Supplemental Digital Content is available in the text.
Abstract
Background:
Postparalysis facial synkinesis (PPFS) can occur after any cause of facial palsy. Current treatments are still inadequate. Surgical intervention, instead of Botox and rehabilitation only, for different degrees of PPFS was proposed.
Methods:
Seventy patients (43 females and 27 males) with PPFS were enrolled since 1986. They were divided into 4 patterns based on quality of smile and severity of synkinesis. Data collection for clinically various presentations was made: pattern I (n = 14) with good smile but synkinesis, pattern II (n = 17) with acceptable smile but dominant synkinesis, pattern III (n = 34) unacceptable smile and dominant synkinesis, and pattern IV (n = 5) poor smile and synkinesis. Surgical interventions were based on patterns of PPFS. Selective myectomy and some cosmetic procedures were performed for pattern I and II patients. Extensive myectomy and neurectomy of the involved muscles and nerves followed by functioning free-muscle transplantation for facial reanimation in 1- or 2-stage procedure were performed for pattern III and many pattern II patients. A classic 2-stage procedure for facial reanimation was performed for pattern IV patients.
Results:
Minor aesthetic procedures provided some help to pattern I patients but did not cure the problem. They all had short follow-up. Most patients in patterns II (14/17, 82%) and III (34/34, 100%) showed a significant improvement of eye and smile appearance and significant decrease in synkinetic movements following the aggressively major surgical intervention. Nearly, all of the patients treated by the authors did not need repeated botulinum toxin A injection nor require a profound rehabilitation program in the follow-up period.
Conclusions:
Treatment of PPFS remains a challenging problem. Major surgical reconstruction showed more promising and long-lasting results than botulinum toxin A and/or rehabilitation on pattern III and II patients.
Postparalysis facial synkinesis (PPFS) represents a wide spectrum of unwanted facial movements after recovery of facial palsy from any etiology. The possible pathogenesis of synkinesis is cross-talks in brain cortex, facial motor neurons, or facial nerve fibers.1–6 Hypothesis of aberrant reinnervation among facial nerve fibers following nerve injury is more preferred.1,3,6 PPFS should be clearly differentiated from hemifacial spasm,7,8 which is caused by vascular loop compression of the facial nerve at facial nerve exit zone from the brain stem, and facial muscle tethering after facial trauma, which causes adhesion between eye and cheek muscles.
PPFS occurs not only always during facial expression but also at rest (intermittent blinking reflex causes intermittent cheek muscles twitching) and at sleeping (upper and lower lip constantly retracted), which are often not noticed by the patients. Long-term unresolved synkinesis may result in permanent contracture, such as hypertrophy of corrugator muscle, deep nasolabial fold, lower lip retraction, chin skin dimples, and neck bands. (See Video 1, Supplemental Digital Content 1, which shows a patient who had right facial palsy caused by parotid gland tumor resection for 2 years and developed right PPFS. The video showed her preoperative facial expression and will be available in the “Related Videos” section of the full-text article on http://links.lww.com/PRSGO/A92.) These will disrupt the resting facial posture and the involved face and neck will become tight and sore. Those symptoms and signs make patients with PPFS distressed with severe emotional sequelae.
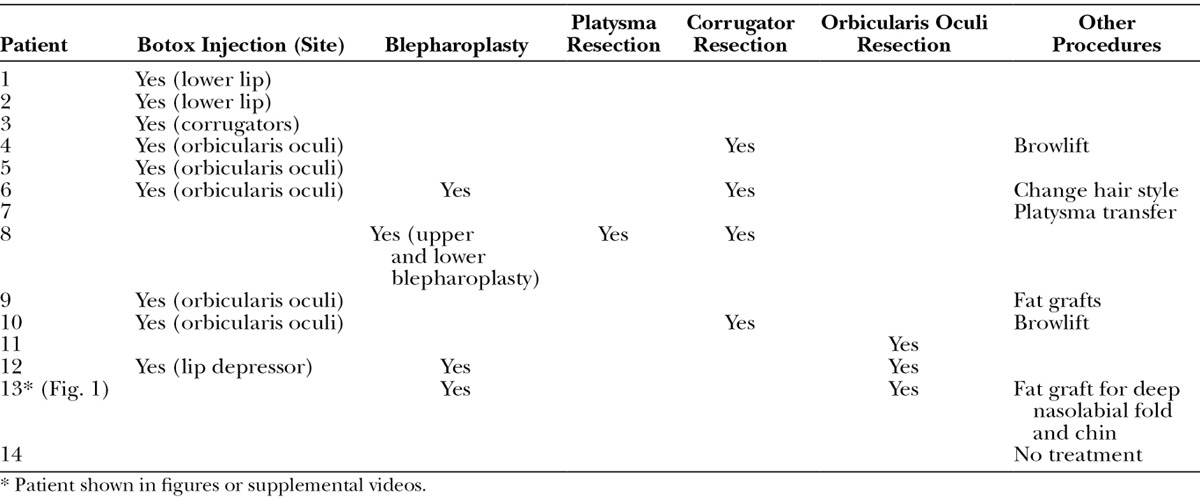
Fig. 1.
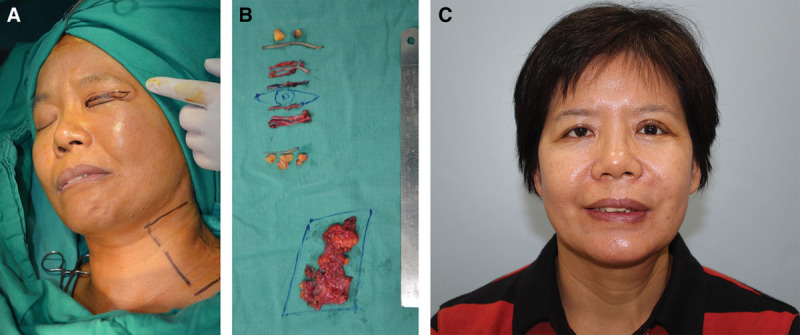
A, A 47-year-old woman (patient 13, Table 4) has developed left PPFPS, pattern I and II, due to Bell’s palsy for 2 years. She presented left face with narrow eye, dominant nasolabial fold, chin dimples, and neck bands when she was smiling. B, She received myectomy of the orbicularis oculi (upper and lower lids) and platysma, blepharoplasty, and late rigotomy and fat grafts for nasolabial fold and chin. C, The results showed much improvement of smile and decreased left facial synkinesis 1 year postoperatively.
Video 1.

See video, Supplemental Digital Content 1, which shows a patient who had right facial palsy caused by parotid gland tumor resection for 2 years and developed right PPFPS. The video showed her preoperative facial expression and will be available in the “Related Videos” section of the full-text article on http://www.PRSGO.com or available at http://links.lww.com/PRSGO/A92.
Several treatments have been proposed, including facial rehabilitation,9–12 botulinum toxin A (BTX-A) injections,13–17 and minor surgical procedures, such as selective myectomy17–21 and neurectomy.19,22,23 However, these treatments were all inadequate and not convincing. The aim of this article was to give a comprehensive overview of our surgical interventions based on different patterns of PPFS.
MATERIALS AND METHODS
A retrospective review was performed from 1986 to 2012 (a 27-year period). A total of 343 facial palsy patients were reconstructed by functioning free-muscle transplantation (FFMT) for facial reanimation. Ninety-nine percent of FFMT utilized gracilis muscle. They all were operated on by the senior surgeon (D.C.-C.C.). Fifty-three were patients with PPFS. Incidence of PPFS was at least 15% (53/343).
Seventy patients with PPFS were randomly enrolled for clinical evaluation and classification. Patients’ demographics including mean age at time of first clinic visit, sex, involved side, and etiology are shown in Table 1.
Table 1.
Patient Demographics

Clinically, PPFS involves 2 movements: trigger and synkinesis movements.
The trigger muscle(s) may be active or paralytic, but synkinetic muscle(s) is always active. There are basically 5 trigger and 6 synkinesis movements. The 5 trigger movements, which were regularly ordered to the patients, include forehead raise, eye closure, smile, lower lip pulled down, and lip pout or whistling. The 6 synkinetic movements, following the ordered movements, involve corrugators, eye, cheek, lower lip, chin skin, and platysma synkinesis. Since 2002, we have designed an examination sheet for PPFS, which was renewed in 2010 (Table 2).
Table 2.
PPFS Examination Sheet (Chang Gung Memorial Hospital, 2010)
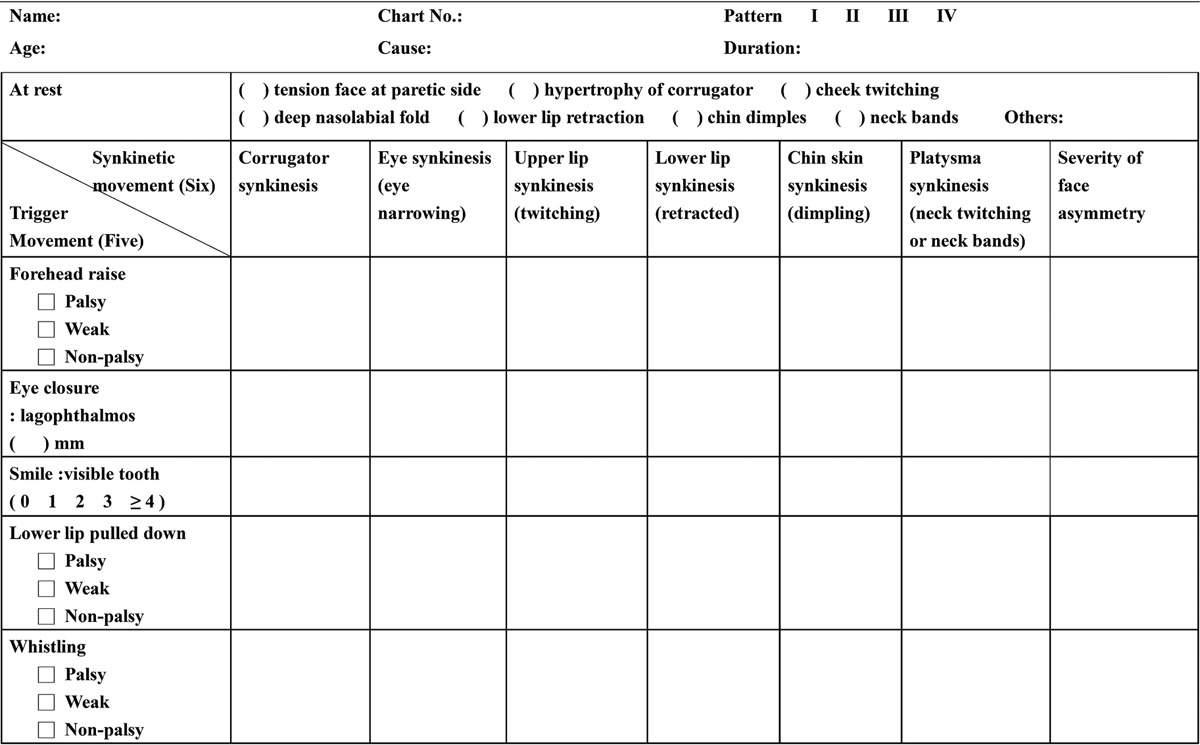
Through that, patients were divided into 4 patterns based on quality of smile and degree of synkinesis. Table 3 shows the characteristics of different patterns of PPFS following the above patients’ evaluation.
Table 3.
Characteristics of Different Patterns of Patients with PPFS
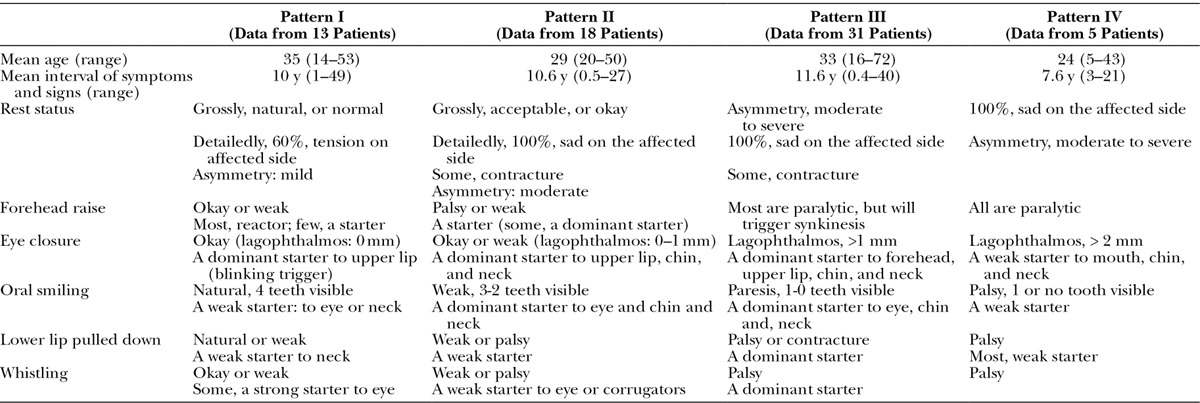
Pattern I: Good Smile but Synkinesis
In pattern I, patients were aware of their altered facial expression, but observers including physicians might not fully recognize the problems due to their mildness. Patients themselves were even not aware that they had synkinesis. Their big smile could always show at least 4 teeth. However, they always had intermittent perioral twitching following eye blinking. Some patients had an unique synkinesis with bizarre facial expression, such as lip pouting–induced eye narrowing on the affected side (oral-eye synkinesis) during spitting or whistling. Pattern I patients were mostly concerned more with cosmetic problems than synkinesis.
Pattern II: Acceptable Smile but Moderate-to-severe Synkinesis
In pattern II, patients were classified as having an acceptable smile but with moderate-to-severe synkinesis. (See Video 1, Supplemental Digital Content 1, which shows a patient who had right facial palsy caused by parotid gland tumor resection for 2 years and developed right PPFPS. The video showed her preoperative facial expression and will be available in the “Related Videos” section of the full-text article on http://www.PRSGO.com or available at http://links.lww.com/PRSGO/A92.) Facial disfiguration in pattern II majority came from synkinesis. Their big smile could usually show 0-1 tooth. Such synkinesis could not be cured or improved by cosmetic surgery. Some aesthetic surgery might even make the appearance worse.
Pattern III: Unacceptable Smile and Severe Synkinesis
In pattern III, patients were classified as having a poor smile and severe synkinesis. They had lagophthalmos with weak eye closure, poor smile with one or no tooth visible, hypertrophic corrugators, narrow eye, dominant nasolabial fold, chin skin dimples, and neck bands on the affected side. Patients in this pattern all demonstrated more courage to accept more aggressive procedures to correct their disfigures.
Pattern IV: Poor Smile and Mild Synkinesis
In pattern IV, patients were classified as having a poor smile and synkinesis. The patients’ complaints in this pattern were focused on the paralytic face, less on the synkinetic movements. All patients in this pattern requested more aggressive procedures for correction.
SURGICAL PROCEDURES
Myectomy
Orbicularis Oculi Muscle
In PPFS, orbicularis oculi muscle is the most trigger and/or synkinetic muscle. Myectomy of the orbicularis oculi muscle, upper and lower eyelids, can reduce the synkinesis (Fig. 1). There were 2 incisions, upper incision along the upper lid crease if present and lower incision along and 2 mm below the lower lid margin, to expose the orbicularis oculi muscle. Orbicularis oculi muscle has 3 components: pretarsus, preseptum, and preorbital portions. Only segmental resection of the former 2 portions in the upper and lower eyelids is enough to decrease synkinesis. It was a new procedure performed in recent 2 years.
Platysma Muscle
Platysma muscle on the affected side is quite often a synkinetic muscle. Myectomy of the platysma can decrease synkinesis. Two parallel incisions, 1 submandibular and 1 above clavicle, to nearly total resection of the platysma were regularly performed (Fig. 1).
Corrugator Muscle
Hypertrophy or involuntary movement of the corrugators would cause bizarre appearance when smiling. BTX-A injection is effective, but more patients will request myectomy for one procedure with permanent result. Suprabrow incision along the medial upper margin of the eyebrow to expose the corrugator muscle and nearly total resection of the muscle were performed.
A 2-stage Procedure
First stage involves short cross-face nerve graft (CFNG); second stage (6–9 months later) involves extensive myectomy of the cheek and platysma muscles, neurectomy of the zygomatic, buccal and cervical facial nerve branches, and gracilis FFMT for facial reanimation.
In the first stage, short CFNG has been evolved (since 2001).24,25 Sural nerve was placed subcutaneously from the preauricular wound on the intact side to the nasolabial fold small wound on the affected side. One stump was coapted to the facial nerve branches under microscopy, and the other stump was sutured to the dermis of the nasolabial fold. (See Video 2, Supplemental Digital Content 2, which shows a patient who received extensive myectomy and neurectomy and reconstruction by CFNG-innervated gracilis, a 2-stage procedure. The video showed excellent results of 1 year after free-muscle transplantation and is available in the “Related Videos” section of the full-text article on http://links.lww.com/PRSGO/A93.)
Fig. 2.
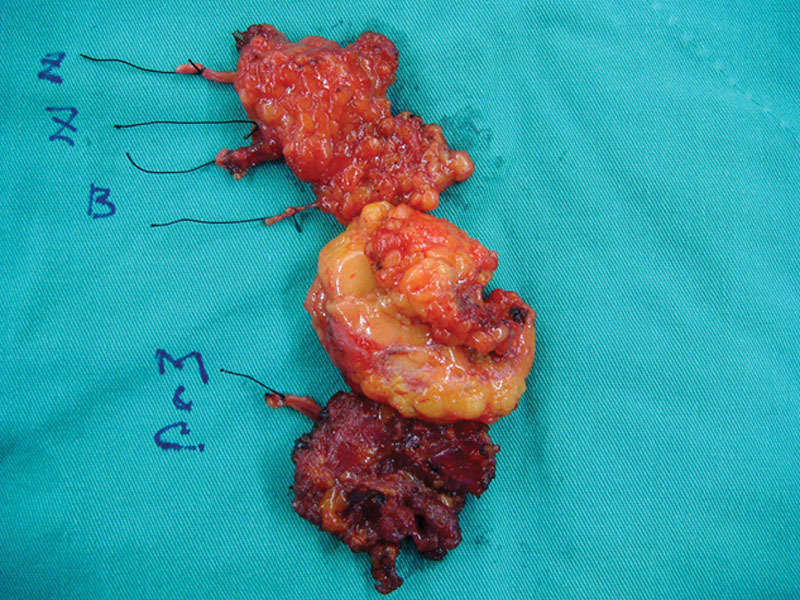
Sample of myectomy of cheek muscles and platysma and neurectomy of whole zygomatic and buccal facial nerve branches. B indicates buccal; C&M, cervical and mandibular branch; Z, upper and lower zygomatic.
Video 2.

See video, Supplemental Digital Content 2, which shows a patient who received extensive myectomy and neurectomy and reconstruction by CFNG-innervated gracilis, a 2-stage procedure. The video showed excellent results of 1 year after free-muscle transplantation and is available in the “Related Videos” section of the full-text article on http://www.PRSGO.com or available at http://links.lww.com/PRSGO/A93.
In the second stage, 1 small upper lip wound was made to identify the CFNG stump. A long second preauricular wound down to submandibular extension was made, and subcutaneous facelift from the upper zygomatic arch to the upper and lower lip was performed. All peripheral branches of the facial nerve should be identified. All zygomatic and buccal branches and their innervated muscles in the cheek area were excised en bloc (Fig. 2). The excision should be as extensive as possible down to the oral mucosa attachment to avoid late residual synkinetic movements. The neck cervical branch of the facial nerve and platysma were removed simultaneously. Stenson’s duct should be preserved. The frontal and mandibular branches were preserved if possible to preserve some function on eye and lower lip.
A gracilis muscle from the contralateral thigh was trimmed,25 harvested, and transferred to the face. Vessels were anastomosed to the facial artery and vein. The motor nerve of the gracilis muscle which was passing under the muscle to the upper lip wound was coapted to the previous CFNG under the operative microscope.
A 1-stage Procedure
Extensive Myectomy and Neurectomy and Gracilis FFMT Innervated by the Spinal Accessory or Masseter (V3) Nerve Simultaneously
The spinal accessory (XI) nerve was found on the anterior margin of the trapezius muscle through a posterior neck incision.26 Dissection of the XI nerve should be as distal as possible, divided, and transferred to the mandibular angle.
The V3 nerve was found at the posterior margin of the masseter muscle just below the zygomatic arch.27 A 1–1.5 cm of the nerve was dissected, divided, and transferred for nerve coaptation. Facelift, myectomy and neurectomy, gracilis FFMT, vessel anastomoses, and nerve coaptation were performed as described.
Postoperative Rehabilitation
CFNG-innervated and V3-innervated gracilis needed no splint immobilization. However, XI-innervated gracilis required face and neck splint immobilization for 3 weeks. Three weeks after surgery, electric stimulation was started. At approximately 4 months postoperatively, “induction exercise”3,26 was initiated. Once the transferred muscle achieved upper lip movement, “smile training”26 was prescribed to achieve independent and spontaneous movement. This smile training should be performed in front of a mirror.
OUTCOME ASSESSMENT
All treated patients were closely followed a minimum of 1 year. Outcomes were assessed in terms of success of decreasing synkinesis and achieving facial symmetry at rest and smiling. There were 4 methods that were used for outcome assessment26,28,29:
Smiling Score is a score system (0–4) based on how many teeth expose during smile: score 0, no or partial first incisor visible; score 1, the full first incisor visible; score 2, 2 teeth visible, and so forth. A maximum score of 4 is given if at least 4 teeth are visible. If reaching ≥score 2, it was acceptable.
Cortical Adaption Stage System (I–V) is a system to evaluate functional recovery of an FFMT: no movement (stage I), dependent movement (stage II), independent movement (stage III), spontaneous but with involuntary movement (stage IV), and spontaneous with little or no involuntary movement (stage V). If reaching ≥stage III, it was acceptable.
Synkinesis Grading System is utilized: mild, moderate, and severe synkinesis.30 Only the mild one was acceptable in outcome result.
Satisfactory Score, a questionnaire to evaluate patient satisfaction was performed, including patient’s complaint, self-image, use of the newly acquired smile, the possible complications, and symptoms by donor nerve transfer.26 Finally, a “satisfactory score” was made: score 1, not acceptable and regret surgery; score 2, not acceptable but did not regret surgery; score 3, acceptable but required further refinement surgery; score 4, satisfied but request minor procedure to improve the results; and score 5, completely satisfied.
RESULTS
Pattern I (n = 14)
BTX-A injection at eyelid muscle (1 shot at upper, 2 shots at lower eyelid; Botox, 0.025 ml = 1.0 IU/shot, 100 IU/2.5 ml), lower lip depressors (affected side or nonaffected side, 5–6 shots, 0.05 ml = 2 IU/shot), and corrugator muscle (2 shots, 0.05 ml = 2 IU/shot) was the most common procedure for pattern I patients (Table 4). They all achieved temporary decrease of synkinesis, but were not completely satisfied. Changing hair style or wearing glasses to cover the synkinesis was advised with some help. Myectomy of the orbicularis oculi showed some help to decrease synkinesis and decrease requirement of the repeated BTX-A injection (Fig. 1) (patient 13, Table 4). One patient (patient 7, Table 4) had platysma muscle transfer to the ipsilateral weak lower lip for balance. Many pattern I patients knew that the synkinesis could not be cured by such minor procedures and tended to stop clinical follow-up after a short time of visiting. A few patients chose no treatment at all (patient 14, Table 4).
Table 4.
Pattern I Patients
Pattern II (n = 17)
Fourteen patients (82%) requested more aggressive surgical procedures after explanation (Table 5). All were treated with extensive myectomy and neurectomy and reconstructed by CFNG-innervated gracilis. They achieved a more symmetric smile (smile score from average 2.6 preoperatively to 4.0 postoperatively, Table 7) and significantly decreased synkinetic movements (from moderate or severe down to mild, P < 0.001; Table 7). The cortical adaptation was all scored at stage V (spontaneous smile). Clinically, it was more witnessed in eye opening and teeth visibility (Fig. 3). Adjuvant surgeries included lip revision, blepharoplasty, tensor fasciae latae sling, or fat grafts to improve results. Most of them received no more BT-A injection. Satisfactory Score showed at least score 3. Six patients had lip contracture and required release procedure. Two patients (patients 2 and 6, Table 5) required lip adhesion (Fig. 4) for 1 year to overcome the lip contracture. Two patients (patients 15 and 16, Table 5) only accepted minor procedures with repeated BT-A injections. One patient (patient 17, Table 5) refused the treatment after explanation.
Table 5.
Pattern II Patients

Fig. 3.
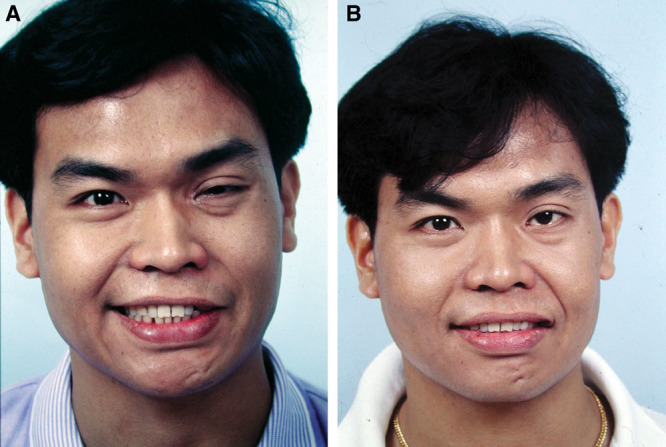
A, A 31-year-old man (patient 1, Table 5) had left Bell’s palsy for 6 years and developed left PPFPS, pattern II. He received extensive myectomy and neurectomy and reconstruction by CFNG-innervated gracilis, a 2-stage procedure. B, Results at 2 years after showed more natural smile.
Fig. 4.
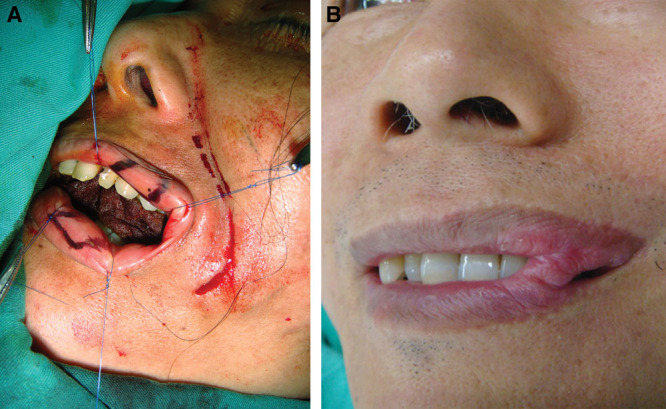
A, A double opposing lip mucosa flap is designed: one inner-based and another outer-based. The muscles of the both upper and lower lips should be sutured together before both mucosal flaps are adhesive. B, The healed lip adhesion.
Pattern III (n = 34)
All patients accepted and received the extensive surgical procedures for treatment (Table 6). Similar to the pattern II surgical-treated patients, the most improved parts with less synkinesis were eye opening and teeth visibility (Figs. 5–7). The smile score improved from an average 0.86 preoperatively to 3.57 postoperatively in the CFNG-innervated gracilis series (21 patients) and from an average 0.6 preoperatively to 3.5 postoperatively in the XI-innervated gracilis series (12 patients). The cortical adaptation stage showed at least stage III. The synkinesis was significantly improved in all from grade II or III down to I (P < 0.001, Table 7). One patient was an exceptional child, 6 years old when he was surgically treated by CFNG-innervated gracilis, with near 10-year follow-up (patient 8, Table 6; Fig. 6). In 1 girl patient (patient 23, Table 6) after CFNG-innervated gracilis, although showing little movement of the transferred muscle (smile score 1) after 2-year follow-up with unknown reason, her synkinesis grading improved. Another patient (patient 29, Table 6) who had failed CFNG-innervated gracilis for 1 year received masseter-innervated second gracilis for reconstruction and showed improving results (Fig. 7). From Satisfactory Score, 33 patients showed significant improvement of their synkinesis (P < 0.001, Table 7) and more tooth exposure when smiling. The Satisfactory Score was at least 3. The eye appeared as the most improved part with a more natural look. One patient (patient 14, Table 6) was considered as a fair result due to upper lip contracture. Four patients had lip contracture and required lip adhesion (patients 8, 10, 14, and 17, Table 6). No patient regretted having such complex surgical procedures.
Table 6.
Pattern III Patients
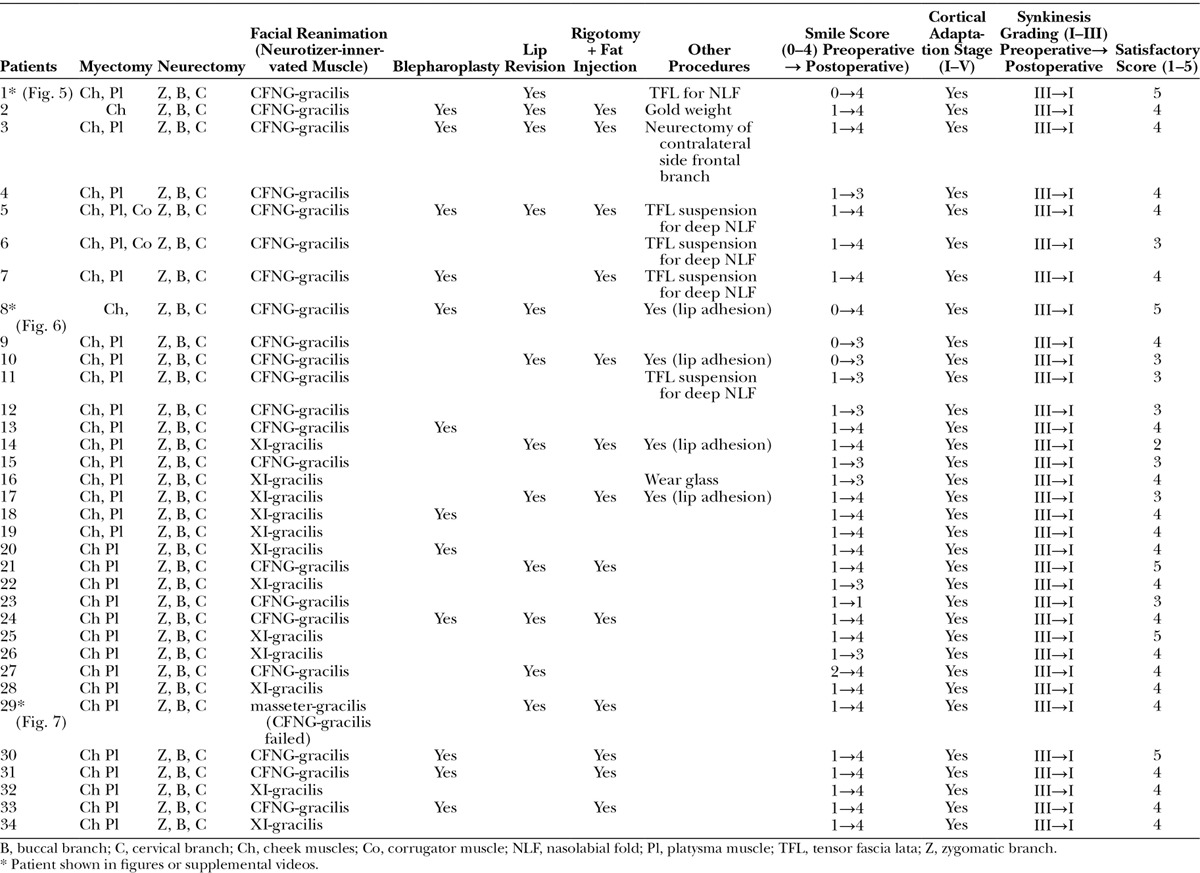
Fig. 5.
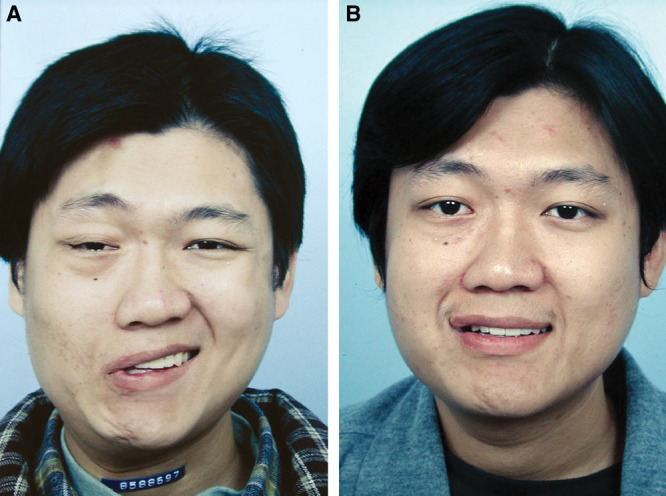
A, An 18-year-old man (patient 1, Table 6) had Bell’s palsy for 14 years and developed right PPFPS, pattern III. He received extensive myectomy and neurectomy and reconstruction by CFNG-innervated gracilis, a 2-stage procedure. B, Results at 2 years after showed excellence.
Fig. 7.
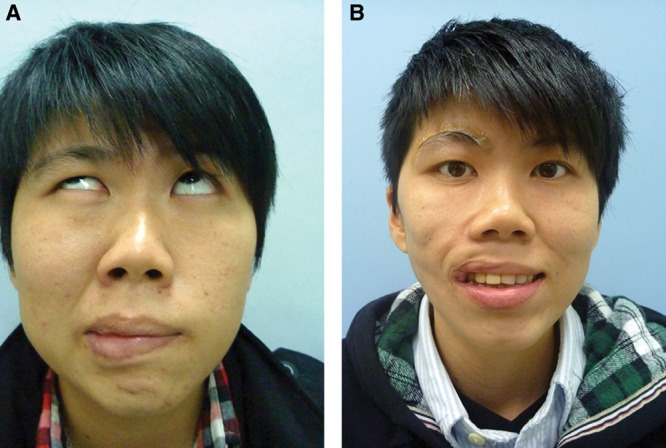
A, A 25-year-old solider (patient 29, Table 6) had right facial palsy for 1 year due to a falling accident and developed right PPFS, pattern III. She had failed CFNG-innervated gracilis (left gracilis) 1 year ago due to infection and muscle necrosis. After 11 months, she had masseter-innervated second gracilis (right gracilis) for facial reanimation. B, Good facial smile score with mild synkinesis was achieved. She needs rigotomy and fat grafts for uneven surface of the reconstructed face later for refined surgery.
Table 7.
Improvement of Synkinesis Grading and Smile Score after Aggressive Surgical Procedures
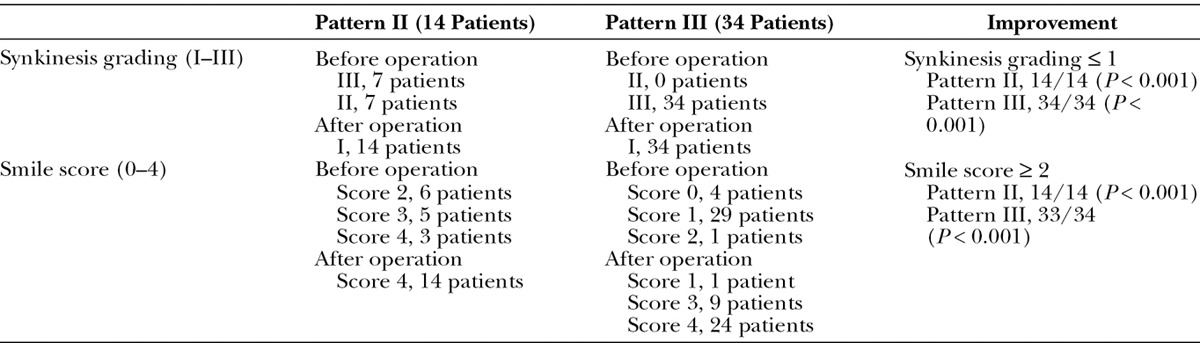
Fig. 6.
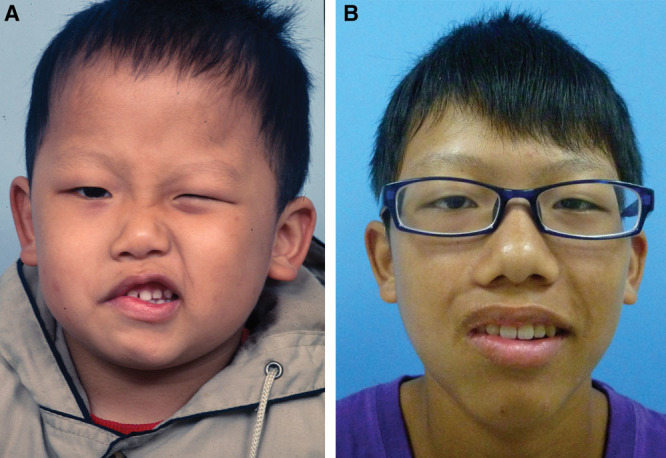
A, A 5-year-old boy (patient 8, Table 6), who suffered a dog bite to the face and received facial nerve repair, developed right PPFPS for 1 year, pattern III. He received extensive myectomy and neurectomy and reconstruction by CFNG-innervated gracilis in a 2-stage procedure. B, Results at 9 years after showed excellence. He had lip adhesion for 1 year when developed contracture.
Pattern IV (n = 5)
In pattern IV, all 5 patients underwent CFNG-innervated gracilis, a classic 2-stage procedure for reconstruction. Four patients had good results, but 2 developed upper lip contracture, requiring lip revision. The cortical adaptation stage was ≥stage III in all, and the smile score improved from average 0.65 preoperatively to 3.0 postoperatively.
DISCUSSION
Aberrant reinnervation, for motor it is synkinesis, may occur after nerve injury and repair, such as cases of obstetric brachial plexus palsy.31,32 Deformities at shoulder and elbow after recovery are seen quite often. The major treatment is surgical intervention31,32 not BTX-A and/or rehabilitation.
The philosophy of surgical treatment of PPFS is similar to the treatment of scars. PPFS is a healing process of facial nerve injury, similar to scar, a healing process of wound. Scars have many different clinical presentations. Not all scars can be treated simply by injection, massage, and pressure. Some scars, hypertrophic scars and keloids, should be treated aggressively, such as radical excision and resurface by free tissue transfer. Similarly, not all PPFS can be treated effectively and permanently by BTX-A and/or rehabilitation. The goal of PPFS treatment is not only to decrease synkinesis but also to achieve a natural and symmetric smile permanently.
Rehabilitation therapy is attempting to inhibit the trigger movement or change the intended voluntary movements to decrease synkinesis.9–12 It has involved exercises (eg, mirror exercises, electromyography feedback training), learning (neuromuscular reeducation), relaxation training, mime therapy, electric stimulation, and BTX-A injection. However, there has been little support showing it could significantly and permanently reduce synkinesis.
BTX-A injection has been a well-established benefit in the control of facial hyperkinesis in the literatures,13–16 mostly due to lack of effective surgical treatment. The authors have been taught that BTX-A can treat the whole PPFS for years (1990–2000) and used it for treatment in the beginning. Quick effects have been noted, but with many variations in the procedure, including injection sites, injection dosage, timing of injection, frequency of injection, result evaluation, and follow-up period. In addition, the major disadvantages of BTX-A were many injection sites (on the paralyzed and healthy face), repeated injections, anaphylaxis, blepharoptosis, diplopia, and accelerating the paralysis. The effect of BTX-A will also be decreasing following the repeated injections. Those will usually exhaust patients. Most patients will lose their patience and finally disappear from clinic after a short follow-up period (usually less than 2 years), but they were not cured yet.
In PPFS, except pattern IV, 2 phenomena have been seen: less synkinesis will tend to be more symmetric in smile; more muscle paralysis is associated with less synkinesis. Our philosophy to treat PPFS is to remove the trigger and synkinetic muscles as much as possible to decrease the synkinesis and to remove the innervated nerves to avoid its recurrence. The effect is permanent. Our treatment protocol is based on patterns of PPFS and patient’s desire. BT-A injection, rehabilitation, and other additional aesthetic surgeries were only our adjuvant therapy to improve the result.
VanSwearingen and Brach9 described the importance of treatment-based classification system for facial synkinesis. As PPFS has many presentations and patient expectations are usually high, pattern classification is necessary, which can be used for explanation and strategy of treatment. Narrow eye, dominant nasolabial fold, dominant lower lip pulled down, and dominant neck bands are all important signs for judging degree of synkinesis. Tooth exposure when smiling is a good sign for judging quality of facial palsy. Most patients with PPFS are more concerned with facial asymmetry than synkinesis. But to the physician, correction of synkinesis should always be done before treatment of asymmetry. This is especially true in pattern II and III patients with PPFS.
Treatment for pattern I patients will be much closer to aesthetic surgery. BT-A injection, rehabilitation, and distraction procedures (hair style changes and eye glasses wearing), and minor facial contouring surgeries can be helpful. However, those conservative treatments cannot cure synkinesis. The best treatment for pattern II and pattern III patients is to treat synkinesis first and then aesthetics. Extensive removal of the involved muscles and nerves, followed by FFMT for facial reanimation, is our treatment of choice. All patients with PPFS who received such aggressive procedures should be aware that additional minor procedures such as blepharoplasty and fat grafts might be required later to enhance the results. The advantage of such extensive procedures is to avoid insufficient treatment and recurrence.
Performing aggressive surgical procedures to treat pattern II and III patients with PPFS is a challenging task. For a safe practice and to avoid potential law suit, some prerequisites are essentially necessary:
The surgeon should have experiences in facial palsy reconstruction and skills in aesthetic judgment and management.
Extensive preoperative explanation to both the patient and patient’s family is required, including mechanism, 4 patterns of PPFS and their treatment options, and conservative vs surgical intervention. The patient should recognize that the disfiguring involved is due to synkinesis effects. Surgical risks, postoperative rehabilitation, potential sequelae, and further surgical procedures should be explained for decision making. Showing photographs or videos of results from previously treated patients are also helpful. The patient and parents should take the messages home and think about them. The final decision will be made by the patient not by a driven surgeon. The patient should be given enough time for consideration of such invasive surgery.
The patient should have close follow-up postoperatively.
Indication and contraindication: patient selection is also crucial. The age of the patient should not be too young (before 14 years old) or too old (after 60). Patients with low education level and high expectation for results or hesitation in decision making should not be considered for radical procedures.
Until now, for more than 25 years, the authors have never met 1 case with law suit, even though 1 fair result (patient 23, pattern III) and 1 failed first gracilis (patient 29, pattern III) were encountered. The patients did not need repeated BTX-A injection or require a profound rehabilitation program during the follow-up period.
CONCLUSIONS
Treatment of PPFS remains a challenging problem. Aggressive surgical procedures for pattern II and III patients including myectomy, neurectomy, and functioning muscle transplantation showed more promising results than minor procedures. The 4 patterns of classification and treatment strategies mentioned above are still constantly followed.
PATIENT CONSENT
Patients or their parents or guardians provided written consent for the use of the patients’ image
Supplementary Material
Footnotes
Presented, in part, at the Annual Meeting of the American Society for Reconstructive Microsurgery, January 13, 2013, Naples, Fla.; the 12th International Facial Nerve Symposium, June 29, 2013, Boston, Mass.; and the 7th World Society for Reconstructive Microsurgery, July 13, 2013, Chicago, Ill.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by PRS GO at the discretion of the Editor-in-Chief.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
REFERENCES
- 1.Valls-Solé J, Montero J. Movement disorders in patients with peripheral facial palsy. Mov Disord. 2003;18:1424–1435. doi: 10.1002/mds.10605. [DOI] [PubMed] [Google Scholar]
- 2.Oge AE, Yayla V, Demir GA, et al. Excitability of facial nucleus and related brain-stem reflexes in hemifacial spasm, post-facial palsy synkinesis and facial myokymia. Clin Neurophysiol. 2005;116:1542–1554. doi: 10.1016/j.clinph.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 3.Chuang DC. Adult brachial plexus injuries. In: Mathes S, Hentz VR, editors. In: Plastic Surgery. 2nd ed. Philadelphia, Pa.: Saunders Elsevier Inc.;; 2006. pp. 515–538. [Google Scholar]
- 4.Rijntjes M, Tegenthoff M, Liepert J, et al. Cortical reorganization in patients with facial palsy. Ann Neurol. 1997;41:621–630. doi: 10.1002/ana.410410511. [DOI] [PubMed] [Google Scholar]
- 5.Cossu G, Valls-Solé J, Valldeoriola F, et al. Reflex excitability of facial motoneurons at onset of muscle reinnervation after facial nerve palsy. Muscle Nerve. 1999;22:614–620. doi: 10.1002/(sici)1097-4598(199905)22:5<614::aid-mus10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 6.Baker RS, Stava MW, Nelson KR, et al. Aberrant reinnervation of facial musculature in a subhuman primate: a correlative analysis of eyelid kinematics, muscle synkinesis, and motoneuron localization. Neurology. 1994;44:2165–2173. doi: 10.1212/wnl.44.11.2165. [DOI] [PubMed] [Google Scholar]
- 7.Colosimo C, Bologna M, Lamberti S, et al. A comparative study of primary and secondary hemifacial spasm. Arch Neurol. 2006;63:441–444. doi: 10.1001/archneur.63.3.441. [DOI] [PubMed] [Google Scholar]
- 8.Valls-Solé J. Electrodiagnostic studies of the facial nerve in peripheral facial palsy and hemifacial spasm. Muscle Nerve. 2007;36:14–20. doi: 10.1002/mus.20770. [DOI] [PubMed] [Google Scholar]
- 9.VanSwearingen JM, Brach JS. Changes in facial movement and synkinesis with facial neuromuscular reeducation. Plast Reconstr Surg. 2003;111:2370–2375. doi: 10.1097/01.PRS.0000061007.36637.88. [DOI] [PubMed] [Google Scholar]
- 10.Novak CB, Mackinnon SE. Facial neuromuscular retraining for oral synkinesis. Plast Reconstr Surg. 1997;99:1932–1933. doi: 10.1097/00006534-199706000-00017. [DOI] [PubMed] [Google Scholar]
- 11.Ross B, Nedzelski JM, McLean JA. Efficacy of feedback training in long-standing facial nerve paresis. Laryngoscope. 1991;101(7 Pt 1):744–750. doi: 10.1288/00005537-199107000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Diels HJ, Combs D. Neuromuscular retraining for facial paralysis. Otolaryngol Clin North Am. 1997;30:727–743. [PubMed] [Google Scholar]
- 13.Borodic G, Bartley M, Slattery W, et al. Botulinum toxin for aberrant facial nerve regeneration: double-blind, placebo-controlled trial using subjective endpoints. Plast Reconstr Surg. 2005;116:36–43. doi: 10.1097/01.prs.0000169689.27829.c4. [DOI] [PubMed] [Google Scholar]
- 14.Putterman AM. Botulinum toxin injections in the treatment of seventh nerve misdirection. Am J Ophthalmol. 1990;110:205–206. doi: 10.1016/s0002-9394(14)76994-6. [DOI] [PubMed] [Google Scholar]
- 15.Rogers CR, Schmidt KL, VanSwearingen JM, et al. Automated facial image analysis: detecting improvement in abnormal facial movement after treatment with botulinum toxin A. Ann Plast Surg. 2007;58:39–47. doi: 10.1097/01.sap.0000250761.26824.4f. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong MW, Mountain RE, Murray JA. Treatment of facial synkinesis and facial asymmetry with botulinum toxin type A following facial nerve palsy. Clin Otolaryngol Allied Sci. 1996;21:15–20. doi: 10.1111/j.1365-2273.1996.tb01018.x. [DOI] [PubMed] [Google Scholar]
- 17.Husseman J, Mehta RP. Management of synkinesis. Facial Plast Surg. 2008;24:242–249. doi: 10.1055/s-2008-1075840. [DOI] [PubMed] [Google Scholar]
- 18.Terzis JK, Karypidis D. Therapeutic strategies in post-facial paralysis synkinesis in adult patients. Plast Reconstr Surg. 2012;129:925e–939e. doi: 10.1097/PRS.0b013e318230e758. [DOI] [PubMed] [Google Scholar]
- 19.Guerrissi JO. Selective myectomy for postparetic facial synkinesis. Plast Reconstr Surg. 1991;87:459–466. doi: 10.1097/00006534-199103000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Henstrom DK, Malo JS, Cheney ML, et al. Platysmectomy: an effective intervention for facial synkinesis and hypertonicity. Arch Facial Plast Surg. 2011;13:239–243. doi: 10.1001/archfacial.2011.43. [DOI] [PubMed] [Google Scholar]
- 21.Lai CS, Lu SR, Yang SF, et al. Surgical treatment of the synkinetic eyelid closure in Marin-Amat syndrome. Ann Plast Surg. 2011;67:498–501. doi: 10.1097/SAP.0b013e318201fdc3. [DOI] [PubMed] [Google Scholar]
- 22.Zhang B, Yang C, Wang W, et al. Repair of ocular-oral synkinesis of postfacial paralysis using cross-facial nerve grafting. J Reconstr Microsurg. 2010;26:375–380. doi: 10.1055/s-0030-1249603. [DOI] [PubMed] [Google Scholar]
- 23.Iwakuma T, Matsumoto A, Nakamura N. Surgical results of hemifacial spasm: comparison of partial selectioning of the facial nerve, selective neurectomy and microvascular decompression. In: Graham MD, House WF, editors. Disorders of the Facial Nerve. New York, N.Y.: Raven Press; 1982. pp. 323–329. [Google Scholar]
- 24.Chuang DC. Free tissue transfer for the treatment of facial paralysis. Facial Plast Surg. 2008;24:194–203. doi: 10.1055/s-2008-1075834. [DOI] [PubMed] [Google Scholar]
- 25.Chuang DC. Technique evolution for facial paralysis reconstruction using functioning free muscle transplantation–experience of Chang Gung Memorial Hospital. Clin Plast Surg. 2002;29:449–459, v. doi: 10.1016/s0094-1298(02)00021-4. [DOI] [PubMed] [Google Scholar]
- 26.Chuang DC, Lu JC, Anesti K. One-stage procedure using spinal accessory nerve (XI)-innervated free muscle for facial paralysis reconstruction. Plast Reconstr Surg. 2013;132:117e–129e. doi: 10.1097/PRS.0b013e318290f8cd. [DOI] [PubMed] [Google Scholar]
- 27.Manktelow RT, Tomat LR, Zuker RM, et al. Smile reconstruction in adults with free muscle transfer innervated by the masseter motor nerve: effectiveness and cerebral adaptation. Plast Reconstr Surg. 2006;118:885–899. doi: 10.1097/01.prs.0000232195.20293.bd. [DOI] [PubMed] [Google Scholar]
- 28.Tzou CHJ, Chuang DCC, Chen HH. Facial paralysis grading system: a new and simple smile excursion score for evaluating facial reanimation surgery. Ann Plast Surg. 2015;74:210–213. doi: 10.1097/SAP.0b013e318295dec2. [DOI] [PubMed] [Google Scholar]
- 29.Tzou CH, Chuang DC, Chen HY. Cortical adaptation staging system: a new and simple staging for result evaluation of functioning free-muscle transplantation for facial reanimation. Ann Plast Surg. 2014;73:50–53. doi: 10.1097/sap.0000000000000064. [DOI] [PubMed] [Google Scholar]
- 30.Ross BG, Fradet G, Nedzelski JM. Development of a sensitive clinical facial grading system. Otolaryngol Head Neck Surg. 1996;114:380–386. doi: 10.1016/S0194-59989670206-1. [DOI] [PubMed] [Google Scholar]
- 31.Chuang DC, Ma HS, Wei FC. A new strategy of muscle transposition for treatment of shoulder deformity caused by obstetric brachial plexus palsy. Plast Reconstr Surg. 1998;101:686–694. doi: 10.1097/00006534-199803000-00015. [DOI] [PubMed] [Google Scholar]
- 32.Chuang DC, Hattori Y, Ma And HS, et al. The reconstructive strategy for improving elbow function in late obstetric brachial plexus palsy. Plast Reconstr Surg. 2002;109:116–126; discussion 127. doi: 10.1097/00006534-200201000-00020. [DOI] [PubMed] [Google Scholar]


