Abstract
Acute myeloid leukemia (AML) is an aggressive disease with a poor 5-year survival of 21% that is characterized by a differentiation arrest of immature myeloid cells. For a rare subtype of AML (acute promyeloctyic leukemia, 5-10% of cases) all-trans retinoic acid therapy removes the differentiation block, yielding over a 90% cure rate. However, this treatment is not effective for the other 90-95% of AML patients, suggesting new differentiation strategies are needed. Interestingly, differentiation is induced in normal hematopoietic cells through Toll-like receptor (TLR) stimulation and TLRs are expressed on AML cells. We present evidence that the TLR8 activation promotes AML differentiation and growth inhibition in a TLR8/MyD88/p38 dependent manner. We also show that that TLR7/TLR8 agonist, R848, considerably impairs the growth of human AML cells in immunodeficient mice. Our data suggests TLR8 activation has direct anti-leukemic effects independent of its immunomodulating properties that are currently under investigation for cancer therapy. Taken together, our results suggest that treatment with TLR8 agonists may be a promising new therapeutic strategy for AML.
Introduction
Acute myeloid leukemia (AML), the most common acute leukemia in adults, is characterized by an arrest of maturation and aggressive proliferation of immature myeloid progenitor cells. The current AML therapeutics, which target rapidly dividing cells and not proteins causing maturation arrest have poor efficacy and high toxicities. For example, only 20% of patients over age 56 survive two years.1 Therefore there is an enormous unmet need for novel agents to improve the morbidity and mortality of these patients. Unfortunately even with these challenges, there has not been any change in the standard AML clinical agents in over 40 years.
By targeting proteins that cause maturation arrest instead of trying to kill rapidly dividing cells, a more efficacious and less toxic therapeutic regimen may be developed. AML differentiation can result in cells that are irreversibly growth arrested and eventually die without the necessity for overt cytotoxicity. In fact, all-trans retinoic acid (ATRA) is an AML differentiation agent that has revolutionized the treatment of acute promyelocytic leukemia (APL, 5-10% of AML), a subtype of AML expressing t(15:17) yielding the fusion protein PML/RAR, leading to the long term survival and presumed cure of well over 85% of patients.2 Unfortunately ATRA is not clinically useful for the other subtypes of AML. The enormous success of differentiation therapy for APL has led to numerous efforts to identify other differentiation agents that may exhibit clinical benefit for non-APL leukemia.3, 4
One pathway that is well known to lead to the differentiation of normal hematopoietic cells is Toll-like Receptor (TLR) signaling. TLRs are a key family of receptors linked to inflammation.5 In humans, 13 TLRs have been identified that recognize pathogen associated molecular patterns (PAMPs), leading to the induction of proinflammatory cytokines and an immune response.6 Specific TLRs have been shown to respond to distinct PAMPs. For example TLR4 responds to bacterial LPS, TLR5 to flagellin and TLR9 to unmethylated bacterial DNA.6 Most TLRs undergo defined signaling through predominantly MyD88-dependent pathways.7 After ligand binding, TLRs dimerize and undergo conformational changes that lead to the recruitment of the adapter protein, Myd88.7 Myd88 then recruits IL-1 receptor associated kinases (IRAKs) that lead to the activation of downstream signaling that involves mitogen associated protein kinases (MAPKs) and the transcription factors NF-κB and AP1.7 Interestingly, previous studies suggest that there are shared signaling components between TLR signaling and AML differentiation pathways. For example, p38 isoforms potentiate vitamin D and hydroxyurea mediated AML differentiation.8, 9
Previous work suggested TLR stimulation may not drive AML differentiation, but several TLRs have been implicated in normal hematopoietic cell differentiation.10 For example, both TLR2 and TLR4 stimulation of normal hematopoietic cells promotes hematopoietic stem cell and myeloid progenitor differentiation.5 In addition, TLR7/8 activation has been shown to drive CD34+ hematopoietic progenitor differentiation into macrophages and dendritic cell precursors.11 AML cells are known to express a wide range of TLRs.10 In addition, TLR stimulation of cells from other hematopoietic malignancies such as chronic lymphoid leukemia and multiple myeloma have been shown to modulate their growth and drug sensitivity.12, 13 Finally, while TLR-mediated AML differentiation has not been reported, a variety of TLR agonists are being developed to promote anti-cancer effects through immunomodulation such as the activation of cytotoxic T cell responses.14-18
In this study, we tested the role of TLR activation in AML differentiation. We show that the TLR7/8 agonist R848 promotes AML differentiation in a TLR8/Myd88/p38 dependent fashion. As TLR8 agonists are being developed for clinical use, TLR8-targeted differentiation therapy offers a potential new strategy for AML treatment.
Methods
Reagents and cells
R848, MyD88 knockdown constructs, and noble agar were from Sigma (St Louis, MO, USA). SB203580, PD08959, and SP600125 were obtained from Selleck Chem (Boston MA, USA). MTT reagent was obtained in the CellTiter 96 Aqueous One Solution Cell Proliferation Assay (Promega Madison WI, USA). p-p38, MyD88, α-tubulin, and actin antibodies were obtained from Cell Signaling Technologies (Beverly MA, USA). GAPDH was from Santa Cruz (Carlsbad, CA, USA). CD11b and CD14 antibodies were obtained from EBiosciences (San Diego CA, USA). Anti-Il-8 antibody was obtained from Abcam (Cambridge MA, USA). OCI-AML3 was obtained from DSMZ (Braunschweig, Germany) and 293T, HCT116, HL60, THP-1, and U937 cells were obtained from ATCC (Manassas, VA, USA). Primary AML human bone marrow cells were obtained from the CWRU Hematopoietic Stem Cell Core Facility (Cleveland, OH, USA). The cDNA for TLR8 was cloned into the pLVX-EF1alpha-IRES-mCherry vector obtained from Clontech (Mountain View, CA, USA)
Cell culture
Cells were cultured in RPMI 1640 media (Hyclone, Waltham, MA, USA). All media were supplemented with 10% FBS and 1% penicillin and streptomycin. Cells were cultured in a humidified atmosphere of 95% (v/v) air and 5% (v/v) CO2 at 37 °C.
Differentiation
NBT reduction assay was performed as previously described.19 Immunophenotyping was performed by staining cells with CD11b-PE or CD14-PE (eBiosciences San Diego CA, USA). The stained samples were analyzed using an EpicsXL cytometer (Beckman Coulter, Brea, CA, USA). For morphology assessments, cell slides were prepped using cytospin and then stained with a standard Wright-Giemsa stain.
Cytokine Array
Cytokine secretion was assessed using the Proteome Profiler Array: Human Cytokine Array Panel A (R & D Systems, Minneapolis MN, USA) as described by the manufacturer.
Microscope imaging
Slides were viewed using an EVOS XL core microscope with a 100x oil objective. Images were taken at room temperature using the built in EVOS XL core camera and acquisition software. Pictures were changed to grey scale and modified for brightness using PowerPoint.
Colony Assay
Cells were treated for at least 72 hours with 25 μg/ml R848, the drug was washed away, and then an equal number of viable cells were suspended in 0.03% noble agar (Sigma, St Louis, MO USA). Cells were allowed to grow for one week and colonies were counted.
Proliferation Assay
Equal numbers of HL60, THP1, OCI-AML3, HCT116, or 293T cells were plated and treated with 25 μg/ml R848 for at least 72 hours. Cells were analyzed for proliferation using the CellTiter 96 Aqueous One Solution Cell Proliferation Assay (Promega Madison WI, USA) as recommended by the manufacturer using a Molecular Devices Spectramax M2 plate reader.
Transfections and lentivirus infections
293T cells were co-transfected with either shMyD88 or empty vector pLKO (Sigma) and the packaging plasmids pCMV R8.74 and pMD.G or VSVG and ΔR84.1 using Turbofect (Thermo Fisher Pittsburgh PA, USA). AML cells were infected with the virus containing supernatant concentrated overnight in 12.5% PEG in the presence of 6 μg/ml of polybrene and stable cell lines were generated by selection with puromycin (1 μg/ml).
Western blot analysis
Cells treated as indicated were washed with PBS, centrifuged and lysed with a Triton-X containing lysis buffer for whole cell extracts. Protein lysates (50 μg/lane) were resolved on appropriate SDS-PAGE gel and transferred to PVDF membrane (Millipore, Billerica, MA, USA) using Bio-Rad transfer apparatus (Bio-Rad, Hercules, CA, USA). The membranes were blocked, incubated with the indicated primary antibodies at 4°C overnight, and then the appropriate horseradish peroxidase-conjugated secondary antibodies. Immunoreactive protein bands were detected by enhanced chemiluminescence (Pierce, Waltham, MA, USA) using XAR-5 film.
Mouse xenograft study
Six-week-old female Nod/SCID/IL2Rγ-/- (NSG) mice were injected bilaterally subcutaneously (sc) with 5×106 HL60 cells or 5×106 OCI-AML3 cells (N=5 per treatment group). Drug treatment was started when tumors were palpable, one week after tumor cell injection. R848 (20 μg or 40 μg, approximately 0.65 mg/kg or 1.3 mg/kg) or vehicle (30 μl of DMSO and 70 μl of water/PBS) were injected intraperitoneally (ip) 3 days a week for 3 weeks. Mouse tumor measurements were made at the indicated times after treatment was started. C57/BL6 mice were treated with R848 or vehicle intraperitoneally (ip) 3 days a week for 1 week using the same dosing as the NSG mouse study and then mouse blood was harvested and analyzed using a Drew Scientific Hemavet Hematology Analyzer (Dallas TX, USA). The Case Western Reserve University Animal Research Committee approved all of the animal protocols used in this study.
Statistics
Figures are reported as means +/− one standard error. P values were obtained using a one-tailed student’s T test to compare differences in means.
Results
R848 induces AMI differentiation
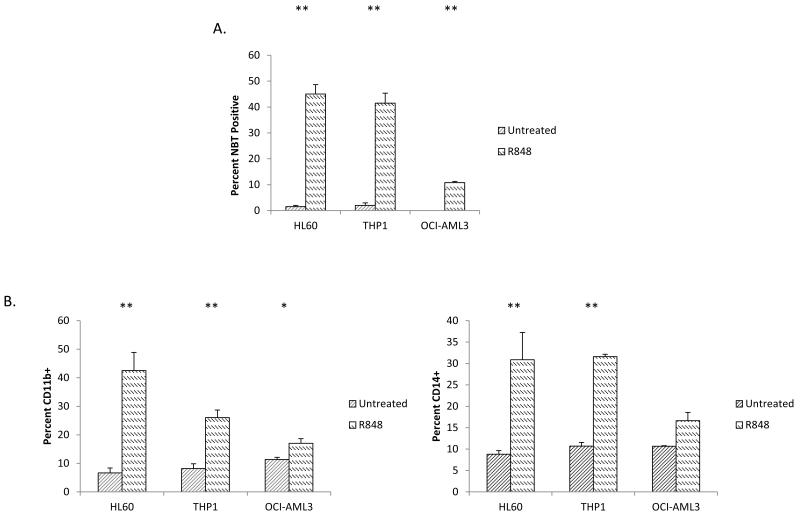
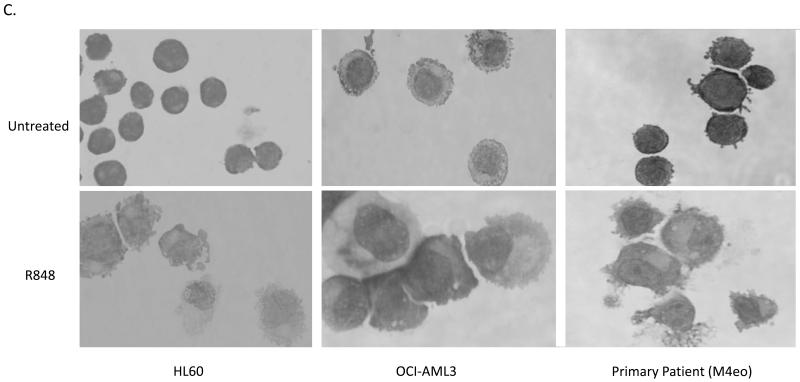
In order to test if the activation of TLRs can induce AML differentiation, we screened a panel of TLR ligands on HL60 cells (M2 subtype, t(15;17) negative) using the nitrotetrazolium blue (NBT) reduction assay. The NBT assay is a test that is highly specific to and has been used extensively as a measure of functional myelomonocytic differentiation.20-23 The NBT reduction test measures the ability of cells to generate a respiratory burst, a function that is only present in differentiated cells. While differentiation activity was not observed using TLR2 (Pam3Cys), TLR3 (PolyI:C), TLR4 [LPS], TLR5 (flagellin), imiquimod (TLR7) or TLR9 (CpG DNA) ligands (data not shown), stimulation with the prototypical TLR7/8 ligand, R848, was found to induce significant NBT reduction in HL60 cells, increasing the percentage of NBT positive cells from 1.5% to 47% (Figure 1A). Besides NBT reduction, R848-mediated differentiation was further evidenced by immunophenotyping (Figure 1B) and morphology (Figure 1C). These assays not only confirm the induction of differentiation, but also demonstrate that R848 induces monocytic differentiation as evidenced by the induction of the cell surface markers CDllb and CD14. While CDllb is induced by myelomonocytic differentiation, CD14 is a specific marker for monocytic differentiation. Clear morphologic monocytic differentiation is demonstrated by such features as condensed nuclei, increased cell size, abundant cytoplasm, and vacuoles (Figure 1C).
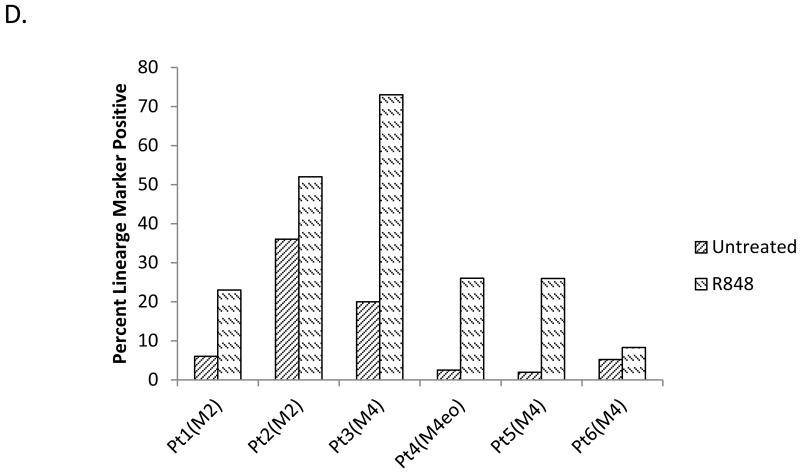
Figure 1. R848 promotes AML differentiation in vitro in AML cell lines and primary patient samples.
(A) The TLR7/8 ligand R848 increases the percentage of AML cells capable of respiratory burst. HL60, THP1 and OCI-AML3 cells were treated with the TLR7/8 ligand R848 (25 μg/ml unless otherwise stated) for at least 72 hours and then tested for differentiation using the NBT assay. ** indicates p<0.01. (B) R848 promotes lineage marker expression in AML lines. HL60, THP1, and OCI-AML3 cells were treated with R848 for at least 72 hours and stained for the lineage markers CD11b and CD14. * indicates p<0.05 ** indicates p<0.01 OCI. (C) R848 treatment induces morphological changes in AML cell lines and patient samples. Original magnification 100×. (D) R848 increases lineage marker expression in primary AML patient samples. Primary patient samples were treated with R848 for at least 72 hours and then lineage markers were assessed. All patients were tested for CD14 except for Patient 1 (CD11b+) and with 25 μg/ml R848 except for Patient 2 (10 μg/ml).
R848 induces differentiation in non-promeylocytic leukemia cell lines and patient samples
In order to explore the ability of R848 to induce differentiation in other AML cells besides HL60 cells, additional AML cell lines as well as primary patient samples were treated with R848. Importantly, like HL-60, none of these cell lines possess the t(15;17) PML/RARα translocation, the molecular target that makes ATRA a clinically useful differentiation agent. R848 exhibited AML differentiation activity on 0CI-AML3 (M4 subtype) and THP-1 (M5 subtype) cells as measured by NBT reduction and immunophenotyping (Figure 1A-B).
Besides AML cell lines, R848 can also induce clear evidence of differentiation in non-promyelocytic primary AML patient samples (M2, M4 and M4Eo subtypes). Testing of primary samples is important, as many reported differentiation agents have been found to exhibit high activity only in cell lines. Of 6 primary AML samples tested, R848 induced clear evidence of differentiation in 5 samples (Figure 1D). Morphological changes are also observed in patient samples (Figure 1C and data not shown).
R848 induces terminal differentiation and inhibits AML proliferation
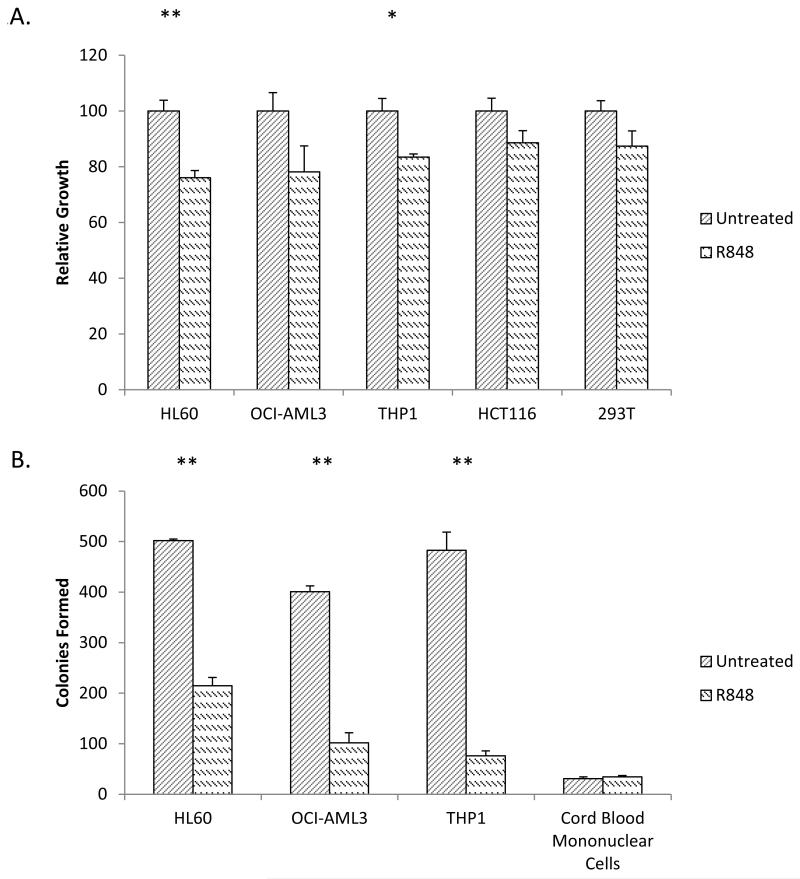
To assess the ability of R848 to affect leukemic cell growth, its effects on HL60, THP1, and OCI-AML3 cell proliferation were measured using the MTT assay. R848 led to 24%, 22%, and 17% less growth of these three cell lines respectively as compared to untreated cells after four days, suggesting R848 is impairing leukemia cell proliferation (Figure 2A).
Figure 2. R848 induces terminal differentiation in AML cell lines.
(A) R848 slows the growth rate of AML cell lines. Equal numbers of HL60, OCI-AML3, THP1, HCT116 and 293T cell lines were treated with R848 for at least 72 hours and monitored by MTT assay for proliferation. MTT signal was normalized to untreated controls. * indicates p< 0.05 ** indicates p<0.01. (B) R848 inhibits colony formation of AML cell lines. HL60, OCI-AML3, THP1 and normal cord blood cells were treated with R848. After at least 72 hours of R848 treatment, the drug was washed away and an equal number of viable cells were cultured in a semisolid soft agar medium for 7 days. ** indicates p<0.01.
To test if R848 is specifically impairing the growth of AML cells, the non-AML cell lines, HCT116 and 293T, were also treated with R848. HCT116 and 293T cells exhibited no statistically significant difference in growth, supporting R848 mediated growth inhibitory effects may have some specificity to AML cells (Figure 2B).
In order for a differentiation-inducing compound to be clinically desirable, it must induce terminal differentiation. Terminally differentiated cells irreversibly lose their capacity to divide and therefore to cause disease. To assess for evidence of irreversible growth arrest, AML cells were treated with R848 for at least 72 hours and the drug was washed away. The cells were then plated in soft agar and assessed for colony growth after 7 days. The R848 treatment led to a marked decrease in colony formation in all cell lines tested. We observed on average 42%, 25%, and 15% colony formation for the HL60, OCI-AML3 and THP1 cell lines, respectively, as compared to untreated cells (Figure 2B). This dramatic reduction in colony formation suggests a significant portion of cells have undergone terminal differentiation and have likely lost the capacity to divide and drive disease.
To further test the specificity of R848 effects on the colony formation of leukemic cells, colony assays were also performed using healthy hematopoietic progenitor cells from cord blood. Importantly, R848 did not cause statistically significant differences in colony formation using normal cord blood (Figure 2B).
R848-driven differentiation is mediated through TLR8
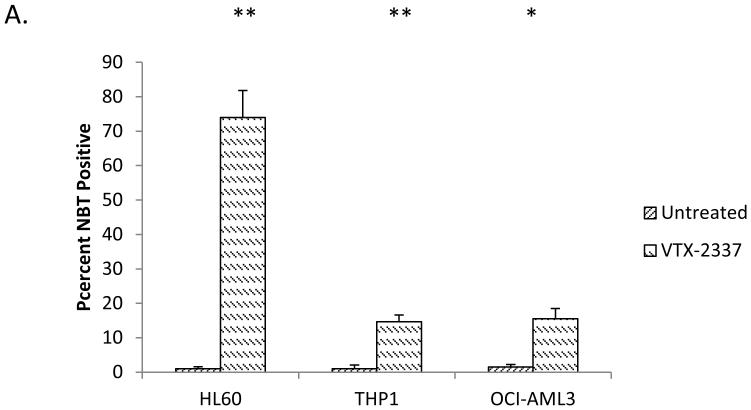
In order to further explore the mechanisms through which R848 induces differentiation, we tested if the differentiation effects were mediated through TLR7 as R848 activates both TLR7 and TLR8 in human cells. While R848 induces differentiation, no increase in NBT reduction capacity was observed utilizing the TLR7-specific ligand, imiquimod, suggesting that TLR8 is the key mediator of the differentiation effects (Data not shown). To further support a TLR8-driven differentiation mechanism, HL60, THP1, and OCI-AML3 cells were treated with VTX-2337. VTX-2337 is a potent TLR8-specific agonist currently in development.24 Similar to R848, VTX-2337 promoted AML differentiation as measured by NBT reduction in HL-60, THP1, and 0CI-AML3 cells, supporting a TLR8-mediated pathway (Figure 3A).
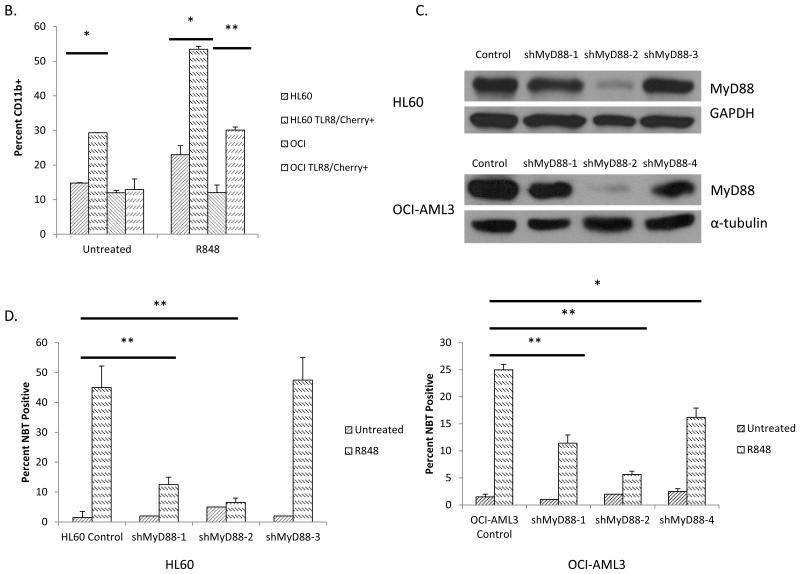
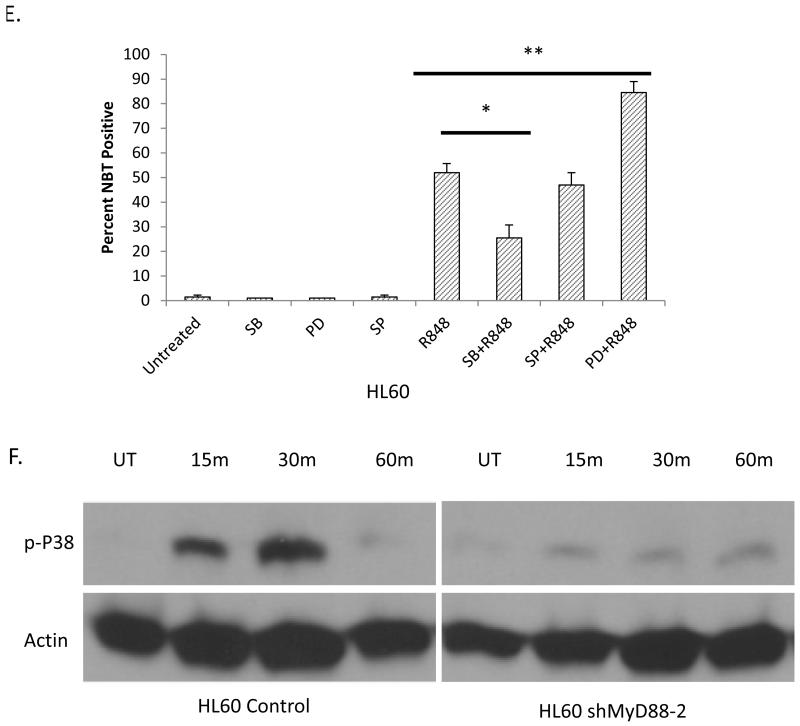
Figure 3. R848 promotes AML differentiation in a TLR8/MyD88/p38 dependent manner.
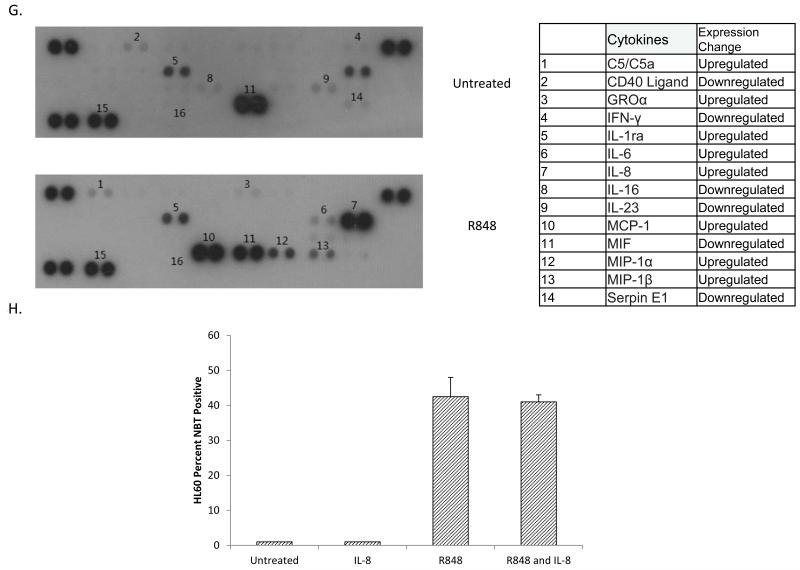
(A) VTX-2337 promotes AML differentiation. HL60, THP1, and OCI-AML3 were treated with 10 nM VTX-2337 for at least 72 hours and assessed for differentiation by the NBT reduction assay.(B) TLR8 overexpression increases R848 driven increase of the CD11b lineage marker. HL60 cells and OCI-AML3 were transfected with a TLR8-mCherry plasmid and treated with R848. After at least 72 hours, cells were stained for CD11b and gated based upon the presence or absence of mCherry. TLR8 overexpression yielded statistically significant differences in CD11b expression in untreated HL60 and treated HL60 and OCI-AML3 cells. * indicates p< 0.05 ** indicates p<0.01. (C) Myd88 knockdown in HL60 and OCI-AML3 cells. Cell lysate from the indicated cell lines was analyzed by western blot. (D) MyD88 knockdown impairs R848-mediated differentiation. The indicated cell lines were treated with R848 for at least 72 hours and assessed for differentiation using NBT assay. shMyD88-1 and shMyD88-2 yield statistically significant differences in NBT reduction on treatment in HL60 cells compared to parental HL60 controls. The difference between shMyD88-3 and HL60 was not significant. shMyD88-1, shMyD88-2 and shMyD88-4 yield statistically significant differences in NBT reduction in OCI-AML3 cells as compared to an empty vector control. * indicates p < 0.05 ** indicates p<0.01. (E) p38 inhibition impairs R848-mediated HL60 cell differentiation. HL60 cells were treated with 10 μM of SB203580 (p38), PD98059 (ERK1/2), or SP600125 (JNK) and/or R848 for at least 72 hours and assessed for differentiation using the NBT assay. * indicates p< 0.05 ** indicates p<0.01. (F) R848 activates p38 in a MyD88-dependent manner in HL60 cells. The indicated cells were treated with R848 and assessed for p-P38 status over time by western blot. (G) R848 changes the cytokine secretion profile of HL60 cells. HL60 cells were treated with R848 for at least 72 hours and cytokines were analyzed using a human cytokine array. (h) R848-mediated differentiation of AML cell lines is not driven by IL-8. HL60 cells were treated with R848, anti-IL-8 neutralizing antibody (50 ng/ml), or both for at least 72 hours and assessed by NBT assay.
To further confirm the role of TLR8 in AML differentiation, we overexpressed TLR8 in HL60 and 0CI-AML3 cells utilizing a TLR8-mcheriy expression construct. The overexpression of TLR8 led to both an increase in the basal level of differentiation in HL60 cells as well as sensitized both HL60 and 0CI-AML3 cells to R848-mediated differentiation as measured by immunophenotyping (Figure 3B). On R848 treatment, we observed 55% and 30% CDllb+ expression in HL60 and OCI-AML3 cells overexpressing TLR8 as compared to 25% and 12% in HL60 and 0CI-AML3 cells not overexpressing TLR8 (Figure 3B).
R848-driven differentiation is mediated through MyD88
As the adapter protein, MyD88, is known to be an essential upstream mediator of TLR8 signaling pathways, we explored the role of this protein in R848-mediated differentiation. For this study we knocked down the expression of Myd88 in HL60 and OCI-AML3 cells using four different lentiviral shRNA constructs that led to varying levels of Myd88 knockdown (Figure 3C). The Myd88 knockdown cells were treated with R848, and differentiation was assessed using the NBT assay. We observed a dramatic reduction in R848-mediated differentiation in the cell lines that had significantly reduced levels of Myd88. For example, the shMyD88-2 transfected HL60 cells exhibited 8% differentiation as compared to parental HL60 cells that exhibit 45% differentiation. (Figure 3D) Similar results were observed in the OCI-AML3 line, yielding 5% differentiation of the shMyD88-2 transfected 0CI-AML3 cells as compared to 22% in parental 0CI-AML3 cells. (Figure 3D). Importantly, even modest knockdown levels of MyD88 leads to impaired AML differentiation, suggesting this differentiation effect is highly dependent on MyD88. This study further confirms that R848-mediated AML differentiation is dependent upon the activation of Toll-like receptor signaling as Myd88 is a necessary and relatively specific mediator of TLR signaling.
R848-driven differentiation is mediated through p38
In order to further elucidate mechanisms through which R848 induces AML differentiation, we investigated the role of downstream mediators of TLR signaling. TLR ligands are known to activate the MAP kinase pathways, which are key regulators of cell growth, division and differentiation.6,7,25 Utilizing chemical inhibitors for the ERK1/2, JNK, and p38 kinases, we assessed changes in R848-mediated differentiation in the HL60 cells. Interestingly, we found that the p38 inhibitor, SB203580, significantly impaired differentiation induction in response to R848 as only 30% of HL60 cells can reduce NBT on R848 and SB203580 treatment compared to 52% on R848 treatment alone (Figure 3E). p38 activation has been shown to promote differentiation in other malignancies, and our results suggest it promotes R848-mediated AML differentiation 26-28. Interestingly, ERK1/2 inhibition with PD08959 enhances R848-mediated differentiation exhibiting 83% NBT positive cells on R848 and PD08959 treatment versus 52% on R848 treatment alone, suggesting an appropriate balance of MAPK activation may be necessary for TLR-driven AML differentiation (Figure 3E). R848 treatment of HL60 cells was found to activate p38 in a MyD88 dependent manner, suggesting that p38 is downstream of Myd88 in the TLR8-mediated AML differentiation pathway (Figure 3F).
Cytokine signaling has been shown to promote AML differentiation. For example, CD44 stimulated AML differentiation is IL-8 dependent.29 To assess changes in cytokine secretion, we treated HL60 cells with R848 and assessed cytokine secretion, observing a diverse change in cytokine profile on R848 treatment, including marked IL-8 induction (Figure 3G). As IL-8 has been previously shown to promote differentiation, and p38 has been shown to promote IL-8 secretion, we tested if IL-8 secretion promoted R848-mediated AML differentiation. 29,30 HL60 cells were treated with R848, anti-IL-8 neutralizing antibody, or a combination and assessed for differentiation. No change in NBT reduction capacity was observed between R848 and R848/anti-IL-8 treated cells, suggesting IL-8 does not play a role in R848-mediated AML differentiation (Figure 3H).
R848 demonstrates potent efficacy in an AML NSG mouse xenograft model
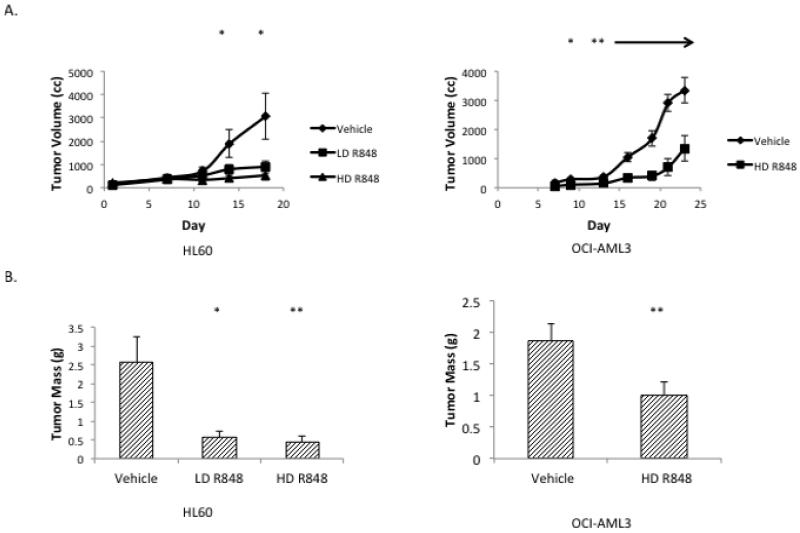
To determine the in vivo activity of utilizing R848 for AML treatment, a mouse AML xenograft study was performed. As TLR7/8 agonists are known to promote immunomodulatory effects such as cytotoxic T cell anti-tumor responses, we used severely immunodeficient mice (NSG) for this study.15-17 As these mice lack mature B, T and NK cells and have multiple defects in the innate immune response, it is highly likely that tumor responses to R848 in this model are due to a direct anti-leukemic effect and not an inflammatory response. In addition, it is important to note that mouse cells do not respond to R848 through TLR8 signaling.31 In this established tumor model, the HL60 and 0CI-AML3 tumors in the R848-treated mice grew at a significantly reduced rate as compared to vehicle-treated mice [Figure 4A). At the end of the treatment period, the HL60 tumors weighed 2.56 g +/− 0.68 g in the vehicle treated mice compared to 0.45 g +/− 0.145 g for the high dose R848-treated mice and the OCI-AML3 tumors weighed 2.03 g +/− 0.24 g for vehicle-treated mice as compared to 0.81 g +/− 0.14 g for the R848-treated mice (Figure 4B). Importantly, there were no obvious signs of toxicity from the dosing used suggesting that this therapeutic strategy may be safe, and it is worthy of further development for AML.
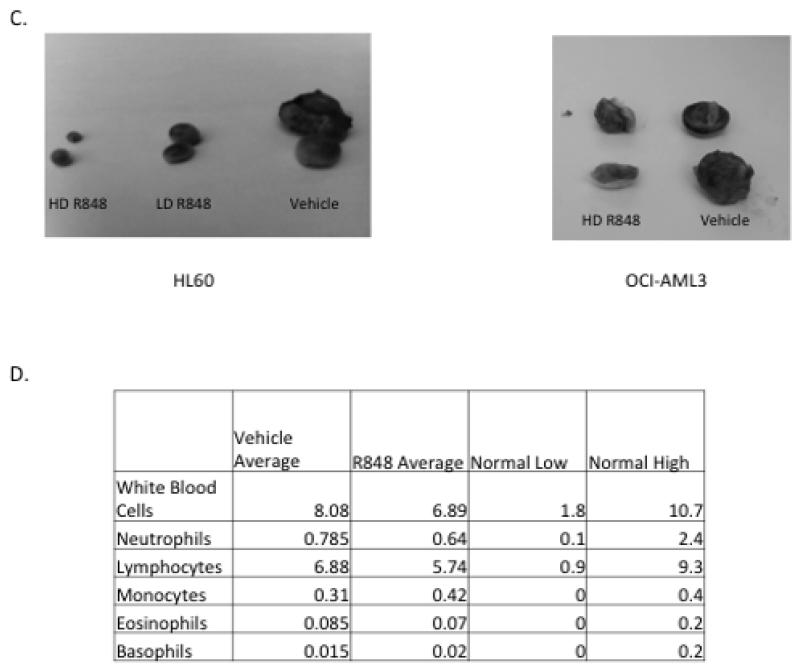
Figure 4. R848 treatment slows AML tumor growth in an NSG mouse model.
(A) R848 treatment leads to a significant reduction in HL60 and OCI-AML3 tumor growth over time. NSG mice were injected subcutaneously with HL60 or OCI-AML3 cells and then treated with 20 μg (Low dose, LD) or 40 μg (High dose, HD) of R848, approximately 0.65 mg/kg or 1.3 mg/kg (OCI-AML3 mice were only treated with HD). Tumor volume was assessed over time. * indicates p< 0.05, comparing HL60 vehicle and high dose at day 14 and vehicle to high or low dose at day 18. ** indicates p<0.01 (B) R848 treated mice exhibit significantly smaller tumors than vehicle treated mice. * indicates p<0.05 ** indicates p<0.01. (C) Picture of representative tumors. (D) C57BL/6 mice were treated with 40 μg R848 (N=3) or vehicle (N=2) 3 times a week for 1 week, and white blood cell counts were assessed. Differences between treatment groups were not significant.
To demonstrate the specificity of R848 for leukemic cells over normal hematopoietic cells in the in vivo setting, we treated wild-type C57/BL6 mice with R848 or vehicle for one week using the same dosing as the efficacy study. These mice demonstrated normal white blood cell counts (Figure 4D).
Discussion
Here we present the first evidence demonstrating TLR8 simulation induces AML differentiation, revealing a novel therapeutic strategy for AML, a disease for which novel approaches are urgently needed. TLRs are a family of proteins (TLR1-13) that play important roles in the immune system.7 TLRs recognize broad pathogen associated molecular patterns, activating signaling pathways leading to the induction of NF-κB and AP1 transcription factors and inflammation.7
TLR stimulation is linked to normal hematopoietic differentiation. Specifically, TLR2 or TLR4 stimulation of normal hematopoietic cells drive hematopoietic stem cell and myeloid progenitor growth and differentiation, leading to increased myeloid cell counts, and TLR7/8 have been shown to drive CD34+ differentiation into macrophages and dendritic cell precursors.5, 11 While TLRs are expressed on AML cells, TLR stimulation previously has not been shown to promote AML differentiation.10 Thus, our work suggests TLR8 stimulation may activate a novel AML differentiation pathway.
In addition to demonstrating TLR8 can lead to AML differentiation in cell lines and primary patient samples, we also show that it impairs AML cell proliferation in vitro, colony growth, and in vivo tumor formation, suggesting R848 mediated differentiation effects impairs leukemia growth. Of note, while R848 induces only modest AML growth inhibition in vitro using proliferation assays, it leads to potent effects on colony formation and mouse tumor growth. These findings are likely due to the fact that AML cells typically undergo several rounds of division after differentiation is induced. For example, these results are very similar to the effects on AML proliferation mediated by ATRA, the prototypical AML differentiation agent. 19 The marked reduction in colony formation and impaired growth of AML tumors in mice at a sub-toxic dose, suggests a potential clinical utility of a TLR8 agonist for leukemia therapy.
TLR stimulation has been previously proposed as an anti-cancer strategy as TLR stimulation activates immune cells to kill cancer cells. The major anti-cancer mechanism through which TLR agonists are thought to act is through T cell activation.15-17 In addition, TLR activation via bacterial products such as LPS and bacterial have been shown to promote antitumor effects through cytokine induction.18 TLR8, specifically, has been shown to downregulate regulatory T cell function.17
Our results demonstrate that TLR8 stimulation can exert direct anti-leukemia activity. We have shown R848 has anti-leukemic effects in severely immunodeficient NSG mice that lack T, B cells and NK cells. This study demonstrates R848’s in vivo anti-leukemic activity does not require T cells and is likely not mediated by its immunomodulatory effects in this model. In immunocompetent individuals, TLR8 stimulation may lead to anti-leukemic activities through both direct and immunomodulatory effects that would likely complement each other. Importantly, our results suggest that R848 treatment does not impact the colony forming capacity of normal hematopoietic progenitor cells nor does it significantly modify white blood cell counts in control mice, suggesting R848 treatment is specifically driving AML differentiation with limited effects on normal cells (Figure 2B, 4D). These results support developing TLR8 stimulation as an AML therapy.
We show that R848-mediated differentiation occurs through a TLR8, MyD88 and p38 dependent pathway. To demonstrate the direct role of TLR8 on AML cells, we overexpressed TLR8 in HL60 and OCI-AML3 cells and found that the HL60 cells overexpressing TLR8 exhibit significantly higher level of AML differentiation alone, and both HL60 and OCI-AML3 cells exhibit higher levels of AML differentiation upon R848 treatment. The increased level of differentiation after TLR8 transfection alone is expected as TLR8 overexpression leads to constitutive signaling. To further support the direct role of TLR8 in impacting AML differentiation, we explored the requirement of key components of its signaling pathway. The AML differentiation was found to be dependent upon a critical TLR adapter protein, Myd88, and at least partially dependent upon a downstream kinase p38. Interestingly, p38 activation has been linked to differentiation in several malignancies such as rhabdosarcoma, glioma, and colon carcinoma.26-28 This p38-mediated effect may partially explain TLR8 specificity as although the TLRs all activate MAPK pathways, the extent and pattern of these activations vary.25, 32
Though we have identified a novel direct anti-leukemia activity of R848 on AML cells, importantly TLR agonists are already widely in development for cancer therapy due to their immunomodulatory effects. For example, TLR7/8 ligands are currently being used topically to treat skin cancers and actinic keratosis.14 The use of a cocktail of dead S pyrogenes and S marcescens has been clinically used in sarcoma treatment as early as the 19th century which would lead to the activation of a multitude of cell surface and intracellular TLRs.14, 18
Interestingly, there is a long history of clinical reports that support the role of TLR stimulation in specifically controlling AML. In total, approximately 100 spontaneous AML remissions have been reported, predominately associated with patients with infections or other inflammatory conditions.33-36 It was previously thought that an immune response against the leukemic cells led to these remissions.33-36 Though TLR stimulation leads to immune activation, our results suggest that in addition to these well-known and clinically relevant host-versus-leukemia effects, TLR8 activation can directly promote leukemia differentiation and growth inhibition. This finding provides a potential alternative or complementary mechanism for these cases of spontaneous AML remission.14, 15, 17
In addition to our work, a previous study also probed TLR ligand mediated differentiation as well as TLR expression patterns in AML cell lines.10 This group probed a small panel of TLR ligands (not including R848) and did not observe any evidence of AML differentiation. Consistent with this report, we did not identify AML differentiation effects by activating any TLR except for a TLR8-mediated effect, and this group did not test R848. They did test a small RNA ligand of the type that has been found to activate TLR7 and/or TLR8, however this type of stimuli is known to be a relatively weak inducer of TLR8 compared to R848.37, 38 Though no evidence of differentiation was observed, they did demonstrate that TLRs, including TLR8, are widely expressed in AML cells.10 Consistent with our findings, another study has also reported that a different TLR8 ligand (ssRNA40) leads to growth inhibition of a leukemia cell line, though no differentiation studies were performed.39 Our work, as well as these previous studies, suggest that the TLR-mediated AML differentiation effects are likely only activated by a limited number of TLRs. While we show R848-mediated differentiation was TLR8 dependent, we cannot rule out the possibility that co-stimulation of the TLR7 pathway also plays a role as TLR7 is also activated by R848. However, it should be noted that VTX-2337 also exhibited significant AML differentiation and it is a TLR8 specific agonist without significant TLR7 activity.
In summary, here we present evidence that R848 treatment of AML cells can promote differentiation in a TLR8/MyD88/p38 dependent manner and exhibits strong anti-leukemia activity in a mouse model system, suggesting TLR8 stimulation may be a novel AML therapy. The same dosing we used for our mouse studies has been shown to cause no organ damage or major toxicities, with the major change noted by others being a shift in hematopoietic profile towards more myeloid cells although our studies do not reveal such a shift in control mice.32 As TLR7/8 ligands are currently used clinically as topical treatments for dermatological malignancies and are being tested as anti-cancer immune adjuvants in other settings, the clinical potential of these agents as anti-leukemic agents could be rapidly assessed.
Acknowledgements
This research was supported by the Athymic Animal and Xenograft Core Facility and the Cytometery and Imaging Microscopy Core Facility of the Case Comprehensive Cancer Center (P30CA043703) as well as the following grants: NIH T32 CA59366-17 /GM007250 (J.I-H.) and National Natural Science Foundation of China 30400520 (H.W.).
Footnotes
Conflict of Interest:
The authors declare no conflicts of interest.
References
- 1.Estey EH. General approach to, and perspectives on clinical research in, older patients with newly diagnosed acute myeloid leukemia. Semin Hematol. 2006 Apr;43(2):89–95. doi: 10.1053/j.seminhematol.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Tallmann MS. Curative therapeutic approaches to APL. Annals of hematology. 2004;83(Suppl 1):S81–82. doi: 10.1007/s00277-004-0850-2. [DOI] [PubMed] [Google Scholar]
- 3.Gupta K, Gulen F, Sun L, Aguilera R, Chakrabarti A, Kiselar J, et al. GSK3 is a regulator of RAR-mediated differentiation. Leukemia. 2012 Jun;26(6):1277–1285. doi: 10.1038/leu.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Z, Smith KS, Murphy M, Piloto O, Somervaille TC, Cleary ML. Glycogen synthase kinase 3 in MLL leukaemia maintenance and targeted therapy. Nature. 2008 Oct 30;455(7217):1205–1209. doi: 10.1038/nature07284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagai Y, Garrett KP, Ohta S, Bahrun U, Kouro T, Akira S, et al. Toll-like Receptors on Hematopoietic Progenitor Cells Stimulate Innate Immune System Replenishment. Immunity. 2006;24(6):801–812. doi: 10.1016/j.immuni.2006.04.008. 6// [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akira S TK. Toll-like receptor signalling. Nat Rev Immunol. 2004;4(7):499–511. doi: 10.1038/nri1391. 2004. [DOI] [PubMed] [Google Scholar]
- 7.Akira S. Toll-like Receptor Signaling. Journal of Biological Chemistry. 2003;278(40):38105–38108. doi: 10.1074/jbc.R300028200. [DOI] [PubMed] [Google Scholar]
- 8.Milella M, Kornblau SM, Estrov Z, Carter BZ, Lapillonne H xE, et al. Therapeutic targeting of the MEK/MAPK signal transduction module in acute myeloid leukemia. The Journal of Clinical Investigation. 2001;108(6):851–859. doi: 10.1172/JCI12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park J CH, Jeong J, Han J, Kim I. Invovlement of p38 kinase in Hydroxyurea-induced Differentiation of K562 Cells. Cell Growth Differ. 2001;12(9):481–486. [PubMed] [Google Scholar]
- 10.Okamoto M, Hirai H, Taniguchi K, Shimura K, Inaba T, Shimazaki C, et al. Toll-like Receptors (TLRs) are expressed by myeloid leukaemia cell lines, but fail to trigger differentiation in response to the respective TLR ligands. British Journal of Haematology. 2009;147(4):585–587. doi: 10.1111/j.1365-2141.2009.07858.x. [DOI] [PubMed] [Google Scholar]
- 11.Sioud M, Fløisand Y. TLR agonists induce the differentiation of human bone marrow CD34+ progenitors into CD11c+ CD80/86+ DC capable of inducing a Th1-type response. European Journal of Immunology. 2007;37(10):2834–2846. doi: 10.1002/eji.200737112. [DOI] [PubMed] [Google Scholar]
- 12.Spaner DE, Masellis A. Toll-like receptor agonists in the treatment of chronic lymphocytic leukemia. Leukemia. 2006;21(1):53–60. doi: 10.1038/sj.leu.2404456. 10/26/online. [DOI] [PubMed] [Google Scholar]
- 13.Bohnhorst J, Rasmussen T, Moen SH, Flottum M, Knudsen L, Borset M, et al. Toll-like receptors mediate proliferation and survival of multiple myeloma cells. Leukemia. 2006;20(6):1138–1144. doi: 10.1038/sj.leu.2404225. 04/13/online. [DOI] [PubMed] [Google Scholar]
- 14.Hennessy EJ, Parker AE, O’Neill LAJ. Targeting Toll-like receptors: emerging therapeutics? Nat Rev Drug Discov. 2010;9(4):293–307. doi: 10.1038/nrd3203. 04//print. [DOI] [PubMed] [Google Scholar]
- 15.Larangé A, Antonios D, Pallardy M, Kerdin-Römere S. TLR7 and TLR8 agonists trigger different signaling pathways for human dendritic cell maturation. Journal of Leukocyte Biology. 2009;85(4):673–683. doi: 10.1189/jlb.0808504. [DOI] [PubMed] [Google Scholar]
- 16.Oberg HH JM, Kabelitz D, Wesch D. Regulation of T cell activation by TLR ligands. European Journal of Cell Biology. 2011;90(6-7):582–592. doi: 10.1016/j.ejcb.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 17.Peng G, Guo Z, Kiniwa Y, Voo Ks, Peng W, Fu T, et al. Toll-Like Receptor 8-Mediated Reversal of CD4+ Regulatory T Cell Function. Science. 2005;309(5739):1380–1384. doi: 10.1126/science.1113401. [DOI] [PubMed] [Google Scholar]
- 18.Wei MQ, Mengesha A, Good D, Anné J. Bacterial targeted tumour therapy-dawn of a new era. Cancer Letters. 2008;259(1):16–27. doi: 10.1016/j.canlet.2007.10.034. 1/18/ [DOI] [PubMed] [Google Scholar]
- 19.Wald DN, Vermaat HM, Zang S, Lavik A, Kang Z, Peleg G, et al. Identification of 6-Benzylthioinosine as a Myeloid Leukemia Differentiation-Inducing Compound. Cancer Research. 2008 Jun 1;68(11):4369–4376. doi: 10.1158/0008-5472.CAN-07-6559. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collins SJ, Ruscetti FW, Gallagher RE, Gallo RC. Normal functional characteristics of cultured human promyelocytic leukemia cells (HL-60) after induction of differentiation by dimethylsulfoxide. The Journal of Experimental Medicine. 1979 Apr 1;149(4):969–974. doi: 10.1084/jem.149.4.969. 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collins SJ BA, Ting R, Gallo RC. Induction of morphological and functional differentiation of human promyelocytic leukemia cells (HL-60) by compounds which induce differentiation of murine leukemia cells. Int J Cancer. 1980;25(15):213–218. doi: 10.1002/ijc.2910250208. [DOI] [PubMed] [Google Scholar]
- 22.Newburger P, Chovaniec M, Greenberger J, Cohen H. Functional changes in human leukemic cell line HL-60. A model for myeloid differentiation. The Journal of Cell Biology. 1979 Aug 1;82(2):315–322. doi: 10.1083/jcb.82.2.315. 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piedfer M, Bouchet S, Tang R, Billard C, Dauzonne D, Bauvois B. p70S6 kinase is a target of the novel proteasome inhibitor 3,3′-diamino-4′-methoxyflavone during apoptosis in human myeloid tumor cells. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2013;1833(6):1316–1328. doi: 10.1016/j.bbamcr.2013.02.016. 6// [DOI] [PubMed] [Google Scholar]
- 24.Lu H, Dietsch GN, Matthews M-AH, Yang Y, Ghanekar S, Inokuma M, et al. VTX-2337 Is a Novel TLR8 Agonist That Activates NK Cells and Augments ADCC. Clinical Cancer Research. 2012;18(2):499–509. doi: 10.1158/1078-0432.CCR-11-1625. [DOI] [PubMed] [Google Scholar]
- 25.Dziarski R, Jin Y-p, Gupta D. Differential Activation of Extracellular Signal-Regulated Kinase (ERK) 1, ERK2, p38, and c-Jun NH2-Terminal Kinase Mitogen-Activated Protein Kinases by Bacterial Peptidoglycan. Journal of Infectious Diseases. 1996;174(4):777–785. doi: 10.1093/infdis/174.4.777. [DOI] [PubMed] [Google Scholar]
- 26.Chiacchiera F, Matrone A, Ferrari E, Ingravallo G, Lo Sasso G, Murzilli S, et al. p38[alpha] blockade inhibits colorectal cancer growth in vivo by inducing a switch from HIF1[alpha]- to FoxO-dependent transcription. Cell Death Differ. 2009;16(9):1203–1214. doi: 10.1038/cdd.2009.36. 04/03/online. [DOI] [PubMed] [Google Scholar]
- 27.Puri PL, Wu Z, Zhang P, Wood LD, Bhakta KS, Han J, et al. Induction of terminal differentiation by constitutive activation of p38 MAP kinase in human rhabdomyosarcoma cells. Genes & Development. 2000 Mar 1;14(5):574–584. 2000. [PMC free article] [PubMed] [Google Scholar]
- 28.Sato A, Okada M, Shibuya K, Watanabe E, Seino S, Narita Y, et al. Pivotal role for ROS activation of p38 MAPK in the control of differentiation and tumor-initiating capacity of glioma-initiating cells. Stem Cell Research. 2014;12(1):119–131. doi: 10.1016/j.scr.2013.09.012. 1// [DOI] [PubMed] [Google Scholar]
- 29.Delaunay J, Lecomte N, Bourcier S, Qi J, Gadhoum Z, Durand L, et al. Contribution of GM-CSF and IL-8 to the CD44-induced differentiation of acute monoblastic leukemia. Leukemia. 2007;22(4):873–876. doi: 10.1038/sj.leu.2404976. 10/04/online. [DOI] [PubMed] [Google Scholar]
- 30.Islam Z, Gray JS, Pestka JJ. p38 mitogen-activated protein kinase mediates IL-8 induction by the ribotoxin deoxynivalenol in human monocytes. Toxicology and Applied Pharmacology. 2006;213(3):235–244. doi: 10.1016/j.taap.2005.11.001. 6/15/ [DOI] [PubMed] [Google Scholar]
- 31.Jurk M HF, Vollmer J, Schetter C, Kreig AM, Wagner H, Lipford G, Bauer S. Human TLR7 or TLR8 indepednently confer responsiveness to the antiviral compound R-848. Nature Immunology. 2002;3:499. doi: 10.1038/ni0602-499. [DOI] [PubMed] [Google Scholar]
- 32.Baenziger S, Heikenwalder M, Johansen P, Schlaepfer E, Hofer U, Miller RC, et al. Triggering TLR7 in mice induces immune activation and lymphoid system disruption, resembling HIV-mediated pathology. Blood. 2009 Jan 8;113(2):377–388. doi: 10.1182/blood-2008-04-151712. 2009. [DOI] [PubMed] [Google Scholar]
- 33.Ifrah N, James J-M, Viguie F, Marie J-P, Zittoun R. Spontaneous remission in adult acute leukemia. Cancer. 1985;56(5):1187–1190. doi: 10.1002/1097-0142(19850901)56:5<1187::aid-cncr2820560536>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 34.Kazmierczak M SA, Czyz A, Rupa-Matysek J, Komarnicki M. Spontaneous hematological remission of acute myeloid leukemia. Contemp Oncol. 2014;18(1):67–69. doi: 10.5114/wo.2013.38915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lachant NA, Goldberg J, Nelson DA, Gottlieb AJ. Spontaneous remission in acute myelogenous leukemia in the adult. The American journal of medicine. 1979;67(4):687–692. doi: 10.1016/0002-9343(79)90266-3. [DOI] [PubMed] [Google Scholar]
- 36.Maywald O, Buchheidt D, Bergmann J, Schoch C, Ludwig WD, Reiter A, et al. Spontaneous remission in adult acute myeloid leukemia in association with systemic bacterial infection—case report and review of the literature. Ann Hematol. 2004;83(3):189–194. doi: 10.1007/s00277-003-0741-y. 2004/03/01. [DOI] [PubMed] [Google Scholar]
- 37.Forsbach A, Nemorin J-G, Montino C, Müller C, Samulowitz U, Vicari AP, et al. Identification of RNA Sequence Motifs Stimulating Sequence-Specific TLR8-Dependent Immune Responses. The Journal of Immunology. 2008 Mar 15;180(6):3729–3738. doi: 10.4049/jimmunol.180.6.3729. 2008. [DOI] [PubMed] [Google Scholar]
- 38.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, et al. Species-Specific Recognition of Single-Stranded RNA via Toll-like Receptor 7 and 8. Science. 2004;303(5663):1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 39.Xiong F WX, Zhang JH, Liu W, Sun S, Liu LQ, Wang P, Huang SA. Expression and role of toll-like receptors in U937 cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2007;15(3):449–453. [PubMed] [Google Scholar]