Abstract
Background:
Our understanding of the pathophysiology of venous thromboembolism is largely based on the experience of orthopedic patients undergoing total joint replacement. Little is known regarding the natural history of venous thromboembolism in plastic surgery outpatients. Today, ultrasound screening, including compression and Doppler color flow imaging, represents the standard for detecting deep venous thromboses.
Methods:
Ultrasound screening was offered to 200 consecutive plastic surgery outpatients undergoing 205 operations. Patients were scanned before surgery, on the day after surgery, and approximately 1 week after surgery. No patient declined to participate (inclusion rate, 100%). Spontaneous breathing, Avoid gas, Face up, Extremities mobile anesthesia was used, with no chemoprophylaxis. Patient surveys were administered.
Results:
Six hundred ultrasound screening tests were performed. All scans performed the day after surgery were negative. Only one examination was positive, 8 days after a lipoabdominoplasty. Subsequent scans revealed complete resolution of the thrombosis with anticoagulation. Ninety percent of surveyed patients would choose to have ultrasound screening in the future.
Conclusions:
The natural history of thromboembolism in plastic surgery outpatients differs from orthopedic patients. The risk of a deep venous thrombosis in a patient treated with Spontaneous breathing, Avoid gas, Face up, Extremities mobile anesthesia is approximately 0.5%. Thromboses are unlikely to develop intraoperatively. In the single affected patient, the thrombosis was located distally, in a location that is less prone to embolism and highly susceptible to anticoagulation. Ultrasound screening is an effective and highly feasible method to identify affected patients for treatment.
Deep venous thrombosis is a serious surgical complication that can lead to fatal pulmonary emboli.1 The author recently reported the feasibility of Doppler ultrasound screening in plastic surgery outpatients.2
Clinical diagnosis of venous thromboembolism is known to be unreliable.3–10 A clinical diagnosis is confirmed by objective testing using ultrasound or venography in only about 20–35% of patients,4,5,7,10 making objective confirmation mandatory.4 Noninvasive ultrasound technology has replaced venography as the standard for screening.9 When compression ultrasound is complemented by Doppler color flow evaluation (“duplex” sonography), the sensitivity for thrombosis detection is about 96%, with a high negative predictive value (99%).11
Only one large study12 [the Venous Thromboembolism Prevention (VTEP) study] compares the incidence of venous thromboembolism in plastic surgery inpatients treated with or without postoperative enoxaparin. The incidence of this complication was 1.2% in both groups.13 The VTEP study12 did not include screening examinations and did not provide information on the timing of deep venous thromboses.
Anticoagulation carries a risk of bleeding and hematomas.14,15 In an effort to improve safety and reduce risk, the author advocates a Spontaneous breathing, Avoid gas, Face up, Extremities mobile (SAFE) anesthesia method, foregoing individual risk stratification and chemoprophylaxis.13
Objective data are needed regarding the natural history of deep venous thrombosis in plastic surgery patients, so as to better inform patient management.13 The study hypothesis was that deep venous thromboses likely develop during surgery and that subclinical thromboses may go undetected and untreated.
PATIENTS AND METHODS
Doppler ultrasound screening was offered to all plastic surgery patients undergoing surgery performed by the author. The only inclusion requirement was patient consent. Institutional review board approval was obtained from Chesapeake Institutional Review Board Services, Inc. There was no charge or reimbursement for taking part in this clinical trial.16 To date, all patients have consented to take part in the study, making the inclusion rate 100%. One patient reported a venous thromboembolism after previous surgery, and another patient had a known factor V Leiden clotting disorder. Scans were scheduled before surgery, the day after surgery, and approximately 1 week (range, 6–10 days) after surgery. Patients were surveyed regarding their experience with ultrasound screening tests. A power analysis and sample size calculation were not performed because this study was not intended to evaluate a treatment effect.17 This study was undertaken to gain an understanding of the frequency and timing of deep venous thromboses in plastic surgery outpatients.
Ultrasound Scans
The Terason t3200 Ultrasound System Vascular series (Terason Ultrasound, Burlington, Mass.) was used to image the deep veins of both lower extremities, including the calf veins, at each visit. The imaged vessels included the common femoral, great saphenous, superficial femoral, deep femoral, popliteal, posterior tibial, and peroneal veins. Part-time sonographers, qualified to perform vascular ultrasound studies and also employed at local hospitals, were recruited to perform the studies in the plastic surgery clinic; no scans were performed by the author.
Surgery and Anesthesia
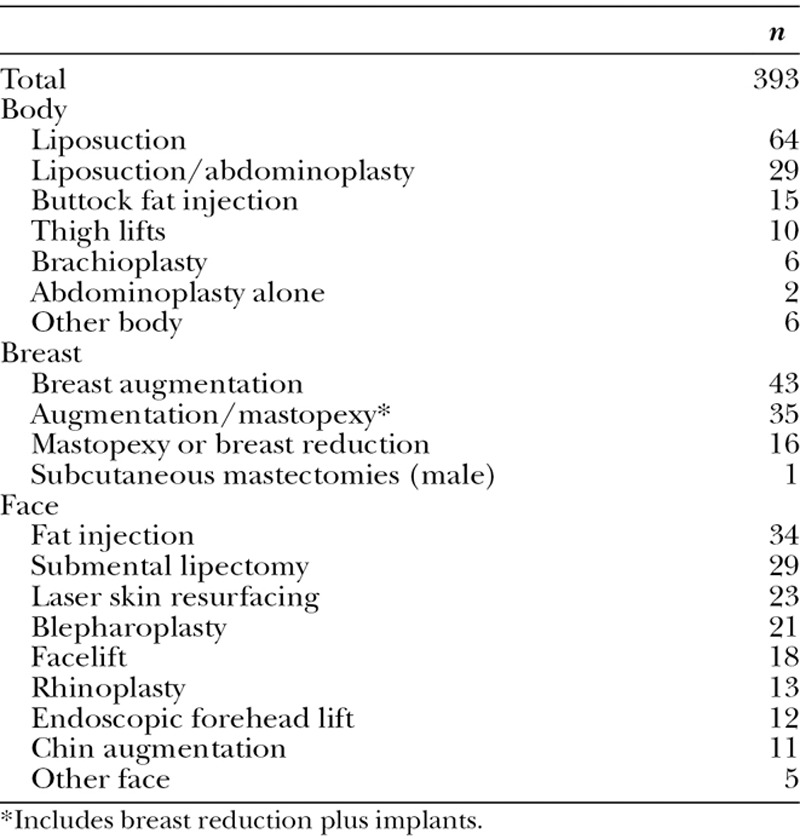
Two hundred patients underwent a total of 205 operations (Tables 1 and 2). Total intravenous anesthesia18 was administered to all patients. “SAFE” principles13 were observed, consisting of (1) Spontaneous breathing, (2) Avoid gas, (3) Face up, and (4) Extremities mobile. Sequential compression devices were used.
Table 1.
Patient Data
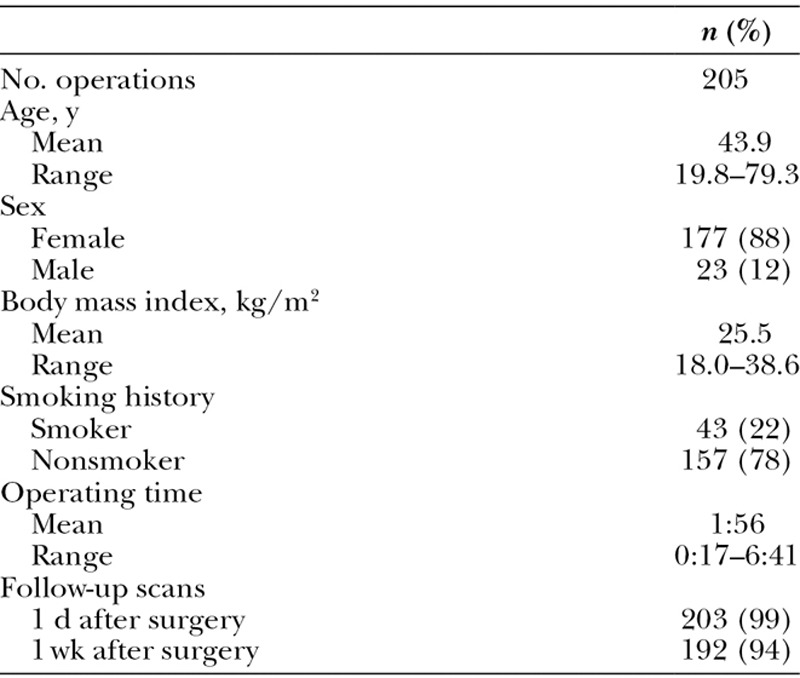
Table 2.
Procedures in 205 Consecutive Cases
All patients underwent surgery in a licensed ambulatory surgery center as outpatients and were ambulatory before leaving the recovery room. No patient was admitted to hospital or was immobilized postoperatively. Patients undergoing liposuction and abdominoplasty were positioned supine and then turned from side to side during the superwet infusion.18 The sequence was repeated for liposuction, ensuring mobility of the lower extremities.
RESULTS
The average patient age was 44 years and 88% of patients were women. There were no deaths, hospitalizations, or patients with symptoms or signs of pulmonary emboli. Only one screening examination revealed a deep venous thrombosis in a patient who underwent a lipoabdominoplasty and augmentation/mastopexy. The affected patient had no personal history of venous thromboembolism and no history of a clotting disorder. Her ultrasound scans before surgery and the day after surgery were negative. A thrombosis was detected in her left calf veins. The popliteal vein was not involved. At the time of her scan, she reported a discomfort in her left calf that she noticed just the day before, 7 days after surgery. She was not admitted. A consulting hematologist prescribed enoxaparin 80 mg subcutaneously b.i.d. for 3 days and (concurrently) rivaroxaban 15 mg p.o. b.i.d. for 2 weeks, followed by rivaroxaban 20 mg p.o. daily for 3 months. This was the only patient in the series to receive anticoagulation. Five weeks after surgery, there was no sonographic evidence of a thrombosis. She had the usual degree of swelling expected after liposuction of the thighs and knees.
Surveys
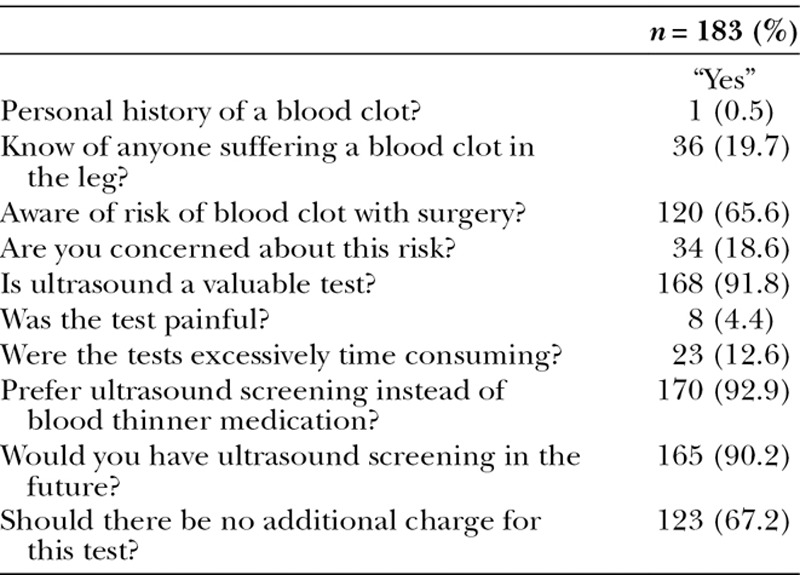
Only 8 patients (4.4%) reported discomfort and 165 patients (90%) would choose to undergo perioperative ultrasound screening examination in the future (Table 3).
Table 3.
Patient Surveys
DISCUSSION
SAFE anesthesia aims to reduce the risk of venous stasis.13 Venous stasis is the final common pathway that is believed to be responsible for causing deep venous thrombosis.13 The reduced risk of total intravenous anesthesia (ie, spontaneous breathing and no gas) as opposed to traditional general endotracheal anesthesia is supported by strong empirical evidence.13 Avoiding prone positioning eliminates pelvic pressure from a bolster that might impair venous return.14 Undue pressure on the face, the need for mechanical ventilation, and more difficult airway access are also avoided.13 Moving the extremities during surgery with supine and side-to-side positioning mimics the normal movement of patients when sleeping, avoiding prolonged immobility, which is a known risk factor for venous thromboembolism.13
Ultrasound Screening for Detection of Deep Venous Thrombosis
Today, venous ultrasound evaluation, including compression and color flow Doppler imaging, represents the standard for the diagnosis of deep venous thrombosis.19,20 Two different approaches are recommended: (1) serial compression ultrasonography of the proximal veins, based on the belief that thrombosis of the distal veins (ie, distal to the popliteal vein) are not dangerous unless they extend proximally, or (2) complete compression ultrasonography of the deep veins of the lower extremity, including the calves.20 Color-flow Doppler imaging improves the accuracy of ultrasonography of the calves.21 d-dimer assays and impedance plethysmography are not sufficiently sensitive for detecting distal thromboses.19,21 Whenever possible (marked calf swelling can interfere with calf evaluation),21 the proximal and distal veins should be examined.19
Some investigators consider compression testing of the proximal veins sufficient,4,5,22 saving time, and expense. These concerns may be misplaced. A complete ultrasound screening examination of both lower extremities, including the calf veins, takes about 20 minutes for an experienced sonographer.
Existing Knowledge
The risk of venous thromboembolism is related to the type of surgery performed.1 Much of the present knowledge base derives from studies of orthopedic patients.8,23,24 in whom the risk of deep venous thrombosis is as high as 60% in patients undergoing hip replacement.8 Dahl et al8 suggest that local vascular injury and both local and systemic activation of coagulation and suppression of fibrinolysis are responsible for the increased risk after hip replacement. Temporary interruption of blood flow is likely to induce venous stasis.25 Maynard et al23 report deep vein thrombosis in 47% of patients undergoing total knee arthroplasty, as detected by venography performed on the day of surgery or on the first postoperative day.
Venous Thromboembolism after Plastic Surgery
Unlike orthopedic surgery, little is known regarding the natural history of deep venous thrombosis occurring after plastic surgery.2 Lemaine et al17 used duplex sonography to evaluate 118 breast reconstruction inpatients (average operating time, 10.5 hours) who were treated postoperatively with low-molecular-weight heparin. Their patients17 were scanned before discharge from hospital, which took place on average 4.7 days after surgery. Four patients (3.4%) were identified with asymptomatic distal deep venous thromboses.17 Ironically, in the study by Lemaine et al,17 the scans were negative in the 9 patients clinically suspected of having a deep venous thrombosis, underscoring the unreliability of clinical examination. No patient developed a known symptomatic venous thromboembolism after discharge.17 The findings of the present study and the experience of Lemaine et al17 suggest that deep venous thromboses developing within the first week after surgery in plastic surgery patients tend to be limited to the calf veins.
Origin of Deep Venous Thrombosis
Virchow’s26 original triad implicates changes in blood flow, the state of the endothelium, and the composition of the blood. Severe hypoxia from prolonged venous stasis has been documented in the venous valvular sinuses of dogs in the absence of calf muscle-driven pulsatile blood flow.27 Pathologic studies suggest that thrombosis initiation also occurs in the valve sinus in humans.28,29 Impaired blood flow in the pocket of a valve and low oxygen tension are believed to precipitate activation of a coagulation cascade involving tissue factor, P-selectin, platelets, microparticles, monocytes, and granulocytes.25,29,30 Small thrombi forming within the valve pocket grow slowly over days or weeks.29,30 It is generally believed that most deep venous thromboses start within the calf.3,5,9,31 After forming in the calf, the thrombosis may extend proximally, where it is more likely to cause a pulmonary embolus.1,9,23,31,32 Proximal extension precedes embolization.32
The natural history of a deep venous thrombosis isolated to the calf is difficult to study because many patients receive anticoagulation.20 Patients with isolated calf thrombi are frequently asymptomatic.1,33 Distal thromboses represent approximately 11% of deep venous thromboses diagnosed in the community,34 but isolated distal thromboses are the prevalent finding in asymptomatic patients.20 The rate of pulmonary embolism occurring in association with thromboses limited to the calf is about 2%,34,35 and fatal emboli are rare.32,33 Palareti et al35 report that >90% of untreated distal thromboses monitored by serial compression ultrasound went on to complete resolution. By contrast, it is estimated that about 50% of patients with untreated proximal deep venous thrombosis will develop symptomatic pulmonary embolism within 3 months.1,31
A recent literature review33 reports an 8% rate of thrombus propagation to the popliteal vein in patients treated with surveillance only. Two forms of treatment are recognized, either anticoagulation or imaging surveillance with selective anticoagulation.33,36 The 2012 American College of Chest Physicians guidelines36 allow for surveillance using ultrasound with no anticoagulation in a patient with a postsurgical distal venous thrombosis, mild symptoms, and no other risk factors. There is no widely accepted protocol for surveillance ultrasound testing.36 A recent multicenter study37 reports no propagation of distal deep venous thromboses and no adverse events in 110 patients treated with nadroparin and compression therapy and monitored with serial duplex scanning. A meta-analysis38 reveals a significantly lower incidence of thrombus propagation and pulmonary embolism in patients with a distal venous thrombosis who received anticoagulation.
Duration of Anticoagulation
Patients who develop a deep venous thrombosis after surgery (a “transient” risk factor) are less likely to experience recurrences than patients with idiopathic thromboembolism or persistent risk factors such as malignancy or prolonged immobilization.39–43 For these low-risk patients, some investigators recommend 4 weeks of anticoagulation rather than the traditional 3 months.39–41
Bleeding
Many investigators emphasize the need to balance the risk of thrombosis with bleeding,20,33,41 which is a complication of anticoagulation in numerous case series7,14,15,39,40,42 and one that is occasionally fatal.7 Anticoagulation should not be used when the benefit does not clearly compensate for the additional risk.13,44 The annual risk of a venous thromboembolism in adults in industrialized countries is about 0.1–0.3%.25,30 The risk of major bleeding from anticoagulation is about 3% annually.42,45 Recently, plaintiff’s attorneys in the United States have started advertising their services for patients who develop bleeding while being treated with the new oral anticoagulant, rivaroxaban.46
Timing of Scans
In a study using venograms23 to screen consecutive patients for the presence of a deep venous thrombosis, 86% of eventually positive limbs were already positive within 1 day after surgery. However, this study evaluated patients receiving total knee replacements, who are exposed to local conditions that increase risk,8,23 such as vessel injury and hypercoagulability.
The absence of positive findings on scans performed on the day after surgery (0/203 scans) in the present study suggests that (1) the development of venous thromboses in plastic surgery patients differs from orthopedic patients undergoing joint replacement, who are more likely to develop thrombi intraoperatively, and (2) clinical adjustments to reduce the risk of venous stasis13 may be effective. Evidence from the present study suggests that 1-week postoperative scans are sufficient for plastic surgery outpatients. No patient developed a known venous thromboembolism more than 1 week after surgery. For patients in whom a thrombosis is detected, or with ongoing risk factors (eg, immobilization or a cancer diagnosis), subsequent scans may be indicated.
Number Needed to Screen
The number needed to screen47 is the number of people who need to be screened for a given duration to prevent 1 death or adverse event. If a calf vein thrombosis causes a pulmonary embolus in 2% of patients,34,35 screening followed by selective anticoagulation is effective (and the evidence suggests it is),37 and the prevalence of a distal thrombosis is 0.5% (as found in the present study), the number of patients needed to screen to avoid 1 case of pulmonary embolism is 10,000. However, if the rate of venous thromboembolism is 5% among high-risk patients,14 the number needed to screen to avoid 1 case of pulmonary embolism drops to 1000 and may drop lower if more dangerous proximal thromboses are more prevalent among these patients (this information is presently unavailable).
Individual risk stratification is presently recommended as part of thromboembolism prevention.12,48 However, it is of limited value in identifying affected individuals.13,17 As a screening method, risk stratification has a sensitivity of only 52% and a false-positive rate of 97%.13
Chemoprophylaxis
There is no evidence that anticoagulant medication prevents venous thromboses from developing in plastic surgery patients.13 Anticoagulation does not affect the factors comprising Virchow’s26 triad. Venous thromboembolism still occurs in anticoagulated patients.8,12–14,23,33,38 Its value is in preventing further thrombus deposition30 and in facilitating spontaneous lysis1 that may already be underway.35
Is There a Role for Testing for Clotting Disorders?
There are 6 moderately strong genetic risk factors for venous thromboembolism.25 Three are rare (combined prevalence, <1%)45,49 heterozygous deficiencies of the natural anticoagulants, antithrombin, protein C, and protein S.25 Venous thrombotic risk may be increased up to 10-fold in these deficiency states.45,49 The other 3 genetic factors are factor V Leiden (3- to 5-fold increase), prothrombin G20201A (2- to 3-fold increase), and blood group non-O (2-fold increase).25,45,49 Approximately 5% of people of mixed European descent carry factor V Leiden.25,30,45,49–51 Patients with factor V Leiden or prothrombin G20201A do not have a significantly increased risk of a recurrent venous thromboembolism.1,42 The majority of carriers never develop a thrombosis.49,50 A multicenter study50 evaluating factor V Leiden and prothrombin G20210A mutations as risk factors for patients undergoing total hip and knee replacement surgery concludes that preoperative genotyping is of questionable value. Joseph et al51 recommend against routine preoperative blood screening for a potential hypercoagulable state. Any additional risk is likely to be small in comparison with the risk of surgery itself.45,51
A history of a previous venous thromboembolism is not a significant risk factor for patients undergoing lower limb arthroplasty.51 Individuals with a family history of thrombosis affecting a first-degree relative have a 2- to 3-fold increased risk of venous thromboembolism.45 A high plasma level of factor VIII may increase risk 5-fold, but a genetic basis has not been identified.45 High levels of prothrombin, factor IX, and factor XI impart only a 2-fold increase in thrombotic risk.45 Measurement of clotting factor levels is not routinely included in a thrombophilia evaluation.45 Moreover, the assays are not standardized and threshold values for identifying high-risk patients vary considerably.45 There is no evidence that identification of thrombophilia in asymptomatic patients reduces the risk of venous thromboembolism.45
By comparison, aging (the strongest risk factor for venous thromboembolism)29,45,51 raises the risk of venous thrombosis exponentially from an annual incidence of 0.01% in people under 45 years old to 0.9% by 80 years of age, a difference of 90-fold.29,52 Changes in compliance of the vein wall and thickening of the valve leaflets may disrupt the normal flow of blood during the valvular cycle.29 Several large series53–56 of plastic surgery outpatients that are statistically likely to contain numerous individuals with inherited thrombophilias report no cases of a known postoperative venous thromboembolism.
Financial Commitment
The cost of the system used by the author was approximately $30,000, including a 5-year warranty, or $6000 per year. The cost of employing part-time sonographers over the course of a year is about $20,000, which is similar to the cost of a single hospitalization for treatment of deep venous thrombosis.57 Such an effective “early warning system” compares favorably to the cost of many other plastic surgery devices in the marketplace. Any plastic surgeon who has encountered a patient death from a pulmonary embolism understands the enormity of this complication and is unlikely to find the cost prohibitive. Other useful clinical applications include diagnosing and treating seromas and preoperative abdominal imaging to identify abdominal wall defects in liposuction and abdominoplasty patients.58 The scans provide increased opportunity for patient interaction with the office staff.
Underscoring Our Commitment to Safety
Patients understand that their surgeon is genuinely concerned about their safety and willing to take the extra steps needed to reduce the risk of venous thromboembolism. Such a safety measure is likely to reduce medicolegal liability.
Limitations of the Study
Although 200 patients (600 scans) is a substantial volume, it is still a small number for investigation of a complication that occurs in <1% of plastic surgery outpatients.53–56 Hopefully, other investigators will adopt this noninvasive measure in their practices so as to gather more experience and data. The study findings should not be extrapolated to plastic surgery inpatients,12 patients with cancer, traumatized patients, or patients subjected to long operations (eg, microsurgical reconstructions),45 factors that are known to increase risk. There is no suggestion that ultrasound screening is part of the standard of practice. This method is not presently included in published practice guidelines.48
Strengths of the Study
This study provides needed data regarding the natural history and prevalence of this complication among plastic surgery outpatients. The findings support ultrasound surveillance and selective anticoagulation in affected patients, consistent with the time-honored medical practice of performing tests, making a diagnosis, and then recommending treatment based on the findings.
CONCLUSIONS
The natural history of venous thromboembolism in plastic surgery outpatients differs from the experience of orthopedic patients undergoing joint replacement. Thromboses are unlikely to develop intraoperatively. The risk of a deep venous thrombosis in plastic surgery outpatients treated with SAFE anesthesia is approximately 0.5%. Ultrasound screening is an effective and feasible method to identify affected patients for treatment.
ACKNOWLEDGMENTS
The author thanks Lindsey Kroenke, BSN, and Sarah Maxwell, RN, for data collection.
Footnotes
Disclosure: The author has no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the author.
REFERENCES
- 1.Kearon C. Natural history of venous thromboembolism. Circulation. 2003;107(23 Suppl 1):I22–I30. doi: 10.1161/01.CIR.0000078464.82671.78. [DOI] [PubMed] [Google Scholar]
- 2.Swanson E. Doppler ultrasound imaging for DVT detection in plastic surgery. Presented at Meeting of the American Society for Aesthetic Plastic Surgery; April 24–29, 2014; San Francisco, Calif.. [Google Scholar]
- 3.Nicolaides AN, Kakkar VV, Field ES, et al. The origin of deep vein thrombosis: a venographic study. Br J Radiol. 1971;44:653–663. doi: 10.1259/0007-1285-44-525-653. [DOI] [PubMed] [Google Scholar]
- 4.Heijboer H, Büller HR, Lensing AW, et al. A comparison of real-time compression ultrasonography with impedance plethysmography for the diagnosis of deep-vein thrombosis in symptomatic outpatients. N Engl J Med. 1993;329:1365–1369. doi: 10.1056/NEJM199311043291901. [DOI] [PubMed] [Google Scholar]
- 5.Cogo A, Lensing AW, Prandoni P, et al. Distribution of thrombosis in patients with symptomatic deep vein thrombosis. Implications for simplifying the diagnostic process with compression ultrasound. Arch Intern Med. 1993;153:2777–2780. [PubMed] [Google Scholar]
- 6.Wells PS, Hirsh J, Anderson DR, et al. Accuracy of clinical assessment of deep-vein thrombosis. Lancet. 1995;345:1326–1330. doi: 10.1016/s0140-6736(95)92535-x. [DOI] [PubMed] [Google Scholar]
- 7.Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125:1–7. doi: 10.7326/0003-4819-125-1-199607010-00001. [DOI] [PubMed] [Google Scholar]
- 8.Dahl OE, Andreassen G, Aspelin T, et al. Prolonged thromboprophylaxis following hip replacement surgery–results of a double-blind, prospective, randomised, placebo-controlled study with dalteparin (Fragmin) Thromb Haemost. 1997;77:26–31. [PubMed] [Google Scholar]
- 9.Kearon C, Julian JA, Newman TE, et al. Noninvasive diagnosis of deep venous thrombosis. McMaster Diagnostic Imaging Practice Guidelines Initiative. Ann Intern Med. 1998;128:663–677. doi: 10.7326/0003-4819-128-8-199804150-00011. [DOI] [PubMed] [Google Scholar]
- 10.Elias A, Mallard L, Elias M, et al. A single complete ultrasound investigation of the venous network for the diagnostic management of patients with a clinically suspected first episode of deep venous thrombosis of the lower limbs. Thromb Haemost. 2003;89:221–227. [PubMed] [Google Scholar]
- 11.Lapidus L, de Bri E, Ponzer S, et al. High sensitivity with color duplex sonography in thrombosis screening after ankle fracture surgery. J Thromb Haemost. 2006;4:807–812. doi: 10.1111/j.1538-7836.2006.01832.x. [DOI] [PubMed] [Google Scholar]
- 12.Pannucci CJ, Dreszer G, Wachtman CF, et al. Postoperative enoxaparin prevents symptomatic venous thromboembolism in high-risk plastic surgery patients. Plast Reconstr Surg. 2011;128:1093–1103. doi: 10.1097/PRS.0b013e31822b6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swanson E. The case against chemoprophylaxis for venous thromboembolism prevention and the rationale for SAFE anesthesia. Plast Reconstr Surg Glob Open. 2014;2:e160. doi: 10.1097/GOX.0000000000000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hatef DA, Kenkel JM, Nguyen MQ, et al. Thromboembolic risk assessment and the efficacy of enoxaparin prophylaxis in excisional body contouring surgery. Plast Reconstr Surg. 2008;122:269–279. doi: 10.1097/PRS.0b013e3181773d4a. [DOI] [PubMed] [Google Scholar]
- 15.Dini GM, Ferreira MC, Albuquerque LG, et al. How safe is thromboprophylaxis in abdominoplasty? Plast Reconstr Surg. 2012;130:851e–857e. doi: 10.1097/PRS.0b013e31826d9fc0. [DOI] [PubMed] [Google Scholar]
- 16.Doppler Ultrasound Imaging of Plastic Surgery Patients for DVT Detection. Available at: http://clinicaltrials.gov/ct2/show/NCT02123550?term=Doppler+ultrasound+plastic+surgery+Swanson&rank=1. Accessed October 28, 2014. [Google Scholar]
- 17.Lemaine V, McCarthy C, Kaplan K, et al. Venous thromboembolism following microsurgical breast reconstruction: an objective analysis in 225 consecutive patients using low-molecular-weight heparin prophylaxis. Plast Reconstr Surg. 2011;127:1399–1406. doi: 10.1097/PRS.0b013e318208d025. [DOI] [PubMed] [Google Scholar]
- 18.Swanson E. Prospective study of lidocaine, bupivacaine and epinephrine levels and blood loss in patients undergoing liposuction and abdominoplasty. Plast Reconstr Surg. 2012;130:702–722; discussion 723–725. doi: 10.1097/PRS.0b013e31825dc408. [DOI] [PubMed] [Google Scholar]
- 19.Zierler BK. Diagnosis of venous thromboembolism. Circulation. 2004;109:I9–I14. doi: 10.1161/01.CIR.0000122870.22669.4a. [DOI] [PubMed] [Google Scholar]
- 20.Palareti G. How I treat isolated distal deep vein thrombosis (IDDVT). Blood. 2014;123:1802–1809. doi: 10.1182/blood-2013-10-512616. [DOI] [PubMed] [Google Scholar]
- 21.Rose SC, Zwiebel WJ, Nelson BD, et al. Symptomatic lower extremity deep venous thrombosis: accuracy, limitations, and role of color duplex flow imaging in diagnosis. Radiology. 1990;175:639–644. doi: 10.1148/radiology.175.3.2188293. [DOI] [PubMed] [Google Scholar]
- 22.Birdwell BG, Raskob GE, Whitsett TL, et al. The clinical validity of normal compression ultrasonography in outpatients suspected of having deep venous thrombosis. Ann Intern Med. 1998;128:1–7. doi: 10.7326/0003-4819-128-1-199801010-00001. [DOI] [PubMed] [Google Scholar]
- 23.Maynard MJ, Sculco TP, Ghelman B. Progression and regression of deep vein thrombosis after total knee arthroplasty. Clin Orthop Related Res. 1991;273:125–130. [PubMed] [Google Scholar]
- 24.Planes A, Vochelle N, Darmon JY, et al. Risk of deep-venous thrombosis after hospital discharge in patients having undergone total hip replacement: double-blind randomised comparison of enoxaparin versus placebo. Lancet. 1996;348:224–228. doi: 10.1016/s0140-6736(96)01453-5. [DOI] [PubMed] [Google Scholar]
- 25.Reitsma PH, Versteeg HH, Middeldorp S. Mechanistic view of risk factors for venous thromboembolism. Arterioscler Thromb Vasc Biol. 2012;32:563–568. doi: 10.1161/ATVBAHA.111.242818. [DOI] [PubMed] [Google Scholar]
- 26.Virchow R. In: Gesammelte Abhandlungen zur wissenschaftlichen Medicin. Frankfurt am Main: Von Meidinger & Sohn; 1856. Thrombose und Embolie. Gefässentzündung und septische Infektion. pp. 219–732. [Google Scholar]
- 27.Hamer JD, Malone PC, Silver IA. The PO2 in venous valve pockets: its possible bearing on thrombogenesis. Br J Surg. 1981;68:166–170. doi: 10.1002/bjs.1800680308. [DOI] [PubMed] [Google Scholar]
- 28.Sevitt S. The structure and growth of valve-pocket thrombi in femoral veins. J Clin Pathol. 1974;27:517–528. doi: 10.1136/jcp.27.7.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bovill EG, van der Vliet A. Venous valvular stasis-associated hypoxia and thrombosis: what is the link? Annu Rev Physiol. 2011;73:527–545. doi: 10.1146/annurev-physiol-012110-142305. [DOI] [PubMed] [Google Scholar]
- 30.Mackman N. New insights into the mechanisms of venous thrombosis. J Clin Invest. 2012;122:2331–2336. doi: 10.1172/JCI60229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kakkar VV, Howe CT, Flanc C, et al. Natural history of postoperative deep-vein thrombosis. Lancet. 1969;2:230–232. doi: 10.1016/s0140-6736(69)90002-6. [DOI] [PubMed] [Google Scholar]
- 32.Philbrick JT, Becker DM. Calf deep venous thrombosis. A wolf in sheep’s clothing? Arch Intern Med. 1988;148:2131–2138. [PubMed] [Google Scholar]
- 33.Masuda EM, Kistner RL, Musikasinthorn C, et al. The controversy of managing calf vein thrombosis. J Vasc Surg. 2012;55:550–561. doi: 10.1016/j.jvs.2011.05.092. [DOI] [PubMed] [Google Scholar]
- 34.Spencer FA, Kroll A, Lessard D, et al. Isolated calf deep vein thrombosis in the community setting: the Worcester Venous Thromboembolism study. J Thromb Thrombolysis. 2012;33:211–217. doi: 10.1007/s11239-011-0670-x. [DOI] [PubMed] [Google Scholar]
- 35.Palareti G, Cosmi B, Lessiani G, et al. Evolution of untreated calf deep-vein thrombosis in high risk symptomatic outpatients: the blind, prospective CALTHRO study. Thromb Haemost. 2010;104:1063–1070. doi: 10.1160/TH10-06-0351. [DOI] [PubMed] [Google Scholar]
- 36.Kearon C, Akl EA, Comerota AJ, et al. American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e419S–e494S. doi: 10.1378/chest.11-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guarnera G, Abeni D, Antignani PL, et al. News on distal deep venous thrombosis. Reports of a multicenter study. Int Angiol. 2014;33:560–564. [PubMed] [Google Scholar]
- 38.De Martino RR, Wallaert JB, Rossi AP, et al. A meta-analysis of anticoagulation for calf deep venous thrombosis. J Vasc Surg. 2012;56:228–237.e1. doi: 10.1016/j.jvs.2011.09.087. [DOI] [PubMed] [Google Scholar]
- 39.Research Committee of the British Thoracic Society. Optimum duration of anticoagulation for deep-vein thrombosis and pulmonary embolism. Lancet. 1992;340:873–876. [PubMed] [Google Scholar]
- 40.Pini M, Aiello S, Manotti C, et al. Low molecular weight heparin versus warfarin in the prevention of recurrences after deep vein thrombosis. Thromb Haemost. 1994;72:191–197. [PubMed] [Google Scholar]
- 41.Levine MN, Hirsh J, Gent M, et al. Optimal duration of oral anticoagulant therapy: a randomized trial comparing four weeks with three months of warfarin in patients with proximal deep vein thrombosis. Thromb Haemost. 1995;74:606–611. [PubMed] [Google Scholar]
- 42.Kearon C, Gent M, Hirsh J, et al. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N Engl J Med. 1999;340:901–907. doi: 10.1056/NEJM199903253401201. [DOI] [PubMed] [Google Scholar]
- 43.Pinede L, Duhaut P, Cucherat M, et al. Comparison of long versus short duration of anticoagulant therapy after a first episode of venous thromboembolism: a meta-analysis of randomized, controlled trials. J Intern Med. 2000;247:553–562. doi: 10.1046/j.1365-2796.2000.00631.x. [DOI] [PubMed] [Google Scholar]
- 44.Swanson E. Chemoprophylaxis for venous thromboembolism prevention: concerns regarding efficacy and ethics. Plast Reconstr Surg Glob Open. 2013;1:e23. doi: 10.1097/GOX.0b013e318299fa26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Varga EA, Kujovich JL. Management of inherited thrombophilia: guide for genetics professionals. Clin Genet. 2012;81:7–17. doi: 10.1111/j.1399-0004.2011.01746.x. [DOI] [PubMed] [Google Scholar]
- 46.The Fox Law Firm TV Commercial, ‘Xarelto.’. Available at: http://www.ispot.tv/ad/7Cx6/the-fox-law-firm-xarelto. Accessed November 13, 2014. [Google Scholar]
- 47.Rembold CM. Number needed to screen: development of a statistic for disease screening. BMJ. 1998;317:307–312. doi: 10.1136/bmj.317.7154.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murphy RX, Jr, Alderman A, Gutowski K, et al. Evidence-based practices for thromboembolism prevention: summary of the ASPS Venous Thromboembolism Task Force Report. Plast Reconstr Surg. 2012;130:168e–175e. doi: 10.1097/PRS.0b013e318254b4ee. [DOI] [PubMed] [Google Scholar]
- 49.Rosendaal FR, Reitsma PH. Genetics of venous thrombosis. J Thromb Haemost. 2009;7(Suppl 1):301–304. doi: 10.1111/j.1538-7836.2009.03394.x. [DOI] [PubMed] [Google Scholar]
- 50.Wåhlander K, Larson G, Lindahl TL, et al. Factor V Leiden (G1691A) and prothrombin gene G20210A mutations as potential risk factors for venous thromboembolism after total hip or total knee replacement surgery. Thromb Haemost. 2002;87:580–585. [PubMed] [Google Scholar]
- 51.Joseph JE, Low J, Courtenay B, et al. A single-centre prospective study of clinical and haemostatic risk factors for venous thromboembolism following lower limb arthroplasty. Br J Haematol. 2005;129:87–92. doi: 10.1111/j.1365-2141.2005.05419.x. [DOI] [PubMed] [Google Scholar]
- 52.Silverstein RL, Bauer KA, Cushman M, et al. Venous thrombosis in the elderly: more questions than answers. Blood. 2007;110:3097–3101. doi: 10.1182/blood-2007-06-096545. [DOI] [PubMed] [Google Scholar]
- 53.Stuzin JM, Baker TJ, Baker TM. Deep venous thrombosis and pulmonary embolus after face lift: a study of incidence and prophylaxis (Discussion). Plast Reconstr Surg. 2001;(107):1576–1577. doi: 10.1097/00006534-200105000-00044. [DOI] [PubMed] [Google Scholar]
- 54.Bitar G, Mullis W, Jacobs W, et al. Safety and efficacy of office-based surgery with monitored anesthesia care/sedation in 4778 consecutive plastic surgery procedures. Plast Reconstr Surg. 2003;111:150–156; discussion 157. doi: 10.1097/01.PRS.0000037756.88297.BC. [DOI] [PubMed] [Google Scholar]
- 55.Ersek RA. Dissociative anesthesia for safety’s sake: ketamine and diazepam–a 35-year personal experience. Plast Reconstr Surg. 2004;113:1955–1959. doi: 10.1097/01.prs.0000122402.52595.10. [DOI] [PubMed] [Google Scholar]
- 56.Mustoe TA, Buck DW, II, Lalonde DH. The safe management of anesthesia, sedation, and pain in plastic surgery. Plast Reconstr Surg. 2010;126:165e–176e. doi: 10.1097/PRS.0b013e3181ebe5e9. [DOI] [PubMed] [Google Scholar]
- 57.Elting LS, Escalante CP, Cooksley C, et al. Outcomes and cost of deep venous thrombosis among patients with cancer. Arch Intern Med. 2004;164:1653–1661. doi: 10.1001/archinte.164.15.1653. [DOI] [PubMed] [Google Scholar]
- 58.Marins JRB. Ultrasound in lipoabdominoplasty, 3rd edition. In: Saldanha O, editor. In: Lipoabdominoplasty. Boca Raton: CRC Press; 2006. pp. 153–157. [Google Scholar]


