SUMMARY
Regulation of corticotropin-releasing hormone (CRH) activity is critical for the animal’s adaptation to stressful challenges, and its dysregulation is associated with psychiatric disorders in humans. However, the molecular mechanism underlying this transcriptional response to stress is not well understood. Using various stress paradigms in mouse and zebrafish, we show that the hypothalamic transcription factor Orthopedia modulates the expression of CRH as well as the splicing factor Ataxin 2-Binding Protein-1 (A2BP1/Rbfox-1). We further show that the G protein coupled receptor PAC1, which is a known A2BP1/Rbfox-1 splicing target and an important mediator of CRH activity, is alternatively spliced in response to a stressful challenge. The generation of PAC1-hop messenger RNA isoform by alternative splicing is required for termination of CRH transcription, normal activation of the hypothalamic-pituitary-adrenal axis and adaptive anxiety-like behavior. Our study identifies an evolutionarily conserved biochemical pathway that modulates the neuronal adaptation to stress through transcriptional activation and alternative splicing.
INTRODUCTION
Stress is defined as an animal’s state of threatened homeostasis, which triggers the activation of the hypothalamic-pituitaryadrenal (HPA) axis (Chrousos, 1998; Selye, 1936). The hypothalamus regulates stress responses by affecting endocrine, metabolic, and behavioral processes to restore homeostasis (Chrousos, 2009). Prolonged and repeated exposure to physical or psychological stressors can cause a chronic state of distress that may lead to stress-associated pathologies such as anxiety disorders and depression (Chrousos, 2009; de Kloet et al., 2005; McEwen, 2003).
Stress is sensed by multiple neuronal circuits, whose major outputs feed into corticotropin-releasing hormone (CRH)-containing neurons located in the paraventricular nucleus (PVN) of mammals or the preoptic area (PO) in fish. CRH (also known as CRF) controls various responses to stress, including immediate sympathetic and behavioral “fight-or-flight” responses followed by a delayed adaptive response that is associated with the activation of the HPA axis (de Kloet et al., 2005; Ulrich-Lai and Herman, 2009). The activation of the HPA axis by the neuropeptide CRH is the major adaptive response to threats on homeostasis (Chrousos, 1998). CRH is rapidly released in response to real or perceived stress challenges; it is transported to the anterior pituitary gland, where it activates CRH receptors leading to increased production of adrenocorticotrophic hormone (ACTH) (Vale et al., 1981). ACTH is then released from the pituitary into the general circulation, where it promotes synthesis and secretion of corticosteroids from the adrenal cortex (de Kloet et al., 2005; Ulrich-Lai and Herman, 2009). Secreted corticosteroids trigger a range of immune and cardiovascular responses, redirection of energy, and behavioral responses (Chrousos, 1998; de Kloet et al., 2005; Ulrich-Lai and Herman, 2009).
Stressor-induced release of CRH is always followed by its de novo synthesis during a period of recovery from stress. Exposure to various physical, physiological, and psychological stressors leads to rapid changes in crh transcription in the PVN of the hypothalamus (Herman et al., 1989; Herman et al., 1992; Ma et al., 1997). Similar stressor-induced changes in crh transcription have been reported in frogs and fish, indicating that stress-dependent crh gene activation is evolutionarily conserved (Fuzzen et al., 2010; Yao and Denver, 2007). Emotional and physiological stressors such as forced swimming, foot shock, restraint, and increased blood osmolality all lead to a rapid increase in the level of the crh messenger RNA (mRNA), which decreases with time (Herman et al., 1989; Herman et al., 1992; Ma et al., 1997; Yao and Denver, 2007). This transcriptional regulation of the crh gene is critical for neuronal adaptation to stress. The activation and termination of crh transcription are both critical for reestablishing the homeostatic state. Failure to either activate or terminate the crh response may lead to a chronic hypoor hyperactivation of the HPA axis, which is associated with pathological conditions such as anxiety, depression, and affective spectrum disorders (Chrousos, 2009; de Kloet et al., 2005; McEwen, 2003).
Despite the wealth of information regarding the physiological role of crh in mediating stress response, the molecular mechanism(s) by which the expression of crh is regulated during stress adaptation has remained largely elusive. Here, we have identified an intracellular signaling pathway that controls stress-induced crh mRNA induction and its subsequent downregulation.
RESULTS
Orthopedia Is Required for Stress Adaptation
The homeodomain-containing protein Orthopedia (Otp) is involved in the embryonic development of a distinct subset of hypothalamic neurons (Acampora et al., 1999; Blechman et al., 2007; Ryu et al., 2007; Wang and Lufkin, 2000). However, Otp expression is maintained in the mature hypothalamus of mouse (Bardet et al., 2008) and zebrafish (Blechman et al., 2007; Ryu et al., 2007). A prominent area expressing Otp in CRH-containing neurons is the PVN in mouse as well as the equivalent PO in fish (Figure 1A; see also Figures S1, S2A, and S2B available online). Given the importance of the CRH-positive PVN/PO as a major hypothalamic region, which allows all vertebrates to adapt to challenges and restore homeostasis, we hypothesized that Otp might be involved in the stressor-mediated response of crh neurons. To explore this possibility, we set out to analyze the induction of crh transcription by stressors in Otp mutant animals. Otp-deficient mice die shortly after birth (Acampora et al., 1999; Wang and Lufkin, 2000), precluding such analysis. The zebrafish genome contains two Otp orthologs, otpa and otpb, which display functional redundancy during hypothalamic development (Blechman et al., 2007; Ryu et al., 2007). Zebrafish homozygous for the otpa null mutant allele otpam866 are viable through adulthood (Ryu et al., 2007), and importantly, CRH-expressing neurons develop normally in otpam866−/− fish larvae, allowing functional analysis of these neurons in the mature brain (Figures 1B–1F). otpam866−/− fish mutants also display normal development of hypothalamic neurons producing the neuropeptides somatostatin, hypocretin, oxytocin, vasopressin, and proopiomelanocortin (POMC) as well as pituitary secretory cells expressing POMC, prolactin, and growth hormone (data not shown).
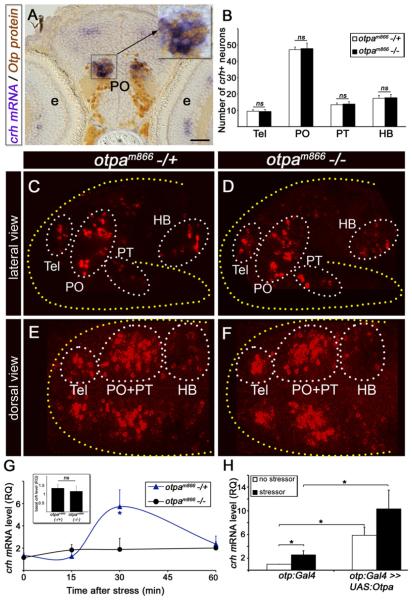
Figure 1. Otp Mediates Stress Response.
(A) Coronal sections (6 μm) through the PO showing colocalization of Otp and crh in a 6-day-old zebrafish larvae. Larvae were subjected to whole-mount in situ hybridization with a crh-directed probe followed by sectioning and immunostaining with an anti-Otp antibody. High magnification of the crh+;Otp+ area (black rectangle) is shown in the inset.
(B) Histogram showing average cell counts in individual CRH+ neuronal clusters of a null zebrafish mutant allele, otpam866−/− (n = 20) compared to heterozygous otpam866−/+ siblings (n = 19). ns, not significant.
(C–F) Projected confocal z stack images of crh neurons in otpam866−/− (D and F) and otpam866−/+ siblings (C and E), acquired from lateral (C and D) and dorsal (E and F) angles. Neurons were visualized by fluorescent in situ hybridization of crh mRNA.
(G) Induction of crh mRNA by homeostatic challenge. Physical stressor was applied to 6-day-old progenies of an otpam866−/+ cross for a period of 4 min. The amount of crh mRNA was measured in individual fish larvae at different time points of recovery using quantitative PCR (qPCR). Each tested larva was then genotyped by sequencing and crh mRNA levels of mutant (−/−) and heterozygous (−/+) animals were plotted accordingly. *p < 0.05; n = 8.
(H) Gain of function of Otpa in Otp-positive neurons. Physical stress challenge was applied to either a transgenic otp:Gal4 embryos expressing Gal4 in Otp+ cells or their siblings, which were injected with a transposonbased plasmid vector containing the full-length cDNA encoding the otpa gene under the control of ten UAS elements (otp:Gal4 >> UAS:otpa). The amount of crh mRNA was measured using qPCR. *p < 0.05, n = 8. The following abbreviations are used: e, eye; HB, hindbrain; PO, preoptic area; PT, posterior tuberculum; RQ, relative quantity; Tel, telencephalon. Scale bar represents 50 μm in (A) and 100 μm in (C)–(F).
The involvement of Otp in CRH-mediated stress adaptation was studied by analyzing stressor-induced crh transcription in 6-day-old otpam866 mutant larvae compared to their heterozygous siblings. At this age, the larvae already exhibit complex behaviors providing an opportunity to understand genetically specified behaviors (Wolman and Granato, 2011). We employed two independent, acute, and robust homeostatic challenges, which we term as “physical” and “osmotic” stress. These paradigms were previously employed in larval fish and elicited rapid increase in cortisol levels in response to the stress challenge (Barry et al., 1995; Stouthart et al., 1998). Physical stress was induced by netting the larvae, and osmotic stress was elicited by transferring the animals to 50% artificial seawater. Both stressors were acutely induced for a period of 4 min, and the levels of crh mRNA were measured during the initiation and the recovery phases of the stress response. We found that 6-day- old zebrafish larvae display a robust change in crh levels during the recovery phase, which follows the exposure to stressor. otpam866 heterozygous larvae follow a typical adaptive stress response found in other animal models: a rapid increase in crh mRNA level, which decreases with time (Figure 1G). In contrast, no stress-induced increase was observed in crh levels in the otpam866 mutants (Figure 1G; Figure S2C). To further support this finding, we undertook a genetic approach for tissue-specific gain of function of Otpa using a transgenic zebrafish line (otp:Gal4) expressing the Gal4 protein in Otp-positive neurons (Fujimoto et al., 2011). We used the Tol2 transposon-based vectors (Kawakami et al., 2004) that efficiently integrate into the genome ensuring stable expression in 6-day-old larvae (Figure S2E). Injection of otp:Gal4 transgenic driver line with a plasmid harboring the otpa complementary DNA (cDNA) under the control of multiple Gal4 upstream activation sequence (UAS) significantly increases both basal and stress-induced crh mRNA levels, suggesting that Otpa regulates crh transcription in vivo (Figure 1H).
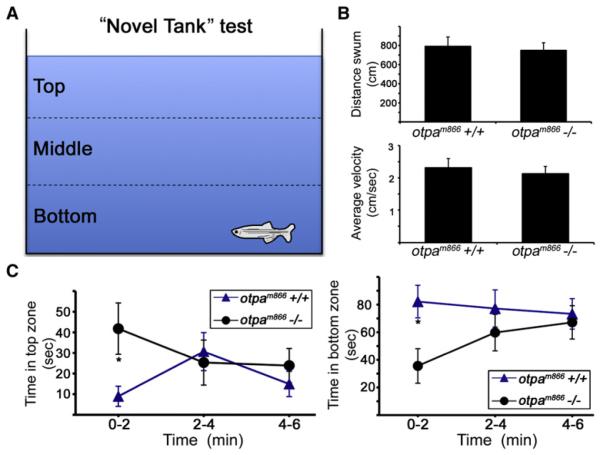
The effect of Otp on stress-related behavioral activity was tested in adult (4-month-old) otpam866 mutant animals. We performed a “novel tank-diving test,” which is the most extensively studied model measuring novelty stress in adult zebrafish (Bencan and Levin, 2008; Bencan et al., 2009; Egan et al., 2009; Levin et al., 2007; Wong et al., 2010). Following exposure of zebrafish to a novel tank environment, they have a clear preference toward the bottom third of the tank in the first 1–2 min, a tendency that is reduced to approximately chance levels by the end of a 6 min test (Figure 2A). We first showed that adult otpam866 mutants display normal locomotor activity, as judged by measuring their average velocity and distance traveled over the course of the test (Figure 2B, n = 11). We next measured the time spent in the top, middle, and bottom tank zones. No difference in the place preference to the middle zone was observed (data not shown). However, analysis of the top and bottom zones clearly showed that within the first 2 min following exposure to a novel environment, otpam866−/− animals spend significantly less time in the bottom tank zone and more time in the top zone when compared to their wild-type (WT) siblings, indicating that Otpa is necessary for normal behavioral response to novelty stress (Figure 2C). Taken together, these results show that the adaptive response to stress is impaired in the absence of otpa gene activity.
Figure 2. Otp Is Necessary for Novelty Stress Response.
(A) The “novel tank” test measures the vertical place preference of adult zebrafish following exposure to a novel environment. Four-month-old otpam866−/− and their WT siblings were moved from their home tank into a new tank with dissimilar dimensions, and their behavior was monitored for a period of 6 min using an automated video tracking system.
(B and C) Quantified behavioral parameters showing locomotor activity (B) and the time spent in the top and bottom thirds of the test tank (C) within consecutive time intervals (2 min, each). *p < 0.05; n = 11.
Otp Is Recruited to Stress Promoters
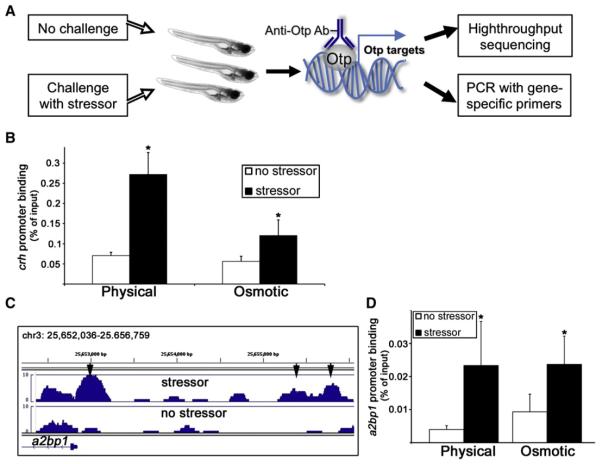
We next explored the mechanism underlying the effect of Otp on stress adaptation. In order to identify the prime targets of Otp regulation in response to homeostatic challenge, we performed chromatin immunoprecipitation (ChIP) assay using an anti-Otp antibody, followed by either promoter-specific quantitative PCR or high-throughput sequencing (ChIP-seq) (Figure 3A). We looked for genomic promoter regions that showed enrichment of Otp binding following either physical or osmotic stress. A complete analysis of the ChIP-seq experiment will be published elsewhere (L.A.-Z. and G.L. in preparation). Our ChIP analyses showed that the Otp protein is recruited to the fish crh promoter following exposure to physical and osmotic stressors (Figure 3B; Figure S3A). Otp was also found to form a complex with the crh promoter in the hypothalamic paraventricular nuclei dissected from mice that were subjected to a psychological stressor (Figure S4A). In agreement with the impaired stress response we observed in the otpam866 mutant (Figure 1G), the association of Otp with the crh promoter was significantly diminished in these animals (Figure S3B). Low-level enrichment of Otp binding to crh promoter in the otpam866 mutants is likely due to Otpa’s paralog Otpb, which is recognized by our polyclonal antibody. These experiments demonstrate that recruitment of Otp to the crh promoter is triggered by stress challenges and that this process is conserved in fish and mammals.
Figure 3. ChIP Assay Reveals Otp Association with Stress Promoters.
(A) Schematic representation of the experimental setup used for the ChIP-seq screen. A pool of fish (600 larvae per lane) were subjected to stress challenges in bulk or left unchallenged followed by anti-Otp ChIP. Putative Otp targets induced by stress were identified either by high-throughput sequencing or by qPCR with promoter-specific primers.
(B and D) Histograms showing quantitative ChIP analyses of the recruitment Otp to crh (*p < 0.05, n = 3) and a2bp1 (*p % 0.1, n = 3) promoters. Chromatin was extracted from a pool of 50 larvae per treatment 30 min after physical or osmotic challenges, followed by anti-Otp ChIP. Recruitment of Otp to the respective promoter was calculated relative to the amount of input chromatin.
(C) Stressor-induced Otp binding peaks (arrowheads), which were mapped at the vicinities of a2bp1 gene loci. Bar histograms represent the abundances of high-throughput sequencing reads in relation to their chromosomal location.
Another Otp target revealed by the ChIP-seq screen is the promoter of the a2bp1 gene (also known as rbfox1), which encodes a splicing factor known to regulate the alternative splicing of several neuronal transcripts linked to neuronal plasticity (Lee et al., 2009). As with crh, Otp forms a complex with the a2bp1 promoter following physical and osmotic stress in fish and in response to psychological stress in mice (Figures 3C and 3D; Figures S3C and S4B).
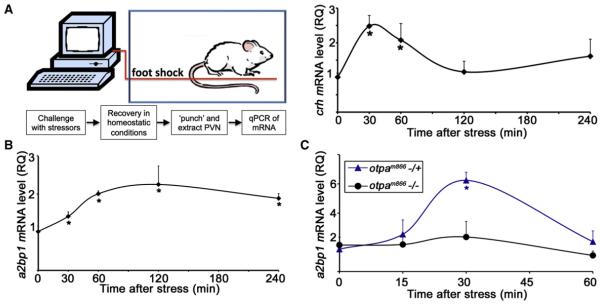
In agreement with this finding, an acute foot shock stressor in mice, which induced a stereotypical crh transcription, led to a rapid increase in the levels of a2bp1 mRNA (Figures 4A and 4B). Similar induction of a2bp1 mRNA expression was induced following exposure to stressors in the fish (Figure 4C; Figure S2D). In contrast, the stressor-induced increase in a2bp1 mRNA was significantly reduced in zebrafish larvae homozygous for the otpam866 mutant allele (Figure 4C; Figure S2D). As was the case with the crh promoter, ChIP analysis of otpam866−/− fish showed only residual binding of Otp to the a2bp1 promoter (Figure S3D). These results suggest that the splicing factor Ataxin 2-Binding Protein-1 (A2BP1) is involved in neuronal adaptation to stress, downstream of Otp. It remains to be determined whether the neurons in which Otp forms a complex with a2bp1 promoter are the same as the ones in which the protein binds to the crh promoter.
Figure 4. Splicing Factor A2BP1/Fox-1 Is Induced by Stressors.
(A and B) Acute stress paradigm performed on mice. A computer-controlled system was used to deliver a foot shock stress challenge to mice placed in a chamber. Mice were taken out of the chamber, PVN punches were dissected from mice at different time points following stress initiation, and crh (A) or a2bp1 (B) mRNA levels were analyzed by qPCR. *p < 0.05, n = 5. RQ, relative quantity.
(C) Physical stress challenges were applied, in bulk, to 6-day-old zebrafish larvae derived from an otpam866−/+ cross. The amount of a2bp1 mRNA was measured in individual fish larva at different time points of recovery using qPCR. Each tested larva was then genotyped by sequencing and a2bp1 mRNA levels of mutant (−/−) and heterozygous (−/+) animals were plotted accordingly. *p < 0.05; n = 4.
Otp Is Associated with the Stress Coactivator CREB
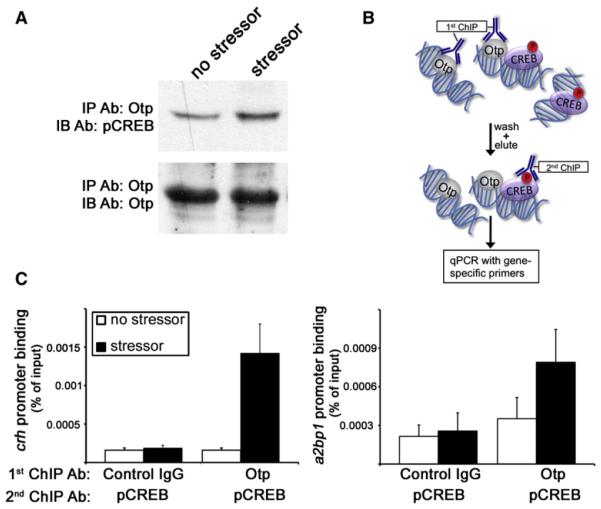
An important question is how, in response to stress, Otp protein is recruited to the crh and a2bp1 promoters. It has been shown that the phosphorylated form of the cyclic AMP (cAMP) response element-binding protein (CREB) is essential for activation of crh transcription (Brunson et al., 2001; Liu et al., 2008; Wöfl et al., 1999). We have therefore tested whether Otp is complexed with phospho-CREB (pCREB) in response to a stressful stimulus. Immunoprecipitation (IP) of larval protein extracts with an antiOtp antibody followed by immunoblotting with a pCREB-specific antibody showed that Otp forms a complex with pCREB, and this protein-protein interaction is enhanced by approximately 2-fold in response to stress (2.2 ± 0.34, n = 3; Figure 5A). We then performed sequential ChIP-reChIP assay, in which Otp-DNA complexes were isolated and then subjected to a second ChIP to detect the presence of the pCREB protein (Figure 5B). In agreement with the observed protein-protein association between Otp and pCREB, our ChIP-reChIP analysis demonstrated stress-induced co-occupancy of Otp and pCREB on the crh and a2bp1 promoters (Figures 5B and 5C). Taken together, these findings suggest that an association between Otp and pCREB proteins promotes the binding of this complex to crh and a2bp1 promoters.
Figure 5. pCREB-Otp Protein Complex Is Recruited to crh and a2bp1 Promoters.
(A) A pool of 100 larvae per lane were subjected to stress challenges in bulk or left unchallenged. Larvae were lysed and subjected to IP with a resin-coupled anti-Otp antibody (Ab) followed by gel electrophoresis and protein electrotransfer to a membrane. Nitrocellulose filters were immunoblotted with an antibody directed to pCREB, stripped, and reblotted with an anti-Otp antibody.
(B) Schematic representation of the sequential ChIP- reChIP procedure in which larvae lysates were first subjected to anti-Otp ChIP and the resulting Otp-DNA complex were eluted and subjected to a second ChIP with a pCREB antibody.
(C) A pool of fish (300 larvae per lane) were subjected to stress challenges in bulk or left unchallenged followed by the ChIP-reChIP procedure using control IgG, anti-Otp, and anti-pCREB antibodies. The histograms show promoter co-occupancy analysis of Otp and pCREB (n = 2).
Alternative Splicing of PAC1 Is Induced by Stress
A2BP1 is a sequence-specific RNA-binding protein that regulates alternative splicing of specific genes by either promoting or repressing the inclusion of alternatively spliced target exons (Lee et al., 2009; Zhang et al., 2008). a2bp1 and its targets are known to be expressed throughout the nervous system and in muscle tissues (Zhang et al., 2008). We searched for specific candidate a2bp1 targets that might be involved in the stress response. A recent study reported that several a2bp1 target exons are regulated by chronic neuronal depolarization (Lee et al., 2009). Notably, the alternative splicing of pac1 (also known as adcyap1r1), which encodes the receptor for the pituitary adenylate cyclase-activating peptide (PACAP) (Vaudry et al., 2009) has been shown to be regulated by a2bp1 in neurons (Lee et al., 2009; Zhang et al., 2008). Activation of PAC1 by PACAP, which leads to increased cAMP levels and recruitment of the phosphorylated CREB protein to crh promoter, is required for stress-induced crh transcription in vivo and in vitro (Agarwal et al., 2005; Kageyama et al., 2007; Stroth and Eiden, 2010). Moreover, the splice variants of PAC1 display differential intracellular signal transduction (Lyu et al., 2000; Mustafa et al., 2007; Mustafa et al., 2010; Pisegna and Wank, 1996; Spengler et al., 1993).
Because of the known involvement of PACAP and PAC1 in the stress response, we hypothesized that activity-dependent alternative splicing of PAC1, which alters its intracellular signaling mode, may be a unique mechanism for neuronal adaptation to stress. a2bp1 regulates the alternative splicing of pac1’s exon 14 (dubbed the “hop cassette”), which encodes 28 amino acids of the third intracellular loop of the mouse PAC1 protein (Lee et al., 2009; Vaudry et al., 2009; Zhang et al., 2008). We tested whether alternative splicing of the pac1 hop cassette is regulated by homeostatic challenge. Given that PAC1 is broadly expressed in the zebrafish brain (data not shown), it was difficult to analyze its alternative splicing in the PO of fish. We therefore analyzed whether a stressful challenge induces alternative splicing of PAC1 in the PVN, the major CRH-expressing hypothalamic component of the HPA axis, which can be surgically isolated from the mouse brain. The expression of both isoforms increased during the early stress recovery phase. At the late recovery phase of the stress response, the short pac1 isoform returned to its basal level, whereas long splice isoform, pac1hop, was retained at a significantly higher expression level (Figure 6A). Examining the ratio between the two splice isoforms throughout the recovery period revealed a consistent stress-induced increase in the long/short ratio, indicating a clear shift in the balance between these isoforms (Figure 6B). These results suggest that alternative splicing of the hop cassette, an a2bp1 target exon, may be involved in the adaptive response to stress.
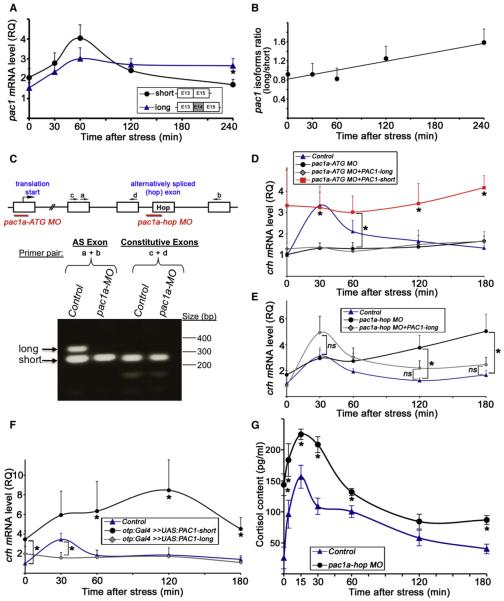
Figure 6. Modulation of Stress Response by Alternative Splicing of PAC1.
(A) Quantitative PCR analysis of the two pac1 splice isoforms in mice that were subjected to a foot shock challenge. PVN punches were harvested at different time points following stress initiation. The analyzed pac1 cDNA fragments corresponding to the short and long (hop) splice isoform are schematically shown. The hop cassette is encoded by exon 14 (E14). *p < 0.05, n = 11.
(B) Calculated ratio between the amounts of the long and short pac1 isoforms shown in (A) as a function of time after the initiation of the foot shock stressor showing a positive linear correlation. R2 = 0.9, n = 11.
(C) The top part shows a scheme depicting pac1a gene structure including the respective binding sites for two antisense MO oligonucleotides designed either to block expression of both PAC1 isoforms (pac1a-ATG MO) or prevent the inclusion of the hop exon by alternative splicing (pac1a-hop MO). The bottom part shows a RT analysis of 6-day-old larvae showing the effect of the pac1a-hop MO. The long (hop) and short splice variants (arrows) are visualized in the control larvae, whereas only the short isoform is present in pac1a-hop MO-injected animals. Inclusion of adjacent constitutive exons is not affected by the pac1a-hop MO.
(D–F) Quantitative PCR analyses of crh mRNA following a physical stress challenge of 6-day-old larva. The amount of crh mRNA was measured in individual fish larvae at different time points of recovery.
(D) Stress challenge was applied to either mock-treated 6-day-old larvae (control, n = 14) or their siblings that were injected with antisense pac1a-ATG MO (n = 12), which prevents the formation of both PAC1 isoforms. Alternatively, transgenic otp:Gal4 embryos expressing Gal4 in Otp+ cells were coinjected with pac1a-ATG MO together with transposon-based vector constructs harboring PAC1’s short (pac1a-ATG MO+PAC1-short, n = 4) and long (pac1a-ATG MO+PAC1-long, n = 4) isoforms under the control of ten UAS elements (UAS:PAC1-short or UAS:PAC1-long, respectively). *p % 0.01.
(E) Stress challenge was applied to either mock-treated 6-day-old transgenic otp:Gal4 larvae (control, n = 11) or their siblings, which were injected with a pac1ahop MO (n = 12) or pac1a-ATG MO together with UAS:PAC1-long construct (pac1a-ATG MO+PAC1-long, n = 6). *p % 0.05.
(F) Stress challenge was applied to either a otp:Gal4 transgenic line larvae (control, n = 10) or their siblings, which were injected with constructs containing the short (n = 7) or long (n = 6) PAC1 isoform under the control of ten UAS elements (otp:Gal4 >> UAS:PAC1-short or otp:Gal4 >> UAS:PAC1-long, respectively). The amount of crh mRNA was measured as described above. *p < 0.05.
(G) Analysis of cortisol content following a physical stress challenge applied to either mock-treated 6-day-old larvae (control) or their siblings, which were injected with pac1a-hop MO. Whole-body cortisol levels were measured at different time points of recovery in tissue extracts derived from pools of ten larvae. *p < 0.05, n = 3.
The following abbreviations are used: AS, alternative spliced exon; MO, morpholino; ns, not significant; RQ, relative quantity.
Alternative Splicing of PAC1 Terminates Stress Response
In view of the above, we examined whether formation of the PAC1-hop mRNA isoform might modulate the animal’s transcriptional response to stressors. To test this hypothesis, we designed two types of antisense morpholino (MO) knockdown reagents (Figure 6C): the first (pac1a-ATG MO) was designed to block expression of all PAC1 isoforms by directing it to PAC1’s translation start site. The second (pac1a-hop MO) was directed to the exon-intron boundary of the hop encoding exon of the zebrafish pac1a gene. This reagent caused exon skipping of the hop cassette in pac1a, preventing the formation of the long PAC1 isoform without affecting the short variant (Figure 6C; Figure S5).
Complete knockdown of all pac1 isoforms, using pac1a-ATG MO antisense oligonucleotide, led to a marked reduction in the stressor-induced activation of crh transcription (Figure 6D). This result is in agreement with the importance of PAC1/PACAP pathway for stress-induced crh transcription in vivo and in vitro (Agarwal et al., 2005; Kageyama et al., 2007; Stroth and Eiden, 2010). Reexpressing PAC1-short, but not PAC1-long, overrides the deficits in crh transcription that were caused by complete knockdown (pac1a-ATG MO) of all PAC1 isoforms, suggesting that the two isoforms mediate differential effect on crh transcription (Figure 6D).
We next examined whether blocking the alternative splicing of pac1a will affect crh transcription, by analyzing crh mRNA levels during the recovery phase of the stress response. We observed that whereas the amount of crh mRNA in mock-treated fish larvae had decreased to a low basal level by 120–180 min after stress initiation, pac1a-hop MO-injected larvae displayed significantly higher levels of crh mRNA at this late stress adaptation phase (Figure 6E; Figure S6A). Injection of an unrelated control MO did not affect the stress-induced crh response when compared to uninjected larvae (Figure S6B). The effect of pac1ahop MO was rescued by reexpressing the long (hop) isoform in Otp+ neurons (Figure 6E; pac1a-hop MO+PAC1-long). This was achieved by using the otp:Gal4 transgenic zebrafish line to drive the expression of pac1-hop, which was placed under the control of Gal4-responsive UAS elements.
As an alternative manner to examine the role of PAC1 splice variants in the stress response, we overexpressed either the short or the long PAC1 isoforms in the fish hypothalamus, thereby shifting the balance between the two proteins. As shown above, we injected either UAS:PAC1-short or UAS:PAC1-long constructs into the otp:Gal4 transgenic zebrafish line, expressing the Gal4 protein in the PO, which is the fish equivalent of the mammalian PVN (Fujimoto et al., 2011). Gain of function of PAC1-short resulted in a constitutive increase in crh levels, whereas overexpressing the long isoform prevented the stress- induced activation of crh transcription (Figure 6F).
These results suggest that the short PAC1 variant positively affects crh transcription, whereas stressor-induced formation of the PAC1-hop mRNA specie leads to an intracellular signaling switch that mitigates crh synthesis during the recovery phase of the stress response, thereby terminating the ongoing stress reaction.
PACAP signaling controls corticosterone secretion in response to a psychological stressor in the mouse (Stroth and Eiden, 2010). We examined whether perturbation of PAC1 splicing might influence the physiological stress response by measuring cortisol levels, the main biomarker for the activation of the HPA axis in mammals and fish. Similar to its effect on crh mRNA levels, injection of pac1a-hop MO led to significant changes in whole larva cortisol content including an increased basal level and a heightened kinetic response (Figure 6G).
Inhibition of PAC1 Splicing Causes Delayed Adaptation to Anxiogenic Stress
Similar to other animals, zebrafish larvae exhibit a stress-related anxiety-like behavior that can be measured using a light-dark preference test (Steenbergen et al., 2011). We placed 6-day- old larvae in a two-compartment light-dark measuring arena and recorded the amount of time spent on the dark side (Figure 7A). In agreement with Steenbergen et al. (2011), larvae showed a strong aversion to the dark side of the arena, spending 95.5% of time on white and only 4.5% of time on dark (Figure 7B). Six-day-old pac1a-hop MO-injected larvae, in which the formation of the long PAC1 isoform is blocked, initially showed a less-pronounced phenotype and spent longer (8.4% of their time) on the dark side of the box. Larvae injected with an unrelated MO did not behave differently than uninjected controls, ruling out an effect of injection per se (data not shown).
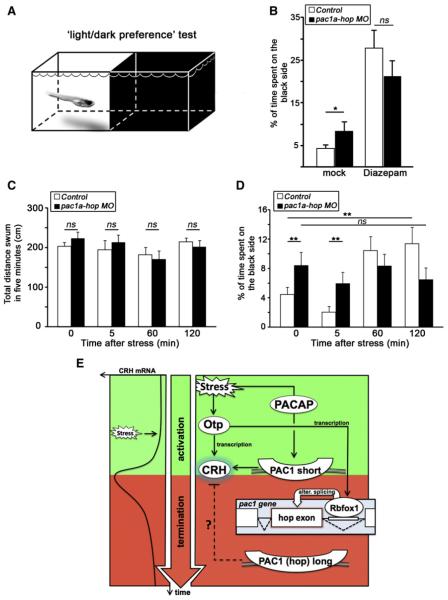
Figure 7. Anxiety-like Behavior following Inhibition of PAC1 Splicing.
(A) Schematic representation of the two-compartment arena used for the “light-dark preference” behavioral test of 6-day-old larvae (adopted from [Steenbergen et al., 2011]). The assay measures anxiety-like dark avoidance behavior.
(B) Histogram showing the percentage of time spent on the black side of the two-compartment light-dark measuring arena. Treatment of larvae with 5 μm Diazepam decreases dark avoidance in a light-dark box. *p < 0.05, n = 24.
(C and D) Control (n = 36) and pac1a-hop morphant (n = 33) larvae were challenged with an osmotic stressor for 4 min, and their total distance swum in the test arena (C) and dark avoidance (D) were measured for 5 min at 0, 5, 60, and 120 min time points of recovery from osmotic shock.
**p < 0.01; ns, not significant.
(E) A model summarizing the roles of Otp and alternative splicing in stress adaptation. The stereotypic kinetics of stress-induced crh mRNA levels is shown on the left, whereas the genetic and biochemical interactions are illustrated on the right. Otp and the short PAC1 isoform (a receptor for the PACAP neuropeptide) promote crh transcription, whereas the generation of the long PAC1 (hop) alternative (alter.) splice variant terminates crh transcription at the late recovery phase (see text).
In order to confirm that place preference in this setup measures an aspect of stress-related anxiety-like behavior, we treated both WT and pac1a-hop MO-injected larvae with 5 μm Diazepam and remeasured the amount of time spent in the dark (Figure 7B). Diazepam treatment increased the amount of time spent in the dark side of the arena for both treatment groups (Figure 7B; n = 24, p < 0.0001) and normalized the behavior of morphants, confirming a link to anxiety-like behavior.
pac1a-hop MO-injected larvae show an abnormal termination of the response to stress, as revealed by measuring crh mRNA and cortisol levels (Figure 6). To test for any possible behavioral phenotype correlated to this, we next analyzed the behavioral response to osmotic stress in both control WT and pac1a-hop morphant larvae. Larvae from both groups were given an osmotic stress, and their dark avoidance was measured during recovery from stress at 0, 5, 60, and 120 min time points. We first showed that inhibition of PAC1 splicing does not affect larval locomotion by demonstrating that there is no significant difference in the total distance swum for either control or morphant group at each of the measurement periods (Figure 7C). We next found that the early response of both WT and morphant larvae to osmotic stress was indistinguishable, with a trend reduction of dark avoidance (and thus only a mild increase in anxiety-like behavior) in both treatment groups (p < 0.12, Figure 7D). However, the recovery of WT larvae was rapid, and they quickly became less anxious over time, decreasing their dark avoidance at both 1 and 2 hr time points (Figure 7D). Conversely, in keeping with the altered recovery of crh mRNA and cortisol levels, pac1a-hop-injected larvae showed an abnormal recovery from osmotic shock: at both 1 and 2 hr time points, their avoidance of the dark side of the arena was not significantly different than the initial measures and showed a trend increase compared to their uninjected siblings.
Although we cannot as yet directly connect this behavior to our crh and cortisol measurements, the delayed behavioral response of pac1a-hop morphant larvae at the recovery phase correlates with their respective failure to terminate crh levels following stressors (Figure 6).
Taken together, these findings indicate that the formation of the PAC1-hop splice variant is necessary for termination of stress-induced crh synthesis, normal activation of the HPA axis, and adaptive anxiety-like behavioral response. The underlying mechanism, by which the two PAC1 splice isoforms affect crh synthesis and anxiety-like behavior during stress adaptation, remains to be determined.
In summary, we have identified a regulatory network linking stress stimuli with crh transcription. As depicted in Figure 7E, our model suggests that in response to various stressors, Otp and the short PAC1 splice variant modulate transcriptional activation of crh to adapt to the changes in homeostasis. Otp may contribute to the termination of crh transcription by regulating the splicing factor A2BP1, which in turn promotes the formation of the long PAC1-hop splice variant. Generation of the long PAC1-hop splice variant terminates both stress-induced crh transcription and HPA activation by means yet to be uncovered.
DISCUSSION
Stress occurs when an animal’s state of homeostasis is threatened or perceived to be so (Chrousos, 1998, 2009; Engelmann et al., 2004; Selye, 1936). The adaptive response to most stressors involves the release of crh followed by rapid changes in its transcription (Aguilera, 1998; Vale et al., 1981; Yao and Denver, 2007). However, the exact intracellular signaling pathways that modulate crh synthesis during stress adaptation remain unclear. To date, regulation of crh transcription has only been addressed using either in vitro cell transfection assays or application of pharmacological agents in animal models. Our study provides pioneering in vivo evidence for a new molecular mechanism of stress adaptation. We show that stress-induced crh levels are regulated by the transcription factor Otp and the transmembrane neuropeptide receptor PAC1. We have also demonstrated that the generation of a PAC1 splice variant by means of alternative splicing causes a signaling switch that terminates crh transcription in response to stress and leads to dysregulated HPA axis response.
The activation of crh transcription following stressful stimuli is a biological response shared by all vertebrate species (Bernier et al., 2009; Burbach, 2002; Yao and Denver, 2007). This speaks to the importance of this pathway and implies that an evolutionarily conserved biochemical cascade controls the synthesis of CRH. In this respect, the hypothalamic neuroendocrine-specific factor Otp was an obvious candidate mediator of stress. Otp is expressed in the mature crh neurons of fish and mouse. Otp deficient mice display impaired development of all hypothalamic neuroendocrine cell types (Acampora et al., 1999; Wang and Lufkin, 2000). In contrast, we found that the zebrafish otpam866 mutant fish, which carries the partially redundant otpb duplicated paralog, displays normal development of CRH-containing neurons. This has allowed us to examine the role of Otp in mediation of stress response and to demonstrate that it regulates crh synthesis during stress adaptation.
A major finding of this study is that stress response is modu- lated by a mechanism that involves activity-dependent alternative splicing. Specifically, we found that the splicing factor A2BP1/Rbfox-1, the mammalian homolog of the Caenorhabditis elegans Feminizing gene 1 on X (Jin et al., 2003; Nakahata and Kawamoto, 2005; Underwood et al., 2005), is induced by stress in an Otp-dependent manner. a2bp1 is known to regulate the alternative splicing of several genes involved in neuronal and synaptic plasticity, including the hop cassette of the trans- membrane receptor PAC1/Adcyap1r1 (Lee et al., 2009). Notably, dysregulated splicing of A2BP1-dependent alternative exons was observed in the Autism spectrum disorder (ASD) brain (Voineagu et al., 2011). In the present study, we focused on PAC1, whose high-affinity ligand, PACAP/Adcyap, is an important mediator of crh synthesis in the hypothalamus (Agarwal et al., 2005; Kageyama et al., 2007; Stroth and Eiden, 2010). We now show that the alternative splicing of PAC1 receptor is induced by stress and that the termination of stress-induced crh synthesis, as well as normal stress-induced cortisol levels and adaptation to an anxiogenic stimulus, require the generation of the PAC1-hop splice variant. Previous studies performed in cell culture systems have revealed that inclusion of the hop cassette alters PAC1’s intracellular mode of signaling, including diverse coupling to G proteins (Pisegna and Wank, 1996; Spengler et al., 1993), changes in Ca2+ mobilization and neurosecretion (Mustafa et al., 2007; Mustafa et al., 2010), and/or different rate of endocytosis (Lyu et al., 2000). Our study now links the alternative splicing of PAC1 with animal physiology. It remains to be determined which of the above biochemical mechanisms is relevant to the regulation of crh synthesis and adaptive anxiety-like behavior in an in vivo setting.
As discussed above, we have implicated Otp in both stress- induced crh mRNA induction and its subsequent downregulation. It should be noted that there are other known mechanisms involved in termination of ongoing stress response. For example, corticosteroids can repress stress-induced crh transcription through the action of the glucocorticoid and mineralocorticoid receptors (de Kloet et al., 2005; Joëls and Baram, 2009; Ulrich-Lai and Herman, 2009). Further analysis is necessary to examine the possible interactions between corticosteroids, PAC1-hop, and/or the homeodomain protein Otp.
Stress is a major contributor to psychosocial pathologies in humans (Joëls and Baram, 2009; Lupien et al., 2009; McEwen, 2003; Ulrich-Lai and Herman, 2009). The signaling pathway presented here may be relevant to neurodevelopmental and psychiatric disorders exhibiting dysregulated stress responses. a2bp1 is associated with disorders such as epilepsy, mental retardation, bipolar disorder, and autism (Bhalla et al., 2004; Elia et al., 2009; Hamshere et al., 2009; Le-Niculescu et al., 2009; Martin et al., 2007). PACAP, the high-affinity ligand for PAC1, is associated with schizophrenia and major depressive disorder, and PACAP- deficient mice display psychobehavioral abnormalities (Hashimoto et al., 2007; Ishiguro et al., 2001; Ishihama et al., 2010; Stroth and Eiden, 2010). PACAP-PAC1 pathway was recently implicated in abnormal stress responses underlying post-traumatic stress disorder (Ressler et al., 2011). Otp, PAC1, and crh are not only expressed in the PVN but also in several nuclei of the extended amygdala, which are associated with such stress-related behaviors as anxiety, fear, and memory (Bardet et al., 2008; Chrousos, 2009; Garcá-Moreno et al., 2010; Hashimoto et al., 1996; Regev et al., 2010). Finally, both crh and a2bp1 are repressed by the methyl-CpG binding protein 2 (MeCP2), which causes the neurodevelopmental disorder Rett syndrome (Chahrour et al., 2008; McGill et al., 2006). Because Rett syndrome patients often display abnormal stress responses, manifested in episodes of heightened anxiety, it is worth exploring whether a biochemical mechanism of stress adaptation similar to the one described here is shared by MeCP2 and Otp.
EXPERIMENTAL PROCEDURES
Antibodies Transgenes and Plasmid Constructs
The anti-Otp antibody was previously described (Blechman et al., 2007). Rabbit polyclonal antibody directed to phospho-CREB(Ser133) (Cat. #9191) was purchased from Cell Signaling Technology (Boston, MA). The otp:Gal4 transgenic line [Tg(otpb:Gal4, myl7:EGFP)zc57] was previously described (Fujimoto et al., 2011). We used the Tol2kit transposon-based transgenic vectors system (Kwan et al., 2007) for site-specific recombination-based cloning of the otpa, pac1a-short, and pac1a-long cDNAs downstream of 103 UAS element and basal promoter for Gal4 response. This system efficiently integrates into the genome, ensuring stable expression in 6-day-old larvae (Kawakami et al., 2004).
Stress Paradigms
Experiments were performed in accordance with protocols approved by the Weizmann Institute’s Institutional Animal Care and Use Committee.
Physical and Osmotic Stress in Fish
Stress challenges in fish were applied to either the zebrafish Tupfel long fin (TL) line or the progenies of otpam866−/+ outbred to TL. In all experiments, zebrafish were allowed to acclimatize to the test vessel (90 3 15 μm MINIPLAST dish, Ein-Shemer, Israel) overnight at 28.5° C (100 larvae/dish) in Danieau’s medium [1.74 μm NaCl, 0.21 μm KCl, 0.12 μm MgSO4, 0.18 μmM Ca(NO3)2, 0.15 μm HEPES pH 7.4]. Exposure to stressor was performed on 6-day-old larvae at 8:30 AM. Control unchallenged fish were kept in a separate dish without further handling.
Exposure to osmotic stressor was done by transferring the fish to 50% artificial seawater (3.5 gr instant ocean salt in 200 ml Danieau’s buffer) for a period of 4 min and then washed and returned to Danieau’s medium at 28.5° C. Physical stress was induced by netting the larvae for a period of 4 min. In short, larvae were transferred to a cell strainer (Falcon Blue 40 UM, BD Biosceinces, NJ, USA) and excess water drops were absorbed. After a period of 4 min, the embryos were washed back with Danieau’s medium at 28.5° C. Fish were collected at different time points during a recovery period, which followed the exposure to stressors, transferred to ice, collected in centrifuge tubes, and immediately frozen at -80° C.
Novelty Stress in Adult Zebrafish
Adult zebrafish (4 months old) were habituated to the test room for 1 hr before the test. For the assessment of anxiety-like behavior we used the “novel tank diving test” as previously described (Cachat et al., 2010), with some modifications concerning the tank dimensions. The parameters measured in the test were quantified using an automated video tracking system (EthoVision XT7; Noldus Information Technology, The Netherlands).
Analysis of Stress-Related Anxiety in Zebrafish Larvae
Stress-related anxiety-like behavior was measured by recording the preference of 6-day-old zebrafish larvae for either side of a custom-made light-dark arena. Behavior was recorded by video tracking larvae for a 5 min time period using Videotrack software (Videotrack; ViewPoint Life Sciences, Lyon, France). Injected larvae were placed into separate light-dark arenas (modified from Rechteckdosen, Neo- Lab) inside a Zebrabox (ViewPoint Life Sciences) and were allowed to habituate for 5 min before recording. The arenas were divided into two equal compartments by covering the sides with either white or black opaque tape. The bottom of the dark was side was covered with a neutral gray ND8 photography filter (Cokin, France), and the light side was covered with clear plexi- glass. The arenas were illuminated from below with both infrared and white light. The distance swum was calculated by tracking the larvae for 5 min using a high-speed infrared camera with the Videotrack software set to a detection threshold of 11, inactive-small threshold of 5, and small-large threshold of 10. Preference for either side of the arena was measured by defining two separate recording areas using the ViewPoint software. The total distance swum was also calculated for each larva. Any animals that stopped swimming for periods of 60 s or more, a behavior never observed in WT larvae, were removed from the analysis. For the Diazepam experiment, larvae were incubated in 5 μm Diazepam (Ratiopharm) for 1 hr before recording.
Foot Shock and Restrain Stress in Mice
PVN “punches” (see below) were taken from mice before (basal) and 30, 60, 120, and 240 min following stress initiation. Foot shock was delivered using a computer-controlled fear conditioning system (TSE Systems, Bad Homburg, Germany). Mice were placed in the chamber, a clear Plexiglas cage (21 cm 3 20 cm 3 height 36 cm) with a stainless steel floor grid. During a 3 min session, two shocks (0.7 mA, 2 s, constant current) with an interval of 1 min were delivered to the mice through the metal grid floor. Mice were then taken out of the chamber 1 min following the last shock. The restraint stress was induced as described (Regev et al., 2010) by confining the mice to a cut 50 ml plastic conical tube for a period of 30 min such that they were held immobile. Care was taken to assure unobstructed breathing of the animals.
Paraventricular Nucleus Punch Method
The PVN of the hypothalamus was microdissected using the Palkovits technique as described (Sztainberg et al., 2010). Immediately after the decapitation, the brain was removed and placed into a 1 μm metal matrix (Stoelting Co., Wood Dale, IL, USA, cat# 51386). The brain was sliced using standard razor blades (GEM, Personna American Safety Razor Co., Cedar Knolls, NJ, USA; cat# 62–0165) into 2 μm slices. PVN were either quickly frozen for quantitative PCR or immediately fixed in 2.2% formaldehyde for the ChIP analyses. The PVN was punched using a microdissecting needle of an appropriate size.
RNA Preparation and Gene Expression Analyses
In situ hybridization and immunostaining were performed as we previously described (Blechman et al., 2007). Total RNA was prepared from fish larvae and mouse PVN tissues using the TRI-REAGENT (Molecular Research Center, Cincinnati, OH, USA) according to the manufacturer’s instructions. RNA preparations were treated with DNase recombinant I, RNase free (Roche, Basel, Switzerland) to avoid contamination of genomic DNA. First-strand cDNA was synthesized from 0.5–1 mg of total RNA using an oligo(dT)15 primer and SuperScript II reverse transcriptase (RT) (Invitrogen, Blackrock, Co. Dublin, Ireland). The PCR was performed in 96 plates using a 7300 real time PCR system (Applied Biosystem, Foster City, CA, USA). The total cDNAs were amplified using a SYBR Green qPCR kit (Finnzymes, Espoo, Finland) according to the manufacturer’s instructions. The relative quantity (RQ) was calculated using the Relative Quantification program (Sequence Detection System software version 1.2.2) according to the comparative method. Samples were normalized according an endogenous gene (b-actin for zebrafish fish or hprt for mouse) and to a control time zero sample, which served as a calibrator.
Chromatin Immunoprecipitation, Immunoprecipitation, and Immunoblot Analyses
ChIP analysis was performed on 6-day-old larvae (pools of 50 larvae per treatment) or adult mouse PVNs (pools of five punches per treatment) as described in Blechman et al. (2011). For IP, we used 4 ml of affinity-purified anti-Otp, 1 ml of anti-Pol II, or control Rabbit IgG. Sequential ChIP-reChIP analysis was performed using a previously described protocol (Furlan-Magaril et al., 2009).
Proteins for IP were extracted from a pool of 100 fish larvae per treatment as we previously described (Blechman et al., 2007). IP was performed using affinity-purified anti-Otp antibody, which was covalently bound to an Affi-Gel 10 beads (Bio-Rad, Hercules, CA, USA) and incubated with the protein extracts for 3 hr at 4° C. Thereafter, beads were extensively washed and protein complexes were eluted (200 μm Glycine-HCl pH 2.7, 150 μm NaCl) for 15 min at room temperature. The resulting protein complexes were subjected to 12% SDS-PAGE under nonreducing conditions, followed by immunoblotting with an antibody directed to phospho-CREB(Ser133). Nitrocellulose membranes were stripped and reblotted with an anti-Otp antibody.
Cortisol Measurements
Pools of 6-day-old zebrafish (ten larvae) were used for cortisol measurements. Cortisol was extracted as previously described (Alsop and Vijayan, 2008), and whole-larvae cortisol content was quantified using the cortisol enzyme immunoassay (EIA) kit (Cayman Chemical, Ann Arbor, MI, USA) according to the manufacturer’s instructions.
Statistical Analysis
For the behavioral analyses, p values were calculated using a one-way analysis of variance followed by an unpaired t test if applicable. Elsewhere, statistical significance was determined using unpaired t test. All error bars indicate SEM. All data was analyzed using Microsoft Excel.
Supplementary Material
ACKNOWLEDGMENTS
Thanks are due to Shifra Ben Dor and Dena Leshkowitz for sequence, phylogenetic, and genomic analyses; Raya Eilam for assisting with the immunohistochemistry of Otp; Limor Ziv and Berta Levavi-Sivan for help with the cortisol measurements; Wolfgang Driever and Soojin Ryu for kindly providing the otpam866 mutant line; Amos Gutnick for graphic illustrations; Chi-Bin Chien for the Tol2kit plasmid vectors; Giselbert Hauptmann for the crh probe; and Mike Fainzilber, Elior Peles, Marnie Halpern, Avraham Yaron, and Amos Gutnick for comments on this manuscript. The research in the Levkowitz laboratory is supported by the German-Israeli Foundation, Israel Science Foundation, Kirk Center for Childhood Cancer and Immunological Disorders, and Irvin Green Alzheimer’s Research Fund. G.L. is an incumbent of the Tauro Career Development Chair in Biomedical Research. L.A.-Z designed and performed most of the experiments and collected and analyzed the data. J.B. performed the coimmunoprecipitation and cortisol measurements and participated in PAC1 gain-of-function experiments. A.R., N.B., and M.T. performed the in situ hybridization and immunostaining. J.L.B. generated the transgenic otpb:Gal4 transgenic line. Y.S., A.R., and G.L. performed the novelty stress assay in fish. W.H.J.N. and L.B.-C. designed, performed, and analyzed the larval anxiety-like behavior. Y.S. and A.C. performed stress challenges and PVN dissection procedures in mice. G.L. initiated and headed the project and prepared the figures and the manuscript. All authors discussed the results and contributed to the data interpretation.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes six figures and Supplemental Experimental Procedures and can be found with this article online at doi:10.1016/j.neuron. 2011.11.019.
REFERENCES
- Acampora D, Postiglione MP, Avantaggiato V, Di Bonito M, Vaccarino FM, Michaud J, Simeone A. Progressive impairment of developing neuroendocrine cell lineages in the hypothalamus of mice lacking the Orthopedia gene. Genes Dev. 1999;13:2787–2800. doi: 10.1101/gad.13.21.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A, Halvorson LM, Legradi G. Pituitary adenylate cyclase-activating polypeptide (PACAP) mimics neuroendocrine and behavioral manifestations of stress: Evidence for PKA-mediated expression of the corticotropin-releasing hormone (CRH) gene. Brain Res. Mol. Brain Res. 2005;138:45–57. doi: 10.1016/j.molbrainres.2005.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilera G. Corticotropin releasing hormone, receptor regulation and the stress response. Trends Endocrinol. Metab. 1998;9:329–336. doi: 10.1016/s1043-2760(98)00079-4. [DOI] [PubMed] [Google Scholar]
- Alsop D, Vijayan MM. Development of the corticosteroid stress axis and receptor expression in zebrafish. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;294:R711–R719. doi: 10.1152/ajpregu.00671.2007. [DOI] [PubMed] [Google Scholar]
- Bardet SM, Martinez-de-la-Torre M, Northcutt RG, Rubenstein JL, Puelles L. Conserved pattern of Otp-positive cells in the paraventricular nucleus and other hypothalamic sites of tetrapods. Brain Res. Bull. 2008;75:231–235. doi: 10.1016/j.brainresbull.2007.10.037. [DOI] [PubMed] [Google Scholar]
- Barry TP, Malison JA, Held JA, Parrish JJ. Ontogeny of the cortisol stress response in larval rainbow trout. Gen. Comp. Endocrinol. 1995;97:57–65. doi: 10.1006/gcen.1995.1006. [DOI] [PubMed] [Google Scholar]
- Bencan Z, Levin ED. The role of alpha7 and alpha4beta2 nicotinic receptors in the nicotine-induced anxiolytic effect in zebrafish. Physiol. Behav. 2008;95:408–412. doi: 10.1016/j.physbeh.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bencan Z, Sledge D, Levin ED. Buspirone, chlordiazepoxide and diazepam effects in a zebrafish model of anxiety. Pharmacol. Biochem. Behav. 2009;94:75–80. doi: 10.1016/j.pbb.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier NJ, Flik G, Klaren PHM. Regulation and contribution of the corticotropic, melanotropic and thyrotropic axes to the stress response in fishes. In: Bernier NJ, Van Der Kraak G, Farrell AP, Brauner CJ, editors. Fish Neuroendocrinology. Elsevier Inc.; London: 2009. pp. 235–311. [Google Scholar]
- Bhalla K, Phillips HA, Crawford J, McKenzie OL, Mulley JC, Eyre H, Gardner AE, Kremmidiotis G, Callen DF. The de novo chromosome 16 translocations of two patients with abnormal phenotypes (mental retardation and epilepsy) disrupt the a2bp1 gene. J. Hum. Genet. 2004;49:308–311. doi: 10.1007/s10038-004-0145-4. [DOI] [PubMed] [Google Scholar]
- Blechman J, Borodovsky N, Eisenberg M, Nabel-Rosen H, Grimm J, Levkowitz G. Specification of hypothalamic neurons by dual regulation of the homeodomain protein Orthopedia. Development. 2007;134:4417–4426. doi: 10.1242/dev.011262. [DOI] [PubMed] [Google Scholar]
- Blechman J, Amir-Zilberstein L, Gutnick A, Ben-Dor S, Levkowitz G. The metabolic regulator PGC-1a directly controls the expression of the hypothalamic neuropeptide oxytocin. J. Neurosci. 2011;31:14835–14840. doi: 10.1523/JNEUROSCI.1798-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunson KL, Avishai-Eliner S, Hatalski CG, Baram TZ. Neurobiology of the stress response early in life: evolution of a concept and the role of corticotropin releasing hormone. Mol. Psychiatry. 2001;6:647–656. doi: 10.1038/sj.mp.4000942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbach JP. Regulation of gene promoters of hypothalamic peptides. Front. Neuroendocrinol. 2002;23:342–369. doi: 10.1016/s0091-3022(02)00005-5. [DOI] [PubMed] [Google Scholar]
- Cachat J, Stewart A, Grossman L, Gaikwad S, Kadri F, Chung KM, Wu N, Wong K, Roy S, Suciu C, et al. Measuring behavioral and endocrine responses to novelty stress in adult zebrafish. Nat. Protoc. 2010;5:1786–1799. doi: 10.1038/nprot.2010.140. [DOI] [PubMed] [Google Scholar]
- Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J, Zoghbi HY. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320:1224–1229. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP. Stressors, stress, and neuroendocrine integration of the adaptive response. The 1997 Hans Selye Memorial Lecture. Ann. N Y Acad. Sci. 1998;851:311–335. doi: 10.1111/j.1749-6632.1998.tb09006.x. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5:374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat. Rev. Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Egan RJ, Bergner CL, Hart PC, Cachat JM, Canavello PR, Elegante MF, Elkhayat SI, Bartels BK, Tien AK, Tien DH, et al. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav. Brain Res. 2009;205:38–44. doi: 10.1016/j.bbr.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elia J, Gai X, Xie HM, Perin JC, Geiger E, Glessner JT, D’Arcy M, Deberardinis R, Frackelton E, Kim C, et al. Rare structural variants found in attention-deficit hyperactivity disorder are preferentially associated with neurodevelopmental genes. Mol Psychiatry. 2009;15:637–646. doi: 10.1038/mp.2009.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann M, Landgraf R, Wotjak CT. The hypothalamic- neurohypophysial system regulates the hypothalamic-pituitary-adrenal axis under stress: an old concept revisited. Front. Neuroendocrinol. 2004;25:132–149. doi: 10.1016/j.yfrne.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Fujimoto E, Stevenson TJ, Chien CB, Bonkowsky JL. Identification of a dopaminergic enhancer indicates complexity in vertebrate dopamine neuron phenotype specification. Dev Biol. 2011;352:393–404. doi: 10.1016/j.ydbio.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlan-Magaril M, Rincön-Arano H, Recillas-Targa F. Sequential chromatin immunoprecipitation protocol: ChIP-reChIP. Methods Mol. Biol. 2009;543:253–266. doi: 10.1007/978-1-60327-015-1_17. [DOI] [PubMed] [Google Scholar]
- Fuzzen ML, Van Der Kraak G, Bernier NJ. Stirring up new ideas about the regulation of the hypothalamic-pituitary-interrenal axis in zebrafish (Danio rerio) Zebrafish. 2010;7:349–358. doi: 10.1089/zeb.2010.0662. [DOI] [PubMed] [Google Scholar]
- García-Moreno F, Pedraza M, Di Giovannantonio LG, Di Salvio M, Löpez-Mascaraque L, Simeone A, De Carlos JA. A neuronal migratory pathway crossing from diencephalon to telencephalon populates amygdala nuclei. Nat. Neurosci. 2010;13:680–689. doi: 10.1038/nn.2556. [DOI] [PubMed] [Google Scholar]
- Hamshere ML, Green EK, Jones IR, Jones L, Moskvina V, Kirov G, Grozeva D, Nikolov I, Vukcevic D, Caesar S, et al. Wellcome Trust Case Control Consortium Genetic utility of broadly defined bipolar schizoaffective disorder as a diagnostic concept. Br. J. Psychiatry. 2009;195:23–29. doi: 10.1192/bjp.bp.108.061424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto H, Nogi H, Mori K, Ohishi H, Shigemoto R, Yamamoto K, Matsuda T, Mizuno N, Nagata S, Baba A. Distribution of the mRNA for a pituitary adenylate cyclase-activating polypeptide receptor in the rat brain: an in situ hybridization study. J. Comp. Neurol. 1996;371:567–577. doi: 10.1002/(SICI)1096-9861(19960805)371:4<567::AID-CNE6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Hashimoto H, Shintani N, Chiba S, Hattori S, Okada T, Nakajima M, Tanaka K, Kawagishi N, Nemoto K, et al. Pituitary adenylate cyclase-activating polypeptide is associated with schizophrenia. Mol. Psychiatry. 2007;12:1026–1032. doi: 10.1038/sj.mp.4001982. [DOI] [PubMed] [Google Scholar]
- Herman JP, Scháfer KH, Sladek CD, Day R, Young EA, Akil H, Watson SJ. Chronic electroconvulsive shock treatment elicits upregulation of CRF and AVP mRNA in select populations of neuroendocrine neurons. Brain Res. 1989;501:235–246. doi: 10.1016/0006-8993(89)90641-0. [DOI] [PubMed] [Google Scholar]
- Herman JP, Schafer MK, Thompson RC, Watson SJ. Rapid regulation of corticotropin-releasing hormone gene transcription in vivo. Mol. Endocrinol. 1992;6:1061–1069. doi: 10.1210/mend.6.7.1324419. [DOI] [PubMed] [Google Scholar]
- Ishiguro H, Ohtsuki T, Okubo Y, Kurumaji A, Arinami T. Association analysis of the pituitary adenyl cyclase activating peptide gene (PACAP) on chromosome 18p11 with schizophrenia and bipolar disorders. J. Neural Transm. 2001;108:849–854. doi: 10.1007/s007020170034. [DOI] [PubMed] [Google Scholar]
- Ishihama T, Ago Y, Shintani N, Hashimoto H, Baba A, Takuma K, Matsuda T. Environmental factors during early developmental period influence psychobehavioral abnormalities in adult PACAP-deficient mice. Behav. Brain Res. 2010;209:274–280. doi: 10.1016/j.bbr.2010.02.009. [DOI] [PubMed] [Google Scholar]
- Jin Y, Suzuki H, Maegawa S, Endo H, Sugano S, Hashimoto K, Yasuda K, Inoue K. A vertebrate RNA-binding protein Fox-1 regulates tissue-specific splicing via the pentanucleotide GCAUG. EMBO J. 2003;22:905–912. doi: 10.1093/emboj/cdg089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joëls M, Baram TZ. The neuro-symphony of stress. Nat. Rev. Neurosci. 2009;10:459–466. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama K, Hanada K, Iwasaki Y, Sakihara S, Nigawara T, Kasckow J, Suda T. Pituitary adenylate cyclase-activating polypeptide stimulates corticotropin-releasing factor, vasopressin and interleukin-6 gene transcription in hypothalamic 4B cells. J. Endocrinol. 2007;195:199–211. doi: 10.1677/JOE-07-0125. [DOI] [PubMed] [Google Scholar]
- Kawakami K, Takeda H, Kawakami N, Kobayashi M, Matsuda N, Mishina M. A transposon-mediated gene trap approach identifies developmentally regulated genes in zebrafish. Dev. Cell. 2004;7:133–144. doi: 10.1016/j.devcel.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Kwan KM, Fujimoto E, Grabher C, Mangum BD, Hardy ME, Campbell DS, Parant JM, Yost HJ, Kanki JP, Chien CB. The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev. Dyn. 2007;236:3088–3099. doi: 10.1002/dvdy.21343. [DOI] [PubMed] [Google Scholar]
- Le-Niculescu H, Patel SD, Bhat M, Kuczenski R, Faraone SV, Tsuang MT, McMahon FJ, Schork NJ, Nurnberger JI, Jr., Niculescu AB., 3rd. Convergent functional genomics of genome-wide association data for bipolar disorder: comprehensive identification of candidate genes, pathways and mechanisms. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2009;150B:155–181. doi: 10.1002/ajmg.b.30887. [DOI] [PubMed] [Google Scholar]
- Lee JA, Tang ZZ, Black DL. An inducible change in Fox-1/ a2bp1 splicing modulates the alternative splicing of downstream neuronal target exons. Genes Dev. 2009;23:2284–2293. doi: 10.1101/gad.1837009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Bencan Z, Cerutti DT. Anxiolytic effects of nicotine in zebrafish. Physiol. Behav. 2007;90:54–58. doi: 10.1016/j.physbeh.2006.08.026. [DOI] [PubMed] [Google Scholar]
- Liu Y, Kamitakahara A, Kim AJ, Aguilera G. Cyclic adenosine 30 ,50 -monophosphate responsive element binding protein phosphorylation is required but not sufficient for activation of corticotropin-releasing hormone transcription. Endocrinology. 2008;149:3512–3520. doi: 10.1210/en.2008-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Lyu RM, Germano PM, Choi JK, Le SV, Pisegna JR. Identification of an essential amino acid motif within the C terminus of the pituitary adenylate cyclase-activating polypeptide type I receptor that is critical for signal transduction but not for receptor internalization. J. Biol. Chem. 2000;275:36134–36142. doi: 10.1074/jbc.M004612200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XM, Levy A, Lightman SL. Rapid changes in heteronuclear RNA for corticotropin-releasing hormone and arginine vasopressin in response to acute stress. J. Endocrinol. 1997;152:81–89. doi: 10.1677/joe.0.1520081. [DOI] [PubMed] [Google Scholar]
- Martin CL, Duvall JA, Ilkin Y, Simon JS, Arreaza MG, Wilkes K, Alvarez-Retuerto A, Whichello A, Powell CM, Rao K, et al. Cytogenetic and molecular characterization of A2BP1/FOX1 as a candidate gene for autism. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2007;144B:869–876. doi: 10.1002/ajmg.b.30530. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Mood disorders and allostatic load. Biol. Psychiatry. 2003;54:200–207. doi: 10.1016/s0006-3223(03)00177-x. [DOI] [PubMed] [Google Scholar]
- McGill BE, Bundle SF, Yaylaoglu MB, Carson JP, Thaller C, Zoghbi HY. Enhanced anxiety and stress-induced corticosterone release are associated with increased crh expression in a mouse model of Rett syndrome. Proc. Natl. Acad. Sci. USA. 2006;103:18267–18272. doi: 10.1073/pnas.0608702103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa T, Grimaldi M, Eiden LE. The hop cassette of the PAC1 receptor confers coupling to Ca2+ elevation required for pituitary adenylate cyclase-activating polypeptide-evoked neurosecretion. J. Biol. Chem. 2007;282:8079–8091. doi: 10.1074/jbc.M609638200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa T, Walsh J, Grimaldi M, Eiden LE. PAC1hop receptor activation facilitates catecholamine secretion selectively through 2-APB- sensitive Ca(2+) channels in PC12 cells. Cell. Signal. 2010;22:1420–1426. doi: 10.1016/j.cellsig.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahata S, Kawamoto S. Tissue-dependent isoforms of mammalian Fox-1 homologs are associated with tissue-specific splicing activities. Nucleic Acids Res. 2005;33:2078–2089. doi: 10.1093/nar/gki338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisegna JR, Wank SA. Cloning and characterization of the signal transduction of four splice variants of the human pituitary adenylate cyclase activating polypeptide receptor. Evidence for dual coupling to adenylate cyclase and phospholipase C. J. Biol. Chem. 1996;271:17267–17274. doi: 10.1074/jbc.271.29.17267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regev L, Neufeld-Cohen A, Tsoory M, Kuperman Y, Getselter D, Gil S, Chen A. Prolonged and site-specific over-expression of corticotropin-releasing factor reveals differential roles for extended amygdala nuclei in emotional regulation. Mol Psychiatry. 2010;16:714–728. doi: 10.1038/mp.2010.64. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, Norrholm SD, Kilaru V, Smith AK, Myers AJ, et al. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 2011;470:492–497. doi: 10.1038/nature09856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu S, Mahler J, Acampora D, Holzschuh J, Erhardt S, Omodei D, Simeone A, Driever W. Orthopedia homeodomain protein is essential for diencephalic dopaminergic neuron development. Curr. Biol. 2007;17:873–880. doi: 10.1016/j.cub.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Selye H. A syndrome produced by diverse nocuous agents. Nature. 1936;138:32. doi: 10.1176/jnp.10.2.230a. [DOI] [PubMed] [Google Scholar]
- Spengler D, Waeber C, Pantaloni C, Holsboer F, Bockaert J, Seeburg PH, Journot L. Differential signal transduction by five splice variants of the PACAP receptor. Nature. 1993;365:170–175. doi: 10.1038/365170a0. [DOI] [PubMed] [Google Scholar]
- Steenbergen PJ, Richardson MK, Champagne DL. Patterns of avoidance behaviours in the light/dark preference test in young juvenile zebrafish: a pharmacological study. Behav. Brain Res. 2011;222:15–25. doi: 10.1016/j.bbr.2011.03.025. [DOI] [PubMed] [Google Scholar]
- Stouthart AJ, Lucassen EC, van Strien FJ, Balm PH, Lock RA, Wendelaar Bonga SE. Stress responsiveness of the pituitary-interrenal axis during early life stages of common carp (Cyprinus carpio) J. Endocrinol. 1998;157:127–137. doi: 10.1677/joe.0.1570127. [DOI] [PubMed] [Google Scholar]
- Stroth N, Eiden LE. Stress hormone synthesis in mouse hypothalamus and adrenal gland triggered by restraint is dependent on pituitary adenylate cyclase-activating polypeptide signaling. Neuroscience. 2010;165:1025–1030. doi: 10.1016/j.neuroscience.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztainberg Y, Kuperman Y, Tsoory M, Lebow M, Chen A. The anxiolytic effect of environmental enrichment is mediated via amygdalar CRF receptor type 1. Mol Psychiatry. 2010;15:905–917. doi: 10.1038/mp.2009.151. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood JG, Boutz PL, Dougherty JD, Stoilov P, Black DL. Homologues of the Caenorhabditis elegans Fox-1 protein are neuronal splicing regulators in mammals. Mol. Cell. Biol. 2005;25:10005–10016. doi: 10.1128/MCB.25.22.10005-10016.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, Fournier A, Chow BK, Hashimoto H, Galas L, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol. Rev. 2009;61:283–357. doi: 10.1124/pr.109.001370. [DOI] [PubMed] [Google Scholar]
- Voineagu I, Wang X, Johnston P, Lowe JK, Tian Y, Horvath S, Mill J, Cantor RM, Blencowe BJ, Geschwind DH. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature. 2011;474:380–384. doi: 10.1038/nature10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Lufkin T. The murine Otp homeobox gene plays an essential role in the specification of neuronal cell lineages in the developing hypothalamus. Dev. Biol. 2000;227:432–449. doi: 10.1006/dbio.2000.9902. [DOI] [PubMed] [Google Scholar]
- Wölfl S, Martinez C, Majzoub JA. Inducible binding of cyclic adenosine 30 ,50 -monophosphate (cAMP)-responsive element binding protein (CREB) to a cAMP-responsive promoter in vivo. Mol. Endocrinol. 1999;13:659–669. doi: 10.1210/mend.13.5.0282. [DOI] [PubMed] [Google Scholar]
- Wolman M, Granato M. Behavioral genetics in larval zebrafish- learning from the young. Dev. Neurobiol. 2011 doi: 10.1002/dneu.20872. in press. Published online January 10, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K, Elegante M, Bartels B, Elkhayat S, Tien D, Roy S, Goodspeed J, Suciu C, Tan J, Grimes C, et al. Analyzing habituation responses to novelty in zebrafish (Danio rerio) Behav. Brain Res. 2010;208:450–457. doi: 10.1016/j.bbr.2009.12.023. [DOI] [PubMed] [Google Scholar]
- Yao M, Denver RJ. Regulation of vertebrate corticotropin- releasing factor genes. Gen. Comp. Endocrinol. 2007;153:200–216. doi: 10.1016/j.ygcen.2007.01.046. [DOI] [PubMed] [Google Scholar]
- Zhang C, Zhang Z, Castle J, Sun S, Johnson J, Krainer AR, Zhang MQ. Defining the regulatory network of the tissue-specific splicing factors Fox-1 and Fox-2. Genes Dev. 2008;22:2550–2563. doi: 10.1101/gad.1703108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.