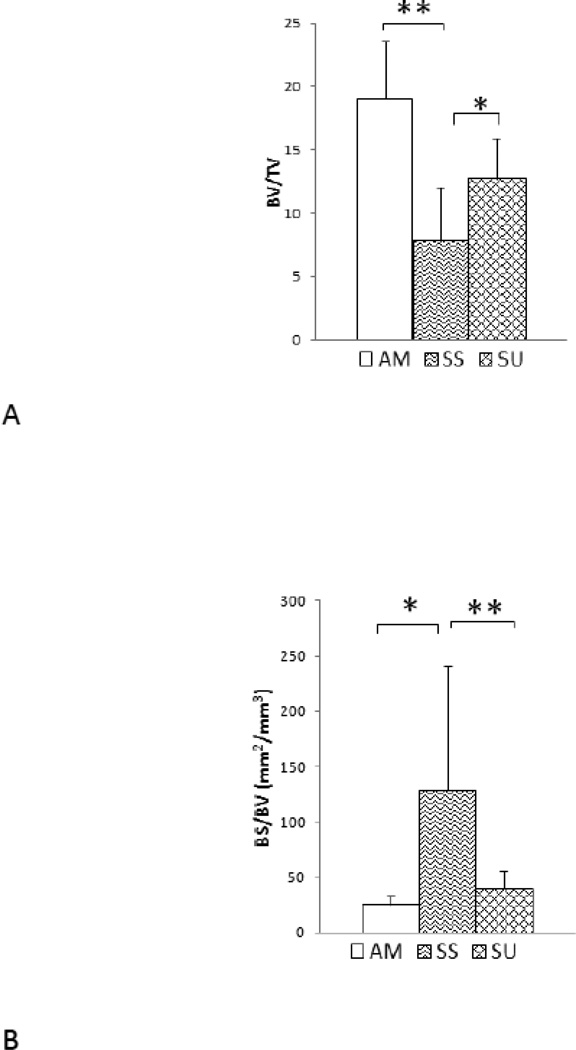
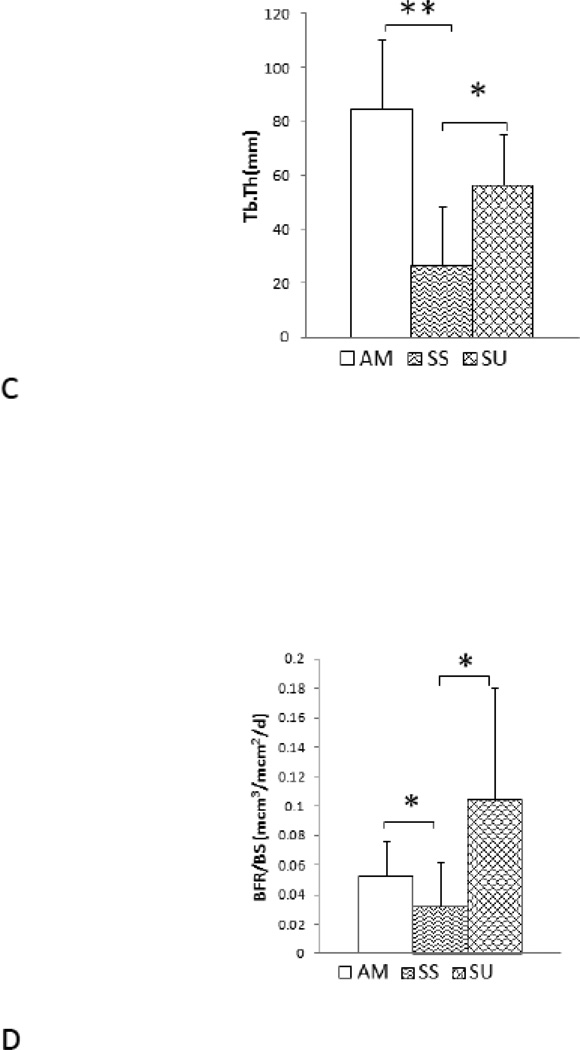
The histomorphometry data were consistent with those from µCT scanning (Table 3). As compared to age-matched controls, SS mice had −41% BV/TV (p<0.05, Figure 8 a) and −31% Tb.Th (p<0.05, Figure 8). LIPUS treatment had anabolic effects as SU mice showed significant increases of +38% in BV/TV (p<0.05) and +53% in Tb.Th (p<0.05) in comparison to SS mice in the metaphyseal region. In addition, BS/BV and Tb.Sp showed significant increases in SS mice compared to SU, and LIPUS exposure significantly decreased BS/TV (p<0.05, Figure 6 c). The trabecular bone formation rate decreased in SS mice, while treatment with LIPUS significantly increased BFR/BS (p<0.05, Figure 6d) in proximal tibia metaphysis. Furthermore MAR, and mineralization surface was reduced after 4 weeks of suspension. However, application of LIPUS significantly increased BFR/BS and mineralization surface, while MAR values showed positive trends.
Table 3.
Histomorphometry analyses of disuse proximal tibia metaphysis. This table shows significantly decreased trabecular BV/TV, Tb.Th, MS and BFR/BS and increase BS/BV and Tb.Sp in SS mice relative to AM mice. SU mice showed significantly increased BV/TV, Tb.Th, MA and BF/BS and decreased BS/BV and Tb.Sp in comparison to SS mice.
| Index | AM | SS | SU |
|---|---|---|---|
| BV/TV | 19.04±4.54 | 7.81±4.24* | 12.72±3.21† |
| Tb.Th(µm) | 84.70±25.91 | 26.58±22.02* | 56.64±18.75† |
| BS/BV | 25.42±7.43 | 128.78±112.09* | 35.46±16.19† |
| Tb.Sp(µm) | 317.21±67.50 | 427.42±211.35* | 369.88±160.71 |
| BFR/BS (µm3/(µm2)/d) | 0.052±0.024 | 0.031±0.30* | 0.104±0.07† |
| MS | 4.01±1.42 | 3.00±0.84 | 5.90±2.37 |
| MAR (µm/day) | 1.41±1.01 | 0.75±0.55 | 1.57±0.92† |
P<0.05 compared to age-matched
p< 0.05 compare to SS
Figure 6.
Histomorphometric analyses were performed using Osteomeasure. Bar graphs showing the results of this analysis for a, b, c, d are shown. SS mice proximal metaphysis show significant decreases in BV/TV, Tb.Th and BFR/BS. LIPUS treatment practically retains BV/TV and Tb.Th while significantly enhancing BFR and mineralization surface.
a) BV/TV: −41% decreases in SS tibia metaphysis, application of LIPUS mitigated(SU) BV/TV by +38% relative to SS mice
b) BS/BV: Greater in SS mice, SU metaphysis show significant reduction in BS/BV ratio.
c) Tb.Th lowered by −31% in SS mice relative to AM animals. Application of LIPUS metaphysis retains +53% more Tb.Th then SS mice.
d) BFR/BS: significant lowers the rate of bone formation in SS mice, LIPUS exposure induces greater rate of bone formation in SU metaphysis.
*P < 0.05, **P<0.01, ***P<0.001
LIPUS improving femoral mechanical properties under disuse
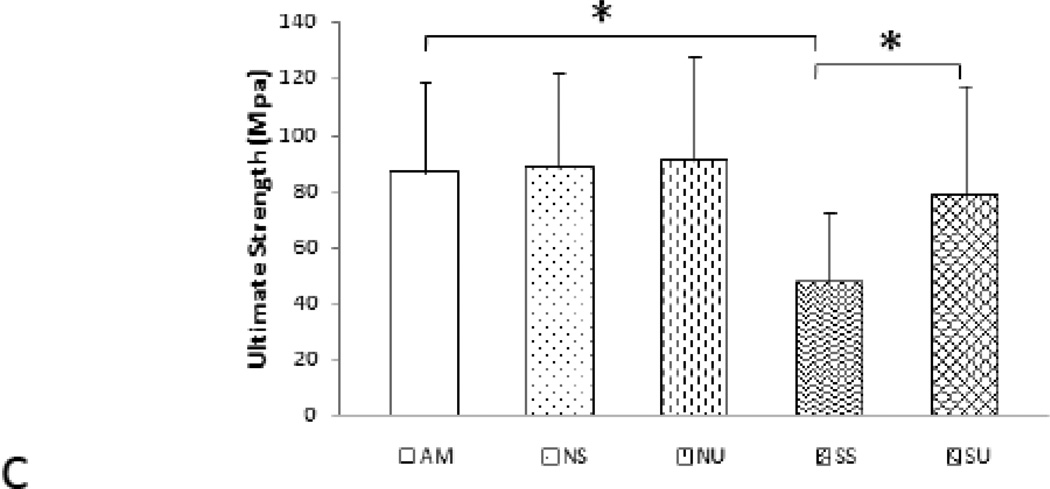
Although µCT analyses of femoral mid-diaphysis showed no significant decreases in cortical thickness in SS mice compared to AM mice, significant differences in elastic modulus, yield strength, and ultimate strength were seen for the SS mice in comparison to AM, NS, and NU mice (Table 4). Suspension significantly compromised femoral mechanical properties as SS femurs had a −53% elastic modulus (p<0.05, Figure 7a), −33% yield strength, (p<0.05, Figure 7b) and −45% ultimate bone strength when compared to AM femurs (Figure 7). LIPUS treatment significantly improved the mechanical properties of the mid-diaphysis. Femoral elastic modulus enhanced by +42% (p< 0.05), yield stress by +29% (p<0.05), and ultimate strength by +39% (p<0.05), in SU compared to SS. Contralateral femora of SU mice confirmed the increase in elastic modulus, yield strength, and ultimate strength in LIPUS treated femurs. Finally, compared to AM mice, SU mice showed an 18% decrease in elastic modulus, 6% decrease in yield strength, and 10 % decrease in ultimate strength (Figure 7 b, c). Table 4, shows the comparison between the groups and contralateral controls.
Table 4.
(a) Four Point Mechanical testing analyzing bone elastic modulus and strength in disuse condition and anabolic effects of LIPUS of femoral mid-diaphysis, TMD (Tissue Mineral Density). Gray row represent treated (left) tibia, Clear rows represent un-treated (right) tibia
(b) Mechanical property units.
| (a) | |||||
|---|---|---|---|---|---|
| Index | AM | NS | NU | SS | SU |
| Cortical Thickness (mm) | 0.149017±0.008 | 0.14381±0.009 | 0.14182±0.007 | 0.13205±0.012* | 0.13295±0.008 |
| 0.146117±0.009 | 0.14824±0.017 | 0.14039±0.014 | 0.13553±0.010* | 0.13997±0.012 | |
| TMD (mmhg/cc) | 1102.703±22.82 | 1099.54±54.05 | 1091.68±11.70 | 1088.49±32.83 | 1092.29±19.68 |
| 1084.59±24.29 | 1089.04±28.46 | 1073.99±26.16 | 1069.35±17.36 | 1075.57±25.57 | |
| Elastic Modulus (GPa) | 1730.66± 631.94 | 1581.32±737.71 | 1578.52±712.35 | 811.86±344.52* | 1407.64±581.01† |
| 1772.12±682.38 | 1793.27±619.87 | 1881.03±642.78 | 726.48±316.18 | 792.76±310.41‡‡ | |
| Yield Strength (MPa) | 69.79 ±25.77 | 74.94±37.65 | 75.28±27.86 | 46.41±18.99* | 65.44±26.98† |
| 74.38±25.27 | 75.52±30.98 | 69.12±12.99 | 33.07±12.20 | 34.18±12.67‡ | |
| Ultimate Strength (MPa) | 86.66±31.22 | 88.68±32.45 | 91.19±35.80 | 47.49±25.02* | 78.07±38.78† |
| 92.03±35.22 | 90.40±44.66 | 81.21±22.01 | 36.42±13.29 | 45.45±15.60‡‡ | |
| (b) | ||||||
|---|---|---|---|---|---|---|
| Parameter | Bone Length | Cross Section Area |
Young’s Modulus (E) |
Strain (ε) | Stress (σ) | Mechanical Strength |
| Unit | mm | mm^2 | GPa | ε or με | MPa | MPa |
P<0.05,
P<0.01,
P<0.001 relative to AM
P < 0.05,
P< 0.01 relative to SS,
P<0.05
P<0.01 relative to left tibia (contra-lateral control)
Figure 7.
Four point bending bar charts showing significantly decrease in mechanical properties of SS mice femur (Elastic modulus, yield and ultimate strength). LIPUS exposure retains mechanical integrity of femoral bone.
a) Elastic modulus: SS mice showed a significant decrease in elastic modulus relative to AM. LIPUS exposure increased elastic modulus in SU mice.
b) Ultimate strength: decreased significantly in SS femurs in comparison to AM mice. SU showed a significant increase in ultimate strength relative to SS mice.
c) Yield stress: significantly decreased in SS mice. LIPUS exposure increased yield stress relative to SS mice.
Discussion
Disuse osteoporosis is a degenerative bone pathology that compromises bone strength and leads to an increased risk for fractures. The bone loss is concentrated on weight bearing limbs. The localized pathology of disuse osteoporosis favors a targeted and non-invasive approach to treatment, which can provide the essential mechanical stimuli required to maintain bone quality. Since LIPUS has shown encouraging results in providing an anabolic stimulus that promotes bone healing, this study examined LIPUS as a potential countermeasure for disuse induced bone loss. The µCT results confirmed the detrimental effects of disuse on trabecular and cortical bone, with trabecular bone showing more sensitivity to disuse than cortical bone. Trabecular bone showed significant decrease in, TMD, Tb.Th, and Tb.N along with an increase in BS/BV. Cortical bone showed significant losses in thickness with lesser effects on TMD, BA, MOI, Endo S, and Peri S. Application of LIPUS mitigated the decreases in trabecular TMD, Tb.Th, and showed increases in BS/BV. LIPUS treatment partially mitigated the losses in cortical thickness and bone area. The contralateral untreated right tibiae (SU) did not show changes in bone microstructure relative to treated left tibiae indicative of the targeted nature of LIPUS stimulation.
Histomorphometry results confirmed µCT results, showing that LIPUS treated mice retained bone microstructural integrity and had increased bone formation rates. Furthermore, the histomorphometry data showed a significant increase in proximal tibiae BV/TV which was not apparent in the µCT analyses. This is likely due to the higher sensitivity of histomorphometry compared to µCT because of low resolution. The use of 12 micron resolution in the microCT measurement may be potentially one of the limitations in current study, while higher resolution, e.g., 6 micron or less, may yield more significant results. This may be implemented into future continuous study and may provide more insightful results into bone quality and quantity assessment. In addition, the histomorphometry images showed fluorochrome labels concentrated on endosteal and trabecular bone surfaces with little or no labeling at the periosteal surface. As the endosteal and trabecular surfaces are lined with osteoblasts, increased labeling is indicative of increased rates of bone formation by osteoblasts. Previous studies have shown increased bone loss on the endosteal surface and reduced trabecular thickness in response to disuse due lack of bone formation [38, 39]Our data indicate that LIPUS treatment can induce bone formation on endosteal and trabecular surfaces to counteract the adverse effects of disuse.
The biomechanical effects of tail suspension and LIPUS treatment were assessed using four point bending. Euler-Bernoulli-Navier beam theory equations were used to calculate the elastic modulus and strength at mid-diaphysis. Calculation errors due to assumptions of beam theory were minimized by testing bone mechanical properties at mid-diaphysis and four-point bending were preferred to minimize shear induced deflection [35, 40]. Femoral mechanical properties were severely compromised in SS animals despite no differences being found for TMD and cortical thickness of AM and SS. This suggests that other factors such as collagen alignment, collagen cross-linking, porosity, microcracks etc. may play an important role in determining bone mechanical properties. Application of LIPUS partially restored the mechanical properties of bone without significantly affecting cortical thickness and TMD. In vitro studies have shown that application of LIPUS improves collagen cross-linking in calcified matrix by increasing prostaglandin E2 activity in osteoblast cell cultures [41]. It is speculated that the absence of mechanical stimulus when under disuse may adversely affect collagen cross-linking, leading to compromised mechanical properties, and LIPUS stimulation partially mitigates this effect to improve biomechanical properties.
The results of current study indicate that LIPUS has the strong potential to be used as a targeted, non-invasive and non-pharmacological countermeasure for disuse induced bone loss. Increased calcein and alizarin red staining on endosteal and trabecular surfaces indicated that LIPUS was increasing bone formation, but the current study did not examine the effects of LIPUS on osteoclast activity and/or numbers. Bone homeostasis is preserved by the coupled activities of osteoblasts and osteoclasts. Increased osteoblast activity can reduce osteoclast activity and differentiation. Studies have shown that LIPUS stimulation increases osteoblast activity, resulting in increased production of TGF-β3 and reduced levels of TNF-α and IL-6 [42], which combine to decrease osteoclast activity [43, 44]. Furthermore, LIPUS decreases RANKL expression in osteoblasts, which is essential for osteoclast differentiation (unpublished data). Considering the effects of LIPUS stimulation from in vitro studies it is possible that LIPUS decreases osteoclastogenesis and osteoclast activity, leading to reduced bone resorption. However, more detailed in vivo studies are required to fully determine the effects of LIPUS on osteoclast activity and differentiation.
These results indicate localized anabolic effects of LIPUS in disuse induced bone loss. LIPUS has the potential to be used in clinical settings as in non-invasive, targeted therapy for age related, post-menopausal and long term bed rest induced osteopenia and osteoporosis. Furthermore, LIPUS provides a portable and easy-to-comply-with therapy for microgravity induced bone loss in astronauts and can be a pivotal therapy to increase the duration of time astronauts can spend in microgravity, as well as speeding rehabilitation upon returning to earth. Data from contralateral controls showed no effects due to LIPUS treatment thus indicating localized and targeted stimulation of bone cells without any adverse effects on healthy bone tissue.
The exact mechanism through which LIPUS enhances osteoblast activity and increases the rate of bone formation remains unknown. Ultrasound is speculated to induce acoustic streaming in interstitial fluid and localized mechanical vibrations in the extracellular matrix [45]. This results in local deformation of cell membrane and the induction of shear stresses and strains in osteoblasts [16, 17, 46–49]. These mechanical deformations activate receptors on the cell membrane such as integrin, mechanosensitive-calcium channels, G-proteins, IGF, TGF-β/BMP, and gap junctions, all of which activate different downstream pathways [48, 50–54].
This study explored the potential of LIPUS as a non-invasive, non-pharmacological targeted therapy for disuse osteoporosis. The results suggest that LIPUS has very strong potential as a non-invasive and targeted anabolic agent for disuse osteoporosis. The study did not evaluate the effects of LIPUS on osteoclast and osteocytes activity in vivo and futures studies will be required to study these responses as well as perform in-depth analyses of alterations in bone matrix and gene expression in order to begin to understand the underlying mechanism.
Highlights.
Low-intensity ultrasound stimulation mitigated the adverse effects of disuse on bone density and elastic modulus, and ultimate strength.
The mitigation effect of LIPUS on disuse trabecular bone was highly related to the structural integrity.
This study provides valuable information for acoustic radiation force as therapeutic agent to countermeasure disuse osteopenia.
Acknowledgements
This work is kindly supported by the National Institute of Health (R01 AR52379 and R01AR61821), the US Army Medical Research and Materiel Command, and the National Space Biomedical Research Institute through NASA contract NCC 9-58. The authors would like to thank all the members in the Orthopaedic Bioengineering Research Lab, particularly for Drs. Jiqi Cheng, Wei Lin, Liangjun Lin, and Suzanne Ferreri, for their assistance and discussion in this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: All authors state that they have no conflicts of interest.
References
- 1.Simske SJ, Guerra KM, Greenberg AR, Luttges MW. The physical and mechanical effects of suspension-induced osteopenia on mouse long bones. J Biomech. 1992;25:489–499. doi: 10.1016/0021-9290(92)90089-j. [DOI] [PubMed] [Google Scholar]
- 2.Oppl B, Michitsch G, Misof B, Kudlacek S, Donis J, Klaushofer K, Zwerina J, Zwettler E. Low bone mineral density and fragility fractures in permanent vegetative state patients. J Bone Miner Res. 2014;29:1096–1100. doi: 10.1002/jbmr.2122. [DOI] [PubMed] [Google Scholar]
- 3.Broe KE, Hannan MT, Kiely DK, Cali CM, Cupples LA, Kiel DP. Predicting fractures using bone mineral density: a prospective study of long-term care residents. Osteoporos Int. 2000;11:765–771. doi: 10.1007/s001980070055. [DOI] [PubMed] [Google Scholar]
- 4.Thomsen JS, Christensen LL, Vegger JB, Nyengaard JR, Bruel A. Loss of bone strength is dependent on skeletal site in disuse osteoporosis in rats. Calcif Tissue Int. 2012;90:294–306. doi: 10.1007/s00223-012-9576-7. [DOI] [PubMed] [Google Scholar]
- 5.Lanyon LE. The Influence of Function on the Development of Bone Curvature - an Experimental-Study on the Rat Tibia. Journal of Zoology. 1980;192:457–466. [Google Scholar]
- 6.Leblanc AD, Schneider VS, Evans HJ, Engelbretson DA, Krebs JM. Bone mineral loss and recovery after 17 weeks of bed rest. J Bone Miner Res. 1990;5:843–850. doi: 10.1002/jbmr.5650050807. [DOI] [PubMed] [Google Scholar]
- 7.Kiratli BJ, Smith AE, Nauenberg T, Kallfelz CF, Perkash I. Bone mineral and geometric changes through the femur with immobilization due to spinal cord injury. J Rehabil Res Dev. 2000;37:225–233. [PubMed] [Google Scholar]
- 8.Vico L, Collet P, Guignandon A, Lafage-Proust MH, Thomas T, Rehaillia M, Alexandre C. Effects of long-term microgravity exposure on cancellous and cortical weight-bearing bones of cosmonauts. Lancet. 2000;355:1607–1611. doi: 10.1016/s0140-6736(00)02217-0. [DOI] [PubMed] [Google Scholar]
- 9.Little DG, Cornell MS, Hile MS, Briody J, Cowell CT, Bilston L. Effect of pamidronate on distraction osteogenesis and fixator-related osteoporosis. Injury. 2001;32(Suppl 4):SD14–SD20. doi: 10.1016/s0020-1383(01)00161-9. [DOI] [PubMed] [Google Scholar]
- 10.Gerstenfeld LC, Sacks DJ, Pelis M, Mason ZD, Graves DT, Barrero M, Ominsky MS, Kostenuik PJ, Morgan EF, Einhorn TA. Comparison of effects of the bisphosphonate alendronate versus the RANKL inhibitor denosumab on murine fracture healing. J Bone Miner Res. 2009;24:196–208. doi: 10.1359/jbmr.081113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hock JM, Gera I, Fonseca J, Raisz LG. Human parathyroid hormone-(1–34) increases bone mass in ovariectomized and orchidectomized rats. Endocrinology. 1988;122:2899–2904. doi: 10.1210/endo-122-6-2899. [DOI] [PubMed] [Google Scholar]
- 12.Li X, Ominsky MS, Warmington KS, Morony S, Gong J, Cao J, Gao Y, Shalhoub V, Tipton B, Haldankar R, Chen Q, Winters A, Boone T, Geng Z, Niu QT, Ke HZ, Kostenuik PJ, Simonet WS, Lacey DL, Paszty C. Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J Bone Miner Res. 2009;24:578–588. doi: 10.1359/jbmr.081206. [DOI] [PubMed] [Google Scholar]
- 13.Lems WF, Geusens P. Established and forthcoming drugs for the treatment of osteoporosis. Curr Opin Rheumatol. 2014;26:245–251. doi: 10.1097/BOR.0000000000000057. [DOI] [PubMed] [Google Scholar]
- 14.Varenna M, Bertoldo F, Di Monaco M, Giusti A, Martini G, Rossini M. Safety profile of drugs used in the treatment of osteoporosis: a systematical review of the literature. Reumatismo. 2013;65:143–166. doi: 10.4081/reumatismo.2013.143. [DOI] [PubMed] [Google Scholar]
- 15.O'Brien M. Exercise and osteoporosis. Ir J Med Sci. 2001;170:58–62. doi: 10.1007/BF03167724. [DOI] [PubMed] [Google Scholar]
- 16.Boutahar N, Guignandon A, Vico L, Lafage-Proust MH. Mechanical strain on osteoblasts activates autophosphorylation of focal adhesion kinase and proline-rich tyrosine kinase 2 tyrosine sites involved in ERK activation. J Biol Chem. 2004;279:30588–30599. doi: 10.1074/jbc.M313244200. [DOI] [PubMed] [Google Scholar]
- 17.Buckley MJ, Banes AJ, Levin LG, Sumpio BE, Sato M, Jordan R, Gilbert J, Link GW, Tran Son Tay R. Osteoblasts increase their rate of division and align in response to cyclic, mechanical tension in vitro. Bone Miner. 1988;4:225–236. [PubMed] [Google Scholar]
- 18.Jagodzinski M, Drescher M, Zeichen J, Hankemeier S, Krettek C, Bosch U, van Griensven M. Effects of cyclic longitudinal mechanical strain and dexamethasone on osteogenic differentiation of human bone marrow stromal cells. Eur Cell Mater. 2004;7:35–41. doi: 10.22203/ecm.v007a04. discussion 41. [DOI] [PubMed] [Google Scholar]
- 19.Jessop HL, Rawlinson SC, Pitsillides AA, Lanyon LE. Mechanical strain and fluid movement both activate extracellular regulated kinase (ERK) in osteoblast-like cells but via different signaling pathways. Bone. 2002;31:186–194. doi: 10.1016/s8756-3282(02)00797-4. [DOI] [PubMed] [Google Scholar]
- 20.Brouwers JE, van Rietbergen B, Ito K, Huiskes R. Effects of vibration treatment on tibial bone of ovariectomized rats analyzed by in vivo micro-CT. J Orthop Res. 2010;28:62–69. doi: 10.1002/jor.20951. [DOI] [PubMed] [Google Scholar]
- 21.Chow DH, Leung KS, Qin L, Leung AH, Cheung WH. Low-magnitude high-frequency vibration (LMHFV) enhances bone remodeling in osteoporotic rat femoral fracture healing. J Orthop Res. 2010 doi: 10.1002/jor.21303. [DOI] [PubMed] [Google Scholar]
- 22.Rubin C, Judex S, Qin YX. Low-level mechanical signals and their potential as a non-pharmacological intervention for osteoporosis. Age Ageing. 2006;35(Suppl 2):ii32–ii36. doi: 10.1093/ageing/afl082. [DOI] [PubMed] [Google Scholar]
- 23.Boyd JL, Holcomb JP, Rothenberg RJ. Physician treatment of osteoporosis in response to heel ultrasound bone mineral density reports. J Clin Densitom. 2002;5:375–381. doi: 10.1385/jcd:5:4:375. [DOI] [PubMed] [Google Scholar]
- 24.Carvalho DC, Cliquet Junior A. The action of low-intensity pulsed ultrasound in bones of osteopenic rats. Artif Organs. 2004;28:114–118. doi: 10.1111/j.1525-1594.2004.07091.x. [DOI] [PubMed] [Google Scholar]
- 25.Ferreri SL, Talish R, Trandafir T, Qin YX. Mitigation of bone loss with ultrasound induced dynamic mechanical signals in an OVX induced rat model of osteopenia. Bone. 2011 doi: 10.1016/j.bone.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agnusdei D, Camporeale A, Gennari C. Ultrasound transmission velocity in the patella of normal subjects and in patients with postmenopausal osteoporosis. Minerva Endocrinol. 1991;16:73–77. [PubMed] [Google Scholar]
- 27.Warden SJ, Bennell KL, Forwood MR, McMeeken JM, Wark JD. Skeletal effects of low-intensity pulsed ultrasound on the ovariectomized rodent. Ultrasound Med Biol. 2001;27:989–998. doi: 10.1016/s0301-5629(01)00376-3. [DOI] [PubMed] [Google Scholar]
- 28.Pohl P, Rosenfeld E, Millner R. Effects of ultrasound on the steady-state transmembrane pH gradient and the permeability of acetic acid through bilayer lipid membranes. Biochim Biophys Acta. 1993;1145:279–283. doi: 10.1016/0005-2736(93)90300-o. [DOI] [PubMed] [Google Scholar]
- 29.Heckman JD, Ryaby JP, McCabe J, Frey JJ, Kilcoyne RF. Acceleration of tibial fracture-healing by non-invasive, low-intensity pulsed ultrasound. J Bone Joint Surg Am. 1994;76:26–34. doi: 10.2106/00004623-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Azuma Y, Ito M, Harada Y, Takagi H, Ohta T, Jingushi S. Low-intensity pulsed ultrasound accelerates rat femoral fracture healing by acting on the various cellular reactions in the fracture callus. J Bone Miner Res. 2001;16:671–680. doi: 10.1359/jbmr.2001.16.4.671. [DOI] [PubMed] [Google Scholar]
- 31.Jingushi S. Bone fracture and the healing mechanisms. Fracture treatment by low-intensity pulsed ultrasound. Clin Calcium. 2009;19:704–708. [PubMed] [Google Scholar]
- 32.Jingushi S, Mizuno K, Matsushita T, Itoman M. Low-intensity pulsed ultrasound treatment for postoperative delayed union or nonunion of long bone fractures. J Orthop Sci. 2007;12:35–41. doi: 10.1007/s00776-006-1080-3. [DOI] [PubMed] [Google Scholar]
- 33.Cook SD, Ryaby JP, McCabe J, Frey JJ, Heckman JD, Kristiansen TK. Acceleration of tibia and distal radius fracture healing in patients who smoke. Clin Orthop Relat Res. 1997:198–207. doi: 10.1097/00003086-199704000-00022. [DOI] [PubMed] [Google Scholar]
- 34.Cook SD, Salkeld SL, Mse, Patron LP, Ryaby JP, Whitecloud TS. Low-intensity pulsed ultrasound improves spinal fusion. Spine J. 2001;1:246–254. doi: 10.1016/s1529-9430(01)00086-9. [DOI] [PubMed] [Google Scholar]
- 35.M P, Haron YHA. Wiley Encyclopedia of Biomedical Engineering. 2006 [Google Scholar]
- 36.Gross TS, Edwards JL, McLeod KJ, Rubin CT. Strain gradients correlate with sites of periosteal bone formation. J Bone Miner Res. 1997;12:982–988. doi: 10.1359/jbmr.1997.12.6.982. [DOI] [PubMed] [Google Scholar]
- 37.Gross TS, McLeod KJ, Rubin CT. Characterizing bone strain distributions in vivo using three triple rosette strain gages. J Biomech. 1992;25:1081–1087. doi: 10.1016/0021-9290(92)90044-2. [DOI] [PubMed] [Google Scholar]
- 38.Jaworski ZF, Liskova-Kiar M, Uhthoff HK. Effect of long-term immobilisation on the pattern of bone loss in older dogs. J Bone Joint Surg Br. 1980;62-B:104–110. doi: 10.1302/0301-620X.62B1.6985912. [DOI] [PubMed] [Google Scholar]
- 39.Uhthoff HK, Jaworski ZF. Bone loss in response to long-term immobilisation. J Bone Joint Surg Br. 1978;60-B:420–429. doi: 10.1302/0301-620X.60B3.681422. [DOI] [PubMed] [Google Scholar]
- 40.Wallace R, Pankaj P, Simpson A. Major Source of Error When Calculating Bone Mechanical Properties. J Bone Miner Res. 2014 doi: 10.1002/jbmr.2304. [DOI] [PubMed] [Google Scholar]
- 41.Saito M, Fujii K, Tanaka T, Soshi S. Effect of low- and high-intensity pulsed ultrasound on collagen post-translational modifications in MC3T3-E1 osteoblasts. Calcif Tissue Int. 2004;75:384–395. doi: 10.1007/s00223-004-0292-9. [DOI] [PubMed] [Google Scholar]
- 42.Li JK, Chang WH, Lin JC, Ruaan RC, Liu HC, Sun JS. Cytokine release from osteoblasts in response to ultrasound stimulation. Biomaterials. 2003;24:2379–2385. doi: 10.1016/s0142-9612(03)00033-4. [DOI] [PubMed] [Google Scholar]
- 43.Fuller K, Murphy C, Kirstein B, Fox SW, Chambers TJ. TNFalpha potently activates osteoclasts, through a direct action independent of and strongly synergistic with RANKL. Endocrinology. 2002;143:1108–1118. doi: 10.1210/endo.143.3.8701. [DOI] [PubMed] [Google Scholar]
- 44.Yoshitake F, Itoh S, Narita H, Ishihara K, Ebisu S. Interleukin-6 directly inhibits osteoclast differentiation by suppressing receptor activator of NF-kappaB signaling pathways. J Biol Chem. 2008;283:11535–11540. doi: 10.1074/jbc.M607999200. [DOI] [PubMed] [Google Scholar]
- 45.Baker KG, Robertson VJ, Duck FA. A review of therapeutic ultrasound: biophysical effects. Phys Ther. 2001;81:1351–1358. [PubMed] [Google Scholar]
- 46.Guignandon A, Akhouayri O, Usson Y, Rattner A, Laroche N, Lafage-Proust MH, Alexandre C, Vico L. Focal contact clustering in osteoblastic cells under mechanical stresses: microgravity and cyclic deformation. Cell Commun Adhes. 2003;10:69–83. [PubMed] [Google Scholar]
- 47.Jansen JH, Jahr H, Verhaar JA, Pols HA, Chiba H, Weinans H, van Leeuwen JP. Stretch-induced modulation of matrix metalloproteinases in mineralizing osteoblasts via extracellular signal-regulated kinase-1/2. J Orthop Res. 2006;24:1480–1488. doi: 10.1002/jor.20186. [DOI] [PubMed] [Google Scholar]
- 48.Plotkin LI, Mathov I, Aguirre JI, Parfitt AM, Manolagas SC, Bellido T. Mechanical stimulation prevents osteocyte apoptosis: requirement of integrins, Src kinases, and ERKs. Am J Physiol Cell Physiol. 2005;289:C633–C643. doi: 10.1152/ajpcell.00278.2004. [DOI] [PubMed] [Google Scholar]
- 49.Tang L, Lin Z, Li YM. Effects of different magnitudes of mechanical strain on Osteoblasts in vitro. Biochem Biophys Res Commun. 2006;344:122–128. doi: 10.1016/j.bbrc.2006.03.123. [DOI] [PubMed] [Google Scholar]
- 50.Hsu HC, Fong YC, Chang CS, Hsu CJ, Hsu SF, Lin JG, Fu WM, Yang RS, Tang CH. Ultrasound induces cyclooxygenase-2 expression through integrin, integrin-linked kinase, Akt, NF-kappaB and p300 pathway in human chondrocytes. Cell Signal. 2007;19:2317–2328. doi: 10.1016/j.cellsig.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 51.Takeuchi R, Ryo A, Komitsu N, Mikuni-Takagaki Y, Fukui A, Takagi Y, Shiraishi T, Morishita S, Yamazaki Y, Kumagai K, Aoki I, Saito T. Low-intensity pulsed ultrasound activates the phosphatidylinositol 3 kinase/Akt pathway and stimulates the growth of chondrocytes in three-dimensional cultures: a basic science study. Arthritis Res Ther. 2008;10:R77. doi: 10.1186/ar2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tang CH, Lu DY, Tan TW, Fu WM, Yang RS. Ultrasound induces hypoxia-inducible factor-1 activation and inducible nitric-oxide synthase expression through the integrin/integrin-linked kinase/Akt/mammalian target of rapamycin pathway in osteoblasts. J Biol Chem. 2007;282:25406–25415. doi: 10.1074/jbc.M701001200. [DOI] [PubMed] [Google Scholar]
- 53.Tang CH, Yang RS, Huang TH, Lu DY, Chuang WJ, Huang TF, Fu WM. Ultrasound stimulates cyclooxygenase-2 expression and increases bone formation through integrin, focal adhesion kinase, phosphatidylinositol 3-kinase, and Akt pathway in osteoblasts. Mol Pharmacol. 2006;69:2047–2057. doi: 10.1124/mol.105.022160. [DOI] [PubMed] [Google Scholar]
- 54.Yang RS, Lin WL, Chen YZ, Tang CH, Huang TH, Lu BY, Fu WM. Regulation by ultrasound treatment on the integrin expression and differentiation of osteoblasts. Bone. 2005;36:276–283. doi: 10.1016/j.bone.2004.10.009. [DOI] [PubMed] [Google Scholar]