Abstract
Objective(s):
The aim of this open label crossover study was to investigate the effects of Nigella sativa on reproductive health and metabolic profile of perimenopausal women in Rawang, Malaysia.
Materials and Methods:
Sixty nine perimenopausal women aged 45 to 65 were allocated into the experimental group treated orally with 1600mg/day of encapsulated pure powdered N. sativa compared to control groups treated with placebo for 12 weeks. At the end of study, participants underwent washout period for fourteen days before being crossed over and continued for another cycle of treatment. Participants were abstained from taking any other drugs, herbal preparations or food supplements throughout the study. Body weight, height, waist circumference, blood pressure, biochemical parameters and hormonal levels were measured at baseline and at the end of experiment for both cycles. Face to face interview was carried out at baseline and every week to check for compliance, minimize dropouts and to record reproductive health and quality of life indicators using Greene climacteric and SF-36 instruments.
Results:
The treatment groups in both cycles showed significant improvement with reference to low density lipoprotein cholesterol and blood glucose (P<0.05). There were no significant differences between groups in total cholesterol, high density lipoprotein and triglyceride concentration. Treatment with N. sativa induced a significant reduction of prevalence and severity of menopausal symptomsas well as significant improvement in some components of quality of life (P<0.05).
Conclusion:
These results suggested that treatment with N. sativa exert a therapeutic and protective effect by modifying weight gain, improving lipid profile and blood glucose as well as hormonal level which is believed to play an important role in the pathogenesis of metabolic syndrome during menopause.
Keywords: Metabolic syndrome, Nigella sativa, Perimenopausal, Reproductive health
Introduction
Menopause is recognized by the cessation of menses for at least one year and, although it is not a disease, it is often accompanied by debilitating symptoms. Menopausal symptoms can affect women both physically and psychologically, and vary in frequency as well as intensity (1). Depending on the disorder of ovarian function and, therefore, lack of oestrogen in the postmenopausal period, symptoms such as hot flushes, irritability, sleeping disorders, fatigue, anxiety, loss of concentration are observed in the early period. Risk of coronary artery disease and incidence rate of osteoporosis increase due to the loss of protective effects of oestrogen will occur in the late period. These symptoms in the postmenopausal period adversely affect the quality of life (QOL) of woman (2). The impact of menopausal symptoms has gained in importance as the lifespan of women has increased throughout the world since women can expect to spend a significant portion of their lives after menopause (3). This period should be a highly productive time for women, and maintaining functional ability and a good QOL is of utmost importance. Accordingly, it is important to understand the QOL impacts of menopausal symptoms as well as the most recent therapeutic options of different approaches to managing symptoms (4).
Current treatments for symptoms of menopause include hormone replacement therapy (HRT) with conjugated equine estrogens and selective oestrogen receptor modulators (5). However, the standard practice of HRT is not free of risks or side effects and does not always alleviate the symptoms (6) and has been shown to increase the risk of stroke and cardiovascular disease (7). With concerns over the safety of long-term conjugated equine estrogens, women are seeking alternatives for the treatment of menopausal symptoms (8). The effects of complementary alternative medicines (CAMs) in preventing the postmenopausal symptoms as another aspect of treatment are widely accepted today. In the context of menopause, CAMs have the potential to alleviate symptoms as well as improve general health status and QOL. In the last years, there has been growing interest in alternative therapies and the therapeutic use of natural products, especially those derived from plants (9). The treatment of choice in complementary medicine is supplementation with herbal remedies (10). The identification of an alternative agent, which has the beneficial effects of oestrogen but has low cancer risk and side effects, would, therefore, be of considerable value.
Despite a lack of data on the majority of CAM therapies for menopause, many women are seeking “natural” therapies as an alternative to relieve menopausal symptoms. A review showed that over $600 million is spent annually on CAM products for menopause (11). With some controversies regarding HRT and the risk of cardiovascular disease and breast cancer, there is more interest in alternative therapies that commonly include vitamins and minerals, soy isoflavones, herbs such as Cimicifugaracemosa, and custom compounded hormones. Micronized progesterone derived from plant sources and identical to physiologic progesterone has also been shown to be beneficial in relieving menopausal symptoms (12, 13). Numerous studies suggest that Nigella sativa (N. sativa) as an amazing and multidimensional herb has beneficial effects on various body systems (14-16) and according to its traditional use for promoting lactation and menstrual disorders; N. sativa isthought to be effective in hormonal deficiency such as menopausal symptoms.
Despite the wide use of N. sativa in clinical application and studies on its efficacy on animal reproductive systems (17, 18), the scientific literature on its efficacy and safety for the treatment of menopause is not available. The aim of this study, therefore, was to assess the effects of supplied product N. sativa capsule (BijirinHitam or Al-Habbatus As-Sauda’) on menopausal symptoms and consequently quality of life among menopausal women suffering climacteric symptoms in an open label trial.
Materials and methods
Participants
Non-hysterectomized menopausal and perimenopausal women, aged between 45 and 65 years old, with complaints of climacteric symptoms were included. The women included in this study were recruited from urban areas of Rawang, Malaysia. No HRT had been given within the last 3-month period. Women taking any herbal and chemical drugs possibly affecting climacteric symptoms were excluded from the study. Subjects with a history of uncontrolled hypertension, stroke or transient ischemic attack, cancer diagnosed less than 5 years ago, or previous myocardial infarction were excluded. Other exclusion criteria were women currently using lipid-lowering drugs, anti-diabetic or anti hypertensive medications.
Study design
The aim of this study, an open label single arm comparative trial, was to compare the effect of l2-week treatment with N. sativa capsule or placebo in primenopausal women with climacteric symptoms. Sixty nine primenopausal women were enrolled into this study comparing the effects of N. sativa capsule and placebo on menopausal symptoms, psychological well being and QOL. Participants were first enrolled for the order of treatments and assigned to a 3-month treatment period with N. sativa, at a daily dosage of 1600 mg, as first drug. The capsules were manufactured in a local GMP compliant pharmaceutical company (SabitBananiSdnBhd, Malaysia). Study medication was taken twice daily, morning and evening. After completion of the 3-month period and, following by 2 weeks washout period, participants were assigned to a placebo (600 mg/day elemental Calcium Dietary Supplement) for another 3 months (Figure 1). Before the commencementof the study, the protocol of the study was approved by theClinical Research Ethics Committee of Faculty of Medicine and Health Sciences, Universiti Putra Malaysia (UPM). All participants gave their written informed consent prior to the start of the study. Before inclusion in the trial, a general medical and gynecologic examination was performed, including weight, height, and heart rate, systolic and diastolic blood pressure. The general examinations were repeated biweekly. At baseline and after 12 weeks intervention, laboratory measures were taken. For the women who completed the study, a second assessment of FSH and E2 levels were done at the end of the study. Any events or side effects reported by the patient during the study were checked by general physician and graded according to severity (mild, moderate, and severe). Subjects were followed with weekly telephone calls and biweekly general examination during the treatments, to record adverse events and study compliance. Fortnightly check was conducted to ensure full compliance by subjects.
Figure 1.
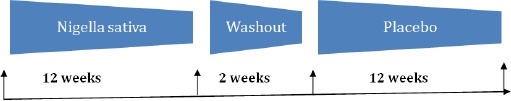
Study design of an open label single arm comparative trial (Nigella sativa vs. placebo) groups of premenopausal women
Clinical assessment
The primary endpoints for menopause-specific symptoms were evaluated with a Greene Climacteric Scale. Climacteric scale independently measures psychological, somatic and vasomotor symptoms; each symptom is rated by the subject according to its severity using a four point rating scale. The psychological, somatic and vasomotor scales are a sum of Greene Scale symptoms 1-/11, 12-/18 and 19-20, respectively. The psychological scale can be further subdivided to give measures of anxiety (a sum of symptoms 1-6) and depression (a sum of symptoms 7-11). In addition, symptom 21 is a probe for sexual dysfunction. The scale was completed at the start of the run-in period, at the initiation and after 12 weeks of treatment. After 2 weeks washout period, the scale was completed before second trial (placebo) and was repeated after 12 weeks treatment. Results were analyzed from women who had a complete set of Greene Scale recordings for all visits.
The secondary endpoint was the overall health-related QOL assessed using the standard SF-36 short form health survey derived from the Medical Outcomes Study (19, 20). The SF-36 was chosen for its multidimensionality, brevity, and its previous successful application in a variety of conditions (21). Responses to the 36 items are categorized into 8 domains: physical functioning, role physical, bodily pain, general health, vitality, social functioning, role emotional and mental health. Scores range from 0 to 100 with higher scores indicating better states of health and QOL, and lower scores indicating poorer states of health. The SF-36 has been used as a clinical outcome indicator in menopausal symptoms treatment efficacy studies (22, 23) and monitoring QOL changes in menopausal women (24-26). To assess the response to the botanicals based on non disease-specific but overall QOL in menopausal women with vasomotor symptoms, the SF-36 was evaluated at baseline and at 3 months.
Statistical analysis
Analysis of the primary and secondary end-point was performed using the SPSS 18 program. Primary outcome variable, improvement in symptom relief, expressed as the changes of the mean overall scores of the Greene Climacteric Scale and SF 36 index, were analyzed using paired Student’s t-test for comparisons within the treatment group. Unpaired Student’s t -test for independent samples was used to analyze the differences between the groups. The continuous secondary outcome variables (weight, height, BMI, waist/hip ratio) and other clinical parameters (fasting blood sugar, total cholesterol, LDL, HDL, triglyceride, creatinine, total billirobin) were also studied using the paired t - test for the evolution within the treatment groupand Student’s t-test for independent samples wasused for the comparison between groups.
Results
Out of 120 screened subjects, 69 eligible women were enrolled. Out of sixty nine women who agreed to participate in the trial, 55 initial recruits satisfied all inclusion and exclusion criteria and continued to the study until the end of trial. Fourteen subjects prematurely withdrew from the study because of protocol violation, adverse events, unusual vaginal bleeding and family holiday before the evaluation at the third month of the first study period (Table 1).
Table 1.
Reasons for early discontinuation
| Reasons for withdrown from study | No | % |
|---|---|---|
| Protocol violation | 5 | 7.24 |
| Adverse events (constipation after starting the placebo) | 3 | 4.34 |
| Unusual vaginal bleeding | 2 | 2.89 |
| Other reasons(went out for holidays abroad) | 4 | 5.79 |
| Total | 14 | 20.28 |
The characteristics of respondents are presented in Table 2. The ages of the subjects ranged from 37 to 71 years, with a mean of 50.1 years. Almost of the respondents were not menopause (65.2%) and majority of them have passed secondary school.
Table 2.
Baseline demographic characteristics of respondents
| N (%) | Mean±SD | Range | |
|---|---|---|---|
| Menopause woman | |||
| Yes | 24 (34.8) | ||
| No | 45 (65.2) | ||
| Duration of menopause (years) | *7.0±12.5 | 0.75–32 | |
| <5 | 7 (29.2) | ||
| 5 – 9 | 8 (33.3) | ||
| 10 – 14 | 2 (8.3) | ||
| ≥15 | 7 (29.2) | ||
| Age of menarche (years) | 13.3±1.5 | 9–16 | |
| Ever use oral contraceptives | |||
| Yes | 24 (34.8) | ||
| No | 45 (65.2) | ||
| Duration of oral contraceptives use (years) | *3.5±10 | 0.25–25 | |
| Number of children | 3.6±1.8 | 0–9 | |
| None | 1 (1.4) | ||
| 1-2 | 16 (23.2) | ||
| 3-4 | 33 (47.8) | ||
| ≥5 | 19 (27.5) | ||
| Age (year) | 50.1±7.6 | 37–71 | |
| <45 | 19 (27.5) | ||
| 45-54 | 31 (44.9) | ||
| ≥55 | 19 (27.5) | ||
| Marital status | |||
| Married | 59 (85.5) | ||
| Single | 1 (1.4) | ||
| Widowed | 8 (11.6) | ||
| Divorced/Separated | 1 (1.4) | ||
| Highest education attained | |||
| No formal education | 3 (4.3) | ||
| Primary school | 14 (20.3) | ||
| Secondary school | 44 (57.8) | ||
| College degree or higher | 8 (11.6) |
No clinically significant findings were found from the results of clinical parameters during treatment in both groups. Except that the significant changes of LDL mean scores were found between N. sativa and placebo (P=0.02). With comparisons of principal clinical parameters, no significant changes were observed for the levels ofwaist/hip ratio, FBS, LDL and, triglyceride in N. sativa groups (before and after the treatment), but other parameters including BMI, total cholesterol, HDL, creatinine, total billirobine and blood pressure (systolic and diastolic) improved significantly from baseline (P<0.05). On the other hand within placebo group there were no significant mean changes from baseline except systolic and diastolic blood pressure (Table 3).
Table 3.
Clinical parameters before and after 12-weeks’ treatment with Nigella sativa or placebo
| Nigella sativa | Control | Differences between groups | |||||
|---|---|---|---|---|---|---|---|
| Baseline | After 12 weeks | Mean change | Baseline | After 12 weeks | Mean change | ||
| BMI | 26.31 (4.89) | 25.78 (4.73) | -0.53 * (1.10) | 26.03 (4.66) | 26.29 (4.87) | -0.28 (1.12) | NS |
| Waist/Hip ratio | 0.82 (0.06) | 0.80 (0.06) | -0.02 (0.05) | 0.87 (0.06) | 0.81 (0.06) | -0.006 (0.05) | NS |
| FBS | 5.64 (1.64) | 5.55 (0.87) | -0.09 (1.35) | 5.65 (1.55) | 5.61 (1.32) | -0.02 (1.78) | NS |
| Total cholesterol | 5.82 (1.22) | 5.56 (1.17) | -0.25 * (0.61) | 5.84 (1.19) | 5.76 (1.26) | 0.08 (0.58) | NS |
| HDL | 1.56 (0.38) | 1.73 (0.47) | 0.17 * (0.33) | 1.52 (0.45) | 1.58 (0.42) | 0.08 (0.28) | NS |
| LDL | 4.29 (5.28) | 3.35 (1.09) | -0.93 (4.98) | 4.30)5.12) | 3.39 (1.25) | -0.91 (4.95) | P=0.02 |
| TG | 1.42 (0.84) | 1.37 (0.75) | -0.05 (0.64) | 1.41 (0.79) | 1.45 (0.72) | 0.04 (0.59) | NS |
| Creatinine | 67.31 (24.87) | 77.29 (27.87) | 9.98 * (10.53) | 65.76 (25.32) | 69.72 (20.89) | 4.96 (17.22) | NS |
| Total billirobine | 12.80 (5.97) | 11.27 (4.41) | -1.52* (3.41) | 12.74 (5.36) | 12.68 (5.97) | -0.61 (3.31) | NS |
| B/P systolic | 122.32 (15.25) | 117.63 (14.65) | -4.69 * (11.15) | 121.42 (14.89) | 118.18 (15.40) | 3.23 * (12.16) | NS |
| B/P diastolic | 79.07 (7.76) | 73.96 (9.48) | -5.10 * (6.96) | 78.42 (8.48) | 74.50 (9.30) | -3.90 * (8.61) | NS |
Data are presented as the Mean values (SD)
NS: not significant
* P<0.05 (paired t-test for the evolution within the treatment group)
Treatment with N. sativa induced a significant reduction of prevalence and severity of menopausal symptoms. The mean total Greene Climacteric Scores and the mean scores of primary endpoints, psychological and somatic, decreased markedly with NS treatments. The significant changes of mean scoresin vasomotor symptoms and sexual dysfunctionwere found with the treatment of NS. No significant changes were found in Greene climacteric scale among the control group. In general, statistically significant differences between treatments were observed in most of Greene climacteric scales, except for depression, somatic and vasomotor score (Table 4).
Table 4.
Quality of life as assessed by the GreeneClimacteric scale before and after 12-weeks’ treatment with Nigella sativa or placebo
| Nigella sativa | Control | Differences between groups | |||||
|---|---|---|---|---|---|---|---|
| Baseline | After 12 weeks | Mean change | Baseline | After 12 weeks | Mean change | ||
| Total score of GCS | 16.29 (10.60) | 11.81 (9.50) | -4.47 * (9.03) | 15.12 (9.37) | 15.90 (9.23) | 0.78 (1.11) | P=0.024 |
| Psychological score | 8.49 (6.06) | 5.80 (5.10) | -2.69 * (5.02) | 7.81 (5.24) | 8.14 (5.14) | 0.32 (0.63) | P=0.018 |
| Anxiety score | 3.89 (3.38) | 2.40 (2.94) | -1.49 * (2.90) | 3.52 (2.94) | 3.85 (2.85) | 0.33 (1.62) | P=0.010 |
| Depression score | 4.60 (3.24) | 3.40 (2.97) | -1.20 * (3.15) | 4.29 (2.82) | 4.25 (2.76) | -0.04 (1.24) | NS |
| Somatic score | 6.11 (4.19) | 4.78 (4.20) | -1.32 * (4.27) | 5.76 (3.92) | 6.05 (3.90) | 0.29 (0.59) | NS |
| Vasomotor score | 0.92 (1.19) | 0.90 (1.19) | -0.02 * (1.42) | 0.83 (1.21) | 0.94 (1.22) | 0.11 (0.36) | NS |
GCS: Greeneclimacteric scale data are presented as the mean values (SD). NS: not significant
* P<0.05 (paired t-test for the evolution within the treatment group)
From SF-36 components, four of the eight domains of health in response to 12 weeks treatment with NSimproved significantly (P<0.05), including; social function, role emotional, vitality and mental component summary. The mean changes in the four domains indicate improved overall health-related QoL with respect to social status and emotional health dimensions in the perimenopausal women. The changes in the other four domains were not significant. The inter-group comparison revealed the significant differences in some dimensions (P<0.05) including general health, role emotional, vitality, mental health and mental component summary (Table 5).
Table 5.
Quality of life as assessed by the SF-36 Index before and after 12-weeks’ treatment with Nigella sativa or placebo
| Nigella sativa | Control | Differences between groups | |||||
|---|---|---|---|---|---|---|---|
| Baseline | After 12 weeks | Mean change | Baseline | After 12 weeks | Mean change | ||
| PF | 27.92 (2.67) | 27.38 (3.02) | -0.54 (3.16) | 27.54 (2.26) | 27.09 (2.22) | -0.45 * (1.01) | NS |
| BP | 5.85 (0.40) | 5.74 (0.69) | -0.10 (0.71) | 5.72 (0.52) | 5.61 (0.52) | 0.10 (0.49) | NS |
| GH | 8.85 (1.74) | 9.16 (1.42) | 0.31 (1.71) | 8.61 (1.69) | 8.38 (1.59) | -0.23 * (0.63) | P=0.008 |
| RP | 7.45 (1.52) | 7.38 (1.69) | -0.07 (1.70) | 7.27 (1.39) | 7.07 (1.45) | -0.02 * (0.59) | NS |
| PCS | 50.09 (4.67) | 49.67 (4.21) | -0.41 (4.51) | 49.16 (4.27) | 48.16 (4.11) | -1.00 * (1.55) | NS |
| SF | 4.43 (1.47) | 3.98 (1.39) | -0.45 * (1.89) | 4.27 (1.31) | 4.10 (1.25) | -0.16 * (0.53) | NS |
| RE | 12.69 (1.30) | 13.29 (1.59) | 0.60 * (1.47) | 12.34 (1.35) | 12.16 (1.25) | -0.18 * (0.47) | P=0.000 |
| VT | 14.58 (2.14) | 15.23 (1.96) | 0.65 * (2.49) | 14.01 (1.91) | 13.78 (1.92) | -0.23 * (0.71) | P=0.000 |
| MH | 17.78 (2.92) | 18.96 (2.21) | 1.18 (3.37) | 17.14 (2.77) | 16.76 (2.78) | -0.38 (0.99) | P=0.000 |
| MCS | 49.49 (4.41) | 51.74 (3.45) | 1.98 * (4.43) | 47.78 (4.19) | 46.81 (3.89) | -0.96 * (1.76) | P=0.000 |
Data are presented as the Mean values (SD). NS: not significant
P<0.05 (paired t -test for the evolution within the treatment group)
PF: physical function; RP: role physical; BP: bodily pain; GH: general health; PCS: Physical Components Summary; SF: social function;
RE: role emotional; VT: vitality; MH: mental health; MCS: Mental components Summary
Discussion
Despite widespread use of alternative treatments, scientific evidence supporting the efficacy and safety of most complementary treatments for relief of menopausal symptoms is sparse (27). Therefore, randomized controlled trials demonstrating the short- and long-term effects of these treatments are needed. The present study is the first controlled trial to compare the clinical effects of the N. sativa, and a placebo on climacteric symptoms in non-hysterectomized perimenopausal women. This study proved clearly that N. sativa could help women with their menopausal problems. Moreover, N. sativa caused less unusual vaginal bleeding or spotting episodes and absence of breast tenderness during the study period. In this study, N. sativa showed significant improvements in overall symptomatic relief of the primary endpoint (total score of the Greene Climacteric Scale). Moreover, the significant reductions of psychological (anxiety, depression) and somatic symptom scores on the Greene Climacteric Scale were also noted.
The 3-month intervention period was used to assess changes, if any, produced by the N. sativa capsule (BijirinHitam or Al-Habbatus -Sauda’)in menopausal subjects. Recent studies have used comparable short-term time periods to determine efficacy of therapies in menopausal subjects, including studies of CAM therapies for menopausal symptom control, such as phytoestrogens and isoflavones, and combination herbs (28, 29). Despite of preliminary nature of the pilot study, clinical measurement (e.g. weight, height, BMI, waist/hip ratio) and laboratory markers (e.g. fasting blood sugar, total cholesterol, LDL, HDL, triglyceride, creatinine, total billirobin) as well as qualitative endpoints (e.g. Greene Climacteric Scale and SF 36 Index) were examined.
As expected, the biochemical parameters such as total cholesterol, HDL, creatinine, total billirobine improve significantly in response to 12 weeks N. sativa treatment as well as some clinical measurement including BMI, systolic and diastolic blood pressure. Other studies on human and animal subjects consistently supported current study (14, 30-37). Ibrahim and his colleagues also reported improvement of lipid profile and blood glucose of menopausal women in response to 8 weeks supplementation with N. sativa capsule (38).
Randomized controlled trials in larger patient populations and longer intervention periods with positive and negative control groups will provide more evidence and better understanding of the value of N. sativa for controlling menopausal symptoms. With current concerns about the use of HRT to control menopausal symptoms, safer alternative choices are urgently needed. More extensive data on efficacy, safety, toxicity, and long-term consequences of botanicals, particularly N. sativa, focusing on quality assurance and quality control must also be evaluated.
Conclusion
Generally, the preliminary results suggested that N. sativa was a safe and efficacious therapy and might be an alternative choice for relief of climacteric symptoms in postmenopausal women who refuse or have contraindications for HRT. However, the exact efficacy and clinical roles of N. sativa have not been convincingly demonstrated in this study because of lack of the blinding approach and the lack of positive control group, and only the possibility of its efficacy has been raised. Therefore, a blinding trial with more patient numbers to evaluate the efficacy of N. sativa deserves further study.
Acknowledgment
The authors would like to thank SabitBananiSdnBhd for sponsoring the community trial.
References
- 1.Curcio JJ, Kim LS, Wollner D, Pockaj BA. The potential role of 5-hydroxytryptophan for hot flash reduction: a hypothesis. Altern Med Rev. 2005;10:216–221. [PubMed] [Google Scholar]
- 2.Teomana N, Özcana A, Acarb B. The effect of exercise on physical fitness and quality of life in postmenopausal women. Maturitas. 2004;47:71–77. doi: 10.1016/s0378-5122(03)00241-x. [DOI] [PubMed] [Google Scholar]
- 3.Utian WH. Psychosocial and socioeconomic burden of vasomotor symptoms in menopause: A comprehensive review. Health Qual Life Out. 2009;3:39–48. doi: 10.1186/1477-7525-3-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Speroff L, Gass M, Constantine G, Olivier S. Efficacy and tolerability of desvenlafaxinesuccinate treatment for menopausal vasomotor symptoms. Obstet Gynaecol. 2008;111:77–87. doi: 10.1097/01.AOG.0000297371.89129.b3. [DOI] [PubMed] [Google Scholar]
- 5.Roe EB, Chiu KM, Arnaud CD. Selective estrogen receptor modulators and postmenopausal health. Adv Intern Med. 2000;45:259–278. [PubMed] [Google Scholar]
- 6.Smolinski D, Wollner D, Orlowski J, Curcio J, Nevels J, Kim LS. A pilot study to examine a combination botanical for the treatment of menopausal symptoms. J Altern Complement Med. 2005;11:483–489. doi: 10.1089/acm.2005.11.483. [DOI] [PubMed] [Google Scholar]
- 7.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 8.Parhizkar S, Latiff AL, Dollah MA, Hassan STS, Rashid I, Hanachi P. A review of the menopausal symptoms management with herbal remedies. Eur J Sci Res. 2008;21:46–63. [Google Scholar]
- 9.Schwartsmann G, Ratain MJ, Cragg GM, Wong JE, Saijo N, Parkinson D, et al. Anticancer drug discovery and development throughout the world. J Clin Oncol. 2002;20:47s–59s. [PubMed] [Google Scholar]
- 10.Boronditsky RS. Balancing safety and efficacy focuse on endometrial protection. J Reprod Med. 2000;45:273–84. [PubMed] [Google Scholar]
- 11.Taylor M. Alternatives to conventional hormone replacement therapy. Compr Ther. 1997;23:514–532. [PubMed] [Google Scholar]
- 12.Lieberman S. A review of the effectiveness of Cimicifugaracemosa (black cohosh) for the symptoms of menopause. J Women's Health. 1998;7:525–529. doi: 10.1089/jwh.1998.7.525. [DOI] [PubMed] [Google Scholar]
- 13.Kronenberg F, Fugh-Berman A. Complementary and alternative medicine for menopausal symptoms: A review of randomized, controlled trials. Ann Intern Med. 2002;137:805–813. doi: 10.7326/0003-4819-137-10-200211190-00009. [DOI] [PubMed] [Google Scholar]
- 14.Haq A, Lobo PL, Al-Tufail M, Rama NR, Al-Sedairy S. Immunomodulatory effect of Nigella sativa proteins fractionated by ion exchange chromatography. J Immunopharmacol. 1995;21:283–295. doi: 10.1016/s0192-0561(99)00010-7. [DOI] [PubMed] [Google Scholar]
- 15.Bamosa AO, Ali BA, Sawayan SA. Effect of oral ingestion of Nigella sativa seed on some blood parameters. J Saudi Pharm. 1997;5:126–129. [Google Scholar]
- 16.Buriro MA, Tayyab M. Effect of Nigella sativa on lipid profile in albino rats. Gomal J Med Sci. 2007;5:28–31. [Google Scholar]
- 17.Parhizkar S, Latiff LA, Rahman SA, Dollah MA, Hanachi P. Assessing estrogenic activity of Nigella sativa in ovariectomized rats using vaginal cornification assay. Afr J Pharm Pharmacol. 2011;5:137–142. [Google Scholar]
- 18.Parhizkar S, Latiff AL, Rahman SA, Dollah MA. Evaluation of estrogen-like activity of Nigella sativa inovariectomized rats. Afr J Pharm Pharmacol. 2011;5:1006–1011. [Google Scholar]
- 19.Wee CC, Davis RB, Hamel MB. Comparing the SF-12 and SF-36 health status questionnaires in patients with and without obesity. Health Qual Life Out. 2008;6:11. doi: 10.1186/1477-7525-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michalak EE, Yatham LN, Lam RW. Quality of life in bipolar disorder: a review of the literature. Health Qual Life Out. 2005;3:72. doi: 10.1186/1477-7525-3-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brazier JE, Harper R, Jones NM, O’Cathain A, Thomas KJ, Usherwood T, Westlake L. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992;305:160–164. doi: 10.1136/bmj.305.6846.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryan N, Rosner A. Quality of life and costs associated with micronized progesterone and medroxyprogesterone acetate in hormone replacement therapy for nonhysterectomized, postmenopausal women. Clin Ther. 2001;23:1099–1115. doi: 10.1016/s0149-2918(01)80094-1. [DOI] [PubMed] [Google Scholar]
- 23.Tinker LF, Perri MG, Patterson RE, Bowen DJ, McIntosh M, Parker LM, Sevick MA, Wodarski LA. The effects of physical and emotional status on adherence to a low-fat dietary pattern in the Women's Health Initiative. J Am Diet Assoc. 2002;102:789–800. doi: 10.1016/s0002-8223(02)90178-1. [DOI] [PubMed] [Google Scholar]
- 24.Elit L, Esplen MJ, Butler K, Narod S. Quality of life and psychosexual adjustment after prophylactic oophorectomy for a family history of ovarian cancer. Fam Cancer. 2001;1:149–156. doi: 10.1023/a:1021119405814. [DOI] [PubMed] [Google Scholar]
- 25.Mishra GD, Brown WJ, Dobson AJ. Physical and mental health: Changes during menopause transition. Qual Life Res. 2003;12:405–412. doi: 10.1023/a:1023421128141. [DOI] [PubMed] [Google Scholar]
- 26.Schneider HP, Heinemann LA, Rosemeier HP, Potthoff P, Behre HM. The menopause rating scale (MRS): comparison with Kupperman index and quality-of-life scale SF-36. Climacteric. 2000;3:50–58. doi: 10.3109/13697130009167599. [DOI] [PubMed] [Google Scholar]
- 27.Guo R, Canter PH, Ernst E. A systematic review of randomised clinical trials of individualised herbal medicine in any indication. Postgrad Med J. 2007;83:633–637. doi: 10.1136/pgmj.2007.060202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kessel B, Kronenberg F. The role of complementary and alternative medicine in management of menopausal symptoms. Endocrinol Metab Clin N Am. 2004;33:717–739. doi: 10.1016/j.ecl.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Lioyd KB, Hornsby LB. Complementary and alternative medications for women's health issues. Nutr Clin Pract. 2009;24:589–608. doi: 10.1177/0884533609343001. [DOI] [PubMed] [Google Scholar]
- 30.Tissera MHA, B1, Serasinghe P, Karunarathna C. Preliminary Study on the effect of Baraka Oil on Cholesterol in Human Blood. Baraka. 2014. Available from: http://www.baraka.lk/research/clinical-trials-of-black-seed .
- 31.El Dakhakhny M, Mady NL, Halim MA. Nigella sativa L. Oil protects against induced hepatotoxicity and improves serum lipid profile in rats. Arzneimittelforschung. 2000;50:832–836. doi: 10.1055/s-0031-1300297. [DOI] [PubMed] [Google Scholar]
- 32.Zandi PR, Carlson MC, Plassman BL, Welsh-bohmer KA, Mayer LS, Steffens DC, et al. Hormone replacement therapy and incidence of Alzheimer disease in older women. The cache county study. JAMA. 2002;288:2123–2129. doi: 10.1001/jama.288.17.2123. [DOI] [PubMed] [Google Scholar]
- 33.Houcher Z, Boudiaf Kh, Benboubetra M, Houcher B. Effects of Methanolic Extract and Commercial Oil of Nigella sativa L. on Blood Glucose and Antioxidant Capacity in Alloxan-Induced Diabetic Rats. Pteridines. 2007;18:8–18. [Google Scholar]
- 34.Najmi A, Haque SF, Khan RA, Nasiruddin M. Therapeutic Effect of Nigella sativa Oil on different clinical andbiochemical parameters inmetabolic syndrome. Inter J Pharmacol. 2008;5:1531–2976. [Google Scholar]
- 35.Qidwai W, Hamza HB, Qureshi R, Gilani A. Effectiveness, Safety, and Tolerability of powdered Nigella sativa (Kalonji) seed in capsules on serum lipid levels, blood sugar, blood pressure, and body weight in adults: Results of a randomized, double-blind controlled trial. J Altern Complement Med. 2009;15:639–644. doi: 10.1089/acm.2008.0367. [DOI] [PubMed] [Google Scholar]
- 36.El-Bagir NM, Farah ITO, Alhaidary A, Mohamed HE, Beynen AC. Clinical loboratory serum values in rabbits fed diets containing black cumin seed. J Anim Vet Adv. 2010;9:2532–2536. [Google Scholar]
- 37.Parhizkar S, Latiff AL, Rahman SA, Dollah MA. Preventive effect of Nigella sativa on metabolic syndrome in menopause induced rats. J Med Plants Res. 2011;5:1478–1484. [Google Scholar]
- 38.Ibrahim RM, Hamdan NS, Ismail M, Saini SM, Rashid SNA, Latiff LA, et al. Protective effect of Nigella sativa on metabolic syndrome in menopausal women. Adv Pharm Bull. 2014;4:29–33. doi: 10.5681/apb.2014.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


