Abstract
Long days (LDs) stimulate and short days (SDs) inhibit reproduction in photoperiodic rodents by modifying nocturnal pineal melatonin secretion. In LD Turkish hamsters, unlike other rodents, pinealectomy induces reproductive quiescence comparable to that produced by SDs. We assessed whether SDs and pinealectomy induce similar or different patterns of kisspeptin and gonadotropin-inhibitory hormone (also known as RFamide-related peptide-3 [RFRP-3] in mammals) expression, important mediators of seasonal reproductive changes in other species. Brains were harvested from sham-operated female Turkish hamsters maintained in LDs and SDs and LD-pealectomized (pinx) females, all housed in their respective photoperiods for 12 weeks. Uterine weights were substantially higher in LD-sham than in LD-pinx and SD-sham females. RFRP-3-immunoreactive(-ir) cells in the dorsomedial hypothalamic nucleus were greater in number and size in the reproductively competent LD-sham hamsters than in both reproductively suppressed SD-sham and LD-pinx hamsters. LD-sham hamsters had more kisspeptin-ir cells in the anteroventral periventricular nucleus than did LD-pinx hamsters. Reproductive quiescence, whether induced by short-day lengths or pinealectomy, was generally accompanied by comparable changes in RFRP-3 and kisspeptin, suggesting that long-duration melatonin signaling and withdrawal of melatonin by pinealectomy may act through the same neural substrates to induce gonadal quiescence.
Keywords: RFRP-3, RFamide-related peptide, KiSS-1, GRP54, GPR147, photoperiod, female reproduction
The Turkish hamster, Mesocricetus brandti, a cricetid rodent indigenous to Turkey, northern Syria, and Iraq, has been a useful model species for studies of hibernation and photoperiodism (Lyman and Obrien, 1977; Hall et al., 1982; Carter and Goldman, 1983; Batavia et al., 2013a, 2013b). In common with all other photoperiodic rodents (Bartness et al., 1993; Prendergast et al., 2002), Turkish hamsters cease reproductive activities when day lengths fall below a critical minimum; values below the critical duration induce the short-day reproductive phenotype (Hong et al., 1986). In Syrian and Siberian hamsters, all day lengths longer than the critical day length support reproduction (Elliott, 1976; Hoffmann, 1982); in marked contrast, only a narrow range of long photoperiods (15–17 h light per day) sustains the long-day reproductive phenotype in Turkish hamsters; day lengths outside this range, whether longer or shorter, induce reproductive arrest (Hong et al., 1986).
Day length is transduced into a pineal melatonin signal that acts on neural substrates to control reproduction (Carter and Goldman, 1983; Goldman, 2001). Long durations of nightly melatonin secretion are associated with short, winter-like days, whereas short melatonin durations accompany long, summer-like photoperiods. In photoperiodic rodents, removal of the pineal gland sustains the long-day reproductive phenotype. Pinealectomy accelerates recrudescence or premature growth of the quiescent gonads of adult and juvenile short-day individuals, respectively (Syrian hamsters, Siberian hamsters, meadow voles; Bartness et al., 1993; Kelly et al., 1994; Smale et al., 1988); activation of the reproductive axis in long days does not require pineal mediation in these species. In sharp contrast, suppression of melatonin secretion by pinealectomy or constant light induces testicular regression in long-day Turkish hamsters (Carter et al., 1982; Butler et al., 2008; Jarjisian and Zucker, 2011); pineal melatonin is necessary to sustain reproduction in long day lengths. The neural circuits and neurochemical systems underlying this atypical response to the absence of melatonin have not been explored.
Gonadotropin-releasing hormone (GnRH) neurons represent the final common pathway at which internal and external reproductively relevant stimuli converge to control reproduction (Bronson, 1989). In most rodents, GnRH cell bodies reside most densely in the nucleus of the diagonal band of Broca (NDB) and medial preoptic area (mPOA). GnRH neurons stimulate pituitary gonadotropin secretion that maintains gametogenesis and sex steroid secretion. Short days inhibit the release of GnRH, but not its synthesis, leading to increased numbers of GnRH-immunoreactive neurons in short-day Syrian hamsters (Ronchi et al., 1992a; Shiotani et al., 1985), white-footed mice (Glass, 1986), and prairie voles (Kriegsfeld and Nelson, 1999); some studies report no differences in the number of these neurons in short-day (SD) versus long-day (LD) Syrian hamsters (Urbanski et al., 1991) and Siberian hamsters (Yellon, 1994).
Two RFamide (Arg-Phe-NH2) neuropeptides upstream of the GnRH system, kisspeptin and gonadotropin-inhibitory hormone (also known as RFamide-related peptide-3 [RFRP-3] in mammals), drive seasonal changes in reproduction (Greives et al., 2008; Simonneaux et al., 2013; Tsutsui et al., 2013). During the reproductive season, RFRP-3 potently inhibits the reproductive axis across most species and conditions (Kriegsfeld et al., 2006; Ubuka et al., 2012; Tsutsui et al., 2000; but see Caraty et al., 2012). Interestingly, exogenous RFRP-3 administration during reproductive quiescence stimulates reproductive behavior and physiology (Ancel et al., 2012; Ubuka et al., 2012). In fitting with this stimulatory effect, RFRP-3 peptide and mRNA expression are reduced in Syrian and Siberian hamsters exposed to SDs or long-duration melatonin signals (Mason et al., 2010; Revel et al., 2008; Ubuka et al., 2012).
Kisspeptin, on the other hand, stimulates gonadotropin secretion during both short and long day lengths (de Roux et al., 2003; Funes et al., 2003; Seminara et al., 2003; Gottsch et al., 2004; Navarro et al., 2005). The primary populations of kisspeptin neurons reside in the anteroventral periventricular (AVPV) and arcuate (ARC) nuclei (reviewed in Lehman et al., 2013). A long-duration melatonin signal induced by short day lengths reduces kisspeptin mRNA and the number of kisspeptin-positive cells in the ARC in male Syrian hamsters; this effect is eliminated by pinealectomy (Revel et al., 2006a; Simonneaux et al., 2013). In male and female Siberian hamsters, kisspeptin-ir is reduced in the AVPV and increased in the ARC of SD compared to LD hamsters (Greives et al., 2007; Mason et al., 2007). Kisspeptin replacement reactivates the reproductive axis and breaks photoinhibition in male Syrian hamsters (Revel et al., 2006a; Ansel et al., 2011).
We determined the pattern of neuropeptide expression in reproductively competent and quiescent female Turkish hamsters; few studies have addressed this question in female photoperiodic rodents (but see Ansel et al., 2010; Mason et al., 2007; Shahed and Young, 2009). We assessed whether reproductive quiescence following removal of melatonin in long-day females is accompanied by changes in kisspeptin and RFRP-3 comparable to those associated with short day lengths. The withdrawal of melatonin via pinealectomy could result in a pattern of neuropeptide expression similar to that of intact SD hamsters, suggesting that the neural mechanism(s) that decodes day length in Turkish hamsters lies upstream of hypothalamic neuropeptides that control the reproductive axis. By contrast, in the Syrian hamster (Revel et al., 2006a), pinealectomy and the absence of melatonin counteract the effects of short days on RFRP-3 and kisspeptin neurons, sustaining the long-day phenotype. Alternatively, RFRP-3 and kisspeptin patterns may be controlled by day length in a pineal-independent manner and remain unchanged in pinealectomized Turkish hamsters, with unspecified neuroendocrine mechanisms overriding the effects of kisspeptin and RFRP-3. To evaluate these possibilities, we manipulated photoperiod and melatonin and found that, as in other species, GnRH, RFRP-3, and kisspeptin are affected by photoperiod, with similar change in these neuropeptides in long-day Turkish hamsters with complete melatonin withdrawal and in SD females. The complete withdrawal of melatonin after pinealectomy and increased duration of melatonin signaling in short days appear to act through similar neural substrates to inhibit reproduction.
MATERIALS AND METHODS
Animals
Twenty-four female Turkish hamsters (M. brandti) from our colony, between 120 and 150 days of age at the start of the experiment, were housed individually in translucent polypropylene cages (48 × 27 × 20 cm) on Tek-Fresh Lab Animal Bedding (Harlan Teklab, Madison, WI) at 22 ± 2 °C in an LD 16:8 light:dark (L:D) cycle (lights on at 0200 h PST). Some hamsters were transferred to SD lengths at the start of the experiment (8:16 L:D). Harlan 8664 Teklad Rodent Diet and tap water were available ad libitum throughout the study. All procedures were approved by the Animal Care and Use Committee of the University of California at Berkeley and conformed to principles enunciated in the National Institutes of Health (NIH) guide for the use and care of laboratory animals.
Experimental Procedures and Design
Estrous cycles were monitored by gently palpating the hamster’s vagina to detect the sticky vaginal discharge also characteristic of cycling Syrian hamsters (Orsini, 1961). Only females that displayed at least 3 consecutive 4-day estrous cycles immediately prior to the beginning of the experiment were retained for study.
Hamsters were either pinealectomized (pinx; n = 11) or sham-pinx (n = 13) and remained housed in LD or were transferred to SD photoperiod to form LD-sham (n = 5), LD-pinx (n = 11), or SD-sham (n = 8) groups. Twelve weeks after surgery, brains were collected as described below.
Surgical Procedures
Pinealectomies were performed under isoflurane vapor anesthesia (Baxter Healthcare, Deerfield, IL) by exposing the skull and drilling a small opening above the bregma. The pineal gland was excised with fine forceps and examined under a surgical microscope to verify completeness of the pinealectomy. The skull opening was filled with Gelfoam (Upjohn Company Kalamazoo, MI), the skin sutured, and wound clips applied (Mikron Auto Clip 9 mm; Becton Dickinson, Franklin Lakes, NJ). Hamsters were injected subcutaneously with the analgesic buprenorphine (5.0%, 0.2 mL/animal) postoperatively (Hospira, Inc., Lake Forest, IL). For the first week after surgery, the diet was supplemented with fresh fruit and mush made from the daily feed. All hamsters were inspected during daily monitoring of estrus for the duration of the experiment.
Perfusion and Histology
Hamsters were deeply anesthetized with sodium pentobarbital solution (200 mg/kg) and perfused transcardially with 150 mL 0.9% saline followed by 300 mL 4% paraformaldehyde in 0.1 M PBS (pH 7.4). Brains were postfixed for 6 h in 4% paraformaldehyde followed by cryoprotection in 30% sucrose in 0.1 M PBS for 2 days and then frozen at −80 °C until processing.
Coronal brain sections 40 µm thick were collected on a cryostat at −20 °C. Slices were stored at −20 °C in an ethylene glycol/sucrose-based antifreeze until immunohistochemistry was performed. Each brain was labeled for GnRH, RFRP-3, and kisspeptin in separate series of brain slices. Within each series, every fourth slice was washed in phosphate buffer (PB), followed by 0.5% hydrogen peroxide. Brain sections were then washed in PB before incubating for 1 h in normal goat serum in PB with 0.1% Triton X-100 (PBT). For kisspeptin-labeled brains, sections were incubated for 48 h at 4 °C in rabbit polyclonal antikisspeptin-10 antiserum (Abcam, Cambridge, MA) at a concentration of 1:4000 for ARC sections and 1:1000 for AVPV sections. To label RFRP-3 and GnRH, brains were incubated in either white crown sparrow polyclonal anti-RFRP-3 antiserum (1:10,000; gift from Dr. George Bentley) or rabbit anti-GnRH antiserum (1:10,000; LR5, gift from Dr. Robert Benoit) diluted in PBT for 48 h at 4 °C. After incubation with the primary antibody, sections were washed in PBT followed by 1 h in biotinylated goat-anti-rabbit serum (1:300 Vector Laboratories, Burlingame, CA), washed in PBT, and incubated in avidin–biotin– horseradish peroxidase complex (ABC Elite Kit; Vector Laboratories). Brains were then washed in PBT and labeled cells visualized with 3,3′-diaminobenzidine. Slices were mounted onto gelatin-coated slides, dehydrated in a graded series of ethanol, and cleared in xylenes before coverslips were applied.
Light Microscopy
Brain sections were examined under bright-field illumination on a Zeiss Axioimager M1 microscope (Carl Zeiss, Oberkochen, Germany) by observers uninformed about the hamsters’ treatments. All cells in every fourth section were counted. Kisspeptin expression was quantified in the AVPV and ARC, RFRP-3 in the dorsomedial nucleus of the hypothalamus (DMH), and GnRH in the NDB and mPOA. Soma size and optical density measurements were determined for each positively labeled neuron from the slice expressing the highest number of labeled cells for each brain region. Each cell was photographed with a Zeiss Axiocam Cooled CCD camera at 400× magnification. A single mean optical density and cell size was calculated by taking the mean value of all measured neurons for each hamster and brain region. Cell bodies were outlined and the 2-dimensional area calculated using ImageJ, version 1.45s (NIH, Bethesda, MD). Each pixel in the grayscale image capture has a measurable specific intensity. The mean value for all pixels in an outlined area defined the mean intensity of staining for a given region of the image. Optical density measures were normalized by taking a background measurement by placing a square outline, 4 times, on nonoverlapping, nonspecifically labeled areas of each section. The mean of these 4 measures provided the background optical density for each section. The optical density for each cell body was assessed by outlining the cell body, obtaining a density measure using ImageJ, and subtracting the background optical density from the optical density of each cell.
Statistics
Our a priori hypotheses allowed for individual 1 degree-of-freedom comparisons between each of the 3 groups, resulting in 3 comparisons per measure. These were conducted with a between-groups t test. All analyses were conducted in R (R 2.12.1; R Foundation for Statistical Computing, Vienna, Austria). Results were considered significant if p < 0.05, 2-tailed tests.
RESULTS
Reproductive Measures
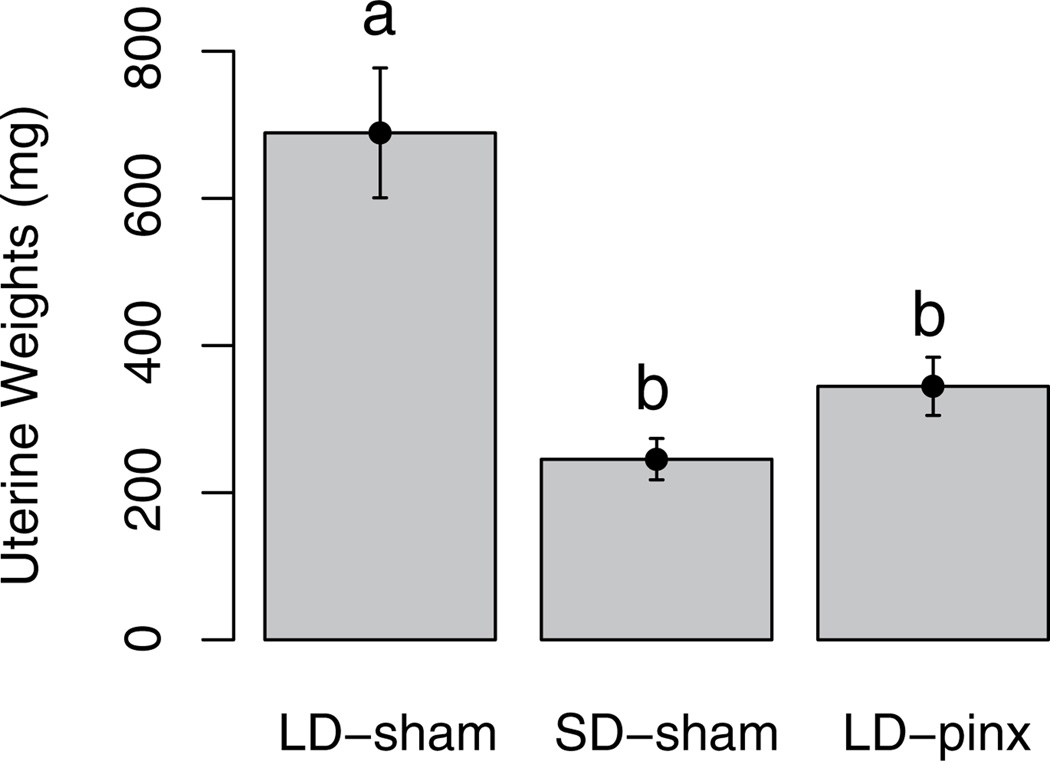
Whereas most female Turkish hamsters exposed to short day lengths or melatonin withdrawal become acyclic (e.g., Okulicz et al., 1988; Ogilvie et al., 1992) and uterine weights decrease (Darrow et al., 1986), a subset continues to undergo estrous cycles (Okulicz et al., 1988; Ogilvie et al., 1992). In the present study, 4 of these so-called nonresponders in the SD-sham group and 2 in the LD-pinx group were eliminated from further consideration. In the remaining hamsters, uterine weights were significantly higher in the LD-sham than in both the SD-sham (t7 = 4.3; p < 0.05) and LD-pinx groups (t12 = 4.13; p < 0.05) (Fig. 1). The SD-sham and LD-pinx groups did not differ from each other (t11 = 1.57; p > 0.05).
Figure 1.
Mean ± SEM of uterine weights. LD-sham hamsters had significantly higher uterine weights than either SD-sham or LD-pinx hamsters, verifying that both short photoperiod and pinealectomy induced the short-day phenotype. Letters different from each other denote significant differences (p < 0.05).
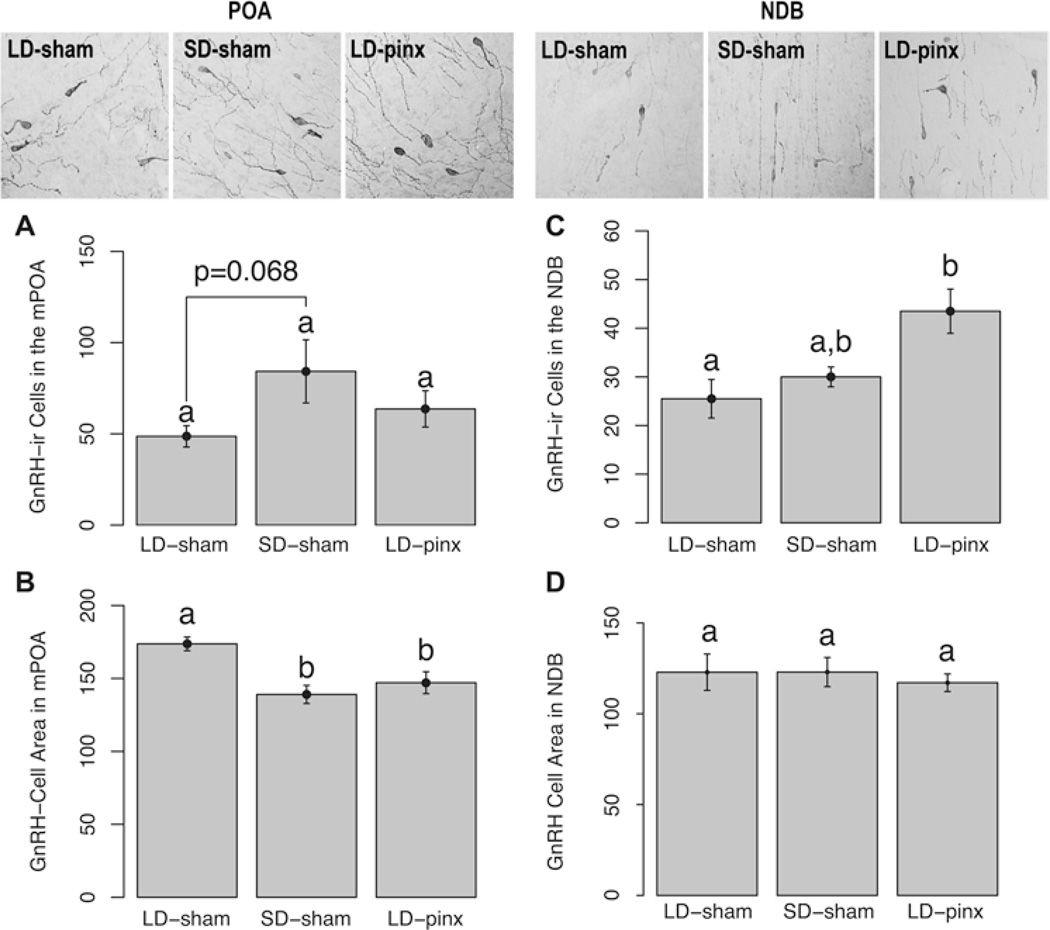
GnRH Cell Counts, Size, and Optical Density
In the mPOA, there was not a significant difference between LD-sham hamsters and SD-sham hamsters (t7 = −2.16; p = 0.068). Likewise the LD-sham and LD-pinx groups (t12 = −1.06; p > 0.05) exhibited similar numbers of cells. Finally, the SD-sham and LD-pinx groups did not differ (t11 = 1.10; p > 0.05) (Fig. 2A). In contrast, the number of GnRH cells in the NDB did not differ between LD-sham and SD-sham hamsters (t7 = −1.26; p > 0.05) (Fig. 2C), whereas LD-sham hamsters had fewer cells than did LD-pinx hamsters (t12 = −2.59; p < 0.05); SD-sham and LD-pinx groups did not differ (t11 = −1.85; p > 0.05) (Fig. 2C).
Figure 2.
Mean ± SEM GnRH-ir cell numbers in the (A) mPOA and (C) NDB and GnRH-ir cell areas in the (B) mPOA and (D) NDB. Representative photomicrographs of GnRH-ir cells are above each bar. LD-sham hamsters displayed a trend toward fewer GnRH cells in the mPOA compared to SD-sham but not LD-pinx hamsters. LD-sham hamsters exhibited a larger cell area in the mPOA than either SD-sham or LD-pinx hamsters. LD-sham hamsters displayed a lower number of immunoreactive cells in the NDB than did LD-pinx hamsters, but SD-sham hamsters did not differ from either group. Letters different from each other denote significant differences (p < 0.05).
In the mPOA, GnRH immunoreactive cells of LD-sham females were significantly larger than those of SD-sham (t6 = 4.45; p < 0.05) and LD-pinx hamsters (t10 = 2.35; p < 0.05). Cell size did not differ between SD-sham and LD-pinx hamsters (t10 = 0.69; p > 0.05) (Fig. 2B). In the NDB, there were no significant differences among groups in GnRH cell size (p > 0.05 for all comparisons) (Fig. 2D). Likewise, GnRH cells in the mPOA and NDB did not differ among groups in optical density (p > 0.05 for all comparisons; data not shown).
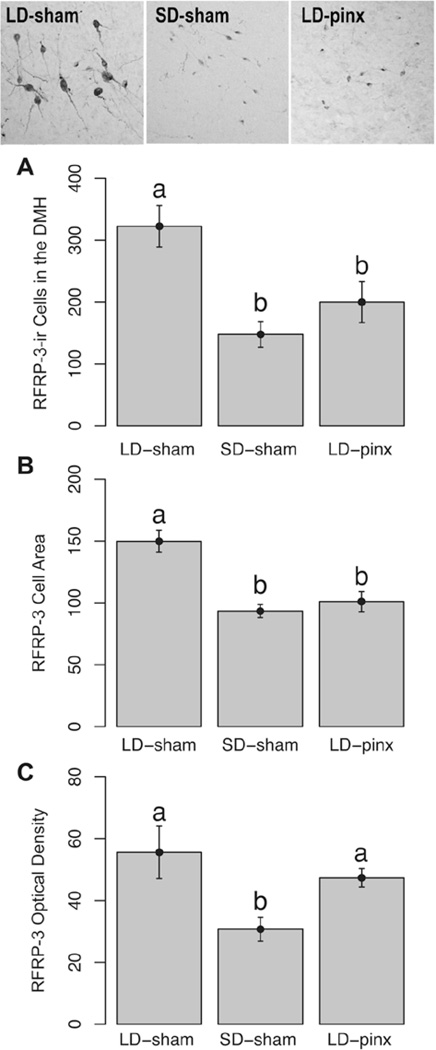
RFRP-3 Cell Counts, Size, and Optical Density
The number of RFRP-3 DMH immunoreactive cells in the LD-sham group was greater than in both SD-sham (t7 = 4.13; p < 0.05) and LD-pinx hamsters (t12 = 2.39; p < 0.05); SD-sham and LD-pinx groups did not differ (t11 = 0.99; p > 0.05) (Fig. 3A). In common with RFRP-3 cell numbers, RFRP-3 immunoreactive cells were larger in LD-sham than in SD-sham (t7 = 5.07; p < 0.05) and LD-pinx groups (t12 = 3.79; p < 0.05) but did not differ between SD-sham and LD-pinx hamsters (t11 = 0.59; p > 0.05) (Fig. 3B). Unlike cell numbers and size, RFRP-3 cellular optical density in LD-sham hamsters was significantly higher than that of the SD-sham group (t7 = 2.43; p <0.05) but not the LD-pinx group (t12 = 1.12; p > 0.05). RFRP-3 optical density in the SD-sham group was lower than in the LD-pinx hamsters (t11 = 3.19; p < 0.05) (Fig. 3C).
Figure 3.
Mean ± SEM (A) number of RFRP-3-ir cells (B) area of RFRP-3-ir cells and (C) optical density of RFRP-3-ir cells in the DMH. Representative photomicrographs of RFRP-3-ir cells are at the top of the figure. LD-sham hamsters had significantly more RFRP-3-ir cells and a larger cell area than both the SD-sham and LD-pinx hamsters. LD-sham hamsters also had a significantly higher optical density than SD-sham but not LD-pinx hamsters. Letters different from each other denote significant differences (p < 0.05).
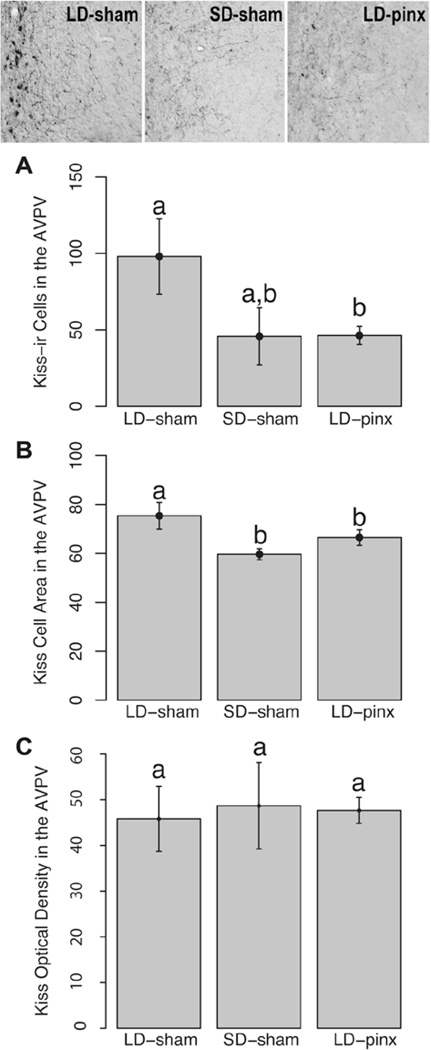
Kisspeptin Cell Counts, Size, and Optical Density
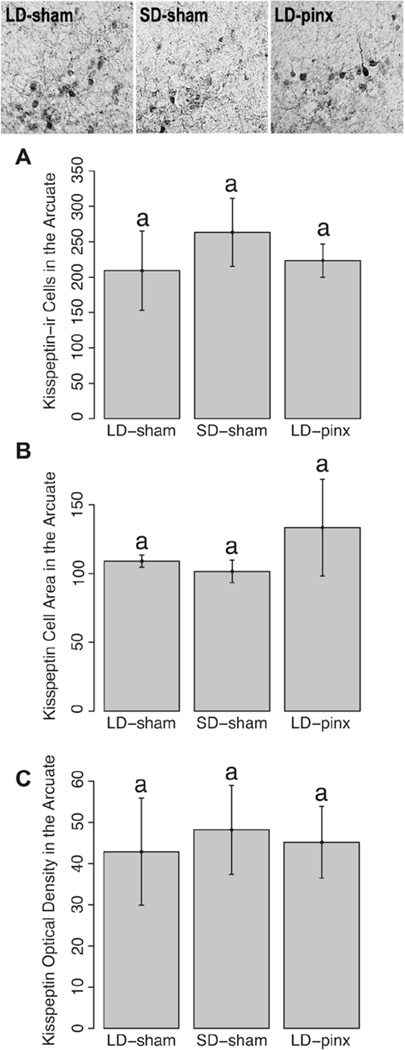
The LD-sham group had more kisspeptin-positive cells in the AVPV than did the LD-pinx hamsters (t12 = 2.65, p < 0.05) but not the SD-sham animals (t7 = 0.71; p > 0.05). SD-sham and LD-pinx groups did not differ on this measure (t11 = 0.04; p > 0.05) (Fig. 4A). Groups did not differ from each other with respect to the number of kisspeptin-ir cell numbers in the ARC (p > 0.05 for all comparisons) (Fig. 5A).
Figure 4.
Mean ± SEM (A) kisspeptin-ir cells in the AVPV and (B) area of kisspeptin-ir cells in the AVPV. Representative photomicrographs of kisspeptin-ir cells at the top of the figure. LD-sham hamsters had more kisspeptin cells than did LD-pinx hamsters, but not SD-sham hamsters. However, LD-sham hamsters had significantly larger kisspeptin cells than both SD-sham and LD-pinx hamsters. Letters different from each other denote significant differences (p < 0.05).
Figure 5.
Mean ± SEM (A) kisspeptin-ir cells in the ARC, (B) area of kisspeptin-ir cells, and (C) optical density in the ARC. Representative photomicrographs of kisspeptin-ir cells are at the top of the figure. No differences were observed among groups for any measure.
AVPV kisspeptin neurons of LD-sham hamsters were also larger than those of SD-sham hamsters (t6 = 2.66; p < 0.05) (Fig. 4B). In contrast to findings for AVPV kisspeptin cells, there were no significant differences among groups in cell size in ARC kisspeptin cells (p > 0.05 for all comparisons) (Fig. 5B). Likewise, there were no significant differences among the groups in cellular optical density in either AVPV or ARC kisspeptin-ir cells (p > 0.05 for all comparisons) (Fig. 4C and Fig. 5C).
DISCUSSION
Turkish hamsters are unique in that gonadal function is maintained only by a narrow range of day lengths (15–17 h), signaled by nocturnal melatonin secretion of 3 to 4 h duration (Darrow et al., 1986); withdrawal of melatonin via pinealectomy or by long durations of nocturnal melatonin in SDs induces gonadal quiescence (Carter et al., 1982). Whether these two contrasting melatonin profiles act through the same effector systems to inhibit reproduction was unknown. We found that reproductive quiescence, whether induced by short day lengths or pinealectomy, induced parallel changes in some measures of GnRH, RFRP-3 and kisspeptin expression. These peptide changes may be causally related to the reproductive responses, but we cannot discount the possibility that they are a consequence rather than a cause of reproductive arrest; nor can we discount the possibility that there may be direct effects of day length independent of melatonin. In Turkish hamsters, 3 quite different melatonin profiles (either very short or very long pulses each night, or the complete absence of melatonin) produce the same effects on the reproductive axis. We tentatively conclude that long-duration melatonin signaling as day lengths decrease in the late summer and early autumn in the field, and withdrawal of melatonin by pinealectomy, act through the same neural substrates to induce gonadal quiescence. As noted (Jarjisian and Zucker, 2011), the biological meaning of the absence of melatonin after pinealectomy remains obscure and unlikely to be encountered in nature, except during hibernation (Darrow et al., 1986).
Across species, GnRH, kisspeptin, and RFRP-3 exhibit predictable changes in response to photoperiod or melatonin-induced gonadal quiescence, implicating these neuropeptides as key mediators of seasonal reproduction (Simmonneaux et al., 2013). In the present study, GnRH expression in the NDB exhibits a pattern of expression consistent with that noted for other photoperiodic rodents (Syrian hamsters, white-footed mice, prairie voles; Ronchi et al., 1992b; Shiotani et al., 1985; Glass, 1986; Heideman et al., 2010; Kriegsfeld and Nelson, 1999). Specifically, in the NDB, pinealectomy induced an increase in the number of GnRH-ir cells but did not affect cell area. In Syrian hamsters and prairie voles, GnRH mRNA production is unchanged by photoperiod (Kriegsfeld et al., 2000; Ronchi et al., 1992b), whereas GnRH-ir cell numbers are increased (Kriegsfeld and Nelson, 1999; Rochi et al., 1992a), suggesting that reproductive regression results from inhibited GnRH release, rather than reduced synthesis. Likewise, pituitary responsiveness to GnRH is unchanged in SD relative to LD animals across rodent species (Pickard and Silverman, 1979; Ansel et al., 2011; Kriegsfeld et al., 1999), further implicating reduced GnRH signaling in the transition to reproductive quiescence and its maintenance. In the mPOA, in contrast, the total number of immunoreactive cells was not affected by melatonin manipulation, but cell size was decreased in reproductively quiescent hamsters. Whether this pattern of labeling represents a decrease in GnRH production or accelerated release remains to be determined. The differential effect of photoperiod and pinx on NDB and mPOA populations of GnRH neurons suggests that these 2 cell populations might serve distinct roles in basal GnRH secretion and luteinizing hormone surge induction, as in Syrian hamsters (Berriman et al., 1992).
In reproductively active hamsters, RFRP-3 inhibits reproductive behavior (Piekarski et al., 2013) and GnRH release (Kriegsfeld et al., 2006; Ubuka et al., 2012). However, RFRP-3 peptide and mRNA were reduced in SDs in Syrian and Siberian hamsters (Mason et al., 2010; Revel et al., 2008; Ubuka et al., 2012), and central administration of RFRP-3 is stimulatory to the reproductive axis of SD hamsters (Ubuka et al., 2012; Ancel et al., 2012; Tsutsui et al., 2013). In the present study, SDs or pinealectomy led to decreased RFRP-3-ir cell numbers and size, suggesting a reduction in RFRP-3 release, consistent with previous findings in other hamster species.
The present findings reveal a unique pattern of kisspeptin expression compared to those seen in Syrian and Siberian hamsters. In male and female Syrian hamsters, SDs decrease the number of cells expressing KiSS-1 mRNA in the ARC and AVPV; this decrease is prevented by pinealectomy (Revel et al., 2006a; Ansel et al., 2010), suggesting that melatonin mediates the SD-induced downregulation of KiSS-1 expression. Additionally, injection of estradiol to photoregressed female Syrian hamsters leads to an increase in the number of kisspeptin cells in the AVPV but a reduction in the ARC (Ansel et al., 2010). In contrast to Syrian hamsters, kisspeptin is reduced in the AVPV and increased in the ARC of SD male and female Siberian hamsters, compared to LD animals (Greives et al., 2007; Mason et al., 2007, respectively); this effect is abolished in hamsters unresponsive to short day lengths (Greives et al., 2007). Kisspeptin replacement restores the LD phenotype in photoregressed male Syrian hamsters (Revel et al., 2006a; Ansel et al., 2011) but not male Siberian hamsters (Greives et al., 2008), with the latter finding presumably due to kisspeptin receptor downregulation following continuous peptide infusion. In the present investigation, SDs decreased AVPV kisspeptin cell area but not cell number in Turkish hamsters. However, pinealectomy significantly reduced AVPV kisspeptin cell number and size. Additionally, in contrast to other hamster species, ARC kisspeptin cells are not affected by melatonin signaling in Turkish hamsters. Future studies investigating the means by which these 2 populations of kisspeptin cells respond to melatonin and resulting changes in gonadal steroids will help to elucidate the species differences herein identified.
In the current study, GnRH, RFRP-3 and kisspeptin profiles matched reproductive phenotype more closely than did the melatonin profile. Because hamsters with either long-duration melatonin profiles or complete absence of melatonin display comparable neural changes, decoding of the melatonin signal likely occurs upstream of these neuropeptidergic systems. The specific loci contributing to seasonal changes in reproduction vary across species. Siberian hamsters exhibit strong melatonin binding in the suprachiasmatic nucleus, paraventricular nucleus, and nucleus reuniens of the thalamus; each of these sites can act independently to regulate gonadal responses to photoperiod (Badura and Goldman, 1992; Freeman and Zucker, 2001; Kriegsfeld and Bittman, 2010). In Syrian hamsters, the DMH is essential for the transition to reproductive quiescence following SD or long-duration melatonin (Maywood et al., 1996; Lewis et al., 2002). In Syrian and Siberian hamsters, melatonin also acts on the pars tuberalis to alter thyroid-stimulating hormone (TSH) secretion, which, in turn, acts on tanycytes in the hypothalamic ependymal layer to alter deiodinase activity and, consequently, the conversion of local thyroxine (T4) to triiodothyronine (T3) (Barrett et al., 2007; Hanon et al., 2008; Revel et al., 2006b; Stevenson and Prendergast, 2013). Additionally, chronic central administration of TSH to SD Syrian and Siberian hamsters for several weeks restores the LD pattern of RFRP-3 and kisspeptin expression (Klosen et al., 2013). Whether or not long-duration melatonin signaling or its withdrawal acts to induce similar patterns of pars tuberalis TSH expression represents an intriguing possibility for future exploration. Most rodents are long-day breeders and respond to long-duration melatonin signals with reproductive arrest, whereas the reproductive axis of SD breeders, such as sheep, is stimulated by long-duration melatonin (Wagner et al., 2008; Malpaux et al., 1997). These differences in melatonin decoding strategies serve to phase breeding to the appropriate time of year. The means by which different species interpret the same melatonin signal differentially remain to be deciphered. That Turkish hamsters respond to both signaling events with alterations in kisspeptin, RFRP-3, and GnRH signaling, and that these neuropeptides exhibit marked seasonal changes in both LD and SD breeders, suggests an important role for their collective contribution to reproductive axis regulation downstream of melatonin signaling.
In summary, 3 reproductively relevant neuropeptides are affected similarly by SD treatment and pinealectomy. This suggests that these effectors of photoperiod-induced reproductive quiescence are not controlled by melatonin directly; rather, mechanisms upstream of these substrates decode melatonin-signal durations and relay information to hypothalamic neuropeptidergic systems that control seasonal rhythms of reproduction.
ACKNOWLEDGMENTS
The authors thank Chris Tuthill and Daniel Yu for technical assistance. This study was supported by NIH R01 grant HD-050470 (to L.J.K.).
Footnotes
CONFLICT OF INTEREST STATEMENT
The author(s) have no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
REFERENCES
- Ancel C, Bentsen AH, Sébert ME, Tena-Sempere M, Mikkelsen JD, Simonneaux V. Stimulatory effect of RFRP-3 on the gonadotrophic axis in the male Syrian hamster: The exception proves the rule. Endocrinology. 2012;153:1352–1363. doi: 10.1210/en.2011-1622. [DOI] [PubMed] [Google Scholar]
- Ansel L, Bentsen AH, Ancel C, Bolborea M, Klosen P, Mikkelsen JD, Simonneaux V. Peripheral kisspeptin reverses short photoperiod-induced gonadal regression in Syrian hamsters by promoting GNRH release. Reproduction. 2011;142:417–425. doi: 10.1530/REP-10-0313. [DOI] [PubMed] [Google Scholar]
- Ansel L, Bolborea M, Bentsen AH, Klosen P, Mikkelsen JD, Simonneaux V. Differential regulation of kiss1 expression by melatonin and gonadal hormones in male and female Syrian hamsters. J Biol Rhythms. 2010;25:81–91. doi: 10.1177/0748730410361918. [DOI] [PubMed] [Google Scholar]
- Badura LL, Goldman BD. Central sites mediating reproductive responses to melatonin in juvenile male Siberian hamsters. Brain Res. 1992;598:98–106. doi: 10.1016/0006-8993(92)90172-6. [DOI] [PubMed] [Google Scholar]
- Barrett P, Ebling FJ, Schuhler S, Wilson D, Ross AW, Warner A, Jethwa P, Boelen A, Visser TJ, Ozanne DM, et al. Hypothalamic thyroid hormone catabolism acts as a gatekeeper for the seasonal control of body weight and reproduction. Endocrinology. 2007;148:3608–3617. doi: 10.1210/en.2007-0316. [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Powers JB, Hastings MH, Bittman EL, Goldman BD. The timed infusion paradigm for melatonin delivery: What has it taught us about the melatonin signal, its reception, and the photoperiodic control of seasonal responses? J Pineal Res. 1993;15:161–190. doi: 10.1111/j.1600-079x.1993.tb00903.x. [DOI] [PubMed] [Google Scholar]
- Batavia M, Nguyen G, Harman K, Zucker I. Hibernation patterns of Turkish hamsters: Influence of sex and ambient temperature. J Comp Physiol B. 2013a;183:269–277. doi: 10.1007/s00360-012-0706-3. [DOI] [PubMed] [Google Scholar]
- Batavia M, Nguyen G, Zucker I. The effects of day length, hibernation, and ambient temperature on incisor dentin in the Turkish hamster (Mesocricetus brandti) J Comp Physiol B. 2013b;183:557–566. doi: 10.1007/s00360-012-0729-9. [DOI] [PubMed] [Google Scholar]
- Berriman SJ, Wade GN, Blaustein JD. Expression of Fos-like proteins in gonadotropin-releasing hormone neurons of Syrian hamsters: Effects of estrous cycles and metabolic fuels. Endocrinology. 1992;131:2222–2228. doi: 10.1210/endo.131.5.1425420. [DOI] [PubMed] [Google Scholar]
- Bronson FH. Mammalian Reproductive Biology. Chicago: University of Chicago Press; 1989. [Google Scholar]
- Butler MP, Turner KW, Zucker I. A melatonin-independent seasonal timer induces neuroendocrine refractoriness to short day lengths. J Biol Rhythms. 2008;23:242–251. doi: 10.1177/0748730408317135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraty A, Blomenröhr M, Vogel GM, Lomet D, Briant C, Beltramo M. RF9 powerfully stimulates gonadotrophin secretion in the ewe: Evidence for a seasonal threshold of sensitivity. J Neuroendocrinol. 2012;24:725–736. doi: 10.1111/j.1365-2826.2012.02283.x. [DOI] [PubMed] [Google Scholar]
- Carter DS, Goldman BD. Antigonadal effects of timed melatonin infusion in pinealectomized male Djungarian hamsters (Phodopus sungorus sungorus): Duration is the critical parameter. Endocrinology. 1983;113:1261–1267. doi: 10.1210/endo-113-4-1261. [DOI] [PubMed] [Google Scholar]
- Carter DS, Hall VD, Tamarkin L, Goldman BD. Pineal is required for testicular maintenance in the Turkish hamster (Mesocricetus brandti) Endocrinology. 1982;111:863–871. doi: 10.1210/endo-111-3-863. [DOI] [PubMed] [Google Scholar]
- Darrow JM, Tamarkin L, Duncan MJ, Goldman BD. Pineal melatonin rhythms in female Turkish hamsters: Effects of photoperiod and hibernation. Biol Reprod. 1986;35:74–83. doi: 10.1095/biolreprod35.1.74. [DOI] [PubMed] [Google Scholar]
- de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott JA. Circadian rhythms and photoperiodic time measurement in mammals. Fed Proc. 1976;35:2339–2346. [PubMed] [Google Scholar]
- Freeman DA, Zucker I. Refractoriness to melatonin occurs independently at multiple brain sites in Siberian hamsters. Proc Natl Acad Sci U S A. 2001;98:6447–6452. doi: 10.1073/pnas.111140398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funes S, Hedrick JA, Vassileva G, Markowitz L, Abbondanzo S, Golovko A, Yang S, Monsma FJ, Gustafson EL. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun. 2003;312:1357–1363. doi: 10.1016/j.bbrc.2003.11.066. [DOI] [PubMed] [Google Scholar]
- Glass JD. Short photoperiod-induced gonadal regression: Effects on the gonadotropin-releasing hormone (GnRH) neuronal system of the white-footed mouse, Peromyscus leucopus . Biol Reprod. 1986;35:733–743. doi: 10.1095/biolreprod35.3.733. [DOI] [PubMed] [Google Scholar]
- Goldman BD. Mammalian photoperiodic system: Formal properties and neuroendocrine mechanisms of photoperiodic time measurement. J Biol Rhythms. 2001;16:283–301. doi: 10.1177/074873001129001980. [DOI] [PubMed] [Google Scholar]
- Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145:4073–4077. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- Greives TJ, Humber SA, Goldstein AN, Scotti MA, Demas GE, Kriegsfeld LJ. Photoperiod and testosterone interact to drive seasonal changes in kisspeptin expression in Siberian hamsters (Phodopus sungorus) J Neuroendocrinol. 2008;20:1339–1347. doi: 10.1111/j.1365-2826.2008.01790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greives TJ, Mason AO, Scotti MA, Levine J, Ketterson ED, Kriegsfeld LJ, Demas GE. Environmental control of kisspeptin: Implications for seasonal reproduction. Endocrinology. 2007;148:1158–1166. doi: 10.1210/en.2006-1249. [DOI] [PubMed] [Google Scholar]
- Hall VD, Bartke A, Goldman BD. Role of the testis in regulating the duration of hibernation in the Turkish hamster, Mesocricetus brandti . Biol Reprod. 1982;27:802–810. doi: 10.1095/biolreprod27.4.802. [DOI] [PubMed] [Google Scholar]
- Hanon EA, Lincoln GA, Fustin JM, Dardente H, Masson-Pévet M, Morgan PJ, Hazlerigg DG. Ancestral TSH mechanism signals summer in a photoperiodic mammal. Curr Biol. 2008;18:1147–1152. doi: 10.1016/j.cub.2008.06.076. [DOI] [PubMed] [Google Scholar]
- Heideman PD, Pittman JT, Schubert KA, Dubois CM, Bowles J, Lowe SM, Price MR. Variation in levels of luteinizing hormone and reproductive photoresponsiveness in a population of white-footed mice (Peromyscus leucopus) Am J Physiol Regul Integr Comp Physiol. 2010;298:R1543–R1548. doi: 10.1152/ajpregu.00686.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann K. The critical photoperiod in the Djungarian hamster Phodopus sungorus. In: Aschoff J, Daan S, Groos G, editors. Vertebrate Circadian Systems. Berlin: Springer Verlag; 1982. pp. 297–304. [Google Scholar]
- Hong SM, Rollag MD, Stetson MH. Maintenance of testicular function in Turkish hamsters: Interaction of photoperiod and the pineal gland. Biol Reprod. 1986;34:527–531. doi: 10.1095/biolreprod34.3.527. [DOI] [PubMed] [Google Scholar]
- Jarjisian SG, Zucker I. Elimination of short-day melatonin signaling accelerates gonadal recrudescence but does not break refractoriness in male Turkish hamsters. J Biol Rhythms. 2011;26:130–135. doi: 10.1177/0748730410395481. [DOI] [PubMed] [Google Scholar]
- Kelly KK, Goldman BD, Zucker I. Gonadal growth and hormone concentrations in photoregressed Siberian hamsters: Pinealectomy versus photostimulation. Biol Reprod. 1994;51:1046–1050. doi: 10.1095/biolreprod51.5.1046. [DOI] [PubMed] [Google Scholar]
- Klosen P, Sébert ME, Rasri K, Laran-Chich MP, Simonneaux V. TSH restores a summer phenotype in photoinhibited mammals via the RF-amides RFRP3 and kisspeptin. FASEB J. 2013;27:2677–2686. doi: 10.1096/fj.13-229559. [DOI] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Bittman EL. Photoperiodism in mammals: Reproduction. In: Nelson RJ, Denlinger D, Sommers D, editors. Photoperiodism: Biological Calendar. New York: Oxford University Press; 2010. pp. 503–542. [Google Scholar]
- Kriegsfeld LJ, Drazen DL, Nelson RJ. Effects of photoperiod and reproductive responsiveness on pituitary sensitivity to GnRH in male prairie voles (Microtus ochrogaster) Gen Comp Endocrinol. 1999;116:221–228. doi: 10.1006/gcen.1999.7414. [DOI] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Mei DF, Bentley GE, Ubuka T, Mason AO, Inoue K, Ukena K, Tsutsui K, Silver R. Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals. Proc Natl Acad Sci U S A. 2006;103:2410–2415. doi: 10.1073/pnas.0511003103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Nelson RJ. Photoperiod affects the gonadotropin-releasing hormone neuronal system of male prairie voles (Microtus ochrogaster) Neuroendocrinology. 1999;69:238–244. doi: 10.1159/000054424. [DOI] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Trasy AG, Nelson RJ. Temperature and photoperiod interact to affect reproduction and GnRH synthesis in male prairie voles. J Neuroendocrinol. 2000;12:553–558. doi: 10.1046/j.1365-2826.2000.00485.x. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Hileman SM, Goodman RL. Neuroanatomy of the kisspeptin signaling system in mammals: Comparative and developmental aspects. Adv Exp Med Biol. 2013;784:27–62. doi: 10.1007/978-1-4614-6199-9_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D, Freeman DA, Dark J, Winne-Edwards KE, Zucker I. Photoperiodic control of oestrous cycles in Syrian hamsters: Mediation by the mediobasal hypothalamus. J Neuroendocrinol. 2002;14:294–299. doi: 10.1046/j.1365-2826.2002.00779.x. [DOI] [PubMed] [Google Scholar]
- Lyman CP, O’Brien RC. A laboratory study of the Turkish hamster Mesocricetus brandti . Breviora. 1977;442:1–27. [Google Scholar]
- Malpaux B, Viguié C, Skinner DC, Thiéry JC, Chemineau P. Control of the circannual rhythm of reproduction by melatonin in the ewe. Brain Res Bull. 1997;44:431–438. doi: 10.1016/s0361-9230(97)00223-2. [DOI] [PubMed] [Google Scholar]
- Mason AO, Duffy S, Zhao S, Ubuka T, Bentley GE, Tsutsui K, Silver R, Kriegsfeld LJ. Photoperiod and reproductive condition are associated with changes in RFamide-related peptide (RFRP) expression in Syrian hamsters (Mesocricetus auratus) J Biol Rhythms. 2010;25:176–185. doi: 10.1177/0748730410368821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason AO, Greives TJ, Scotti MA, Levine J, Frommeyer S, Ketterson ED, Demas GE, Kriegsfeld LJ. Suppression of kisspeptin expression and gonadotropic axis sensitivity following exposure to inhibitory day lengths in female Siberian hamsters. Horm Behav. 2007;52:492–498. doi: 10.1016/j.yhbeh.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maywood ES, Bittman EL, Hastings MH. Lesions of the melatonin- and androgen-responsive tissue of the dorsomedial nucleus of the hypothalamus block the gonadal response of male Syrian hamsters to programmed infusions of melatonin. Biol Reprod. 1996;54:470–477. doi: 10.1095/biolreprod54.2.470. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Castellano JM, Fernández-Fernández R, Tovar S, Roa J, Mayen A, Nogueiras R, Vazquez MJ, Barreiro ML, Magni P, et al. Characterization of the potent luteinizing hormone-releasing activity of KiSS-1 peptide, the natural ligand of GPR54. Endocrinology. 2005;146:156–163. doi: 10.1210/en.2004-0836. [DOI] [PubMed] [Google Scholar]
- Ogilvie KM, Donham RS, Stetson MH. Daily rhythms of follicle-stimulating hormone in adult anestrous and prepubertal female Turkish hamsters (Mesocricetus brandti) Biol Reprod. 1992;46:279–283. doi: 10.1095/biolreprod46.2.279. [DOI] [PubMed] [Google Scholar]
- Okulicz WC, Darrow JM, Goldman BD. Uterine steroid hormone receptors during the estrous cycle and during hibernation in the Turkish hamster (Mesocricetus brandti) Biol Reprod. 1988;38:597–604. doi: 10.1095/biolreprod38.3.597. [DOI] [PubMed] [Google Scholar]
- Orsini MW. The external vaginal phenomena characterizing the stages of the estrous cycle, pregnancy, pseudopregnancy, lactation, and the anestrous hamster, Mesocricetus auratus . Proc Anim Care Panel. 1961;11:193–206. [Google Scholar]
- Pickard GE, Silverman AJ. Effects of photoperiod on hypothalamic luteinizing hormone releasing hormone in the male hamster. J Endocrinol. 1979;83:421–428. doi: 10.1677/joe.0.0830421. [DOI] [PubMed] [Google Scholar]
- Piekarski DJ, Zhao S, Jennings KJ, Iwasa T, Legan SJ, Mikkelsen JD, Tsutsui K, Kriegsfeld LJ. Gonadotropin-inhibitory hormone reduces sexual motivation but not lordosis behavior in female Syrian hamsters (Mesocricetus auratus) Horm Behav. 2013;64:501–510. doi: 10.1016/j.yhbeh.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast BJ, Nelson RJ, Zucker I. Seasonal rhythms of mammals: Behavior and neuroendocrine substrates. In: Pfaff D, Arnold A, Etgen A, Fahrbach S, Moss R, Rubin R, editors. Hormones, Brain, and behavior. Vol. 1. San Diego: Academic Press; 2002. pp. 93–156. [Google Scholar]
- Revel FG, Saboureau M, Masson-Pévet M, Pévet P, Mikkelsen JD, Simonneaux V. Kisspeptin mediates the photoperiodic control of reproduction in hamsters. Curr Biol. 2006a;16:1730–1735. doi: 10.1016/j.cub.2006.07.025. [DOI] [PubMed] [Google Scholar]
- Revel FG, Saboureau M, Pévet P, Mikkelsen JD, Simonneaux V. Melatonin regulates type 2 deiodinase gene expression in the Syrian hamster. Endocrinology. 2006b;147:4680–4687. doi: 10.1210/en.2006-0606. [DOI] [PubMed] [Google Scholar]
- Revel FG, Saboureau M, Pévet P, Simonneaux V, Mikkelsen JD. RFamide-related peptide gene is a melatonin-driven photoperiodic gene. Endocrinology. 2008;149:902–912. doi: 10.1210/en.2007-0848. [DOI] [PubMed] [Google Scholar]
- Ronchi E, Aoki C, Krey LC, Pfaff DW. Immunocytochemical study of GnRH and GnRH-associated peptide in male Syrian hamsters as a function of photoperiod and gonadal alterations. Neuroendocrinology. 1992a;55:134–145. doi: 10.1159/000126108. [DOI] [PubMed] [Google Scholar]
- Ronchi E, Krey LC, Pfaff DW. Steady state analysis of hypothalamic GnRH mRNA levels in male Syrian hamsters: Influences of photoperiod and androgen. Neuroendocrinology. 1992b;55:146–155. doi: 10.1159/000126109. [DOI] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- Shahed A, Young KA. Differential ovarian expression of KiSS-1 and GPR-54 during the estrous cycle and photoperiod induced recrudescence in Siberian hamsters (Phodopus sungorus) Mol Reprod Dev. 2009;76:444–452. doi: 10.1002/mrd.20972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiotani Y, Cho HJ, Shiosaka S, Tasaka K, Miyake A, Aona T. Changes in the pineal gland, LH-RH neuron system and pituitary-gonadal axis in golden hamsters under artificial winter conditions. Biomed Res. 1985;6:297–305. [Google Scholar]
- Simonneaux V, Ancel C, Poirel VJ, Gauer F. Kisspeptins and RFRP-3 act in concert to synchronize rodent reproduction with seasons. Front Neurosci. 2013;7:22. doi: 10.3389/fnins.2013.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smale L, Dark J, Zucker I. Pineal and photoperiodic influences on fat deposition, pelage, and testicular activity in male meadow voles. J Biol Rhythms. 1988;3:349–355. doi: 10.1177/074873048800300404. [DOI] [PubMed] [Google Scholar]
- Stevenson TJ, Prendergast BJ. Reversible DNA methylation regulates seasonal photoperiodic time measurement. Proc Natl Acad Sci U S A. 2013;110:16651–16656. doi: 10.1073/pnas.1310643110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui K, Saigoh E, Ukena K, Teranishi H, Fujisawa Y, Kikuchi M, Ishii S, Sharp PJ. A novel avian hypothalamic peptide inhibiting gonadotropin release. Biochem Biophys Res Commun. 2000;275:661–667. doi: 10.1006/bbrc.2000.3350. [DOI] [PubMed] [Google Scholar]
- Tsutsui K, Ubuka T, Bentley GE, Kriegsfeld LJ. Regulatory mechanisms of gonadotropin-inhibitory hormone (RFRP-3) synthesis and release in photoperiodic animals. Front Neurosci. 2013;7:60. doi: 10.3389/fnins.2013.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubuka T, Inoue K, Fukuda Y, Mizuno T, Ukena K, Kriegsfeld LJ, Tsutsui K. Identification, expression, and physiological functions of Siberian hamster gonadotropin-inhibitory hormone. Endocrinology. 2012;153:373–385. doi: 10.1210/en.2011-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanski HF, Doan A, Pierce M. Immunocytochemical investigation of luteinizing hormone-releasing hormone neurons in Syrian hamsters maintained under long or short days. Biol Reprod. 1991;44:687–692. doi: 10.1095/biolreprod44.4.687. [DOI] [PubMed] [Google Scholar]
- Wagner GC, Johnston JD, Clarke IJ, Lincoln GA, Hazlerigg DG. Redefining the limits of day length responsiveness in a seasonal mammal. Endocrinology. 2008;149:32–39. doi: 10.1210/en.2007-0658. [DOI] [PubMed] [Google Scholar]
- Yellon SM. Effects of photoperiod on reproduction and the gonadotropin-releasing hormone-immunoreactive neuron system in the postpubertal male Djungarian hamster. Biol Reprod. 1994;50:368–372. doi: 10.1095/biolreprod50.2.368. [DOI] [PubMed] [Google Scholar]