Abstract
Nigella sativa (Family Ranunculaceae) is a widely used medicinal plant throughout the world. N. sativa is referred in the Middle East as a part of an overall holistic approach to health. Pharmacological properties of N. sativa including immune stimulant, hypotensive, anti-inflammatory, anti-cancer, antioxidant, hypoglycemic, spasmolytic and bronchodilator have been shown. Reactive oxygen species (ROS) and oxidative stress are known as the major causes of many diseases such as liver injury and many substances and drugs can induce oxidative damage by generation of ROS in the body. Many pharmacological properties of N. sativa are known to be attributed to the presence of thymoquinone and its antioxidant effects. Thymoquinone protects liver from injury via different mechanisms including inhibition of iron-dependent lipid peroxidation, elevation in total thiol content and glutathione level, radical scavengering, increasing the activity of quinone reductase, catalase, superoxide dismutase and glutathione transferase, inhibition of NF-κB activity and inhibition of both cyclooxygenase and lipoxygenase. Therefore, this review aimed to highlight the roles of ROS in liver diseases and the mechanisms of N. sativa in prevention of liver injury.
Keywords: Black cumin, Hepatitis, Liver injury, Nigella sativa, Thymoquinone
Introduction
Despite all the considerable improvement in modern medicine, traditional herbal medical profession has always been practiced (1). Plants have organized the basis of sophisticated traditional medicine systems which have given rise to some important drugs that are still in use today (2). Many plants are in use today, but their potential mechanism of action, medicinal properties, toxicological studies and safety evaluation are not fully known to us (3).
Nigella sativa Linneaus (N. sativa) (Family Ranunculaceae) is a herbal plant which is popularly called with different names of black cumin, black seed and the seed of blessing (Habatul-barakah in Arabic countries). The seeds have traditionally been used for thousands of years in the Middle East, Far East and Asia as a food additive and as a herbal health aid (4). The seed or its oil has been used as a diuretic, lactagogue, vermifuge and carminative. It has also been used in the treatment of fever, common cold, rheumatic diseases, asthma, headache, warts, and stings of scorpions and bites of snake (5-10). Recently, it is suggested that black seed, its oil and extracts act as antimicrobial, immune stimulant (11), hypotensive (12), anti-inflammatory (13, 14), anti-cancer (15, 16), antioxidant (17-19), hypoglycemic (20, 21), spasmolytic and bronchodilator (22-24) (Figure 1).
Figure 1.
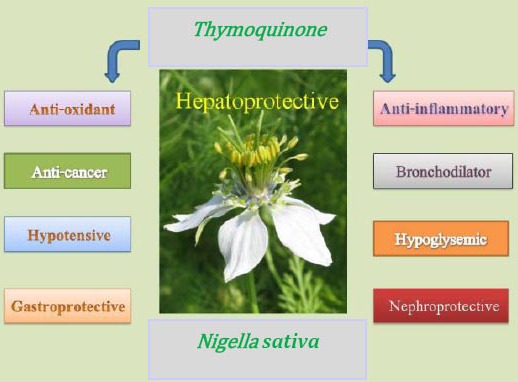
Pharmacological effects of Nigella sativa and its constituent, thymoquinone
Pharmacognostical characteristics
N. sativa is a 20-90 cm tall plant with finely divided leaves and the leaf segments narrowly linear to threadlike. Flowers are pale, blue on solitary long peduncles. N. sativa is a bisexual and forms a fruit capsule which consists of many white trigonal seeds. The fruit is a large and inflated capsule composed of 3-7 united follicles (3). Matured fruit capsule opens up and the seeds contained within are exposed to the air, becoming black in color (black seeds). The seeds are trigonous and black in color (25).
Chemical composition of N. sativa seeds
Seeds of N. sativa contain numerous esters of structurally unusual unsaturated fatty acids with terpene alcohols (7%). Furthermore, traces of alkaloids are found which belong to two different types: isochinoline alkaloids and pyrazol alkaloids (see Table 1 for additional information) (26).
Table 1.
The general compositions of Nigella sativa seeds
| Constituent | % Range (w/w) |
|---|---|
| Oil | 31-35.5 |
| Protein | 16-19.9 |
| Carbohydrate | 33-34 |
| Fiber | 4.5-6.5 |
| Saponin | 0.013 |
| Moisture | 5-7 |
In the essential oil (average 0.5%, max. 1.5%), thymoquinone was identified as the main component (up to 50%) besides p-cymene (40%), pinene (up to 15%), thymohydroquinone and dithymoquinone. Other terpene derivatives were found only in trace amounts: carvone, carvacrol, limonene, 4-terpineol and citronellol (see Table 2 for additional informations) (26).
Table 2.
The general compositions of Nigella sativa oil
| Constituent | % Range (w/w) |
|---|---|
| Linoleic acid | 44.7-56 |
| Oleic acid | 20.7-24.6 |
| Linolenic acid | 0.6-1.8 |
| Arachidic acid | 2-3 |
| Palmitoleic acid | 3 |
| Eicosadienoic acid | 2-2.5 |
| Palmitic acid | 12-14.3 |
| Stearic acid | 2.7-3 |
| Myristic acid | 0.16 |
| Stroles | 0.5 |
The essential oil contains significant (10%) amounts of fatty acid ethyl esters. On storage, thymoquinone turns into the dithymoquinonene and higher oligocondensation products. Fatty oil rich in unsaturated fatty acids, such as linoleic acid (50 60%), oleic acid (20%), eicodadienoic acid (3%) and dihomolinoleic acid (10%) are also find in the seeds. Saturated fatty acids (stearic acid and palmitic acid) amount is approximately 30% or less. Furthermore contain parts of the essential oil, mostly thymoquinone that is responsible for an aromatic flavor. The oil contains d -limonene, carvone, and a carbonyl compound, nigellone (26).
N. sativa seeds contain 36-38% fixed oils, saponin, proteins, alkaloids and 0.4-2.5% essential oil. By high performance liquid chromatography analysis of N. sativa essential oil, thymoquinone, thymohydroquinone, dithymoquinone, and thymol are considered the main active ingredients. The most prominent activity of thymoquinone is its anti-oxidant effect (26).
Traditional uses of N. sativa
N. sativa seeds are used as a stimulant, aromatic, carminative, anthelmintic, diuretic, galactagogue and diaphoretic. They are used as a spice in curries. A tincture provided from the seeds is useful in diarrhea, indigestion, dropsy, loss of appetite, dysmenorrhea and amenorrhea and in the treatment of worms and skin eruptions. To arrest vomiting, seeds are roasted and given internally (26-28). Externally, the oil is used as an antiseptic (27). N. sativa has been used as an antitussive for many years (29).
Reactive oxygen species and the liver diseases
Oxidative stress is one of the main causes of liver injury that depletes the antioxidant enzymes sources and decreases the ability of cells in functioning against injury (30).
Nicotinamide adenine dinucleotide phosphate (NAD(P)H) oxidases, nitric oxide synthases (NOS), and myeloperoxidases are three main enzymes that produce ROS in the body. ROS, including super-oxide anion radical (O2-), hydrogen peroxide (H2O2), peroxynitrite (ONOO), hydroxyl radical (OH-) and hypochlorous acid (HOCl-) are by-products of the normal metabolic processes in the cells. Beneficial effects of ROS disrupt when the balance between amount of ROS and antioxidants is diminished. This imbalance state defines as oxidative stress. Polyunsaturated lipids and sulfhydryl groups of proteins are the main constituents of cells which have essential roles in the functions of the cells. Disruption of their structural properties by free radicals may cause cellular functions aborted (30).
The liver is a very important organ with high rate of metabolism in the body. It is very sensitive to oxidative stress and damages caused by free radicals. Preventing the production of free radicals, scavenging free radicals or enhancement in antioxidant defense can minimize the adverse effects of them (31).
The majors liver damages mediated by oxidative stress are including: a) alcoholic liver disease, b) fibrosis/cirrhosis, c) hepatic stellate cells, d) hepatocellular carcinoma, e) ischemic/reperfusion liver injury, f) paracetamol-induced liver damage, g) viral hepatitis, h) fatty liver and i) chemical pollutant-induced liver damage (30).
a) Alcoholic liver disease
Ethanol is a potent inducer of ROS production in the body. This characteristic of ethanol can lead to oxidative stress and the liver is the main organ that is affected by that. Lipid peroxidation, depletion of glutathione and formation of lipid radicals can induce liver damages (31). Administration of antioxidants, such as superoxide dismutase (SOD), vitamin E, ebselen, and precursors of glutathione prevented alcohol-induced hepatic damage in rats (32).
The role of free radicals in hepatic injury induced by alcohol and the protective effects of antioxidant therapy in reducing the toxic effects showed that oxidative stress is the main cause in ethanol-induce liver damages (32).
b) Fibrosis/cirrhosis
Production of ROS and lipid peroxidation is associated with liver fibrosis and cirrhosis (33). This effect can lead to the death of hepatocytes and many researchers have shown that oxidative stress has a critical role in fibrosis and cirrhosis of liver (34). Free radicals initiate cell damage by binding to the membrane proteins. These effects are partially prevented by antioxidants (33).
c) Hepatic stellate cells
The source of hepatic stellate cells (HSC) in liver injury is not completely understood but many investigations have shown that ROS are involved in necrosis and apoptosis of hepatocytes and HSC activation (35, 36). ROS induces the production of platelet-derived growth factor that is the most potent mitogen of HSC and is, therefore, likely to be a major mediator during liver fibrogenesis. The usage of antioxidant was able to reduce of these states (37, 38).
d) Hepatocellular carcinoma
Oxidative stress and chronic inflammation are the common mechanisms for hepatocellular carcinoma (39). ROS interacts directly with DNA and changes expression of specific genes which are responsive for cell proliferation and apoptosis (40). ROS can directly stimulate the growth of cancer cells. Chronic inflammation can worsen this state. The formation of 5-hydroxylmethyluracil, 8-hydroxydeoxyguanosine, thymine and thymidine glycol in this state is a main evidence that ROS is a cause of hepatocellular carcinoma (41).
e) Ischemic/reperfusion (I/R) liver injury
Periods of ischemia can occur in hepatic surgeries. Reperfusion increases the damage induced during the ischemic period when the flow of oxygen and blood is re-established. In this state ATP depletion, decline in calcium homeostasis with decline in cytoprotective compounds such as prostacycline, nitric oxide (NO), and elevation of ROS are present (42, 43). ROS is responsible for many processes in I/R (formation of xanthine oxidase, induction of NADPH oxidase and NO formation). At this state lipid peroxidation, inactivation of heme group and nitrosylation of iron-sulfur group induced by ROS worsen I/R conditions (44, 45). According to initiate mechanism of I/R the use of antioxidant can ameliorates the adverse effect of this condition. Administration of antioxidants, especially in the early stages of reperfusion, may significantly diminish I/R injury in transplanted livers (30).
f) Paracetamol-induced liver damage
Paracetamol-induced liver damage is the most prominent drug toxicity in the world. The reactive metabolite of paracetamol (N-acetyl-p-benzoquinone imine) can bind to cellular proteins and cause hepatocyte death. The formation of peroxynitrite and depletion of glutathione resources can lead to liver damages. Oxidant stress of mitochondria triggers the mitochondrial membrane permeability transition pore, depletion of ATP and release of cytokines that are responsible for DNA fragmentation (30).
Taking antioxidant or compounds that restore glutathione resources in the body can protect the liver from toxicity of paracetamol overdoses (46).
g) Viral hepatitis
Hepatitis C virus (HCV) and oxidative stress are parallel to each other. Lipid peroxidation and antioxidant levels are increased in patients with HCV (47). Decreased content of glutathione in the blood, liver, and lymphatic system causes increase in GSSG level, indicating a high glutathione turnover (48).
Inflammatory cytokines and oxidative stress play important roles in the mediation of hepatic injury in HCV. The expressions of cyclooxygenase-2 and iNOS which can increase ROS are increased in HCV patients (30). Use of antioxidants with current treatments of HCV can enhance the beneficial outcomes in patients with HCV (49).
h) Fatty liver
Accumulation of fat in the liver can result in steatosis and steatohepatitis. This condition can progress to cirrhosis. Up to 10% of cirrhotic fatty liver develop hepatocellular carcinoma. Free fatty acids are the main sources of production of free radicals. With progression of stage of illness, oxidative stress level is increased and biomarker (malondialdehyde) is rises in the body. Elevated plasma malondialdehyde and tumor necrosis factor α (TNFα) reflected an increase of oxidative stress and inflammation, respectively (50).
i) Chemical pollutant-induced liver damage
Industrialization in today’s world increases the environmental pollutant sources, such as mercury, carbon tetrachloride, arsenic, cadmium, thioacetamide etc. Mechanism of these chemicals is peroxidation of the hepatocyte lipids causing destruction of the cells and their intracellular organelles and covalent binding to the membrane proteins (51). Many researches have shown that chemical pollutants increase oxidative stress and then the effects of this condition on the body organelles such as liver (52-53).
Mechanisms mention above revealed that many liver damage causes are mediates by oxidative stress and production of ROS. Thus, the enhancement of antioxidant defense and increase of the glutathione resources in the body can attenuates oxidative damages to hepatocytes.
Mechanisms of hepatoprotection of N. sativa
Studies on N. sativa have been confirmed the potential therapeutic effects of it. One of the most important effects is hepatoprotective which is shown in many research projects (54-55).
In this article we summarized the mechanisms of hepatoprotection of N. sativa, and its main constituents, such as thymoquinone (Table 3).
Table 3.
Mechanism of hepatoprotective effects of thymoquinone (TQ)
| Effect | Mechanism | Reference | |
|---|---|---|---|
| Hepatoprotection | Antioxidant | TQ inhibited iron-dependent lipid peroxidation | 59 |
| TQ increased total thiol content and GSH level | 61 | ||
| TQ was O-2 and OH radical scavenger | 60-63 | ||
| TQ inhibited the activity of hepatic CYP1A1/A2 isozymes | 64 | ||
| TQ inhibited expression of inducible nitric oxide synthetase | 50 | ||
| TQ increased the activities of quinone reductase, catalase, SOD and glutathione transferase | 66-68 | ||
| TQ inhibited lipogenesis in the hepatocytes | 70 | ||
| Anti-inflammatory | TQ inhibited both cyclooxygenase and lipoxygenase | 62 | |
| TQ increased the ratio of helper to suppressor T cells, enhanced natural killer cell activity, enhanced production of IL-3 and had a stimulatory effect on macrophages | 69 | ||
| TQ inhibited of NF-Kβ reduction of cytochrome c production | 75 | ||
| TQ inhibition PG E2 formation | 72 |
Antioxidant properties
Antioxidant properties of N. sativa have shown in many studies (57-60). Thymoquinone has the ability to inhibit iron-dependent lipid peroxidation in concentration-dependent manner (59). It is a potent O-2 scavenger activity (60-62). With this characteristic, thymoquinone can decrease oxidative stress and increase antioxidant defense in the body. Decrease in malondialdehyde and other biomarkers of oxidative stress in parallel with increase in total thiol content and glutathione level are the results of thymoquinone treatment (48, 61, 62). The content of glutathione in the liver is found particularly in high concentration in the liver and is known to have key functions in cellular protective mechanisms. Depletion in total thiol content caused by oxidative stress can result in protein inactivation, protein oxidation, lipid peroxidation, perturbation in calcium homeostasis and resultful loss of cell viability (62). Depletion in free radicals with thymoquinone can decrease the risk of them attacking to DNA and decrease the risk of cancers (63, 64). Thymoquinone inhibits the activity of hepatic CYP1A1/A2 isozymes involved in biotransformation of many xenobiotics into reactive genotoxic radical derivatives (64).
The antioxidant properties of thymoquinone are responsible for the antischistosomi characteristic of thymoquinone and reduce in liver injury caused by parasites (65). The antioxidant properties of thymoquinone can reduce the adverse effects of ROS that produced in I/R state. Thymoquinone increased catalase (CAT) activity, and this is consistent with its protective effect on liver tissue against I/R injury (66). In addition, thymoquinone can protect against renal I/R induced damage through an antioxidant mechanism as well as the decrease of CYP3A1 and SSAT gene expression. CYP3A1 mRNA expression was induced significantly by I/R in both liver and kidney tissues. I/R caused induction of mRNA expression of spermidine/spermine N-1-acetyl-transferase (SSAT), a catabolic enzyme that partakes in polyamine alteration, in kidney and liver tissues. Thymoquinone reduces SSAT mRNA expression significantly in liver and markedly in kidney (67). Oral administration of thymoquinone is a promising prophylactic agent against chemical carcinogenesis and toxicity in liver tissues by increasing the activities of quinone reductase and glutathione transferase (68).
Exposure of isolated rat hepatocytes to oxidative stress induced by tert-butyl hydroperoxide, as an oxidative agent, lead to rapid depletion in intracellular glutathione due to its oxidation by glutathione peroxidase (GSHPx), and ultimately oxidation of pyridine nucleotides, which has been associated with the impairment of calcium sequestration by endoplasmic reticulum and mitochondria. This is followed by formation of surface blebs in the plasma membrane as well as the leakage of cytosolic enzymes. Thymoquinone can inhibit formation of blebs and it can preserve the integrity of cell membrane of hepatocyte (69).
Thymoquinone can inhibit the expression of iNOS, that is participated in oxidative stress state and can increase the expression of antioxidant enzymes such as GSHPx and SOD (50). Thymoquinone may be able to reduce NADH, thereby reducing the NADH-NAD+ changes, which lead to inhibition lipogenesis in the hepatocytes (70).
Many studies have evaluated antioxidant properties of thymoquinone that mentioned before.
Anti-inflammatory properties
The use of thymoquinone has also been shown to have anti-inflammatory effects in several inflammatory diseases (71-73). Inflammatory cytokines in hepatocytes can promote signaling pathways that induce cell injury. Thymoquinone is a potent inhibitor of eicosanoid generation namely thromboxane B2 and leukotriene B4, by inhibiting both cyclooxygenase and lipoxygenase enzymes, respectively. The roles of these mediators are formation of bleb in cell membrane of hepatocytes and activation of free radicals production (62).
Antioxidant and anti-inflammatory actions of thymoquinone are two main mechanisms that parallel to each other preserve hepatocytes from injury (46). Thymoquinone increased in the ratio of helper to suppressor T cells, enhanced natural killer cell activity, enhanced the production of IL-3 and had a stimulatory effect on macrophages (69). Inflammatory responses and activated neutrophils can increase myeloperoxidase activity in the liver tissue. Myeloperoxidase increases lipid peroxidation and free radicals formation. This state worsens the liver injury (74).
Thymoquinone has a designation in reducing inflammation by decreasing malondialdehyde and lipid peroxidation products, reduction in amount of cytokines via inhibiting activity of NF-κB and to reducing cytochrome c production from mitochondria via inhibition of generates ROS in the liver (75).
Anticancer properties
Oxidative stress, nitrosative stress and inflammation are three major concepts in cancer. With two mechanisms thymoquinone preserve cells from cancer: 1) with antioxidant and anti-inflammatory effect thymoquinone decreases oxidative stress and preserves the activity and expression of antioxidant enzymes (68, 76) and 2) induces apoptosis (77).
Thymoquinone inhibits the ROS-induced oxidative DNA damage and therefore reduces the risk of cancer. Oxidative DNA cleavages reactions were mediated by polyphenolic antioxidants in the presence of copper (Cu) ions. Cancer cells have higher content of copper in comparison to normal cells. Thymoquinone may generate superoxide anion radicals. It also exhibit prooxidant DNA-damaging properties. Thymoquinone is capable of binding to DNA and operates thymoquinone –Cu (II)-mediated DNA cleavage (76).
Thymoquinone causes a time- and dose-dependent formation of cytoplasmic vacuoles. Upon exposure to cytotoxic compounds cells will attempt to sequester the compounds into vacuoles to protect themselves. Thymoquinone causes lysosome membrane permeability and lead to leakage of lysosomal proteases, such as cathepsin B and D that induce apoptotic cell death (77).
Animal studies
Sixty days treatment with carbon tetrachloride (CCl4) followed by sixty days treating with N. sativa, in fifty-six healthy male Wistar albino rats, was done. CCl4 increased the lipid peroxidation and liver enzymes, and reduced the antioxidant enzyme levels. N. sativa treatment decreased the elevated levels of lipid peroxidation and reduced the liver enzyme levels, also increased antioxidant enzyme levels (33).
According to mechanism of paracetamol-induced hepatotoxicity Yesmin and co-workers described hepatoprotection of N. sativa with antioxidant and anti-inflammatory properties against paracetamol toxicity. N. sativa improved histopathological changes such as centrilobular necrosis, pyknosis of hepatocytes and neutrophils infiltration that were induced by oxidative stress in the liver in rats (46).
The effect of N. sativa on inflammatory fatty liver was shown by Al-Okby and co-workers. Induction of inflammatory fatty liver in rats with feeding high fructose diet exhibited significant dyslipidemia, high plasma TNF-α and malondialdehyde along with significant high liver triglycerides and cholesterol and liver dysfunction. N. sativa produced significant improvement of all parameters (50).
Thioacetamide is an experimental hepatotoxic agent. A study was conducted to evaluate its hepatotoxicity, in this research intraperitoneal injection of thioacetamide at a concentration of 20 mg/kg body weight for a period of eight week, in male albino rat leads to cirrhosis. N. sativa oil (10 ml/kg body weight) improved the altered levels of albumin, bilirubin, total protein, γ-glutamyltransferase, alanine transaminase and alkaline phosphatase also significant improvement in SOD, CAT, GSHPx, and reduced glutathione and reduction in thiobarbituric acid reactive substances was seen, as well as the histological tissue damage of liver decreased in N. sativa treated group (53). In another study, after 24 hr of CCl4 administration (0.625 ml/kg IP), for 2 weeks the rats were treated with N. sativa, then sacrificed and alkaline phosphatase, Alanine aminotransferase, and aspartate aminotransferase in serum of rats was measured, the changes that produced by CCl4 on hepatic cells and enzymes revised with thymoquinone treatment (62).
The effect of N. sativa on ischemia/reperfusion injury on liver in rat was performed, after 45 min hepatic ischemia followed by 60 min reperfusion. In the group that received intraperitoneal N. sativa (0.2 ml/kg) prior to ischemia and before reperfusion, liver enzymes were significantly lower than control group which received 0.9% saline solution. Total antioxidant capacity was higher in N. sativa group compared to control group, myeloperoxidase, total oxidative status, and oxidative stress index in hepatic tissue were significantly lower in N. sativa group than in the control group. The histological tissue damage in the N. sativa group was milder compared to control group (66).
Diethylnitrosamine is a hepatocarcinogen. In liver tissue diethylnitrosamine induced severe histopathological lesions and lead to increase the level of total nitrate/nitrite, total bilirubin, thiobarbituric acid reactive substances, alanine transaminase, alkaline phosphatase and reduced CAT, GSHPx, glutathione, glutathione-s-transferase (GST) as well as diminished the gene expression of GST, GSHPx and CAT, These changes completely corrected by thymoquinone supplementation (68).
One study was performed to evaluate the hepatotoxicity of N. sativa, the result showed that up to the dose of 1 g/kg, no significant change in serum alanine aminotransferase and aspartate aminotransferase in comparison between the N. sativa treated and non-treatment groups in rat seen. Histopathological examination showed mild and very low changes in fatty degeneration in non-treatment group and treated with high doses of N. sativa also there was no inflammation and necrosis (78).
Industrialization in today’s world increases the environmental pollutant exposures that they can generate ROS. Benzene, toluene and heavy metals are the leaders of them. They can deplete glutathione contents and destroy intracellular proteins especially in the liver. Salman Ashraf and coworkers showed that injection of toluene with dose 250 mg/kg body weight in dimethyl sulfoxide could significantly deplete hepatic glutathione content and could increase protein carbonyl formation in hepatocytes. The use of 100 μl, oral administration of black seed extract 1 hr after toluene injection could significantly elevate hepatic glutathione content. The application of different concentration of N. sativa extracts showed that protection against oxidative damage and elevate of hepatic glutathione was dependent to thymoquinone concentration and the method of extraction. Studies with partial purification and fractionation of N. sativa oil showed that although fractions rich in thymoquinone were most potent in terms of their antioxidant capacity (79).
Suddek showed that the hepatotoxicity induced by tamoxifen resulted in elevated serum levels of liver enzymes such as alanine transaminase, gamma glutamyl transferase, lactate dehydrogenase, alkaline phosphatase, and total bilirubin, plus diminution of reduced glutathione in the liver, reduced super oxide dismutase activity and accumulation of lipid peroxides. Pretreatment with thymoquinone significantly inhibited tamoxifen-induced hepatic glutathione depletion and normalized the activity of SOD, inhibited the rise in TNFα (80).
Human studies
Marked induction of ROS in infected cells leading to oxidative stress is responsive for induced HCV-related fibrosis, cirrhosis and liver failure. Abdel-Moneim and co-workers showed that N. sativa in HCV patients exhibited potential therapeutic benefits via decreasing viral load and alleviating the altered liver function (47).
The most common childhood malignancy is acute lymphoblastic leukemia, and the choice drug for treatment in children is methotrexate. Long-term treatment with methotrexate may cause hepatotoxicity. N. sativa oil administered orally prevented liver damage that induced by methotrexate in leukemic children as well as improved the survival of this malignancy (81).
Conclusion
Antioxidant and anti-inflammatory properties of N. sativa are the main features of preventing and protecting liver from injury. Several studies have shown the protective effects of N. sativa against liver injury produced by ROS with its free radical scavenger properties and enhance antioxidant defenses in body. Thymoquinone is the main active ingredients of N. sativa responsible for it. Also, none of the studies reported that the use of thymoquinone in moderate doses had significant toxic effects. The efficacy of N. sativa to postpone progression in chronic liver diseases should be considered as preventive medicine in patients with hepatic disorders.
References
- 1.Shaikh B, Hatcher J. Complementary and alterna-tive medicine in pakistan: prospects and limitations. Evidence-Based Compl Altern Med. 2005;2:139–142. doi: 10.1093/ecam/neh088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boroushaki MT, Sadeghnia HR, Banihashem M, Yavari S. Protective effect of pomegranate seed oil on hexachlorobutadien-induced nephrotoxicity in rats. J Renal Failur. 2010;32:612–617. doi: 10.3109/08860221003778056. [DOI] [PubMed] [Google Scholar]
- 3.Ahmad A, Husain A, Mujeeb M, Khan SA, Najmi AK, Siddikue NA, et al. A review on therapeutic potential of Nigella sativa: A miracle herb. Asian Pac J Trop Biomed. 2013;3:337–352. doi: 10.1016/S2221-1691(13)60075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halawani E. Antibactrial activity of Thymoquinone and Thymohydroquinone of Nigella sativa L. and their interaction with some antibiotics. Advan in Biol Res. 2009;3:148–152. [Google Scholar]
- 5.Arici M, Sagdic O, Gecgel U. Antibactrial effect of Turkish black cumin (Nigella sativa L.) oils. Grasas y Aceites. 2005;56:259–262. [Google Scholar]
- 6.Boskabady NH, Mohsenpur N, Takaloo L. Anti asthmatic effect of Nigella sativa in airways of asthmatic patients. Phytomedicine. 2010;17:707–713. doi: 10.1016/j.phymed.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Goreja WG. New York, NY: Amazing Herbs Press; 2003. Black seed: nature`s miracle remedy. [Google Scholar]
- 8.Abdel-Sater KA. Gastroprotective effect of Nigella sativa oil on the formation of stress gastritis in hypothyroidal rats. Int J Physiol Pathophysiol Pharmacol. 2009;1:143–149. [PMC free article] [PubMed] [Google Scholar]
- 9.Yarnell E, Abascal K. Nigella sativa: holy herb of the middle East. Altern Compl Therap. 2011;17:99–105. [Google Scholar]
- 10.Padhye S, Banerjee S, Ahmad A, Mohammad R, Sarkar FH. Fromhere to eternity-the secret of Pharaohs: Therapeutic potential of black cumin seeds and beyond. Cancer Ther. 2008;6:495–510. [PMC free article] [PubMed] [Google Scholar]
- 11.Abel-Salam BK. Immunomodulatory effects of black seedsand garlic on alloxan-induced diabetes in albino rat. Allergol Immunopathol (Madr) 2012;40:336–340. doi: 10.1016/j.aller.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Ali BH, Blunden G. Pharmacological and toxicological properties of Nigella sativa. Phytother Res. 2003;17:299–305. doi: 10.1002/ptr.1309. [DOI] [PubMed] [Google Scholar]
- 13.Shuid AN, Mohamed N, Mohamed IN, Othman F, Suhaimi F, Mohd Ramli ES, et al. Nigella sativa: A potential antiosteoporotic agent. Evid Based Compl Altern Med 2012. 2012 doi: 10.1155/2012/696230. 696230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El Mezayen R, El Gazzar M, Nicolls MR, Marecki JC, Dreskin SC, Nomiyama H. Effect of thymoquinone on cyclooxygenase expression and prostaglandin production in a mouse model of allergic airway inflammation. Immunol Lett. 2006;106:72–81. doi: 10.1016/j.imlet.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 15.Mahmoud SS, Torchilin VP. Hormetic/cytotoxic effects of Nigella sativa seed alcoholic and aqueous extracts on MCF-7 breast cancer cells alone or in combination with doxorubicin. Cell Biochem Biophys. 2013;66:451–460. doi: 10.1007/s12013-012-9493-4. [DOI] [PubMed] [Google Scholar]
- 16.Peng L, Liu A, Shen Y, Xu HZ, Yang SZ, Ying XZ, et al. Antitumor and anti-angiogenesis effects of thymoquinone on osteosarcoma through the NF-κB pathway. Oncol Rep. 2013;29:571–578. doi: 10.3892/or.2012.2165. [DOI] [PubMed] [Google Scholar]
- 17.Umar S, Zargan J, Umar K, Ahmad S, Katiyar CK, Khan HA. Modulation of the oxidative stress and inflammatory cytokine response by thymoquinone in the collagen induced arthritis in Wistar rats. Chem Biol Interact. 2012;197:40–46. doi: 10.1016/j.cbi.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Bourgou S, Pichette A, Marzouk B, Legault J. Antioxidant, anti-inflammatory, anticancer and antibacterial activities of extracts from Nigella sativa (black cumin) plant parts. J Food Biochem. 2012;36:539–546. [Google Scholar]
- 19.Hosseinzadeh H, Parvardeh S, Asl MN, Sadeghnia HR, Ziaee T. Effect of thymoquinone and Nigella sativa seeds oil on lipid peroxidation level during global cerebral ischemia-reperfusion injury in rat hippocampus. Phytomedicine. 2007;14:621–627. doi: 10.1016/j.phymed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Salama RH. Hypoglycemic effect of lipoic acid, carnitine and Nigella sativa in diabetic rat model. Int J Health Sci (Qassim) 2011;5:126–134. [PMC free article] [PubMed] [Google Scholar]
- 21.Abdelmeguid NE, Fakhoury R, Kamal SM, Al Wafai RJ. Effects of Nigella sativa and thymoquinone on biochemical and subcellular changes in pancreatic β-cells of streptozotocin-induced diabetic rats. J Diabetes. 2010;2:256–266. doi: 10.1111/j.1753-0407.2010.00091.x. [DOI] [PubMed] [Google Scholar]
- 22.Lei X, Liu M, Yang Z, Ji M, Guo X, Dong W. Thymoquinone Prevents and ameliorates dextran sulfate sodium-induced colitis in mice. Dig Dis Sci. 2012;57:2296–2303. doi: 10.1007/s10620-012-2156-x. [DOI] [PubMed] [Google Scholar]
- 23.Hosseinzadeh H, Tafaghodi M, Mosavi MJ, Taghiabadi E. Effect of aqueous and ethanolic extracts of Nigella sativa seeds on milk production in rats. J Acupuncture Meridian Stud. 2013;6:18–23. doi: 10.1016/j.jams.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 24.Boskabady MH, Keyhanmanesh R, Saadatloo MA. Relaxant effects of different fractions from Nigella sativa L. on guinea pig tracheal chains and its possible mechanism(s) Indian J Exp Biol. 2008;46:805–810. [PubMed] [Google Scholar]
- 25.Nazari N. Universiti Malaysia Pahang; 2006. Extraction of pharmacologically active thymoquinone in Nigella sativa L. Doctoral dissertation. [Google Scholar]
- 26.El-Tahir K E H, Bakeet DM. The black seed Nigella sativa L. a min for multi cure: a plea for urgent clinical evaluation of its volatile oil. J Taibah Uni Med Sci. 2006;1:1–19. [Google Scholar]
- 27.Hosseinzadeh H, Fazly Bazzaz BS, Haghi MM. Antibacterial activity of total extracts and essential oil of Nigella sativa L. seeds in mice. Pharmacologyonline. 2007;2:429–435. [Google Scholar]
- 28.Ziaee T, Moharreri N, Hosseinzadeh H. Review of pharmacological and toxicological effects of Nigella sativa and its active constituents. J Medicinal Plants. 2012;11:16–42. [Google Scholar]
- 29.Hosseinzadeh H, Eskandari M, Ziaee T. Antitussive effect of thymoquinone, a constituent of Nigella sativa seeds, in guinea pigs. Pharmacologyonline. 2008;2:480–484. [Google Scholar]
- 30.Muriel P. Roles of free radicals in liver deseases. Hepatol Int. 2009;3:526–536. doi: 10.1007/s12072-009-9158-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsukamoto H, Lu SC. Current concepts in the pathogenesis of alcoholic liver injury. FASEB J. 2001;15:1335–1349. doi: 10.1096/fj.00-0650rev. [DOI] [PubMed] [Google Scholar]
- 32.Iimuro Y, Bradford BU, Yamashina S, Rusyn I, Nakagami M, Enomoto N, et al. The glutathione precursor l-2-oxothiazolidine-4-carboxilic acid protects against liver injury due to chronic enteral ethanol exposure in the rat. Hepatology. 2000;31:391–398. doi: 10.1002/hep.510310219. [DOI] [PubMed] [Google Scholar]
- 33.Kanter M, Coskun O, Budancamanac M. Hepatoprotective effects of Nigella sativa L and Urtica dioica L on lipid peroxidation, antioxidant enzyme systems and liver enzymes in carbon tetrachloride-treated rats. World J Gastroenterol. 2005;11:6684–6688. doi: 10.3748/wjg.v11.i42.6684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parola M, Leonarduzzi G, Biasi F, Albano E, Biocca ME, Poli G, et al. Vitamin E dietary supplementation protects against carbon tetrachloride-induced chronic liver damage and cirrhosis. Hepatology. 1992;16:1014–1021. doi: 10.1002/hep.1840160426. [DOI] [PubMed] [Google Scholar]
- 35.Wu J, Zern MA. Hepatic stellate cells: a target for the treatment of liver fibrosis. J Gastroenterol. 2000;35:665–672. doi: 10.1007/s005350070045. [DOI] [PubMed] [Google Scholar]
- 36.Bataller R, Schwabe RF, Choi YH, Yang L, Paik YH, Lindquist J, et al. NADPH oxidase signal transduces angiotensin II in hepatic stellate cells and is critical in hepatic fibrosis. J Clin Invest. 2003;112:1383–1394. doi: 10.1172/JCI18212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pinzani M, Gesualdo L, Sabbah GM, Abboud HE. Effects of platelet-derived growth factor polypeptide mitogens on DNA synthesis and growth of cultured rat liver fat-storing cells. J Clin Invest. 1989;84:1786–1793. doi: 10.1172/JCI114363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adachi T, Togashi H, Suzuki A, Kasai S, Ito J, Sugahara K, et al. NAD(P)H oxidase plays a crucial role in PDGF-induced proliferation of hepatic stellate cells. Hepatology. 2005;41:1272–1281. doi: 10.1002/hep.20719. [DOI] [PubMed] [Google Scholar]
- 39.Seitz HK, Stickel F. Risk factors and mechanisms of hepatocarcinogenesis with special emphasis on alcohol and oxidative stress. Biol Chem. 2006;387:349–360. doi: 10.1515/BC.2006.047. [DOI] [PubMed] [Google Scholar]
- 40.Adelman R, Saul RL, Ames BN. Oxidative damage to DNA: relation to species metabolic rate and life span. Proc Natl Acad Sci USA. 1988;85:2706–2708. doi: 10.1073/pnas.85.8.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marx J. Inflammation and cancer: the link grows stronger. Science. 2004;36:966–968. doi: 10.1126/science.306.5698.966. [DOI] [PubMed] [Google Scholar]
- 42.de y H, Rauen U. Ischemia–reperfusion injury: processes in pathogenetic networks: a review. Transplant Proc. 2007;39:481–484. doi: 10.1016/j.transproceed.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 43.Jaeschke H. Molecular mechanism of hepatic ischemia–reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol. 2003;284:G15–G26. doi: 10.1152/ajpgi.00342.2002. [DOI] [PubMed] [Google Scholar]
- 44.Montalvo-Jave EE, Escalante-Tattersfield T, Ortega-Salgado JA, Piña E, Geller DA. Factors in the pathophysiology of the liver ischemia–reperfusion injury. J Surg Res. 2008;147:153–159. doi: 10.1016/j.jss.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hosseinzadeh H, Moghim FF, Mansouri SMT. Effect of Nigella sativa seed extracts on ischemia-reperfusion in rat skeletal muscle. Pharmacologyonline. 2007;2:326–335. [Google Scholar]
- 46.Yesmin F, Rahman Z, Dewan JF, Helali AM, Islam Z, Rahman AM, et al. Hepatoprotective effect of aqueous and N-hexane extract of Nigella sativa in paracetamol (acetaminophen) induced liver disease of rats: a histopathological evaluation. J Pharm. 2013;4:90–94. [Google Scholar]
- 47.Abdel-Moneim A, Morsy BM, Mahmoud AM, Abo-Seif MA, Zanaty MI. Beneficial therapeutic effects of Nigella sativa and/or Zingiber officinale in HCV patients in Egypt. EXCLI J. 2013;12:943–955. [PMC free article] [PubMed] [Google Scholar]
- 48.Seronello S, Sheikh MY, Choi J. Redox regulation of hepatitis C in nonalcoholic and alcoholic liver. Free Radic Biol Med. 2007;43:869–882. doi: 10.1016/j.freeradbiomed.2007.05.036. [DOI] [PubMed] [Google Scholar]
- 49.Melhem A, Stern M, Shibolet O, Israeli E, Ackerman Z, Pappo O, et al. Treatment of chronic hepatitis C virus infection via antioxidants: results of a phase I clinical trial. J Clin Gastroenterol. 2005;39:737–742. doi: 10.1097/01.mcg.0000174023.73472.29. [DOI] [PubMed] [Google Scholar]
- 50.Al-Okbi SY, Mohamed DA, Hamed TE, Edris AE. Potential protective effect of Nigella sativa crude oils towards fatty liver in rats. Eur J Lipid Sci Technol. 2013;115:774–782. [Google Scholar]
- 51.Danladi J, Abdusalam A, Timbuak JA, Miriga AA, Dahiru AU. Hepatoprotective effect of black seed (Nigella sativa) oil on carbon tetrachloride (ccl4) induced liver toxicity in adult wistar rats. J Dental Med Sci. 2013;4:56–62. [Google Scholar]
- 52.Zafeer MF, Waseem M, Chaudhari S, Parvez s. Cadmium-Induced hepatotoxicity and its abrogation by thymoquinone. J Bio Mole Toxico. 2012;26:199–205. doi: 10.1002/jbt.21402. [DOI] [PubMed] [Google Scholar]
- 53.Nehar S, Kumari M. Ameliorating effect of Nigella sativa oil on Thioacetamide-induced liver cirrohsis in albino rats. Ind J Pharm Edu Res. 2013;47:135–139. [Google Scholar]
- 54.Salem ML. Immunomodulatory and therapeutic effects of Nigella sativa L. seed. Int Immunopharmacol. 2005;5:1749–1770. doi: 10.1016/j.intimp.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 55.Calvino J, Lens XM, Romero R, Sanchez Guisande D. Long-term anti-proteinuric effect of Losartan in renal transplant recipients treated for hypertension. Nephrol Dial Transplant. 2000;15:82–86. doi: 10.1093/ndt/15.1.82. [DOI] [PubMed] [Google Scholar]
- 56.Aboul-Ela EI. Cytogenetic studies on Nigella sativa seeds extract and thymoquinone on mouse cells infected with schistosomiasis using karyotyping. Mutat Res. 2002;516:11–17. doi: 10.1016/s1383-5718(01)00333-3. [DOI] [PubMed] [Google Scholar]
- 57.Al Rowais NA. Herbal medicine in the treatment of diabetes mellitus. Saudi Med J. 2002;23:1327–1331. [PubMed] [Google Scholar]
- 58.Kalus U, Pruss A, Bystron J, Jurecka M, Smekalova A, Lichius JJ, et al. Effect of Nigella sativa (black seed) on subjective feeling in patients with allergic diseases. Phytother Res. 2003;17:1209–1214. doi: 10.1002/ptr.1356. [DOI] [PubMed] [Google Scholar]
- 59.Nagi MN, Mansour MA. Protective effect of thymoquinone against doxorubicin-induced cardiotoxicity in rats: a possible mechanism of protection. Pharmacol Res. 2000;41:283–289. doi: 10.1006/phrs.1999.0585. [DOI] [PubMed] [Google Scholar]
- 60.Badary OA, Taha RA, Gamal el-Din AM, Abdel-Wahab MH. Thymoquinone is a potent superoxide anion scavenger. Drug Chem Toxicol. 2003;26:87–98. doi: 10.1081/dct-120020404. [DOI] [PubMed] [Google Scholar]
- 61.Mohamed A, Afridi DM, Garani O, Tucci M. Thymoquinone inhibites the activation of NF-kappaB in the bain and spinal cord of experimental autoimmune encephalomyelitis. Biomed Sci Instrum. 2005;41:388–393. [PubMed] [Google Scholar]
- 62.El-Tawil O, Moussa SZ. Antioxidant and hepatoprotective effects of thymoquinone against carbon tetrachloride-induced hepatotoxicity in isolated rat hepatocyte. J Egypt Soc Toxicol. 2006;34:33–41. [Google Scholar]
- 63.Burits M, Bucar F. Antioxidant activity of Nigella sativa essential oil. Phytother Res. 2000;14:323–328. doi: 10.1002/1099-1573(200008)14:5<323::aid-ptr621>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 64.Fouda AMM, Daba MHY, Yousef Ahmed AR. Antigenotoxic effects of thymoquinone against benzo[a]pyrene and mitomycin C -induced genotoxicity in cultured human lymphocytes. Research in Immunology: An International Journal 2014. 2014 Articl ID 5352.79. [Google Scholar]
- 65.Mahmoud MR, El-Abhar HS, Saleh S. The effect of Nigella sati_a oil against the liver damage induced by Schistosoma mansoni infection in mice. J Ethnopharmacol. 2002;79:1–11. doi: 10.1016/s0378-8741(01)00310-5. [DOI] [PubMed] [Google Scholar]
- 66.Yildiz F, Coban S, Terzi A, Ates M, Aksoy N, Cakir H, et al. Nigella sativa relieves the deleterious effects of ischemia reperfusion injury on liver. World J Gastroenterol. 2008;14:5204–5209. doi: 10.3748/wjg.14.5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Awad A, Kamel R, Elsayed Sherief MA. Effect of thymoquinone on hepatorenal dysfunction and alteration of CYP3A1 and spermidine/spermine N-1-acetyl-transferase gene expression induced by renal ischaemia–reperfusion in rats. J Pharm Pharmacol. 2011;63:1037–42. doi: 10.1111/j.2042-7158.2011.01303.x. [DOI] [PubMed] [Google Scholar]
- 68.Sayed-Ahmed MM, Aleisa AM, Al-Rejaie SS, Al-Yahya AA, Al-Shabanah OA, Hafez MM, et al. Thymoquinone attenuates diethylnitrosamine induction of hepatic carcinogenesis through antioxidant signaling. Oxid Med Cell Longev. 2010;3:254–261. doi: 10.4161/oxim.3.4.12714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Daba MH, Abdel-Rahman MS. Hepatoprotective activity of thymoquinone in isolated rat hepatocytes. Toxicol Lett. 1998;95:23–29. doi: 10.1016/s0378-4274(98)00012-5. [DOI] [PubMed] [Google Scholar]
- 70.Khalife KH, Lupidi G. Nonenzymatic reduction of thymoquinone in physiological conditions. Free Radic Res. 2007;41:153–161. doi: 10.1080/10715760600978815. [DOI] [PubMed] [Google Scholar]
- 71.Mansour M, Tornhamre S. Inhibition of 5 lipoxygenase and leukotriene C4 synthase in human blood cells by thymoquinone. J Enzyme Inhib Med Chem. 2004;19:431–436. doi: 10.1080/14756360400002072. [DOI] [PubMed] [Google Scholar]
- 72.Mahgoub AA. Thymoquinone protects against experimental colitis in rats. Toxicol Lett. 2003;143:133–143. doi: 10.1016/s0378-4274(03)00173-5. [DOI] [PubMed] [Google Scholar]
- 73.Tekeoglu I, Dogan A, Ediz L, Budancamanak M, Demirel A. Effects of thymoquinone (volatile oil of black cumin) on rheumatoid arthritis in rat models. Phytother Res. 2007;21:895–897. doi: 10.1002/ptr.2143. [DOI] [PubMed] [Google Scholar]
- 74.Cetinkaya A, Bulbuloglu E, Kurutas EB, Kantarceken B. N-acetylcysteine ameliorates methotrexate-induced oxidative liver damage in rats. Med Sci Monit. 2006;12:BR274–BR278. [PubMed] [Google Scholar]
- 75.Badary OA, Bdel-Naim AB, Bdel-Wahab MH, Hamada FM. The influence of thymoquinone on doxorubicine-induced hyperlipidemic nephropathy in rats. Toxicology. 2000;143:219–226. doi: 10.1016/s0300-483x(99)00179-1. [DOI] [PubMed] [Google Scholar]
- 76.Zubair H, Khan HY, Sohail A, Azim S, Ullah MF, Ahmad A, et al. Redox cycling of endogenous copper by thymoquinone leads to ROS-mediated DNA breakage and consequent cell death: putative anticancer mechanism of antioxidants. Cell Death Dis. 2013;4:e660. doi: 10.1038/cddis.2013.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Racoma IO, Meisen WH, Wang QE, Kaur B, Wani AA. Thymoquinone Inhibits Autophagy and Induces Cathepsin-Mediated, Caspase-Independent Cell Death in Glioblastoma Cells. PLoS ONE. 2013;8:e72882. doi: 10.1371/journal.pone.0072882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dollah MA, Parhizkar S, Latifi LA, Binhasan MH. Toxicity effect of Nigella sativa on the liver functions of rats. Adv Pharm Bull. 2013;3:97–102. doi: 10.5681/apb.2013.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ashraf SS, Rao MV, Kaneez FS, Qadri S, Al-Marzouqi AH, Chandranath IS, et al. Nigella sativa Extract as a Potent Antioxidant for Petrochemical-Induced Oxidative Stress. J Chromatogr Sci. 2011;49:321–326. doi: 10.1093/chrsci/49.4.321. [DOI] [PubMed] [Google Scholar]
- 80.Shuddek GM. Protective roles of thymoquinone against liver damage induced by tamoxifen in female rats. Can J Physiol Pharmacol. 2014;92:640–644. doi: 10.1139/cjpp-2014-0148. [DOI] [PubMed] [Google Scholar]
- 81.Hagag AA, Abd Elal AM, Elsheik A, Elzamarany EA. Protective Effect of Nigella sativa Oil against Methotrexate Induced Hepatotoxicity in Children with Acute Lymphoblastic Leukemia. J Leuk. 2013;1:1–7. [Google Scholar]


