Abstract
A record number of 39 209 HSCT in 34 809 patients (14 950 allogeneic (43%) and 19 859 autologous (57%)) were reported by 658 centers in 48 countries to the 2013 survey. Trends include: more growth in allogeneic than in autologous HSCT, increasing use of sibling and unrelated donors and a pronounced increase in haploidentical family donors when compared with cord blood donors for those patients without a matched related or unrelated donor. Main indications were leukemias, 11 190 (32% 96% allogeneic); lymphoid neoplasias, 19 958 (57% 11% allogeneic); solid tumors, 1543 (4% 4% allogeneic); and nonmalignant disorders, 1975 (6% 91% allogeneic). In patients without a matched sibling or unrelated donor, alternative donors are used. Since 2010 there has been a marked increase of 96% in the number of transplants performed from haploidentical relatives (802 in 2010 to 1571 in 2013), whereas the number of unrelated cord blood transplants has slightly decreased (789 in 2010 to 666 in 2013). The use of donor type varies greatly throughout Europe.
Introduction
Hematopoietic SCT (HSCT) is an established procedure for many acquired and congenital disorders of the hematopoietic system, including disorders of the immune system, and as enzyme replacement in metabolic disorders.1, 2, 3, 4 The annual activity survey of the European Society of Blood and Marrow Transplantation (EBMT), describing the status of HSCT in Europe and affiliated countries, has become an instrument used to observe trends and to monitor changes in technology use.5, 6, 7, 8, 9, 10 The survey captures the numbers of HSCT performed in the preceding year from each participating team, divided by indication, donor type and stem cell source. The standardized structure of the survey over many years and the excellent commitment of the participating teams allow us to observe changes over time and to evaluate factors associated with these changes. More recently, the survey has included additional information on novel cell therapies with hematopoietic stem cells for non-hematopoietic use, as well as on the use of non-hematopoietic stem and progenitor cells.11 This coincides with the recent interest of the WHO (www.who.org) in cell and tissue transplants and further stresses the need for adequate and timely information.12 The analysis of the survey data spanning over 20 years has shown a continued and constant increase in the annual numbers of HSCT and transplant rates (number of HSCT per 10 million inhabitants) for both allogeneic and autologous HSCT.
This report is based on the 2013 survey data. In addition to transplant rates and indications, this report focuses on the use of donors other than HLA-identical siblings and matched unrelated donors for allogeneic HSCT.
Patients and Methods
Data collection and validation
Participating teams were invited to report data for 2013 by indication, stem cell source and donor type as listed in Table 1. The survey allows the possibility to report additional information on the numbers of subsequent transplants performed as a result of relapse, rejection or those that are part of a planned sequential transplant protocol. Supplementary information on the numbers of DLIs, reduced intensity HSCT and the numbers of pediatric HSCT is also collected. Quality control measures included several independent systems: confirmation of validity of the entered data by the reporting team, selective comparison of the survey data with MED-A data sets in the EBMT Registry database, cross-checking with the National Registries.
Table 1. Numbers of hematopoietic SCTs in Europe 2013 by indication, donor type and stem cell source.
|
Allogeneic |
Autologous |
Total |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Family |
Total family |
Unrelated |
Total unrelated | ||||||||||||||||
|
HLA identical |
Non-identical |
Twin |
BM | BM+ | |||||||||||||||
| BM | PB | Cord | BM | PB | Cord | BM | PB | BM | PB | cord | only | PB | cord | Allo | Auto | Total | |||
| Leukemias | 725 | 3140 | 15 | 332 | 557 | 2 | 5 | 24 | 4800 | 795 | 4675 | 420 | 5890 | 10 | 489 | 1 | 10 690 | 500 | 11 190 |
| AML | 309 | 1599 | 5 | 169 | 271 | 1 | 2 | 9 | 2365 | 315 | 2337 | 211 | 2863 | 7 | 373 | 0 | 5228 | 380 | 5608 |
| 1st CR | 232 | 1123 | 2 | 88 | 118 | 1 | 1 | 9 | 1574 | 209 | 1340 | 114 | 1663 | 6 | 313 | 0 | 3237 | 319 | 3556 |
| Not 1st CR | 77 | 476 | 3 | 81 | 153 | 0 | 1 | 0 | 791 | 106 | 997 | 97 | 1200 | 1 | 60 | 0 | 1991 | 61 | 2052 |
| Acute lymphatic leukemia | 276 | 688 | 9 | 86 | 185 | 1 | 2 | 8 | 1255 | 271 | 767 | 112 | 1150 | 3 | 72 | 0 | 2405 | 75 | 2480 |
| 1st CR | 148 | 486 | 3 | 41 | 77 | 1 | 1 | 6 | 763 | 141 | 477 | 60 | 678 | 0 | 57 | 0 | 1441 | 57 | 1498 |
| Not 1st CR | 128 | 202 | 6 | 45 | 108 | 0 | 1 | 2 | 492 | 130 | 290 | 52 | 472 | 3 | 15 | 0 | 964 | 18 | 982 |
| CML | 31 | 107 | 0 | 18 | 20 | 0 | 0 | 0 | 176 | 36 | 173 | 13 | 222 | 0 | 3 | 0 | 398 | 3 | 401 |
| Chronic phase | 20 | 51 | 0 | 6 | 6 | 0 | 0 | 0 | 83 | 21 | 65 | 5 | 91 | 0 | 0 | 0 | 174 | 0 | 174 |
| Not 1st chronic phase | 11 | 56 | 0 | 12 | 14 | 0 | 0 | 0 | 93 | 15 | 108 | 8 | 131 | 0 | 3 | 0 | 224 | 3 | 227 |
| MDS, MDS/MPN overlap | 93 | 476 | 1 | 42 | 69 | 0 | 1 | 6 | 688 | 134 | 882 | 58 | 1074 | 0 | 11 | 1 | 1762 | 12 | 1774 |
| MPN | 11 | 129 | 0 | 9 | 8 | 0 | 0 | 0 | 157 | 22 | 257 | 16 | 295 | 0 | 5 | 0 | 452 | 5 | 457 |
| CLL | 5 | 141 | 0 | 8 | 4 | 0 | 0 | 1 | 159 | 17 | 259 | 10 | 286 | 0 | 25 | 0 | 445 | 25 | 470 |
| Lymphoproliferative disorders | 94 | 721 | 0 | 80 | 111 | 0 | 1 | 17 | 1024 | 108 | 1097 | 55 | 1260 | 51 | 17 623 | 0 | 2284 | 17 674 | 19 958 |
| Plasma cell disorders—MM | 18 | 220 | 0 | 6 | 16 | 0 | 1 | 10 | 271 | 29 | 283 | 3 | 315 | 4 | 9532 | 0 | 586 | 9536 | 10 122 |
| Plasma cell disorders—other | 1 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 0 | 12 | 1 | 13 | 2 | 256 | 0 | 20 | 258 | 278 |
| Hodgkin's lymphoma | 14 | 102 | 0 | 34 | 55 | 0 | 0 | 1 | 206 | 20 | 161 | 23 | 204 | 21 | 1859 | 0 | 410 | 1880 | 2290 |
| Non-Hodgkin lymphoma | 61 | 393 | 0 | 40 | 40 | 0 | 0 | 6 | 540 | 59 | 641 | 28 | 728 | 24 | 5976 | 0 | 1268 | 6000 | 7268 |
| Solid tumors | 2 | 5 | 0 | 4 | 34 | 0 | 0 | 0 | 45 | 7 | 8 | 0 | 15 | 54 | 1428 | 1 | 60 | 1483 | 1543 |
| Neuroblastoma | 0 | 2 | 0 | 2 | 26 | 0 | 0 | 0 | 30 | 2 | 5 | 0 | 7 | 22 | 464 | 1 | 37 | 487 | 524 |
| Soft tissue sarcoma | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 3 | 1 | 0 | 0 | 1 | 5 | 19 | 0 | 4 | 24 | 28 |
| Germinal tumors | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 353 | 0 | 0 | 355 | 355 |
| Breast cancer | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 42 | 0 | 1 | 42 | 43 |
| Ewing | 1 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 4 | 1 | 1 | 0 | 2 | 15 | 200 | 0 | 6 | 215 | 221 |
| Other solid tumors | 1 | 2 | 0 | 1 | 3 | 0 | 0 | 0 | 7 | 3 | 2 | 0 | 5 | 10 | 350 | 0 | 12 | 360 | 372 |
| Non malignant disorders | 601 | 227 | 47 | 86 | 111 | 1 | 1 | 3 | 1077 | 355 | 245 | 119 | 719 | 4 | 175 | 0 | 1796 | 179 | 1975 |
| BM failure—SAA | 165 | 124 | 4 | 13 | 20 | 0 | 1 | 3 | 330 | 117 | 84 | 19 | 220 | 0 | 0 | 0 | 550 | 0 | 550 |
| BM failure—other | 72 | 17 | 8 | 17 | 9 | 1 | 0 | 0 | 124 | 51 | 37 | 10 | 98 | 0 | 1 | 0 | 222 | 1 | 223 |
| Hemoglobinopathies—thalassemia | 161 | 55 | 17 | 19 | 8 | 0 | 0 | 0 | 260 | 27 | 13 | 1 | 41 | 0 | 1 | 0 | 301 | 1 | 302 |
| Hemoglobinopathies—other | 55 | 7 | 5 | 5 | 3 | 0 | 0 | 0 | 75 | 7 | 1 | 0 | 8 | 0 | 0 | 0 | 83 | 0 | 83 |
| Primary Immune deficiencies | 120 | 18 | 10 | 28 | 56 | 0 | 0 | 0 | 232 | 118 | 89 | 57 | 264 | 2 | 2 | 0 | 496 | 4 | 500 |
| Inherited disorders of metabolism | 25 | 6 | 1 | 3 | 11 | 0 | 0 | 0 | 46 | 28 | 16 | 31 | 75 | 1 | 1 | 0 | 121 | 2 | 123 |
| Auto immune disease | 3 | 0 | 2 | 1 | 4 | 0 | 0 | 0 | 10 | 7 | 5 | 1 | 13 | 1 | 170 | 0 | 23 | 171 | 194 |
| Others | 23 | 20 | 0 | 5 | 7 | 0 | 0 | 0 | 55 | 27 | 26 | 12 | 65 | 0 | 23 | 0 | 120 | 23 | 143 |
| Total patients | 1445 | 4113 | 62 | 507 | 820 | 3 | 7 | 44 | 7001 | 1292 | 6051 | 606 | 7949 | 119 | 19 738 | 2 | 14 950 | 19 859 | 34 809 |
| Retransplants | 73 | 265 | 2 | 54 | 165 | 2 | 1 | 5 | 567 | 69 | 468 | 58 | 595 | 5 | 1543 | 1162 | 1548 | 2710 | |
| Additional transplants | 11 | 25 | 13 | 7 | 56 | 41 | 2 | 43 | 5 | 1586 | 99 | 1591 | 1690 | ||||||
| Total ALL transplants | 1529 | 4403 | 64 | 574 | 992 | 5 | 8 | 49 | 7624 | 1361 | 6560 | 666 | 8587 | 129 | 22 867 | 2 | 16 211 | 22 998 | 39 209 |
| Pediatric transplants | 878 | 241 | 62 | 166 | 292 | 1 | 2 | 6 | 1648 | 728 | 475 | 254 | 1457 | 65 | 1064 | 2 | 3105 | 1131 | 4236 |
Abbreviations: MDS=myelodysplastic; MPN=myeloproliferative neoplasm; PBSC=peripheral blood; SAA=severe aplastic anemia.
Teams
Six hundred and eighty-seven centers from 48 countries were contacted for the 2013 survey (39 European and 9 affiliated countries); of which 658 teams reported. This corresponds to a 96% return rate and includes 551 active EBMT member teams. Twenty nine teams failed to report in 2013.
Contacted teams are listed in the Supplementary appendix in alphabetical order by country, city, EBMT centre code, with their reported numbers of first and total HSCT, and of first allogeneic and autologous HSCT. The WHO regional office definitions (www.who.org) were used to classify countries as European or Non-European. Nine non-European countries participated in the 2013 EBMT survey: Algeria, Iran, Israel, Jordan, Lebanon, Nigeria, Saudi Arabia, South Africa and Tunisia. Their data from 26 actively transplanting teams make up 6.2% of the total data set and is included in all analyses.
Definitions
Patient and transplant numbers. Wherever appropriate, patient numbers corresponding to the number of patients receiving a first transplant and transplant numbers reflecting the total number of transplants performed are listed.
The term sibling donor includes HLA-identical siblings and twins but not siblings with HLA mismatches. Unrelated donor transplants includes HSCT from unrelated donors with PB and bone marrow as a stem cell source but not cord blood HSCT, these are shown as cord blood HSCT in Figures 3. Mismatched family donors are termed ‘haploidentical' for the purpose of this analysis but this category includes also mismatched related donors that are mismatched to a lesser degree than a full haplotype. As the haplotype mismatched donors are the vast majority in this category, the term ‘haploidentical' is used for the entire group.
Multiple transplants may include multiple transplants defined as subsequent transplants within a planned double or triple autologous or allogeneic transplant protocol, and retransplants (autologous or allogeneic) defined as unplanned HSCT for rejection or relapse after a previous HSCT.
Information on additional cellular therapies was subdivided into: HSC for non-hematopoietic use; non-hematopoietic stem cell therapies; MSC therapies for rejection or GVHD prevention/treatment; and DLIs. Collection of information was validated by cross-checking with a similar more detailed survey carried out by TERMIS-EU (Tissue Engineering and Regenerative Medicine International Society; www.termis.org), EULAR (European League against Rheumatism; www.eular.org), ICRS-EU (International Cartilage Repair Society; www.cartilage.org) and ISCT (International Society of Cellular Therapy; www.celltherapysociety.org).11
Transplant rates. Transplant rates, defined as the total number of HSCT per 10 million inhabitants, were computed for each country without adjustments for patients who crossed borders and received their HSCT in a foreign country. Population numbers were obtained from Eurostats for the European countries (http://epp.eurostat.ec.europa.eu/portal/page/portal/statistics/search_database) and the US census bureau database for the non-European countries (http://www.census.gov/population/international/data/idb/rank.php).
Analysis. Wherever appropriate, absolute numbers of transplanted patients, transplants or transplant rates are shown for specific countries, indications or transplant techniques.
Results
2013 data
Participating teams in 2013. Of the 658 teams, 406 (62%) performed both allogeneic and autologous transplants; 225 (34%) restricted their activity to autologous HSCT only, and 17 teams (3%) to allogeneic transplants only. Ten teams (1%) reported having performed no transplants in 2013 owing to renovation or temporary closure of the transplant unit. Of the 648 active centers, 120 (19%) centers performed transplants on both adult and pediatric patients. An additional 105 (16%) centers were dedicated pediatric transplant centers, and 423 (65%) centers performed transplants on adults only.
Numbers of patients and transplants. A total of 34 809 patients received their first transplant in 2013. Of these, 14 950 (43%) were allogeneic and 19 859 (57%) autologous. When compared with 2012, the total number of patients transplanted increased by 3.4% (5.5% allogeneic HSCT and 1.8% autologous HSCT).10 Furthermore, there were 2710 retransplants (1162 allogeneic and 1548 autologous) and 1690 multiple transplants (99 allogeneic and 1591 autologous), bringing the total to 39 209 HSCT procedures, 16 211 allogeneic (41%) and 22 998 autologous (59%) performed in 2013, which is an increase of 26% compared with 5 years and 88% compared with 15 years previously.
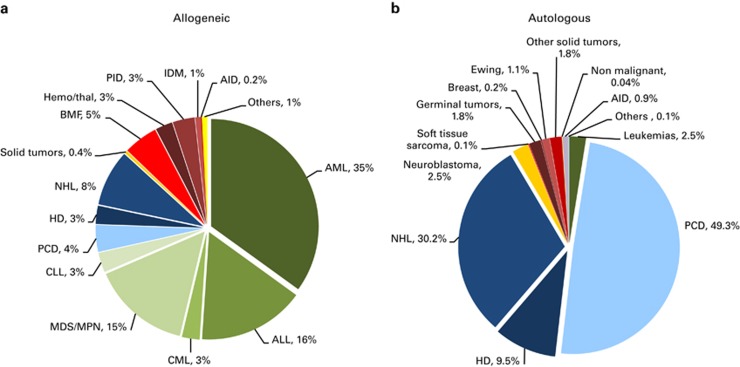
Indications for HSCT in 2013 are listed in detail in Table 1. The main indications were leukemias; 11 190 (32% of total; 96% of which were allogeneic); lymphoid neoplasias including Non-Hodgkin lymphoma, Hodgkin lymphoma and plasma cell disorders, 19 958 (57% 11% allogeneic); solid tumors, 1543 (4% 4% allogeneic); and nonmalignant disorders, 1975 (6% 91% allogeneic). As seen in previous years, the majority of HSCT for lymphoid malignancies were autologous, whereas most transplants for leukemia were performed using stem cells from allogeneic donors. Autologous HSCT for nonmalignant disorders predominantly include patients with autoimmune disorders.
Distributions of indications for HSCT are shown in Figures 1a and b for allogeneic and autologous HSCT, respectively. Compared with 2012, there were increases in allogeneic HSCT for AML in CR1 (10.7%), MPN (11.1%) and NHL (12.5%). For autologous HSCT, there was a decrease in activity for AML (18%) and HD (10%) but an increase for plasma cell disorders by 6.1%.
Figure 1.
Relative proportions of indications for an HSCT in Europe in 2013. (a) Proportions of disease indications for an allogeneic HSCT in Europe in 2013. (b) Proportions of disease indications for an autologous HSCT in Europe in 2013.
DLIs. 2513 patients received treatment with DLIs, a 12% increase since 2012.
Reduced intensity conditioning. 6534 of the total allogeneic HSCT were performed using non myeloablative conditioning. This is an increase of 11.4% since 2012 and is 40% of all allogeneic HSCT.
Fifteen year trends
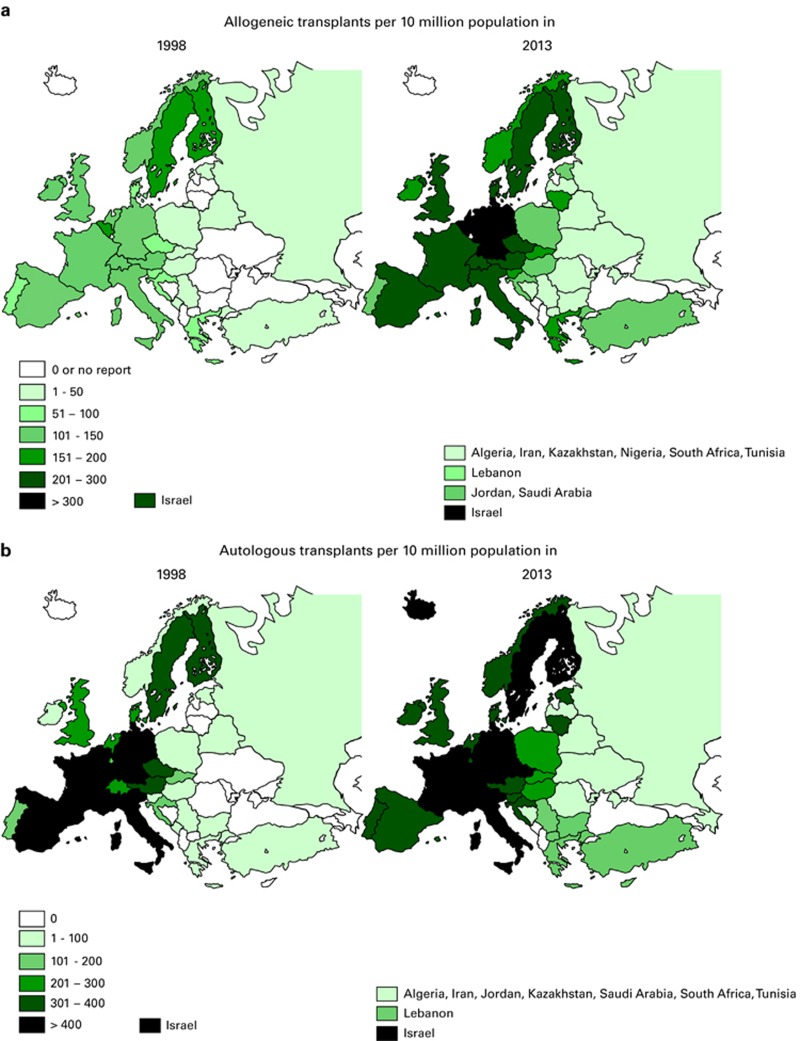
Figures 2a and b show transplant rates by country for allogeneic and autologous HSCT comparing rates in 2013 to rates 15 years ago, to 1998. Median transplant rates per 10 million inhabitants were 124 (range, 0.1–493) for allogeneic HSCT and 233 (range, 1.0–538) for autologous HSCT in 2013 as compared with 89 and 164 in 1998.
Figure 2.
Transplant rates in Europe (= total number of HSCT per 10 million inhabitants) by participating country, showing 15-year trends 1998–2013. (a) Allogeneic transplant rates per 10 million population in 1998–2013 (b) Autologous transplant rates per 10 million population in 1998–2013.
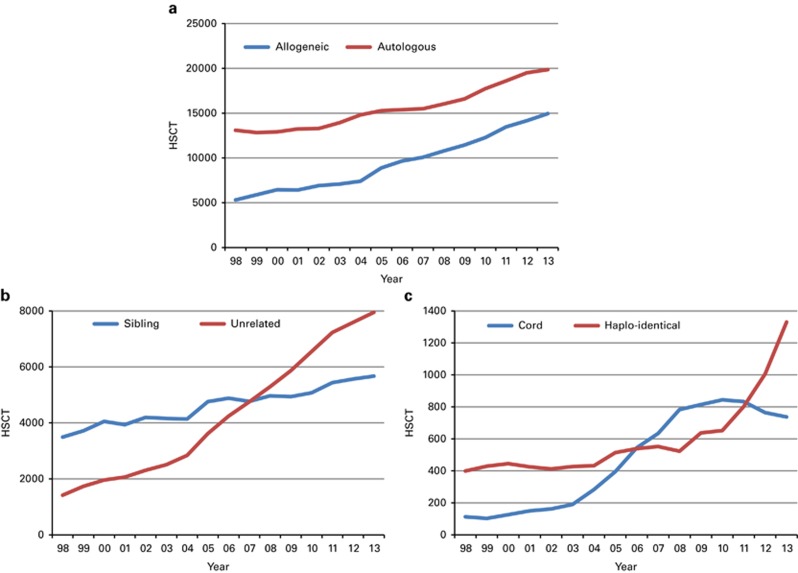
Figure 3a shows the 15-year trends for allogeneic and autologous HSCT showing some narrowing in the difference between autologous and allogeneic HSCT performed.
Figure 3.
Absolute numbers by transplant and donor type 1998–2013. (a) Absolute numbers of allogeneic and autologous HSCT in Europe in 1998–2013. (b) Absolute numbers of sibling donor and unrelated donor HSCT in Europe 1998–2013. (c) Absolute numbers of haploidentical and cord blood HSCT in Europe 1998–2013.
Figure 3b shows trends for allogeneic HSCT over the past 15 years for sibling donor and unrelated donor transplantation, with more unrelated donor HSCT since 2007 as compared with sibling donor HSCT, unrelated donor HSCT accounting for (53% of all allogeneic HSCT)% in 2013.13 Figure 3c shows trends in the use of alternative donor transplantation, separately for cord blood and for haploidentical family donor HSCT. It is obvious that the use of cord blood has stabilized in 2011 and is going down slightly; the number of haploidentical HSCT has more than doubled since 2010. For haploidentical HSCT, marrow is used in 37% and PB in 63% as a stem cell source14, 15 in 2013.
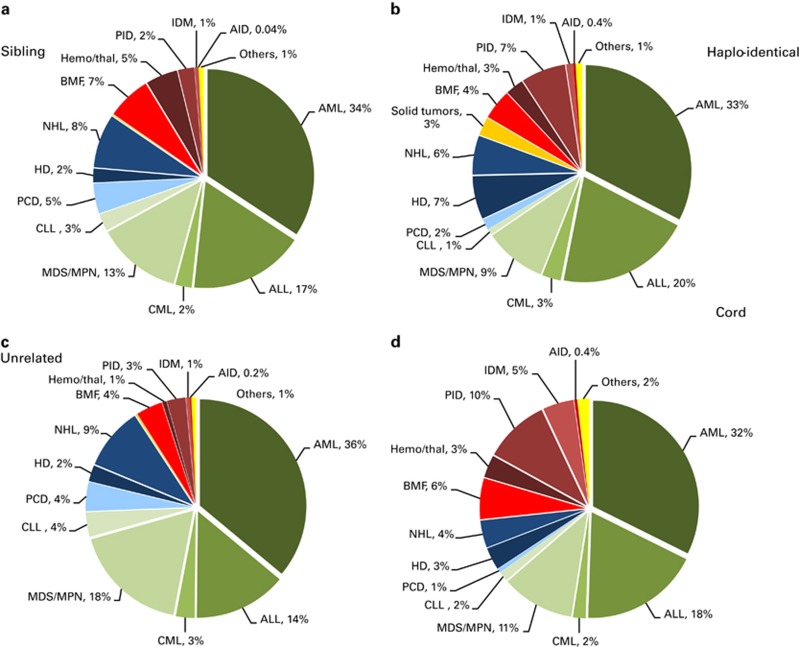
Figures 4a–d depict the indications for allogeneic HSCT separately for sibling donor, unrelated donor, cord blood donor and haploidentical donor HSCT in 2013. When comparing leukemias, lymphoid neoplasias and nonmalignant disorders, distributions of indications do not differ greatly; except for nonmalignant disorders receiving more commonly cord blood transplants, reflecting the younger age of these patients and the preference for cord blood in children.
Figure 4.
Disease indications by donor type in 2013. (a) Proportions of disease indications in 2013 for sibling donor HSCT. (b) Proportions of disease indications in 2013 for haplo-identical donor HSCT. (c) Proportions of disease indications in 2013 for unrelated donor HSCT. (d) Proportions of disease indications in 2013 for cord blood HSCT.
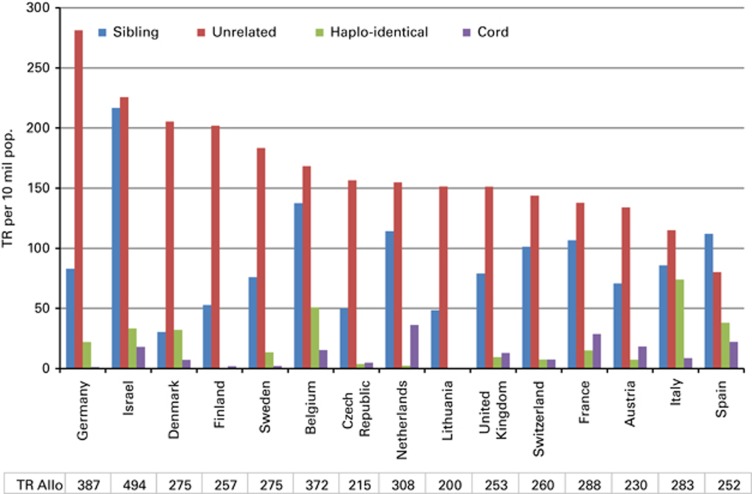
Figure 5 shows transplant rates for the 15 countries with the highest rates of allogeneic HSCT in Europe ordered by decreasing rate of unrelated donor HSCT. This figure shows considerable heterogeneity in the use of HSCT technology among countries.
Figure 5.
Transplant rates for sibling donor, unrelated donor, haploidentical donor and cord blood HSCT in Europe in 2013 for the 15 countries with the highest transplant rates.
Additional cellular therapies
Seventeen teams from 11 countries reported having treated 130 patients with hematopoietic stem cells for non-hematopoietic use in 2013. All therapies were performed using autologous HSC's. The main indications were cardiovascular, 75; neurological, 32; tissue repair, 20; and epithelial, 3. In addition, 405 patients in 86 teams and 21 countries received mesenchymal stromal cells for prevention/treatment of GVHD (344), prevention/treatment of graft failure (34) and for unspecified reasons (27).11
Discussion
The EBMT activity survey has been conducted annually since 1990.6 The 2010 survey reported for the first time in more than 30 000 patients transplanted in a given year.16 This trend continues with an additional increase by 3.6% in 2013, suggesting that HSCT remains an increasingly important treatment modality in the era of targeted antibody and molecular therapy. The present 2013 report focuses on allogeneic transplants using different types of donors.
HSCT for some indications continues to increase but not for others. Of interest is growth of allogeneic HSCT for AML in CR1, MPN and lymphoma. For autologous HSCT, the number of transplants for myeloma continues to increase. In autologous HSCT, the numbers of procedures for AML in CR1 and for Hodgkin's lymphoma dropped slightly. The decrease in autologous HSCT for Hodgkins Lymphoma of 10% may be related to the availability of monoclonal antibodies in this disease.
Notable in this year's survey is the increase in the use of allogeneic HSCT more than autologous HSCT and the increasing use of alternative donor transplants, where an impressive trend for more haploidentical HSCT has been observed. The category of haploidentical HSCT as used for this analysis includes haploidentical HSCTs as well as mismatched family donor HSCTs, where the mismatch does not include a full haplotype, for example, 1 or 2 allele mismatched relatives. Unfortunately we do not have the data to separate these categories but we think it is unlikely that the increase observed could be explained by one allele mismatched siblings. The increase in haploidentical HSCT coincides with the publications of the post-transplant CY GVHD prophylaxis in haploidentical HSCT.14 Again we do not have the data within this survey to separate haploidentical HSCTs using this strategy from other ways to perform haploidentical HSCT.
This is accompanied by a slight decrease in HSCT using cord blood pointing to the fact that mismatched unrelated cord blood and haploidentical donors are in competition for patients in whom no sibling or matched unrelated donor has been identified.14, 5, 17
When comparing the use of donors for allogeneic HSCT in countries with high transplant rates, it is obvious that there are important differences. Some may be explained by availability of sibling donors as there are differences in family size across Europe. There are, however, threefold differences in transplant rates for sibling and unrelated donor HSCT among countries and even larger differences in the use of unrelated cord blood and haploidentical donors probably reflecting availability, financial issues as well as differences in the interpretation of results of recent studies and local experience.
Overall, this paper reflects current practice, and results may be useful to health care planning and health policy makers.
Acknowledgments
The cooperation of all participating teams and their staff (listed in the Appendix), the EBMT Co-ordination office; Barcelona, Paris, London (C Ruiz de Elvira), the Austrian Registry (ASCTR) (H Greinix, B Lindner, CWagner), the Belgium Registry (Yves Beguin, M Van Spauwen) the Czech Registry (P Zak, M Trnkova), the French Registry (SFGM) (N Milpied, N Raus ), the German Registry (DRST) (H Ottinger, K Fuchs, C Müller, H Neidlinger, F Strehle), the Italian Registry (GITMO) (A Rambaldi, B Bruno, A Camboni), the Dutch Registry (JJ. Cornelissen, M Groenendijk), the Spanish Registry (GETH) (J Diez Martin, A Cedillo), the Swiss Registry (SBST) (U Schanz, H Baldomero), the Turkish Registry (G Gurman, M Arat) and the British Registry (BSBMT) (K Kirkland, J Perry) is greatly appreciated. We also thank D John for database support. EBMT is supported by grants from the corporate sponsors: Gentium S.p.A, Molmed S.p.A, AstellasPharma Europe Ltd, Celgene International SARL, Clinigen Group Ltd, Gilead Sciences Europe Ltd, GlaxoSmithKline plc, Hospira Inc., Medac Hematology GmbH, MiltenyiBiotec GmbH, MSD Sharp&Dohme GmbH, Neovii Biotech GmbH, Sanofi Oncology, Takeda, Terumo BCT, Therakos Photopheresis, Alexion, Amgen Oncology GmbH, Exem Consulting SA, Kiadispharma, Macropharma, Mundipharma and Pierre Fabre Médicament SAS.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Bone Marrow Transplantation website (http://www.nature.com/bmt)
Supplementary Material
References
- Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- Appelbaum FR. Hematopoietic-cell transplantation at 50. N Engl J Med. 2007;357:1472–1475. doi: 10.1056/NEJMp078166. [DOI] [PubMed] [Google Scholar]
- Ljungman P, Bregni M, Brune M, Cornelissen J, deWitte T, Dini G, et al. European Group for Blood and Marrow. Allogeneic and autologous transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe 2009. Bone Marrow Transplant. 2010;45:219–234. doi: 10.1038/bmt.2009.141. [DOI] [PubMed] [Google Scholar]
- Gratwohl A, Baldomero H, Aljurf M, Pasquini MC, Bouzas LF, Yoshimi A, et al. Hematopoietic stem cell transplantation: a global perspective. JAMA. 2010;303:1617–1624. doi: 10.1001/jama.2010.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratwohl A, Baldomero H, Schwendener A, Gratwohl M, Apperley J, Frauendorfer K, et al. The EBMT activity survey 2008 impact of team size, team density and new trends. Bone Marrow Transplant. 2011;46:174–191. doi: 10.1038/bmt.2010.69. [DOI] [PubMed] [Google Scholar]
- Gratwohl A. Bone marrow transplantation activity in Europe 1990. Report from the European Group for. Bone Marrow Transplantation (EBMT) Bone Marrow Transplant. 1991;8:197–201. [PubMed] [Google Scholar]
- Gratwohl A, Baldomero H, Horisberger B, Schmid C, Passweg J, Urbano-Ispizua A, Accreditation Committee of the European Group for Blood and Marrow Transplantation (EBMT) Current trends in haematopoietic stem cell transplantation in Europe. Blood. 2002;100:2374–2386. doi: 10.1182/blood-2002-03-0675. [DOI] [PubMed] [Google Scholar]
- Gratwohl A, Baldomero H, Schwendener A, Rocha V, Apperley J, Frauendorfer K, et al. The EBMT activity survey 2007 with focus on allogeneic HSCT for AML and novel cellular therapies. Bone Marrow Transplant. 2009;43:275–291. doi: 10.1038/bmt.2009.7. [DOI] [PubMed] [Google Scholar]
- Gratwohl A, Schwendener A, Baldomero H, Gratwohl M, Apperley J, Niederwieser D, et al. Changes in use of hematopoietic stem cell transplantation; a model for diffusion of medical technology. Haematologica. 2010;95:637–643. doi: 10.3324/haematol.2009.015586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passweg JR, Baldomero H, Peters C, Gaspar HB, Cesaro S, Dreger P, et al. Hematopoietic SCT in Europe: data and trends in 2012 with special consideration of pediatric transplantation. Bone Marrow Transplant. 2014;49:744–750. doi: 10.1038/bmt.2014.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin I, Ireland H, Baldomero H, Passweg JR.The survey on cellular and engineered tissue therapies in Europe in 2012 Tissue Eng Part A 2014(e-pub ahead of print). [DOI] [PMC free article] [PubMed]
- World Health Organisation, WHO( ( http://www.who.int/topics/transplantation/en/ ).
- Foeken LM, Green A, Hurley CK, Marry E, Wiegand T, Oudshoorn M. Monitoring the international use of unrelated donors for transplantation: the WMDA annual reports. Bone Marrow Transplant. 2010;45:811–818. doi: 10.1038/bmt.2010.9. [DOI] [PubMed] [Google Scholar]
- Brunstein CG, Fuchs EJ, Carter SL, Karanes C, Costa LJ, Wu J, et al. Blood and Marrow Transplant Clinical Trials Network. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011;118:282–288. doi: 10.1182/blood-2011-03-344853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luznik L, O'Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passweg JR, Baldomero H, Gratwohl A, Bregni M, Cesaro S, Dreger P, et al. The EBMT activity survey: 1990-2010. Bone Marrow Transplant. 2012;47:906–923. doi: 10.1038/bmt.2012.66. [DOI] [PubMed] [Google Scholar]
- Eapen M, O'Donnell P, Brunstein CG, Wu J, Barowski K, Mendizabal A, et al. Mismatched related and unrelated donors for allogeneic hematopoietic cell transplantation for adults with hematologic malignancies. Biol Blood Marrow Transplant. 2014;20:1485–1492. doi: 10.1016/j.bbmt.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.