Abstract
Cyclin-dependent kinase (Cdk) activation and RNA polymerase II transcription are linked by the Cdk7 kinase, which phosphorylates Cdks as a trimeric Cdk-activating kinase (CAK) complex, and serine 5 within the polymerase II (Pol II) C-terminal domain (CTD) as transcription factor TFIIH-bound CAK. However, the physiological importance of integrating these processes is not understood. Besides the Cdk7 ortholog Mcs6, fission yeast possesses a second CAK, Csk1. The two enzymes have been proposed to act redundantly to activate Cdc2. Using an improved analogue-sensitive Mcs6-as kinase, we show that Csk1 is not a relevant CAK for Cdc2. Further analyses revealed that Csk1 lacks a 20-amino-acid sequence required for its budding yeast counterpart, Cak1, to bind Cdc2. Transcriptome profiling of the Mcs6-as mutant in the presence or absence of the budding yeast Cak1 kinase, in order to uncouple the CTD kinase and CAK activities of Mcs6, revealed an unanticipated role of the CAK branch in the transcriptional control of the cluster of genes implicated in ribosome biogenesis and cell growth. The analysis of a Cdc2 CAK site mutant confirmed these data. Our data show that the Cdk7 kinase modulates transcription through its well-described RNA Pol II CTD kinase activity and also through the Cdc2-activating kinase activity.
INTRODUCTION
Cyclin-dependent kinases (Cdk) coordinate timely progression through the cell cycle. Cdk activity is modulated by the association of regulatory subunits (cyclins, inhibitors, and assembly factors) and by activating and inhibiting phosphorylation at conserved residues (1). Phosphorylation within the activating segment, referred to as the T loop, is essential for maximal activity and is catalyzed by a Cdk-activating kinase (CAK) (2, 3). Genetic and biochemical studies in several model organisms pointed to Cdk7-cyclin H-Mat1 as the in vivo CAK in metazoans. Cdk7 specifically phosphorylates both Cdk1 and Cdk2 in human cells (4–6), and elegant experiments using chemical genetics showed that Cdk7 is required for the assembly of Cdk1-cyclin B (7). CAK activity is decreased in cdk7 mutants in either Drosophila (8) or worms (9). However, correlations between biochemical data and phenotypes have always been complicated by the additional role of Cdk7 in transcription (10). Indeed, the trimeric CAK is also a component of the RNA polymerase II (Pol II) general transcription factor TFIIH, where it phosphorylates the C-terminal domain (CTD) of Rpb1, the largest subunit of the Pol II enzyme (11, 12). Within the Cdk family, Cdk7 also is distinct in being activated by either a phosphorylation on its T loop or by the assembly factor Mat1 (13). These functions and regulations of Cdk7 are conserved in the budding yeast Saccharomyces cerevisiae, where its ortholog, Kin28, is well described as a CTD kinase regulating transcription (14, 15). Intriguingly, Kin28 is devoid of CAK activity (16), and Cak1, a divergent, single-subunit kinase distantly related to Cdk (17, 18), instead catalyzes Cdk activation at both transitions of the budding yeast cell cycle.
The fission yeast Schizosaccharomyces pombe possesses two CAKs, the nonessential Csk1 and the essential Mcs6 kinases, corresponding to the yeast Cak1 and the metazoan Cdk7, respectively (19–25). Csk1 activates Mcs6 by phosphorylation of the T loop on serine 165 (20), and a widely held view is that the two CAKs act redundantly for Cdc2 activation (22, 24), implying that inactivation of Mcs6 reveals the sole role of Cdk7 in transcription. However, strong genetic data argue against the idea that Csk1 contributes significantly to Cdc2 activation in vivo. First, the original mutant alleles of both the mcs6 (mcs6-13) and mcs2 (mcs2-75) genes, which, respectively, encode Cdk7 and cyclin H, were independently identified in a screen for suppressor of mitotic catastrophe, a phenotype resulting from hyperactive Cdc2, and both show allele-specific interaction with cdc2 (23). This is inconsistent with a functional redundancy with Csk1, as the strain was otherwise wild type for csk1. Moreover, another allele of fission yeast cdk7 (mcs6 S165A L238R [the S-to-A change at position 165 and the L-to-R change at position 238 encoded by mcs6]) leads to thermosensitivity associated with decreased Cdc2 phosphorylation (24), and the growth defect is suppressed by Cdc2 activators or by budding yeast Cak1 but not by Csk1 (21). Another complication comes from the fact that none of the attempts to mimic the uniform elongated cdc phenotype usually resulting from Cdc2 inactivation, by interfering with one or both CAKs from fission yeast, were satisfactory.
Considering these discrepancies, we analyzed the effects of Mcs6 inactivation on Cdc2 phosphorylation and activity by chemical genetics using an improved analogue-sensitive mutant. The results indicated that Mcs6 is the genuine Cdc2-activating kinase in fission yeast, in a manner reminiscent of metazoans. Surprisingly, genome-wide expression profiling revealed that Mcs6 affects transcription through both its CAK and its CTD kinase activities. Specifically, we show that a group of mRNAs implicated in ribosome biogenesis is downregulated when Cdc2 activation by CAK is impeded, which results in slower growth.
We propose that Mcs6 (Cdk7) modulates gene expression through both its CAK and CTD kinase activities and that Cdc2 activation by the Mcs6 CAK is required for the timely expression of growth-related genes.
MATERIALS AND METHODS
Fission yeast methods.
All classical fission yeast methods were as described previously (26–31). Cell numbers were measured with a Bio-Rad cell counter.
Generation of the analogue-sensitive mutants of mcs6, CAK1, and csk1.
The cdk9-as mutant was previously described (32). To generate the analogue-sensitive mcs6-as2, mcs6-as3, mcs6-as4, csk1-as, and cak1-as mutants, a QuikChange kit (Stratagene) was used following the instructions of the manufacturer. The sequences of all the oligonucleotides are available upon request. The mcs6 mutants were integrated in a diploid strain harboring the mcs6::ura4 deletion. One-step 5-fluoroorotic acid (5-FOA) selection was used to select gene replacements. The locus was sequenced in all cases. The csk1 and cak1 mutants were constructed in either pAHA, a fission yeast vector allowing expression of hemagglutinin (HA)-tagged proteins (21), or in pMET25, a budding yeast vector (21, 33) used to complement the civ1-4 temperature-sensitive (TS) strain (17). The ATP analogues used were either the 1-NM-PP1 {1-(1,1- dimethylethyl)-3-(1-naphthalenylmethyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine} or 3-MB-PP1 {4-amino-1-tert-butyl-3-(3-methylbenzyl)pyrazolo[3,4-d]pyrimidine} compound (both obtained from Toronto Research Chemical).
Synthesis of radiolabeled N6(benzyl)-ATP and kinase assay.
Labeling of N6(benzyl)-ADP was performed as described previously (34) with the following modifications: 200 U of nucleoside diphosphate kinase (Sigma N0379) and 2 μl of N6(benzyl)-ADP (Biolog B 023 at 0.5 nmol/μl) were used. The cpm/μl ratio of the final labeled molecule was estimated with a scintillation counter. Kinase assays were performed as described previously (20). Glutathione S-transferase (GST)–Cdk2 (a k33R mutant devoid of kinase activity [20]) and GST-CTD were purified using the pGEX4T1 system (GE Healthcare), and 1.5 μg was used in the assay. One microgram of histone H1 (Calbiochem) was used in the Cdc2 kinase assay.
Structural analysis.
Structure predictions and analysis of Mcs6 and Cdk7 were performed with the SWISS-MODEL workspace version 8.05 (35).
Microarray experiments.
Transcriptome analyses were performed on customized 4×44K Agilent microarrays. The previously described fission yeast tiling arrays (36), consisting of 60-mer oligonucleotides tiled every 55 bp on both strands, were used as a probe source. The new array consisted of about three probes per gene on the sense strand and tiled probes in the intergenic regions covering both strands. For each sample, 500 μg of total RNA was converted into labeled cDNA with nucleotides coupled to a fluorescent dye (Cy3 or Cy5) using the low RNA input linear amplification kit (Agilent Technologies). Equal amounts of differentially labeled cRNAs (750 ng) from mutants and wild-type (wt) strains were used for hybridizations. Two biological samples were hybridized for each mutant strain, with two dye swap technical replicates per sample. The microarrays were scanned using a GenePix 4000B laser scanner (Axon Instrument), and spot quantification was carried out using Imagene 7.5 (Biodiscovery, El Segundo, CA). The microarray data were analyzed as previously described (37).
Immunoprecipitation and Western blotting.
Immunoprecipitations were performed as described previously (20). In short, cells were disrupted with a Fastprep (MP Biochemical), and proteins were precipitated on appropriately coated Dynabeads (Invitrogen) following the instructions of the manufacturer (38). Cdc2 was precipitated using p13-Suc1 beads (Upstate Biotechnology). Anti-HA (Covance), anti-Pol II phospho-Ser5 (Covance), antitubulin (Sigma), anti-Cdc2 (a kind gift of Paul Nurse), and anti-Cdc2 phospho-T167 (Cell Signaling) were all used at 1/1,000 and detected following incubation with the appropriate secondary antibody and a chemiluminescence kit (PerkinElmer).
Relative quantification of mRNAs using the comparative threshold cycle (ΔΔCT) method.
Total RNA was prepared as described previously (39) and purified with a Qiagen RNeasy kit. Quantitative reverse transcription (RT)-PCR was performed using the ABI high-capacity RNA-to-cDNA kit following the instructions of the manufacturer. For each strain, the untreated sample was used as a reference, and the actin or tubulin gene was used for normalization. All primers are available upon request.
Generation of the cdc2 T167A, cak1Δ29, and csk1+29 mutants.
The cdc2 cDNA was cloned in pREP-1, and the QuikChange kit (Stratagene) was used following the instructions of the manufacturer to generate the T167A mutation.
The cak1Δ29 and csk1+29 mutants were generated by PCR using the following oligonucleotides and cloned in pAHA (see above): cak1 PCR1, ACGCGTCGACACCACCATGGGGTACCC and CATCTCTTCAAAGGGAAACAAC; cak1 PCR2, ATTGTTGCGGACCCCC and CGGGGTACCTTATGGCTTTTCTAATTCTTGCAAG; csk1 PCR1, CCGCCGCTCGAGACCACCATGGGGTACCC and TCTATCTCTTTTATAGTGCATTTGCATGAACTCATAAAGGTTCGATTTAAAACTCGTTATAAGGTAAAC; csk1 PCR2, AGAAAAAAAAATCCCTATTACGATTTGCTAAATCCCAGTATCCCATTTGTTCTATCAGATGTGATGG and CGGGGTACCTTATGCATATTGTGAAAGCC.
Microarray data accession number.
The microarray data have been deposited in the Gene Expression Omnibus (NCBI-GEO) database under accession no. GSE27425.
RESULTS
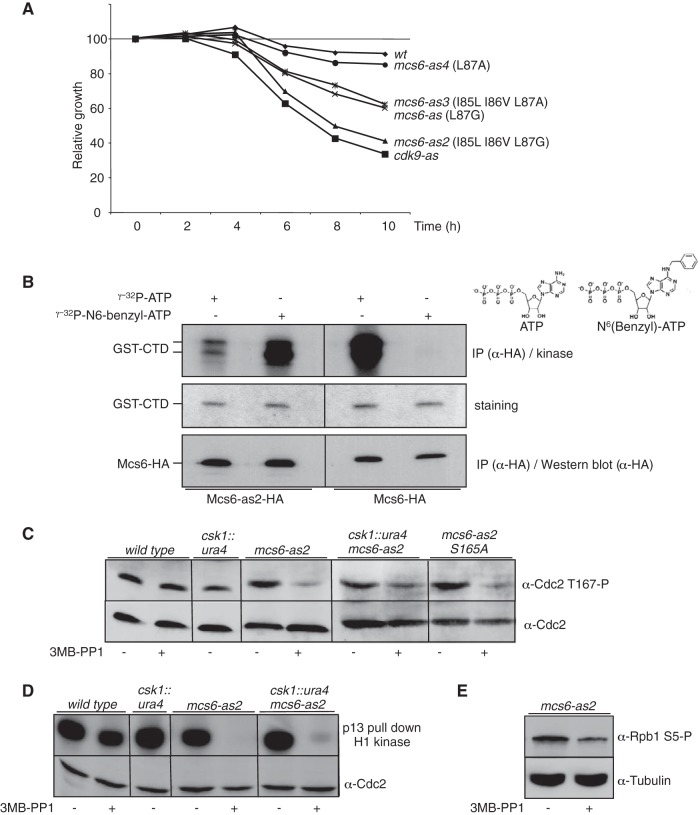
Fission yeast possesses two unrelated Cdc2-activating kinases, Csk1 and Mcs6 (Cdk7), that have been proposed to redundantly phosphorylate Cdc2 in vivo (24). Because genetic data argue against that possibility (20, 21), we analyzed the previously described (40) analogue-sensitive mcs6-as mutant (L87A) that showed a decrease in viability in the presence of micromolar concentrations of 3-MB-PP1 (Fig. 1A). However, the effect was modest compared to a cdk9-as strain (32), which was unexpected, as both proteins are encoded by essential genes and proposed to have connected functions during transcription (40). We reasoned that Mcs6-as might not be fully inhibited by the addition of 3-MB-PP1, and an alignment of the region containing the gatekeeper residue (L87) in Mcs6 and other kinases known to respond very efficiently to chemical genetics (Cdc2 [41], Cdc28 [41], and Cdk7 [7]) revealed that Mcs6 possesses two isoleucines (I85 and I86) upstream of the gatekeeper (L87), while the other kinases all have a leucine-valine sequence (see Fig. S1A in the supplemental material). Structural analysis of the Mcs6 and Cdk7 proteins suggested that an isoleucine in position 85 generates more bulk than the corresponding leucine present in the other kinases, potentially preventing efficient binding of the ATP analogue (see Fig. S1B in the supplemental material). Supporting this hypothesis, changing I85-I86-L87 to L85-V86-G87 in the mcs6-as2 mutant resulted in increased sensitivity to the inhibitor 3-MB-PP1 (Fig. 1A) without affecting the growth rate of mcs6-as2 in the absence of drug (data not shown). An in vitro kinase assay confirmed that the combined mutations allowed the modified kinase to use either labeled ATP or N6(benzyl)-ATP, while the wild type showed strict preference for ATP (Fig. 1B). Other combinations, such as replacement of L87 by an alanine, did not improve Mcs6-as sensitivity further and actually made the enzyme less sensitive (Fig. 1A).
FIG 1.
Mcs6 is the genuine Cdc2-activating kinase in fission yeast. (A) Growth defect resulting from the inhibition of Mcs6-as. The indicated strains were grown in the presence or absence of 30 μM 3-MB-PP1. Cell numbers were measured and plotted relative to that of dimethyl sulfoxide (DMSO)-treated cells of the same genotype, defined as 100%. (B) In vitro kinase activity assay of the wt and analogue-sensitive (as) versions of Mcs6 in the presence of the wt or a bulky ATP analogue. HA-tagged versions of the Mcs6 or Mcs6-as2 kinases were precipitated (IP) using anti-HA antibodies and used on beads for kinase assays with either ATP or N6(benzyl)-ATP, with GST-CTD as the substrate. After separation on SDS-PAGE, the kinase gel was exposed (top), stained with Coomassie blue (middle), and transferred to a membrane for anti-HA (α-HA) Western blotting (bottom). Note that the exposure time of the left side of the kinase gel was shorter in order to compare the use of ATP versus N6(benzyl)-ATP. (C) In vivo effect of Mcs6 inactivation on Cdc2 T167 phosphorylation. The indicated strains were treated or not with the 3-MB-PP1 inhibitor, and total protein was extracted and separated on SDS-PAGE. Western blot analyses were performed with anti-Cdc2 T167P or anti-Cdc2, as indicated. (D) In vitro kinase assay of Cdc2 after inhibition of Mcs6. The indicated strains were treated or not with the 3-MB-PP1 inhibitor, and soluble proteins were extracted and immunoprecipitated using p13 beads. Kinase assays were performed using histone H1 as the substrate. After separation on SDS-PAGE, the kinase gel was exposed (top) and transferred to a membrane for anti-Cdc2 Western blotting (bottom). (E) Same as panel C, except that anti-S5P and anti tubulin were used in Western blotting.
In the presence of the inhibitor, a marked decrease in Cdc2 phosphorylation on the CAK site (T167) was observed, and deletion of csk1, or mutation of the S165 activation site of Mcs6, which renders Mcs6 insensitive to Csk1, did not decrease it further. The deletion of csk1 alone, or the addition of the inhibitor to a wild-type strain, had no detectable effect on Cdc2 phosphorylation (Fig. 1C). The abolition of activating phosphorylation on Cdc2 was directly reflected in an in vitro Cdc2 activity assay using histone H1 as the substrate (Fig. 1D). The second known target of Mcs6, namely, serine 5 within the CTD of Pol II, was similarly affected when Mcs6 activity was inhibited (Fig. 1E).
We concluded that specific inhibition of Mcs6 abolished Cdc2 phosphorylation on the CAK site even in the presence of Csk1, supporting the idea that Mcs6 is the genuine Cdc2-activating kinase.
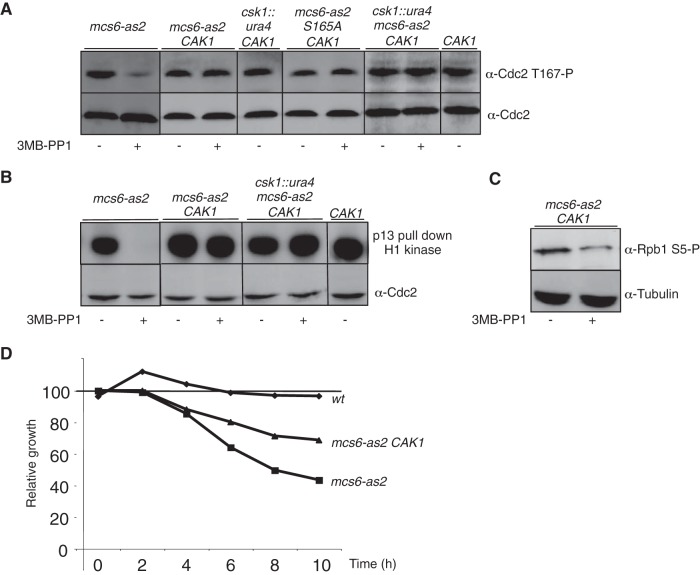
The availability of the budding yeast monomeric Cak1 kinase allowed us to specifically complement the Cdc2 activation defect seen in Mcs6-as2. When integrated at the fission yeast ura4 locus, the CAK1 gene fully restored Cdc2 phosphorylation and activity after Mcs6-as2 inhibition (Fig. 2A and B) while the decreased phosphorylation of the CTD on serine 5 was still observed, indicating that Cak1 directly phosphorylated Cdc2 but not the CTD of Pol II under these conditions (Fig. 2C). Importantly, the presence of Cak1 partially suppressed the reduction in the growth rate that resulted from Mcs6-as2 inhibition (Fig. 2D), suggesting that this defect did not exclusively result from impaired transcription but rather reflected the combined alteration of Mcs6 CAK and CTD kinase activities.
FIG 2.
Cak1 complements the CAK defect resulting from Mcs6 inactivation. (A) In vivo effects of Mcs6 inactivation on Cdc2 T167 phosphorylation are complemented by expression of Cak1. The indicated strains were grown in the presence or absence of 3-MB-PP1 inhibitor, and total protein was extracted and separated on SDS-PAGE. Western blot analyses were performed with anti-Cdc2 T167P or anti-Cdc2, as indicated. Note that the msc6-as2 data are identical to those in Fig. 1C and were duplicated here for clarity. All samples were run on the same gel. (B) In vitro kinase assay of Cdc2 after inhibition of Mcs6 in the presence of Cak1. The indicated strains were grown in the presence or absence of 3-MB-PP1 inhibitor, and soluble proteins were extracted and immunoprecipitated using p13 beads. Kinase assays were performed using histone H1 as the substrate. After separation on SDS-PAGE, the kinase gel was exposed (top) and transferred to a membrane for the anti-Cdc2 Western blotting (bottom). Note that the msc6-as2 data are identical to those in Fig. 1D and were duplicated here for clarity. All samples were run on the same gel. (C) The in vivo effect of Mcs6 inactivation on CTD phosphorylation is not complemented by expression of Cak1. Same as panel B, except that Western blot analysis was performed with anti-S5P or anti tubulin, as indicated. (D) The growth defect resulting from the inhibition of Mcs6-as is partially suppressed by Cak1. The indicated strains were grown in the presence or absence of 30 μM 3-MB-PP1. Cell numbers were measured and plotted relative to that of DMSO-treated cells of the same genotype, defined as 100%.
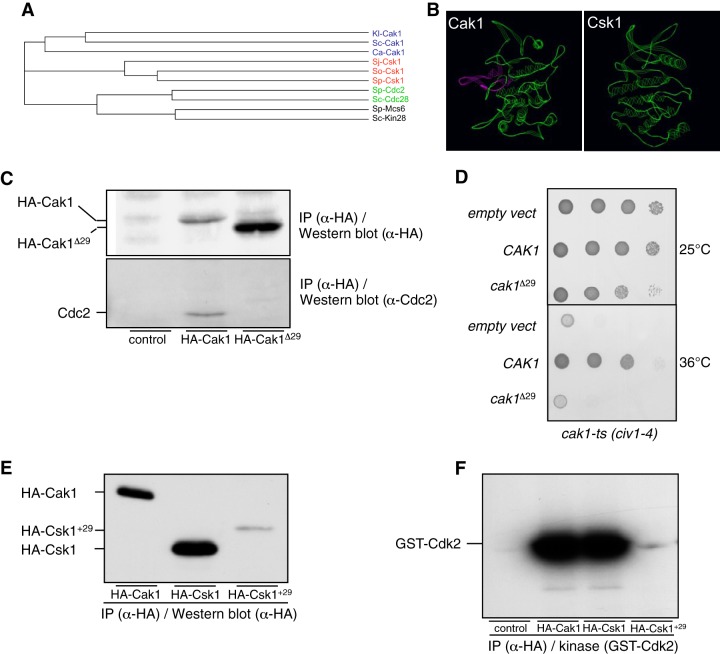
To further differentiate the specificities of Csk1 and Cak1 in Cdc2 activation by in vivo labeling of their substrates, we generated an analogue-sensitive mutant of each kinase and expressed them from plasmids in fission yeast. The Csk1 T89G and Cak1 F79G mutants were properly expressed, but unlike Mcs6 (Fig. 1B) or Cdk9-as (Fig. 3A), they showed poor in vitro affinity and specificity for the N6(benzyl)-ATP (Fig. 3B), suggesting that the ATP-binding pocket of these kinases has unusual properties, as noted previously (42, 43). Moreover, the analogue-sensitive kinases showed modest (Cak1) or negligible (Csk1) sensitivity to various inhibitors when tested in vivo (see Fig. S2 in the supplemental material), altogether precluding their use. However, when expressed in fission yeast and immunoprecipitated from cell extracts, wild-type Cak1 could precipitate and phosphorylate [using either ATP or N6(benzyl)-ATP as a substrate]. A Western blot analysis indicated that the substrate is likely Cdc2 (Fig. 3C and 4C). In an identical experimental setup, Csk1 could not associate with and phosphorylate this substrate (Fig. 3C), which is reminiscent of previous work showing that purified GST-Cak1 bound and phosphorylated Cdc2 while GST-Csk1 did not (21).
FIG 3.
In vivo interaction between Cak1 and Cdc2 in fission yeast. (A) In vitro kinase activity assay of the wt and analogue-sensitive (as) versions of Cdk9 in the presence of wt or bulky ATP. TAP-tagged versions of the Cdk9 or Cdk9-as kinase were precipitated using anti-TAP antibodies and used on beads for kinase assays with either ATP or N6(benzyl)-ATP, with GST-CTD as the substrate. After separation on SDS-PAGE, the kinase gel was exposed. Note that the exposure time of the left side of the kinase gel was shorter in order to compare the use of ATP versus N6(benzyl)-ATP. (B) In vitro kinase activity assay of the wt and analogue-sensitive versions of Csk1 and Cak1 in the presence of wt or bulky ATP. HA-tagged versions of the indicated kinases were precipitated using anti-HA antibodies and used on beads for kinase assays with either ATP or N6(benzyl)-ATP, with GST-Cdk2 as the substrate. After separation on SDS-PAGE, the kinase gel was exposed (top and middle, with different exposure [exp] times) and transferred to a membrane for anti-HA Western blotting (bottom). (C) In vitro kinase activity assay of the wt versions of Csk1 and Cak1 in the presence of wt or bulky ATP. HA-tagged versions of the indicated kinases were precipitated using anti-HA antibodies and used on beads for kinase assays with either ATP or N6(benzyl)-ATP in the absence of exogenous substrate. After separation on SDS-PAGE, the kinase gel was exposed (middle) and transferred to a membrane for anti-HA Western blotting (top) or anti-Cdc2 Western blotting (bottom). The phosphorylated-substrate band was superimposed on the Cdc2 band.
FIG 4.
A conserved insertion within the budding yeast Cak1 sequence family is required to bind Cdc2. (A) Phylogram tree based on a multiple alignment of the indicated protein sequences performed in ClustalW (not shown). Kl, K. lactis; Sc, S. cerevisiae; Ca, C. albicans; Sj, S. japonicus; So, S. octosporus; Sp, S. pombe. (B) Three-dimensional homology-based models of S. cerevisiae Cak1 (ScCak1) (left) and S. pombe Csk1 (SpCsk1) (right) built using SWISS-MODEL (http://swissmodel.expasy.org/?pid=smh01&uid=&token=). Only the backbone is represented. The conserved insertion present in Cak1 (see Fig. S3 in the supplemental material) is highlighted. The images were prepared with the Swiss-Pdb Viewer software. (C) Cdc2-binding assay of wt Cak1 and a truncated version lacking the sequence highlighted in panel B (Cak1Δ29). HA-tagged versions of the indicated kinases were precipitated using anti-HA antibodies. After SDS-PAGE and transfer to a membrane, they were processed for anti-HA Western blotting (top) and anti-Cdc2 Western blotting (bottom). (D) Complementation of a cak1 TS strain. The civ1-4 strain was transformed with pMET25 vectors expressing either CAK1 or cak1Δ29, and serial dilutions were plated at the indicated temperatures. (E) The Csk1+29 protein is expressed at a very low level. HA-tagged versions of the indicated kinases were precipitated using anti-HA antibodies. After SDS-PAGE and transfer to a membrane, they were processed for anti-HA Western blotting. (F) The Csk1+29 protein has low kinase activity. HA-tagged versions of the indicated kinases were precipitated using anti-HA antibodies and used on beads for kinase assays with GST-Cdk2 as the substrate. After separation on SDS-PAGE, the kinase gel was exposed.
Taken together, these data further strengthened the conclusion that Cdc2 activation results from the linear activation pathway Csk1→Mcs6→Cdc2.
We next investigated if the substrate specificities of Csk1 versus Cak1 could result from structural properties. As noted previously, the sequence conservation between the members of the monomeric CAK family is low (43). The recent sequencing of additional species within the fission yeast genus, Schizosaccharomyces japonicus and Schizosaccharomyces octosporus, allowed us to perform a broader alignment, which revealed the absence of a stretch of about 25 amino acids in the Cak1-related sequences of the Schizosaccharomyces species (see Fig. S3 in the supplemental material). Interestingly, structural analyses indicated that the insertion was located close to the catalytic site (Fig. 4A and B), suggesting that it may play a role in the substrate specificity. To test this possibility, we deleted the corresponding sequence in the budding yeast Cak1 kinase. Although the protein was properly expressed in fission yeast, it failed to coprecipitate Cdc2 (Fig. 4C) and could not complement a cak1 TS (civ1-4) mutant (Fig. 4D). This prompted us to generate a chimeric Csk1 kinase in which the same 29 amino acids were inserted in the corresponding location. However, the resulting protein was expressed at a very low level, being barely detectable, and the associated kinase activity was accordingly very low, which impeded further analyses (Fig. 4E and F). In conclusion, we have identified a short sequence present only in the budding yeast Cak1 homologues that is required for their efficient binding to Cdc2.
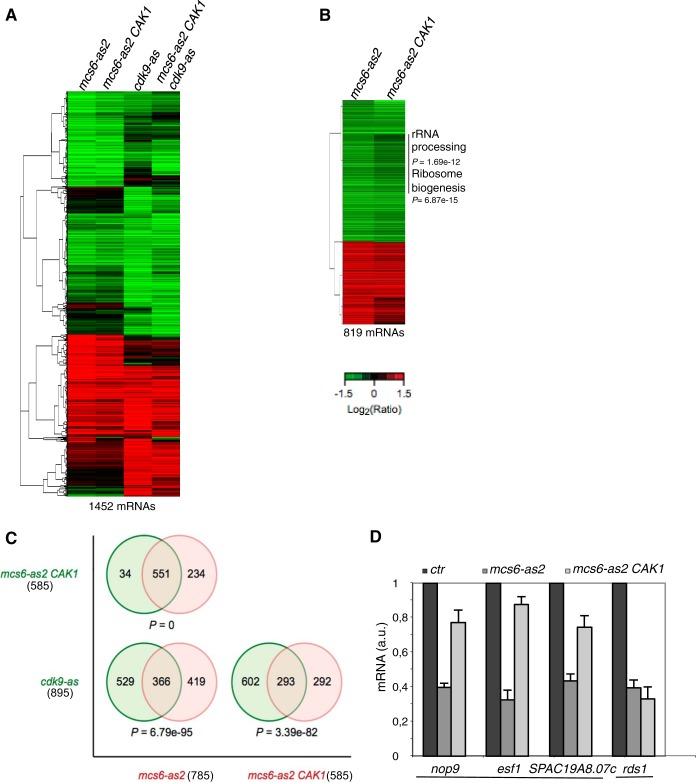
The fact that the S. cerevisiae Cak1 kinase partially restored growth after Mcs6-as2 inhibition (Fig. 2D) suggested that the inactivation of Mcs6 resulted in phenotypes linked to two independent pathways: Cdc2 activation and Pol II transcription. To test this assumption, we analyzed gene expression profiles in the mcs6-as2 and the mcs6-as2 CAK1 strains by using microarrays and compared them to the cdk9-as and cdk9-as mcs6-as2 CAK1 strains (see Table S1 in the supplemental material). All the strains were treated for 120 min with 30 μM 3-MB-PP1, a concentration that did not affect the growth of the wild-type strain, and expression profiling was performed relative to mock-treated controls (Fig. 5A and B). A similar global profile was observed in each case, indicating that Mcs6 and Cdk9 target a large common set of genes, as previously reported (40).
FIG 5.
Activation of Cdc2 by CAK is required for expression of the ribocluster. (A) Hierarchical clustering of 1,452 mRNAs whose expression is significantly affected in the mcs6-as2, mcs6-as2 CAK1, cdk9-as, and mcs6-as2 cdk9-as CAK1 mutant strains. The strains were cultured in yeast extract-supplemented medium, and the inhibitor 3-MB-PP1 or the solvent DMSO was added for 120 minutes. The data are presented as log2 30 μM 3-MB-PP1/DMSO ratios of hybridization intensity and are color coded as indicated in the key (a P value of ≤0.05 and ≥1.5-fold change). (B) Hierarchical clustering of the 819 mRNAs whose expression is significantly affected in the mcs6-as2 or the mcs6-as2 CAK1 strain (a P value of ≤0.05 and ≥1.5-fold change). The gene cluster whose expression is significantly decreased only in the absence of CAK1 is highlighted, and the enriched GO categories are indicated, with the associated P values. (C) Venn diagrams of the overlap in expression of mcs6-as2, mcs6-as2 CAK1, and cdk9-as with associated P values for the degree of overlap calculated by a hypergeometric test. The numbers of mRNAs are indicated in parentheses. (D) Relative quantification (RQ) of the nop9, esf1, SPAC19A8.07c, and rds1 mRNAs determined by quantitative RT-PCR using the ΔΔCT method in mcs6-as2 and mcs6-as2 CAK1 strains. Each strain was grown in the absence or presence of 3-MB-PP1 for 120 minutes, and treated samples were compared to untreated samples (ctr), set as 1. a.u., arbitrary units. Note that for clarity, the untreated control is shown only once per mRNA analyzed. The nop9 gene belongs to the rRNA-processing GO category (0006364), and the SPAC19A8.07c and esf1 genes belong to the ribosome biogenesis GO category (0042254). The error bars indicate standard deviations of the means from 3 independent experiments.
Upon inhibition of Mcs6, most of the genes behaved similarly regardless of the presence of Cak1, but a subset of about 200 genes were affected only when Cak1 was absent, indicating that they mainly responded to the inhibition of the CAK activity on Cdc2, rather than a defect in Pol II CTD phosphorylation. More precisely, about 95% (551 genes) of the differentially expressed genes in mcs6-as2 CAK1 (which comprise 585 genes) overlapped with those of the mcs6-as2 strain (Fig. 5C), but this common set represented only 71% of the differentially expressed genes in mcs6-as2 (785 genes) (Fig. 5A and B).
This effect did not result from a cell cycle arrest, as indicated by fluorescence-activated cell sorting (FACS) (data not shown). Gene ontology (GO) analysis of the CAK activity-dependent genes (234 genes with a P value of ≤0.05 and ≥1.5-fold change) revealed enrichment in this category for genes required for ribosome biogenesis and rRNA processing (see Fig. S4 in the supplemental material), which could not be attributed to an artifactual effect of the presence of CAK1 because these categories were not overrepresented when expression profiling of a wild-type strain expressing CAK1 was performed (data not shown). We confirmed these data by independent quantitative RT-PCR on nop9, esf1, and SPAC19A8.07c, three genes that appeared to be specifically downregulated by the defect in Mcs6 CAK activity (Fig. 5D). In the presence of CAK1, the expression levels of all three genes were improved but never reached wild-type levels, since the CTD kinase activity of Mcs6 was still compromised. As a control, we also chose the rds1 gene, which did not respond to the presence of Cak1 based on the microarray data, which we could confirm (Fig. 5D).
Based on these data, we predicted that a cdc2 T167A mutant may affect the expression of the group of genes identified in Fig. 5B. We failed to generate a heterozygous strain harboring the cdc2 T167A mutant and therefore expressed it from a plasmid under the control of the nmt1 promoter, which is downregulated by thiamine. As previously reported, the transformation efficiency of the mutant was very low compared to that of a plasmid expressing wild-type cdc2 or an empty vector (44). Even in the presence of thiamine, a marked growth defect was observed in the strain expressing cdc2 T167A (Fig. 6A), indicating a dominant-negative effect of the mutant. Surprisingly, no cell elongation was observed, which suggested that the slow growth did not result from cell cycle arrest. Only in the absence of thiamine, when the mutant was expressed at a much higher level, did some colonies clearly show a typical cdc phenotype (Fig. 6A). Together with the microarray analysis discussed above, these data indicate that the activating phosphorylation of Cdc2 by the Mcs6 (Cdk7) CAK is required to promote cell growth, most likely by regulating the expression of a group of genes implicated in ribosome biogenesis. This hypothesis was supported by the specific downregulation of nop9, esf1, and SPAC19A8.07c when the cdc2 T167A mutant was expressed, while rds1 remained unaffected (Fig. 6B).
FIG 6.

Activation of Cdc2 by CAK is required for the transcription of some ribosome-associated genes. (A) Dominant growth defect resulting from the expression of the cdc2 T167A mutant. (Top) A wt strain was transformed with plasmids expressing cdc2, cdc2 T167A (cdc2 TA), or empty vector, and serial dilutions were incubated at 32°C for 3 days. (Bottom) Colonies expressing the cdc2 TA mutant in the presence or absence of thiamine. Elongated cdc phenotypes (asterisk) appear only in the absence of thiamine. EMM, Edinburgh minimal medium. (B) RQ of the nop9, esf1, SPAC19A8.07c, and rds1 mRNAs determined by quantitative RT-PCR using the ΔΔCT method in the same strains as in panel A, grown in the presence of thiamine. The strain harboring the empty vector was set as 1. Note that for clarity, the untreated control is shown only once per mRNA analyzed. The nop9 gene belongs to the rRNA-processing GO category (0006364), and the SPAC19A8.07c and esf1 genes belong to the ribosome biogenesis GO category (0042254). The error bars indicate standard deviations of the means from 3 independent experiments.
We conclude that, in addition to its well-documented RNA polymerase II CTD kinase activity, Mcs6 also regulates gene expression through its CAK activity.
DISCUSSION
A linear Cdc2 activation cascade in fission yeast.
Using an improved analogue-sensitive allele of mcs6, we demonstrate that Mcs6 is the genuine CAK of Cdc2, the cyclin-dependent kinase controlling both the G1-S and the G2-M transitions in fission yeast. Upon inhibition of Mcs6, both the T167 phosphorylation of Cdc2 and Cdc2 kinase activity are decreased independently of the presence of Csk1, the second CAK in the organism. Together with previous data (21), this indicates that a linear Csk1→Mcs6→Cdc2 CAK pathway exists in vivo in S. pombe, as anticipated based on early genetic analyses (23). The fission yeast CAK pathway is therefore very reminiscent of that of higher eukaryotes, unlike budding yeast, where the Cdk7 ortholog Kin28 plays no role in CDK activation and is required solely for CTD phosphorylation.
Here, we provide evidence that a structural difference, the presence of an insertion of about 20 amino acids close to the catalytic site, is responsible for the differences in substrate specificity observed between the Cak1 kinases from several species of budding yeast (S. cerevisiae, Kluyveromyces lactis, and Candida albicans) and the Csk1 kinases from species of fission yeast (S. pombe, S. japonicus, and S. octosporus). Indeed, this insertion is required both for binding to Cdc2 and for the ability of Cak1 to complement a cak1 TS mutant. It was reported that Csk1 can phosphorylate Cdc2 in vitro (22), and it remains possible that under these conditions, the structural difference between Csk1 and Cak1 becomes irrelevant. The fact that strong overexpression of Csk1 complements a cak1 TS mutant (21) (see Fig. S2 in the supplemental material) supports this possibility.
Besides this structural feature, the sequence alignment of additional Cak1-related sequences from the fission yeast genus confirms the high divergence that occurred within the monomeric CAK kinase group (see Fig. S3 in the supplemental material). Our attempt to generate analogue-sensitive alleles of either Cak1 or Csk1 revealed that the wild-type kinases already have a significant capacity to use the bulky N6(benzyl)-ATP. In parallel, the analogue-sensitive mutants are barely sensitive to high doses of ATP analogue, altogether confirming that the monomeric CAKs have an unusual ATP-binding pocket, as previously proposed (42).
The Mcs6 (Cdk7) kinase regulates gene expression through both its CAK and Pol II CTD kinase activities.
Expression profiling of Cdk7 mutants from various species presupposed that the inactivation of Cdk7 solely affected mRNA transcription due to a defect in serine 5 phosphorylation within the RNA polymerase II CTD. Although our data generally support this view, the specific rescue by Cak1 of the CAK defect following the inhibition of Mcs6 revealed an unanticipated role of Cdc2 phosphorylation in the expression of a group of genes implicated in ribosome biogenesis. Although most of the genes belonging to that group are slightly affected by the inhibition of serine 5 phosphorylation, the concomitant inhibition of Cdc2 activation further decreases the expression level of the corresponding mRNAs, which suggests that Cdc2 plays a direct role in their regulation.
In support of this possibility, we show that the expression of a cdc2 mutant that cannot be phosphorylated on the CAK site (cdc2 T167A) results in a dominant-negative phenotype characterized by slow growth and associated with decreased expression of the ribosome biogenesis genes that we have tested. As it was previously reported that the Cdc2 T167A mutant can associate with cyclin B (44), albeit to a lesser extent than the wild type, it is likely that the presence of a Cdc2 T167A-cyclin B complex interferes with the expression of the ribosome biogenesis cluster, which results in dominant slow growth. Only when the Cdc2 T167A mutant is strongly overexpressed from the nmt1 promoter (45) is the elongated cdc phenotype, typical of cdc2 mutants, observed. Most likely, in this case, the mutant titrates the complete cyclin B pool, leading to a mitotic block.
The growth defect observed when Cdc2 activation is impaired also explains why downregulation of Mcs6 similarly does not result in the cdc phenotype expected if Cdc2 is inactivated but rather leads to slower growth. As expected based on the above data, the expression of Cak1 during Mcs6 inactivation restored normal Cdc2 activation (Fig. 2A and B) and significantly counteracted the growth defect (Fig. 2D).
A previous genome-wide analysis of the cell cycle-regulated genes in fission yeast identified an early/mid-G2 wave of genes peaking with a moderate amplitude (46). This wave included genes involved in ribosome biogenesis (the ribocluster) and the cdc2 gene itself. Our data suggest that Cdc2 activation by CAK in early to mid-G2 is responsible for the increased expression of this cluster during the same window of the cell cycle.
Could Cdc2 activation by CAK couple cell growth to cell division?
In respect to whether Cdc2 activation by CAK couples cell growth to cell division, it is interesting that a link between CDK activity and ribosome synthesis has been established in other species (47–49). Candidate substrates have been proposed (50, 51), and the large-scale analysis of Cdc2 substrates by chemical genetics identified a group of substrates related to ribosome metabolism (52). Further work is needed to fully characterize how these potential Cdc2 substrates regulate the expression of the ribocluster. Our unpublished data show that Cdc2 phosphorylates the Sfp1 transcription factor, a key regulator of the ribocluster in budding yeast, on various sites. Therefore, one possibility is that Cdc2 phosphorylation by CAK in early G2, when the level of B-type cyclin is low, is required for the efficient activation of a master regulator of the ribocluster.
It is also known that the conserved Xpd protein (Rad15 in fission yeast), which is part of TFIIH, negatively regulates the CAK activity of Cdk7 and relocalizes it to different subcellular compartments in Drosophila (53, 54). The regulation of Xpd could therefore play a pivotal role in the putative link between Cdk activation and growth.
The data presented here have additional important implications. Earlier studies in fission yeast have documented a cell cycle oscillation in the rate of protein synthesis (the rate change point [RCP]) and a corresponding acceleration of the growth rate in mid-G2 in fission yeast (55–58). The wave of transcription of the ribocluster in early to mid-G2 correlates with the RCP and occurs at about the time when H1 kinase activity starts to rise, as noted previously (46). Based on the data presented in this study, we raise the hypothesis that the G2 RCP is defined by Cdc2 phosphorylation and activation by CAK, which increases the transcription of the ribosome biogenesis cluster and accelerates protein synthesis (RCP). Because full Cdc2 activity is also required later in the cell cycle to promote entry into mitosis, CAK activity could therefore couple cell growth to cell division.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Paul Nurse for the Cdc2 antibodies. We thank Tomi Makela and Katja Helenius for critical reading of the manuscript.
M.D. was an FNRS Research Fellow. H.V.B. was supported by grants from the Netherlands Organization for Scientific Research (NOW) (grant no. 825.06.033) and the Canadian Institutes of Health Research (CIHR) (grant no. 193588). D.H. was supported by grants FRFC 2.4510.10, Credit aux chercheurs 1.5.013.09, and MIS F.4523.11 and by Ceruna and Marie Curie Action. D.H. is an FNRS Research Associate.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/MCB.00024-15.
REFERENCES
- 1.Morgan DO. 1997. Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu Rev Cell Dev Biol 13:261–291. doi: 10.1146/annurev.cellbio.13.1.261. [DOI] [PubMed] [Google Scholar]
- 2.Kaldis P. 1999. The cdk-activating kinase (CAK): from yeast to mammals. Cell Mol Life Sci 55:284–296. doi: 10.1007/s000180050290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solomon MJ, Kaldis P. 1998. Regulation of CDKs by phosphorylation. Results Probl Cell Differ 22:79–109. doi: 10.1007/978-3-540-69686-5_4. [DOI] [PubMed] [Google Scholar]
- 4.Fisher RP. 2012. The CDK network: linking cycles of cell division and gene expression. Genes Cancer 3:731–738. doi: 10.1177/1947601912473308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larochelle S, Batliner J, Gamble MJ, Barboza NM, Kraybill BC, Blethrow JD, Shokat KM, Fisher RP. 2006. Dichotomous but stringent substrate selection by the dual-function Cdk7 complex revealed by chemical genetics. Nat Struct Mol Biol 13:55–62. doi: 10.1038/nsmb1028. [DOI] [PubMed] [Google Scholar]
- 6.Merrick KA, Larochelle S, Zhang C, Allen JJ, Shokat KM, Fisher RP. 2008. Distinct activation pathways confer cyclin-binding specificity on Cdk1 and Cdk2 in human cells. Mol Cell 32:662–672. doi: 10.1016/j.molcel.2008.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larochelle S, Merrick KA, Terret ME, Wohlbold L, Barboza NM, Zhang C, Shokat KM, Jallepalli PV, Fisher RP. 2007. Requirements for cdk7 in the assembly of cdk1/cyclin B and activation of cdk2 revealed by chemical genetics in human cells. Mol Cell 25:839–850. doi: 10.1016/j.molcel.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larochelle S, Pandur J, Fisher RP, Salz HK, Suter B. 1998. Cdk7 is essential for mitosis and for in vivo Cdk-activating kinase activity. Genes Dev 12:370–381. doi: 10.1101/gad.12.3.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallenfang MR, Seydoux G. 2002. cdk-7 is required for mRNA transcription and cell cycle progression in Caenorhabditis elegans embryos. Proc Natl Acad Sci U S A 99:5527–5532. doi: 10.1073/pnas.082618399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rossi DJ, Londesborough A, Korsisaari N, Pihlak A, Lehtonen E, Henkemeyer M, Makela TP. 2001. Inability to enter S phase and defective RNA polymerase II CTD phosphorylation in mice lacking Mat1. EMBO J 20:2844–2856. doi: 10.1093/emboj/20.11.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roy R, Adamczewski JP, Seroz T, Vermeulen W, Tassan JP, Schaeffer L, Nigg EA, Hoeijmakers JH, Egly JM. 1994. The MO15 cell cycle kinase is associated with the TFIIH transcription-DNA repair factor. Cell 79:1093–1101. doi: 10.1016/0092-8674(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 12.Serizawa H, Makela TP, Conaway JW, Conaway RC, Weinberg RA, Young RA. 1995. Association of Cdk-activating kinase subunits with transcription factor TFIIH. Nature 374:280–282. doi: 10.1038/374280a0. [DOI] [PubMed] [Google Scholar]
- 13.Fisher RP, Jin P, Chamberlin HM, Morgan DO. 1995. Alternative mechanisms of CAK assembly require an assembly factor or an activating kinase. Cell 83:47–57. doi: 10.1016/0092-8674(95)90233-3. [DOI] [PubMed] [Google Scholar]
- 14.Holstege FC, Jennings EG, Wyrick JJ, Lee TI, Hengartner CJ, Green MR, Golub TR, Lander ES, Young RA. 1998. Dissecting the regulatory circuitry of a eukaryotic genome. Cell 95:717–728. doi: 10.1016/S0092-8674(00)81641-4. [DOI] [PubMed] [Google Scholar]
- 15.Kanin EI, Kipp RT, Kung C, Slattery M, Viale A, Hahn S, Shokat KM, Ansari AZ. 2007. Chemical inhibition of the TFIIH-associated kinase Cdk7/Kin28 does not impair global mRNA synthesis. Proc Natl Acad Sci U S A 104:5812–5817. doi: 10.1073/pnas.0611505104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cismowski MJ, Laff GM, Solomon MJ, Reed SI. 1995. KIN28 encodes a C-terminal domain kinase that controls mRNA transcription in Saccharomyces cerevisiae but lacks cyclin-dependent kinase-activating kinase (CAK) activity. Mol Cell Biol 15:2983–2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thuret JY, Valay JG, Faye G, Mann C. 1996. Civ1 (CAK in vivo), a novel Cdk-activating kinase. Cell 86:565–576. doi: 10.1016/S0092-8674(00)80130-0. [DOI] [PubMed] [Google Scholar]
- 18.Kaldis P, Sutton A, Solomon MJ. 1996. The Cdk-activating kinase (CAK) from budding yeast. Cell 86:553–564. doi: 10.1016/S0092-8674(00)80129-4. [DOI] [PubMed] [Google Scholar]
- 19.Damagnez V, Makela TP, Cottarel G. 1995. Schizosaccharomyces pombe Mop1-Mcs2 is related to mammalian CAK. EMBO J 14:6164–6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hermand D, Pihlak A, Westerling T, Damagnez V, Vandenhaute J, Cottarel G, Makela TP. 1998. Fission yeast Csk1 is a CAK-activating kinase (CAKAK). EMBO J 17:7230–7238. doi: 10.1093/emboj/17.24.7230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hermand D, Westerling T, Pihlak A, Thuret JY, Vallenius T, Tiainen M, Vandenhaute J, Cottarel G, Mann C, Makela TP. 2001. Specificity of Cdk activation in vivo by the two Caks Mcs6 and Csk1 in fission yeast. EMBO J 20:82–90. doi: 10.1093/emboj/20.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee KM, Saiz JE, Barton WA, Fisher RP. 1999. Cdc2 activation in fission yeast depends on Mcs6 and Csk1, two partially redundant Cdk-activating kinases (CAKs). Curr Biol 9:441–444. doi: 10.1016/S0960-9822(99)80194-8. [DOI] [PubMed] [Google Scholar]
- 23.Molz L, Booher R, Young P, Beach D. 1989. cdc2 and the regulation of mitosis: six interacting mcs genes. Genetics 122:773–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saiz JE, Fisher RP. 2002. A CDK-activating kinase network is required in cell cycle control and transcription in fission yeast. Curr Biol 12:1100–1105. doi: 10.1016/S0960-9822(02)00903-X. [DOI] [PubMed] [Google Scholar]
- 25.Molz L, Beach D. 1993. Characterization of the fission yeast mcs2 cyclin and its associated protein kinase activity. EMBO J 12:1723–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bamps S, Westerling T, Pihlak A, Tafforeau L, Vandenhaute J, Makela TP, Hermand D. 2004. Mcs2 and a novel CAK subunit Pmh1 associate with Skp1 in fission yeast. Biochem Biophys Res Commun 325:1424–1432. doi: 10.1016/j.bbrc.2004.10.190. [DOI] [PubMed] [Google Scholar]
- 27.Bauer F, Matsuyama A, Yoshida M, Hermand D. 2012. Determining proteome-wide expression levels using reverse protein arrays in fission yeast. Nat Protoc 7:1830–1835. doi: 10.1038/nprot.2012.114. [DOI] [PubMed] [Google Scholar]
- 28.Cassart C, Drogat J, Migeot V, Hermand D. 2012. Distinct requirement of RNA polymerase II CTD phosphorylations in budding and fission yeast. Transcription 3:231–234. doi: 10.4161/trns.21066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drogat J, Hermand D. 2012. Gene-specific requirement of RNA polymerase II CTD phosphorylation. Mol Microbiol 84:995–1004. doi: 10.1111/j.1365-2958.2012.08071.x. [DOI] [PubMed] [Google Scholar]
- 30.Drogat J, Migeot V, Mommaerts E, Mullier C, Dieu M, van Bakel H, Hermand D. 2012. Cdk11-cyclinL controls the assembly of the RNA polymerase II mediator complex. Cell Rep 2:1068–1076. doi: 10.1016/j.celrep.2012.09.027. [DOI] [PubMed] [Google Scholar]
- 31.Fersht N, Hermand D, Nurse P. 2007. Cdc18/CDC6 activates the Rad3-dependent checkpoint in the fission yeast. Nucleic Acids Res 35:5323–5337. doi: 10.1093/nar/gkm527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guiguen A, Soutourina J, Dewez M, Tafforeau L, Dieu M, Raes M, Vandenhaute J, Werner M, Hermand D. 2007. Recruitment of P-TEFb (Cdk9-Pch1) to chromatin by the cap-methyl transferase Pcm1 in fission yeast. EMBO J 26:1552–1559. doi: 10.1038/sj.emboj.7601627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mumberg D, Muller R, Funk M. 1994. Regulatable promoters of Saccharomyces cerevisiae: comparison of transcriptional activity and their use for heterologous expression. Nucleic Acids Res 22:5767–5768. doi: 10.1093/nar/22.25.5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eblen ST, Kumar NV, Shah K, Henderson MJ, Watts CK, Shokat KM, Weber MJ. 2003. Identification of novel ERK2 substrates through use of an engineered kinase and ATP analogs. J Biol Chem 278:14926–14935. doi: 10.1074/jbc.M300485200. [DOI] [PubMed] [Google Scholar]
- 35.Bordoli L, Kiefer F, Arnold K, Benkert P, Battey J, Schwede T. 2009. Protein structure homology modeling using SWISS-MODEL workspace. Nat Protoc 4:1–13. doi: 10.1038/nprot.2008.197. [DOI] [PubMed] [Google Scholar]
- 36.Dutrow N, Nix DA, Holt D, Milash B, Dalley B, Westbroek E, Parnell TJ, Cairns BR. 2008. Dynamic transcriptome of Schizosaccharomyces pombe shown by RNA-DNA hybrid mapping. Nat Genet 40:977–986. doi: 10.1038/ng.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coudreuse D, van Bakel H, Dewez M, Soutourina J, Parnell T, Vandenhaute J, Cairns B, Werner M, Hermand D. 2010. A gene-specific requirement of RNA polymerase II CTD phosphorylation for sexual differentiation in S. pombe. Curr Biol 20:1053–1064. doi: 10.1016/j.cub.2010.04.054. [DOI] [PubMed] [Google Scholar]
- 38.Tafforeau L, Le Blastier S, Bamps S, Dewez M, Vandenhaute J, Hermand D. 2006. Repression of ergosterol level during oxidative stress by fission yeast F-box protein Pof14 independently of SCF. EMBO J 25:4547–4556. doi: 10.1038/sj.emboj.7601329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lyne R, Burns G, Mata J, Penkett CJ, Rustici G, Chen D, Langford C, Vetrie D, Bahler J. 2003. Whole-genome microarrays of fission yeast: characteristics, accuracy, reproducibility, and processing of array data. BMC Genomics 4:27. doi: 10.1186/1471-2164-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Viladevall L, St Amour CV, Rosebrock A, Schneider S, Zhang C, Allen JJ, Shokat KM, Schwer B, Leatherwood JK, Fisher RP. 2009. TFIIH and P-TEFb coordinate transcription with capping enzyme recruitment at specific genes in fission yeast. Mol Cell 33:738–751. doi: 10.1016/j.molcel.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dischinger S, Krapp A, Xie L, Paulson JR, Simanis V. 2008. Chemical genetic analysis of the regulatory role of Cdc2p in the S. pombe septation initiation network. J Cell Sci 121:843–853. doi: 10.1242/jcs.021584. [DOI] [PubMed] [Google Scholar]
- 42.Enke DA, Kaldis P, Holmes JK, Solomon MJ. 1999. The CDK-activating kinase (Cak1p) from budding yeast has an unusual ATP-binding pocket. J Biol Chem 274:1949–1956. doi: 10.1074/jbc.274.4.1949. [DOI] [PubMed] [Google Scholar]
- 43.Tsakraklides V, Solomon MJ. 2002. Comparison of Cak1p-like cyclin-dependent kinase-activating kinases. J Biol Chem 277:33482–33489. doi: 10.1074/jbc.M205537200. [DOI] [PubMed] [Google Scholar]
- 44.Gould KL, Moreno S, Owen DJ, Sazer S, Nurse P. 1991. Phosphorylation at Thr167 is required for Schizosaccharomyces pombe p34cdc2 function. EMBO J 10:3297–3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maundrell K. 1990. nmt1 of fission yeast. A highly transcribed gene completely repressed by thiamine. J Biol Chem 265:10857–10864. [PubMed] [Google Scholar]
- 46.Oliva A, Rosebrock A, Ferrezuelo F, Pyne S, Chen H, Skiena S, Futcher B, Leatherwood J. 2005. The cell cycle-regulated genes of Schizosaccharomyces pombe. PLoS Biol 3:e225. doi: 10.1371/journal.pbio.0030225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sirri V, Hernandez-Verdun D, Roussel P. 2002. Cyclin-dependent kinases govern formation and maintenance of the nucleolus. J Cell Biol 156:969–981. doi: 10.1083/jcb.200201024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Voit R, Grummt I. 2001. Phosphorylation of UBF at serine 388 is required for interaction with RNA polymerase I and activation of rDNA transcription. Proc Natl Acad Sci U S A 98:13631–13636. doi: 10.1073/pnas.231071698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Voit R, Hoffmann M, Grummt I. 1999. Phosphorylation by G1-specific cdk-cyclin complexes activates the nucleolar transcription factor UBF. EMBO J 18:1891–1899. doi: 10.1093/emboj/18.7.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gulli MP, Faubladier M, Sicard H, Caizergues-Ferrer M. 1997. Mitosis-specific phosphorylation of gar2, a fission yeast nucleolar protein structurally related to nucleolin. Chromosoma 105:532–541. doi: 10.1007/BF02510490. [DOI] [PubMed] [Google Scholar]
- 51.Gulli MP, Girard JP, Zabetakis D, Lapeyre B, Melese T, Caizergues-Ferrer M. 1995. gar2 is a nucleolar protein from Schizosaccharomyces pombe required for 18S rRNA and 40S ribosomal subunit accumulation. Nucleic Acids Res 23:1912–1918. doi: 10.1093/nar/23.11.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blethrow JD, Glavy JS, Morgan DO, Shokat KM. 2008. Covalent capture of kinase-specific phosphopeptides reveals Cdk1-cyclin B substrates. Proc Natl Acad Sci U S A 105:1442–1447. doi: 10.1073/pnas.0708966105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen J, Larochelle S, Li X, Suter B. 2003. Xpd/Ercc2 regulates CAK activity and mitotic progression. Nature 424:228–232. doi: 10.1038/nature01746. [DOI] [PubMed] [Google Scholar]
- 54.Li X, Urwyler O, Suter B. 2010. Drosophila Xpd regulates Cdk7 localization, mitotic kinase activity, spindle dynamics, and chromosome segregation. PLoS Genet 6:e1000876. doi: 10.1371/journal.pgen.1000876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mitchison JM, Nurse P. 1985. Growth in cell length in the fission yeast Schizosaccharomyces pombe. J Cell Sci 75:357–376. [DOI] [PubMed] [Google Scholar]
- 56.Creanor J, Mitchison JM. 1982. Patterns of protein synthesis during the cell cycle of the fission yeast Schizosaccharomyces pombe. J Cell Sci 58:263–285. [DOI] [PubMed] [Google Scholar]
- 57.Creanor J, Mitchison JM. 1984. Protein synthesis and its relation to the DNA-division cycle in the fission yeast Schizosaccharomyces pombe. J Cell Sci 69:199–210. [DOI] [PubMed] [Google Scholar]
- 58.Sveiczer A, Novak B, Mitchison JM. 1996. The size control of fission yeast revisited. J Cell Sci 109:2947–2957. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.