Abstract
Objective(s):
There are several scolicidal agents for inactivation of hydatid cyst protoscolices during surgery, but most of them are associated with adverse side effects such as sclerosing cholangitis and liver necrosis. The present study was aimed to evaluate scolicidal effects of various extracts of Nigella sativa seeds against protoscoleces of hydatid cyst in an in vitro model.
Materials and Methods:
Protoscoleces were aseptically aspirated from naturally infected livers of sheep and goats. Various concentrations of the different extracts of N. sativa (5 to 50 mg/ml) were used for 5 to 60 min. Viability of protoscoleces was confirmed by 0.1% eosin staining.
Results:
The findings exhibited that methanolic extract at the concentration of 50 mg/ml after 10 min of incubation, and aqueous extract at the concentration of 50 mg/ml after 30 min of incubation can kill 100% of protoscoleces. In addition, all of experiments revealed dose-dependent and also time-dependent scolicidal effect of various extracts of N. sativa on the protoscoleces of hydatid cyst.
Conclusion:
The results of the present study demonstrated that N. sativa may be a natural source for the production of new scolicidal agent for use in hydatid cyst surgery. However, further studies will be required to evaluate scolicidal effects of N. sativa in the in vivo model.
Keywords: Hydatid cyst, Echinococcus granulosus, Nigella sativa
Introduction
Hydatidosis or cystic echinococcosis (CE) is a chronic zoonosis affecting humans as well as domestic animals caused by the larval stage of a cosmopolitan parasitic cestode Echinococcus granulosus. It has been identified as a global public health and economic problem particularly in developing regions including Iran, which causes serious morbidity and death if left untreated (1). The dog is the definitive host, in which adult tapeworms attached to the intestinal epithelium undergo sexual reproduction, leading to the development of eggs. Intermediate hosts such as humans as well as domestic livestock including cattle, sheep, camels, pigs become infected following ingestion of eggs through direct contact with dog or indirectly through food, water or soil contaminated with eggs. The released embryos penetrate the intestinal wall and via the portal system enter mainly into the liver (50-70%), lungs (20-30%), or any other organs where the hydatid cysts grow up (2). At present, surgical removal is still the ideal and preferred treatment for CE in many parts of the world, including Iran (3). In addition, chemotherapy with benzimidazoles (albendazole, mebendazole) and PAIR (puncture, aspiration, injection, re-aspiration) are also recommended as alternative treatments to surgery, especially for the patients who do not suffer from complicated cases of CE (3, 4). To reduce the risk of intraoperative spillage of the cyst contents (scolices) during surgery and subsequently recurrence of CE and secondary infection, which is observed in nearly 10% of the postoperative cases, the use of effective scolicidal agents are necessary (4, 5). Existing scolicidal agents including hypertonic saline, silver nitrate, cetrimide, and ethanol, which commonly used as scolicidal agents, are associated with adverse effects such as sclerosing colangititis, liver necrosis and methaemoglobinaemia (6-8). For these reasons, the development of new scolicidal agents especially from natural products due to having fewer side effects, low cost, high availability and higher efficacy is an urgent need for surgeons (9).
Nigella sativa Linn. (Ranunculaceae), black seed commonly grows in the Southern Europe, North Africa, Middle East and Western Asia. N. sativa called “Siah Daneh” in Persian, has long been traditionally used as a natural medicine for treatment of many acute, as well as, chronic conditions include hypertension, diabetes, cough, bronchitis, headache, eczema, fever and dizziness in worldwide (10). Reviews have reported N. sativa as having antioxidant and neuroprotective effects in addition to many other therapeutic activities such as antitumor, immunopotentiation, anti-inflammatory, antiasthmatic and antimicrobial properties (10, 11). Moreover, studies have revealed antibacterial, antifungal, antiviral and antiparasitic effects of N. sativa and its derivatives (12-16). To our knowledge, there is no study on the effect of N. sativa on protoscoleces of hydatid cyst in an in vitro model. Therefore, this study was aimed to evaluate scolicidal effects of various extracts of N. sativa against protoscoleces of E. granulosus in an in vitro model.
Materials and Methods
Collection and identifying the plant materials
The seeds of N. sativa as a source of the active ingredients of this plan were collected from rural [d1]regions of Bam in September 2012, Kerman province, Iran. The plant was identified by a botanist of the Botany Department of Shahid Bahonar University, Kerman, Iran. Voucher specimen (KF575) has been deposited in the Herbarium of Department of Pharmacognosy, School of Pharmacy, Kerman University of Medical Sciences, Kerman, Iran.
Preparation of various extracts
The dried seeds (100 g) of N. sativa were grinded and extracted by percolation method with methanol and water successively for 72 hr in room temperature. The extracts were passed through filter paper (Whatman No.3, Sigma, Germany) to remove plant debris. The extracts were finally concentrated in vacuum at 50°C using a rotary evaporator (Heidolph, Germany) and stored at -20°C, until use).
Drug dilutions
To prepare the dilutions of the various extracts of N. sativa, 1 g of various extracts dissolved in 9.9 ml of normal saline. In addition, to enhance the dispersal of the extracts in normal saline, 0.1 ml of dimethyl sulphoxide (DMSO) was added to the test tube and serial dilution was subsequently made to obtain extracts at 5, 10, 25 and 50 mg/ml. The selection of dilutions of the various extracts of N. sativa was based on initial experiments, which showed that DMSO below 1.5% had no effect on the growth of protoscoleces. In this study, the concentration of DMSO in all of the various dilutions was 1.5% and below.
Collection of protoscolices
The protoscoleces of E. granulosus were collected from livers of naturally infected sheep and goats slaughtered at Kerman abattoir, Southeast of Iran. Infected livers transferred to the Parasitology Laboratory of Kerman University of Medical Sciences, Kerman, Iran. The fluid of hydatid cysts aspirated by a 50 ml syringe and aseptically transferred into the glass cylinders. After 30 min, the supernatant was discarded and the settled protoscoleces were collected and washed three times with PBS (pH 7.2). The concentration of protoscoleces was adjusted as 2× 103 protoscoleces in 0.9% NaCl solution with at least 90% viability rate. The viability of the protoscolices was confirmed by their flame cell motility and impermeability to 0.1% eosin solution under a light microscope.
Scolicidal effects of N. sativa
To determine the scolicidal activity of various extracts of N. sativa upon the protoscoleces of hydatid cysts, various concentrations of the extracts were used for 10, 20, 30 and 60 min. Initially, 0.5 ml of the protoscoleces (2× 103/ml) solution was placed in test tubes. In the next step, 0.5 ml of various concentrations of the extracts was added to each test tube, separately. The contents of the tubes were slowly mixed and then incubated at 37°C for 10, 20, 30 and 60 min. At the end of each incubation time, the upper phase was carefully removed so as not to interrupt the protoscoleces. Fifty µl of 0.1% eosin stain (Sigma-Aldrich, St Louis, MO, USA) was then added to the remaining settled protoscoleces and mixed gently. The upper portion of the solution was discarded after 10 min of incubation. The remaining pellet of protoscoleces was then smeared on a glass slide, covered with a cover glass and examined under a light microscope. The percentages of dead protoscoleces were determined by counting 200 protoscoleces. Furthermore, normal saline and 20% hypertonic saline were used as negative and positive control, respectively.
Viability test
For evaluation of the viability of protoscoleces, we used eosin exclusion test. In this test, eosin solution with a concentration of 0.1% (1 g of eosin powder in 1000 ml distilled water) was used. A few minutes after exposure to the eosin stain, dead protoscolices absorbed eosin and colored red (Figure 1), whereas, alive protoscolices remained colorless and showed characteristic muscular movements and flame cell activity (Figure 2) (17).
Figure 1.

Dead protoscolece of hydatid cysts after exposure to N. sativa with 0.1% eosin
Figure 2.

Live protoscolex of hydatid cysts after exposure with 0.1% eosin
Statistical analysis
In this investigation, all experiments were carried out in triplicate. Data analysis was performed by using SPSS statistical package (version 17.0) (SPSS Inc., Chicago, IL, USA). Differences between test and control groups were analyzed by t-test. P<0.05 was considered statistically significant.
Results
Scolicidal effects upon protoscoleces
As shown in Figures 3 and 4, various extracts of N. sativa at different concentrations following different exposure times had potent scolicidal effects against protoscoleces of hydatid cysts. The findings revealed that methanolic extract of N. sativa in comparison with the aqueous extract had strong scolicidal effects on protoscoleces of hydatid cysts. While, the mortality rate of protoscoleces in the negative control group was 7.1% after 60 min of exposure, and 100% scolicidal effect was observed with methanolic extract at the concentrations of 50 and 25 mg/ml after 10 and 30 min of incubation, respectively. The scolicidal effect of the methanolic extract of N. sativa at concentration of 10 mg/ml was 21.3, 41.6, 66.3, 83.6 and 98.3% after 5, 10, 20, 30 and 60 min of incubation, respectively. These values for the concentration of 5 mg/ml were 12, 28.3, 44.6, 67.3 and 86% respectively. In contrast, aqueous extract at the concentration of 50 and 25 mg/ml killed 100 and 96% of protoscoleces after 30 and 60 min of incubation, respectively. The scolicidal activity of the aqueous extract of N. sativa at concentration of 10 mg/ml was 10.3, 24, 37.6, 52.6 and 68.3% after 5, 10, 20, 30 and 60 min of incubation, respectively. The above values for the concentration of 5 mg/ml were only 5.3, 16.6, 27.3, 32.3 and 46.6%, respectively. The scolicidal power of 20% hypertonic saline as the positive control was 100% after 10 min of application. Therefore, the scolicidal activity of methanolic and aqueous extract at the concentrations of 50 mg/ml after 10 and 30 min of incubation respectively was similar to the scolicidal activity of positive control. The scolicidal effect of various concentrations of the N. sativa extracts particularly methanolic extract was extremely significant (P<0.05) compared to the control group (normal saline) at all exposure times. In the present study, all of experiments revealed dose-dependent and also time-dependent scolicidal effect of various extracts of N. sativa on the protoscoleces of hydatid cyst.
Figure 3.
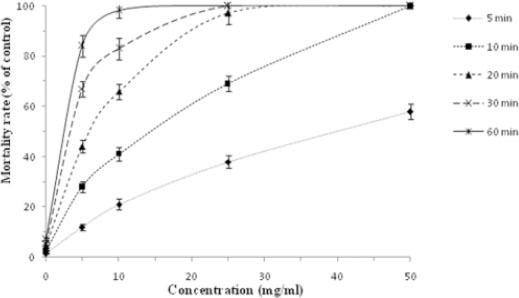
In vitro lethal effects of methanolic extract of Nigella sativa against protoscoleces of hydatid cyst at the various concentrations following various exposure times
Figure 4.
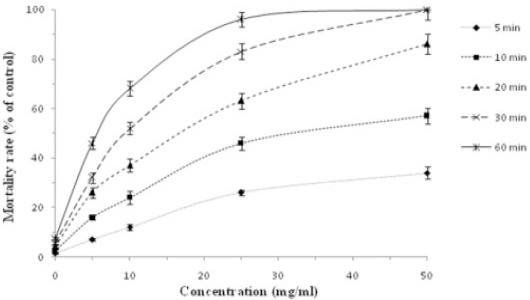
In vitro lethal effects of aqueous extract of Nigella sativa against protoscoleces of hydatid cyst at the various concentrations following various exposure times
Discussion
The results of this study demonstrated that the various extracts of N. sativa seed, especially methanolic extract possess potent scolicidal activity and might be used as a natural scolicidal agent in CE surgery. Although, in the various studies the scolicidal effects of hypertonic saline (18), silver nitrate (19), cetrimide (20), ethyl alcohol (95%) (21), H2O2 and 10% povidone iodine (22), albendazole (23), chlorhexidine gluconate (24), honey (25) and some plant extracts (26-28) have been shown. However, it has been proven that existing scolicidal agents have dangerous adverse effects and their efficacy is controversial (5). Therefore, development of new scolicidal agents with no local or systemic side effects and higher efficacy is an urgent need for surgeons for surgical success of hydatid cysts. From the past centuries, plant-derived natural products have been widely used as a valuable source of antimicrobial agents in folk medicine (29). In the recent decades, development of synthetic antimicrobial drugs caused disaffection toward natural products as an attractive resource for antimicrobial agents (30). However, emergence of some adverse effects in the use of these synthetic drugs caused shift in situation and interest in field of ethnobotanical research (31). Results of the present study revealed that the methanolic extract of N. sativa at the concentration of 50 mg/ml after 10 min of incubation and its aqueous extract at the concentration of 50 mg/ml after 30 min of incubation was able to kill 100% of protoscoleces of E. granulosus. These results are comparable with the scolicidal effects of some existing scolicidal agents such as 20% hypertonic saline (15 min), 20% silver nitrate (20 min), 0.5 to 1% cetrimide (10 min), H2O2 3% (15 min) and 95% ethyl alcohol (15 min). So far, in the various studies, antibacterial, antifungal and antiviral effects of N. sativa seeds have been demonstrated (12, 14-16). In addition, antiparasitic effects of N. sativa extracts to treat some parasitic infections have been investigated. Agrawal et al, (1979) demonstrated significant antihelmintic [AGA2]activity of N. sativa seeds against some pathogenic cestode and nematode parasites compared to those of piperazine (12). In another study, Mahmoud et al (2002) showed that N. sativa oil can significantly reduce the number of Schistosoma mansoni worms in the liver and can decrease the total number of ova deposited in both the liver and the intestine (13). A study conducted by Nilforoushzadeh et al (2010) exhibited that combination of honey and N. sativa extract in patients with cutaneous leishmaniasis (CL) receiving glucantime is more effective to treat and improve the clinical signs as compared to honey alone (32). Fatahi Bafghi et al (2013) also reported that alcoholic extract of N. sativa possess good efficacy against cutaneous leishmaniasis in BALB/c mice (33). In addition, Okeola et al (2011) showed that N. sativa seeds had a significant antioxidant property and might be a good phytotherapeutic agent against Plasmodium infection in malaria (34). However, some studies indicated that N. sativa extracts had no significant effect to treat some parasitic infections such as balantidiasis in equines and Cryptosporidium parvum infection in calves (35, 36). The exact mechanism of the antiparasitic effects of N. sativa is not clear and further studies are required to clarify these mechanisms. Nevertheless, Suthar et al (2010) demonstrated that N. sativa oil could inhibit DNA synthesis by inhibiting histone deacetylase (HDAC) enzyme interacting with the chromosomes (37). Cisplatin (a widely used chemotherapeutic drug) is toxic to the kidney. Administration of N. sativa can reduced the cisplatin toxic side effects in rats including nephrotoxicity (38). Thymoquinone (TQ), the main constituent of the volatile oil of the seeds of N. sativa, has significant cytoprotective properties (39). Badary et al (1998) administrated thymoquinone in the drinking water of mice at concentrations of 0.01, 0.02, and 0.03% for 90 days with no resulting mortality or signs of toxicity. The average daily intake of the compound was approximately 30, 60, or 90 mg/kg/day. They observed no changes of toxicological significance in body and organ weights, food and water intake, or urine and feces output. Tissue GSH, plasma concentrations of TP, urea, creatinine and triglycerides, and enzyme activities of ALT, LDH, and CPK were also not affected. Histological examination revealed no gross or microscopic tissue damage. The results indicate that the acute oral toxicity of TQ in mice is of a low order and it is generally well tolerated when given subchronically at doses previously shown to have cytoprotective activity (40). Thus, according to these evidences, we can conclude that the seed extract of N. sativa could be considered as a safe scolicidal agent.
Conclusion
The findings of the present study demonstrated a potent scolicidal activity of various extracts of N. sativa especially for its methanolic extract against protoscoleces of hydatid cyst, so N. sativa can be considered as a natural source for the production of new scolicidal agent for use in hydatid cyst surgery. However, further studies will be required to confirm these findings by checking the other derivatives of N. sativa such as essential oil and its active components in the in vitro model. Moreover, more research is needed to evaluate mode of actions and in vivo effects of this plant extracts.
Acknowledgment
We would like to thank Mr. sadoghian and Mr. mirbadie for collection of hydatid cysts.
The authors declare that there is no conflict of interest in this study.
References
- 1.Fasihi Harandi M, Budke CM, Rostami S. The Monetary Burden of Cystic Echinococcosis in Iran. PLOS Negl Trop Dis. 2012;6:e1915. doi: 10.1371/journal.pntd.0001915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eckert J, Deplazes P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin Microbiol Rev. 2004;17:107–135. doi: 10.1128/CMR.17.1.107-135.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunetti E, Kern P, Vuitton DA. Writing Panel for the WHO-IWGE. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 2010;114:1–16. doi: 10.1016/j.actatropica.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Junghanss T, da Silva AM, Horton J, Chiodini PL, Brunetti E. Clinical management of cystic echinococcosis: state of the art, problems, and perspectives. Am J Trop Med Hyg. 2008;79:301–311. [PubMed] [Google Scholar]
- 5.McManus DP, Zhang W, Li J, Bartley PB. Echinococcosis. Lancet. 2003;362:1295–1304. doi: 10.1016/S0140-6736(03)14573-4. [DOI] [PubMed] [Google Scholar]
- 6.Besim H, Karayalcin K, Hamamci O, Güngör C, Korkmaz A. Scolicidal agents in hydatid cyst surgery. HPB Surg. 1998;10:347–351. doi: 10.1155/1998/78170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hosseini SV, Ghanbarzadeh K, Barzin J, Sadjjadi SM, Tanideh N, Mehrabani D. In vitro protoscolicidal effects of hypertonic glucose on protoscolices of hydatid cyst. Korean J Parasitol. 2006;44:239–242. doi: 10.3347/kjp.2006.44.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajabi MA. Fatal reactions and methaemoglobinaemia after silver nitrate irrigation of hydatid cyst. Surgical Practice. 2009;13:2–7. [Google Scholar]
- 9.Adas G, Arikan S, Kemik O, Oner A, Sahip N, Karatepe O. Use of albendazole sulfoxide, and combined solutions as scolicidal agents on hydatid cysts (in vitro study) World J Gastroenterol. 2009;15:112–116. doi: 10.3748/wjg.15.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ali BH, Blunden G. Pharmacological and toxicological properties of Nigella sativa. Phytother Res. 2003;17:299–305. doi: 10.1002/ptr.1309. [DOI] [PubMed] [Google Scholar]
- 11.Randhawa MA, Al-Ghamdi MS. A review of the pharmacotherapeutic effects of Nigella sativa. Pak J Med Res. 2002;41:1–10. [Google Scholar]
- 12.Agrawal R, Kharya MD, Shrivastava R. Antimicrobial and anthelmintic activities of the essential oil of Nigella sativa Linn. Indian J Exp Biol. 1979;17:1264–1265. [PubMed] [Google Scholar]
- 13.Mahmoud MR, El-Abhar HS, Saleh S. The effects of Nigella sativa oil against the liver damage induced by Schistosoma mansoni in mice. J Ethnopharmacol. 2002;79:1–11. doi: 10.1016/s0378-8741(01)00310-5. [DOI] [PubMed] [Google Scholar]
- 14.Khan MA, Ashfaq MK, Zuberi HS, Mahmood MS, Gilani AH. The in vivo antifungal activity of the aqueous extract from Nigella sativa seed. Phytother Res. 2003;17:183–186. doi: 10.1002/ptr.1146. [DOI] [PubMed] [Google Scholar]
- 15.Morsi NM. Antimicrobial effect of crude extracts of Nigella sativa on multiple antibiotics-resistant bacteria. Acta Microbiol Pol. 2000;49:63–74. [PubMed] [Google Scholar]
- 16.Salem ML, Hossain MS. Protective effect of black seed oil from Nigella sativa against murine cytomegalovirus infection. Int J Immunopharmacol. 2000;22:729–740. doi: 10.1016/s0192-0561(00)00036-9. [DOI] [PubMed] [Google Scholar]
- 17.Smyth JD, Barrett NJ. Procedures for testing the viability of human hydatid cysts following surgical removal, especially after chemotherapy. Trans R Soc Trop Med Hyg. 1980;74:649–652. doi: 10.1016/0035-9203(80)90157-1. [DOI] [PubMed] [Google Scholar]
- 18.Kayaalp C, Balkan M, Aydin C, Ozgurtas T, Tanyuksel M, Kirimlioglu V, et al. Hypertonic saline in hydatid disease. World J Surg. 2001;25:975–979. doi: 10.1007/s00268-001-0065-9. [DOI] [PubMed] [Google Scholar]
- 19.Caglar R, Yuzbasioglu MF, Bulbuloglu E, Gul M, Ezberci F, Kale I. In vitro effectiveness of different chemical agents on scolices of hydatid cyst. J Invest Surg. 2008;21:71–75. doi: 10.1080/08941930701883640. [DOI] [PubMed] [Google Scholar]
- 20.Besim H, Karayalcin K, Hamamci O, Güngör C, Korkmaz A. Scolicidal agents in hydatid cyst surgery. HPB Surg. 1998;10:347–351. doi: 10.1155/1998/78170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erzurumlu K, Hokelek M, Baris S, Sahin M, Birinci A, Amanvermez R, et al. Effect of albendazolesulfoxide solution on the scolices and the hepatobiliary system. Eur Surg Res. 1998;30:433–438. doi: 10.1159/000008610. [DOI] [PubMed] [Google Scholar]
- 22.Landa Garcı´a JI, Alonso E, Gonzalez-Uriarte J, Rodriguez Romano D. Evaluation of scolicidal agents in an experimental hydatid disease model. Eur Surg Res. 1997;29:202–208. doi: 10.1159/000129525. [DOI] [PubMed] [Google Scholar]
- 23.Paksoy Y, Odev K, Sahin M, Arslan A, Koç O. Percutaneous treatment of hydatid cysts: comparison of direct injection of albendazole and hypertonic saline solution. AJR Am J Roentgenol. 2005;185:727–734. doi: 10.2214/ajr.185.3.01850727. [DOI] [PubMed] [Google Scholar]
- 24.Puryan K, Karadayi K, Topcu O, Canbay E, Sumer Z, Turan M, et al. Chlorhexidine gluconate: an ideal scolicidal agent in the treatment of intraperitoneal hydatidosis. World J Surg. 2005;29:227–230. doi: 10.1007/s00268-004-7587-x. [DOI] [PubMed] [Google Scholar]
- 25.Kilicoglu B, Kismet K, Koru O, Tanyuksel M, Oruc MT, Sorkun K, et al. The scolicidal effects of honey. Adv Ther. 2006;23:1077–1083. doi: 10.1007/BF02850228. [DOI] [PubMed] [Google Scholar]
- 26.Moazeni M, Nazer A. In vitro effectiveness of garlic (Allium sativum) extract on scolices of hydatid cyst. World J Surg. 2010;34:2677–2681. doi: 10.1007/s00268-010-0718-7. [DOI] [PubMed] [Google Scholar]
- 27.Moazeni M, Saharkhiz MJ, Hosseini AA. In vitro lethal effect of ajowan (Trachyspermum ammi L.). essential oil on hydatid cyst protoscoleces. Vet Parasitol. 2012;187:203–208. doi: 10.1016/j.vetpar.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 28.Zibaei M, Sarlak A, Delfan B, Ezatpour B, Azargoon A. Scolicidal effects of Olea europaea and Satureja khuzestanica extracts on protoscolices of hydatid cysts. Korean J Parasitol. 2012;50:53–56. doi: 10.3347/kjp.2012.50.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rocha LG, Almeida JR, Macedo RO, Barbosa-Filho JM. A review of natural products with antileishmanial activity. Phytomedicine. 2005;12:514–535. doi: 10.1016/j.phymed.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 30.Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12:564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCutcheon AR, Ellis SM, Hancock REW, Tower GN. Antibiotic screening of medicinal plants of the British Columbian native peoples. J Ethnopharmacol. 1992;37:213–223. doi: 10.1016/0378-8741(92)90036-q. [DOI] [PubMed] [Google Scholar]
- 32.Nilforoushzadeh MA, Hejazi SH, Zarkoob H, Shirani-Bidabadi L, Jaffary F. Efficacy of adding topical honey-based hydroalcoholic extract Nigella Sativa 60% compared to honey alone in patients with cutaneous leishmaniasis receiving intralesional glucantime. J Skin Leishmaniasis. 2010;1:26–31. [Google Scholar]
- 33.Fattahi Bafghi A, Vahidi AR, Anvari MH, Barzegar K, Ghafourzadeh M. The in vivo antileishmanial activity of alcoholic extract from Nigella sativa seeds. Afr J Microb Res. 2011;5:1504–1510. [Google Scholar]
- 34.Okeola VO, Adaramoye OA, Nneji CM, Falade CO, Farombi EO, Ademowo OG. Antimalarial and antioxidant activities of methanolic extract of Nigella sativa seeds (black cumin) in mice infected with Plasmodium yoelli nigeriensis. Parasitol Res. 2011;108:1507–1512. doi: 10.1007/s00436-010-2204-4. [DOI] [PubMed] [Google Scholar]
- 35.Nasir A, Avais M, Khan MS, Khan JA, Hameed S, Reichel MP. Treating Cryptosporidium parvum infection in calves. J Parasitol. 2013;99:715–717. doi: 10.1645/12-42.1. [DOI] [PubMed] [Google Scholar]
- 36.Khan A, Khan MS, Avais M, Ijaz M, Ali MM, Abbas T. Prevalence, hematology, and treatment of balantidiasis among donkeys in and around Lahore, Pakistan. Vet Parasitol. 2013;196:203–205. doi: 10.1016/j.vetpar.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 37.Suthar MP, Patel PN, Shah TG, Patel RK. In vitro screening of Nigella sativa seeds for antifungal activity. Inter J Pharm App Sci. 2010;1:84–91. [Google Scholar]
- 38.el Daly ES. Protective effect of cysteine and vitamin E Crocus sativus and Nigella sativa extracts on cisplatininduced toxicity in rats. J Pharm Belg. 1998;53:87–95. [PubMed] [Google Scholar]
- 39.Mansour MA. Protective effects of thymoquinone and desferrioxamine against hepatotoxicity of carbon tetrachloride in mice. Life Sci. 2000;66:2583–2591. doi: 10.1016/s0024-3205(00)00592-0. [DOI] [PubMed] [Google Scholar]
- 40.Badary OA, Al-Shabanah OA, Nagi MN, Al-Bekairi AM, Almazar MMA. Acute and subchronic toxicity of thymoquinone in mice. Drug Dev Res. 1998;44:56–61. [Google Scholar]


