Abstract
Background:
Allogeneic skin grafts onto C57BL/6 mice are rejected, and the rejected skin is replaced by surrounding skin with black hair. In contrast, syngeneic skin grafts are tolerated, and gray hair grows on the grafts.
Methods:
To explore the mechanism of gray hair growing on the tolerated skin grafts, we prepared full-thickness skin (2-cm square) autografts, 2 (2 cm + 2 cm) horizontal or vertical parallel incisions, and U-shaped (2 cm × 2 cm × 2 cm) flaps with or without pedicle vessels. The grafts, incisions, and flaps were fixed by suturing with string and protected by a transparent bandage. On day 14 after the operation, the bandages were removed to observe the color of the hair growing on the skin.
Results:
Skin autografts from wild-type or hepatocyte growth factor-transgenic (Tg) C57BL/6 mice survived with gray hair, whereas those from steel factor (Kitl)-Tg C57BL/6 mice survived with black hair. In addition, U-shaped flaps lacking both of the 2 main feeding vessels of wild-type mice had gray hair at the tip of the flaps. Light microscopy after staining with hematoxylin and eosin or dihydroxyphenylalanine showed that the formation of melanin pigment in the follicles, but not in the interadnexal skin, was susceptible to the blood supply.
Conclusions:
Melanin pigment formation in the hair bulb melanocytes appeared to be susceptible to the blood supply, and melanocytosis was promoted in the follicles and in the epidermis of Kitl-Tg C57BL/6 mice.
Skin grafts from syngeneic strains of mice onto C57BL/6 mice (ie, isografts) are tolerated, whereas those from allogeneic (eg, BALB/c) strains of mice (ie, allografts) are rejected.1 The alloreactive immune response is predominantly directed at the major histocompatibility complex (H-2 in mice) class I molecules,2–11 and the rejected skin is replaced by surrounding skin with black hair.5,12 Unexpectedly, however, mostly gray-colored hair grows on the syngeneic skin grafts to cover the original size of the grafts.12
Mature melanocytes are melanin-synthesizing dendritic cells located within the basal layer of the epidermis, hair bulb, and outer root sheath of hair follicles.13 Hair melanin is formed by melanocytes situated in the hair bulb epithelium around the upper half of the dermal papilla among the cells destined to form the hair cortex.14 Melanogenic activity in the hair follicle is closely linked to the hair cycle. In the telogen follicle, nonmelanogenic melanocytes are found in the basal layer of the outer root sheath and in the secondary germ region.15,16 These cells are assumed to be the precursors of active melanocytes during the next anagen phase, and they either migrate or are carried into the hair bulb in early anagen development. Melanogenic (ie, tyrosinase) activity becomes apparent in Anagen 3, pigment transfer to the cortical epithelium begins in the Anagen 4 stage of development, and melanogenesis ceases with the onset of catagen.17–19 The fate of hair bulb melanocytes during catagen is uncertain.20,21
The wide range of genetically determined variations in coat color of the laboratory mouse provides an excellent model for the study of gene action within many biological processes.22 More than 150 distinct mutations that affect pigmentation either directly or indirectly have been identified in murine models23: for example, Steel, c-Kit, Splotch, Microphthalmia, and Dom for melanocyte embryogenesis; Silver, Beige, and OA-1 for melanosome structure and function; Ashen and Dilute for transport; Albino/platinum, Brown, and Slaty for melanogenic enzyme; and Extension and Agouti for regulation of melanogenesis. Many of these gene products have been shown to be involved in various clinical pigmentary diseases in man. In particular, the tyrosine kinase receptor Kit and its ligand (Steel factor, mast cell/stem cell growth factor, or Kitl) play a critical role in melanocyte physiology (eg, up-regulation of melanoblast proliferation and action as a survival factor for active migrating and proliferating melanoblasts).24–26 Piebaldism, a genetic disorder, results from Kit receptor defects secondary to mutation of the c-Kit proto-oncogene, and patients with piebaldism present at birth with white patches of skin and hair that lack melanocytes.27 Transgene expression of steel factor (Kitl) in the basal layer of the epidermis in a mouse model used for the study of melanocyte development promotes survival, proliferation, differentiation, and migration of melanocyte precursors,28 and keratinocyte expression of transgenic (Tg) hepatocyte growth factor (HGF) affects melanocyte development, leading to dermal melanocytosis.29
Establishment of cultures of human melanocytes has led to an explosion of knowledge concerning the biology of melanocytes: in gray and white hair, the melanocytes in the basal layer of the hair matrix are greatly reduced in number or are absent.30 However, cellular and molecular events that are responsible for the loss of melanogenesis in skin or hair after birth are nearly unknown. In the present study, we investigated the mechanisms of gray hair growth in autologous skin grafted onto the backs of C57BL/6 mice. The hair color did not change after making 2 horizontal or vertical parallel incisions or U-shaped flaps with pedicle vessels. However, U-shaped flaps lacking both of the 2 main feeding vessels had gray hair at the tip of the flaps, and skin autografts from wild-type C57BL/6 mice survived in the original size of the grafts with gray hair, implying blood supply-dependent melanin pigment formation. To explore which step(s) in the melanin pigment formation or which type(s) of cells was susceptible to the blood supply, we prepared skin autografts from HGF-Tg or Kitl-Tg C57BL/6 mice. Hematoxylin and eosin (H&E) and dihydroxyphenylalanine (DOPA) stainings showed that dermal (ie, interadnexal skin) melanocytosis was mainly stimulated in HGF-Tg C57BL/6 mice and that epidermal and follicular melanocytosis was induced in the Kitl-Tg C57BL/6 mice. Furthermore, there was mixture of black and gray hair growing on the tolerated skin grafts from HGF-Tg C57BL/6 mice, whereas there was unchanged accumulation of melanin granules in the upper half of dermal papilla of the grafts from Kitl-Tg C57BL/6 mice. These results showed that melanin pigment formation in the follicular melanocytes appeared to be susceptible to blood supply and that follicular melanocytosis was also stimulated in the Kitl-Tg C57BL/6 mice.
MATERIALS AND METHODS
Animals
Specific pathogen-free male C57BL/6 mice (7–12 weeks of age) were purchased from Japan SLC (Hamamatsu, Japan). KRT14-Kitl-Tg (Kitl-Tg) mice and KRT14-HGF-Tg (HGF-Tg) mice expressing mouse Kitl and human HGF driven by KRT14 show hyperaccumulation of melanin granules in the epidermis and the dermis, respectively.28,29 All experiments were carried out in accordance with The Guidelines on Animal Experiments of Osaka Medical College and the Japanese Government Notification on Feeding and Safekeeping of Animals (Notification No. 6 of the Prime Minister’s Office), and the experimental protocol was approved by The Review Committee for Animal Experiments of Osaka Medical College.
Skin Grafts
C57BL/6 mice were anesthetized with inhaled isoflurane. We shaved off the dorsal hair with a razor and made four 2-cm incisions in the shape of a square into the dorsal wall. Full-thickness dorsal skin (2-cm square) was removed and then grafted back onto the original location by suturing it at intervals of 5 mm with a 5-0 nylon string, as described previously.12 On day 14 after grafting, the grafts were scored as having survived when gray hair had grown to cover the original size of the grafts.
Two Horizontal or Vertical Parallel Incisions
We anesthetized C57BL/6 mice, shaved off the dorsal hair with a razor, and made 2 (2 cm + 2 cm) horizontal or vertical parallel incisions in the dorsal walls. The skin (2-cm square) was raised and then sutured back.
Skin Flaps
We prepared 3 types of flaps in the dorsal skin of C57BL/6 mice. (1) Axial pattern flap with 2 main feeding vessels: we made full-thickness incisions to prepare U-shaped flaps by making 3 incisions in different directions in the dorsal wall. The flap (2 cm × 2 cm × 2 cm) was raised and then sutured back. (2) Flap with 1 of 2 main feeding vessels: we raised the U-shaped flap and cut 1 of the 2 main feeding vessels with a needle knife. (3) Random pattern flap lacking the 2 main feeding vessels: we raised the U-shaped flap and cut both main feeding vessels. Blood flow in this type of flap depends on capillaries in the skin.
DOPA and H&E Staining
Specimens of skin grafts were embedded in optimal cutting temperature compound and quick frozen in liquid nitrogen. Frozen serial sections (10-μm thickness) were cut, placed on poly-l-Lysine-coated glass slides, and air-dried. One section was stained with H&E, and the adjacent one was used for DOPA staining, as described previously.31 These sections were fixed in 10% formalin solution for several hours and thereafter dehydrated in alcohol and xylol.
RESULTS
We removed full-thickness dorsal skin (2-cm square) from C57BL/6 mice and then grafted it back onto the original location (autografts). The autografts in C57BL/6 mice survived as the original size of the grafts with gray hair on day 21 after transplantation, and the gray hair was maintained for more than 5 months (data not shown). Similarly, 21 days after autologous skin transplantation, gray hair grew on the tolerated grafts (Fig. 1A) and was shaved off with a razor, and by day 37 after shaving, gray hair had regrown on the grafts (Fig. 1B).
Fig. 1.
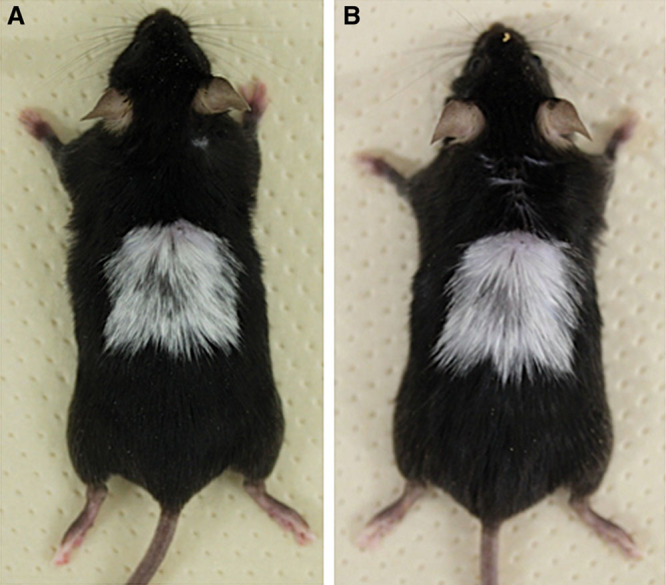
Autologous skin of a C57BL/6 mouse was engrafted. A, On day 21 after autologous skin grafting on the dorsum of a C57BL/6 mouse, gray hair had grown on the graft. When the gray hair was shaved off on the same day, 37 days later the gray hair had regrown on the graft (B).
To explore the mechanism of the change in the color of the hair growing on the tolerated skin grafts, we made 2 (2 cm + 2 cm) vertical or horizontal parallel incisions in the dorsal wall of C57BL/6 mice, raised the skin (2-cm square), and then sutured it back. As expected, the hair color did not change significantly (data not shown). Next, we made U-shaped skin flaps (2 cm × 2 cm × 2 cm) in the dorsal wall, raised the skin (2-cm square), and then sutured it back. Again, there was no change in the color of hair after making these U-shaped skin flaps (data not shown), demonstrating that 4 incisions (2-cm square) were essential for the growth of gray hair on the skin autografts.
Since the U-shaped skin flaps had their blood supply mainly from 2 feeding vessels coming from the underlying muscles (Fig. 2), we next examined the effects of cutting (with a needle knife) one or both of these main feeding vessels on the change in the hair color of the skin flap. When we made U-shaped skin flaps possessing 1 of these 2 main feeding vessels, the mice had black hair on the flaps (Fig. 3A). In contrast, U-shaped skin flaps lacking both vessels showed the growth of gray hair at the tip of the flaps (Fig. 3B), indicating that an adequate blood supply to the flaps might be essential for the growth of pigmented hair at the tip of the flaps.
Fig. 2.
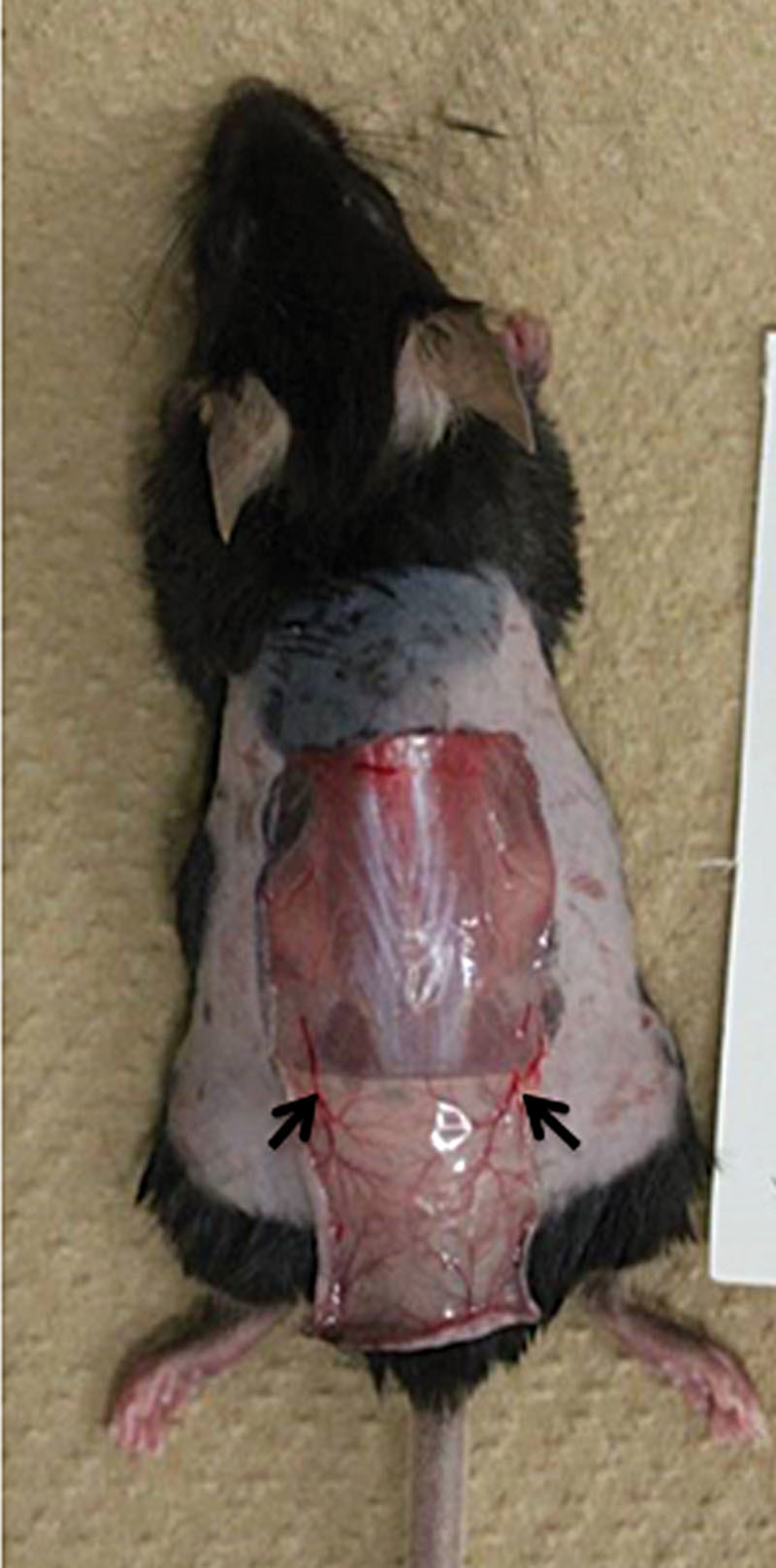
A U-shaped incision was made in the dorsal wall of a C57BL/6 mouse. Arrows, 2 main feeding vessels.
Fig. 3.
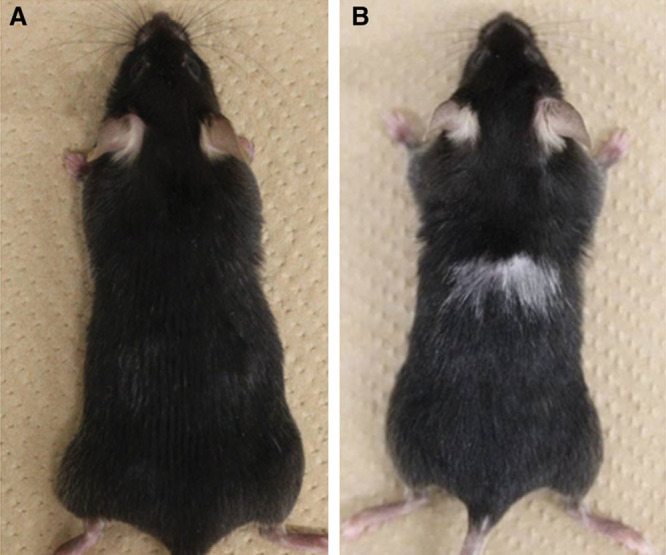
Effect of cutting of 1 or both main feeding vessels of the U-shaped skin flap made in the dorsal walls of C57BL/6 mice. A, One of the 2 main feeding vessels was cut. B, Both vessels were cut. On day 21, the color of hair growing on the flap was determined.
To examine histologically the mechanism of gray hair growth on the tolerated skin autografts, we made serial frozen sections of dorsal skin from C57BL/6 mice and stained them in alternate fashion, that is, with H&E for one section and with DOPA for the next one (Fig. 4). On light microscopy, the H&E-stained sections (Fig. 4A) showed almost constant numbers of follicles in the dermis (10.4 ± 1.5 follicles/[×100] field [mean ± SD, n = 5], 7.8 ± 1.3, 7.5 ± 2.4, 7.8 ± 0.8, and 6.2 ± 1.1 for untreated skin, the tip of the U-shaped skin flaps possessing both, 1, or neither of the 2 main feeding vessels, and tolerated autografts, respectively). It also showed more clear deposition of melanin in the dermal (ie, interadnexal skin) layer in the tip of U-shaped skin flaps possessing both (107.0 ± 17.6 melanin+ granules/[×100] field [mean ± SD, n = 5]), 1 (159.8 ± 15.3), or neither (130.4 ± 20.6) of the 2 main feeding vessels or in the tolerated autografts (29.8 ± 9.1) than in the untreated skin (10.6 ± 1.1). By contrast, a few (1.8 ± 1.3) or very few (0.6 ± 0.9) hair shafts had melanin pigments in the tip of U-shaped skin flaps lacking the 2 main feeding vessels or in the tolerated autografts, respectively.
Fig. 4.
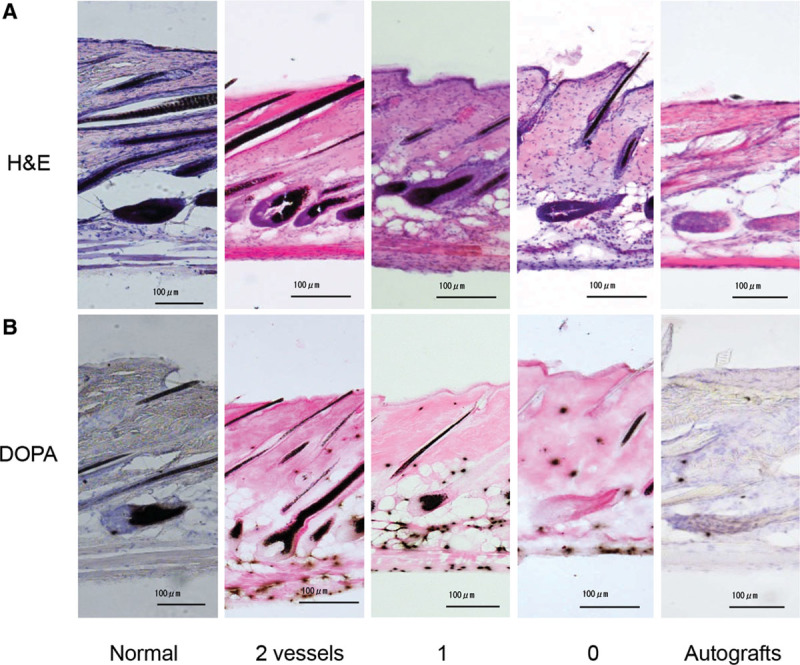
H&E (A)- and DOPA (B)-stained sections of normal dorsal skin, U-shaped skin flaps possessing 2, 1, or no feeding vessels, or autografts from C57BL/6 mice. Scale bars = 100 μm. The difference in the numbers of DOPA+ follicles between “2 feeding vessels” and “no feeding vessels” and between “1 feeding vessel” and “no feeding vessels” is significant (P = 0.002105 and 0.003756, respectively), whereas the difference between “2 feeding vessels” and “1 feeding vessel” is not significant (P = 0.7978) according to Student’s t test.
To explore whether the melanocytes from U-shaped skin flaps lacking both main feeding vessels or tolerated autografts exhibited normal melanogenic activity, we used other adjacent sections for DOPA staining. Light microscopy after DOPA staining (Fig. 4B) confirmed melanin pigment production (10.4 ± 1.5 DOPA+ follicles [n = 5]) in skin sections from untreated mice and in most of the hair follicles at the tip of the U-shaped skin flaps possessing both (7.0 ± 0.7 DOPA+ follicles [n = 5]) or 1 (6.8 ± 1.7 DOPA+ follicles [n = 4]) of the 2 main feeding vessels. In contrast, melanin pigment was not produced in some hair follicles (1.8 ± 1.3 DOPA+ follicles [n = 5]) at the tip of the U-shaped skin flaps lacking both vessels. In the case of tolerated autografts, there was almost no melanin pigment production (0.4 ± 0.5 DOPA+ follicles [n = 5]) in the hair follicles, revealing that melanin pigment formation in the follicular melanocytes was blood supply susceptible.
To examine which step(s) in the melanin pigment formation or which type(s) of cells was susceptible to blood supply, we performed skin autografting in Kitl-Tg and HGF-Tg C57BL/6 mice. An HGF-Tg C57BL/6 mouse with dermal melanocytosis had black hair before transplantation, and dermal melanocytosis was observed after shaving (data not shown). However, the mouse produced gray hair covering the original size of the grafts (Fig. 5A), suggesting specific impairment in the follicular melanocytosis after skin autografting. In the case of the Kitl-Tg C57BL/6 mice with epidermal melanocytosis, these mice without transplantation had black hair; and marked epidermal melanocytosis was observed after shaving (data not shown). In addition, there was no gray hair on the grafts after autologous skin transplantation (Fig. 5B), suggesting that melanocytosis in the epidermis and the follicles of the Kitl-Tg C57BL/6 mice might have become resistant to insufficient blood supply after skin autografting.
Fig. 5.
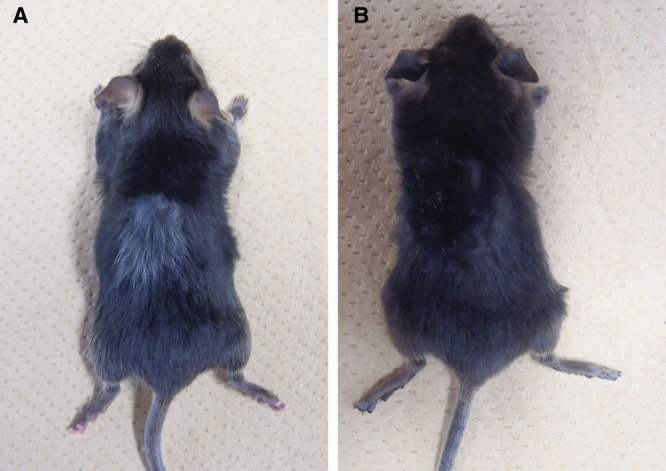
HGF-Tg (A) and Kitl-Tg (B) C57BL/6 mice after skin autografting.
Light microscopy after staining with H&E (Fig. 6A) or DOPA (Fig. 6B) clearly showed that dermal (ie, interadnexal skin) melanocytosis was stimulated in HGF-Tg C57BL/6 mice before (1388.8 ± 239.3 melanin+ granules [n = 5]) and after (1414.4 ± 214.8 melanin+ granules [n = 5]) skin autografting, whereas follicular melanocytosis on the tolerated grafts was reduced after it: before (7.0 ± 0.7 DOPA+ follicles [n = 5]) and after (2.6 ± 0.9 DOPA+ follicles [n = 5]). In contrast, there was enhanced epidermal melanocytosis in the Kitl-Tg mice before transplantation, and there was unchanged accumulation of melanin granules in the epidermis and in the hair follicles: before (7.8 ± 1.3 DOPA+ follicles [n = 5]) and after (4.6 ± 1.7 DOPA+ follicles [n = 5]).
Fig. 6.
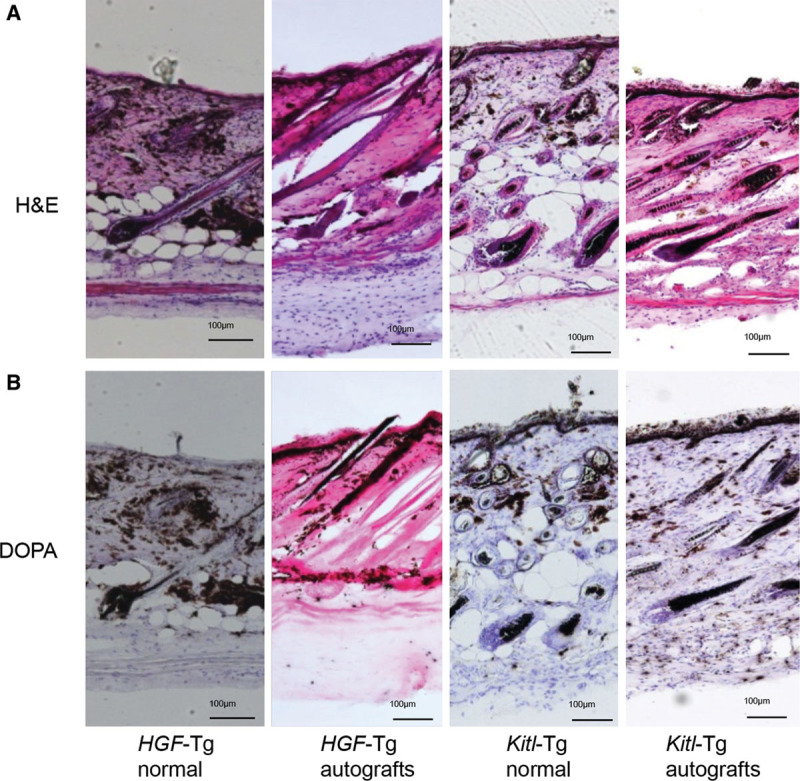
H&E (A)- and DOPA (B)-stained sections of normal dorsal skin or skin autografts from HGF-Tg and Kitl-Tg C57BL/6 mice. Scale bars = 100 μm. The difference in the numbers of DOPA+ follicles between “HGF-Tg normal” and “HGF-Tg autografts” is significant (P = 0.0001), whereas the difference between “Kitl-Tg normal” and “Kitl-Tg autografts” is not significant (P = 0.0723) according to Student’s t test.
DISCUSSION
As observed with skin isografts,12 skin autografts in C57BL/6 mice survived in the original size of the grafts and developed gray hair (Fig. 1). U-shaped flaps with 1 or both of the 2 main feeding vessels had black hair, whereas U-shaped flaps lacking these 2 main feeding vessels had gray hair at the tip of the flaps (Figs. 2, 3), implying that an adequate blood supply to melanocytes or their progenitor melanoblasts might have been essential to the growth of pigmented hair. In fact, the numbers of DOPA+ follicles increased in a blood supply-dependent manner (Fig. 4): tolerated autografts (0.4 ± 0.5); tip (1.8 ± 1.3), middle (7.2 ± 0.8), or bottom (7.4 ± 0.9) part of U-shaped flaps lacking the 2 main feeding vessels; U-shaped flaps with 1 (6.8 ± 1.7) or both (7.0 ± 0.7) of 2 main feeding vessels; and untreated skin (10.4 ± 1.5).
Keratinocyte expression of Tg HGF and epidermal expression of Tg Kitl promote the survival and proliferation of melanoblasts in the dermis29 and in the basal layer of the epidermis,28 respectively. Therefore, we expected the growth of black and gray hair on the tolerated skin autografts of HGF-Tg and Kitl-Tg C57BL/6 mice, respectively. Unexpectedly, however, the hair color on tolerated skin grafts from Kitl-Tg mice was black and that from HGF-Tg mice, a mixture of black and gray (Fig. 5). Melanocytosis in the interadnexal skin was stimulated in HGF-Tg C57BL/6 mice before and after skin autografting, whereas follicular melanocytosis on the tolerated grafts was reduced after it (Fig. 6). In the case of Kitl-Tg mice, there was unchanged accumulation of melanin granules in the epidermis and the follicles of the tolerated grafts (Fig. 6), demonstrating that melanocytosis was promoted in the follicles and in the epidermis of the Kitl-Tg mice.
The primary distinguishing feature of follicular melanogenesis, compared with the continuous melanogenesis in the epidermis, is the tight coupling of hair follicle melanogenesis to the hair growth cycle15–19: the hair cycle involves melanocyte death via apoptosis during early catagen.32 In addition, follicular melanogenesis (ie, melanogenic activity of follicular melanocytes, transfer of melanin granules into cortical and medullary keratinocytes, and formation of pigmented hair shafts)33 is more sensitive to aging influences than melanocytes in the epidermis, resulting in hair graying/canities.31 The melanogenic activity appeared to be impaired at the tip of U-shaped flaps lacking the 2 main feeding vessels or in the tolerated autografts (Fig. 4), and the hair shafts without pigmentation elongated normally to be maintained for more than 5 months (Fig. 1). Therefore, the nonmelanogenic melanocytes, a progenitor of active melanocytes during the next anagen phase, in the telogen follicle15,16 would appear to be susceptible to the poor blood supply.
Recently, Tanimura et al34 reported that the transmembrane protein collagen XVII is highly expressed in hair follicle stem cells (HFSCs) and is required for the maintenance not only of HFSCs but also of melanocyte stem cells. They also showed that mice lacking collagen XVII show premature hair graying and hair loss and that forced expression of collagen XVII in basal keratinocytes, including HFSCs, in collagen XVII-null mice rescues melanocyte stem cells from premature differentiation and restores transforming growth factor-β signaling. In the present study, however, skin autografts from wild-type or HGF-Tg C57BL/6 mice survived with gray hair and without hair loss and the hair shafts without pigmentation elongated normally suggesting a collagen XVII/transforming growth factor-β-independent mechanism of hair graying (Figs. 1, 3, 5).
Patients with piebaldism, a genetic disorder of the Kit receptor, present at birth with white patches of skin and hair that lack melanocytes.27 In contrast, vitiligo is an acquired idiopathic and, in the majority of cases, a progressive, unpredictable disorder of the skin; skin with such lesions is characterized by the lack of epidermal melanocytes.35–42 Tobin et al43 reported that melanocytes are never completely absent in the depigmented epidermis of vitiligo and that these melanocytes can recover their functionality in vivo and in vitro upon the removal of H2O2. During vitiligo repigmentation, hypertrophic pigmented melanocytes appear in the outer root sheath of the hair follicle and migrate from the infundibulum into the nearby epidermis.44 The melanin pigment formation was stimulated mainly in the epidermis of Kitl-Tg C57BL/6 mice (Figs. 5, 6); the follicular melanocytes from vitiligo patients are to some extent intact at the first onset of the disease.35 These results imply a possibility that oxidative stress by an insufficient blood supply and impairment in c-Kit and/or its ligand expression might induce melanocyte-specific lesions in the epidermal (at the first onset) and/or follicular (at the later stages) melanin unit.
U-shaped skin flaps possessing 1 of the 2 main feeding vessels had black-colored hair, whereas those lacking both vessels showed the growth of gray-colored hair at the tip of the flaps (Fig. 3). In addition, as inhibition of the c-Kit function, by injection of an anti-c-Kit monoclonal antibody, results in the growth of unpigmented hair45 and as there was unchanged follicular melanocytosis on the tolerated skin grafts from Kitl-Tg mice (Fig. 6), the c-Kit gene might play a critical role in this process in mice. These results suggest that generation of at least 1 feeding vessel at a skin transplantation site might be useful for protection against gray hair growth in mice and that topical treatment with c-Kit and/or its ligand might not only achieve a normal dermal structure and pigmented hair but also induce epidermal melanocytosis for piebaldism, vitiligo, and so forth in humans.
ACKNOWLEDGMENTS
We thank Dr. Takahiro Kunisada and Dr. Shin-Ichi Hayashi for providing the HGF-Tg and Kitl-Tg mice and Mr. Kaname Shimokawa, Mr. Teruo Ueno, and Mr. Yukio Nakahira for their skillful technical assistance.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. This study was supported, in part, by the program Grants-in-aid for Young Scientists (B) (grant no. 22791740 and 22791741) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Counce S, Smith P, Barth R, et al. Strong and weak histocompatibility gene differences in mice and their role in the rejection of homografts of tumors and skin. Ann Surg. 1956;144:198–204. doi: 10.1097/00000658-195608000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoshida R, Park SW, Yasui H, et al. Tryptophan degradation in transplanted tumor cells undergoing rejection. J Immunol. 1988;141:2819–2823. [PubMed] [Google Scholar]
- 3.Yoshida R, Takikawa O, Oku T, et al. Mononuclear phagocytes: a major population of effector cells responsible for rejection of allografted tumor cells in mice. Proc Natl Acad Sci U S A. 1991;88:1526–1530. doi: 10.1073/pnas.88.4.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ushio Y, Yamamoto N, Sanchez-Bueno A, et al. Failure to reject an allografted tumor after elimination of macrophages in mice. Microbiol Immunol. 1996;40:489–498. doi: 10.1111/j.1348-0421.1996.tb01099.x. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto N, Einaga-Naito K, Kuriyama M, et al. Cellular basis of skin allograft rejection in mice: specific lysis of allogeneic skin components by non-T cells. Transplantation. 1998;65:818–825. doi: 10.1097/00007890-199803270-00009. [DOI] [PubMed] [Google Scholar]
- 6.Yoshida R, Sanchez-Bueno A, Yamamoto N, et al. Ca2+-dependent, Fas- and perforin-independent apoptotic death of allografted tumor cells by a type of activated macrophage. J Immunol. 1997;159:15–21. [PubMed] [Google Scholar]
- 7.Yoshida R, Matsuura A, Einaga K, et al. Two distinct populations of primary cytotoxic cells infiltrating into allografted tumor rejection site: infiltration of macrophages cytotoxic against allografted tumor precedes that of multiple sets of cytotoxic T lymphocytes with distinct specificity to alloantigens. Microbiol Immunol. 1997;41:149–159. doi: 10.1111/j.1348-0421.1997.tb01180.x. [DOI] [PubMed] [Google Scholar]
- 8.Yoshida R, Yoneda Y, Kuriyama M, et al. IFN-gamma- and cell-to-cell contact-dependent cytotoxicity of allograft-induced macrophages against syngeneic tumor cells and cell lines: an application of allografting to cancer treatment. J Immunol. 1999;163:148–154. [PubMed] [Google Scholar]
- 9.Lee K, Takenaka H, Yoneda Y, et al. Differential susceptibility of cells expressing allogeneic MHC or viral antigen to killing by antigen-specific CTL. Microbiol Immunol. 2004;48:15–25. doi: 10.1111/j.1348-0421.2004.tb03483.x. [DOI] [PubMed] [Google Scholar]
- 10.Tashiro-Yamaji J, Einaga-Naito K, Kubota T, et al. A novel receptor on allograft (H-2d)-induced macrophage (H-2b) toward an allogeneic major histocompatibility complex class I molecule, H-2Dd, in mice. Microbiol Immunol. 2006;50:105–116. doi: 10.1111/j.1348-0421.2006.tb03775.x. [DOI] [PubMed] [Google Scholar]
- 11.Tashiro-Yamaji J, Kubota T, Yoshida R. Macrophage MHC receptor 2: a novel receptor on allograft (H-2D(d)K(d))-induced macrophage (H-2D(b)K(b)) recognizing an MHC class I molecule, H-2K(d), in mice. Gene. 2006;384:1–8. doi: 10.1016/j.gene.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Inoue Y, Tashiro-Yamaji J, Hayashi M, et al. Transgene number-dependent, gene expression rate-independent rejection of Dd-, Kd-, or DdKd-transgened mouse skin or tumor cells from C57BL/6 (DbKb) mice. Microbiol Immunol. 2011;55:446–453. doi: 10.1111/j.1348-0421.2011.00337.x. [DOI] [PubMed] [Google Scholar]
- 13.Bronner-Fraser M. Mechanisms of neural crest cell migration. Bioessays. 1993;15:221–230. doi: 10.1002/bies.950150402. [DOI] [PubMed] [Google Scholar]
- 14.Cesarini JP. Hair melanin and hair color. In: Orfanos CE, Happle R, editors. In: Hair and Hair Diseases. Berlin: Springer-Verlag; 1990. pp. 165–197. [Google Scholar]
- 15.Cui J, Shen LY, Wang GC. Role of hair follicles in the repigmentation of vitiligo. J Invest Dermatol. 1991;97:410–416. doi: 10.1111/1523-1747.ep12480997. [DOI] [PubMed] [Google Scholar]
- 16.Silver AF, Chase HB, Potten CS. Melanocyte precursor cells in the hair follicle germ during the dormat stage (telogen). Experientia. 1969;25:299–301. doi: 10.1007/BF02034407. [DOI] [PubMed] [Google Scholar]
- 17.Kukita A. Changes in tyrosinase activity during melanocyte proliferation in the hair growth cycle. J Invest Dermatol. 1957;28:273–274. doi: 10.1038/jid.1957.33. [DOI] [PubMed] [Google Scholar]
- 18.Fitzpatrick TB, Brunet P, Kukita A. The nature of hair pigment. In: Montagna W, Ellis RA, editors. In: The Biology of Hair Growth. New York, N.Y.: Academic Press; 1958. pp. 255–303. [Google Scholar]
- 19.Slominski A, Paus R. Melanogenesis is coupled to murine anagen: toward new concepts for the role of melanocytes and the regulation of melanogenesis in hair growth. J Invest Dermatol. 1993;101(1 Suppl):90S–97S. doi: 10.1111/1523-1747.ep12362991. [DOI] [PubMed] [Google Scholar]
- 20.Commo S, Bernard BA. Melanocyte subpopulation turnover during the human hair cycle: an immunohistochemical study. Pigment Cell Res. 2000;13:253–259. doi: 10.1034/j.1600-0749.2000.130407.x. [DOI] [PubMed] [Google Scholar]
- 21.Tobin DJ, Hagen E, Botchkarev VA, et al. Do hair bulb melanocytes undergo apoptosis during hair follicle regression (catagen)? J Invest Dermatol. 1998;111:941–947. doi: 10.1046/j.1523-1747.1998.00417.x. [DOI] [PubMed] [Google Scholar]
- 22.Jackson IJ. Molecular and developmental genetics of mouse coat color. Annu Rev Genet. 1994;28:189–217. doi: 10.1146/annurev.ge.28.120194.001201. [DOI] [PubMed] [Google Scholar]
- 23.Alhaidari Z, Olivry T, Ortonne JP. Melanocytegenesis and melanogenesis: genetic regulation and comparative clinical diseases. Veterinary Dermatol. 1999;10:3–16. doi: 10.1046/j.1365-3164.1999.00132.x. [DOI] [PubMed] [Google Scholar]
- 24.Grabbe J, Welker P, Dippel E, et al. Stem cell factor, a novel cutaneous growth factor for mast cells and melanocytes. Arch Dermatol Res. 1994;287:78–84. doi: 10.1007/BF00370723. [DOI] [PubMed] [Google Scholar]
- 25.Wehrle-Haller B, Weston JA. Soluble and cell-bound forms of steel factor activity play distinct roles in melanocyte precursor dispersal and survival on the lateral neural crest migration pathway. Development. 1995;121:731–742. doi: 10.1242/dev.121.3.731. [DOI] [PubMed] [Google Scholar]
- 26.Grichnik JM. Kit and melanocyte migration. J Invest Dermatol. 2006;126:945–947. doi: 10.1038/sj.jid.5700164. [DOI] [PubMed] [Google Scholar]
- 27.Spritz RA. Molecular basis of human piebaldism. J Invest Dermatol. 1994;103(5 Suppl):137S–140S. doi: 10.1111/1523-1747.ep12399455. [DOI] [PubMed] [Google Scholar]
- 28.Kunisada T, Yoshida H, Yamazaki H, et al. Transgene expression of steel factor in the basal layer of epidermis promotes survival, proliferation, differentiation and migration of melanocyte precursors. Development. 1998;125:2915–2923. doi: 10.1242/dev.125.15.2915. [DOI] [PubMed] [Google Scholar]
- 29.Kunisada T, Yamazaki H, Hirobe T, et al. Keratinocyte expression of transgenic hepatocyte growth factor affects melanocyte development, leading to dermal melanocytosis. Mech Dev. 2000;94:67–78. doi: 10.1016/s0925-4773(00)00308-7. [DOI] [PubMed] [Google Scholar]
- 30.Nishimura EK, Granter SR, Fisher DE. Mechanisms of hair graying: incomplete melanocyte stem cell maintenance in the niche. Science. 2005;307:720–724. doi: 10.1126/science.1099593. [DOI] [PubMed] [Google Scholar]
- 31.Tobin DJ, Paus R. Graying: gerontobiology of the hair follicle pigmentary unit. Exp Gerontol. 2001;36:29–54. doi: 10.1016/s0531-5565(00)00210-2. [DOI] [PubMed] [Google Scholar]
- 32.Stenn KS, Combates NJ, Eilertsen KJ, et al. Hair follicle growth controls. Dermatol Clin. 1996;14:543–558. doi: 10.1016/s0733-8635(05)70383-1. [DOI] [PubMed] [Google Scholar]
- 33.Slominski A, Wortsman J, Plonka PM, et al. Hair follicle pigmentation. J Invest Dermatol. 2005;124:13–21. doi: 10.1111/j.0022-202X.2004.23528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanimura S, Tadokoro Y, Inomata K, et al. Hair follicle stem cells provide a functional niche for melanocyte stem cells. Cell Stem Cell. 2011;8:177–187. doi: 10.1016/j.stem.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 35.Bose JP, Ortonne SK. Vitiligo: where do we stand? Pigment Cell Res. 1993;8:61–72. doi: 10.1111/j.1600-0749.1993.tb00584.x. [DOI] [PubMed] [Google Scholar]
- 36.Cui J, Harning R, Henn M, et al. Identification of pigment cell antigens defined by vitiligo antibodies. J Invest Dermatol. 1992;98:162–165. doi: 10.1111/1523-1747.ep12555773. [DOI] [PubMed] [Google Scholar]
- 37.Gilhar A, Zelickson B, Ulman Y, et al. In vitro destruction of melanocytes by the IgG fraction of serum from patients with vitiligo. J. Invest. Dermatol. 1995;105:683–686. doi: 10.1111/1523-1747.ep12324456. [DOI] [PubMed] [Google Scholar]
- 38.Ogg GS, Rod Dunbar P, Romero P, et al. High frequency of skin-homing melanocyte-specific cytotoxic T lymphocytes in autoimmune vitiligo. J Exp Med. 1998;188:1203–1208. doi: 10.1084/jem.188.6.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pawalek J, Körner A, Bergstrom A. New regulation of melanin biosynthesis and autodestruction of melanoma cells. Nature. 1980;286:617–619. doi: 10.1038/286617a0. [DOI] [PubMed] [Google Scholar]
- 40.Schallreuter KU, Wood JM, Berger J. Low catalase levels in the epidermis of patients with vitiligo. J Invest Dermatol. 1991;97:1081–1085. doi: 10.1111/1523-1747.ep12492612. [DOI] [PubMed] [Google Scholar]
- 41.Maresca V, Roccella M, Roccella F, et al. Increased sensitivity to peroxidative agents as a possible pathogenic factor of melanocyte damage in vitiligo. J Invest Dermatol. 1997;109:310–313. doi: 10.1111/1523-1747.ep12335801. [DOI] [PubMed] [Google Scholar]
- 42.Passi S, Grandinetti M, Maggio F, et al. Epidermal oxidative stress in vitiligo. Pigment Cell Res. 1998;11:81–85. doi: 10.1111/j.1600-0749.1998.tb00714.x. [DOI] [PubMed] [Google Scholar]
- 43.Tobin DJ, Swanson NN, Pittelkow MR, et al. Melanocytes are not absent in lesional skin of long duration vitiligo. J Pathol. 2000;191:407–416. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH659>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 44.Ortonne JP, MacDonald DM, Micoud A, et al. PUVA-induced repigmentation of vitiligo: histoenzymological (split-DOPA) and ultrastructural study. Br J Dermatol. 1979;101:1–13. doi: 10.1111/j.1365-2133.1979.tb15285.x. [DOI] [PubMed] [Google Scholar]
- 45.Nishikawa S, Kusakabe M, Yoshinaga K, et al. In utero manipulation of coat color formation by a monoclonal anti-c-kit antibody: two distinct waves of c-kit-dependency during melanocyte development. EMBO J. 1991;10:2111–2118. doi: 10.1002/j.1460-2075.1991.tb07744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


