Abstract
Somatostatin receptor (SSTR) expressions in neuroblastomas (NBs) have been confirmed employing various methods. High SSTR-2 expression was suggested to be a favorable prognostic marker, though little is known about the relationships between the expressions of SSTR subtypes, other than SSTR-2, and prognosis. We investigated the expressions of all five known SSTR subtypes in 63 neuroblastic tumors (NTs), employing immunohistochemistry, and also conducted quantitative real-time RT-PCR in 37 of these tumors. We evaluated correlations between the expressions of SSTR subtypes and prognosis, based on the International Neuroblastoma Pathology Classification and patient outcomes. More than 90% of cases expressed, at a minimum, SSTR-1 and/or 2. Ganglioneuromas and ganglioneuroblastomas expressed more than two SSTR subtypes. Among NBs, the favorable histology group showed higher SSTR subtype expressions than the unfavorable histology group. The same tendency was observed when surviving and deceased cases were compared, though SSTR-2 expression was well preserved in some of the deceased cases. In conclusion, NTs highly expressed SSTR-1 and/or 2, and expressions of SSTR generally indicate a good prognosis. However, even those in the unfavorable histology group with NBs expressing SSTR are good candidates for molecular targeting therapy using somatostatin analogues.
Keywords: somatostatin receptor, neuroblastoma, immunohistochemistry, quantitative real-time RT-PCR, International Neuroblastoma Pathology Classification
I. Introduction
Somatostatin is a regulatory peptide, with two forms known as somatostatin-14 and somatostatin-28, produced by neuroendocrine cells, inflammatory cells and immune cells, and shows diverse physiological effects, generally exerting inhibitory effects on hormonal products, secretion and cell growth [21]. Its plasma half-life is quite short, such that stable synthetic analogues have been developed for experimental and clinical use. The biological effects of somatostatin are mediated by five somatostatin receptor (SSTR) subtypes, 1, 2, 3, 4 and 5, which are G-protein-coupled plasma membrane receptors, extensively distributed in systemic organs. They are also detected in many tumors including endocrine and non-endocrine tumors, by techniques such as scintigraphy [12], affinity and autoradiography binding with natural ligand somatostatin or synthetic analogues [25], reverse-transcriptase polymerase chain reaction (RT-PCR) [22], western blot, in situ hybridization and immunohistochemistry (IHC) [27]. At present, scintigraphy remains a useful tool not only for in vivo detection of SSTR but also for peptide receptor radionuclide therapy. Qualitative and quantitative RT-PCR techniques are both used extensively in experimental settings. IHC has come into widespread use for routine pathological diagnosis employing commercially available antibodies to evaluate neuroendocrine tumors, since treatment with somatostatin analogues was approved. In recent years, the use of a monoclonal antibody against SSTR has increased and promising results have reportedly been obtained [26].
Neuroblastic tumors (NTs) are the most common solid pediatric malignancy. NTs have unique biology in terms of tumor maturation, a process which mimics differentiation of the embryonal neural crest. The International Neuroblastoma Pathology Classification (INPC) for NTs is now widely accepted [29, 30]. It defines tumor category and prognostic group. Tumor category reflects the morphological differentiation of tumors including ganglioneuroma (GN), ganglioneuroblastoma (GNB) intermixed, neuroblastoma (NB) and GNB nodular. Prognostic group, which takes age and MKI (mitosis-karyorrhexis index, representing cellular turnover) into account in addition to tumor category, correlates well with patient outcomes. Prognostic group may reflect functional differentiation of the tumor. As a practical matter in the clinical setting, it is useful to classify morphologically similar NBs into two prognostically distinct groups. SSTR expressions of NBs have been demonstrated in cell lines and tumor specimens in the last two decades using various methods [15, 18, 19]. High expression of SSTR-2 was shown to correlate with patient survival [24]. Recently, there has been remarkable progress in treatments targeting SSTRs. Peptide receptor radionuclide therapy has been performed for unresectable NBs in clinical trials [6, 10, 16]. New therapeutic techniques are under investigation in light of their demonstrated SSTR selectivity [13, 31]. Novel multi-receptor targeting somatostatin analogues are also being developed [4].
Earlier studies which detected the expressions of SSTRs in NBs focused mostly on SSTR-2, because octreotide, a representative somatostatin analogue and also a very useful tool for study, was found to be highly selective for SSTR-2. Only a few studies have examined the expressions of SSTR subtypes, other than SSTR-2, in NBs. Thus, little is known about the relationships between subtypes other than SSTR-2 and prognosis. However, the profiling of SSTR subtypes is necessary for future effective use of novel multi-receptor targeting somatostatin analogues. In this study, we investigated the expressions of SSTR-1, 2, 3, 4 and 5 in NTs and evaluated the relationships of these expressions with prognosis. We applied the INPC criteria, in addition to patient outcomes as prognostic factors, because the INPC is the standard histological classification used in clinical practice, and it reflects both morphological differentiation and prognosis. One of our interests is the relationships between SSTR expressions and morphology in NTs, because high expressions of SSTRs were observed in differentiated neuroendocrine tumors [17]. For detecting the expressions of SSTR subtypes in NTs, we used IHC and quantitative real-time RT-PCR (qRT-PCR) techniques. IHC results were evaluated employing the immunoreactive score (IRS) which was first developed for IHC assessment of estrogen receptors in breast tumors and has also recently been used for assessing neuroendocrine tumors [8, 9].
II. Materials and Methods
Materials
We studied 63 cases with neuroblastic tumors (NTs) consisting of 54 neuroblastomas (NBs), 4 ganglioneuroblastomas (GNBs) and 5 ganglioneuromas (GNs). The tumors were sampled by open biopsy or surgically removed before chemotherapy and submitted for pathological examination at Nihon University Itabashi Hospital and National Center for Child Health during the period between 1991 and 2012. The selected tumors were reclassified according to the histological classification of the INPC by more than two pathologists. NBs included 27 cases each in the favorable and unfavorable histology groups. GNBs included 2 intermixed types and 2 nodular types. The clinical and pathological information of all cases is summarized in Table 1. Ten patients died, and all deaths were cancer-related. Twelve tumors showed MYCN amplification. Fourteen patients had stage 1, 10 stage 2, 11 stage 3, 26 stage 4 and 2 stage 4S, based on the International Neuroblastoma Staging System (INSS) [3]. Eight normal adrenal medullas and 4 normal sympathetic ganglia, contained in some of the tumor specimens, were also examined as normal control tissues.
Table 1. .
Summary of clinical and pathological profiles
| Histology (INPC category/ prognostic group) |
No. | Age | Sex | Lesion | Histological subtype |
MKI | Stage | MYCN amplification |
Survival |
|---|---|---|---|---|---|---|---|---|---|
| Ganglioneuroma | 1 | 14y | M | R | Mature | 1 | NE | NED 8y | |
| 2 | 19y | M | M | Mature | 1 | NE | Unknown | ||
| 3 | 21y | M | M | Mature | 1 | NE | NED 4y | ||
| 4 | 31y | M | A | Mature | 1 | NE | NED 2y | ||
| 5 | 51y | M | A | Mature | 1 | NE | Unknown | ||
| Ganglioneuroblastoma intermixed | 1 | 2y | F | A | 1 | – | NED 6y | ||
| 2 | 3y | M | R | 2A | – | NED 20y | |||
| Ganglioneuroblastoma nodular | *3 | 2y | M | A | Low | 4 | – | NED 3.5y | |
| **4 | 3y | F | P | 4 | – | AWD 2.5y | |||
| Neuroblastoma/Favorable group | 1 | 6d | F | P | Poorly | Low | 3 | – | NED 7y |
| 2 | 16d | M | R | Poorly | Low | 3 | – | NED 4y | |
| 3 | 3m | M | R | Poorly | Low | 1 | – | NED 2y | |
| 4 | 4m | M | P | Poorly | Low | 1 | – | NED 3y | |
| 5 | 4m | M | A | Poorly | Low | 1 | – | NED 4y | |
| 6 | 6m | F | A | Poorly | Intermediate | 4S | – | NED 4y | |
| 7 | 7m | M | R | Poorly | Low | 2A | – | NED 8y | |
| 8 | 7m | M | A | Poorly | Low | 2B | – | NED 17y | |
| 9 | 7m | M | A | Differentiating | Intermediate | 2B | – | NED 16y | |
| 10 | 7m | F | P | Poorly | Low | 4 | – | DOD 1.5m | |
| 11 | 8m | M | A | Poorly | Intermediate | 3 | – | Unknown | |
| 12 | 8m | M | P | Poorly | Low | 4S | – | NED 19y | |
| 13 | 8m | F | A | Poorly | Low | 1 | – | NED 11y | |
| 14 | 9m | M | R | Poorly | Low | 3 | – | NED 12y | |
| 15 | 9m | F | A | Poorly | Intermediate | 1 | – | NED 11y | |
| 16 | 9m | F | R | Poorly | Low | 2A | – | NED 12y | |
| 17 | 10m | F | A | Poorly | Low | 2B | – | NED 2.5y | |
| 18 | 10m | F | A | Poorly | Low | 2B | – | NED 15y | |
| 19 | 11m | M | A | Poorly | Low | 2B | – | NED 1.5y | |
| 20 | 11m | F | A | Poorly | Low | 1 | – | NED 12y | |
| 21 | 12m | M | P | Poorly | Low | 3 | – | NED 7y after relapse | |
| 22 | 12m | F | R | Differentiating | Low | 1 | – | NED 8y | |
| 23 | 13m | M | R | Poorly | Low | 2 | – | NED 4y | |
| 24 | 14m | F | M | Poorly | Low | 4 | – | NED 3y | |
| 25 | 15m | F | A | Differentiating | Low | 3 | – | NED 13y | |
| 26 | 17m | F | A | Poorly | Low | 4 | – | AWD 2.5y | |
| 27 | 2y | M | A | Differentiating | Low | 1 | – | NED 5y | |
| Neuroblastoma/Unfavorable group | 28 | 9m | F | A | Poorly | High | 2 | + | NED 4y |
| 29 | 16m | M | A | Poorly | High | 4 | + | DOD 1.3y | |
| 30 | 18m | M | A | Poorly | High | 4 | + | NED 5y | |
| 31 | 20m | M | A | Poorly | High | 4 | + | NED 4.5y | |
| 32 | 20m | F | R | Poorly | Low | 3 | – | NED 5y | |
| 33 | 21m | M | R | Undifferentiated | High | 3 | + | DOD 6m | |
| 34 | 23m | M | A | Poorly | High | 4 | + | NED 2y | |
| 35 | 2y | M | R | Poorly | Low | 4 | + | NED 8y | |
| 36 | 2y | M | R | Poorly | Low | 4 | – | AWD 10m | |
| 37 | 2y | M | A | Poorly | Low | 4 | – | NED 9y | |
| 38 | 2y | M | M | Poorly | Intermediate | 3 | + | NED 3.5y | |
| 39 | 2y | F | A | Poorly | Intermediate | 4 | + | NED 6y | |
| 40 | 2y | F | M | Poorly | High | 4 | – | DOD 1.8y | |
| 41 | 3y | M | A | Poorly | Low | 4 | – | DOD 4.3y | |
| 42 | 3y | M | A | Poorly | Intermediate | 4 | – | AWD 4y | |
| 43 | 4y | M | A | Poorly | Low | 4 | – | NED 3.5y | |
| 44 | 4y | M | R | Poorly | High | 4 | + | DOD 1.5y | |
| 45 | 4y | M | R | Poorly | Low | 3 | – | NED 1.5y | |
| 46 | 4y | F | A | Poorly | High | 4 | + | DOD 4m | |
| 47 | 5y | M | A | Poorly | Low | 4 | – | NED 1.8y after relapse | |
| 48 | 5y | M | A | Poorly | High | 4 | + | NED 9y | |
| 49 | 5y | M | A | Poorly | High | 4 | – | DOD 3.3y | |
| 50 | 6y | M | R | Poorly | Intermediate | 4 | – | AWD 3y | |
| 51 | 10y | F | LN | Poorly | Intermediate | 3 | – | DOD 2m | |
| 52 | 11y | M | P | Poorly | Intermediate | 4 | – | NED 1y | |
| 53 | 12y | M | R | Differentiating | Intermediate | 4 | – | AWD 2y | |
| 54 | 12y | F | A | Poorly | Low | 4 | – | DOD 3.3y |
MKI: mitosis-karyorrhexis index, y: years, d: days, m: months, M: male, F: female, R: retroperitoneum, M: mediastinum, A: adrenal gland, P: paravertebral area, LN: lymph node, NE: not examined, NED: no evidence of disease, DOD: died of disease, AWD: alive with disease, *: GNB nodular with mixed features with GNB intermixed and NB poorly differentiated subtype, **: GNB nodular with GN features only in the specimen examined in this study.
IHC for SSTR subtypes
IHC was performed using an automated staining system (Histostainer; Nichirei Bioscience, Tokyo, Japan) with formalin-fixed paraffin-embedded tissue sections cut at a thickness of 4 μm and mounted onto silane-coated glass slides. For antigen retrieval, dewaxed sections were immersed in citrate buffer at pH 6.0 and boiled in a water bath at 95°C for 40 min, then cooled to room temperature. After being washed several times in phosphate buffered saline (PBS), the sections were processed for blocking of the non-specific binding with 5% normal goat serum for 10 min. These steps were omitted for somatostatin immunostaining. Sections were incubated with the primary antibodies for 30 min at room temperature. After washing with PBS, endogenous peroxidase blocking was carried out with 3% hydrogen peroxide for 10 min. Sections were incubated with secondary antibody for 30 min at room temperature. After washing with PBS, sections were incubated with 3,3-diaminobenzidine for 5 min. After washing with running water, the sections were counterstained with hematoxylin for 1 min. The data for the primary and secondary antibodies, as well as the working dilutions, are shown in Table 2. As the positive control, we used normal pancreatic islets. Negative controls were established by omitting the specific primary antibodies followed by the same processing as above.
Table 2. .
Antibodies for IHC
| Primary antibody | Source | Animal | Clone/Code | Dilution ratio | Second antibody |
|---|---|---|---|---|---|
| SSTR 1 | Gramsch | Rabbit | Poly/SS-840 | ×400 | Envision (Dako) |
| SSTR 2 | Abcam | Rabbit | UMB1/ab134152 | ×200 | Envision (Dako) |
| SSTR 3 | Gramsch | Rabbit | Poly/SS-850 | ×400 | Envision (Dako) |
| SSTR 4 | GeneTex | Rabbit | Poly/GTX70677 | ×100 | Envision (Dako) |
| SSTR 5 | Gramsch | Rabbit | Poly/SS-890 | ×400 | Envision (Dako) |
| Somatostatin | Dako | Rabbit | Poly/A566 | ×500 | Histofine (Nichirei) |
Histofine: Histofine Simple Stain MAX PO (MULTI).
For the evaluation of immunohistochemical expressions of SSTR subtypes, we applied a semi-quantitative analysis, employing the IRS, according to the study of Kaemmerer et al. [8, 9]. The IRS is the number of points from the percentage of positive cells multiplied by the intensity of staining. IRS point totals range from 0 to 12. A score of more than one point is considered to be positive (Table 3).
Table 3. .
Immunoreactive score (IRS)
| Percentage of positive cells | 0 | 0% |
| 1 | <10% | |
| 2 | 10–50% | |
| 3 | 51–80% | |
| 4 | >80% | |
| Intensity of staining | 0 | None |
| 1 | Mild | |
| 2 | Moderate | |
| 3 | Intense |
IRS score = Percentage of positive cells × Intensity of staining
IRS scores range from 0 to 12 points, with a score of more than 1 point being considered positive.
Quantitative real-time RT-PCR (qRT-PCR) for measurement SSTR subtypes
For RNA extraction, we used serial sections of the formalin-fixed paraffin-embedded tissues employed for the aforementioned IHC. Eight-μm-thick sections were cut from the paraffin embedded tumor blocks and collected into Eppendorf tubes, and then processed for deparaffinization. In the case of the paraffin blocks including non-tumor tissues, these tissues were removed manually under a microscope and the targeted area was collected into an Eppendorf tube with mineral oil. The samples were mixed with 200 μL of denaturing buffer containing 2% SDS, 0.1 mM EDTA, and 10 mM Tris-HCl, and incubated at 55°C with 5 μL of proteinase K until the sections were completely dissolved. Total RNA was purified with 20 μL of 2 M sodium acetate pH 4.0, 220 μL of citrate saturated phenol pH 4.3, and 60 μL of chloroform-isoamyl alcohol. Following centrifugation at 15,000 rpm for 15 min, the upper aqueous layer was transferred into fresh tubes, and 200 μL of isopropanol and 2 μL of glycogen were added as a carrier. Samples were then frozen at –80°C for more than 30 min. The pellets were collected by centrifugation for 30 min at 14,000 rpm, washed with 70% ethanol, and air-dried on ice. Subsequently, the pellets were dissolved in 5–10 μL of RNase-free water and quantified at an optical density of 260 nm using a Nanodrop 1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, U.S.A.). The total RNA samples were stored at –80°C until use. According to the manufacturer’s instructions, both genomic DNA elimination and cDNA synthesis were performed using the Quantitect Reverse Transcription Kit (QIAGEN, Tokyo, Japan). The mRNA expression levels of SSTR subtypes (SSTR-1, 2, 3, 4 and 5) and the internal control glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were measured by the qRT-PCR method using 1 μL cDNA samples. The qRT-PCR amplification and data analysis were carried out using the ABI Prism 7000 Sequence Detection System (Life Technologies Japan, Tokyo, Japan). The amplification was performed with 20 μL of final reaction mixture containing 900 nmol/L of each primer (Table 4), as previously reported [17], and 1×SYBR® Green PCR Master Mix (Life Technologies Japan). The reaction mixture was preliminarily heated at 95°C for 10 min, followed by 45 cycles at 95°C for 15 sec and 60°C for 1 min. Each SSTR subtype mRNA relative value was measured by the ΔΔCT method with threshold cycle times of each target SSTR and GAPDH [14].
Table 4. .
Primer sequences for real-time RT-PCR assay for SSTR subtypes
| Target | Sequences | Products (bp) | |
|---|---|---|---|
| SSTR 1 | forward | tgagtcagctgtcggtcatc | 93 |
| reverse | ggaaagagcgcttgaagttg | ||
| SSTR 2 | forward | ctttgtggtggtcctcacct | 100 |
| reverse | gcagaggacattctggaagc | ||
| SSTR 3 | forward | ttcctctcctaccgcttcaa | 123 |
| reverse | ctcctcctcatcctcctcct | ||
| SSTR 4 | forward | tctttgtgctctgctggatg | 96 |
| reverse | ggataagggacacgtggttg | ||
| SSTR 5 | forward | cccttcttcaccgtcaacat | 102 |
| reverse | gttggcgtaggagaggatga | ||
| GAPDH | forward | ggaaggtgaaggtcggagtca | 101 |
| reverse | gtcattgatggcaacaatatccact |
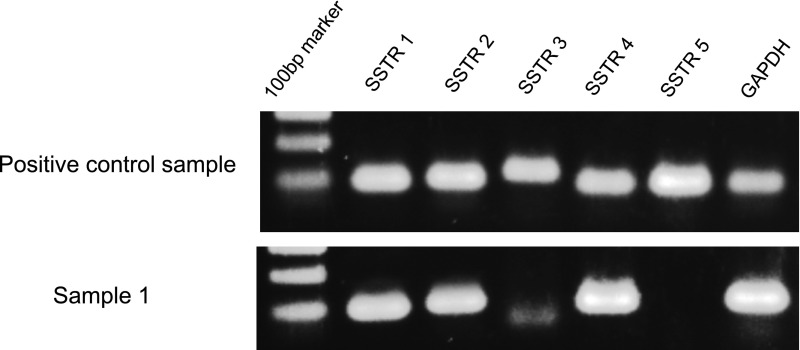
In this study, as the RNA degradation of samples was demonstrated by capillary electrophoresis using Bioanalyser 2100 (Agilent Technologies, Tokyo, Japan), the target amplifications achieved by the qRT-PCR method were confirmed employing agarose gel electrophoresis staining with SYBR® Green (Fig. 1).
Fig. 1. .
The qRT-PCR products were visualized as specific bands (SSTR-1: 93 bp, SSTR-2: 100 bp, SSTR-3: 123 bp, SSTR-4: 96 bp, SSTR-5: 102 bp, GAPDH: 101 bp) by 2% gel electrophoresis. The positive control sample expressed all SSTR subtypes and GAPDH; from the formalin-fixed paraffin-embedded tissue of a neuroendocrine tumor. Sample 1 expressed SSTR-1, 2 and 4; our NB case, from the favorable histology group, corresponds to Case No. 26 in Table 1.
Statistical analysis
Statistical analyses were performed using Ekuseru-Toukei 2012 (Social Survey Research Information, Tokyo, Japan). Each test used is described in the relevant portion of the results section. A value of p<0.05 was considered to indicate a statistically significant difference.
III. Results
IHC expressions of SSTR subtypes and their relationships with INPC
IHC was performed on all 63 NTs and normal tissues (8 adrenal medullas and 4 sympathetic ganglia). The numbers of samples positive for SSTR-1, 2, 3, 4 and 5 in NTs were 45/63 (71.4%), 53/63 (84.1%), 9/63 (14.3%), 29/63 (46.0%) and 0/63 (0%), respectively. Thus, 57/63 (90.5%) of the specimens were positive for expression of SSTR-1 and/or 2. Six cases showed no expression of SSTR subtypes. Co-expression of SSTR-1 and 2 was observed in more than 60% of all cases. None of our cases had tumors expressing SSTR-3 or SSTR-4 alone.
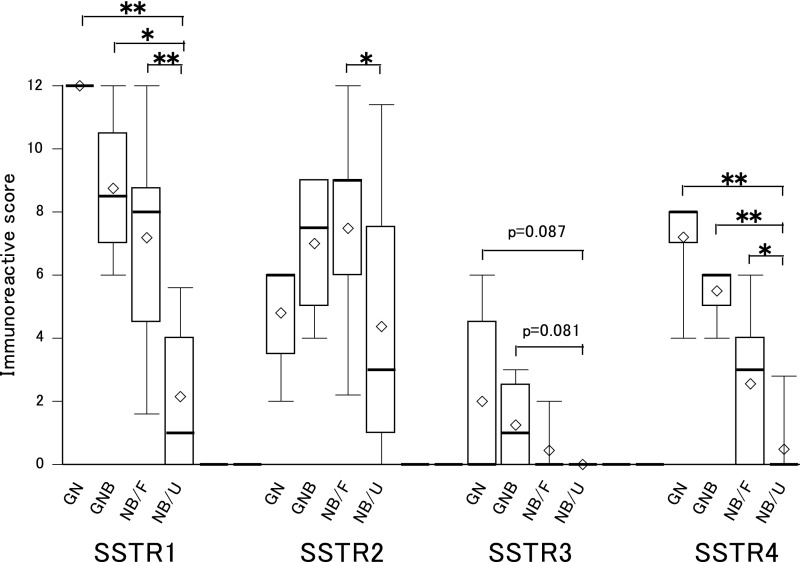
IHC expressions of SSTR subtypes classified by INPC are shown in Table 5. All GNs and GNBs showed 100% SSTR-1, 2 and 4 expressions. In NBs, the rates of positivity for SSTR 1, 2, 3 and 4 were higher in the favorable than in the unfavorable histology group and the differences were statistically significant. These results indicated the tendency for morphologically differentiated tumors and the favorable histology group to express more SSTR subtypes. Semi-quantitative comparison using the IRS system showed GNs and GNBs to have higher scores than NBs with the SSTR-1, 3 and 4 subtypes (Fig. 2). As to SSTR-2, however, the scores were not particularly high for GNs and GNBs. In NBs, the favorable histology group showed higher point totals than the unfavorable histology group, with significant differences in SSTR-1, 2 and 4. Representative positive cases are shown in Fig. 3. In general, large cells which appeared to be more differentiated tended to stain well. Expressions of SSTR subtypes in normal tissues from the adrenal medulla and sympathetic ganglia were quite similar to those of GNs and GNBs, with the expression rates being 100% for SSTR-1, approximately 90% for SSTR-2, approximately 40% for SSTR-3, 100% for SSTR-4 and 0% for SSTR-5.
Table 5. .
IHC results: number of cases positive for SSTR subtype expressions classified by INPC category and prognostic group
| SSTR1 | SSTR2 | SSTR3 | SSTR4 | SSTR5 | |||||
|---|---|---|---|---|---|---|---|---|---|
| GNs (n=5) | 5/5 (100%) | 5/5 (100%) | 2/5 (40%) | 5/5 (100%) | 0/5 (0%) | ||||
| GNBs (n=4) | 4/4 (100%) | 4/4 (100%) | 2/4 (50%) | 4/4 (100%) | 0/4 (0%) | ||||
| NBs (n=54) | 36/54 (66.7%) | 44/54 (81.5%) | 5/54 (9.3%) | 20/54 (37.0%) | 0/54 (0%) | ||||
| Favorable (n=27) | 24/27 (88.9%) |
 ** ** |
25/27 (92.6%) |
 * * |
5/27 (18.5%) |
 * * |
16/27 (59.3%) |
 ** ** |
|
| Unfavorable (n=27) | 12/27 (44.4%) | 19/27 (70.4%) | 0/27 (0%) | 4/27 (14.8%) | |||||
| Total (n=63) | 45/63 (71.4%) | 53/63 (84.1%) | 9/63 (14.3%) | 29/63 (46.0%) | 0/63 (0%) |
** p<0.01, * p<0.05 with Chi-square test for comparison between the favorable and unfavorable histology groups.
Fig. 2. .
Semi-quantitative IHC evaluation of SSTR expressions in NTs (n=63). Immunoreactive scores for SSTR-1, 2, 3 and 4 classified by INPC category and prognostic group are shown as box plots. None of our cases had tumors expressing SSTR-5. Ganglioma (GN, n=5) and ganglioneuroblastoma (GNB, n=4) tended to have higher scores than neuroblastoma (NB) for SSTR-1, 3 and 4. The favorable histology group with NB (NB/F, n=28) had higher scores than the unfavorable histology NB group (NB/U, n=28) for SSTR-1, 2 and 4 and the differences were statistically significant. **p<0.01, *p<0.05 by Kruskal-Wallis test and the Steel multiple comparison procedure. The bottom of each box is the 25th percentile, the top is the 75th percentile and the line in the middle is the 50th percentile. Upper and lower horizontal lines are the 90th and 10th percentiles, respectively. Diamond shapes represent mean values.
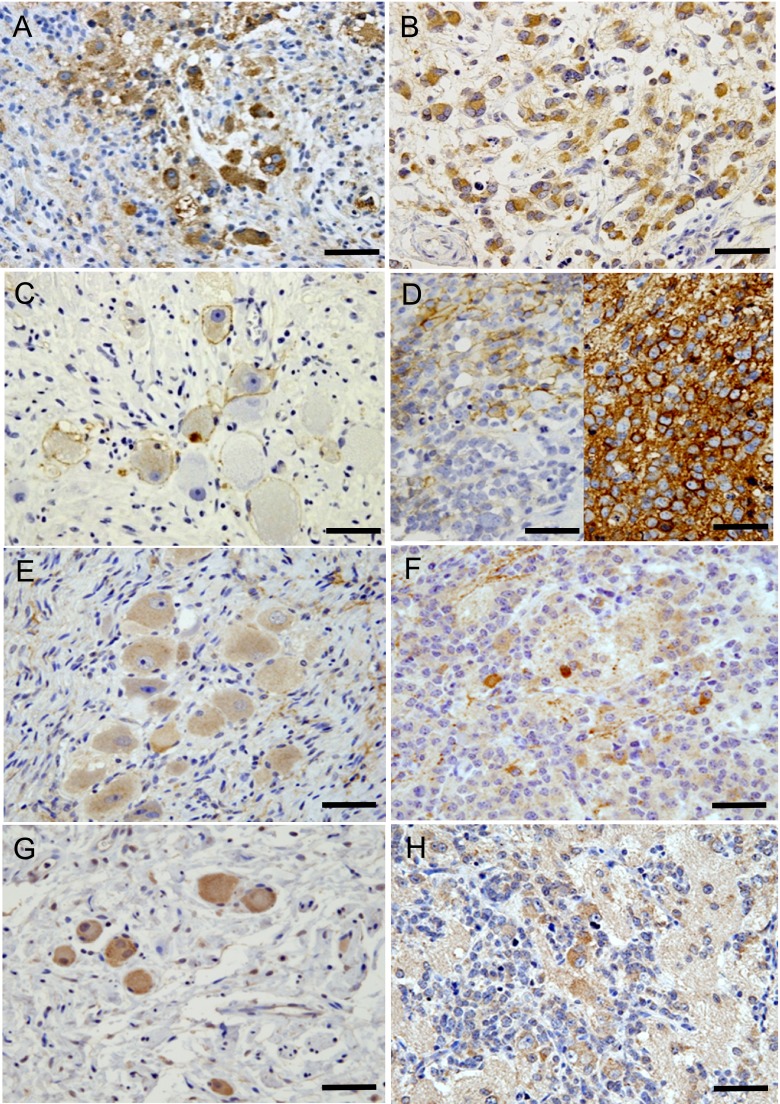
Fig. 3. .
SSTR expressions in NTs; representative cases. A) SSTR-1, GNB, intermixed tumor; strong positive staining identified in cytoplasm of most tumor cells, corresponding to an IRS of 12. B) SSTR-1, NB, favorable histology group; strong diffuse cytoplasmic staining, IRS 12. C) SSTR-2, GN; moderate positive staining in membranes of more than half of all tumor cells, corresponding to an IRS of 6. D) SSTR-2, NB, unfavorable histology group. Left photo: weak to moderate membranous staining in a portion of the tumor cells, given an IRS of 3. Right photo: different case; strong membranous staining in almost all tumor cells, IRS 12. Both cases are deceased. E) SSTR-3, GN; weak diffuse cytoplasmic staining, IRS 4. F) SSTR-3, NB, favorable histology group; moderate staining in scattered tumor cells, IRS 2. G) SSTR-4, GN; moderate diffuse cytoplasmic staining, IRS 8. H) SSTR-4, NB, favorable histology group; moderate positive staining in cytoplasm of more than half of tumor cells, IRS 6. Bars=50 μm.
IHC for somatostatin was performed on all 63 NTs and also the normal tissue specimens. Only 7 of the 63 cases with NTs showed focally positive IHC reactions for somatostatin. As to normal tissues, just one of the 8 adrenal medullas and none of the sympathetic ganglia showed focally positive IHC reactions for somatostatin.
Quantitative mRNA expression of SSTR subtypes and relationships with INPC
qRT-PCR analysis was performed in 37 NTs including 28 NBs, 4 GNBs and 5 GNs. Variable extents of mRNA expressions of SSTRs, other than SSTR-5, were observed. No cases showed expression of SSTR-5, which was consistent with the IHC results. No distinct tendencies were identified according to the INPC category, due to the expression of SSTRs in GNs and GNBs being relatively low. This was attributed to the mRNA levels of SSTRs being relative to the amounts of GAPDH in whole tissues, and tumor cells in GNs and GNBs are very sparse. When limited to NBs, analysis of each of the mRNA levels with corresponding IRS point totals revealed significant correlations, with a value of 0.71 (p<0.01) for SSTR-1, 0.51 (p<0.01) for SSTR-2, 0.40 (p<0.05) for SSTR-3 and 0.66 (p<0.01) for SSTR-4, as determined employing Spearman’s rank correlation analysis. In NBs, mRNA levels of SSTR-1, 2 and 4 tended to be higher in the favorable than in the unfavorable histology group (Fig. 4). Significant differences were, however, noted only in the expression of SSTR-1.
Fig. 4. .
Comparison of mRNA levels of SSTR-1, 2, 3 and 4 between the favorable (NB/F, n=14) and unfavorable (NB/U, n=14) NB histology groups by box plot. NB/F tended to express higher SSTR-1, 2 and 4 levels than NB/U but only the difference for SSTR-1 was statistically significant. The data were widely dispersed without a normal distribution. Thus, the Mann-Whitney U test was employed for analyses. The bottom of each box is the 25th percentile, the top is the 75th percentile and the line in the middle is the 50th percentile. Upper and lower horizontal lines are the 90th and 10th percentiles, respectively. Diamond shapes represent mean values.
SSTR expressions in surviving and deceased cases
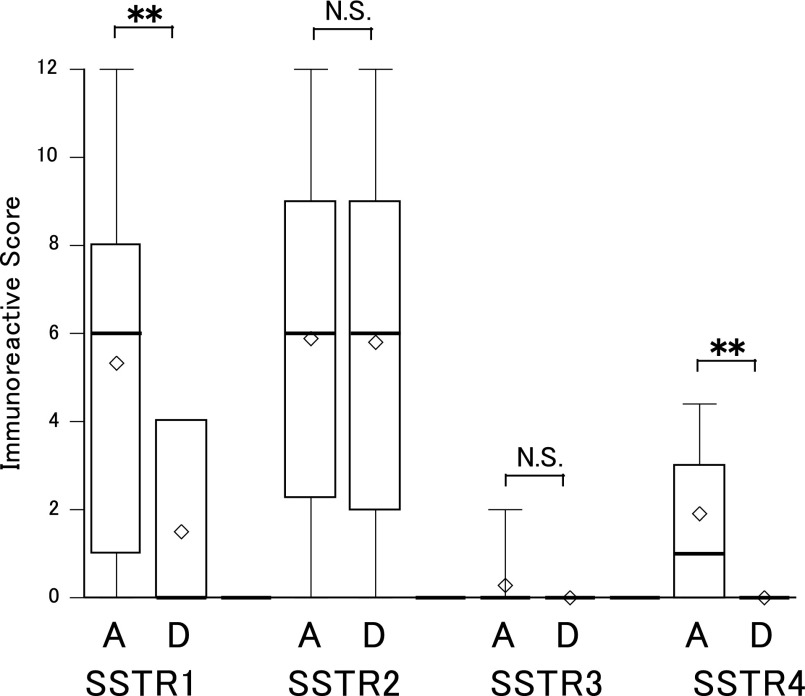
Ten deceased cases were included in this study. All who died had NBs and consisted of 1 case in the favorable and 9 in the unfavorable histology group. No expression of SSTR-3, 4 or 5 had been detected in any of the 10 patients who died. The expressions of SSTR-1 and 2 in the deceased cases were 40% (4/10) and 80% (8/10), respectively, by IHC. Semi-quantitative analysis of IHC results, as assessed by IRS, revealed the expressions of SSTR-1 and 4 to be significantly higher in the surviving cases, while SSTR-2 expression did not differ between the surviving and deceased cases (Fig. 5). mRNA expressions also tended to be higher in surviving cases for SSTR-1, and slightly higher for SSTR-2 and 4, but the differences did not reach statistical significance (Fig. 6). The data obtained from each of the deceased cases are shown in Table 6. The SSTR-2 expression levels varied widely, with some cases showing high expression of SSTR-2 (No. 29, 49 and 54). We investigated the relationships of morphological and clinical factors with the IRS point totals of these cases, but no specific features were identified.
Fig. 5. .
Immunoreactive scores for each SSTR of surviving and deceased cases with NBs. A: alive (n=43), D: dead (n=10), **p<0.01. Higher scores were obtained in surviving than in deceased cases and the differences were statistically significant for SSTR-1 and 4 by the Mann-Whitney U test. The difference in SSTR-2 between surviving and deceased cases was very small. The bottom of each box is the 25th percentile, the top is the 75th percentile and the line in the middle is the 50th percentile. Upper and lower horizontal lines are the 90th and 10th percentiles, respectively. Diamond shapes represent mean values.
Fig. 6. .
mRNA levels of each SSTR in surviving and deceased cases with NBs. A: alive (n=24), D: deceased (n=4). The SSTR-1 values tended to be higher for surviving than for deceased cases. Values were also slightly higher for SSTR-2 and 4 in surviving cases, though the differences did not reach statistical significance with the Mann-Whitney U test. The bottom of each box is the 25th percentile, the top is the 75th percentile and the line in the middle is the 50th percentile. Upper and lower horizontal lines are the 90th and 10th percentiles, respectively. Diamond shapes represents mean values.
Table 6. .
IRS points and mRNA levels of deceased cases
| No | IRS | mRNA level | somatostatin | Histology | MKI | MYCN amplification |
Duration of disease |
||
|---|---|---|---|---|---|---|---|---|---|
| SSTR1 | SSTR2 | SSTR1 | SSTR2 | ||||||
| 10 | 4 | 2 | nd | nd | – | NB, poorly, F | low | – | 1.5m |
| 29 | 0 | 9 | nd | nd | – | NB, poorly, U | high | + | 1.3y |
| 33 | 3 | 0 | 0.12 | 0 | – | NB, undiff, U | high | + | 6m |
| 40 | 0 | 0 | nd | nd | – | NB, poorly, U | high | – | 1.8y |
| 41 | 4 | 6 | nd | nd | + (50%) | NB, poorly, U | low | – | 4.3y |
| 44 | 0 | 6 | nd | nd | – | NB, poorly, U | high | + | 1.5y |
| 46 | 4 | 3 | 0.03 | 0.07 | – | NB, poorly, U | high | + | 4m |
| 49 | 0 | 12 | 0 | 0.18 | – | NB, poorly, U | high | – | 3.3y |
| 51 | 0 | 8 | nd | nd | – | NB, poorly, U | inter | – | 2m |
| 54 | 0 | 12 | 0 | 0.92 | – | NB, poorly, U | low | – | 3.3y |
Numbers correspond to those in Table 1. All cases were negative for SSTR-3, 4 and 5. The SSTR-2 expressions are well preserved in numbers 29, 49 and 54. IRS: Immunoreactive score, nd: not done, NB: neuroblastoma, poorly: poorly differentiated subtype, undiff: undifferentiated subtype, F: favorable histology group, U: unfavorable histology group, MKI: mitosis-karyorrhexis index.
IV. Discussion
In this study, we investigated the expressions of SSTR subtypes in NTs, and their relationships with tumor differentiation based on INPC criteria and patient outcomes. There have been only a few reports examining all known SSTR subtypes in human NB tissues. Albers et al. demonstrated decreasing expressions of SSTR-1, 2, 3, 4 and 5 as the tumor stage advanced [1]. Georgantzi et al. also examined the expressions of all of the SSTR subtypes by IHC [7]. Both reports showed high rates of SSTR-1 and 2 expressions despite the different techniques used. We examined the expressions of SSTR subtypes using formalin-fixed and paraffin-embedded tissues from NTs, and demonstrated relatively high expressions of SSTR-1 (71.4%) and SSTR-2 (84.1%), while nearly 50% of tissue samples showed SSTR-4. On the other hand, the expression of SSTR-3 was quite low, and no SSTR-5 expression was detected. These results support those of former reports demonstrating high SSTR-1 and 2 expression rates in human NB tissues.
The relationship between NBs and somatostatin has already been investigated, and the results suggested that somatostatin induces tumor differentiation [5, 11, 23]. However, somatostatin identification in NBs varies among reports. In our study, only 7 of 63 cases with NTs showed focal positivity for somatostatin. Albers et al. reported the growth of experimental tumors with transfected SSTR-1 and 2 to be inhibited, even in the absence of exogenous somatostatin [1]. Their observations suggest that SSTR might be upregulated by endogenous somatostatin alone, and that even limited expression of somatostatin might be sufficient for SSTRs to exert their effects.
To correlate the expressions of SSTR subtypes with tumor differentiation, we used the criteria established by the INPC tumor category and the prognostic group findings. SSTR-1, 2 and 4 expressions were found in all cases with GNBs and GNs. The semi-quantitative analysis of IHC results showed tendencies for higher expressions of SSTR-1, 3 and 4 in GNBs and GNs, as compared with NBs. These results suggest that expressions of multiple SSTRs might be related to morphological tumor differentiation. The differences were more striking when the prognostic groups were compared, and the expressions of SSTR-1, 2, 3 and 4 were significantly higher in the favorable than in the unfavorable histology group. For INPC the prognostic group is defined employing Shimada’s system, which is based on age and cellular turnover, or otherwise on morphological differentiation. From this standpoint, the expressions of SSTR subtypes may reflect not only morphological differentiation but also characteristics of functional differentiation. In this study, the analysis of mRNA levels showed no specific differences according to the INPC category. This was considered to be attributable to the extremely low cellularity of GNs and GNBs, as compared with NBs. When only NBs were analyzed, the correlations between semi-quantitative assessment employing IHC results and relative mRNA levels of SSTRs were in good accordance.
Previous clinicopathological studies indicated SSTR expression to be a favorable prognostic marker. Moertel et al. reported SSTR expression to correlate well with patient survival [18]. O’Dorisio et al. reported a good correlation between SSTR expression and tumor stages [19]. Raggi et al., using a competitive RT-PCR technique, showed higher SSTR-2 expression to correlate well with patient survival [2, 20, 24, 28]. All of these studies focused mostly on SSTR-2. In our study, expressions of SSTR-1, 2, 3 and 4 tended to be higher in the surviving than in the deceased cases, based on semi-quantitative analyses of both IHC and qRT-PCR results. In the deceased cases, the expressions were limited to SSTR-1 and 2 only. These results suggest that the expressions of SSTR subtypes may serve as favorable prognostic markers. However, there is a discrepant result regarding the expression of SSTR-2 as compared with previous reports. In our study, SSTR-2 expression was well preserved in some of the deceased cases. In other words, while the expressions of SSTR subtypes are generally decreased in NB cases with poor outcomes, SSTR-2 expression seems to be preserved in some of these cases. Thus, NB patients in the unfavorable histology group might benefit from molecular targeting therapy using somatostatin analogues, though whether to use such treatment would have to be determined on a case by case basis.
Somatostatin analogues have been used for acromegaly and certain endocrine tumors for more than two decades. In addition, molecular biological studies have revealed somatostatin analogues to exert antitumor activities in some neuroendocrine tumors. The antitumor activities of these somatostatin analogues include a direct anti-proliferative effect, that involves binding to the SSTRs of tumor cells, and also indirect anti-proliferative effects such as anti-angiogenesis [32]. Recently, peptide receptor radionuclide therapy, using radiolabeled somatostatin analogues, has been established for adult cases with neuroendocrine tumors expressing SSTRs. On the other hand, such treatments for childhood tumors with SSTR expression are still in the clinical trial stage [6, 10, 16]. Octreotide is a well-known representative somatostatin analogue, which has a high affinity for SSTRs, particularly SSTR-2. Against this background, most previous studies focused on SSTR-2, though new somatostatin analogues such as SOM230 with affinity for multiple SSTRs have been developed, and clinical trials using such agents are now underway [4, 33].
In this study, we demonstrated high expressions of SSTRs in NTs, particularly SSTR-1 and 2. High expressions of multiple SSTRs were shown to be present in well differentiated tumors associated with good patient outcomes. The patients with poor outcomes showed very limited SSTR expressions, though even in some of these cases the expression of SSTR-2 was rather high. Thus, the expressions of SSTRs can be used as prognostic markers, predicting a favorable outcome, and patients with NTs would seem to be good candidates for molecular targeting therapy using somatostatin analogues, even those in the unfavorable histology group with NBs expressing SSTRs.
V. Acknowledgments
We are grateful to all staff members of the Pathology Division, Nihon University Itabashi Hospital and National Center for Child Health and Development, for their assistance. This work was supported in part by a Grant from the “Strategic Research Base Development” Program for Private Universities subsidized by MEXT (2010) and by Grants-in-Aid from the National Center for Child Health and Development Fund (21-16).
VI. References
- 1.Albers A. R., O’Dorisio M. S., Balster D. A., Caprara M., Gosh P., Chen F., Hoeger C., Rivier J., Wenger G. D., O’Dorisio T. M. and Qualman S. J. (2000) Somatostatin receptor gene expression in neuroblastoma. Regul. Pept. 88; 61–73. [DOI] [PubMed] [Google Scholar]
- 2.Briganti V., Sestini R., Orlando C., Bernini G., La Cava G., Tamburini A., Raggi C. C., Serio M. and Maggi M. (1997) Imaging of somatostatin receptors by indium-111-pentetreotide correlates with quantitative determination of somatostatin receptor type 2 gene expression in neuroblastoma tumors. Clin. Cancer Res. 3; 2385–2391. [PubMed] [Google Scholar]
- 3.Brodeur G. M., Pritchard J., Berthold F., Carlsen N. L., Castel V., Castelberry R. P., De Bernardi B., Evans A. E., Favrot M., Hedborg F., Kaneko M., Kemshead J., Lampert F., Lee R. E., Look A. T., Pearson A. D., Philip T., Roald B., Sawada T., Seeger R. C., Tsuchida Y. and Voute P. A. (1993) Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J. Clin. Oncol. 11; 1466–1477. [DOI] [PubMed] [Google Scholar]
- 4.Bruns C., Lewis I., Briner U., Meno-Tetang G. and Weckbecker G. (2002) SOM230: a novel somatostatin peptidomimetic with broad somatotropin release inhibiting factor (SRIF) receptor binding and a unique antisecretory profile. Eur. J. Endocrinol. 146; 707–716. [DOI] [PubMed] [Google Scholar]
- 5.Busse E., Bartsch O. and Kornhuber B. (1991) Research on the differentiation of human and murine neuroblastoma cells. Oncology 48; 196–201. [DOI] [PubMed] [Google Scholar]
- 6.Gains J. E., Bomanji J. B., Fersht N. L., Sullivan T., D’Souza D., Sullivan K. P., Aldridge M., Waddington W. and Gaze M. N. (2011) 177Lu-DOTATATE molecular radiotherapy for childhood neuroblastoma. J. Nucl. Med. 52; 1041–1047. [DOI] [PubMed] [Google Scholar]
- 7.Georgantzi K., Tsolakis A. V., Stridsberg M., Jakobson Å., Christofferson R. and Janson E. T. (2011) Differentiated expression of somatostatin receptor subtypes in experimental models and clinical neuroblastoma. Pediatr. Blood Cancer 56; 584–589. [DOI] [PubMed] [Google Scholar]
- 8.Kaemmerer D., Peter L., Lupp A., Schulz S., Sänger J., Baum R. P., Prasad V. and Hommann M. (2012) Comparing of IRS and Her2 as immunohistochemical scoring schemes in gastroenteropancreatic neuroendocrine tumors. Int. J. Clin. Exp. Pathol. 5; 187–194. [PMC free article] [PubMed] [Google Scholar]
- 9.Kaemmerer D., Lupp A., Peter L., Fischer E., Schulz S., Klöppel G. and Hommann M. (2013) Correlation of monoclonal and polyclonal somatostatin receptor 5 antibodies in pancreatic neuroendocrine tumors. Int. J. Clin. Exp. Pathol. 6; 49–54. [PMC free article] [PubMed] [Google Scholar]
- 10.Khanna G., Bushnell D. and O’Dorisio M. S. (2008) Utility of radiolabeled somatostatin receptor analogues for staging/restaging and treatment of somatostatin receptor-positive pediatric tumors. Oncologist 13; 382–389. [DOI] [PubMed] [Google Scholar]
- 11.Kogner P., Borgström P., Bjellerup P., Schilling F. H., Refai E., Jonsson C., Dominici C., Wassberg E., Bihl H., Jacobsson H., Theodorsson E. and Hassan M. (1997) Somatostatin in neuroblastoma and ganglioneuroma. Eur. J. Cancer 33; 2084–2089. [DOI] [PubMed] [Google Scholar]
- 12.Krenning E. P., Kwekkeboom D. J., Bakker W. H., Breeman W. A. P., Kooij P. P. M., Oei H. Y., van Hagen M., Postema P. T. E., de Jong M., Reubi J. C., Visser T. J., Reijs A. E. M., Hofland L. J., Koper J. W. and Lamberts S. W. J. (1993) Somatostatin receptor scintigraphy with [111In-DTPA-d-Phe1]- and [123I-Tyr3]-octreotide: the Rotterdam experience with more than 1000 patients. Eur. J. Nucl. Med. 20; 716–731. [DOI] [PubMed] [Google Scholar]
- 13.Leja J., Yu D., Nilsson B., Gedda L., Zieba A., Hakkarainen T., Åkerström G., Öberg K., Giandomenico V. and Essand M. (2011) Oncolytic adenovirus modified with somatostatin motifs for selective infection of neuroendocrine tumor cells. Gene Ther. 18; 1052–1062. [DOI] [PubMed] [Google Scholar]
- 14.Macabeo-Ong M., Ginzinger D. G., Dekker N., McMillan A., Regezi J. A., Wong D. T. W. and Jordan R. C. K. (2002) Effect of duration of fixation on quantitative reverse transcription polymerase chain reaction analyses. Mod. Pathol. 15; 979–987. [DOI] [PubMed] [Google Scholar]
- 15.Maggi M., Baldi E., Finetti G., Franceschelli F., Brocchi A., Lanzillotti R., Serio M., Camboni M. G. and Thiele C. J. (1994) Identification, characterization, and biological activity of somatostatin receptors in human neuroblastoma cell lines. Cancer Res. 54; 124–133. [PubMed] [Google Scholar]
- 16.Menda Y., O’Dorisio M. S., Kao S., Khanna G., Michael S., Connolly M., Babich J., O’Dorisio T., Bushnell D. and Madsen M. (2010) Phase I trial of 90Y-DOTATOC therapy in children and young adults with refractory solid tumors that express somatostatin receptors. J. Nucl. Med. 51; 1524–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mizutani G., Nakanishi Y., Watanabe N., Honma T., Obana Y., Seki T., Ohni S. and Nemoto N. (2012) Expression of somatostatin receptor (SSTR) subtypes (SSTR-1, 2A, 3, 4 and 5) in neuroendocrine tumors using real-time RT-PCR method and immunohistochemistry. Acta Histochem. Cytochem. 45; 167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moertel C. L., Reubi J. C., Scheithauer B. S., Schaid D. J., Kvols L. K. and Scheithauter B. S. (1994) Expression of somatostatin receptors in childhood neuroblastoma. Am. J. Clin. Pathol. 102; 752–756. [DOI] [PubMed] [Google Scholar]
- 19.O’Dorisio M. S., Chen F., O’Dorisio T. M., Wray D. and Qualman S. J. (1994) Characterization of somatostatin receptors on human neuroblastoma tumors. Cell Growth Differ. 5; 1–8. [PubMed] [Google Scholar]
- 20.Orlando C., Raggi C. C., Bagnoni L., Sestini R., Briganti V., La Cava G., Bernini G., Tonini G. P., Pazzagli M., Serio M. and Maggi M. (2001) Somatostatin receptor type 2 gene expression in neuroblastoma, measured by competitive RT-PCR, is related to patient survival and to somatostatin receptor imaging by indium-111-pentetreotide. Med. Pediatr. Oncol. 36; 224–226. [DOI] [PubMed] [Google Scholar]
- 21.Patel Y. C. (1999) Somatostatin and its receptor family. Front. Neuroendocrinol. 20; 157–198. [DOI] [PubMed] [Google Scholar]
- 22.Pinzani P., Orlando C., Raggi C. C., Distante V., Valanzano R., Tricarico C., Maggi M., Serio M. and Pazzagli M. (2001) Type-2 somatostatin receptor mRNA levels in breast and colon cancer determined by a quantitative RT-PCR assay based on dual label fluorogenic probe and the TaqManTM technology. Regul. Pept. 99; 79–86. [DOI] [PubMed] [Google Scholar]
- 23.Qualman S. J., O’Dorisio M. S., Fleshman D. J., Shimada H. and O’Dorisio T. M. (1992) Neuroblastoma. Correlation of neuropeptide expression in tumor tissue with other prognostic factors. Cancer 70; 2005–2012. [DOI] [PubMed] [Google Scholar]
- 24.Raggi C. C., Maggi M., Renzi D., Calabrò A., Bagnoni M. L., Scaruffi P., Tonini G. P., Pazzagli M., De Bernardi B., Bernini G., Serio M. and Orlando C. (2000) Quantitative determination of sst2 gene expression in neuroblastoma tumor predicts patient outcome. J. Clin. Endorinol. Metab. 85; 3866–3873. [DOI] [PubMed] [Google Scholar]
- 25.Reubi J. C., Schaer J. C., Markwalder R., Waster B., Horisberger U. and Laissue J. (1997) Distribution of somatostatin receptors in normal and neoplastic human tissues: recent advances and potential relevance. Yale J. Biol. Med. 70; 471–479. [PMC free article] [PubMed] [Google Scholar]
- 26.Schmid H. A., Lambertini C., van Vugt H. H., Barzaghi-Rinaudo P., Schäfer J., Hillenbrand R., Sailer A. W., Kaufmann M. and Nuciforo P. (2012) Monoclonal antibodies against the human somatostatin receptor subtypes 1–5: development and immunohistochemical application in neuroendocrine tumors. Neuroendocrinology 95; 232–247. [DOI] [PubMed] [Google Scholar]
- 27.Schulz S., Schulz S., Schmitt J., Wiborny D., Schmidt H., Olbricht S., Weise W., Roessner A., Gramsch C. and Höllt V. (1998) Immunocytochemical detection of somatostatin receptors sst1, sst2A, sst2B, and sst3 in paraffin-embedded breast cancer tissue using subtype-specific antibodies. Clin. Cancer Res. 4; 2047–2052. [PubMed] [Google Scholar]
- 28.Sestini R., Orlando C., Peri A., Tricarico C., Pazzagli M., Serio M., Pagani A., Bussolati G., Granchi S. and Maggi M. (1996) Quantitation of somatostatin receptor type 2 gene expression in neuroblastoma cell lines and primary tumors using competitive reverse transcription-polymerase chain reaction. Clin. Cancer Res. 2; 1757–1765. [PubMed] [Google Scholar]
- 29.Shimada H., Ambros I. M., Dehner L. P., Hata J., Joshi V. V. and Roald B. (1999) Terminology and morphologic criteria of neuroblastic tumors: recommendations by the International Neuroblastoma Pathology Committee. Cancer 86; 349–363. [PubMed] [Google Scholar]
- 30.Shimada H., Umehara S., Monobe Y., Hachitanda Y., Nakagawa A., Goto S., Gerbing R. B., Stram D. O., Lukens J. N. and Matthay K. K. (2001) International neuroblastoma pathology classification for prognostic evaluation of patients with peripheral neuroblastic tumors: a report from the Children’s Cancer Group. Cancer 92; 2451–2461. [DOI] [PubMed] [Google Scholar]
- 31.Sun L. C., Mackey L. V., Luo J., Fuselier J. A. and Coy D. H. (2008) Targeted chemotherapy using a cytotoxic somatostatin conjugate to inhibit tumor growth and metastasis in nude mice. Clin. Med. Oncol. 2; 491–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Susini C. and Buscail L. (2006) Rational for the use of somatostatin analogs as antitumor agents. Ann. Oncol. 17; 1733–1742. [DOI] [PubMed] [Google Scholar]
- 33.Wolin E. M., Hu K., Hughes G., Bouillaud E., Giannone V. and Resendiz K. H. (2013) Safety, tolerability, pharmacokinetics, and pharmacodynamics of a long-acting release (LAR) formulation of pasireotide (SOM230) in patients with gastroenteropancreatic neuroendocrine tumors: results from a randomized, multicenter, open-label, phase I study. Cancer Chemother. Pharmacol. 72; 387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]