Abstract
Spontaneous regression (SR) of human melanoma is a rare, well-documented phenomenon that is not still fully understood. Its detailed study cannot be performed in patients due to ethical reasons. Using the Melanoma-bearing Libechov Minipig (MeLiM) animals of various ages (from 3 weeks to 8 months) we implemented a long-term monitoring of melanoma growth and SR. We focused on immunohistochemical detection of two important extracellular matrix proteins, collagen IV and laminin, which are associated with cancer. We showed that SR of melanoma is a highly dynamic process. The expression of collagen IV and laminin correlated with changes in population of melanoma cells. Tumours of 3-week-old animals consisted primarily of melanoma cells with a granular expression of collagen IV and laminin around them. Thereafter, melanoma cells were gradually destroyed and tumour tissue was rebuilt into the connective tissue. Collagen IV expression slightly increased in tumours of 10-week-old pigs showing extracellular fibrous appearance. In tumours of older animals, areas lacking melanoma cells demonstrated a low expression and areas still containing melanoma cells a high expression of both proteins. We considered the age of 10 weeks as a turning point in the transition between tumour growth and SR of the MeLiM melanoma.
Keywords: collagen IV, laminin, MeLiM, porcine melanoma, spontaneous regression
I. Introduction
Melanoma is the third most common skin malignancy [5], with increasing incidence in the white population during the last decade. Tumours arise by neoplastic transformation of melanocytes. Advanced metastatic melanoma is a disease with a poor prognosis. Median survival of patients is 8.5 months and 5-year survival rate is lower than 5% [35]. In some patients, spontaneous regression (SR) of melanoma is observed. This phenomenon means a partial or complete disappearance of the melanoma cells without any therapeutic intervention. It is related to the ability of melanoma to induce immune response due to recognition of tumour-specific or tumour-associated antigens [11, 36]. Partial SR of melanoma is described in 10% to 35% of all cases. On the contrary, complete SR of melanoma is a very rare event with only around 40 well-documented cases up to now [17, 32]. A thorough understanding of the mechanisms responsible for SR may contribute to creation of new strategies for melanoma therapy. It is clear that particular studies in this field need suitable animal models because they cannot be done in human patients for ethical reasons.
Applying selective breeding in the herd of miniature pigs kept at the Institute of Animal Physiology and Genetics, AS CR, v.v.i in Libechov (Czech Republic) we established an original animal model known as the MeLiM (Melanoma-bearing Libechov Minipig) strain with hereditary melanoma [22]. Cutaneous tumours are usually nodular, multiple and distributed on various parts of the body. They appear in darkly pigmented animals already at birth or during two months thereafter (57% of all animals). Numerous organ metastases mainly into the spleen, lymph nodes, and lungs are regularly present in animals with cutaneous melanoma. About 70% of all affected piglets show a complete SR of melanoma. Remaining animals die for melanoma progression [13, 22]. Thus, minipigs of the MeLiM strain can be used for temporal studies of various aspects of melanoma SR and also for development of new therapeutic procedures.
Reorganization of tumour structure in relationship to supporting framework, i.e. extracellular matrix (ECM), is the main process associated with SR. ECM is a special network composed of collagens, elastin, proteoglycans, and non-collagenous glycoproteins. Various biological processes, such as differentiation or gene expression, are regulated through ECM-cell contacts [25]. In tumour tissues, ECM composition influences tumour growth and metastatic activity [30, 40]. Substantial ECM components are collagens with collagen IV playing an important role. Collagen IV is the major basement membrane protein [38]. It has been demonstrated that collagen IV influences adhesion, proliferation, and migration of melanoma cells. Moreover, collagen IV is involved in tumor angiogenesis, which may be related to tumour proliferation [37]. Laminin is another important ECM component of the basement membrane [38]. Its upregulation is associated with cell signaling, differentiation, and adhesion. Pathologically-increased laminin expression is related to cell migration and formation of metastases [45].
The purpose of this study was to outline the roles of collagen IV and laminin during growth and SR of melanoma. Such a study requires long-term monitoring that cannot be done in humans for ethical reasons. Miniature pigs of the MeLiM strain is a unique model that enables detailed monitoring. In animals at the age of 3 weeks to 8 months, we immunohistochemically analysed changes in the expression and localization of collagen IV and laminin. We chose these two ECM proteins because they are known cancer modulators promoting cell proliferation, growth, adhesion, and migration, and are produced by cancer cells as well as stromal cells. Our results show that the SR of the MeLiM melanoma is a highly dynamic process. Melanoma cells are gradually destroyed and tumour tissue is rebuilt into connective tissue. The expression of collagen IV and laminin correlated with changes in population of melanoma cells. These results contribute to a more detailed understanding of SR that is also known to occur in human patients.
II. Materials and Methods
Animals and tissue sampling
Fifty-eight melanomas of exophytic appearance were taken from MeLiM minipigs of various ages (3 weeks, 4 weeks, 6 weeks, 8 weeks, 10 weeks, 3 months, 5 months, 8 months, with five to nine melanomas in each age; see Table 2) and used for monitoring SR. The process of SR is defined as partial or complete resolution of a tumor without any treatment or as a result of a therapy judged inadequate to alter the course of malignancy [47]. The first macroscopic changes characterizing SR in MeLiM animals are flattening and grey colour of tumours followed by skin depigmentation. It forms a halo effect (whitening) around the tumours. Individual white bristles are sparsely scattered throughout the body or slightly concentrated around melanomas. In later stages, depigmentation expands over the whole body reaching demarcated areas of variable size and number. Overall skin whitening and a regular dispersion of a high number of white bristles among black ones is also observed. Twelve skin samples from healthy (tumour-free) MeLiM animals of equivalent ages were collected for comparison.
Tumours and skin samples were excised under total anaesthesia (premedication with i.m. Atropine (0.5 mg/minipig; Hoechst-Biotika, Slovak Republic) and Stresnil (1 mg/kg of body weight; Janssen Pharmaceutica N.V., Belgium) followed with Narcotan inhalation (Leciva, Czech Republic)). Vetalgin (0.5 mg/kg of body weight; Intervet International, Germany) was applied i.m. for controlling pain after tissue excision and wound suturing. These procedures were performed in accordance with the Project of Experiment approved by the Animal Science Committee of the IAPG AS CR, v.v.i. (Libechov, Czech Republic), following the rules of the European Convention for the Care and Use of Laboratory Animals.
All MeLiM animals utilized in this study developed skin melanomas immediately after birth and demonstrated metastatic lesions as judged on the basis of highly magnified inguinal and/or cervical lymph nodes. Autopsies previously performed in other MeLiM animals demonstrated magnified lymph nodes completely filled with melanoma cells. In addition, MeLiM animals with lymph nodes metastases also showed metastases in other organs, such as lungs and spleen [13]. We observed a clear vertical growth of all analysed melanomas. Melanoma cells were observed in the reticular layer of dermis and in subcutaneous tissue. Taken together with metastatic lesions and tumour thickness, the analysed porcine melanomas corresponded to the Clark V stage used for human melanoma classification.
Immunohistochemistry
Starting from the age of 3 months, small necrotic areas (2–3 mm) were observed macroscopically in the excised porcine melanomas (reaching mean size 35 mm). These local necroses developed spontaneously probably due to the large tumour size and insufficient blood supply. We took tumour samples without clearly visible necrotic area to study intact tumour tissue.
Tissue samples were placed on a piece of cork, covered with Jung Tissue Freezing Medium (Leica Microsystems, Germany) and frozen in liquid nitrogen immediately after excision. Cryosections (6 μm in thickness) were prepared by the Leica CM 1850-Cryostat (Leica, Germany), air dried and processed for detection of collagen IV and laminin by indirect immunofluorescence. Moreover, we applied immunostaining for detection of endothelial cells as a supplementary method to clarify the expression and localization of collagen IV and laminin in relation to the vascular system. Cryosections were fixed with cold acetone (10 min), washed with PBS (3 times, 5 min each), blocked with 10% porcine serum in PBS (1 hr), and incubated with specific primary polyclonal or monoclonal antibodies (overnight in a refrigerator). After washing with PBS (3 times, 5 min each), bound primary antibodies were detected using appropriate secondary Cy3- or FITC-conjugated antibodies (1 hr, in dark). Both the primary and secondary antibodies were diluted in 10% porcine serum in PBS (Table 1). The stained sections were washed with PBS (3 times, 5 min each) and nuclei were counterstained with DAPI (10 min). After final washing in PBS and distilled water, the section were embedded in the Mounting Medium (prepared according to the technical data sheet No. 777; Polysciences, Inc., USA) with Mowiol 4–88 (Calbiochem, Germany) and n-propyl gallate (5 mg/ml final concentration; Sigma-Aldrich, Germany) as anti-fading substance. Immunostaining was evaluated using an AX70 fluorescence microscope (Olympus, Japan). Black and white images of collagen IV and/or laminin plus nuclei (DAPI) staining were taken with the DP30BW Monochrome Microscopy Camera (Olympus, Japan). In addition, the tumour areas evaluated by immunohistochemistry were simultaneously monitored by bright-field microscopy to detect the distribution and morphology of melanoma cells. Both immunohistochemical and bright-field microscopical images were pseudo-coloured and overlapped using Micro Image software (Olympus, Japan). Overall levels of collagen IV and laminin observed in immunohistochemically-stained melanoma sections were semiquantitatively evaluated as very low (+), low (++), moderate (+++), high (++++), and very high (+++++) (see Fig. 1). Expression of these proteins in sebum glands, walls of blood vessels, and connective sheaths of bristles were not taken into account in this evaluation.
Table 1 .
Primary and secondary antibodies used for immunostaining
| Antibody | Company | Catalog number | Dilution |
|---|---|---|---|
| Rabbit anti-collagen IV polyclonal antibody | Acris | BP5031 | 1/100 |
| Rabbit anti-laminin polyclonal antibody | Acris | BP5032 | 1/100 |
| Mouse anti-porcine endothelial cells (clone MIL 11) | AbD Serotec | MCA1752 | 1/300 |
| Goat anti-rabbit immunoglobulins, Cy3 conjugated | Chemicon | AP132C | 1/1000 |
| Swine anti-mouse immunoglobulins, FITC conjugated | Sevac | SwAM/FITC | 1/100 |
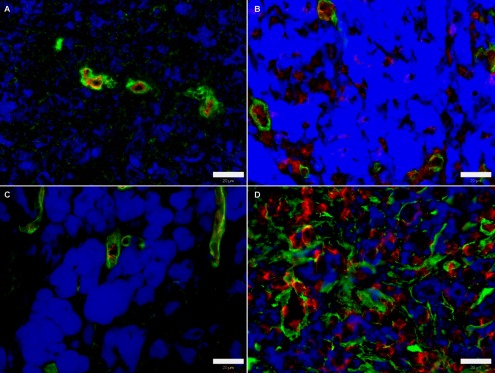
Fig. 1. .
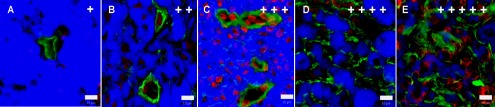
Overall level of collagen IV (A, B, D) and laminin (C, E) expression in immunohistochemically processed cryosections of MeLiM melanomas. Green: collagen IV and/or laminin; blue: melanin; red: nuclei. Bar=20 μm.
Histology
Haemotoxylin-eosin staining was used to detect changes of melanoma structure. Cryosections were fixed with ethanol (20 min), washed with distilled water (3 times, 5 min each) and treated with Weigert’s haematoxylin (20 min) for nuclei staining. Then, running tap water was applied (20 min) followed with distilled water washing (3 times, 5 min each). The cytoplasm was counterstained with 1% eosin alcoholic solution (1 min). After distilled water washing (3 times, 5 min each), the stained sections were embedded in glycerine jelly. We evaluated the slides using an AX70 microscope (Olympus, Japan). Images were taken by SP-820U camera (Olympus, Japan).
III. Results
Macroscopic observation
Changes in size, shape, and colour of cutaneous melanomas, as well as skin and bristle pigmentation, were recorded during the monitored postnatal period. The melanomas of 3-week-old pigs were black, exophytic, and 8–24 mm in diameter (Fig. 2A). The size of tumours gradually increased until the age of 10 weeks reaching 25–60 mm in diameter. Some of the melanomas showed a cracked surface and bleeding during this period. The first macroscopic changes characterizing SR, i.e. flattening and grey colour of tumours, were visible since the 10th week of age. Then, skin depigmentation started to form a halo effect around the tumours. Individual white bristles were sparsely scattered throughout the body or slightly concentrated around melanomas. Depigmentation expanded over the whole body with the increasing age of pigs (Fig. 2B), reaching demarcated areas of variable size and number, along with overall skin whitening and a regular dispersion of a high number of white bristles among the black ones at the 8th month of age.
Fig. 2. .
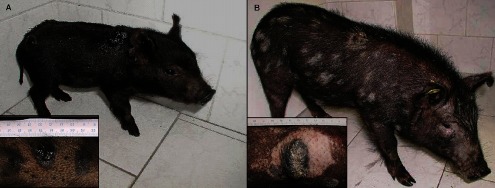
MeLiM pigs with multiple skin melanomas. (A) 4-week-old pig with melanoma in growth phase. (B) 5-month-old pig with spontaneously regressing melanoma. Bristle and skin depigmentation around skin tumours is clearly visible.
Collagen IV expression and localization
Skin melanoma is histologically heterogeneous with regard to melanoma cells, stromal cells, and other tissue components such as blood vessels and bristles. Expression of proteins generally depends on a given cell type. We analyzed stained cryosections immunohistochemically by fluorescence microscopy and simultaneously by bright-field microscopy. It enabled us to observe the localization of collagen IV (or laminin) together with the distribution and appearance of melanoma cells in the same tumour area. Melanoma cells were easily recognized according to the brown-black melanin inside them. Thus, we were able to describe in detail the process of SR in the MeLiM model from the viewpoint of changes in the expression of the studied ECM proteins and tumour morphology.
Melanomas from 3-week-old pigs were almost exclusively comprised of very uniform population of smaller melanoma cells with granular brown-black melanin (Fig. 4A). The clear moderate accumulation of granular collagen IV was observed in the extracellular spaces among the melanoma cells (Table 2A; Fig. 3A). We found very similar collagen IV staining pattern in the melanomas excised from 4-week-old pigs. However, the tumour morphology was slightly changed. In addition to the melanoma cell type observed in the tumours of 3-week-old pigs, a new population of larger melanoma cells with a dense dark brown melanin was observed. In the tumours of 6-week-old pigs, a change from granular to slightly fibrous staining pattern of collagen IV was detected. The protein was found around melanoma cells and its expression was generally reduced. The size of both melanoma cell types increased and short strands of non-pigmented tumour stroma passed through melanoma tissue. The expression of collagen IV further decreased to very low levels in the tumours of 8-week-old pigs. It filled some spaces among melanoma cells. The size of melanoma cells generally increased. However, some of them were destroyed and small particles of melanin were sparsely scattered among the surrounding cells.
Fig. 4. .
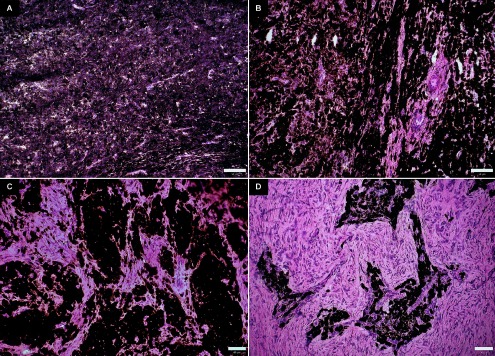
Haematoxylin-eosin staining of swine melanoma cryosections. (A) 3-week-old pig. (B) 10-week-old pig. (C) 3-month-old pig. (D) 8-month-old pig. Bar=50 μm.
Table 2A .
Sizes and immunoreactivity of MeLiM melanomas
| Age of animals | Tumour size (mm) | Collagen IV immunoreactivity | Laminin immunoreactivity | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Individual | Mean | S.D. | Individual tumours | Mean | Individual tumours | Mean | |||
| 3 weeks | 8 | 14.1 | 5.7 | +++ | +++ | +++ | +++ | ||
| 24 | +++ | +++ | |||||||
| 14 | +++ | +++ | |||||||
| 9 | +++ | +++ | |||||||
| 12 | +++ | +++ | |||||||
| 18 | ++++ | +++ | |||||||
| 9 | +++ | +++ | |||||||
| 21 | +++ | ++ | |||||||
| 12 | ++ | +++ | |||||||
| 4 weeks | 21 | 15.8 | 5.8 | ++ | +++ | ++ | ++ | ||
| 24 | +++ | ++ | |||||||
| 16 | +++ | ++ | |||||||
| 14 | +++ | ++ | |||||||
| 9 | +++ | ++ | |||||||
| 11 | +++ | +++ | |||||||
| 6 weeks | 25 | 21.1 | 6.8 | ++ | ++ | ++ | + | ||
| 29 | ++ | + | |||||||
| 12 | ++ | + | |||||||
| 31 | ++ | + | |||||||
| 20 | +++ | + | |||||||
| 17 | +++ | + | |||||||
| 14 | ++ | + | |||||||
| 21 | ++ | + | |||||||
| 8 weeks | 27 | 25.2 | 7.4 | ++ | + | + | + | ||
| 26 | + | + | |||||||
| 15 | + | + | |||||||
| 21 | + | + | |||||||
| 37 | + | + | |||||||
| 10 weeks | 60 | 30.7 | 14.4 | ++ | ++ | + | + | ||
| 23 | ++ | + | |||||||
| 26 | + | + | |||||||
| 25 | ++ | ++ | |||||||
| 24 | ++ | + | |||||||
| 26 | ++ | + | |||||||
Fig. 3. .
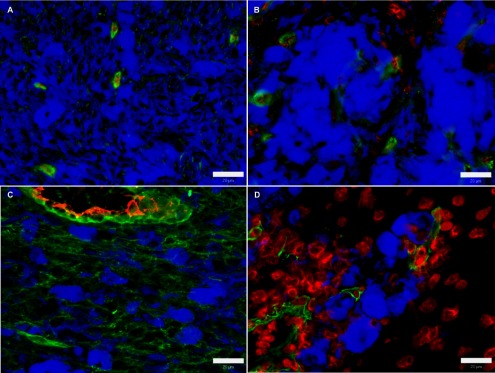
Expression of collagen IV in swine melanoma. (A) 3-week-old pig. (B) 10-week-old pig. (C) 3-month-old pig. (D) 8-month-old pig. Green: collagen IV; blue: melanin; red: blood vessel walls (A, C) and nuclei (B, D). Bar=20 μm.
In the tumours of 10-week-old pigs, the areas devoid of melanoma cells were more numerous and larger, suggesting a continued destruction of tumour cells, extension of tumour stroma, and remodelling of tumour tissues into the connective tissue (Fig. 4B). Larger melanoma cells were highly prevalent whereas smaller ones were almost not detected. The expression of collagen IV showed a mild increase in the extracellular areas (Fig. 3B).
Tumours of 3-month-old pigs were characterized by a further disappearance of melanoma cells. This process led to the formation of areas occupied by melanoma cells that were separated from each other by more prevalent formations of tumour stroma (Fig. 4C). Collagen IV grew significantly to high levels forming short fibres that surrounded some melanoma cells (Table 2B; Fig. 3C). On the other hand, the areas without melanoma cells were characterized by very low occurrence of fibrous collagen IV. Melanomas of 5-month-old and 8-month-old pigs exhibited a continuing gradual destruction of melanoma cells accompanied with tissue remodeling (Fig. 4D). The amount and extent of the areas lacking melanoma cells further increased. Free granular melanin and very sparsely scattered giant melanoma cells were observed in these areas. Remaining tumour regions still showed a high number of big melanoma cells without close contact. At the age of 5 months, the accumulation of collagen IV generally decreased. Low collagen IV positivity was found in the areas with remaining melanoma cells whereas its expression was very low in areas without melanoma cells. The tumours of 8-month-old pigs showed a mild increase of collagen IV (to moderate level) around melanoma cells (Fig. 3D). In contrast, the expression of collagen IV in the tumour regions lacking melanoma cells was similar as in the previous age examined.
Table 2B .
Sizes and immunoreactivity of the MeLiM melanomas (*Data describing individual tunour size, mean, and S.D. of tumour size are not duplicated for clarity sake.)
| Age of animals | Tumour size (mm) | Collagen IV immunoreactivity | Laminin immunoreactivity | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Individual | Mean | S.D. | Individual tumours | Mean | Individual tumours | Mean | ||||
| 3 months | Areas with melanoma cells | 25 | 34.8 | 11.2 | ++++ | ++++ | ++++ | +++ | ||
| 45 | ++++ | +++ | ||||||||
| 58 | ++++ | +++ | ||||||||
| 30 | ++++ | +++ | ||||||||
| 31 | +++ | +++ | ||||||||
| 42 | ++++ | +++ | ||||||||
| 27 | ++++ | +++ | ||||||||
| 29 | +++ | ++ | ||||||||
| 26 | ++++ | +++ | ||||||||
| Areas lacking melanoma cells | * | * | * | + | + | +++ | ++ | |||
| + | ++ | |||||||||
| + | ++ | |||||||||
| + | ++ | |||||||||
| + | ++ | |||||||||
| + | ++ | |||||||||
| + | ++ | |||||||||
| ++ | ++ | |||||||||
| + | ++ | |||||||||
| 5 months | Areas with melanoma cells | 21 | 30.2 | 11.7 | ++ | ++ | + | + | ||
| 26 | ++ | ++ | ||||||||
| 53 | ++ | + | ||||||||
| 27 | + | + | ||||||||
| 23 | ++ | + | ||||||||
| 31 | +++ | + | ||||||||
| Areas lacking melanoma cells | * | * | * | + | + | + | + | |||
| + | + | |||||||||
| + | + | |||||||||
| + | + | |||||||||
| + | + | |||||||||
| + | + | |||||||||
| 8 months | Areas with melanoma cells | 55 | 32.8 | 10.2 | +++ | +++ | +++++ | +++++ | ||
| 26 | +++ | +++++ | ||||||||
| 37 | +++ | ++++ | ||||||||
| 27 | +++ | +++++ | ||||||||
| 25 | ++ | +++++ | ||||||||
| 31 | +++ | ++++ | ||||||||
| 41 | +++ | +++++ | ||||||||
| 29 | +++ | +++++ | ||||||||
| 24 | +++ | +++++ | ||||||||
| Areas lacking melanoma cells | * | * | * | + | + | + | + | |||
| + | + | |||||||||
| + | + | |||||||||
| + | + | |||||||||
| + | + | |||||||||
| + | + | |||||||||
| + | + | |||||||||
| + | + | |||||||||
| + | + | |||||||||
All the melanoma samples showed clear localization of collagen IV in the walls of blood vessels. Collagen IV expression increased with the age of animals corresponding to the growth and development of tumour vascularization. In addition, collagen IV was observed in the sebum glands, and the connective sheaths of muscle fibres and bristles.
Control skin samples demonstrated collagen IV localization in the basement membrane, sebum glands, walls of blood vessels, and connective sheaths of muscle fibres and bristles (Fig. 5A). The intensity of staining increased with the age of pigs and skin differentiation.
Fig. 5. .
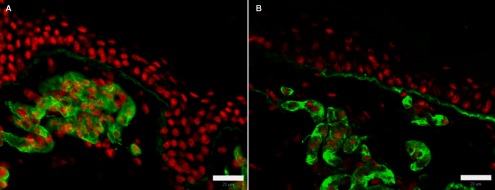
Expression of ECM proteins in normal swine skin. (A) collagen IV. (B) laminin. Green: ECM proteins; red: nuclei. Bar=20 μm.
Laminin expression and localization
Tumours collected from 3-week-old pigs exhibited a moderate granular expression of laminin around melanoma cells (Table 2A; Fig. 6A). Later, laminin expression decreased to low levels in the tumours of 4-week-old pigs. Very low levels of laminin were maintained only in the extracellular spaces of tumours taken from the 6-week-old to 10-week-old pigs (Fig. 6B). At age of 3 months, laminin expression was increased to moderate levels. Granular staining was clearly observed around melanoma cells (Table 2B; Fig. 6C). On the contrary, the tumours of 5-month-old pigs were characterized by a strong reduction of laminin expression to very low levels. The protein was found sparsely among melanoma cells. Very high accumulation of laminin was observed in the areas of tumours with still preserved melanoma cells collected from the 8-month-old pigs (Fig. 6D). Laminin had a fibrous character. It was very weakly detected also in the parts of tumour sections without melanoma cells.
Fig. 6. .
Expression of laminin in swine melanoma. (A) 3-week-old pig. (B) 10-week-old pig. (C) 3-month-old pig. (D) 8-month-old pig. Green: laminin; blue: melanin; red: blood vessel walls (A, C) and nuclei (B, D). Bar=20 μm.
Laminin was also found in various structures of all melanoma samples. Unlike collagen IV, its expression was generally weaker. Laminin was localized in the walls of blood vessels, sebum glands, and connective sheaths of muscle fibres and of bristles. This expression changed with growth and differentiation of these structures showing a lower staining intensity in younger animals.
Control skin samples taken from pigs at different ages displayed laminin in the basement membrane, sebum gland, walls of blood vessels, and connective sheaths of muscle fibres and bristles (Fig. 5B). Similarly, as in collagen IV, the level of laminin expression also increased with the age of the pigs.
IV. Discussion
The MeLiM strain represents a suitable animal cancer model due to the histopathological [13], biochemical [3], and molecular biological similarity [39] of porcine melanoma to human melanoma. The process of SR has already been studied from the histological viewpoint in this model [47]. Moreover, the expression of two ECM proteins, tenascin-C and tenascin-X, has been analysed at the molecular and immunohistochemical levels [15]. However, a temporal study of the expression of tenascincs and other ECM proteins in the MeLiM animals has yet to be performed.
Sinclair miniature swine is another hereditary animal model of melanoma [21, 33, 34]. It originated from the Hormel miniature pig that also participated in the establishment of the MeLiM strain [23]. Thus, the Hormel minipig perhaps brought initial genetic predisposition to melanoma in both Sinclair and MeLiM strains. Of course, further breeding programs giving rise to these models were specific so that their current genetic basis may differ from each other. Melanoma in the MeLiM strain is more aggressive [14] and about one third of melanoma-bearing pigs die due to melanoma progression [22]. On the contrary, a complete tumour SR occurs in most (if not all) affected Sinclair swine [21, 33]. No information about the SR of melanoma in the Sinclair swine from the viewpoint of ECM protein expression has been presented up to now.
Collagen IV and laminin are two important ECM proteins which can highly influence the behaviour of cancer cells, in terms of their invasiveness and formation of metastases. In vitro studies demonstrated that collagen IV induced chemotaxis of the human melanoma cell line [2, 19, 20]. Immunohistochemical detection of collagen IV and laminin in human oral squamous cell carcinomas revealed that expression of these two ECM proteins were correlated with the stage of cancer disease and lymph node metastasis. Deposition of collagen IV and laminin decreased with increased histopathological grade and absence of staining was usually associated with a poor prognosis [12, 18]. A similar finding showed detailed immunohistochemical localization of collagen IV, laminin, and heparan sulfate proteoglycan in human colorectal adenocarcinoma [27]. Immunostaining of basement membrane components was significantly lower in highly invasive pattern at the parenchymal-stromal border and was also clearly related to the incidence of lymph node metastasis. On the contrary, immunohistochemical analysis of urothelial carcinoma showed that the expression of collagen IV, as well as expression of tenascin and fibronectin, were correlated with more aggresive tumour behaviour. Laminin expression had no significant correlation with tumour grade and stage [24]. In benign and malignant naevo-melanocytic lesions, detection of collagen IV and laminin revealed similar basement membrane deposition and therefore it was not helpful in differential diagnosis [16]. It seems that the analysis of collagen IV and laminin expression in various tumour types can have different predictive value as concerns tumour stage, aggressiveness, and metastasis.
In our study, we monitored collagen IV and laminin during initial melanoma growth followed by SR in the MeLiM model. All minipigs developed skin melanomas immediately after birth and also showed lymph node metastases (as judged on the basis of macroscopically magnified inguinal and/or cervical lymph nodes) demonstrating the same tumour stage. For this reason, we used the age of animals as a criterion to trace temporal changes of the ECM protein expression in relationship to tumour morphology. The earliest developmental stages of the porcine melanoma (i.e. the 3- and 4-week-old pigs) were formed mainly by melanoma cells. High granular expression of collagen IV and laminin was observed in extracellular spaces suggesting their production by cancer cells. Strong deposition of collagen IV and laminin found in the early stages of cancer were regularly correlated with tumour cell proliferation [26] and migratory activity of melanoma cells [10, 38]. On the contrary, it was demonstrated that the fibrous form of collagen IV promoted migration and metastasis [6, 49, 50]. It is not clear whether the granular form of collagen IV, which we found in the earliest porcine melanoma stages, could also participate in the formation of metastases that are present in various inner organs of the MeLiM animals with skin melanomas [13, 22].
A gradual destruction of melanoma cells and the rebuilding of skin tumours into fibrous connective tissues were observed in older MeLiM animals. These morphological changes appeared already at the 6- to 8-week-old pigs, although the tumours were still macroscopically exophytic, black, and growing in size. Small areas containing disintegrated melanoma cells were initially observed. Decrease of collagen IV and laminin expression that was observed immunohistochemically during this period corresponded well to the destruction of melanoma cells. Reduction of the number of tumour cells probably resulted in the decreased expression of collagen IV and laminin. It led to a decline of the migration ability of melanoma cells, which is described in a number of cases [37, 45]. Consequently, tumour cells are less invasive and apparently losing their malignant phenotype. Destruction of tumour tissue along with reduced expression of collagen IV and laminin likely represent the initial events of SR.
SR of the MeLiM melanoma is probably caused by an immune response directed against the melanoma cells. This fact is supported by the presence of the halo effect (whitening) in the vicinity of skin tumours of older pigs. Immune cells presumably recognize melanoma antigens which leads to the destruction of melanoma cells. Since melanoma cells arise by neoplastic transformation of melanocytes they share similar antigenic repertoires. Thus, melanocytes around skin tumours are destroyed (due to the immune response initially focused on melanoma cells) creating a halo effect. Evidence to this end has been presented in a number of human cases [1, 4, 28, 31, 43].
In view of this process, larger areas totally devoid of melanoma cells were found in the melanomas of 10-week-old pigs. Slight overall increase of collagen IV expression together with macroscopic (bristle and skin whitening, grey colour, and flattening of tumours) and morphological changes (melanoma cell destruction) indicate the beginning of connective tissue formation. Immune cells attracted to the tumour site were then likely to induce the repair of damaged tissue by specific cytokine secretion (described during healing processes) [46]. This results in the reorganization of ECM proteins. Fibroblasts are the major dermal cells in normal skin as well as in fibrous connective tissue where they enable ECM remodelling [9] and synthesize various ECM proteins including collagen IV [42, 48]. Fibroblasts can migrate into the MeLiM melanomas and partially elevate the level of collagen IV during SR. In tissue rebuilding, collagen IV may serve as a scaffold for other ECM proteins [8]. In accordance with this opinion, we observed the initial rise of collagen IV expression followed by later increase of laminin expression. In the light of the present results, the age of 10 weeks seems to be a crucial turning point between growth phase and SR of melanoma in the MeLiM model. It is obvious that, in a cancer animal model having a similar origin as our MeLiM strain, the timing of SR would be analogous. In agreement with this assumption, the 10th week of age was also considered the beginning of SR in the Sinclair miniature swine [7].
Porcine melanomas of 3-, 5- and 8-month-old pigs were characterized by areas totally lacking melanoma cells (presumably destroyed by immune cells) and areas still containing melanoma cells. The rebuilding of melanoma tissue to the connective tissue proceeded significantly, showing a clear dependence on the age of animals. The expression of collagen IV and laminin was related to the presence of remaining tumour cells. Unlike the previous period (10 weeks), both proteins now showed fibrillar staining pattern. We assumed that both proteins were being synthesized for a longer period and thus complex filiform formations could be created. Their persistent localization in melanomas of older pigs (in areas still containing cancer cells) might increase the chance of melanoma cell survival. This phenomenon was demonstrated in small-cell lung cancer cells. Adhesion of these cells to the ECM proteins was responsible for resistance to apoptosis induced by chemotherapeutic agents [41]. Generally, both collagen IV and laminin showed high expression in blood vessel walls and in various skin structures such as the sheaths of bristles and muscle fibres, and the dermo-epidermal junction during the overall period under examination. Similar observations have already been published for diverse human tumours [8, 29, 41, 44].
In conclusion, this study provides the first long-term monitoring of the expression of two important ECM proteins during SR of cancer. Using the MeLiM model, which allows such detailed observation, we showed that SR of melanoma is a highly dynamic process in terms of ECM remodeling. After a short period of skin tumour growth, melanoma cells were gradually destroyed and tumour tissue was rebuilt into the connective tissue. The expression of collagen IV and laminin correlated well with the changes in population of melanoma cells. Heightened extracellular expression of both proteins in the early postnatal stages is associated with the growth of melanoma cells. Gradual decrease of laminin and collagen IV expression in later stages corresponds to tumour tissue degradation. The age of 10 weeks can be considered as the turning point between growth and SR of porcine melanoma. Subsequent SR leads to the formation of connective tissue in which melanoma cells still retain high expression of both proteins, that probably increases their chances of survival. These results contribute to a more detailed understanding of the SR process that is also known in human patients. Detection of collagen IV and laminin may be used for monitoring of spontaneous regression.
V. Conflicts of interests
The authors declare that they have no conflicts of interests in relation to this article.
VI. Acknowledgements
This work was funded by the MEYS CR (project CZ.1.05/2.1.00/03.0124) and RVO 67985904. We would like to thank Dusan Usvald, Luca Vannucci and Stefan Juhas for tumour and skin excision, Jaroslava Sestakova and Jitka Klucinova for technical assistance, Marie Rabova for help with microscopy imaging and Jozef Janda for useful suggestions during the preparation of this article.
VII. References
- 1.Arpaia N., Cassano N. and Vena G. A. (2006) Regressing cutaneous malignant melanoma and vitiligo-like depigmentation. Int. J. Dermatol. 45; 952–956. [DOI] [PubMed] [Google Scholar]
- 2.Aznavoorian S., Stracke M. L., Krutzsch H., Schiffmann E. and Liotta L. A. (1990) Signal transduction for chemotaxis and haptotaxis by matrix molecules in tumor cells. J. Cell Biol. 110; 1427–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borovansky J., Horak V., Elleder M., Fortyn K., Smit N. P. and Kolb A. M. (2003) Biochemical characterization of a new melanoma model—the minipig MeLiM strain. Melanoma Res. 13; 543–548. [DOI] [PubMed] [Google Scholar]
- 4.Bramhall R. J., Mahady K. and Peach A. H. (2014) Spontaneous regression of metastatic melanoma—clinical evidence of the abscopal effect. Eur. J. Surg. Oncol. 40; 34–41. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y., Chen Y., Huang L. and Yu J. (2012) Evaluation of heparanase and matrix metalloproteinase-9 in patients with cutaneous malignant melanoma. J. Dermatol. 39; 339–343. [DOI] [PubMed] [Google Scholar]
- 6.Condeelis J. and Segall J. E. (2003) Intravital imaging of cell movement in tumours. Nat. Rev. Cancer 3; 921–930. [DOI] [PubMed] [Google Scholar]
- 7.Cui J., Chen D., Misfeldt M. L., Swinfard R. W. and Bystryn J. C. (1995) Antimelanoma antibodies in swine with spontaneously regressing melanoma. Pigment Cell Res. 8; 60–63. [DOI] [PubMed] [Google Scholar]
- 8.Egeblad M., Rasch M. G. and Weaver V. M. (2010) Dynamic interplay between the collagen scaffold and tumor evolution. Curr. Opin. Cell Biol. 22; 697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El Sayegh T. Y., Kapus A. and McCulloch C. A. (2007) Beyond the epithelium: cadherin function in fibrous connective tissues. FEBS Let. 581; 167–174. [DOI] [PubMed] [Google Scholar]
- 10.Engbring J. A. and Kleinman H. K. (2003) The basement membrane matrix in malignancy. J. Pathol. 200; 465–470. [DOI] [PubMed] [Google Scholar]
- 11.Fang L., Lonsdorf A. S. and Hwang S. T. (2008) Immunotherapy for advanced melanoma. J. Invest. Dermatol. 128; 2596–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Firth N. A. and Reade P. C. (1996) The prognosis of oral mucosal squamous cell carcinomas: a comparison of clinical and histopathological grading and of laminin and type IV collagen staining. Aust. Dent. J. 41; 83–86. [DOI] [PubMed] [Google Scholar]
- 13.Fortyn K., Hruban V., Horak V., Hradecky J. and Tichy J. (1994) Melanoblastoma in laboratory minipigs: A model for studying human malignant melanoma. Vet. Med.-Czech 39; 597–604. [PubMed] [Google Scholar]
- 14.Fortyn K., Hruban V., Horak V. and Tichy J. (1998) Exceptional occurrence and extent of malignant melanoma in pig. Vet. Med.-Czech 43; 87–91. [Google Scholar]
- 15.Geffrotin C., Horak V., Crechet F., Tricaud Y., Lethias C., Vincent-Naulleau S. and Vielh P. (2000) Opposite regulation of tenascin-C and tenascin-X in MeLiM swine heritable cutaneous malignant melanoma. Biochim. Biophys. Acta 1524; 196–202. [DOI] [PubMed] [Google Scholar]
- 16.Havenith M. G., van Zandvoort E. H., Cleutjens J. P. and Bosman F. T. (1989) Basement membrane deposition in benign and malignant naevo-melanocytic lesions: an immunohistochemical study with antibodies to type IV collagen and laminin. Histopathology 15; 137–146. [DOI] [PubMed] [Google Scholar]
- 17.High W. A., Stewart D., Wilbers C. R., Cockerell C. J., Hoang M. P. and Fitzpatrick J. E. (2005) Completely regressed primary cutaneous malignant melanoma with nodal and/or visceral metastases: A report of 5 cases and assessment of the literature and diagnostic criteria. J. Am. Acad. Dermatol. 53; 89–100. [DOI] [PubMed] [Google Scholar]
- 18.Hirota J., Yoneda K. and Osaki T. (1990) Basement membrane type IV collagen in oral squamous cell carcinoma. Head Neck 12; 400–405. [DOI] [PubMed] [Google Scholar]
- 19.Hodgson L. and Dong C. (2001) [Ca2+]i as a potential downregulator of alpha2beta1-integrin-mediated A2058 tumor cell migration to type IV collagen. Am. J. Physiol. 281; C106–C113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hodgson L., Henderson A. J. and Dong C. (2003) Melanoma cell migration to type IV collagen requires activation of NK-κB. Oncogene 22; 98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hook R. R. Jr., Berkelhammer J. and Oxenhandler R. W. (1982) Melanoma: Sinclair swine melanoma. Am. J. Pathol. 108; 130–133. [PMC free article] [PubMed] [Google Scholar]
- 22.Horak V., Fortyn K., Hruban V. and Klaudy J. (1999) Hereditary melanoblastoma in miniature pigs and its successful therapy by devitalization technique. Cell. Mol. Biol. 45; 1119–1129. [PubMed] [Google Scholar]
- 23.Hruban V., Horak V., Fortyn K., Hradecky J., Klaudy J., Smith D. M., Reisnerova H. and Majzlik I. (2004) Inheritance of malignant melanoma in the MeLiM strain of miniature pigs. Vet. Med.-Czech 49; 453–459. [Google Scholar]
- 24.Ioachim E., Michael M., Stavropoulos N. E., Kitsiou E., Salmas M. and Malamou-Mitsi V. (2005) A clinicopathological study of the expression of extracellular matrix components in urothelial carcinoma. BJU Int. 95; 655–659. [DOI] [PubMed] [Google Scholar]
- 25.Jarvelainen H., Sainio A., Koulu M., Wight T. N. and Penttinen R. (2009) Extracellular matrix molecules: Potential targets in pharmacotherapy. Pharmacol. Rev. 61; 198–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalluri R. and Cosgrove D. (2000) Assembly of type IV collagen. Insights from alpha3 (IV) collagen-deficient mice. J. Biol. Chem. 275; 12719–12724. [DOI] [PubMed] [Google Scholar]
- 27.Kiyoshima T., Kobayashi I., Matsuo K., Ishibashi Y., Miyoshi A., Akashi Y. and Sakai H. (1998) Immunohistochemical localization of laminin, collagen type IV and heparan sulfate proteoglycan in human colorectal adenocarcinoma: correlation with local invasive pattern and lymph node metastasis. Acta Histochem. Cytochem. 31; 39–47. [Google Scholar]
- 28.Kumagai Y., Miura K., Nishida T., Igari K., Ochiai T., Iida M., Yamazaki S., Odajima H., Kawano T. and Takubo K. (2012) Simultaneous resection of metastatic melanoma in the esophagus and primary cutaneous melanoma showing partial regression: report of a case. Surg. Today 42; 884–890. [DOI] [PubMed] [Google Scholar]
- 29.LeBleu V. S., Macdonald B. and Kalluri R. (2007) Structure and function of basement membranes. Exp. Biol. Med. 232; 1121–1129. [DOI] [PubMed] [Google Scholar]
- 30.Levental K. R., Yu H., Kass L., Lakins J. N., Egeblad M., Erler J. T., Fong S. F., Csiszar K., Giaccia A., Weninger W., Yamauchi M., Gasser D. L. and Weaver V. M. (2009) Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139; 891–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCardle T. W., Messina J. L. and Sondak V. K. (2009) Completely regressed cutaneous melanocytic lesion revisited. Semin. Oncol. 36; 498–503. [DOI] [PubMed] [Google Scholar]
- 32.Menzies S. W. and McCarthy W. H. (1997) Complete regression of primary cutaneous malignant melanoma. Arch. Surg. 132; 553–556. [DOI] [PubMed] [Google Scholar]
- 33.Misfeldt M. L. and Grimm D. R. (1994) Sinclair miniature swine: An animal model of human melanoma. Vet. Immunol. Immunopathol. 43; 167–175. [DOI] [PubMed] [Google Scholar]
- 34.Oxenhandler R. W., Berkelhammer J., Smith G. D. and Hook R. R., Jr. (1982) Growth and regression of cutaneous melanomas in Sinclair miniature swine. Am. J. Pathol. 109; 259–269. [PMC free article] [PubMed] [Google Scholar]
- 35.Palmer S. R., Erickson L. A., Ichetovkin I., Knauer D. J. and Markovic S. N. (2011) Circulating serologic and molecular biomarkers in malignant melanoma. Mayo. Clin. Proc. 86; 981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paredes B. E. (2007) Regression in malignant melanoma. Definition, etiopathogenesis, morphology and differential diagnosis. Pathologe 28; 453–463. [DOI] [PubMed] [Google Scholar]
- 37.Pasco S., Brassart B., Ramont L., Maquart F. X. and Monboisse J. C. (2005) Control of melanoma cell invasion by type IV collagen. Cancer Detect. Prev. 29; 260–266. [DOI] [PubMed] [Google Scholar]
- 38.Pasco S., Ramont L., Maquart F. X. and Monboisse J. C. (2004) Control of melanoma progression by various matrikines from basement membrane macromolecules. Crit. Rev. Oncol. Hematol. 49; 221–233. [DOI] [PubMed] [Google Scholar]
- 39.Pohlreich P., Stribrna J., Kleibl Z., Horak V. and Klaudy J. (2001) Detection of neoplastic cells in blood of miniature pigs with hereditary melanoma. Vet. Med.-Czech 46; 199–204. [Google Scholar]
- 40.Provenzano P. P., Eliceiri K. W., Campbell J. M., Inman D. R., White J. G. and Keely P. J. (2006) Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 4; 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rintoul R. C. and Sethi T. (2002) Extracellular matrix regulation of drug resistance in small-cell lung cancer. Clin. Sci. 102; 417–424. [PubMed] [Google Scholar]
- 42.Soucy P. A. and Romer L. H. (2009) Endothelial cell adhesion, signaling, and morphogenesis in fibroblast-derived matrix. Matrix Biol. 28; 273–283. [DOI] [PubMed] [Google Scholar]
- 43.Speeckaert R., van Geel N., Vermaelen K. V., Lambert J., van Gele M., Speeckaert M. M. and Brochez L. (2011) Immune reactions in benign and malignant melanocytic lesions: lessons for immunotherapy. Pigment Cell Melanoma Res. 24; 334–344. [DOI] [PubMed] [Google Scholar]
- 44.Sund M., Xie L. and Kalluri R. (2004) The contribution of vascular basement membranes and extracellular matrix to the mechanics of tumor angiogenesis. Apmis 112; 450–462. [DOI] [PubMed] [Google Scholar]
- 45.Tzu J. and Marinkovich M. P. (2008) Bridging structure with function: Structural, regulatory, and developmental role of laminins. Int. J. Biochem. Cell Biol. 40; 199–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uitto, J., Mauviel, A. and McGrath, J. (1996) The Molecular and Cellular Biology of Wound Repair, second edition, ed. by R. A. F. Clark, Plenum Publishing Corporation, New York, pp. 513–553. [Google Scholar]
- 47.Vincent-Naulleau S., Le Chalony C., Leplat J. J., Bouet S., Bailly C., Spatz A., Vielh P., Avril M. F., Tricaud Y., Gruand J., Horak V., Frelat G. and Geffrotin C. (2004) Clinical and histopathological characterization of cutaneous melanomas in the melanoblastoma-bearing Libechov minipig model. Pigment Cell. Res. 17; 24–35. [DOI] [PubMed] [Google Scholar]
- 48.Wang T. W., Sun J. S., Huang Y. C., Wu H. C., Chen L. T. and Lin F. H. (2006) Skin basement membrane and extracellular matrix proteins characterization and quantification by real time RT-PCR. Biomaterials 27; 5059–5068. [DOI] [PubMed] [Google Scholar]
- 49.Wang W., Wyckoff J. B., Frohlich V. C., Oleynikov Y., Huttelmaier S., Zavadil J., Cermak L., Bottinger E. P., Singer R. H., White J. G., Segall J. E. and Condeelis J. S. (2002) Single cell behavior in metastatic primary mammary tumors correlated with gene expression patterns revealed by molecular profiling. Cancer Res. 62; 6278–6288. [PubMed] [Google Scholar]
- 50.Wyckoff J. B., Wang Y., Lin E. Y., Li J. F., Goswami S., Stanley E. R., Segall J. E., Pollard J. W. and Condeelis J. (2007) Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 67; 2649–2656. [DOI] [PubMed] [Google Scholar]


