
Insight from (left to right) Amado Quintar, Catherine Hedrick, and Klaus Ley
Monocytes are a heterogeneous population of phagocytic cells that are generated in the bone marrow and released into the bloodstream. There are two main monocyte subsets in mice: “inflammatory” monocytes that are Ly6Chi CCR2hi CX3CR1low and “alternative” or “patrolling” monocytes that are Ly6Clow CCR2low CX3CR1hi. The process of monocyte recruitment and differentiation is still a matter of controversy. In this issue, Dal-Secco et al. report in situ monocyte reprogramming in the liver, from proinflammatory CCR2hi CX3CR1low cells into reparative CCR2low CX3CR1hi cells and show, for the first time, that this occurs at the site of injury.
Dal-Secco et al. harnessed the power of imaging technology and fluorescent reporter mice to track monocytes in a model of sterile inflammation in the liver (induced after a 30 µm3 burn by a thermal probe). In this model, the recruitment of monocytes is dependent on CCR2 present on CCR2hi CX3CR1low monocytes. Some of these cells were observed patrolling the liver sinusoids in the steady state. Within 24–48 hours, CCR2hi CX3CR1low monocytes extravasated and formed a ring structure surrounding the necrotic tissue at the site of the injury. These extravascular monocytes started to differentiate and, by 48–72 hours, had lost CCR2 expression and gained CX3CR1 and MHC class II expression (CCR2low CX3CR1hi CD11bhi MHCIIhi CD64hi F4/80-). Blocking IL-10 and IL-4 delayed this phenotypic transition and impaired wound healing as shown by failure to clear necrotic hepatocytes and failure to deposit collagen.
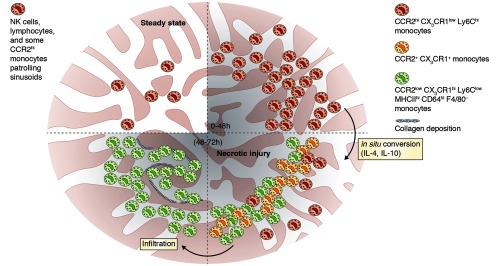
A focal sterile injury induces the recruitment of CCR2hi monocytes (red) to the liver, which form a ring surrounding and “walling off” the necrotic lesion. After 48–72 hours, monocytes start to gain CX3CR1 expression (orange) and undergo an IL-4/IL-10–mediated phenotypic conversion into CCR2low CX3CR1hi CD11bhi MHCIIhi CD64hi F4/80- monocytes (green). These cells infiltrate the necrotic tissue and induce collagen deposition leading to the clearance of dead cells.
These findings reveal a mechanism for monocyte transformation in situ in response to sterile inflammation in the liver, starting with the abundant pool of circulating Ly6Chi CCR2hi CX3CR1low monocytes and exploiting their plasticity for on-site education and conversion. Monocyte conversion from inflammatory to alternative phenotypes has previously been shown to occur in the bone marrow, but this paper shows that phenotypic conversion can also occur in the liver. The dependence on IL-4 and IL-10 for this phenotypic switch is reminiscent of alternatively activated macrophages, similar to wound-healing M2 cells. Further work will be required to investigate the hepatic source of IL-4 and IL-10 and determine how these cytokines induce phenotypic conversion. Investigations that address the nature and source of the CCR2 ligands that recruit inflammatory monocytes to sites of sterile injuries and of the chemokine(s) that facilitate entry of CX3CR1hi monocytes into necrotic lesions may also provide fascinating answers.
These results provide new data on the plasticity of monocytes in the tissue microenvironment which may lead to new opportunities for therapeutic intervention in disease.
References
- Dal-Secco, D., et al. 2015. J. Exp. Med. 10.1084/jem.20141539. [DOI] [Google Scholar]


