SUMMARY
The hypothalamo-neurohypophyseal system (HNS) is the neurovascular structure through which the hypothalamic neuropeptides oxytocin and arginine-vasopressin exit the brain into the bloodstream, where they go on to affect peripheral physiology. Here, we investigate the molecular cues that regulate the neurovascular contact between hypothalamic axons and neurohypophyseal capillaries of the zebrafish. We developed a transgenic system in which both hypothalamic axons and neurohypophyseal vasculature can be analyzed in vivo. We identified the cellular organization of the zebrafish HNS as well as the dynamic processes that contribute to formation of the HNS neurovascular interface. We show that formation of this interface is regulated during development by local release of oxytocin, which affects endothelial morphogenesis. This cell communication process is essential for the establishment of a tight axovasal interface between the neurons and blood vessels of the HNS. We present a unique example of axons affecting endothelial morphogenesis through secretion of a neuropeptide.
INTRODUCTION
The neuroendocrine system is composed of neurosecretory brain cells that transfer hormones into the bloodstream to influence the function of target cells throughout the body. The hypothalamo-neurohypophyseal system (HNS) is a major neuroendocrine conduit through which the brain controls peripheral physiology (Burbach et al., 2001). The anatomy and activities of the HNS are conserved in all vertebrates. Ramon Cajal was the first to provide a description of the nerve fibers that connect the hypothalamus with the posterior pituitary (Cajal, 1911). It has since been established that these hypothalamic neurons themselves secrete neurohormones directly into the blood circulation, a finding that arose from experiments in both fish and mammalian models (Bargmann, 1949; Harris, 1948b; Scharrer, 1928). The hypothalamic neuropeptides arginine-vasopressin (AVP) and oxytocin (OXT) are synthesized in massive magnocellular neurons in the hypothalamus, transported along axons all the way down to the neurohypophysis, where they are secreted (Brownstein et al., 1980). Within the neurohypophysis, AVP and OXT are released from axons of the supraopticohypophyseal tract into fenestrated capillaries, thus leaving the brain and entering the general circulation without disrupting the blood-brain barrier (Burbach et al., 2001).
In the general circulation, secreted AVP regulates water homeostasis by increasing water permeability of the collecting duct of the kidney, and oxytocin regulates labor and milk let down by causing the respective contraction of the smooth muscle of the uterus and of the myoepithelial cells of breast ducts (for review, see Burbach et al., 2001; Gimpl and Fahrenholz, 2001; Verbalis, 2007). These physiological activities are conserved: in teleost fish, the AVP-like neuropeptide (Avpl) (a.k.a. arginine-vasotocin) regulates water balance by affecting filtration in the kidney (Amer and Brown, 1995; Macfarlane and Maetz, 1974; Peter and Fryer, 1983) and oxytocin-like neuropeptide (Oxtl) (a.k.a. isotocin) regulates contraction of smooth muscles in the ovary and oviduct during parturition or oviposition of live bearing and egg laying fish (La Pointe, 1977; Peter and Fryer, 1983). Avpl and Oxtl also regulate blood pressure in the ventral aorta (Chan, 1977; Kulczykowska, 1998; Le Mevel et al., 1993; Peter and Fryer, 1983). The HNS is therefore a central point of interface between the hormonal, neuronal, and vascular systems common to all vertebrate species.
The neurohypophysis is an elaborate three-dimensional structure, which substantially complicates the interpretation of cellular interactions and dynamics based solely on tissue sections. Although the anatomical structures of the neurohypophyseal axons and blood vessels have been the focus of intense study for over a century (Bargmann, 1949; Fink and Smith, 1971; Harris, 1948a; Scharrer, 1928), little progress has been made in uncovering the molecular and cellular processes that underlie formation of the interface between hypothalamic axons and hypophyseal vasculature. Here, we present a unique transgenic approach in which both zebrafish oxytocinergic axonal termini and vascular endothelia cells within the neurohypophysis are labeled. We use this system to analyze the morphogenesis of the neurohypophyseal axonal trajectories and neuroendocrine vasculature in live zebrafish embryos and demonstrate that neurohypophyseal angiogenesis is regulated by the developing hypothalamo-neurohypophyseal nerve termini. Our results suggest that local secretion of oxytocin into the neurohypophysis is an intrinsic developmental event essential for the formation of the hypophyseal neurovascular connection.
RESULTS
Cellular Organization of the Zebrafish HNS
The optically transparent zebrafish embryo offers a unique tool to study the ontogenesis of the HNS in vivo without the need for surgical intervention, although the formation and structure of the zebrafish neurohypophysis is, to date, still poorly characterized. We recently identified the genomic regulatory region of the zebrafish oxtl gene and have generated a transgenic reporter line, oxtl:EGFP, in which zebrafish oxytocinergic cell bodies in the hypothalamus are labeled (Blechman et al., 2011). We first examined whether this line can be used to track axonal projections from the hypothalamus to the neurohypophysis. Three-dimensional reconstitution of optically-sectioned 9-day-old oxtl:EGFP larvae revealed extensive EGFP-positive axonal trajectories of oxytocinergic neurons throughout the nervous system (Figures 1A and 1B; see Movie S1 available online). In particular, we observed a prominent oxtl:EGFP+ tract originating from the two hemispheres of the neurosecretory preoptic areas (NPO) converging into the midline and terminating at the ventral part of the hypothalamus at the presumed location of the pituitary (Figure 1B). Immunostaining of the oxtl:EGFP with an anti-oxytocin antibody confirmed that these ventral hypothalamic axonal termini colocalize with oxytocinergic-positive vesicles of the presumed neurohypophysis of embryonic and adult brains (Figures 1C and 1D and Figure S1). To further validate this observation we crossed the oxtl:EGFP line with another transgenic line in which the adenohypophyseal pituitary cell-type, prolactin (Prl) is labeled (Liu et al., 2003, 2006). Two-color imaging of this double-transgenic line revealed that the ventral hypothalamic oxytocinergic trajectories terminate near the Prlpositive adenohypophyseal cells (Figure 2A). This transgenic system provided us with a new tool to study the morphogenesis of the zebrafish preopticohypophyseal axonal tract analogous to the supraopticohypophyseal tract in mammals.
Figure 1. Oxytocinergic Reporter Transgene Tags HN Projections.
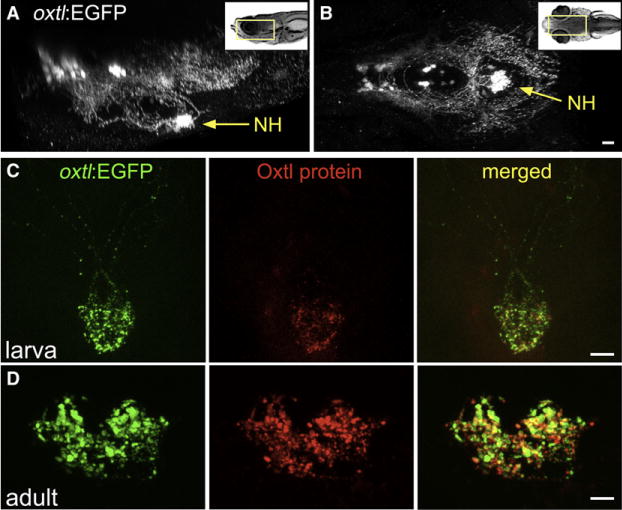
(A and B) 3D reconstruction of the hypothalamic-neurohypophseal system of an optically sectioned 9-day-old zebrafish embryo carrying the oxtl:EGFP transgene (anterior to the left; A, lateral view; B, ventral view). Axonal projections to the neurohypophysis (NH) are highly visible in oxtl:EGFP transgenic animals due to extensive arborization of NH nerve termini.
(C and D) Immunohistochemical analysis of Oxytocin protein in oxtl:EGFP transgenic zebrafish showing colocalization of EGFP+ and Oxytocin+ nerve termini in the neurohypophysis of either 6-day-old larva (C, anterior up) or coronally sectioned adult (D); 14-day-old).
Figure 2. Axovasal Interactions in the Zebrafish Pituitary.
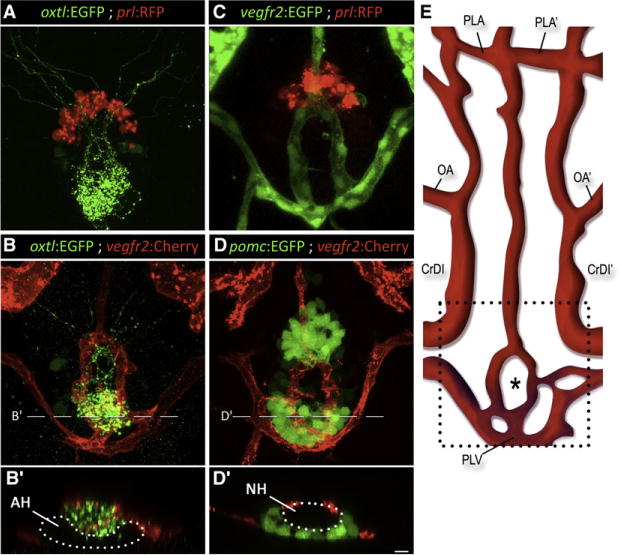
(A–D) Three-dimensional reconstructions of the hypophysis of 9-day-old zebrafish embryos carrying pairsof transgenic fluorescent markers of different hypophyseal components. (A) The oxtl:EGFP transgene (green) marks oxytocinergic axons terminating at the neurohypophysis, just dorsal and posterior to the adenohypophyseal prolactin-producing cells marked with prl:RFP (red).
(B) Double transgenic animals expressing oxtl:EGFP (green) together with the vascular endothelial marker vegfr2:Cherry (red) reveal a distinct, previously unidentified structure of the zebrafish hypophyseal vasculature. (C and D) Hypophyseal vasculature, visualized by either vegfr2:EGFP or vegfr2:Cherry, together with the adenohypophyseal markers prl:RFP and pomc:EGFP. The ring-like vascular structure resided dorsal to the adenohypophysis. (B′) and (D′) show optical Z-slices that demonstrate the position of the NH in an indentation of the adenohypophysis. (E) Schematic map of ventral head vasculature of the zebrafish larvae, including the location of the hypophyseal vasculature. The asterisk marks the location of the neurohypophysis.
AH, adenohypophysis; CrDI, cranial division of the internal carotid artery; NH, neurohypophysis; OA, optic artery; PLA, palatocerebral artery; PLV, palatocerebral vein; pomc, proopiomelanocortin; prl, prolactin; vegfr, vascular endothelial growth factor receptor. Scale bar, 20 μm. See also Movies S2–S4.
We next visualized the neurovascular interface within the neurohypophysis by crossing our HNS specific line with a vascular endothelial reporter expressing mCherry in vegfr2-positive cells (Chi et al., 2008; Jin et al., 2005). Previous work, based on microangiography, has provided an extensive map of the vascular network in the zebrafish head (Isogai et al., 2001). Using our axovasal double transgenic line, we were able to identify GFP+ oxytocinergic projections converging posteriorly and medially into the neurohypophysis, arborizing extensively and interfacing with a formation of blood vessels previously annotated as palatocerebral veins (Figures 2B and 2E and Movies S2 and S3). The hypothalamo-neurohypophyseal (HN) axonal termini project onto the posterior part of this loop-shaped vascular structure. To ascertain the position of this structure in relation to the adenohypophysis, we generated double transgenic lines with labeled blood vessels (vegfr2:EGFP or vegfr2:mCherry) and adenohypophyseal cell types (pomc:GFP or prl:RFP). Three-dimensional image reconstruction of the ventral diencephalon in these embryos revealed that these blood vessels engulf part of the pituitary area abutting the adenohypophysis (Figures 2C and 2D and Movie S4). The NH is positioned in a pocket-like indentation formed by the ventrally located adenohypophysis (Figures 2B′ and 2D′).
The vessels forming the hypophyseal vascular structure has been previously annotated as median palatocerebral vein (MPLV) and palatocerebral vein (PLV) (Isogai et al., 2001). As we now identify these vessels as the zebrafish hypophyseal vessels, we set out to reassess their arterial and venous identity. We performed live imaging of hypophyseal blood flow using time-lapse microscopy of a triple transgenic line with labeled erythrocytes (gata1:dsRed), blood vessels (vegfr2:EGFP) and adenohypophyseal cells for positional reference (prl:RFP). By following individual dsRed+ erythrocytes, we determined the direction of blood flow into and out of the hypophysis to the lateral venous sinuses (Figure 3A and Movie S5). This allowed us to deduce the identity of the hypophyseal artery and veins (Figure 3B and Figure S2).
Figure 3. 3D Structure of the Hypophyseal Neurovascular Interface.
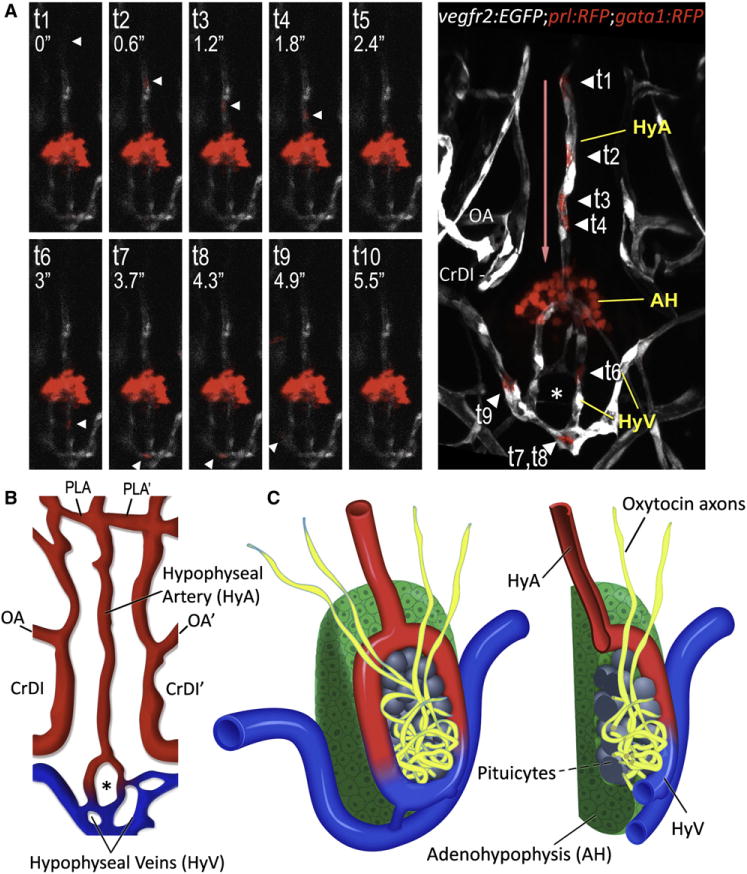
(A and B) Identification of hypophyseal arteries and veins by real-time analysis of hypophyseal blood flow. (A) Confocal time-lapsed images (t1–t10) of a single gata1+ blood cell (arrowhead) traveling from the hypophyseal artery into the neurohypophysis and its outflow through the hypophyseal veins. Superposition of all time points and the direction of blood flow (red arrow) are shown in the right panel. Adjacent Prl-RFP+ adenohypophyseal cells (arrow) served as a positional landmark.
(B) Schematic map of ventral head vasculature of the zebrafish larvae, including the newly annotated hypophyseal artery (red) and veins (blue). AH, adenohypophysis, CrDI, cranial division of the internal carotid artery; HyA, hypophyseal artery; HyV, hypophyseal veins; OA, optic artery; PLA, palatocerebral artery.
(C) Schematic representation of the 3D structure of the zebrafish pituitary depicting the various cellular components of the adeno- and neurohypophysis. A longitudinal section of this structure is shown on the right.
Taken together, our analyses identify the organization of the zebrafish neurovascular HNS structure in relation to the other pituitary cell populations (Figure 3C).
Morphogenesis of the Hypothalamo-Neurohypophyseal System
We set out to delineate the morphogenic events leading to the formation of axovasal contact between hypothalamic axons and neurohypophyseal blood capillaries. Although the expression of oxytocin is apparent from the Prim-15 stage (30–36 hr; Unger and Glasgow, 2003), we found that our oxtl:EGFP did not express sufficient levels of the EGFP protein to detect axonal expression at this embryonic stage (data not shown). To resolve this, we sought an earlier transgenic reporter that would allow detection of the initial stages of hypophyseal innervation. We previously reported that the homeodomain-containing protein Orthopedia (Otp) is expressed in hypothalamic neuronal progenitors, including the magnocellular oxytocinergic neurons of the NPO area (Blechman et al., 2007; Machluf et al., 2011). Moreover, we recently identified a genomic cis-regulatory region driving expression of the zebrafish otpb gene in the NPO (Fujimoto et al., 2011). We made use of this cis-regulatory element to generate a transgenic otpb:EGFP-caax line driving expression of a membrane-tagged EGFP in Otp+ cell bodies and axons, including hypothalamo-neurohypophyseal (HN) projections (Figures 4A–4C). We analyzed neurovascular formation at 36, 54, and 72 hr using an otpb:caax-EGFP;fli:dsRed double transgenic line. This analysis shows that HN axonal projections innervate the neurohypophysis before the vessels are formed and a tight neurovascular interface is established between 2 and 3 days of development (Figures 4D–4F).
Figure 4. Morphogenesis of the Neurohypophysis.
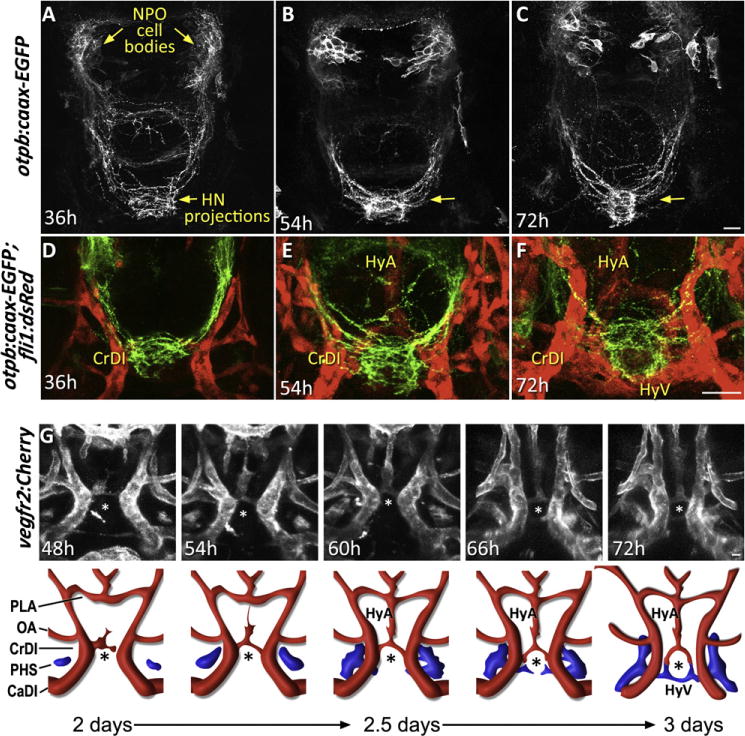
(A–C) Dynamics of hypophyseal innervation. Images of transgenic otpb:caax-EGFP embryos reveal that innervation of the hypophysis by neurons of the neurosecretory preoptic nucleus (NPO) occurs as early as 36 hr postfertilization and becomes well established by 54 hr. Scale bar, 20 μm.
(D–F) Dynamics of neurovascular interface. Images of double transgenic otpb:caax-EGFP;fli1:dsRed embryos at different developmental stages showing innervation of the neurohypophysis prior to hypophyseal vessel formation.
(G) Dynamics of hypophyseal vascularization. Time-lapsed (48–72 hr) confocal microscopy was used to track vascular endothelial cells in the ventral diencephalon of vegfr2:Cherry embryos. The upper panels display snapshots of critical stages in the formation of the hypophyseal loop-like structure. A schematic representation of the corresponding images with arterial (red) and venous (blue) color-coding is shown at the bottom.
CrDI, cranial division of the internal carotid artery; HyA, hypophyseal artery; HyV, hypophyseal veins; OA, optic artery; PHS, primary head sinus; PLA, palatocerebral artery. The asterisk (*) demarcates the location of the neurohypophysis. See also Movie S6.
A dynamic analysis of hypophyseal vascularization/angiogenesis has never been reported. We used time-lapse microscopy to track vascular endothelial cells in the ventral diencephalon of zebrafish embryos carrying the vegfr2:Cherry vascular reporter transgene in which a membrane-localized monomeric Cherry is expressed in endothelial precursors and mature vessels (Chi et al., 2008). The embryos were imaged from 2 days postfertilization (dpf), when the hypophyseal vascular structure is yet absent, through 3 dpf, at which time the vascular loop around the neurohypophysis is fully established. Three-dimensional reconstitution followed by cell tracking of such time-lapse movies (n = 4) showed that formation of the hypophyseal artery begins prior to that of the veins; the artery is initiated at the hypophysis itself and extends anteriorly until it connects with the palatocerebral arteries at day 2.5 (Figure 4G). As the primary lateral vein sinuses extend forward through the diencephalon, they sprout medially and wrap around the posterior edge of the neurohypophysis to form the hypophyseal veins. These fuse bilaterally with one another and with the hypophyseal arteries to form a ring around the neurohypophysis and connect it to the general circulation at approximately 3 days. (Figure 4G and Movie S6). High-resolution 3D rendering of an embryo fixed at this time point clearly shows that the hypophyseal veins connect bilaterally to the primary vein sinuses (Figure S2).
The above analyses of neurohypophyseal axons and blood vessels highlight key cellular events of HNS morphogenesis: (1) HN axons appears at the site of the prospective neurohypophysis as early as 24–36 hr after fertilization. (2) Endothelial cells forming the hypophyseal artery and veins appear at 2 days, approximately 24 hr after the axons start to innervate the neurohypophysis. (3) It takes another 24 hr for the endothelial cells of the artery and veins of the hypophysis to sprout and fuse to form the aforementioned loop-like structure, creating a tight interface with the neurohypophyseal axons.
Hypothalamic Neurons Are Necessary for Pituitary Vascular Organization
During its morphogenesis, the neurohypophysis becomes a point of intersection between axons and vessels. We next examined the interdependence of blood vessels for axon patterning in the neurohypophysis. The zebrafish mutant cloche (clo) has an overall deficiency of endothelial and hematopoietic lineages, including a complete lack of head vasculature (Stainier et al., 1995). We examined whether the neurohypophyseal axonal projections are affected in cloche mutants by comparing oxytocinergic-positive neurohypophyseal axonal termini in mutant and wild-type siblings. This analysis revealed no significant differences in oxytocinergic immune-reactive projections indicating that the presence of endothelial cells/vessels is not required for HN innervation (Figures 5A and 5B; n = 13/15). This result is consistent with the observation that axons begin to innervate the NH before the blood vessel network is established (Figures 4D–4F).
Figure 5. Abnormal Development of Pituitary Vasculature in the Absence of HN Innervation.
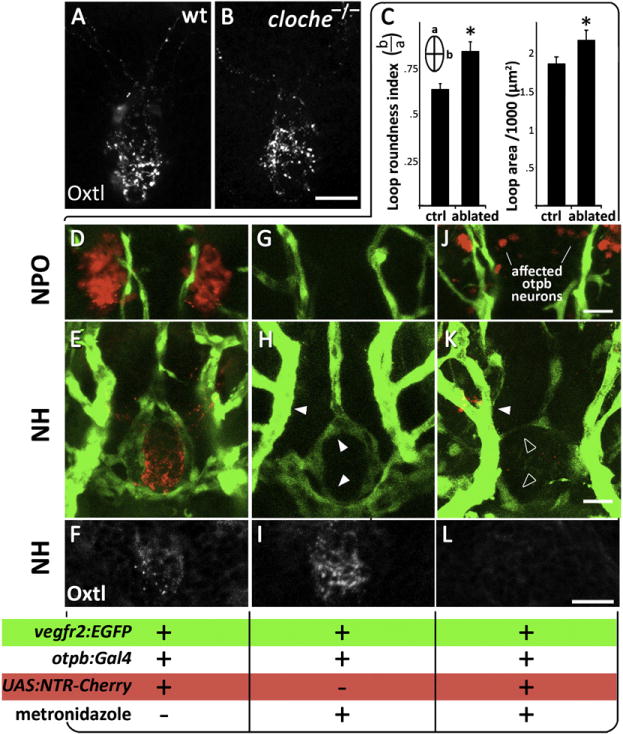
(A and B) Immunostaining of oxytocin (Oxtl) in wild-type (A) and the endothelial-deficient mutant cloche (−/−) (B) mutants, which lack all blood vessels.
(C–L) Genetic cell ablation of Otp+ neurons of the neurosecretory preoptic nucleus (NPO). (C) Quantitative analysis of the vascular phenotype caused by HN neuronal ablation (see text). Using the Gal4/UAS system (otpb:Gal4;UAS:NTR-Cherry) to drive expression of a Nitroreductase-Cherry (NTR-Cherry) fusion protein only in Otp+ neurons make these cells and their projections visible (red) and susceptible to the prodrug metronidazole. This transgenic fish was crossed to the vascular reporter line, vegfr2:EGFP, to generate a triple transgenic line (D–F and J–L). Double transgenic line (otpb:Gal4;vegfr2:EGFP, G–I) expressing vascular EGFP but not the NTR-Cherry protein served as a control for nonspecific drug toxicity. The presence of Cherry+ neurons (D, G, J), EGFP+ blood vessels (E, H, and K) and oxytocin immunoreactivity (F, I, and L) were monitored in three experimental conditions; the respective genotypes as well as the application of the metronidazole killing agent are indicated below. Filled and empty arrowheads indicate normal and abnormal blood vessels, respectively.
See also Figure S3.
We next examined whether the presence of HN neuronal tracts is important for morphogenesis of the neuroendocrine vasculature. We undertook a conditional HN cell ablation strategy using tissue-specific expression of the nitroreductase (NTR), an enzyme that converts the prodrug metronidazole into a cytotoxic agent (Curado et al., 2008; Davison et al., 2007). We chose to target Otp+ neurons as this cell population includes all NPO neurons that innervate the neurohypophysis (Figure 4 and Machluf et al., 2011). To this end, we generated a transgenic line, otpb:Gal4;UAS:NTR-Cherry, in which the NTR-Cherry fusion protein is expressed in Otp+ neurons using a tissue-specific Gal4 transgene to drive an NTR-Cherry that was placed under multiple Gal4 upstream activation sequences (Davison et al., 2007). We crossed the otpb:Gal4;UAS:NTR-Cherry fish with the vascular-specific vegfr2:EGFP line to generate triple transgenic embryos in which vascular development can be monitored in the presence or absence of Otp+ HN cells. The metronidazole drug was applied to embryos at 24–72 hr, from the stage at which the first HN neuronal tracts are detected and until the intact hypophyseal vascular structure is formed and contacts the axons. The efficiency of HN cell ablation was assessed by monitoring NTR-Cherry+ (Figures 5D and 5J) and oxtl+ neurons (Figures S3A and S3B) in control and metronidazole-treated triple transgenic (otpb:Gal4;UAS:NTR-Cherry;vegfr2:EGFP) embryos. To verify the deleterious effects of Otp+ cell ablation on HN neurosecretion, we measured the levels of the Oxytocin protein in the neurohypophysis by anti-oxytocin immunostaining and determined that Otp+ cell ablation causes a marked decrease in neurohypophyseal oxytocin levels (Figures 5F, 5I, and 5L and Figure S3C).
We then went on to analyze the head vasculature in metronidazole-treated vegfr2:EGFP embryos carrying the otpb:Gal4 and UAS:NTR-Cherry transgenes, as compared to siblings not carrying them. As expected, application of the metronidazole prodrug to embryos not carrying the UAS:NTR-Cherry transgene had no significant effect on hypophyseal or other blood vessels, indicating that in the absence of NTR-Cherry, metronidazole treatment is not toxic and does not affect vasculature (Figures 5G–5I, n = 15/15). In contrast, Otp+ cell ablation in metronidazole-treated triple transgenic (vegfr2:EGFP;otpb:Gal4; UAS:NTR-Cherry) embryos was accompanied by a marked impairment of hypophyseal vasculature, indicating that impaired HN neurosecretion is associated with hypophyseal vascular abnormality (Figures 5J–5L, n = 12/15). While the hypophyseal vasculature of normal embryos tightly surrounds the neurohypophysis forming an elliptical loop (Figures 5E and 5H), ablation of Otp+ neurons caused abnormalities characterized by: failure to close the neurohypophyseal vascular loop, incomplete connection of the hypophyseal artery to the PLA and overall thin hypophyseal vascular structures (Figure 5K). We used two measurable parameters to quantify changes to this morphology (Figure 5C): (1) “loop roundness index”– samples with ablated axons display a more round loop as opposed to the ellipsoid shaped loop in the control samples, and (2) “loop area”– axonal ablated embryos exhibit a significant increase in the loop area. In short, ablation of Otp+ neurons led a significantly rounder loop surrounding a larger area (n = 15, *p < 0.05). In all cases, all neighboring head vessels remained unaffected.
These results suggest that a tissue interaction between HN neurons projecting to the neurohypophysis and endothelial cells contributes to the formation of an intact neurohypophyseal vascular structure. We hypothesize that HN axons provide a proangiogenic cue necessary for morphogenesis of the hypophyseal vasculature and establishment of a functional neurovascular connection.
Oxytocin and Its Receptor Are Required for Pituitary Vascular Morphogenesis
We set out to identify a secreted axonal-derived cue that might be involved in pituitary vascular morphogenesis. Previous studies have effectively shown that vascular endothelial cells of many tissues express oxytocin receptor (Jankowski et al., 2004; Thibonnier et al., 1999; Wakasa et al., 2009). Furthermore, recent reports have demonstrated that oxytocin stimulates migration and sprouting of human endothelial cells in vitro (Cassoni et al., 2006; Cattaneo et al., 2008). These findings, together with our finding that the neurohypophysis is innervated before its vascularization (Figure 3) and the coincidence of vascular abnormality with HN oxytocin levels (Figure 5), have led us to hypothesize that hypophyseal secretion of oxytocin acts as a developmental cue that induces angiogenesis of neurohypophyseal vessels during embryonic development.
We went on to test whether specific disruptions of oxytocin signaling cause a hypophyseal vascular phenotype. We injected 1 cell stage embryos with splice-blocking morpholino oligonucliotides targeted against either the oxytocin-like (oxtl) gene or its receptor (oxtlr). These genetic perturbations caused aberrant oxtlr splicing and a dramatic reduction in Oxytocin protein levels (Figures S4A–S4D). Targeted knockdown of either oxtl or oxtl-receptor led to malformation of neurohypophyseal vessels ranging from mild to severe hypoplasia and vessel dysmorphism, but, importantly, did not disrupt nearby internal carotid arteries (Figures 6A–6E). This vascular phenotype appeared to be mediated by oxytocinergic neurons as knockdown of the gene encoding the other neurohypophyseal hormone, arginine-vasopressin (avpl), did not result in any visible vascular phenotype (data not shown). We went on to quantify this effect using double-transgenic embryos that express distinct fluorescent markers in both nuclei and the plasma membranes of vascular endothelial cells (fli1:nuc-EGFP;vegfr2:ras-mCherry) (Figures 6D and 6E). This allowed us to readily identify and count the individual endothelial cells. We found that targeted knockdown of the oxtl-receptor, which affected the neurohypophyseal vasculature, led to marked reduction in the number of endothelial cells that make up the hypophyseal vessel but did not affect the number of cells in the nearby internal carotid arteries (Figure 6F).
Figure 6. Oxytocin and Its Receptor Are Necessary for Pituitary Vascularization.

(A–D) High-resolution confocal images of the head vasculature of 3-day-old embryos following targeted knockdown of the gene encoding for oxytocin (oxtl-MO) or oxytocin receptor (oxtlr-MO) using antisense morpholino oligonucliotides, leads to severe impairment of neurohypophyseal vasculature, but does not impede development of neighboring vessels. A schematic representation of the corresponding phenotype with arterial (red) and venous (blue) color-coding is shown in (A′)–(C′).
(D and E) By crossing nuclear- and membrane-tagged fluorescent vascular reporter lines, we are able to count the number of vascular endothelial cells in each vessel. A dot-plot histogram showing the x,y positions of EGFP+;Cherry+ endothelial cells nuclei of the hypophyseal vesselsas well as the adjacent internal carotid arteries (CrDI, between the PICA and the PLA)isshownin (D′) and (E′). Individual cells belonging to the hypophyseal vessels (red) and CrDI (gray) are color coded.The oxtlr-MO-injected embryos (E) displayamarkedly reduced number of endothelial cells in the hypophyseal vessel but no change in the nearby internal carotid artery. (F) Bar histogram showing EGFP+;Cherry+ cell counts in the internal carotid arteries and hypophyseal vessels in control and following oxtlr knockdown. *p < 0.01, n = 14.
(G) RT-PCR analysis of vegfr2:Cherry-positive and -negative FACS sorted endothelial cells demonstrating vascular expression of the oxytocin receptor (oxtlr). CaDI, caudal division of internal carotid; CrDI, cranial division of the internal carotid artery; MO, morpholino antisense oligonucleotide; OA, optic artery; PICA, primitive internal carotid artery; PLA, palatocerebral artery; PLV, palatocerebral vein. The asterisk (*) demarcates the location of the neurohypophysis. See also Figure S4.
Our findings suggest that the neuropeptide oxytocin serves as a developmental angiogenic cue regulating the morphogenesis of neurohypophyseal vessels during embryonic development.
Oxytocin signaling may affect vascular endothelial cells directly or it may do so by regulating a secondary angiogenic cue that is received by the forming hypophyseal vasculature. For example, blocking oxytocin signaling may interfere with autocrine activation of the oxytocin receptor that could in turn regulate the secretion of a proangiogenic cue, thus affecting hypophyseal vascular morphogenesis. Alternatively, the oxytocin receptor might be autonomously affecting hypophyseal vascular morphogenesis as was shown in the case of cultured human endothelial cells (Cassoni et al., 2006; Cattaneo et al., 2008, 2009; Thibonnier et al., 1999). In agreement with these studies, RT-PCR expression analysis of oxtlr mRNA in FACS-sorted endothelial cells derived from vegfr2:mCherry transgenic embryos clearly shows that the zebrafish oxytocin receptor is expressed in vascular endothelia (Figure 6G and Figure S4E).
To directly address whether oxytocin receptor autonomously affects hypophyseal vascular morphogenesis, we performed a tissue-specific rescue of oxtlr deficient embryos (Figure 7). To this end, we knocked down the oxtlr gene in the entire embryo and simultaneously re-expressed Oxtlr in discrete endothelial clones. We used a fli1:Gal4;UAS:Kaede transgenic line (Zygmunt et al., 2011) to drive endothelial-specific expression of oxytocin receptor. fli:Gal4;UAS:Kaede embryos were microinjected with a transgenic vector harboring the oxytocin receptor cDNA under the control of multiple Gal4 upstream activation sequences (10×UAS), resulting in mosaic vascular clones expressing the oxytocin receptor. To identify these clones, we made use of a multicistronic gene expression cassette in which the viral 2A peptide sequence (Provost et al., 2007) was placed between the oxytocin receptor and EGFP, causing simultaneous expression of EGFP+ oxytocin receptor in discrete clones throughout the Kaede-positive blood vessels (Figures 7A–7C). We analyzed hypophyseal vasculature by scoring two parameters: (1) integrity of the hypophyseal vascular structure, and (2) presence of clones expressing the Oxtlr-2A-EGFP cassette in the hypophyseal vessels. We found that the existence of Oxtlr-expressing clones in the hypophyseal vasculature is significantly associated with a rescued hypophyseal vascular structure (Figure 7C; n = 20/22, p < 0.0001). These results suggest that oxytocin receptor mediates a proangiogenic signal by acting autonomously in vascular endothelial cells.
Figure 7. Cell-Autonomous Effect of the Oxytocin Receptor on the Pituitary Vasculature.
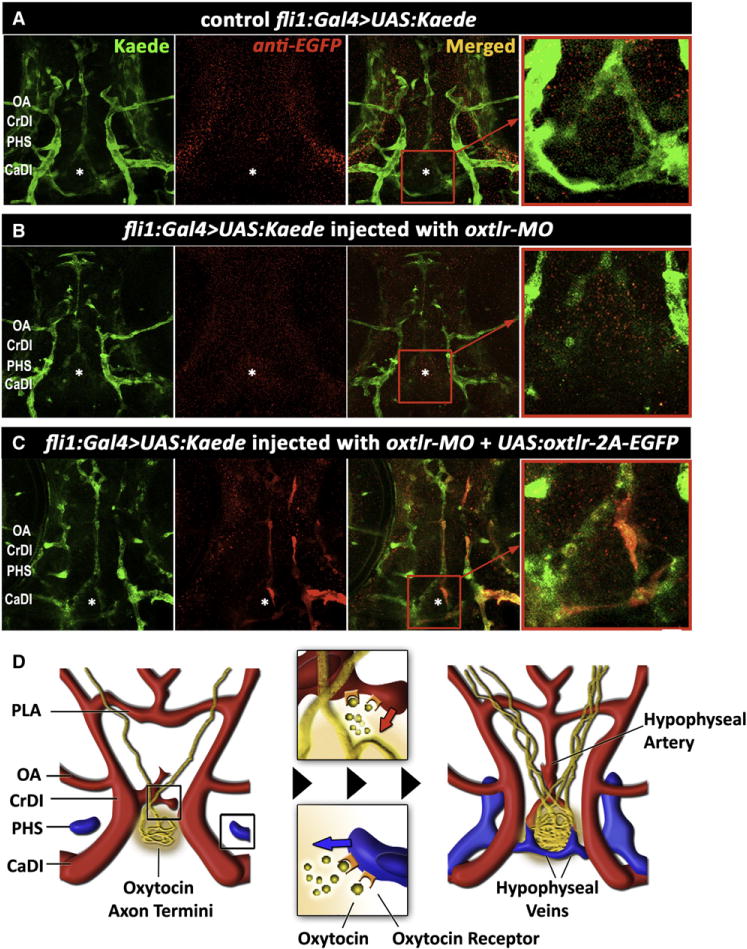
(A–C) Genetic complementation of oxytocin receptor in the vascular endothelia of oxtlr-deficient embryos. Double transgenic embryos expressing an endothelial Gal4 driver (fli1:Gal4) and a fluorescent reporter protein (UAS:Kaede; green) were injected with transposon-based transgenic vector containing a multicistronic gene expression cassette (UAS:oxtlr-2A-EGFP) allowing simultaneous mosaic coexpression of the oxytocin receptor and EGFP in discrete vascular clones (red). These clones were detected by immunostaining with an anti-EGFP antibody that does not react with the Kaede protein followed by a secondary cy3-conjugated antibody. The asterisk (*) demarcates the location of the neurohypophysis.
(D) Schematic model describing the hypothalamohypophyseal neurovascular connection by local secretion of oxytocin (see text).
CaDI, caudal division of internal carotid; CrDI, cranial division of the internal carotid artery; OA, optic artery; PHS, primary head sinus; PLA, palatocerebral artery.
Taken together, this study demonstrates that the formation of the neurovascular interface of the neurohypophysis is regulated during development by the neuropeptide oxytocin and its receptor. Oxytocin secreted from developing NH nerve termini signals to nearby endothelial cells at the site of the neurohypophyseal primordium. Other vascular endothelial cells may also express the receptor but the ligand is not available to them. This axovasal cell communication event is essential for the establishment of a tight interface between HN neurons and blood vessels of the HNS (Figure 7D).
DISCUSSION
The HNS is a main conduit by which the brain exerts control over peripheral organs. The activities of the HNS are conserved in all vertebrates and its main anatomical hallmark is the tight interface between hypothalamic nerve termini and neurohypophyseal blood vessels. In the present study, we have characterized the structure and morphogenesis of the zebrafish HNS and showed that HN axons secrete a localized proangiogenic cue (oxytocin) that is received by endothelial cells leading to the formation of a specialized neurovascular interface.
Morphogenesis of the HN System
The embryonic development of the neurohypophysis has been previously described in other model organisms and in human (Eurenius, 1977; Fink and Smith, 1971; Galabov and Schiebler, 1983; Okado and Yokota, 1980). However, the elaborate three-dimensional structure of the neurohypophysis makes it difficult to study its developmental dynamics. Moreover, the above studies were based on electron microscopy and immunohistological methods, which are useful to study fine structure of the neurovascular interface, but are limited in their ability to resolve the spatiotemporal cellular processes that shape the developing organ. To overcome these limitations, we have utilized a unique transgenic reporter system, in which zebrafish HN cell bodies and neurovascular contacts are labeled. Using this vertebrate model system we were able to monitor the spatiotemporal morphogenesis of neurohypophyseal axons and blood vessels in an intact developing brain. Based these results, we can now conclude that the anatomy of the HNS is largely conserved between fish and mammals.
We show that the zebrafish neurohypophysis is a discernable area, which develops within a concave-shaped indentation formed by the adenohypophysis. The identity of the zebrafish hypophyseal blood vessels has not been reported before (Isogai et al., 2001). Our study provides a structural and functional description of this unique vascular formation. Using real-time imaging, we showed that the hypophyseal artery develops before the veins and separately from them. As the vascular network of the brain is predominantly formed by angiogenesis, it is likely that the hypophyseal vessels we identified sprout from existing vascular network of the head. Future identification of the exact origins of the hypophyseal artery and veins may contribute to elucidation of the factors regulating these vascular identities. Interestingly, HN axons begin to innervate the neurohypophysis 24 hr prior to the appearance of endothelial neurohypophyseal precursors and approximately 48 hr before the establishment of the tight neurovascular contacts between the axon termini and the hypophyseal blood vessels suggesting that the final morphogenesis of these axons and vessels is regulated by temporally distinct pathway(s).
Mechanisms of Neurovascular Connection
The elaborate networks of vertebrate vascular and neuronal systems display striking anatomic similarities. Nerve fibers and blood vessels often follow parallel routes in the body. Furthermore, attraction and repulsion of axons and vascular endothelial cells to/from target tissues were found, in some instances, to be regulated by common guidance molecules (Adams and Eichmann, 2010; Carmeliet and Tessier-Lavigne, 2005). The question we addressed here is: how do hypothalamic axons contact neuroendocrine blood capillaries? The formation of this vital neurovascular interface could potentially be regulated in different ways: neurohypophyseal blood vessels may attract HN neurons to the site of the neurohypophysis similar to the reported guidance of a subset of developing sympathetic axons by vascular-derived endothelins (Makita et al., 2008). We have ruled out this possibility in the present case by showing that oxytocinergic axons display normal hypophyseal innervation and axonal branching in the avascular zebrafish mutant cloche. On the other hand, tissue-specific genetic ablation of HN neurons and knockdown of the HN neuropeptide oxytocin specifically impaired the formation of neurohypophyseal vasculature. These results indicate that neurohypophyseal morphogenesis is regulated by the local release of the neuropeptide oxytocin from the developing nerve termini. A similar type of mechanism, in which axons affect endothelial cells, was shown in the case of peripheral sensory nerves that regulate the pattern of arterial branching via local secretion of VEGF-A (Mukouyama et al., 2002, 2005) and more recently, in the case of motor neurons-derived Netrin-1a that mediates lymphangiogenesis (Lim et al., 2011). A third, nonexclusive possibility is that blood vessels and nerve fibers of the HNS respond to a common attractant that is expressed in the ventral diencephalic midline.
Notably, our data indicate that blocking oxytocinegic signaling does not prevent the guidance of blood vessels to the area of the neurohypophysis. Therefore, it is likely that oxytocin signaling cooperates with other guidance cues to orchestrate vascular morphogenesis in the neurohypophysis, leading to the formation of a tight neurovascular interface.
Role of Oxytocin in Angiogenesis
It has been known for some time that when the HN tract is transected, many of the axons regenerate and re-establish functional neurovascular connections with blood capillaries (Harris, 1948b, 1955). Regenerated areas are characterized by a marked increase in angiogenesis, which was found to occur near large deposits of neurosecretory material derived from the regenerated axon fibers (Moll, 1958). A possible interpretation of these studies is that neurohypophyseal angiogenesis may be regulated by the release of a proangiogenic factor from the developing nerve termini.
Our present finding that the neuropeptide oxytocin has a proangiogenic role during the embryonic development of the HNS receives reinforcement from many studies. Human vascular endothelial cells derived from several organs express the oxytocin but not the arginine-vasopressin receptor (Thibonnier et al., 1999). Oxytocin induces differentiation of embryonic stem cells and adult Sca-1-positive cells to cardiomyocytes (Matsuura et al., 2004; Paquin et al., 2002). Remodeling of uterine blood during human pregnancy involves increased oxytocin receptor expression in vessel walls (Wakasa et al., 2009). Oxytocin and its receptor have been implicated in developmental formation of the coronary vessels and in angiogenic remodeling following myocardial infarction (Jankowski et al., 2004; Kobayashi et al., 2009). Finally, oxytocin stimulates migration and sprouting of cultured human vascular endothelial cells and this angiogenic effect is dependent on oxytocin receptor signaling events (Cassoni et al., 2006; Cattaneo et al., 2008, 2009). Several studies have reported metabolic abnormalities in mice deficient in either oxytocin or its receptor (Kasahara et al., 2007; Takayanagi et al., 2008). As none of these studies have analyzed the integrity of the hypophyseal neurovascular interface it remains to be determined whether the metabolic abnormalities observed in these mice are caused by compromised neurohypophyseal development. Finally, loss of functional Otp disrupts the development of oxytocinergic neurons as well as the neurohypophysis (Acampora et al., 1999; Wang and Lufkin, 2000).
The question of why hypophyseal vasculature responds to oxytocin while other vessels do not remains open. The simplest explanation is that the local secretion of the oxytocin protein by HN termini is a focal attractant that affects the sprouting morphogenesis of only nearby endothelial cells, as shown in our model (Figure 7D) Another nonexclusive possibility is that hypophyseal vascular precursors have a unique molecular composition that renders them responsive to oxytocin signal.
Mechanism of Action of Oxytocin during Neurohypophyseal Morphogenesis
Given that the primary function of the HNS is the mass secretion of oxytocin into the neurovascular interface, we favor the possibility that localized developmental secretion of oxytocin is important for the integrity of neurovascular contacts at the site of the neurohypophysis. We show that endothelial-specific re-expression of the oxytocin receptor rescues the vascular phenotype of in oxtlr-deficient embryos suggesting that this receptor mediates a proangiogenic signal by acting in vascular endothelial cells that receive oxytocin signal from HN neurons. However, we still cannot rule out the possibility that, in addition to the oxytocin-oxytocin receptor-mediated neurovascular signaling event we found here, autocrine activation of the oxytocin receptor regulates another, yet undiscovered, angiogenic cue released by HN axons. So far, we have examined the expression of various isoforms of vegf, semaphorin, and tgfβ/Bmp that could conceivably be involved in HN neurovascular development and found none of them is expressed in HN neurons (data not shown).
Oxytocin receptor belongs to the rhodopsin-type (class I) G protein-coupled receptor family and is coupled to phospholipase C through Gαq11 (Gimpl and Fahrenholz, 2001). Recent reports show that oxytocin stimulates migration and sprouting of human umbilical vein endothelial cells (HUVEC) through a 3D angiogenesis assay, via Gq coupling of the oxytocin receptor leading to phospholipase C activation and phosphorylation of the Pyk2 Src kinase proteins and that knockdown of Pyk2 impaired this effect (Cattaneo et al., 2008, 2009). As these proteins are known to be involved in Semaphorin and/or VEGF signaling in the vascular system, the signal transduction cascade underlying the effect of oxytocin on hypophyseal vascular morphogenesis may feed into known intracellular pathways used for branching morphogenesis of endothelial cells (Gelfand et al., 2009).
Finally, oxytocin-mediated vascular angiogenesis might prove relevant to adult regenerative processes. The regeneration of the HNS following hypophysectomy has been extensively studied in many vertebrate species including fish and humans (Billenstien and Leveque, 1955; Daniel and Prichard, 1972; Harris, 1955; Moll, 1957; Sathyanesan and Gorbman, 1965; Stutinsky, 1951). A strong association has been found between deposition of neurosecretory material and high vascularity in HNS regeneration models (Moll, 1958). The communication between the oxytocinergic neurons and the vasculature reported herein may play a role in this well-documented regeneration of the HNS following local lesion.
In summary, we present a genetic system to study the developmental assembly of the vertebrate HNS. Using this system, we have identified key cellular and biochemical processes required for formation of the neurohypophyseal neurovascular interface, a vital intersection that allows neuropeptidergic hormones from the central nervous system to be transferred directly into the blood and regulate the body’s homeostasis.
EXPERIMENTAL PROCEDURES
Antibodies and Transgenesis Plasmids and Lines
Guinea pig polyclonal antibody directed to the Oxytocin peptide was purchased from Bachem (Bachem California, Torrance, CA, Cat. T-5021.0050). Antibodies made in rabbit and mouse (Molecular Probes, Eugene, OR) were used to detect the transgenic EGFP expression. For all plasmid DNA constructs we used the Tol2kit transposon-based transgenic vectors system for site-specific recombination-based cloning (Kwan et al., 2007). cDNA encoding to the oxtlr (gene locus FJ556870) was cloned downstream of the Gal4 responsive 10×UAS element and basal promoter. We used a multicistronic gene expression cassette in which the viral 2A peptide sequence was placed between the gene of interest and EGFP fluorescent protein, which reports the expression of the transgene (Provost et al., 2007). Genotypes of transgenic lines used in this study are as follows: Tg(oxtl:EGFP); Tg(vegfr2:EGFP) [a.k.a. Tg(kdrl:EGFP)s843]; Tg(vegfr2:mCherry) [a.k.a. Tg(kdrl:HsHRAS-mCherry) s896]; Tg(prl:RFP); Tg(pomc:EGFP) [a.k.a. Tg(−1.0pomca:GFP)zf44]; Tg(gata1: dsRed)sd2; Tg(otpb:caax-EGFP) [a.k.a. = Tg(otpb:1EGFP)zc49]; Tg(fli1: dsRed) [a.k.a. Tg(fli1a.ep:DsRedEx)um13]; Tg(otpb:Gal4) [a.k.a Tg(otpb.A: Gal4, myl7:EGFP)zc67]; Tg(UAS:NTR-Cherry) [a.k.a. Tg(UAS-E1b:NfsB-mCherry)c264]; Tg(fli1:nuc-EGFP) [a.k.a. Tg(fli1a:nEGFP)y7]; Tg(fli1:Gal4) [a.k.a. Tg(fliep:gal4ff)ubs4].
Microinjection of Transgenic DNA and Oligonucleotides
Gene knockdowns were performed using synthetic antisense morpholino oligonucleotides (MO; Gene Tools, LLC, Corvallis, OR) targeted to an RNA-splicing sites (Figure S2) of oxtl, (CACTGCAGATGGTAAGGGAAACCTA) and oxtlr (CATCGCTTTGGAGGAGAAGAAAACA). MOs were microinjected (each at 1.5 ng per 1.7 nl) into 1 cell stage embryos as we previously described (Blechman et al., 2007). The oxtlr cDNA sequence (gene locus FJ556870) used for the rescue experiment lacked the respective MO binding site and was therefore resistant to its effect.
Tissue Preparation and Immunohistochemistry
Immunofluorescent staining of transgenic embryos was performed as we previously described (Russek-Blum et al., 2008). The skulls of Juvenile and adult fish were exposed using sharp forceps and fixed at 4°C overnight in 2%–4% paraformaldehyde/0.1 M phosphate buffer (pH 7.5). Samples were washed twice with phosphate buffer and transferred into 20% sucrose/20% EDTA in 0.1M PB (pH 7.5) for decalcification and cryoprotection. Brains were frozen in 7.5% gelatine/20% sucrose and cut at 14 μm. Sections were stored at −20°C. Primary and secondary antibodies were incubated in PBS with 0.3% Triton X-100 (PBS TX). Primary antibodies were incubated overnight at 4 C° and secondary antibodies for 1 hr at room temperature. The slides were then washed in PBS TX followed by washes in PBS and mounted in PBS:Glycerol (1:1).
Tissue-Specific Cell Ablation
For the nitroreductase-mediated tissue-specific cell ablation we used the analytical-grade metronidazole drug, Vetranal (Sigma-Adridch, Rehovot, Israel Cat. #46461), which was dissolved in E3 medium (1.74 mM NaCl, 0.21 mM KCl, 0.12 mM MgSO4, 0.18 mM Ca[NO3], 0.15 mM HEPES [pH 7.4]). Embryos were manually dechorionated at 22 hpf and treated with 5 mM Vetranal from 24–48 hpf, and then switched into 10 mM Vetranal for additional 30 hr.
FACS and RNA Extraction
Three-day-old vegfr2:mCherry embryos were dissociated by Liberase blendzyme 3 (Roche, Basel, Switzerland) in combination with GIBCO-trypsin (Invitrogen, Carlsbad, CA), each for 15 min at 28°C, with occasional pipetting. Reaction was stopped by three washes of 1% bovine serum. Cells were suspended in L15 medium, and filtered by nylon mesh. Fluorescence activated cell sorting (FACS) was performed using a SORP FACSAriaII cell sorter. RNA was extracted from approximately 50,000 cells and RT-PCR with specific primer pairs (see Supplemental Experimental Methods) were performed as described (Blechman et al., 2007).
Live Imaging and Image Analysis
Embryos growing in E3 solution were embedded in drops of warm 1% Ultra Pure LMP agarose (Invitrogen, Carlsbad, CA) and imaged on a Zeiss 710 inverted confocal microscope with 488 and 561 nm lasers. Data sets were analyzed using Imagej and ZEN (Zeiss). 3D reconstructions were generated and analyzed with Volocity (Improvision, Covington, UK).
Statistical Analyses
Statistical significance between average cell counts was determined using Student’s t test (two-tailed, α = 0.05) on Excel (Microsoft Corp., Redmond, WA). All error bars indicate the standard error of the mean. Correlation between the occurrence of oxtlr-positive clones in ventral head vessels and phenotypic rescue of hypophyseal vasculature was determined by calculating the Phi coefficient of association, then performing a chi-square test of association (p < 0.0001) using the statistical web tool VassarStats.
Supplementary Material
Acknowledgments
Thanks are due to Helit Nabel-Rosen for her initial observation of HN projections; Shifra Ben Dor for phylogenetic and genomic analyses; Genia Brodsky for help with figure graphics; Yossy Machluf and Liron Gibbs for FACS technical advice. Chi-Bin Chien for the Tol2kit plasmid vectors; Didier Stainier, Wiebke Herzog, Nathan Lawson, Michael Parsons, and Shlomo Melmed for transgenic lines. The research in the Levkowitz lab is supported by the Israel Science Foundation; Minerva Foundation; Kirk Center for Childhood Cancer and Immunological Disorders; Irvin Green Alzheimer’s Research Fund. G.L. is an incumbent of the Tauro Career Development Chair in Biomedical Research. A.G. and J.B. designed and performed most of the experiments and collected and analyzed the data. J.K. performed the colocalization experiment of oxytocin and EGFP in adult fish. J.L.B. generated the otpb:Gal4 line and performed the analysis of Otp+ HN projections. L.H., H.-G.B., and M.A. generated the fli1:Gal4;UAS: Kaede line. G.L. initiated and headed the project and helped with data interpretation. G.L. and A.G. prepared the manuscript and figures.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes Supplemental Experimental Procedures, four figures, and six movies and can be found with this article online at doi:10.1016/j.devcel.2011.09.004.
We declare that we have no conflicting interests.
References
- Acampora D, Postiglione MP, Avantaggiato V, Di Bonito M, Vaccarino FM, Michaud J, Simeone A. Progressive impairment of developing neuroendocrine cell lineages in the hypothalamus of mice lacking the Orthopedia gene. Genes Dev. 1999;13:2787–2800. doi: 10.1101/gad.13.21.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams RH, Eichmann A. Axon guidance molecules in vascular patterning. Cold Spring Harb Perspect Biol. 2010;2:a001875. doi: 10.1101/cshperspect.a001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amer S, Brown JA. Glomerular actions of arginine vasotocin in the in situ perfused trout kidney. Am J Physiol. 1995;269:R775–R780. doi: 10.1152/ajpregu.1995.269.4.R775. [DOI] [PubMed] [Google Scholar]
- Bargmann W. J Mol Med. 1949;27:617–622. [Google Scholar]
- Billenstien DC, Leveque TF. The reorganization of the neurohypophyseal stalk following hypophysectomy in the rat. Endocrinology. 1955;56:704–717. doi: 10.1210/endo-56-6-704. [DOI] [PubMed] [Google Scholar]
- Blechman J, Borodovsky N, Eisenberg M, Nabel-Rosen H, Grimm J, Levkowitz G. Specification of hypothalamic neurons by dual regulation of the homeodomain protein Orthopedia. Development. 2007;134:4417–4426. doi: 10.1242/dev.011262. [DOI] [PubMed] [Google Scholar]
- Blechman J, Amir-Zimberstein L, Gutnick A, Ben-Dor S, Levkowitz G. The metabolic regulator PGC-1α directly controls the expression of the hypothalamic neuropeptide oxytocin. J Neurosci. 2011 doi: 10.1523/JNEUROSCI.1798-11.2011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownstein MJ, Russell JT, Gainer H. Synthesis, transport, and release of posterior pituitary hormones. Science. 1980;207:373–378. doi: 10.1126/science.6153132. [DOI] [PubMed] [Google Scholar]
- Burbach JP, Luckman SM, Murphy D, Gainer H. Gene regulation in the magnocellular hypothalamo-neurohypophysial system. Physiol Rev. 2001;81:1197–1267. doi: 10.1152/physrev.2001.81.3.1197. [DOI] [PubMed] [Google Scholar]
- Cajal SR. Histologie du Systeme Neurveux de L’Homme et de Vertebres. Paris: A. Maloine; 1911. [Google Scholar]
- Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436:193–200. doi: 10.1038/nature03875. [DOI] [PubMed] [Google Scholar]
- Cassoni P, Marrocco T, Bussolati B, Allia E, Munaron L, Sapino A, Bussolati G. Oxytocin induces proliferation and migration in immortalized human dermal microvascular endothelial cells and human breast tumorderived endothelial cells. Mol Cancer Res. 2006;4:351–359. doi: 10.1158/1541-7786.MCR-06-0024. [DOI] [PubMed] [Google Scholar]
- Cattaneo MG, Chini B, Vicentini LM. Oxytocin stimulates migration and invasion in human endothelial cells. Br J Pharmacol. 2008;153:728–736. doi: 10.1038/sj.bjp.0707609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo MG, Lucci G, Vicentini LM. Oxytocin stimulates in vitro angiogenesis via a Pyk-2/Src-dependent mechanism. Exp Cell Res. 2009;315:3210–3219. doi: 10.1016/j.yexcr.2009.06.022. [DOI] [PubMed] [Google Scholar]
- Chan DKO. Comparative physiology of the vasomotor effects of neurohypophysial peptides in the vertebrates. Am Zool. 1977;17:751–761. [Google Scholar]
- Chi NC, Shaw RM, De Val S, Kang G, Jan LY, Black BL, Stainier DY. Foxn4 directly regulates tbx2b expression and atrioventricular canal formation. Genes Dev. 2008;22:734–739. doi: 10.1101/gad.1629408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curado S, Stainier DY, Anderson RM. Nitroreductase-mediated cell/tissue ablation in zebrafish: a spatially and temporally controlled ablation method with applications in developmental and regeneration studies. Nat Protoc. 2008;3:948–954. doi: 10.1038/nprot.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel PM, Prichard MM. The human hypothalamus and pituitary stalk after hypophysectomy or pituitary stalk section. Brain. 1972;95:813–824. doi: 10.1093/brain/95.4.813. [DOI] [PubMed] [Google Scholar]
- Davison JM, Akitake CM, Goll MG, Rhee JM, Gosse N, Baier H, Halpern ME, Leach SD, Parsons MJ. Transactivation from Gal4-VP16 transgenic insertions for tissue-specific cell labeling and ablation in zebrafish. Dev Biol. 2007;304:811–824. doi: 10.1016/j.ydbio.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eurenius L. An electron microscope study of the differentiating capillaries of the mouse neurohypophysis. Anat Embryol (Berl) 1977;152:89–108. doi: 10.1007/BF00341437. [DOI] [PubMed] [Google Scholar]
- Fink G, Smith GC. Ultrastructural features of the developing hypothalamo-hypophysial axis in the rat. A correlative study Z Zellforsch Mikrosk Anat. 1971;119:208–226. doi: 10.1007/BF00324522. [DOI] [PubMed] [Google Scholar]
- Fujimoto E, Stevenson TJ, Chien CB, Bonkowsky JL. Identification of a dopaminergic enhancer indicates complexity in vertebrate dopamine neuron phenotype specification. Dev Biol. 2011;352:393–404. doi: 10.1016/j.ydbio.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galabov PG, Schiebler TH. Developmentof the capillary system in the neurohypophysis of the rat. Cell Tissue Res. 1983;228:685–696. doi: 10.1007/BF00211484. [DOI] [PubMed] [Google Scholar]
- Gelfand MV, Hong S, Gu C. Guidance from above: common cues direct distinct signaling outcomes in vascular and neural patterning. Trends Cell Biol. 2009;19:99–110. doi: 10.1016/j.tcb.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Harris GW. Further evidence regarding the endocrine status of the neurohypophysis. J Physiol. 1948a;107:436–448. doi: 10.1113/jphysiol.1948.sp004288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GW. Neural control of the pituitary gland. Physiol Rev. 1948b;28:139–179. doi: 10.1152/physrev.1948.28.2.139. [DOI] [PubMed] [Google Scholar]
- Harris GW. Neural Control of the Pituitary Gland. Vol. 3. London: Edward Arnold & Co.; 1955. [Google Scholar]
- Isogai S, Horiguchi M, Weinstein BM. The vascular anatomy of the developing zebrafish: an atlas of embryonic and early larval development. Dev Biol. 2001;230:278–301. doi: 10.1006/dbio.2000.9995. [DOI] [PubMed] [Google Scholar]
- Jankowski M, Danalache B, Wang D, Bhat P, Hajjar F, Marcinkiewicz M, Paquin J, McCann SM, Gutkowska J. Oxytocin in cardiac ontogeny. Proc Natl Acad Sci USA. 2004;101:13074–13079. doi: 10.1073/pnas.0405324101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SW, Beis D, Mitchell T, Chen JN, Stainier DY. Cellular and molecular analyses of vascular tube and lumen formation in zebrafish. Development. 2005;132:5199–5209. doi: 10.1242/dev.02087. [DOI] [PubMed] [Google Scholar]
- Kasahara Y, Takayanagi Y, Kawada T, Itoi K, Nishimori K. Impaired thermoregulatory ability of oxytocin-deficient mice during cold-exposure. Biosci Biotechnol Biochem. 2007;71:3122–3126. doi: 10.1271/bbb.70498. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Yasuda S, Bao N, Iwasa M, Kawamura I, Yamada Y, Yamaki T, Sumi S, Ushikoshi H, Nishigaki K, et al. Postinfarct treatment with oxytocin improves cardiac function and remodeling via activating cell-survival signals and angiogenesis. J Cardiovasc Pharmacol. 2009;54:510–519. doi: 10.1097/FJC.0b013e3181bfac02. [DOI] [PubMed] [Google Scholar]
- Kulczykowska E. Effects of arginine vasotocin, isotocin and melatonin on blood pressure in the conscious atlantic cod (Gadus morhua): hormonal interactions? Exp Physiol. 1998;83:809–820. doi: 10.1113/expphysiol.1998.sp004161. [DOI] [PubMed] [Google Scholar]
- Kwan KM, Fujimoto E, Grabher C, Mangum BD, Hardy ME, Campbell DS, Parant JM, Yost HJ, Kanki JP, Chien CB. The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev Dyn. 2007;236:3088–3099. doi: 10.1002/dvdy.21343. [DOI] [PubMed] [Google Scholar]
- La Pointe JL. Comparative physiology of neurohypophysial hormone action on vertebrate oviduct uterus. Am Zool. 1977;17:763–773. [Google Scholar]
- Le Mevel JC, Pamantung TF, Mabin D, Vaudry H. Effects of central and peripheral administration of arginine vasotocin and related neuropeptides on blood pressure and heart rate in the conscious trout. Brain Res. 1993;610:82–89. doi: 10.1016/0006-8993(93)91220-m. [DOI] [PubMed] [Google Scholar]
- Lim AH, Suli A, Yaniv K, Weinstein B, Li DY, Chien CB. Motoneurons are essential for vascular pathfinding. Development. 2011;138:3847–3857. doi: 10.1242/dev.068403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu NA, Huang H, Yang Z, Herzog W, Hammerschmidt M, Lin S, Melmed S. Pituitary corticotroph ontogeny and regulation in transgenic zebrafish. Mol Endocrinol. 2003;17:959–966. doi: 10.1210/me.2002-0392. [DOI] [PubMed] [Google Scholar]
- Liu NA, Liu Q, Wawrowsky K, Yang Z, Lin S, Melmed S. Prolactin receptor signaling mediates the osmotic response of embryonic zebrafish lactotrophs. Mol Endocrinol. 2006;20:871–880. doi: 10.1210/me.2005-0403. [DOI] [PubMed] [Google Scholar]
- Macfarlane NA, Maetz J. Effects of hypophysectomy on sodium and water exchanges in the euryhaline flounder, Platichthys flesus (L) Gen Comp Endocrinol. 1974;22:77–89. doi: 10.1016/0016-6480(74)90090-2. [DOI] [PubMed] [Google Scholar]
- Machluf Y, Gutnick A, Levkowitz G. Development of the zebrafish hypothalamus. Ann N Y Acad Sci. 2011;1220:93–105. doi: 10.1111/j.1749-6632.2010.05945.x. [DOI] [PubMed] [Google Scholar]
- Makita T, Sucov HM, Gariepy CE, Yanagisawa M, Ginty DD. Endothelins are vascular-derived axonal guidance cues for developing sympathetic neurons. Nature. 2008;452:759–763. doi: 10.1038/nature06859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura K, Nagai T, Nishigaki N, Oyama T, Nishi J, Wada H, Sano M, Toko H, Akazawa H, Sato T, et al. Adult cardiac Sca-1-positive cells differentiate into beating cardiomyocytes. J Biol Chem. 2004;279:11384–11391. doi: 10.1074/jbc.M310822200. [DOI] [PubMed] [Google Scholar]
- Moll J. Regeneration of the supraoptico-hypophyseal and paraventriculo-hypophyseal tracts in the hypophysectomized rat. Z Zellforsch Mikrosk Anat. 1957;46:686–709. doi: 10.1007/BF00339372. [DOI] [PubMed] [Google Scholar]
- Moll J. The effect of hypophysectomy on the pituitary vascular system of the rat. J Morphol. 1958;102:1–21. [Google Scholar]
- Mukouyama YS, Gerber HP, Ferrara N, Gu C, Anderson DJ. Peripheral nerve-derived VEGF promotes arterial differentiation via neuropilin 1-mediated positive feedback. Development. 2005;132:941–952. doi: 10.1242/dev.01675. [DOI] [PubMed] [Google Scholar]
- Mukouyama YS, Shin D, Britsch S, Taniguchi M, Anderson DJ. Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell. 2002;109:693–705. doi: 10.1016/s0092-8674(02)00757-2. [DOI] [PubMed] [Google Scholar]
- Okado N, Yokota N. An electron microscopic study on the structural development of the neural lobe in the human fetus. Am J Anat. 1980;159:261–273. doi: 10.1002/aja.1001590303. [DOI] [PubMed] [Google Scholar]
- Paquin J, Danalache BA, Jankowski M, McCann SM, Gutkowska J. Oxytocin induces differentiation of P19 embryonic stem cells to cardiomyocytes. Proc Natl Acad Sci USA. 2002;99:9550–9555. doi: 10.1073/pnas.152302499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter RE, Fryer JN. Endocrine Functions of the Hypothalamus of Actinopterygians. Vol. 2. Ann Arbor, MI: University of Michigan Press; 1983. [Google Scholar]
- Provost E, Rhee J, Leach SD. Viral 2A peptides allow expression of multiple proteins from a single ORF in transgenic zebrafish embryos. Genesis. 2007;45:625–629. doi: 10.1002/dvg.20338. [DOI] [PubMed] [Google Scholar]
- Russek-Blum N, Gutnick A, Nabel-Rosen H, Blechman J, Staudt N, Dorsky RI, Houart C, Levkowitz G. Dopaminergic neuronal cluster size is determined during early forebrain patterning. Development. 2008;135:3401–3413. doi: 10.1242/dev.024232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyanesan AG, Gorbman A. Typical and atypical regeneration and overgrowth of hypothalamo-hypophysial neurosecretory tract after partial or complete hypophysectomy in the goldfish. Gen Comp Endocrinol. 1965;5:456–463. doi: 10.1016/0016-6480(65)90106-1. [DOI] [PubMed] [Google Scholar]
- Scharrer E. The light sensitivity of blind minnows (Investigations About the diencephalon of the fish I) Journal of Comparative Physiology A Neuroethology. 1928;7:1–38. [Google Scholar]
- Stainier DY, Weinstein BM, Detrich HW, 3rd, Zon LI, Fishman MC. Cloche, an early acting zebrafish gene, is required by both the endothelial and hematopoietic lineages. Development. 1995;121:3141–3150. doi: 10.1242/dev.121.10.3141. [DOI] [PubMed] [Google Scholar]
- Stutinsky F. Origin of the Gomori-positive substance of the hypothalamo-hypophyseal complex. C R Seances Soc Biol Fil. 1951;145:367–370. [PubMed] [Google Scholar]
- Takayanagi Y, Kasahara Y, Onaka T, Takahashi N, Kawada T, Nishimori K. Oxytocin receptor-deficient mice developed late-onset obesity. Neuroreport. 2008;19:951–955. doi: 10.1097/WNR.0b013e3283021ca9. [DOI] [PubMed] [Google Scholar]
- Thibonnier M, Conarty DM, Preston JA, Plesnicher CL, Dweik RA, Erzurum SC. Human vascular endothelial cells express oxytocin receptors. Endocrinology. 1999;140:1301–1309. doi: 10.1210/endo.140.3.6546. [DOI] [PubMed] [Google Scholar]
- Unger JL, Glasgow E. Expression of isotocin-neurophysin mRNA in developing zebrafish. Gene Expr Patterns. 2003;3:105–108. doi: 10.1016/s1567-133x(02)00064-9. [DOI] [PubMed] [Google Scholar]
- Verbalis JG. How does the brain sense osmolality? J Am Soc Nephrol. 2007;18:3056–3059. doi: 10.1681/ASN.2007070825. [DOI] [PubMed] [Google Scholar]
- Wakasa T, Wakasa K, Nakayama M, Kuwae Y, Matsuoka K, Takeuchi M, Suehara N, Kimura T. Change in morphology and oxytocin receptor expression in the uterine blood vessels during the involution process. Gynecol Obstet Invest. 2009;67:137–144. doi: 10.1159/000172805. [DOI] [PubMed] [Google Scholar]
- Wang W, Lufkin T. The murine Otp homeobox gene plays an essential role in the specification of neuronal cell lineages in the developing hypothalamus. Dev Biol. 2000;227:432–449. doi: 10.1006/dbio.2000.9902. [DOI] [PubMed] [Google Scholar]
- Zygmunt T, Gay CM, Blondelle J, Singh MK, Flaherty KM, Means PC, Herwig L, Krudewig A, Belting HG, Affolter M, et al. Semaphorin-PlexinD1 Signaling Limits Angiogenic Potential via the VEGF Decoy Receptor sFlt1. Dev Cell. 2011;21:301–314. doi: 10.1016/j.devcel.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


