Supplemental Digital Content is Available in the Text.
Key Words: HIV, transmission, viral load, tuberculosis, malaria, helminth, herpes, coinfections
Abstract:
High HIV-1 plasma viral loads (PVLs) in sub-Saharan Africa, partly because of high rates of coinfection, may have been one of the drivers of the “explosive” epidemics seen in that region. Using a previously published framework of infectiousness and survival, we estimate the excess onward HIV-1 transmission events (secondary infections) resulting from coinfection-induced changes in PVL during asymptomatic HIV-1 infection. For every 100 HIV-infected people, each suffering 1 episode of tuberculosis infection, there are 4.9 (2.7th–97.5th percentile: 0.2–21.5) excess onward HIV-1 transmission events attributable to this coinfection. Other estimates are malaria 0.4 (0.0–2.0), soil-transmitted helminths 3.1 (0.1–14.9), schistosomiasis 8.5 (0.2–38.6), filariasis 13.3 (0.3–89.2), syphilis 0.1 (0.0–1.6), herpes simplex virus 4.0 (0.0–24.2), and gonorrhea 2.1 (0.1–8.0) transmissions. If these higher PVLs confer a shorter life expectancy and higher infectiousness, then their impact on transmission is, in general, reduced. For most HIV-1 coinfections, the duration of a single infection is too short and/or the associated PVL elevation is too modest to contribute substantially to onward HIV-1 transmission.
INTRODUCTION
It has recently been suggested that high HIV-1 plasma viral loads (PVLs) in sub-Saharan Africa, partly as a consequence of high rates of coinfection, may have driven the “explosive” epidemics in that region.1 PVL is positively associated with heterosexual transmission,2,3 and systematic reviews have highlighted the increases in PVL that occur in the presence of various coinfections [tuberculosis (TB), malaria, schistosomiasis, other soil-transmitted helminth (STH) infections, filariasis, syphilis, herpes simplex virus (HSV), and gonorrhea] and/or the decreases in PVL when such coinfections are treated.4,5 It has therefore been proposed that treatment and/or prevention of these coinfections could reduce PVL and hence reduce HIV-1 transmission, in addition to the benefits of reducing the burden of these infections.6
We have previously demonstrated that the small reductions in HIV-1 PVL observed in several randomized control trials of HSV treatment of HSV/HIV-1–coinfected individuals7,8 do not necessarily translate into substantial reductions in HIV-1 transmission.7 Indeed, depending on the distribution of PVLs within the population, small reductions in HIV-1 PVL may actually increase the total number of onward HIV-1 transmission events. This range of population-level outcomes is because of the spectrum of PVLs in a population, in response to treatment and the impact of PVL on survival and infectiousness. In general, individuals with high PVL are very infectious but have a relatively fast rate of CD4 decline and therefore a shorter HIV-1 asymptomatic period, whereas those with low PVL are less infectiousness but with a longer asymptomatic period, resulting in a longer window of opportunity for onward HIV-1 transmission.9
Recent trials have shown that treating HSV/HIV-1 coinfection with acyclovir and valacyclovir reduces HIV-1 PVL, which may reduce infectiousness,2,3 and has now also been shown to decrease HIV-1 disease progression by decreasing the rate of CD4 cell count decline during asymptomatic HIV-1 infection.10–12 This delays the point at which antiretroviral therapy (ART) should be initiated but also provides more opportunities for onward HIV-1 spread. The impact of acyclovir/valacyclovir treatment on CD4 decline may be because of it reducing HIV-1 PVL but may also be because of its antiretroviral action reducing HIV-1 replication.13 However, because PVL is a major predictor of survival,14 it is plausible that reducing elevated PVLs because of other coinfections may have similar, if yet unquantified, effects.
Here, we apply our previously published framework7,9 for the impact of changes in PVL on infectiousness and survival to estimate the public health impact of treating or preventing HIV-1 coinfections, and more generally, the impact of interventions such as treatments or vaccines, which reduce HIV-1 PVL to a nonnegligible level to identify at what point such a reduction produces indisputable benefits, without potential deleterious effects on onward HIV-1 transmission. We explore 2 scenarios: (1) the increase in PVL on acquiring a coinfection increases infectiousness during the period of coinfection and (2) this PVL increase additionally reduces individuals' projected durations of asymptomatic HIV-1 infection (by increasing the rate of CD4 decline).
METHODS
During the asymptomatic period of HIV-1 infection, the transmission potential of an infected individual is defined as the mean number of persons that 1 index case can infect over their whole asymptomatic period (the product of infectiousness and duration of asymptomatic infection).9 It is a function of set-point PVL (the PVL steady state reached after the peak PVL in early infection and before progression to AIDS). The concepts we explore are described by Baggaley et al7 and detailed in Figure 1 and the Supplemental Digital Content (http://links.lww.com/QAI/A619). Coinfection is assumed to increase HIV-1 PVL when acquired transiently during asymptomatic infection. We do not look at interactions between HIV-1 and coinfections during acute- or late-stage infection because these are periods where infectiousness changes rapidly and, in the case of acute infection, infectiousness may be far higher than would be predicted on the basis of PVL alone.15
FIGURE 1.
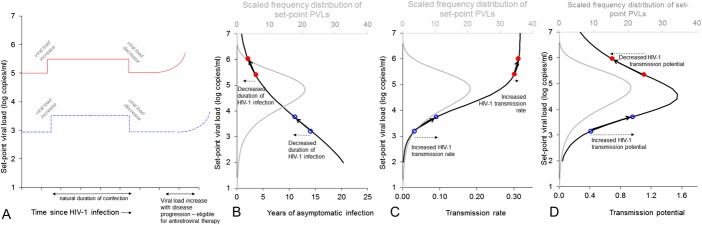
Schematic diagram illustrating the estimated impact of HIV-1 coinfections that increase HIV-1 PVL on (A) set-point PVL, (B) duration of asymptomatic HIV-1 infection, (C) HIV-1 transmission rate (hazard of transmission: probability of HIV-1 transmission per year), and (D) HIV-1 transmission potential (product of hazard of transmission and duration of asymptomatic infection) for 2 hypothetical patients. Time starts at the beginning of each individual's asymptomatic period (ie, after high PVL accompanying primary infection). The individual with lower set-point PVL has a correspondingly longer duration of asymptomatic infection (A). The figure shows that all patients experiencing an increase in PVL as a result of coinfection would experience decreases in duration of asymptomatic infection and increases in HIV-1 infectiousness, but the direction of change to transmission potential depends on baseline set-point PVL and the amount that PVL is augmented on coinfection. See Baggaley et al7 for further explanation. The frequency distribution of set-point PVLs used in the analysis, based on a model fit27 to data from Orange County, South Africa,16 is shown in gray in (B–D) and shows that HIV-1–infected individuals with set-point PVLs typical of South Africa could have an increase or decrease in transmission potential as a result of modest decrease in PVL.
We assessed the impact of coinfections on HIV-1 transmission by modeling a hypothetical population of 10,000 individuals with varying baseline PVLs in a generalized HIV-1 epidemic in sub-Saharan Africa in the absence of ART. We used a frequency distribution of PVLs from a cross-sectional survey of young men in Orange Farm County, South Africa.16
We simulated varying shifts in PVL due to coinfections. Estimated elevations in PVL for each coinfection were sampled from distributions taken from published literature4,5,17 as listed in Figure 2 (for details of sampling, see Supplemental Digital Content, http://links.lww.com/QAI/A619). Durations of each coinfection were also taken from the literature (Fig. 2) and sampled from a uniform distribution of ±50% of the central value. All individuals in the model population experience an episode of coinfection, that is, results are not dependent on prevalence levels of each coinfection, and thus results do not represent any specific geographical area.
FIGURE 2.
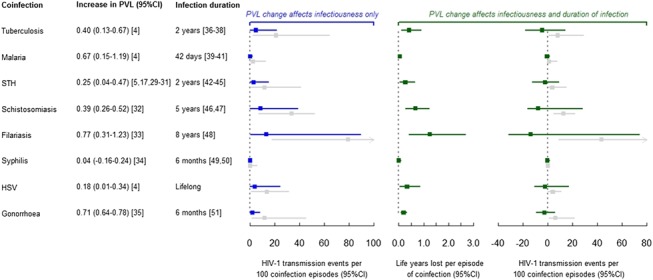
Estimated impact of coinfections on duration of HIV-1 infection and onward HIV-1 transmission. Points represent medians and error bars represent 2.5th and 97.5th percentiles of output from 10,000 simulated individuals. Calculations assume that each coinfection is acquired halfway through the HIV-1 asymptomatic period. Plot in blue shows results under the assumption that changes in PVL only affect HIV-1 infectiousness. Plots in green show results under the assumption that changes in PVL change duration of infectiousness (middle plot of change in life-years) and infectiousness (right-hand plot). Therefore, there is impact in terms of both transmission and life-years gained (preceding initiating ART). Change in HIV-1 transmission events plots additionally show results using the Quinn et al2 PVL-infectiousness relationship (results shown in gray). STH, soil-transmitted helminths (excluding schistosomiasis).
We looked at scenarios where the coinfection was acquired at the start and halfway through the asymptomatic period to explore the maximum impact of coinfection prevention, especially relevant for long duration infections such as HSV. We also explored coinfection-specific attributes such as coinfection-associated TB mortality and the possibility of multiple infection episodes per person for malaria and other macroparasitic infections.
We examined scenarios exploring 2 assumptions: (1) the increase in PVL on acquiring a coinfection increases infectiousness during the period of coinfection, and (2) this PVL increase additionally reduces individuals' projected durations of asymptomatic HIV-1 infection (Fig. 1). We measured the change in the transmission potential due to this change in PVL. Transmission events attributable to each coinfection refer to direct transmission events from a coinfected individual (secondary infections).
The relationship between PVL and HIV-1 infectiousness estimated by Fraser et al9 shows a plateauing of infectiousness at high PVL (Fig. 1C; also see Fig. 3a of Fraser et al, which demonstrates the plateau for Ugandan2 and Zambian3 data). We explore the impact of this assumption by performing the analysis first using this plateauing function and then repeating the analysis using a widely used log-linear relationship estimated from the same Ugandan2 data: each log increment in PVL increases infectiousness by a factor 2.45 (see Fig. S2 for comparison of PVL-infectiousness relationships defined by Fraser9 and Quinn2). The model was programmed and analyzed using Stata version 13.1 (StatCorp LP, College Station, TX).
RESULTS
Under the assumption that changes in PVL only affect infectiousness, for every 100 HIV-1–infected people, each suffering 1 episode of TB, there are 4.9 (0.2–21.5) excess onward HIV-1 transmission events attributable to TB coinfection (2.7th–97.5th percentile; Fig. 2). Excess onward HIV-1 transmissions for other coinfections are 0.4 (0.0–2.0) for malaria, 3.1 (0.1–14.9) for STH, 8.5 (0.2–38.6) for schistosomiasis, 13.3 (0.3–89.2) for filariasis, 0.1 (0.0–1.6) for syphilis, 4.0 (0.0–24.2) for HSV, and 2.1 (0.1–8.0) for gonorrhea. These represent coinfections acquired halfway through the HIV-1 asymptomatic period; coinfections acquired at the start of asymptomatic HIV-1 resulted in larger impacts for the longer duration infections only, such as HSV and filariasis (data not shown).
Under the assumption that changes in PVL due to coinfection affect both infectiousness and duration of infection, the impact of increased PVLs on transmission at the population is reduced (Fig. 2), with all the confidence intervals for number of HIV-1 transmissions crossing zero. In fact, HIV-1 transmission events attributable to coinfection are negative because the impact of increased infectiousness of individuals because of elevated PVL is outweighed by the reduction in survival also conferred by the higher PVL—there is less opportunity to transmit.
Long-lived infections with relatively large impacts on PVL, such as filariasis, conferred the most public health impact but also the greatest uncertainty in impact [filariasis: 1.2 (0.4–2.7) life-years lost per episode of coinfection compared with syphilis: 0.01 (0.00–0.09)]. Averting filariasis and schistosomiasis episodes may have a substantial impact in terms of life-years gained during HIV-1 asymptomatic infection, thus delaying time until ART is required. However, most coinfections are accompanied by too small an increase in PVL to have a substantial impact.
For the shorter lived infections, there is, of course, the possibility of multiple infections, which would increase their impact on PVL and transmission. Annual episodes of malaria infection, representing seasonality among the adult population, increased impact from 0.4 (0.0–2.0) HIV-1 transmissions attributable to 100 coinfection episodes to 2.4 (0.0–21.0) HIV-1 transmissions per 100 people who become infected with malaria annually over their entire duration of asymptomatic HIV-1 infection (results assume PVL change affects infectiousness but not survival). We did not consider endemic continuous infection with malaria or STH and schistosomiasis because endemic infection (ie, constant reinfection) would be rare among adults in high-intensity settings of sub-Saharan Africa, the majority of which would be among young children. Annual acquisition of gonorrhea throughout asymptomatic HIV-1 infection, representing extremely high-risk individuals, would lead to 12.1 (0.3–85.3) HIV-1 transmissions attributable to coinfection over the asymptomatic HIV-1 infection period of 100 people [estimates for syphilis: 0.4 (0.0–13.9) transmissions]. A more realistic level of 3 episodes over the asymptomatic HIV-1 period produces estimates of 6.2 (0.4–24.0) for gonorrhea and 0.3 (0.0–4.9) for syphilis.
We investigated the sensitivity of our findings to different assumptions regarding the PVL-infectiousness relationship. Using the PVL-infectiousness function defined by Quinn et al2 increased the impact of coinfections, predicting more onward HIV-1 transmission events per episode of coinfection (see gray plots in Fig. 2). This is because the Quinn function predicts higher infectiousness than the Fraser function for a given PVL, but this may overestimate infectiousness (see Figure S2, Supplemental Digital Content, http://links.lww.com/QAI/A619).
DISCUSSION
For most HIV-1 coinfections, the duration of coinfection is too short and/or the PVL elevation too modest to reduce HIV-1 transmission or the duration of asymptomatic HIV-1 infection substantially. However, for longer duration infections such as HSV and filariasis, there may be benefit in averting or treating coinfection to delay time to HIV-1 treatment initiation, but the impact of coinfections and their treatment depends on the baseline HIV-1 PVL of the individual.
Scenarios incorporating the impact of TB-associated mortality during HIV-1 asymptomatic infection were investigated (methods and results available on request), which realized the intuitive outcome that life-years saved by preventing TB infection increased, whereas fewer onward transmission events were possible because a proportion of those dually infected would die before transmitting infection. Multiple episodes of coinfection within the same individual during asymptomatic HIV-1 infection increase impact. However, for coinfections where multiple infections are likely, such as malaria and gonorrhea, the PVL elevations per episode remain too small to generate a substantial effect, even when considering annual episodes.
Reductions in HIV-1 PVL can have positive or negative effects on transmission potential depending on individuals' baseline PVL and the effect on survival, for which there is biological plausibility but, as yet, limited empirical evidence. Mugwanya et al18 recently achieved a >1 log10 copies per milliliter reduction in HIV-1 PVL among HSV/HIV-1–coinfected individuals taking high-dose valacyclovir. However, a significant reduction in PVL is insufficient evidence of beneficial public health impact. Figure 2 suggests that such a reduction in HIV-1 PVL would reduce the overall onward HIV-1 transmission of individuals with asymptomatic infection, in the absence of ART, but the error bars indicate that for those with higher set-point PVL, a 1 log10 copies per milliliter reduction would push them to higher transmission potential, not lower. Therefore, an intervention such as high-dose valacyclovir in the absence of ART would not be suitable for targeting to patients with high viremia. Although it may be cost saving in terms of delaying time at which point ART is required on clinical grounds, the potential for patients to transmit HIV-1 to their partners must also be taken into consideration, with suitable counseling and transmission-reducing interventions being essential to avoid negative effects on a longer timescale. Although the prospect of universal ART as treatment-as-prevention may leave this issue redundant, the trial results that will prove the success of this approach at the population level (rather than during clinical trials19) are not yet in our hands, and with rationing of scarce resources, the use of lower cost treatments to delay the time of ART initiation may remain important. Our evaluation of the influence of coinfections on onward HIV-1 transmission among individuals not being treated for their HIV-1 infection remains informative because it would be premature to state that untreated undiagnosed HIV-1 infection is a thing of the past. For example, Public Health England estimated that in 2012, 22% of people living with HIV-1 in the United Kingdom, where there is relatively easy access to testing and treatment, were unaware of their infection.20 Our analysis relates to HIV-1 infectiousness during asymptomatic HIV-1 infection, an infection stage at which fewer patients are treated than during AIDS.
There are a number of limitations to our analysis. For example, the duration of infection for many of these infections may be longer for HIV-1–infected than for uninfected individuals. We could only make predictions for those coinfections where estimates of increases in PVL on coinfection are available in published literature. We also assumed no decrease in sexual activity as a result of coinfection, which would reduce numbers still further and therefore support our conclusion of limited impact of most coinfections on onward HIV-1 transmission events. Evidently, there is benefit to each individual of treating or curing each coinfection apart from the impact on their HIV-1 infection. Data on the duration of HIV-1 infection are from a population of Dutch homosexual men,21 whereas data underlying HIV-1 infectiousness assumptions are from Ugandan2 and Zambian3 populations. However, Fraser et al show a direct side-by-side comparison of survival rates between the Dutch seroconverters data set and a cohort of untreated female sex workers from Nairobi, Kenya, followed since seroconversion,22 which show very similar survival rates for individuals in similar PVL classes (see Figure S6, Supplemental Digital Content, http://links.lww.com/QAI/A619).9 Our assumption that changes in PVL affect survival is tentative, and we know that survival and rates of CD4 decline are affected by factors other than changes in PVL. For example, in Rakai, Uganda, subtypes A and D have very different survival profiles despite similar PVLs.23 Our analysis therefore presents outcomes with and without the assumption that changing PVL can alter HIV-1 disease progression. Our modeling framework does not use a population-level epidemic model of ongoing HIV-1 transmission; further investigation of the effect of coinfections on the time course of HIV-1 epidemics could be devised using dynamical epidemic models.
We have focussed on the long incubation period of HIV-1 infection, during which the PVL-infectiousness relationship holds.2,9 The effect of coinfection during symptomatic (late-stage) HIV-1 will likely be less, as individuals become less sexually active with disease progression. Coinfection during the high viremia accompanying acute infection may increase infectiousness even further, but we postulate the additional impact due to coinfections is small. The Fraser et al9 PVL-infectiousness model provides strong evidence of saturation of infectiousness at high PVLs (Fig. S2 and Fig. 3 of Fraser et al), so a half log augmentation due to coinfection would not markedly affect transmission. During acute infection, the PVL-infectiousness relationship is less clear: transmission rates have been shown to be significantly higher than would be expected on the basis of PVL (see Fig. 3 of Hollingsworth et al15). Authors postulate that sexually transmitted infection (STI) coinfections may in part be responsible for this, but this may be through mechanisms other than PVL (eg, portals of access for ulcerative STIs), and acute HIV-1 virus phenotype may be more transmissible than that which evolves during incubation.
Our analysis focuses on the relationship between PVL and HIV-1 transmission, but an association between genital viral shedding and transmission has also been observed, independent of PVL.24 This relationship is less studied than that for PVL and is less reliable (Baeten et al found that, of 78 transmission events in their study, 11 occurred from persons with undetectable genital HIV-1 RNA despite detection of PVL24). Nonetheless, it is levels of virus in the genital tract at the point of sex which determine likelihood of transmission, and these levels are likely to rise above that observed for PVL in the case of STI, due to local genital inflammation. This may increase the effect of the STIs included in this analysis beyond the effect predicted here through PVL evaluation; ulcerative STIs such as HSV and syphilis will further increase risk. However, the Rakai and Zambia studies show that PVL is the dominant predictor of transmission between discordant couples, and so it is currently the most suitable tool for measuring these effects.
Several research groups have recently suggested that increased PVLs, due in part to coinfection, are responsible for the large HIV-1 epidemics in sub-Saharan Africa.1,25 Our results suggest that some coinfections play a significant role, but it must be noted that although many of these coinfections are very prevalent in regions with high HIV-1 prevalence, they are not necessarily affecting those individuals who play a substantial role in HIV-1 transmission. For example, non-hookworm STH such as Ascaris lumbricoides predominantly affect children.26 The association between HIV and these coinfections has been investigated by a number of authors, but data are rarely routinely collected in a way which would enable a population-specific estimate of the impact of these coinfections on viral load.
Our findings change markedly with the assumptions we make regarding the impact of changes in PVL on survival. The results presented here highlight the importance of understanding the relationship between PVL and duration of infection to estimate the population impact of any coinfection or intervention, which alters the PVL of HIV-1–infected individuals.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Brian G. Williams for providing additional data.
Footnotes
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jaids.com).
R.F.B. conceived and co-wrote the article with substantial inputs from T.D.H. Both authors contributed toward the analysis and interpretation of the data and to the development and critical revision of the manuscript.
REFERENCES
- 1.Abu-Raddad LJ, Barnabas RV, Janes H, et al. Have the explosive HIV epidemics in sub-Saharan Africa been driven by higher community viral load? AIDS. 2013;27:2494–2496. [DOI] [PubMed] [Google Scholar]
- 2.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342:921–929. [DOI] [PubMed] [Google Scholar]
- 3.Fideli US, Allen SA, Musonda R, et al. Virologic and immunologic determinants of heterosexual transmission of human immunodeficiency virus type 1 in Africa. AIDS Res Hum Retroviruses. 2001;17:901–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnabas RV, Webb EL, Weiss HA, et al. The role of coinfections in HIV epidemic trajectory and positive prevention: a systematic review and meta-analysis. AIDS. 2011;25:1559–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walson JL, John-Stewart G. Treatment of helminth co-infection in individuals with HIV-1: a systematic review of the literature. PLoS Negl Trop Dis. 2007;1:e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cuadros DF, Garcia-Ramos G. Variable effect of co-infection on the HIV infectivity: within-host dynamics and epidemiological significance. Theor Biol Med Model. 2012;9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delany S, Mlaba N, Clayton T, Akpomiemie G, Capovilla A, Legoff J, et al. Impact of aciclovir on genital and plasma HIV-1 RNA in HSV-2/HIV-1 co-infected women: a randomized placebo-controlled trial in South Africa. AIDS. 2009;23:461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagot N, Ouedraogo A, Foulongne V, et al. Reduction of HIV-1 RNA levels with therapy to suppress herpes simplex virus. N Engl J Med. 2007;356:790–799. [DOI] [PubMed] [Google Scholar]
- 9.Fraser C, Hollingsworth TD, Chapman R, et al. Variation in HIV-1 set-point viral load: epidemiological analysis and an evolutionary hypothesis. Proc Natl Acad Sci U S A. 2007;104:17441–17446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Celum C, Wald A, Lingappa JR, et al. Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. N Engl J Med. 2010;362:427–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lingappa JR, Baeten JM, Wald A, et al. Daily acyclovir for HIV-1 disease progression in people dually infected with HIV-1 and herpes simplex virus type 2: a randomised placebo-controlled trial. Lancet. 2010;375:824–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reynolds SJ, Makumbi F, Newell K, et al. Effect of daily aciclovir on HIV disease progression in individuals in Rakai, Uganda, co-infected with HIV-1 and herpes simplex virus type 2: a randomised, double-blind placebo-controlled trial. Lancet Infect Dis. 2012;12:441–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McMahon MA, Siliciano JD, Lai J, et al. The antiherpetic drug acyclovir inhibits HIV replication and selects the V75I reverse transcriptase multidrug resistance mutation. J Biol Chem. 2008;283:31289–31293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mellors JW, Rinaldo CR, Jr, Gupta P, et al. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996;272:1167–1170. [DOI] [PubMed] [Google Scholar]
- 15.Hollingsworth TD, Anderson RM, Fraser C. HIV-1 transmission, by stage of infection. J Infect Dis. 2008;198:687–693. [DOI] [PubMed] [Google Scholar]
- 16.Williams BG, Korenromp EL, Gouws E, et al. HIV infection, antiretroviral therapy, and CD4+ cell count distributions in African populations. J Infect Dis. 2006;194:1450–1458. [DOI] [PubMed] [Google Scholar]
- 17.Modjarrad K, Vermund SH. Effect of treating co-infections on HIV-1 viral load: a systematic review. Lancet Infect Dis. 2010;10:455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mugwanya K, Baeten JM, Mugo NR, et al. High-dose valacyclovir HSV-2 suppression results in greater reduction in plasma HIV-1 levels compared with standard dose acyclovir among HIV-1/HSV-2 coinfected persons: a randomized, crossover trial. J Infect Dis. 2011;204:1912–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aghaizu A, Brown AE, Nardone A, et al. HIV in the United Kingdom 2013 Report: Data to End 2012. London, United Kingdom: Public Health England; 2013. [Google Scholar]
- 21.de Wolf F, Spijkerman I, Schellekens PT, et al. AIDS prognosis based on HIV-1 RNA, CD4+ T-cell count and function: markers with reciprocal predictive value over time after seroconversion. AIDS. 1997;11:1799–1806. [DOI] [PubMed] [Google Scholar]
- 22.Lavreys L, Baeten JM, Chohan V, et al. Higher set point plasma viral load and more-severe acute HIV type 1 (HIV-1) illness predict mortality among high-risk HIV-1-infected African women. Clin Infect Dis. 2006;42:1333–1339. [DOI] [PubMed] [Google Scholar]
- 23.Kiwanuka N, Laeyendecker O, Robb M, et al. Effect of human immunodeficiency virus Type 1 (HIV-1) subtype on disease progression in persons from Rakai, Uganda, with incident HIV-1 infection. J Infect Dis. 2008;197:707–713. [DOI] [PubMed] [Google Scholar]
- 24.Baeten JM, Kahle E, Lingappa JR, et al. Genital HIV-1 RNA predicts risk of heterosexual HIV-1 transmission. Sci Transl Med. 2011;3:77ra29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cuadros DF, Crowley PH, Augustine B, et al. Effect of variable transmission rate on the dynamics of HIV in sub-Saharan Africa. BMC Infect Dis. 2011;11:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson RM, Truscott JE, Pullan RL, et al. How effective is school-based deworming for the community-wide control of soil-transmitted helminths? PLoS Negl Trop Dis. 2013;7:e2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams BG. Determinants of sexual transmission of HIV: implications for control. 2011. Available at: http://arxiv.org/ftp/arxiv/papers/1108/1108.4715.pdf. Accessed October 15, 2012.


