Abstract
Human neuropeptide Y Y2 receptors expressed in CHO cells are largely oligomeric, and upon solubilization are recovered by density gradient centrifugation as ~180 kDa complexes of receptor dimers and G-protein heterotrimers. A large fraction of the receptors is inactivated in the presence of pertussis toxin, in parallel with inactivation of Gi α subunits (with half-periods of about 4 h for both). This is accompanied by a very long-lasting loss of receptor dimers and of masked surface Y2 sites (an apparent receptor reserve precoupled mainly to Gi α subunit-containing G-proteins). However, surface Y2 receptors accessible to large peptide agonists are much less sensitive to the toxin. All surface Y2 receptors are rapidly blocked by Y2 antagonist BIIE0246, with a significant loss of the dimers, but with little change of basal Gi activity. However, both dimers and Y2 receptor compartmentalization are restored within 24 hours after removal of the antagonist. In CHO cells, the maintenance and organization of Y2 receptors appear to critically depend on functional pertussis toxin-sensitive G-proteins.
Keywords: G-protein coupled receptor, Receptor dimmer, Receptor masking, Receptor compartmentalization
1. Introduction
Neuropeptide Y (NPY) Y2 receptors can activate the Gi/o type, pertussis toxin-sensitive, fast-loading α subunits of heterotrimeric G-proteins (e.g. [1]), and are inactivated by the toxin in parallel to inhibition of these subunits (ref. [2] and this study). These receptors also form a large masked surface pool that can be exposed to agonist peptides by bridging of protein cysteines, cholesterol depletion, and non-disruptive shearing [2,3]. This compartmentalization could be connected to oligomerization of Y2 receptors, which are known to form dimers [4], and can be isolated as high-affinity aggregates also containing G-proteins [5]. With rod opsin 2 (rhodopsin D), the basic unit of such aggregates is the homodimer [6], as also is found for many other G-protein coupling receptors (GPCRs), including all Y receptors [7, 4]. GPCRs also produce heteromers with other receptors [8], channels [9] and chaperones/adapter proteins [10]. These non-covalent associations of GPCRs could be supported by G-protein aggregates (see [11, 12]). We find that in cells exposed to pertussis toxin the Y2 dimers are lost in parallel to reported decrease of Y2 agonist-stimulated Gi α activity, and of surface compartmentalization of the Y2 sites [2]. The dimers are also reduced at some preference by an irreversible Y2 receptor antagonist, with a much faster recovery, and with little loss of G-protein functionality. The rapid and long-lasting decrease of Y2 dimers after exposure to pertussis toxin points to an extensive coupling with G-proteins containing PTX-sensitive Gα subunits.
2. Experimental procedures
2.1 Materials
The Y peptides were purchased from American Peptide Company (Sunnyvale, CA, USA), or from Bachem (King of Prussia, PA, USA). The Y2 antagonist BIIE0246 ( (S)-N(2)-[[1-[2-[4-[(R,S)-5,11-dihydro-6(6h)-oxodibenz[b, e]azepine-11-yl]-1-piperazinyl]-2-oxoethyl] cyclopentyl] acetyl]-N-[2-[1,2-dihydro-3,5(4H)-dioxo-1,2-diphenyl-3H-1,2, 4-triazol-4-yl]ethyl]-arginineamide;[13]) was from Tocris (Ellisville, MD, USA). Rabbit antibodies against human Gi α subunits were from Upstate (Lake Placid, NY, USA).
Monoiodinated HPLC -purified [125I]-labeled hPYY(3-36) was supplied by Phoenix Pharmaceuticals (Shadyvale, CA, USA). Bovine γ-globulin, human holotransferrin and ovalbumin were iodinated with [125I]NaI by the chloramine T procedure, removing free iodine by gel filtration on Sephadex G-25. Guanosine-5′-O-(γ-thiotriphosphate) (GTP-γ-S ) labeled by 35S (specific activity 1250 Ci/mmole) was purchased from PerkinElmer (Cambridge, MA, USA). Unlabeled GTP-γ-S, GDP-β-S and suramin were from Calbiochem (La Jola, CA, USA).
Digitonin (high purity), sodium cholate and other detergents were obtained from Calbiochem (La Jola, CA). Bacitracin (USP grade), diisopropylfluorodiphosphate, proteinase-free bovine serum albumin (BSA), phenylarsine oxide (PAO), proteinase inhibitors amastatin, aprotinin, bestatin, leupeptin and pepstatin, and other chemicals were from Sigma (St. Louis, MO, USA).
Pertussis toxin (PTX) from List Laboratories (Campbell, CA, USA) was reconstituted in 0.5 M NaCl – 0.1 M Na phosphate pH 7.0 and stored at 4 °C up to 4 months without noticeable change in inhibitory activity.
2.2 Cell cultures and labeling
The cDNAs for human Y1 and Y2 receptors packaged in Invitrogen pcDNA 3.1+ vector were kindly provided by the University of Missouri at Rolla, MO. The cDNA for mouse Y4 receptor was a gift from Dr. Herbert Herzog (Garvan Institute, Sydney, Australia). The K1 line of Chinese hamster ovary cells (CHO-K1; American Type Culture Collection, Baltimore, MD) stably expressing the cloned human Y2 receptor were cultured at 400 μg/ml geneticin in D-MEM/F12 medium (Gibco, Long Island, NY) containing 6% (v/v) of fetal calf serum. The culture medium was always replaced prior to treatments with PTX.
For comparisons of the various treatments in the activation of masked Y2 receptors, the confluent, adherent cells were either lifted by silicone rubber, recovered at 100 x g and resuspended in D-MEM/F12 medium, or incubated in wells for 30 min at 37 °C with D-MEM/F12 with the various additives, as indicated under the respective experiments. The medium with the agents was then removed, and the cells washed. Aliquots of resuspended cells or of cells harvested from appropriate wells were assayed for the total particulate receptor binding. Other aliquots or wells were labeled for 20 min at 37 °C with 50 pM [125I]hPYY(3-36), washed in the cold, and the agonist attached to surface Y2 sites was extracted at 0–4 °C with 0.2 M CH3COOH - 0.5 M NaCl.
2.3 Particle preparation and binding assays
The cells were homogenized in the receptor assay buffer [14] using a Dounce homogenizer (8 strokes of the 0.1 mm-clearance pestle), the debris and nuclei were removed by sedimentation for 5 min at 600 gmax, and the supernatant sedimented for 12 min at 30,000 gmax to obtain particulate fractions which were stored at −80 °C prior to use in assays.
Labeling of the particulate Y2 receptor by [125I]hPYY(3-36) (50 pM final; 30 min at 25 °C) and binding of [35S]GTP-γ-S (200 pM final; 30 min at 27 °C after an activation of 60 min at 27 °C) were done as described [14].
2.4 Immunodetection of G-protein α-subunits
Aliquots of [125I] PYY(3-36)-labeled fractions from sucrose gradients (0.1 ml) were incubated with antibodies to Gi1 and Gi3 α subunits (each at 1:250 final dilution) for 12 h at 4 °C. This was followed by protein A/protein G agarose (50 μl/250 μl final volume). After 6 h of rotation at 4 °C, the mixtures were loaded onto spin columns (Pierce, Rockford, IL) and washed with 2 × 1 ml of the cold receptor assay buffer prior to counting of the gels in a γ-scintillation counter.
2.5 Lysis of particulates for gradient assays
The lysis of particulates at 0 – 4 °C was not complete with up to 20 mM of digitonin, sodium cholate, dodecyl β-maltoside, octyl-β-pyranoside, or CHAPS when applied alone, and some of these detergents promoted dissociation of agonists attached to the Y receptors. However, cholate and digitonin at final 10 mM each produced a more than 85% release of human Y1 and Y2 or mouse Y4 receptor from particulates in the form of respective dimers and monomers (not shown) and without significant dissociation of the respective [125I]-labeled agonists. This mixture was therefore used in all experiments reported here. 30–60 μg particulate protein (corresponding to 5–10×105 original cells) was used per gradient.
2.6 Sucrose gradient assays
Particulates labeled by [125I]hPYY(3-36) or [35S]GTP-γ-S were surface-washed in ice, and dispersed in cold receptor binding buffer. A mixture of sodium cholate and digitonin was added to 10 mM final of each detergent, and the dispersate passed slowly 8 times through a 25-gauge hypodermic needle, at 0 – 4 °C. The homogenates were then sedimented for 5 min at 10,000 × gmax and the supernatants were loaded on linear sucrose gradients (5–20% or 10–30% (w/v); total volume 9.2 ml, made in the receptor binding buffer) and sedimented for 18 h (5–20% gradients) or 24 h (10–30% gradients) at 35,000 rev/min (218,000 × gmax) in SW41 Ti rotor of a Beckman-Spinco M8-80 ultracentrifuge. The approximate molecular weights were deduced from sedimentation positions of [125I]bovine γ-globulin (158 kDa), [125I]human holotransferrin (75 kDa) and [125I]ovalbumin (44 kDa). The gradients were divided in fractions of 0.42 ml, and the polyethyleneglycol-precipitated [15]) Y2 receptor and/or G-protein associated radioactivity determined in aliquots. For a given preparation of Y2-CHO cell particulates, the sedimentation position and the proportion of receptor dimer were the same in the range of 0.5 – 20 fmoles of the labeled receptor per gradient.
2.7 Data evaluation
Calculations of the receptor binding parameters were done in the LIGAND program [16]. The ED50 and IC50 values were estimated from exponential or logistic curve fits with SigmaPlot software (version 8.02). Areas under the curve for radioactivity in gradient peaks were estimated in the ImageJ program (available at the U.S. National Institutes of Health website), employing. TIFF format graphics of the respective gradient profiles.
3. Results
3.1 Compared inactivation of Y2 receptors by pertussis toxin and a blocking antagonist
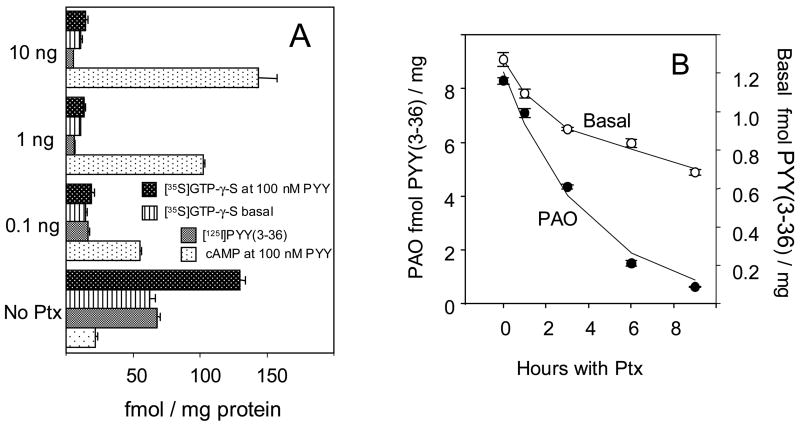
A detailed account of inactivation of CHO cell Y2 receptor and Gi α subunits by PTX was presented in ref. [2]. Fig. 1A summarizes this inactivation. About 85% of either Y2R or Gi α agonist binding is lost to pertussis toxin treatment in steady-state conditions, with a half-period of about 4 h. This decrease of binding activity reflects the loss of Y2 receptors through increased intracellular proteolysis in the absence of functional Gi subunits, and is significantly reduced by co-treatment with acid proteinase inhibitor NH4Cl [2]. There is a strong parallelism in the loss of G-protein nucleotide site activity and of total cellular Y2 binding (Fig. 1A). These changes are also reflected in the decreased inhibition of forskolin-stimulated adenylate cyclase by Y2 agonists (Fig. 1A). The masked surface Y2 complement is reduced by pretreatment with pertussis toxin much faster than either the basal (agonist-accessible) surface Y2 sites (Fig. 1B), or the total cellular Y2 binding. The basal, agonist-accessible surface Y2 binding, however, decreased less than 50% even at steady-state exposure to PTX (Fig. 1B and ref. [2]).
Fig. 1. Effects of pertussis toxin on Y2 receptor and G-protein agonist binding and adenylate cyclase activity in CHO cells expressing the human Y2 receptor. Results are averages of six samples in two independent experiments, shown ± 1 S.E.M.. All changes following PTX treatment were highly significant relative to the corresponding controls in post hoc Scheffé t testing.
A Loss of inhibition of forskolin-stimulated cAMP production, [125I]PYY(3-36) binding and basal or Y2 agonist (100 nM peptide YY) -stimulated binding of [35S]GTP-γ-S over 24 hours of cell culture at 0.1, 1 or 10 ng pertussis toxin.
B Kinetics of decrease of basal and phenylarsine oxide-unmasked surface Y2 binding in cells cultured at 10 ng/ml pertussis toxin. The binding of Y2 agonist [125I]PYY(3-36) was measured with monolayers at 30 μM phenylarsine oxide. The monoexponential half-periods of decrease were 3.2 ± 0.1 h for PAO-unmasked surface Y2 sites, and 5.3 ± 0.6 (68 ± 2.1) for total (particulate) Y2 sites; the basal Y2 binding decreased less than 40%. In the same experiment, the half-period of decrease of [35S]GTP-γ-S binding to particulates was 4.8 ± 0.8 h.
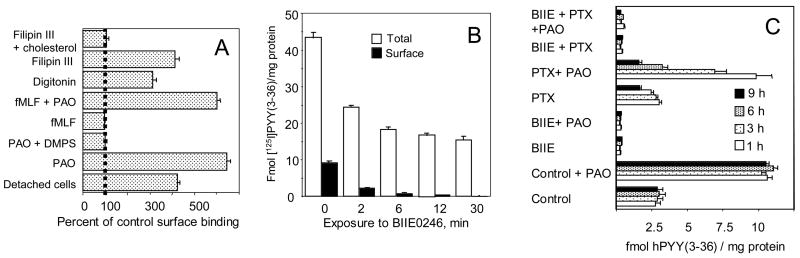
Figure 2A shows properties of the surface Y2 sites in monolayer culture. As shown before [2], the agonist-inaccesible sites could be unmasked, without monolayer disruption or loss of cellular protein, by phenylarsine oxide (PAO) and other alkylators. The unmasking by PAO was prevented by equimolar 2,3-dimercapto-1-propanesulfonate (DMPS), a non-permeating sulfhydryl protector. The masked sites are also exposed by low concentrations of steroid detergent digitonin or of cholesterol-complexing macrolide filipin III (counteracted by cholesterol). There was no unmasking by adhesion protein-shedding peptide formylMet-Leu-Phe (fMLP), indicating lack of a critical dependence on selectin-type adhesion proteins for Y2 receptor masking. The masked sites were however largely exposed by non-disruptive cell detachment by silicone rubber.
Fig. 2. Compartmentalization of CHO cell Y2 receptors and inactivation by antagonist BIIE0246.
A Activation of the masked Y2 surface sites by various agents and treatments. Non-disruptive cell detachment was done by silicone rubber, followed by sedimentation at 100 x g, resuspension and incubation with the labeled agonist. Phenylarsine oxide (PAO) and DMPS were used at 30 μM, fMLP at 100 μM, digitonin at 6 μM, and filipin 3 at 3 μM (without or with 30 μM cholesteryl hemisuccinate). Digitonin at 6 μM exposed, without cell detachment, about 4 fmol [35S]GTP-γ-S sites/100,000 cells, while 30 μM PAO or detachment by rubber exposed less than 1 fmol/100,000 cells. Total [35S]GTP-γ-S sites (as measured with particulates) were about 20 fmol/100,000 cells.
B Kinetics of inactivation of CHO cell receptors by Y2 antagonist BIIE0246. The cell monolayers were exposed to 100 nM of the antagonist for 2, 6, 12 and 30 min, followed by several cycles of washing and then by labeling of total (particulate) and surface (monolayer) receptors for 12 min at 37 °C with 50 pM [125I]PYY(3-36) (the later in the presence of 30 μM PAO, to expose the masked sites). With total particulates from this experiment, the Kdiss values in pM (with Bmax, fmol/mg protein, in parenthesis) were 438 ± 88 (506 ± 39) without the antagonist, and 545 ± 60 (21 ± 12) after 100 nM of the antagonist.
C Compared inactivation of surface Y2 sites by Y2 antagonist BIIE0246 (10 nM) and PTX (10 ng/ml). The inhibitors were applied to CHO cell monolayers separately or together for the indicated periods in the cell culture medium. After washing, the monolayers were labeled with [125I]PYY(3-36) for 20 min at 23 °C without or with 30 μM phenylarsine oxide, extracted with cold acid saline, and the extracts counted. The results are expressed in fmol per mg total cell protein.
A comparison with an agent reducing the Y2 receptor binding by interaction with the receptor itself was of interest in terms of confirming the mechanism of PTX-induced Y2 receptor loss. For this we used the selective Y2 receptor blocker BIIE0246 [13]. It should be noted that this compound contains several nitrogens protonated at physiological pH, and would poorly traverse cell membranes. At ≥ 100 nM, BIIE0246 eliminated all surface Y2 sites (either agonist-accessible or masked) within 30 min (Fig. 2B), while the total Y2 binding was reduced less than 65% even by a 24-h exposure to 1 μM of the antagonist (see Table 2). At 100 nM antagonist, most of the overall decrease in Y2 sites, and a nearly complete blockade of surface sites, occurred in less than 12 min of incubation (Fig. 2B). The antagonist did not significantly modify affinity of the residual Y2 agonist binding to particulates from CHO cells (see the legend of Fig. 2B), which is similar to the lack of affinity change noted in the inactivation of Y2 binding by PTX [2]. Pertussis toxin, acting much slower, eventually eliminated ≥85% of the total cellular Y2 sites (Figures 1A, 3C and Table 1). Loss of the arsenical-unmasked surface sites by treatment with PTX was essentially complete within 9 h, in contrast to a large conservation of the basal surface Y2 binding (ref. [2] and Fig. 1B). BIIE0246 irreversibly blocked >95% of particulate Y2 receptors (the legend of Fig. 2B). The masked surface sites were more than 70% blocked within 2 min of treatment with the antagonist (the legend of Fig. 2B). Reductions of surface Y2 sites due to co-treatment with 100 nM BIIE0246 and 10 ng/ml PTX at any time point did not differ from those caused by the Y2 blocker alone (Fig. 2C).
Table 2.
Effects of Y2 antagonist BIIE0246 on [125I]PYY(3-36) and [35S]GTP-γ-S sites are not modified by NH4Cl
| Group | [125I]PYY(3 -36) | basal [35S] GTP-γ-S | PYY(3-36) stimulated GTP-γ-S | % decrease Y2 binding | % decrease basal GTP- γ-S | % decrease in GTP-γ-S stimulation |
|---|---|---|---|---|---|---|
| Control | 274 ± 3.2 | 62.6 ± 1.36 | 84.5 ± 1.5 | |||
| NH4Cl only | 267 ± 3.4 | 60.7 ± 1.52 | 77.1 ± 1.6 | 2.55 ± 1.3 | 3.04 ± 2.4 | 8.76 ± 1.9 |
| 10 nM BIIE | 136 ± 1.4 | 50.3 ± 0.89 | 72.9 ± 0.8 | 50.4 ± 0.5 | 19.7 ± 1.4 | 13.7 ± 0.9 |
| 10 nM BIIE + NH4Cl | 122 ± 3.2 | 47.4 ± 2.7 | 66.8 ± 3.5 | 55.5 ± 1.2 | 24.3 ± 4.3 | 21.0 ± 4.1 |
| 100 nM BIIE | 115 ± 3.6 | 48.1 ± 0.15 | 74.1 ± 1.9 | 58.0 ± 1.3 | 23.2 ± 0.2 | 12.3 ± 2.3 |
| 100 nM BIIE + NH4Cl | 121 ± 2.9 | 47.9 ± 0.31 | 73.3 ± 1.6 | 55.8 ± 1.1 | 23.5 ± 0.5 | 13.3 ± 1.9 |
| 1 μM BIIE | 108 ± 2.6 | 50.2 ± 2.7 | 68.4 ± 2.7 | 60.6 ± 0.9 | 19.8 ± 4.3 | 19.1 ± 3.2 |
| 1 μM BIIE + NH4Cl | 105 ± 3.4 | 48.9 ± 3.6 | 66.6 ± 3.5 | 61.7 ± 1.2 | 22.0 ± 5.7 | 21.0 ± 4.2 |
The cell monolayers were incubated for 20 h in the growth medium with BIIE0246 (10, 100 or 1000 nM) without or with 30 mM NH4Cl, washed, collected and homogenized, and particulates from all samples assayed for the binding of 50 pM of [125I]PYY(3-36) and 200 pM of [35S]GTP-γ-S (basal and stimulated by 100 nM PYY(3-36)), as specified in Methods. All binding results are in fmol/mg particulate protein. The Y2 agonist –stimulated GTP-γ-S binding is the increase over the basal. All percentages are relative to control values. Cells from four wells were assayed for all PTX-treated groups, and cells from six wells for the control group. Data are shown ± 1 S.E.M..
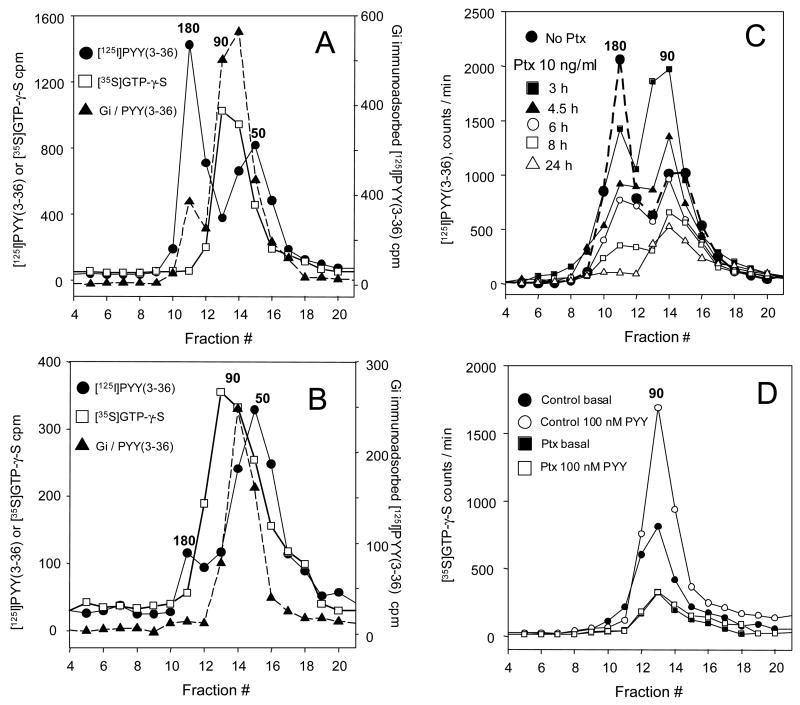
Fig. 3. Y2 receptor dimers are lost along with Y2 agonist binding and the Gi nucleotide site activity in response to CHO cell treatment with pertussis toxin. Particulates from cells exposed to 1 or 10 ng/ml of the toxin for the indicated periods were labeled with [125I]PYY(3-36) or [35S]GTP-γ-S [14], solubilized with 10 mM each cholate and digitonin, and sedimented in 10–30% linear sucrose gradients (9.2 ml) for 24 h at 218,000 × gmax. Fractions of 0.42 ml were then collected. Immunoadsorption of [125I]PYY(3-36)-labeled gradient fractions following 12 h of incubation with a mixture of antibodies to Gi1 and Gi3 was done using protein A+G agarose. Molecular size calibration was with covalently colored myosin (211 kDa), [125I]bovine γ-globulin (158 kDa), [125I]human holotransferrin (75 kDa) and [125I]ovalbumin (44 kDa). Numbers above peaks denote approximate molecular weights in kilodaltons.
A Distribution of Y2 receptor and G-protein labeling in particulates from control cells. The ~180 kDa Y2 dimer represents typically 50% or more of the [125I]hPYY(3-36) labeled receptor and is not significantly labeled by [35S]GTP-γ-S. Immunoadsorption of [125I] Y2 agonist with Giα1-Giα3 antibodies shows peaks at ~180 kDa, and at ~90 kDa (which corresponds to >80% of the total Y2 agonist binding in that region). Labeling by [35S]GTP-γ-S shows a single peak at about 90 kDa, coinciding with Giα1-Giα3 immunoadsorption with the [125I]-Y2 agonist. The [35S] GTP-γ-S -labeled 90 kDa material also strongly immunoreacts with the Gi antibodies (not shown).
B Distribution of Y2 receptor and G-protein labeling in particulates from cells treated with pertussis toxin at 1 ng/ml for 24 hours. The 180 kDa zone represents ≤10% of total Y2 agonist labeling. The 50 kDa labeling by the Y2 agonist, corresponding to Y2 receptor monomer not associated with G-protein, is relatively strong. The [35S] GTP-γ-S –labeled material represents less than 30% of the labeling found with control particulates. The immunoadsorption of the Y2-bound [125I]PYY(3-36) agonist with Gi antibodies shows a similar decrease.
C Time course of decrease of the Y2 receptor dimer at 10 ng PTX/ml culture medium. The dimer counts at 3, 4.5, 6, 8 and 24 h of PTX treatment represented, respectively, 69, 44, 37, 17 and 4.7 % of the control 180 kDa peak counts.
D Compared basal and Y2 agonist-stimulated binding of [35S]GTP-γ-S with particulates exposed to 10 ng/ml pertussis toxin for 24 h. The stimulation was by 100 nM pPYY. The basal and agonist-stimulated binding were respectively reduced 63.3% vs. 74.9% overall, and 61.3 vs. 81.3% at the 90 kDa peak. For other details see the Methods section.
Table 1.
Long-term inhibition of Y2 receptor and G-protein activity in CHO cells by pertussis toxin
| Group | Y2 binding | basal GTP- γ-S binding | PYY- stimulated GTP-γ-S binding | % decrease of Y2 binding | % decrease of basal GTP-γ-S | % decrease of PYY- stimulated GTP-γ-S |
|---|---|---|---|---|---|---|
| Control | 272 ± 1.08 | 84.2 ± 0.11 | 54.3 ± 0.23 | |||
| PTX no recovery | 51.8 ± 0.84 | 35.7 ± 1.2 | 6.53 ± 0.98 | 81 ± 1.3 | 57.6 ± 1.9 | 88 ± 13.2 |
| PTX 2 d recovery | 50.5 ±0.31 | 27.3 ± 1.19 | 4.33 ± 0.77 | 81.4 ± 0.5 | 67.6 ± 3 | 92 ± 16.4 |
| PTX 7 d recovery | 41.4 ± 0.03 | 30.8 ± 0.93 | 7.86 ± 0.68 | 84.8 ± 0.06 | 63.4 ± 1.9 | 85.5 ± 7.4 |
| PTX pass 1 – 11 d recovery | 37.4 ± 3.65 | 37 ±0.71 | 3.55 ± 0.62 | 86.3 ± 8.4 | 56.1 ± 1.1 | 93.5 ± 16.3 |
| PTX pass 2 – 14 d recovery | 30.8 ± 0.27 | 37.6 ±4.6 | 6.93 ±4.08 | 88.7 ± 0.78 | 55.3 ± 6.7 | 87.2 ± 12.8 |
The cells were incubated with PTX (4 ng/ml growth medium) for 24 h, washed, the medium replaced and cells then collected immediately (no recovery) and 2 or 7 days later. Passage 1 was done on recovery day 7, and passage 2 on recovery day 11. Cells from that passage were collected on recovery day 14. Particulates from all samples were assayed for the binding of 50 pM of [125I]PYY(3-36) and 200 pM of [35S]GTP-γ-S (basal and stimulated by 100 nM PYY), as specified in Methods. All binding results are in fmol/mg particulate protein. The Y2 agonist –stimulated GTP-γ-S binding is the increase over the basal. All decrease percentages are relative to control. Cells from four wells were assayed for all PTX-treated groups, and cells from eight wells for the control group. Data are shown ± 1 S.E.M. .
3.2 Y2 receptor dimers are reduced in preference to monomers in the presence of pertussis toxin
Solubilization of particulate Y2 receptors labeled by [125I]PYY(3-36) could be achieved with less than 10% dissociation of the bound agonist by dispersion in a mixture of 10 mM each of cholate and digitonin at 0 – 4 °C. A comparable stability was found for [125I]NPY binding to the Y1 receptor, and for [125I]hPP binding to the Y4 receptor expressed in CHO cells (not shown). The proportion of Y2 receptor dimers was found to be similar (~65%) at expression levels of 35 and 200 fmol/mg particulate protein. The amount of receptor detected in oligomeric form in CHO cell particulates after labeling by [125I]PYY(3-36) was not critically dependent on input of the labeled agonist up to 50 pM. However, a preferential decrease of the dimer was observed after preincubation with 10–100 nM of agonists PYY(3-36) or NPY(22-36) (not shown). The predominant agonist-labeled species in control cells sedimented in sucrose gradients at ~ 180 kDa (Fig. 3A). Average control labeling in the dimer peak assessed as area under the curve was 66 ± 5 % (n = 12). This zone was not labeled by [35S]GTP-γ-S (Fig. 3A), indicating an absence of free Gi α subunits, but the receptor-bound [125I]PYY(3-36) was significantly precipitated by antibodies to Gi α subunits 1–3 (Fig. 3A), or by antibody to Gi α3 used alone (Fig. 4). This material is therefore likely to represent a pentameric association of the receptor dimer with Gi α-βγ heterotrimers, already reported e.g. for leukotriene B4-BLT1 receptor [17] and for the α2A-adrenergic receptor [18]. The labeling by [125I]PYY(3-36) and receptor sedimentation profiles were essentially the same in buffers containing 3 mM Ca2+ + 1 mM Mg2+, or 1 mM EDTA, indicating that divalent cations do not affect Y2 receptor dimerization. A shoulder of Y2 agonist radioactivity was usually observed at about 50 kDa, and should correspond to G-protein -free Y2 receptor monomer.
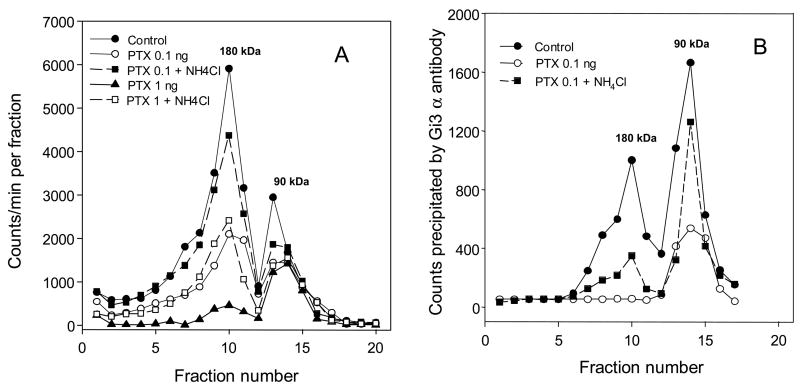
Fig. 4. Ammonium chloride attenuates the reduction of Y2 receptor dimers by pertussis toxin. The cells were treated with 0.1 or 1 ng PTX/ml growth medium for 24 h without or with NH4Cl (30 mM). The particulates were then labeled with [125I]PYY(3-36) and processed as in Fig. 3, except that 5–20% sucrose gradients were used, and the sedimentation time was 18 h at 218,000 × gmax. For other details see Section 2.6.
A Sedimentation profiles of [125I]PYY(3-36) –labeled solubilized receptors.
B Immunoadsorption of Gi3a antibody-coupled labeled material from the corresponding fractions of graph A. Large recovery of the control 90 kDa material labeled by the Y2 agonist also was observed in experiments shown in Fig. 3 and Fig. 5.
Labeling of particulate Y2 receptor by [125I]PYY(3-36) in the presence of 100 nM GTP-γ-S (a concentration saturating >95% of Gi nucleotide sites) was reduced less than 35%, without important change in affinity. This reduction only slightly increased the proportion of the dimer. Labeling in the presence of 30 μM of G-protein antagonist suramin, or 10 μM of Gα nucleotide site antagonist GDP-β-S, produced a similar reduction of Y2 agonist binding, and again only a slight increase in the proportion of the dimer. The specific Y2 antagonist BIIE0246 at 1–100 nM produced a large concentration-dependent decrease in agonist attachment without significant change in affinity or proportion of the dimer. These findings indicate that agonist peptide attachment to the dimer is not dependent on an active Gα nucleotide site, and also that proportion of the dimer detected by our procedure reflects its physiological levels.
The bound [35S] GTP-γ-S sedimented in a somewhat broad zone with a peak at about 90 kDa. As seen in Fig. 3D, the labeling of this zone was more than doubled (without change in the sedimentation rate) by incubation at 100 nM of the Y2 agonist PYY(3-36), indicating a strong Gi activation by agonist-liganded monomeric Y2 receptor. The large stimulation of [35S]GTP-γ-S binding by Y2 agonists indicates that the receptor could be one of the principal partners of Giα subunits in CHO cells expressing the receptor. This should also be important in the constitutive activation of Gi in the absence of Y2 agonists. Indeed, after 24 h of exposure to 4 ng/ml of PTX there was a consistent reduction of basal GTP-γ-S binding of 55–63% over 14 days of further culturing, and two passages (Table 1).
The loss of 180 kDa complex was already observed after 3 h of treatment with the toxin at 10 ng/ml (Fig. 3C), and much more pronounced after 8 h of the treatment (Fig. 3C). The 180 kDa complex was largely lost in response to steady-state (24 h at ≥1 ng/ml) PTX treatment (Fig. 3B, C). This was observed in more than 20 consecutive experiments. The decrease was accompanied by a complete loss of 180 kDa zone immunoreactivity to Gi antibodies (Fig. 3B). At 24 h, there was >80% decrease in labeling of the 90 kDa material by [35S]-GTP-γ-S, and an essentially complete loss of stimulation of GTP-γ-S binding by the Y2 agonist (Fig. 3B, D).
3.3 Reduction of Y2 dimers due to pertussis toxin treatment is attenuated by acid proteinase inhibitor ammonium chloride
As expected from our previous findings [2], co-treatment with 30 mM NH4Cl strongly attenuated the reduction of Y2 binding by PTX. Typical profiles are shown in Fig. 4. At 0.1 ng PTX/ml culture medium, 30 mM NH4Cl reduced the inactivation by more than 70%, and maintained the dimeric receptor above 50% of the total (Fig. 4A). The attenuation was considerable even at 1 ng/ml of the toxin (Fig. 4A). The reduction of dimer loss by NH4Cl was also apparent in profiles of immunoadsorption with Gi3α antibody (Fig. 4B). Rescue by NH4Cl of the dimer reacting with Gi3α antibody, however, was considerably less than that of the corresponding monomer (Fig. 4B). This indicated that a significant portion of the Y2 agonist –labeled dimer could be associated with G-proteins insensitive to PTX. It should be noted that the [125I]agonist-labeled Y2 receptor in the 90 kDa zone was strongly precipitated by the Giα3 antibody, indicating a preferential association of Gαi3 subunit with the Y2 receptor (see also Fig. 5).
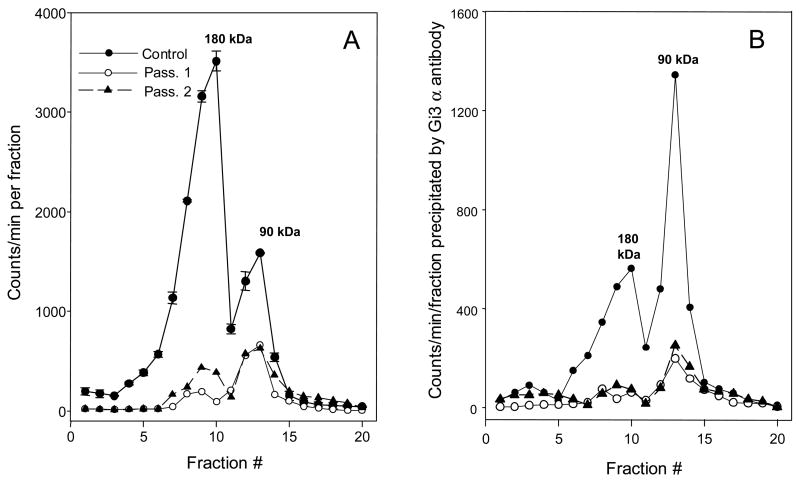
Fig. 5. Slow recovery of Y2 receptors and Gi3 α subunit function after exposure to 4 ng/ml of PTX for 24 h. The cells were collected on days 11 (passage 1) and 14 (passage 2) following removal of the toxin, the particulates were labeled with [125I]PYY(3-36), lysed and sedimented in 5–20% sucrose gradients for 18 h at 218,000 × gmax. For other details see Table 1 and Methods section.
A Sedimentation profiles of [125I]PYY(3-36) –labeled solubilized receptors. The results are averages of two gradients for each condition. For clarity, the standard errors are shown only with the control profile. The percent of dimer was 71 ± 3 in control, 31 ± 1.4 in passage 1, and 37 ± 1.7 in passage 2 gradients.
B Immunoadsorption of Gi3α antibody-coupled labeled material from the corresponding gradient fractions in graph A.
3.4 Recovery of both Y2 receptors and Gi α subunits from pertussis toxin is slow
The recovery from PTX treatment was slow even after exposure to only 0.1 ng/ml for 24 h, and was low even 14 days (and two cell passages) following 24 h of exposure to 4 ng/ml of the toxin (Table 1 and Fig. 5). Most of the receptor labeled by [125I]PYY(3-36) at any time point over this post-treatment period was found in the 90 kDa peak, and was strongly associating with the antibody to Gα i3 subunit (Fig. 5B). With particulates from PTX-pretreated cells there was no association of the Y2 agonist-labeled dimer with Gα i3 antibody at 11 d after treatment with the toxin, and little at post-treatment day 14 (Fig. 5B). The recovery of Y2 agonist-stimulated GTP-γ-S binding was less than 15% even 14 days following removal of external toxin (Table 1). The masked sites at 14 d were only 20% above the basal sites (data not shown).
3.5 Reduction of Y2 receptors due to antagonist BIIE0246 occurs with a lower loss of oligomers and with little loss of G-protein activity
Profiles of oligomerization following cell treatment with Y2 antagonist BIIE0246 did not show a large reduction of dimers at up to 1 μM of the antagonist (Fig 6), and there was only about 30% reduction of basal [35S]GTP-γ-S labeling by the Y2 antagonist at 1 μM (legend of Fig. 6 and Table 2).
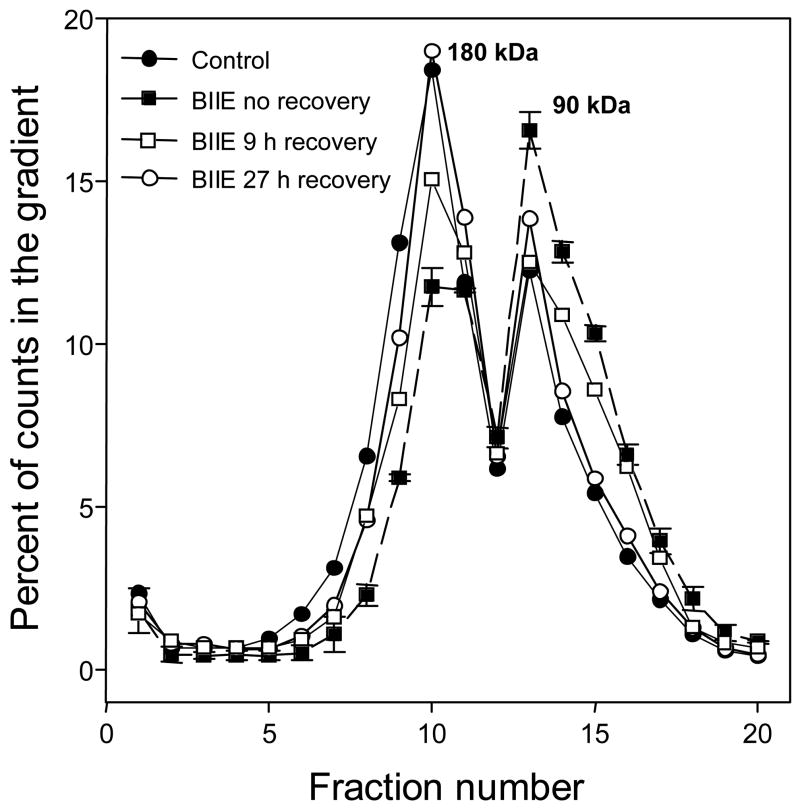
Fig. 6.
Y2 receptor dimers in CHO cell monolayers are significantly reduced by exposure to antagonist BIIE0246. The drug was applied for 30 min at 1 μM. There was a partial recovery at 9 h and a nearly complete recovery at 24 h after removal of the antagonist. Standard errors of counts in duplicate gradients are shown only for BIIE0246 without recovery incubation. The content of dimer, in % total gradient counts, was 56 +/− 2.7 % in control gradients, 54 +/− 3% at 24 h recovery, 44 +/− 2.6% at 9 h recovery, and 33.5 +/− 2.3 with no recovery period.
Since we found an attenuation of PTX-related loss of Y2 dimers by acid proteinase inhibitor ammonium chloride (Fig. 5) it was also of interest to test this agent in co-treatment with the Y2 antagonist BIIE0246. As expected, ammonium chloride at 30 mM did not significantly modify the decrease of Y2 sites by treatment of intact cells with the antagonist in the range of 1 nM –1 μM (Table 2). This experiment also demonstrated a largely saturated effect of the antagonist already at 10 nM in conditions of prolonged (24 h) treatment of the intact cells. It should be noted that no decreases of cell numbers or protein were observed over 24 h even at the highest employed concentration of the antagonist, 1 μM.
At 24 h following removal of the antagonist, there also was a complete recovery of the masked Y2 surface sites (in fmol [125I]PYY(3-36) specifically bound per 100,000 cells: control, 1.25 ± 0.07 basal, 6.6 ± 0.33 at 30 μM PAO; BIIE0246 -treated, 1.41 ± 0.12 basal, 7.4 ± 0.49 at 30 μM PAO (n = 6 for each).
4. Discussion
From our results, association of natural agonists with the Y2 receptor (and also with Y1 and Y4 receptors) is sufficiently stable to allow a direct assessment of interaction with G-protein α-subunits, as well as a reproducible characterization of molecular properties of the receptor-receptor and receptor-G protein complexes. Similar or larger stability of agonist binding is known for endothelin-1 [19] and neuromedin [20] receptors. A sufficient stability of agonist attachment could be present with many other peptide receptors, as well as with receptors that respond to small agonists (e.g. dopamine D2 receptor [21]); however, small agonists could be expected to extensively dissociate upon receptor solubilization. The stability of agonist association with Y receptors, and with the Y2 receptor in particular, could be used to follow receptor interactions without covalent attachment of fluorescent tags (for Y receptors reviewed in [22]), which with large tags presents uncertainty about functional and metabolic equivalence of the tagged and the native receptor.
Our results indicate that in physiological conditions in CHO cells 60% or more of the Y2 receptor complement is oligomeric. Characterization by FRET of Y2 aggregates in BHK cells shows dependence of the apparent fraction of dimer on the size of the fluorescent tag employed (26–100%; Table III in [4]), and the estimates tend to be semi-quantitative. As we show in the Results and in the Supplement, the proportion of oligomers is not affected by long preincubation in the assay buffer, by removal of divalent cations, by shearing in solubilization, and by receptor concentration.
As found previously for the Y4 dimer [7], and for SSTR2 receptor dimer [23], the Y2 dimers are converted to monomers at some stage following the agonist attachment. Since the binding of Y2 agonist peptide to the dimer does not produce attachment of GTP-γ-S to Gα nucleotide site at the level of the 180 kDa complex, activation of the site should be connected to dissociation of the dimer. However, dissociation of the dimer is not a requisite for the attachment of Y2 agonists. In contrast, the binding of large chemokine peptide interleukin-8 to the CXCR1 dimer does require dissociation of the dimer [24].
Dimers are the most stable form of oligomerization for rhodopsin itself [6], and could also be the major oligomeric form for neuropeptide GPCRs, including the Y2 receptor (ref. [4] and this work). Homodimerization of several rhodopsin-like receptors was shown to depend on non-covalent pairing of transmembrane domains [25, 17, 26, 27], in some cases with critical involvement of transmembrane tryptophan residues [26]. The Y2 receptor transmembrane helices 4 and 6 both contain Trp residues [28] that could be used in dimerization [26]. An exclusive homodimerization via the highly anionic N-terminal extracellular domains of the Y2 receptor (see [29]) through metal bridges [30] is not indicated, since protracted exposure to EDTA does not significantly reduce the fraction of the dimer.
Metabolic regulation of GPCR dimers and the possible interdependence of dimer levels and G-protein function do not appear to have been studied thus far. The large decrease of the basal GTP-γ-S binding that we observed upon treatment with PTX should mainly reflect receptor loss, as the Y2 receptor appears to be an important partner of Giα subunit in CHO cells. The small loss of basal Gi activity upon treatment with the irreversible Y2 antagonist could also be connected to inactivation of precoupled receptors, disabling a low-level stimulation of Gα by the receptor in the absence of receptor’s agonist.
The cell surface Y2 monomers prevalent in steady-state exposure to PTX could be largely coupling PTX-insensitive G-proteins, including Gq α subunits, and this can explain their preservation in the presence of the toxin. Internalization of the Y2 receptor, and even of the fast-cycling Y1 receptor, is much less affected by PTX than total receptor numbers [ 2, 31], and could largely be connected to PTX-insensitive G-proteins. We have already presented evidence for an increase in proportion of the Y2 receptor interacting with Gq-like G proteins following PTX treatment [2]. A recent study of β2 adrenergic receptor dimer processing indicates that the addition of G-protein α-subunits enables passage of the functional complex to the cell membrane [32].
Our results show that the parallel downregulation of the Y2 receptor and the Gi α subunit activity in response to pertussis toxin [2] occurs alongside the loss of receptor oligomers associated with heterotrimeric G-proteins. As the very large depletion of Y2 dimers by treatment with PTX in CHO cells develops in the absence of Y2 agonists, the Y2 receptor in CHO cells is obviously regulated by a constitutive turnover that depends on functional Gi α subunits (since Go α subunit is poorly expressed in CHO cells [33]). From our results, Gi3 α subunit could be prominent in this interaction. This regulation appears to be especially pronounced with the large surface reserve of the Y2 receptor, a population likely to be associated with Gi proteins in rafts [34], in a clustered form [35]. This population is lost faster than total dimers, reflecting proximity to the top of gradient of the membrane-ensconced toxin enzyme. The lack of restoration even after two cell passages following a small dose of PTX indicates that the retained toxin is metabolically very stable. This corresponds with previous findings with adipocytes [36] and brain tissues [37]. The toxin could be embedded in rafts constituting the rapidly-cycling endosomal compartment [38].
The coordinated decrease of dimers and of the functional responses of the Y2 receptor and the PTX-sensitive G-proteins probably reflect both a matrix embedding and a precoupling of the receptor and G-protein heterotrimers containing PTX-sensitive α-subunits, as inferred previously [11, 12, 39]. This type of association is known for rod opsin 2 [6] and could be very important to the visual process. The evidently ubiquitous oligomerization of rhodopsin-like GPCRs could be significantly linked to organization in matrices associated with the plasma membrane, and containing PTX-sensitive α subunits [34, 35, 40]. Such associations could serve both organization and control of activity of GPCRs and the GPCR-interacting G-proteins, especially those containing rapidly activated, PTX-sensitive α subunits [40, 41]. Matrix organization of receptors and G-proteins could be connected to propensity of Gi α subunits for concatenation [11], and could be commonplace among GPCRs transducing via these G-protein subunits. Associations of this type may offer, relative to signaling based purely on collision coupling, large advantages in signal transduction.
Supplementary Material
Fig. S1 Typical sedimentation profiles of solubilized human Y1 and Y2 and mouse Y4 receptors labeled by the respective primary agonists. The receptors were labeled by 50 pM [125I]hPYY(3-36) (human Y2), [125I]hNPY(1-36) (human Y1) and [125I]hPP (mouse Y4) in particulates from the respective expressions in CHO cells, solubilized by 10 mM each of cholate and digitonin, and sedimented in 5–20% linear sucrose gradients for 18 h at 218,000 × gmax, as described in Methods. Dimer content as % counts in the gradient was 66.8 ± 2.8 for the Y2, 57 ± 3.2 for the Y1, and 70.4 ± 4.3 for the Y4 receptor (all averages of two gradients, ± 1 S.E.M.).
Fig. S2 Stability of Y2 receptor dimers to incubation at 27 °C and to removal of divalent cations. Duplicate 5–20% gradients were centrifuged for 18 h at 5 °C. All results are average percentages of total counts in the corresponding gradient fractions from two gradients for each condition. The respective standard errors were in most cases below 10%, and for clarity are not shown.
A Y2 receptor dimers in particulates from CHO cells are stable to incubation at 27 °C. The incubation of particulate suspension in the assay buffer at 27 °C was for 1 h (followed by 2 h at 0 – 4 °C) or 3 h, while the control suspension was incubated in ice for 3 h. This was followed by labeling for 20 min at 27 °C by 50 pM [125I]PYY(3-36). The content of dimer, in % total gradient counts, was 66.2 ± 3.7 for control gradients, 63.4 ± 3.4 after preincubation for 1 h at 27 °C, and 64.3 ± 3.4 after preincubation for 3 h at 27 °C.
B Y2 receptor dimers are not significantly disbanded by removal of divalent cations. The profiles are for receptors labeled (30 min at 25 °C), solubilized and sedimented in buffers containing 3 mM CaCl2 + 1 mM MgCl2, or 1 mM Na EDTA (pH 7.4) without CaCl2 and MgCl2, proteinase inhibitors, 0.1% BSA and 10 mM hepes. NaOH, pH 7.4. The dimer content, in % gradient counts, was 65.9 ± 4.7 with Ca2+/Mg2+ buffer, and 60.8 ± 4.6 with EDTA buffer.
Fig. S3 Preferential removal of Y2 receptors dimers by two agonists of the Y2 receptor. NPY(22-36) (Kdiss vs. [125I]PYY(3-36) at hY2 receptor 6.2 ± 1.2 nM) was used at 10 nM, and PYY(3-36) (Kdiss at hY2 0.50 ± 0.06 nM) at 100 nM final, for 45 min at 25 °C prior to resedimentation and labeling with 50 pM [125I]PYY(3-36). After lysis at 10 mM each of digitonin and cholate, duplicate 5–20% gradients for each condition were centrifuged for 18 h at 5 °C. All results are average percentages of total counts in the corresponding gradient fractions. The respective standard errors were in most cases below 10%, and for clarity are shown only for the control profile. The content of dimer, in % total gradient counts, was 67.7 ± 4.8 for control gradients, 42.7 ± 2.3 after pre-treatment with 10 nM NPY(22-36), and 30.5 ± 2 after pre-treatment with 100 nM PYY(3-36).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shigeri Y, Fujimoto M. Y2 receptors for neuropeptide Y are coupled to three intracellular signal transduction pathways in a human neuroblastoma cell line. J Biol Chem. 1994;269:8842–8. [PubMed] [Google Scholar]
- 2.Parker SL, Parker MS, Sah R, Sallee FR, Balasubramaniam A. Parallel inactivation of Y2 receptor and G-proteins in CHO cells by pertussis toxin. Regulatory Peptides. 2007;139:128–35. doi: 10.1016/j.regpep.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 3.Parker SL, Parker MS, Kane JK, Berglund MM. A pool of Y2 neuropeptide Y receptors activated by modifiers of membrane sulfhydryl or cholesterol balance. Eur J Biochem. 2002;269:2315–22. doi: 10.1046/j.1432-1033.2002.02903.x. [DOI] [PubMed] [Google Scholar]
- 4.Dinger MC, Bader JE, Kobor AD, Kretzschmar AK, Beck-Sickinger AG. Homodimerization of neuropeptide y receptors investigated by fluorescence resonance energy transfer in living cells. J Biol Chem. 2003;278:10562–71. doi: 10.1074/jbc.M205747200. [DOI] [PubMed] [Google Scholar]
- 5.Parker SL, Parker MS. Ligand association with the rabbit kidney and brain Y1, Y2 and Y5-like neuropeptide Y (NPY) receptors shows large subtype-related differences in sensitivity to chaotropic and alkylating agents. Regul Pept. 2000;87:59–72. doi: 10.1016/s0167-0115(99)00110-x. [DOI] [PubMed] [Google Scholar]
- 6.Fotiadis D, Jastrzebska B, Philippsen A, Muller DJ, Palczewski K, Engel A. Structure of the rhodopsin dimer: a working model for G-protein-coupled receptors. Curr Opin Struct Biol. 2006;16:252–9. doi: 10.1016/j.sbi.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 7.Berglund MM, Schober DA, Esterman MA, Gehlert DR. Neuropeptide Y Y4 receptor homodimers dissociate upon agonist stimulation. J Pharmacol Exp Ther. 2003;307:1120–6. doi: 10.1124/jpet.103.055673. [DOI] [PubMed] [Google Scholar]
- 8.Gregan B, Jurgensen J, Papsdorf G, Furkert J, Schaefer M, Beyermann M, Rosenthal W, Oksche A. Ligand-dependent differences in the internalization of endothelin A and endothelin B receptor heterodimers. J Biol Chem. 2004;279:27679–87. doi: 10.1074/jbc.M403601200. [DOI] [PubMed] [Google Scholar]
- 9.Scott L, Zelenin S, Malmersjo S, Kowalewski JM, Markus EZ, Nairn AC, Greengard P, Brismar H, Aperia A. Allosteric changes of the NMDA receptor trap diffusible dopamine 1 receptors in spines. Proc Natl Acad Sci U S A. 2006;103:762–7. doi: 10.1073/pnas.0505557103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen BD, Nechamen CA, Dias JA. Human follitropin receptor (FSHR) interacts with the adapter protein 14-3-3tau. Mol Cell Endocrinol. 2004;220:1–7. doi: 10.1016/j.mce.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 11.Mixon MB, Lee E, Coleman DE, Berghuis AM, Gilman AG, Sprang SR. Tertiary and quaternary structural changes in Gi alpha 1 induced by GTP hydrolysis. Science. 1995;270:954–60. doi: 10.1126/science.270.5238.954. [DOI] [PubMed] [Google Scholar]
- 12.Tesmer VM, Kawano T, Shankaranarayanan A, Kozasa T, Tesmer JJ. Snapshot of activated G proteins at the membrane: the Galphaq-GRK2-Gbetagamma complex. Science. 2005;310:1686–90. doi: 10.1126/science.1118890. [DOI] [PubMed] [Google Scholar]
- 13.Doods H, Gaida W, Wieland HA, Dollinger H, Schnorrenberg G, Esser F, Engel W, Eberlein W, Rudolf K. BIIE0246: a selective and high affinity neuropeptide Y Y(2) receptor antagonist. Eur J Pharmacol. 1999;384:R3–5. doi: 10.1016/s0014-2999(99)00650-0. [DOI] [PubMed] [Google Scholar]
- 14.Parker MS, Sah R, Sheriff S, Balasubramaniam A, Parker SL. Internalization of cloned pancreatic polypeptide receptors is accelerated by all types of Y4 agonists. Regulatory Peptides. 2005;132:91–101. doi: 10.1016/j.regpep.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Parker SL, Parker MS, Lundell I, Balasubramaniam A, Buschauer A, Kane JK, Yalcin A, Berglund MM. Agonist internalization by cloned Y1 neuropeptide Y (NPY) receptor in Chinese hamster ovary cells shows strong preference for NPY, endosome- linked entry and fast receptor recycling. Regul Pept. 2002;107:49–62. doi: 10.1016/s0167-0115(02)00094-0. [DOI] [PubMed] [Google Scholar]
- 16.Munson PJ, Rodbard D. LIGAND: a versatile computerized approach for characterization of ligand-binding proteins. Anal Biochem. 1980;107:220–39. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- 17.Baneres JL, Parello J. Structure-based analysis of GPCR function: evidence for a novel pentameric assembly between the dimeric leukotriene B4 receptor BLT1 and the G-protein. J Mol Biol. 2003;329:815–29. doi: 10.1016/s0022-2836(03)00439-x. [DOI] [PubMed] [Google Scholar]
- 18.Nobles M, Benians A, Tinker A. Heterotrimeric G proteins precouple with G protein-coupled receptors in living cells. Proc Natl Acad Sci U S A. 2005;102:18706–11. doi: 10.1073/pnas.0504778102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hilal-Dandan R, Villegas S, Gonzalez A, Brunton LL. The quasi-irreversible nature of endothelin binding and G protein-linked signaling in cardiac myocytes. J Pharmacol Exp Ther. 1997;281:267–73. [PubMed] [Google Scholar]
- 20.Brighton PJ, Szekeres PG, Wise A, Willars GB. Signaling and ligand binding by recombinant neuromedin U receptors: evidence for dual coupling to Galphaq/11 and Galphai and an irreversible ligand-receptor interaction. Mol Pharmacol. 2004;66:1544–56. doi: 10.1124/mol.104.002337. [DOI] [PubMed] [Google Scholar]
- 21.Kapur S, Seeman P. Antipsychotic agents differ in how fast they come off the dopamine D2 receptors. Implications for atypical antipsychotic action. J Psychiatry Neurosci. 2000;25:161–6. [PMC free article] [PubMed] [Google Scholar]
- 22.Bohme I, Morl K, Bamming D, Meyer C, Beck-Sickinger AG. Tracking of human Y receptors in living cells-A fluorescence approach. Peptides. 2007;28:226–34. doi: 10.1016/j.peptides.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 23.Grant M, Collier B, Kumar U. Agonist-dependent dissociation of human somatostatin receptor 2 dimers: a role in receptor trafficking. J Biol Chem. 2004;279:36179–83. doi: 10.1074/jbc.M407310200. [DOI] [PubMed] [Google Scholar]
- 24.Fernando H, Chin C, Rosgen J, Rajarathnam K. Dimer dissociation is essential for interleukin-8 (IL-8) binding to CXCR1 receptor. J Biol Chem. 2004;279:36175–8. doi: 10.1074/jbc.C400283200. [DOI] [PubMed] [Google Scholar]
- 25.Lee SP, Xie Z, Varghese G, Nguyen T, O’Dowd BF, George SR. Oligomerization of dopamine and serotonin receptors. Neuropsychopharmacology. 2000;23:S32–40. doi: 10.1016/S0893-133X(00)00155-X. [DOI] [PubMed] [Google Scholar]
- 26.Guo W, Shi L, Filizola M, Weinstein H, Javitch JA. Crosstalk in G protein-coupled receptors: changes at the transmembrane homodimer interface determine activation. Proc Natl Acad Sci U S A. 2005;102:17495–500. doi: 10.1073/pnas.0508950102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thevenin D, Lazarova T, Roberts MF, Robinson CR. Oligomerization of the fifth transmembrane domain from the adenosine A2A receptor. Protein Sci. 2005;14:2177–86. doi: 10.1110/ps.051409205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rose PM, Fernandes P, Lynch JS, Frazier ST, Fisher SM, Kodukula K, Kienzle B, Seethala R. Cloning and functional expression of a cDNA encoding a human type 2 neuropeptide Y receptor. J Biol Chem. 1995;270:22661–4. doi: 10.1074/jbc.270.39.22661. [DOI] [PubMed] [Google Scholar]
- 29.Parker SL, Parker MS, Sah R, Sallee F. Angiogenesis and rhodopsin-like receptors: A role for N-terminal acidic residues? Biochem Biophys Res Commun. 2005;335:983–92. doi: 10.1016/j.bbrc.2005.06.158. [DOI] [PubMed] [Google Scholar]
- 30.Rieu P, Sugimori T, Griffith DL, Arnaout MA. Solvent-accessible residues on the metal ion-dependent adhesion site face of integrin CR3 mediate its binding to the neutrophil inhibitory factor. J Biol Chem. 1996;271:15858–61. doi: 10.1074/jbc.271.27.15858. [DOI] [PubMed] [Google Scholar]
- 31.Sallee FR, Sah R, Parker MS, Parker SL. Internalization of cloned neuropeptide Y (NPY) Y1 receptors in CHO cells is resistant to pertussis toxin. 2005 Meeting of the Soceity for Neuroscience; 2005. abstract #607.5. [Google Scholar]
- 32.Dupré DJ, Robitaille M, Ethier N, Villeneuve LR, Mamarbachi AM, Hebert TE. Seven transmembrane receptor core signaling complexes are assembled prior to plasma membrane trafficking. J Biol Chem. 2006;281:34561–73. doi: 10.1074/jbc.M605012200. [DOI] [PubMed] [Google Scholar]
- 33.Raymond JR, Olsen CL, Gettys TW. Cell-specific physical and functional coupling of human 5-HT1A receptors to inhibitory G protein alpha-subunits and lack of coupling to Gs alpha. Biochemistry. 1993;32:11064–73. doi: 10.1021/bi00092a016. [DOI] [PubMed] [Google Scholar]
- 34.Oh P, Schnitzer JE. Segregation of heterotrimeric G proteins in cell surface microdomains. G(q) binds caveolin to concentrate in caveolae, whereas G(i) and G(s) target lipid rafts by default. Mol Biol Cell. 2001;12:685–98. doi: 10.1091/mbc.12.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xue M, Vines CM, Buranda T, Cimino DF, Bennett TA, Prossnitz ER. N-formyl peptide receptors cluster in an active raft-associated state prior to phosphorylation. J Biol Chem. 2004;279:45175–84. doi: 10.1074/jbc.M407053200. [DOI] [PubMed] [Google Scholar]
- 36.Denis-Henriot D, de Mazancourt P, Goldsmith PK, Giudicelli Y. G proteins in adipocytes and preadipocytes: characterization, subcellular distribution, and potential roles for Gi2 and/or Gi3 in the control of cell proliferation. Cell Signal. 1996;8:225–34. doi: 10.1016/0898-6568(95)02058-6. [DOI] [PubMed] [Google Scholar]
- 37.Culm KE, Lim AM, Onton JA, Hammer RP., Jr Reduced G(i) and G(o) protein function in the rat nucleus accumbens attenuates sensorimotor gating deficits. Brain Res. 2003;982:12–8. doi: 10.1016/s0006-8993(03)02880-4. [DOI] [PubMed] [Google Scholar]
- 38.Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5:121–32. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 39.Jahangeer S, Rodbell M. The disaggregation theory of signal transduction revisited: further evidence that G proteins are multimeric and disaggregate to monomers when activated. Proc Natl Acad Sci U S A. 1993;90:8782–6. doi: 10.1073/pnas.90.19.8782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nair KS, Balasubramanian N, Slepak VZ. Signal-dependent translocation of transducin, RGS9-1-Gbeta5L complex, and arrestin to detergent-resistant membrane rafts in photoreceptors. Curr Biol. 2002;12:421–5. doi: 10.1016/s0960-9822(02)00691-7. [DOI] [PubMed] [Google Scholar]
- 41.Bari M, Battista N, Fezza F, Finazzi-Agro A, Maccarrone M. Lipid rafts control signaling of type-1 cannabinoid receptors in neuronal cells. Implications for anandamide-induced apoptosis. J Biol Chem. 2005;280:12212–20. doi: 10.1074/jbc.M411642200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Typical sedimentation profiles of solubilized human Y1 and Y2 and mouse Y4 receptors labeled by the respective primary agonists. The receptors were labeled by 50 pM [125I]hPYY(3-36) (human Y2), [125I]hNPY(1-36) (human Y1) and [125I]hPP (mouse Y4) in particulates from the respective expressions in CHO cells, solubilized by 10 mM each of cholate and digitonin, and sedimented in 5–20% linear sucrose gradients for 18 h at 218,000 × gmax, as described in Methods. Dimer content as % counts in the gradient was 66.8 ± 2.8 for the Y2, 57 ± 3.2 for the Y1, and 70.4 ± 4.3 for the Y4 receptor (all averages of two gradients, ± 1 S.E.M.).
Fig. S2 Stability of Y2 receptor dimers to incubation at 27 °C and to removal of divalent cations. Duplicate 5–20% gradients were centrifuged for 18 h at 5 °C. All results are average percentages of total counts in the corresponding gradient fractions from two gradients for each condition. The respective standard errors were in most cases below 10%, and for clarity are not shown.
A Y2 receptor dimers in particulates from CHO cells are stable to incubation at 27 °C. The incubation of particulate suspension in the assay buffer at 27 °C was for 1 h (followed by 2 h at 0 – 4 °C) or 3 h, while the control suspension was incubated in ice for 3 h. This was followed by labeling for 20 min at 27 °C by 50 pM [125I]PYY(3-36). The content of dimer, in % total gradient counts, was 66.2 ± 3.7 for control gradients, 63.4 ± 3.4 after preincubation for 1 h at 27 °C, and 64.3 ± 3.4 after preincubation for 3 h at 27 °C.
B Y2 receptor dimers are not significantly disbanded by removal of divalent cations. The profiles are for receptors labeled (30 min at 25 °C), solubilized and sedimented in buffers containing 3 mM CaCl2 + 1 mM MgCl2, or 1 mM Na EDTA (pH 7.4) without CaCl2 and MgCl2, proteinase inhibitors, 0.1% BSA and 10 mM hepes. NaOH, pH 7.4. The dimer content, in % gradient counts, was 65.9 ± 4.7 with Ca2+/Mg2+ buffer, and 60.8 ± 4.6 with EDTA buffer.
Fig. S3 Preferential removal of Y2 receptors dimers by two agonists of the Y2 receptor. NPY(22-36) (Kdiss vs. [125I]PYY(3-36) at hY2 receptor 6.2 ± 1.2 nM) was used at 10 nM, and PYY(3-36) (Kdiss at hY2 0.50 ± 0.06 nM) at 100 nM final, for 45 min at 25 °C prior to resedimentation and labeling with 50 pM [125I]PYY(3-36). After lysis at 10 mM each of digitonin and cholate, duplicate 5–20% gradients for each condition were centrifuged for 18 h at 5 °C. All results are average percentages of total counts in the corresponding gradient fractions. The respective standard errors were in most cases below 10%, and for clarity are shown only for the control profile. The content of dimer, in % total gradient counts, was 67.7 ± 4.8 for control gradients, 42.7 ± 2.3 after pre-treatment with 10 nM NPY(22-36), and 30.5 ± 2 after pre-treatment with 100 nM PYY(3-36).