Abstract
The known human tumor viruses include the DNA viruses Epstein-Barr virus, Kaposi sarcoma herpesvirus, Merkel cell polyomavirus, human papillomavirus, and hepatitis B virus. RNA tumor viruses include Human T-cell lymphotrophic virus type-1 and hepatitis C virus. The serological identification of antigens/antibodies in plasma serum is a rapidly progressing field with utility for both scientists and clinicians. Serology is useful for conducting seroepidemiology studies and to inform on the pathogenesis and host immune response to a particular viral agent. Clinically, serology is useful for diagnosing current or past infection and for aiding in clinical management decisions. Serology is useful for screening blood donations for infectious agents and for monitoring the outcome of vaccination against these viruses. Serodiagnosis of human tumor viruses has improved in recent years with increased specificity and sensitivity of the assays, as well as reductions in cost and the ability to assess multiple antibody/antigens in single assays. Serodiagnosis of tumor viruses plays an important role in our understanding of the prevalence and transmission of these viruses and ultimately in the ability to develop treatments/preventions for these globally important diseases.
Introduction
Viruses are estimated to be the cause of 12% to 25% of human cancers worldwide.1, 2 Etiological agents of human cancers include the known viruses; (i) Epstein-Barr virus (EBV); (ii) Kaposi sarcoma herpesvirus (KSHV); (iii) viruses of the family Polyomaviridae; (iv) Human papillomavirus (HPV); (v) Human T-cell lymphotrophic virus type-1 (HTLV-1); (vi) hepatitis B virus (HBV); and (vii) hepatitis C virus (HCV) (Figure 1). Although beyond the scope of this review, HIV-1 has also been classified as a carcinogen by the International Agency for Research on Cancer.3 HIV-1 immunosuppression increases the risk of cancers associated with infectious agents. Specifically, Kaposi’s sarcoma, non-Hodgkin’s lymphoma, and cervical cancer are AIDS defining malignancies; moreover HIV infection is associated with increased risk for Hodgkin’s lymphoma, anal cancer, hepatocellular carcinoma and cancer of the conjunctiva, vulva, vagina, and penis. HIV infected individual have also increased risk of malignancies not hitherto associated with infectious agents, such as lung cancer and melanoma. It is anticipated that the list of human tumor viruses will continue to grow. Serological techniques to identify host antibodies reactive against viral antigens is a powerful diagnostic tool that can be used to aid clinical management decisions, inform on the epidemiology of disease (Figure 2), and provide information related to virology and host immunity. Serology is useful for diagnosing current or past infection of a particular viral agent, although it cannot be relied upon for diagnosing the diseases, including cancer, associated with that particular viral agent. This is an especially relevant distinction to make for tumor viruses, as infection with tumor viruses are far more prevalent than the diseases that they cause. This review will discuss the serodiagnosis of each of these human tumor viruses, with an aim to present clinical and epidemiological application of these techniques.
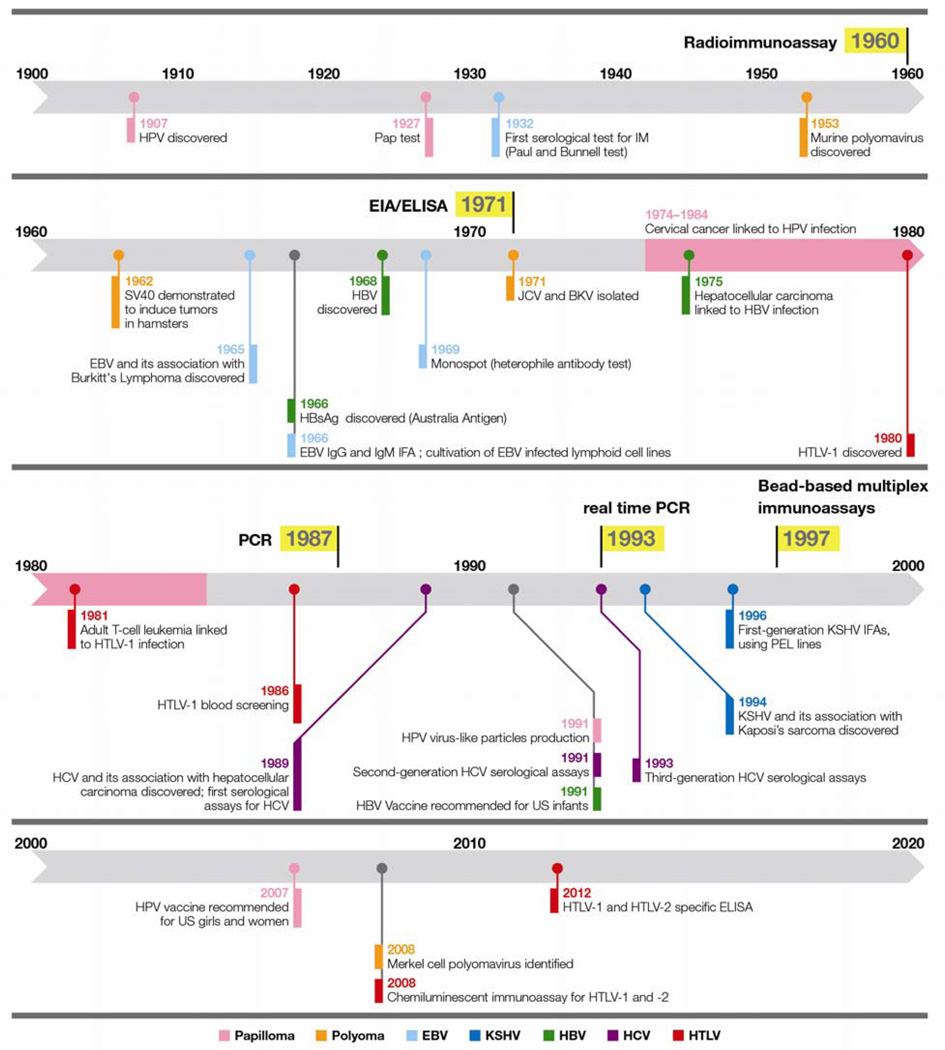
Figure 1. Timeline of tumor viruses and serology.
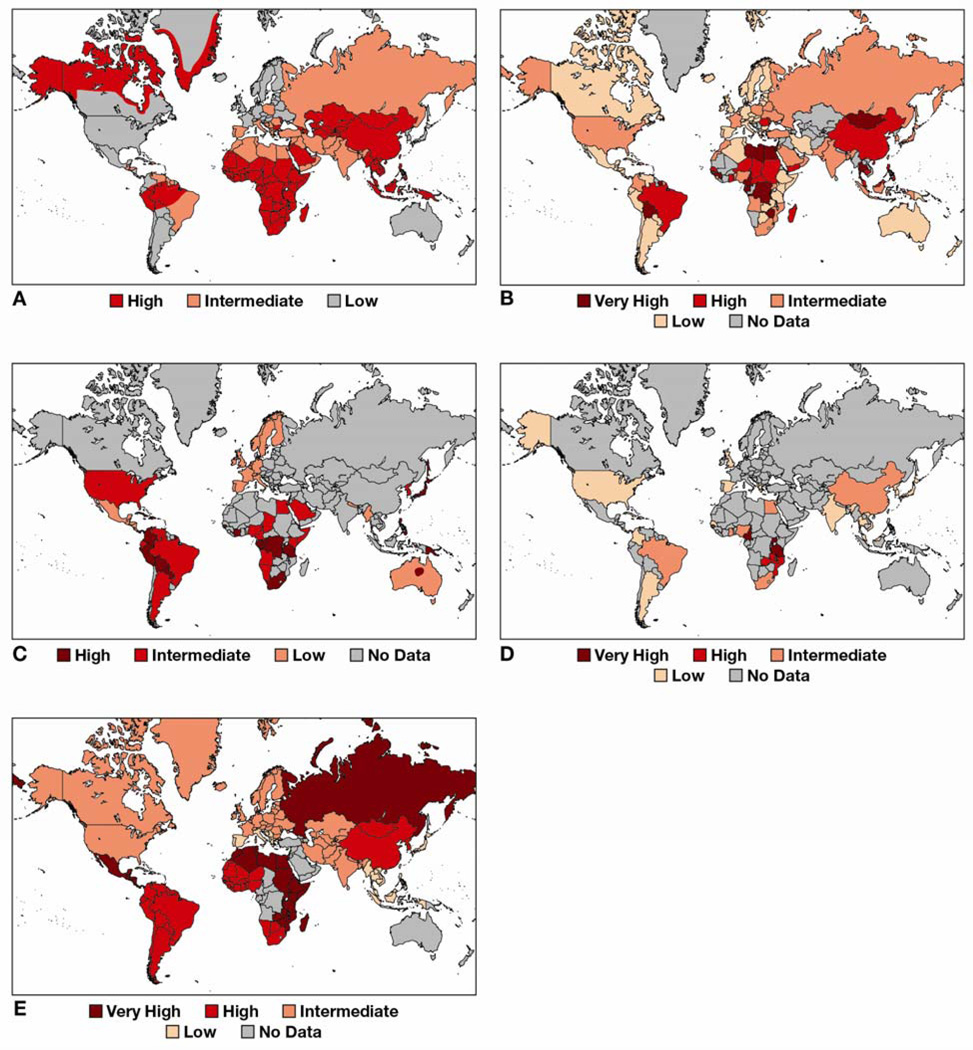
Figure 2. Seroprevalence of tumor viruses.
(A) Worldwide HBV and (B) HCV prevalence derived from CDC and WHO data. (C) Worldwide HTLV-1 prevalence, modified with permission from.110 (D) Worldwide KSHV seroprevalence based on sources cited in a 2012 IARC monograph.3 (E) Estimated HPV DNA prevalence, modified with permission from.111 Unfortunately, for large parts of the world seroprevalence information for many of these viruses is missing. KSHV is a case in point wherein large parts of Eastern Europe, Northern Asia and the Middle Eastern region have limited data.
Herpesviruses
Herpesviridae are a family of large, complex, double-stranded DNA viruses. The subfamily gammaherpesvirus includes two viruses that are oncogenic to humans: Epstein Bar Virus (EBV) and Kaposi’s sarcoma-associated herpesvirus (KSHV). Although there exists preliminary data linking glioblastoma to cytomegalovirus,4 other herpesviruses are not considered carcinogens.
EBV
EBV is a gammaherpesvirus with a tropism for B-lymphocytes and epithelial cells. EBV is highly prevalent around the world: over 90% of adults are seropositive in most populations, although the age of primary infection can vary widely.5 Upon primary infection, the vast majority of subjects develop a life-long asymptomatic latent infection. However, EBV is capable of transforming infected cells and is associated with several cancers including; (i) Burkitt’s lymphoma (BL) and immunosuppression-related non-Hodgkin’s lymphoma; (ii) Hodgkin’s lymphoma; (iii) extranodal NK/T lymphoma, nasal type; (iv) nasopharyngeal carcinoma (NPC); and (v) lymphoepithelioma-like carcinoma.6 Although evidence is less comprehensive, EBV is likely to be associated with gastric carcinoma. In most populations, primary infection with EBV occurring in childhood typically causes no symptoms or symptoms that are indistinguishable from mild infectious illnesses. However, in adolescence or adults as well as in some children, infection may result in infectious mononucleosis (IM) in a variable (35–75%) proportion of cases.5 IM is characterized by clinical manifestations such as fever, fatigue, pharyngitis, and cervical lymphadenopathy, as well as atypical lymphocytosis. Other agents such as cytomegalovirus and Toxoplasma gondii may cause infectious mononucleosis, and other upper respiratory infections and systemic infections may resemble IM clinically. Thus, clinical and hematological findings are not sufficiently specific to perform a diagnosis.
The heterophile antibody test is a standard diagnostic test for EBV IM when clinical features of IM are present.7 The test assesses the presence in serum specimens of heterophile antibodies, IgM antibodies with affinity for horse and sheep red blood cells, by agglutination of these cells or latex depending on the assay. These antibodies typically appear 3–4 weeks after infection, during the first weeks of IM symptoms, and disappear 3–6 months after infection. This test is highly specific, typically 98–100%, but the sensitivity is moderate, typically 81–95%.8 False negatives are especially seen in younger children, who do not produce the heterophile antibody. Serological assays assessing seroconversion to specific EBV antigens should then be employed to aid diagnosis, and are fundamental for diagnosing asymptomatic EBV infection and to infer the timing of infection.
Indirect immunofluorescence assay (IFA), enzyme immunoassay (EIA), and enzyme-linked immunosorbent assays (ELISA) techniques can be used to determine the presence of EBV-specific antibodies. A comparison of six commercially available tests for EBV-specific antibodies using these techniques has calculated specificities between 86–100% and sensitivities between 95–100%.8 IFA and ELISA techniques can be used to determine the presence of IgG against EBV viral capsid antigen (VCA-IgG), and the results of the two techniques are comparable at the different time points of infection; acute, convalescent and previous infection.9–11 VCA-IgM assessed by both methods has been found to decrease over time.11, 12 Considering that symptoms typically occur six to eight weeks after infection,13 the presence of VCA-IgG and VCA-IgM generally indicate infection with EBV for less than four months.11 IgG against EBV nuclear antigen (EBNA) develops weeks or months later.14 Thus, acute infection is characterized by the presence of VCA-IgM and not EBNA-IgG, while post-acute infection is characterized by the presence of EBNA-IgG and not VCA-IgM (Table 1). IgG against the EBV early antigen (early antigen-diffuse, EA-D) appears generally after VCA-IgM and before EBNA-IgG, and tends to disappear after convalescence from the primary infection. However, assays detecting EA-D are not available to all laboratories.
Table 1.
Interpretation of EBV serology results.
| VCA-IgG | EBNA-IgG | EA-IgG | VCA-IgM | Het IgM | |
|---|---|---|---|---|---|
| Naïve | Neg | Neg | Neg | Neg | Neg |
| Primary infection | Neg | Neg | Pos/Neg | Pos | Pos/Neg |
| Latent infection and reactivation |
Pos | Pos/Neg | Pos/Neg | Pos/Neg | Neg |
Disadvantages of the methods described above include that they may be labor intensive and require a separate assay for each analyte. Multiplex flow immunoassays (MFIs) have emerged as an alternative that allows the simultaneous detection and identification of multiple antibodies/antigens in a single reaction.15 MFIs use unique microspheres in suspension, each conjugated with a different capture antibody or antigen for incubation with serum specimens. Addition of a fluorescently labeled reporter molecule whose emission can be measured allows each analyte to be detected and quantified. A recent evaluation of MFI has demonstrated; (i) the simultaneous detection of three EBV serological markers (VCA-IgG, VCA-IgM, and EBNA-IgG); (ii) quicker assay time compared to EIA (2.3 hours for 100 samples versus 4.5 hours); (iii) the incorporation of internal controls into each reaction; and (iv) higher-throughput compared to EIA.15 This technology may enable high-volume clinical laboratories to rapidly and directly test samples for specific EBV antigens.
Serology may find specific limited applications in investigating certain EBV related malignancies. NPC patients often demonstrate elevated titres of IgA directed towards EBV early antigens and virus capsid antigens, making these the classic serological markers for NPC diagnosis. IgG directed against the EBV BRLF1 gene product Rta has also been demonstrated to be elevated in NPC patients compared to healthy volunteers and control patients,16 indicating that this serological technique may find use in NPC screening. Endemic BL, associated with falciparum malaria, is the most common childhood malignancy in equatorial Africa. Its defining feature is the translocation involving chromosomes 8 and 14, 22, or 2. Seroepidemiological studies of endemic BL have demonstrated that Ugandan children with elevated titres of antibody reactive to EBV structural proteins have a greater risk for BL.17 Similarly, patients with Hodgkin’s disease often demonstrate elevated titres of antibodies reactive to EBV structural proteins before the onset of lymphoma compared to the general population.18 Serology may also prove useful in diagnosing chronic active EBV disease (CABV), a lymphoproliferative disorder characterized by markedly elevated levels of antibody to EBV and/or EBV DNA in lymphocytes. However, serology is in general unhelpful for diagnosing of EBV related cancers. Summarizing, serology is useful to diagnose EBV acute and post-acute infections, however, for most EBV-associated lymphoproliferative diseases and cancers, molecular techniques play a major role in diagnosis and management.
Kaposi Sarcoma-associated Herpesvirus
KSHV has a broad viral tropism including peripheral blood B-cells, endothelial cells, keratinocytes, monocytes and epithelial cells19 and is the etiological agent of Kaposi’s sarcoma (KS), primary effusion lymphoma (PEL), and a type of multicentric Castleman’s disease.20 Unlike EBV, KSHV is not ubiquitous. KSHV prevalence is 35–60% or higher in sub-Saharan Africa,21 approximately 10–30% in the Mediterranean region,22 and is low in the United States and Western Europe, where it is however elevated in men who have sex with men (MSM).23
Seroepidemiological studies, have used various assays for the detection of KSHV antibodies. The first serological assays developed for detecting KSHV antibodies were IFAs.24 IFAs that use uninduced PEL cell lines, where KSHV is primarily latent, detect antibodies to KSHV latency-associated nuclear antigen (LANA)/ORF73.25 Induction of KSHV into the lytic phase was subsequently found to increase the sensitivity of IFAs in identifying KS patients. Important lytic phase proteins include K8.1, a surface glycoprotein, and ORF65, a small capsid protein. IFAs have several disadvantages: they are highly subjective and have variable reproducibility that is difficult to monitor. Currently, EIA and ELISAs employing a small number of viral peptides or proteins are also used; for instance our lab has been using ELISA against recombinant K8.1 or ORF7326 in numerous epidemiological studies conducted in many countries. Comparison of various serological assays for detecting KSHV has been conducted.27 In this study, sensitivity in identifying patients with KS varied between 80 and 97%, and between 92 and 100% when investigating patients with a history of KS. The use of assays in combination has also been investigated to improve accuracy compared to single assays.
Because KSHV establishes a lifelong, often-asymptomatic infection, with only occasional reactivations, PCR assays for the detection of KSHV are of limited diagnostic use. KSHV saliva shedding is inconsistent, and viral DNA in peripheral blood mononuclear cells is detected in only approximately 10% of KSHV seropositive asymptomatic individuals.28 Currently there is no Food and Drug Administration (FDA) approved KSHV serological assay, and clinicians who use research laboratory testing should be informed upon the caveats of the assays that are available. The above serological techniques have been fundamental in defining KSHV epidemiology but they are not designed for diagnostic purposes. Serum from KS patients is typically used as the positive control for serological assays. KS patients generally have high antibody levels to KSHV whereas asymptomatic infected subjects often have low levels of antibodies. Therefore sensitivity estimates alone may be too high. Identifying negative controls is also difficult. Blood donors from low prevalence areas are commonly used as negative controls, however, since a small proportion of blood donors are likely to be infected with KSHV specificity estimates may be too low. Another major complication for interpreting KSHV serological data clinically is that seroreactivity to individual antigens may change over time, as shown by longitudinal studies utilizing a variety of techniques.29, 30 For instance, a subject could be reactive to ORF73 many years before demonstrating reactivity to K8.1, or vice versa, and importantly this pattern is not predictable. In some cases, when subjects are followed longitudinally, seroreversion (a negative result following a positive result) can occur.27
These limitations have promoted the development of assays that detect antibody to additional antigens. KSHV encodes more than 85 proteins, the antigenic potential of which had not been systematically investigated. These efforts are being facilitated by newer multiplexed platforms. Luciferase immunoprecipitation system (LIPS) technology has been applied to KSHV serology with considerable success.31 A multiplex bead assay has also been recently developed by our lab.32 Bead-based and other multiplexable assays have advantages over ELISAs. They have a wider dynamic range, which reduces the need to dilute samples, and they can semi-quantitatively determine antibody levels.33 This allows bead assays to be informative clinically, as antibody levels are related to KSHV replication34 and associated with KS disease risk.35 Multiplexed bead assays also reduce sample volume requirement and cost per sample compared to ELISAs.
Future improvements in techniques for serologically diagnosing KSHV will likely lead to better understanding of; (i) KSHV transmission and prevalence variation; (ii) the risk of KSHV-related disease in infected individuals; and (iii) the response of treatments for KSHV and related diseases in clinical settings.
Polyomaviruses
Polyomaviridae are small, icosahedral, non-enveloped DNA viruses that have been found in a large number of vertebrate species. Murine polyomavirus was discovered while investigating mouse leukemia,36 and subsequently was shown to induce tumor formation in mice.37 Subsequently, additional polyomaviridae were discovered; simian virus 40 (SV40) was isolated in 1960 from rhesus monkey kidney cells.38 These agents were the first oncoviruses ever identified in mammals. The first two human polyomaviridae, JCV39 and BKV40 were isolated in 1971. The first human oncogenic polyomavirus, the Merkel cell polyomavirus (MCPyV), was identified in 2008.41 MCPyV is the causative agent of Merkel cell carcinoma (MCC).42 The polyomavirus family is expanding rapidly: eight novel human polyomaviridae (HPyVs) have been discovered over the last five years. Besides MCPyV, no polyomavirus has been causally associated with human cancers, although limited evidence exists for the role of JCV in central nervous system tumors and colon carcinoma,43, 44 and for BKV in prostate cancer.
Human polyomaviridae are widespread (seroprevalence vary from 55 to 80%). Generally speaking, primary polyomaviridae infections are common amongst children and young adults, and are often asymptomatic. In the vast majority of cases, they establish life-long asymptomatic infection in their hosts. However, viral reactivations occur occasionally in healthy hosts and more frequently in immunologically impaired hosts. Infection with JCV and BKV typically occurs in late 45 and early childhood, respectively. BKV infection is associated with hemorrhagic cystitis in bone marrow transplant recipients and nephropathy (PVAN) in renal transplant recipients. In patients with AIDS or iatrogenic immune deficiency, including patients receiving certain recombinant antibodies, JCV infection is associated with the demyelinating disease progressive multifocal leukoencephalopathy (PML). BKV DNA in plasma can be used to diagnose PVAN.46 Identification of JCV DNA in cerebrospinal fluid or brain biopsies is important for diagnosis of PML. Determination of host antibody response to polyomaviridae viruses is useful to understand epidemiology of infection and pathogenesis of the associated diseases.
Hemagglutination inhibition assays (HIAs) have been the standard method for determination of antibody titres for BKV and JCV. The major capsid protein of JCV and BKV (VP1) is responsible for viral attachment to cells and is capable of erythrocyte agglutination.47 In HIAs, serum and antigen are incubated before the addition of human O erythrocytes. Complete inhibition of hemagglutination indicates the presence of antibodies bound to viral antigen and indicates a positive response.48 An English population-based study using HIA results has demonstrated BKV seroprevalence reaching 91% by 5–9 years of age and JCV seroprevalence of 50% by 60–69 years of age.48
More recently EIAs are performed for measuring HPyV antibodies; they are more accurate than HIA, and also more economical for large-scale seroepidemiological studies. Virus-like particles (VLPs) for BKV and JCV can be used as antigens in an EIA measuring serological response for specific IgG or IgM.49 BKV seroprevalence (IgG detection) in healthy blood donors ranged from 87% in 20–29 years old to 71% in 50–59 years olds.49 JCV seroprevalence ranged from 50% in the youngest age group to 71% in the oldest age group.49 Results also indicated that cross-reactivity was not a major concern for BKV and JCV VLP IgG responses.49 A separate study reported a comparison of the antibody titres by HIA and EIA for JCV and BKV, with titres being lower for HIA compared to EIA.50 Results from this study also indicated the production of antibodies that were against species-specific epitopes of the VP1 protein.
Similarly, MCPyV primary infections occur ubiquitously in childhood, so serodiagnosis of MCPyV is not particularly useful in predicting MCC risk. However, a recent large longitudinal study utilizing a MCV neutralization assay and an assay detecting IgG antibodies to MCV pseudovirions, indicated that MCC was associated both with high levels of MCV antibodies and with MCV neutralizing activity, particularly in women.51 MCV T-antigen antibody screening may prove useful in monitoring MCC, as antibody levels are associated with disease burden and risk of recurrence.52
SV40 has controversially been hypothesized as playing a role in a variety of human cancers,53 and SV40 DNA has been detected by PCR in various tumor cells. Human exposure to SV40 occurred on a wide scale between 1955 and 1963 as a result of inadvertent contamination of a poliovirus vaccine,54 however it now appears to also be transmitted interpersonally.55 A case control study has shown that SV40 is unlikely to be associated with cancer in humans.56 VLP-based EIAs are employed in SV40 epidemiology; however, serological cross-reactivity between SV40 and JCV and BKV may complicate interpretations of assay results,57 as there is protein homology amongst these viruses. Development of specific assays for detection of SV40 is necessary for determining the prevalence of SV40 in the human population and for establishing the role of SV40 in carcinogenesis. Recently, an indirect ELISA using synthetic peptides to SV40 capsid viral protein 1–3 epitopes has been utilized to screen healthy children and adolescents for exposure to SV40.58 Results have indicated that; (i) SV40 infection is not widespread (16% prevalence of IgG antibody); (ii) IgM antibodies can be detected in 6–8 month old children, indicating that seroconversion can occur early in life; and (iii) that SV40 is circulating in the human population independent of SV40-contaminated vaccination. One caveat for this is that serological data for SV40 may be due to cross-reactivity to an, as yet undiscovered, human polyomavirus that is closely related to SV40. This highlights the need for increased specificity in serological assays used for detecting closely related viruses.
Human Papillomavirus
HPV are double-stranded, circular DNA viruses that establish infection in keratinocytes of the skin or mucous membranes. Over 120 HPV genotypes have been identified.59 Worldwide, HPV is associated with 5.2% of new cancers.2 Most HPV infections do not cause disease, however HPV infection is the etiological agent of cervical cancer, the second most common cancer of women.60 High-risk HPV types, including HPV16 and HPV18, are associated with cervical cancers61 as well as anal cancer, vulvar cancer, penile cancer, vaginal cancer and HPV-positive oropharyngeal cancer.2, 62 Two prophylactic HPV vaccines are currently available.63
Diagnosis of HPV infection relies mainly on molecular techniques. HPV DNA screening by PCR or hybrid capture, a non-amplification-based nucleic acid detection method, has in recent years supplemented the Papanicolaou test (Pap test) as screening tools for secondary prevention of cervical cancer. HPV DNA has been shown to be detectable in cervical cells from the cervix for less than a year in most infected women.64 Indeed, most HPV infections clear and precancerous lesions also resolve in a proportion of cases depending on the severity of the lesion. HPV infection and replication is limited to peripheral epithelial cells, restricting presentation of viral antigens to the host immune system. This gives rise to a modest but detectable antibody levels in most infected individuals. Persistent HPV infections are more likely than transient infections to cause seroconversion,65 and are more likely to cause high-grade cervical lesions and cancers.66 Nonetheless, in practice HPV serology is not used for risk stratification, nor for diagnosis, although the majority of infected women will develop type-specific HPV antibodies.67 The biology of HPV infection, however, provides the opportunity for the implementation of a novel vaccinal strategy, as VLPs give rise to virus-neutralizing antibodies in the serum. Serology is used to supplement molecular studies of the epidemiology and the pathogenesis of HPV infection, and to assess the immunogenicity of HPV vaccines. Two types of serological assays are routinely employed for screening of anti-HPV antibodies, the neutralization assay and ELISA.
HPV neutralization assays, specifically, secreted alkaline phosphatase neutralization assay (SEAP-NA), are based on neutralization of either; (i) HPV virions; (ii) pseudotyped virions consisting of capsids containing reporter genes on their surface; or (iii) pseudovirions (PsVs) that have encapsidated reporter genes. Neutralization assays have similar sensitivity and better specificity than standard papillomavirus-like particle (VLP)-based ELISAs,68 and detect antibodies of all immunoglobulin classes while most ELISAs detect only IgG. HPV VLP-based ELISAs use type-specific HPV L1 protein capsids as the antigen.69 HPV16 VLP-based ELISAs have a moderate sensitivity of about 50% in detecting current infections identified by HPV DNA.69 A limitation of ELISA is that it requires a large volume of serum to test for antibodies to each HPV type. Thus, high-throughput methods to assess multiple HPV types have been developed. One such multiplexed approach; the competitive Luminex immunoassay (cLIA) is bead-based and uses pseudovirions of eight mucosal HPV types (including HPV16 and HPV18) and two cutaneous HPV types.70 Multiplexed approaches may prove to be useful tools in the continuing investigation of HPV epidemiology.
HPV serology has been instrumental for the development and optimal deployment of HPV vaccines. Results from studies in which both HPV DNA and serology were determined indicated that 81–87% of subjects within the HPV vaccine eligible age group (initially females 9–26 years old) are both HPV16 DNA negative and seronegative worldwide, indicating that the majority of women in the initial target population might obtain protection through vaccination. Serology is also relied upon to investigate immunological correlates of vaccinal protection. HPV type-specific L1 VLP-neutralizing antibodies reach a maximum titre 7 months after the last injection of the vaccine. Titres then decline up to month 24, thereafter remaining stable.71 Three years post-vaccination, antibody titres are 2–20 fold higher than controls.71 A recent study compared cLIA, SEAP-NA, and VLP-based ELISA in a large vaccine trial (HPV16/18): although all three assays detected 100% seroconversion for HPV16 after the third vaccine dose, the assays differed in range and sensitivity, and demonstrated notable differences after one vaccine dose and for HPV18.72 Continued monitoring of antibody levels by serology in immunized individuals will need to be conducted into the future for assessment of vaccine efficacy; however, in this and other applications, it is important to be cognizant of differences that exist between these assays in several technical and biological aspects.
Retroviruses
HTLV-1
HTLV-1, the first human retrovirus discovered, is a single-stranded RNA human type C retrovirus. HTLV-1 is prevalent in Japan, Taiwan, Papua New Guinea, central African coastal regions, and the Caribbean. HTLV-1 has tropism for CD4 T-cells and infection is associated with the development of adult T-cell leukemia (ATL),73 as well as HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP), a chronic, progressive demyelinating disorder.74 It is estimated that 10–25 million people are infected with HTLV-1, however disease is observed in less than 5% of cases.75 A related virus, HTLV-2, has so far no proven etiological role in the development of any human disease. Discrimination of HTLV-1 from HTLV-2 is clinically relevant. Several countries have begun serological screening of HTLV-1 and −2 in blood donors.76
A variety of serological techniques are used to detect HTLV-1 antibodies. EIAs based on viral lysates were among the first developed and frequently resulted in false-positives.77 EIAs based on recombinant proteins or synthetic HTLV-1 peptides perform better, but often still require confirmatory tests to determine false-positives and discriminate between HTLV-1 and −2.76 Confirmatory tests may include IFA techniques, western blots, or line immunoassay. Typically, assays detect antibodies to HTLV-1 proteins encoded by gag (core proteins p19 and p24), env (envelope proteins gp46, and gp61/68), and tax (p40x) genes.78 More recently, an HTLV-1/2 ELISA with high specificity and sensitivity has been developed that may be useful in screening for blood transfusions, tissue transplants and clinical diagnosis.79 Importantly, this technique can discriminate between HTLV-1 and −2.
At present there are two FDA approved tests for the detection of antibodies to HTLV-1/2 in human plasma/serum. One, approved in 2008, is a chemiluminescent immunoassay. This test cannot distinguish between HTLV-1 and −2. The other, approved in 2012, is an ELISA utilizing purified HTLV-1 and HTLV-2 viral lysates as well as recombinant HTLV-1 p21E antigens. In the U.S., donors of leucocyte rich blood products must be screened for HTLV-1/2. In low seroprevalence populations (such as U.S. blood donors) the positive predictive value of any assay is low, so most positive results turn out to be false positives. As such, confirmation assays should follow positive screening tests. U.S. Public Health Service guidelines indicate that positive result tests should be confirmed by western blot or radioimmunoprecipitation assay and these confirmatory tests should include HTLV-1 or −2 type-specific peptides/probes.80 Both assays are highly specific (approximately 99%) and sensitive (approximately 100%). In indeterminate cases, PCR can be used to diagnose infection. PCR is also the method of choice for quantifying the viral load, which is used as a marker for prognosis and disease progression in infected patients.
HTLV-1 remains a poorly recognized infection. Improvements in serological techniques for diagnosing HTLV-1 infection in a disease setting, conducting epidemiological studies, and for screening of blood will be invaluable for improving our understanding of this virus.
Hepatitis viruses
Hepatitis B Virus
HBV is a partially double-stranded DNA virus of the family Hepadnaviridae, and is a major cause of cirrhosis, chronic hepatitis, and hepatocellular carcinoma. HBV has a prevalence of 2–20% of the general population in Africa, Asia, South America and Southern Europe.81 Acute infection can be asymptomatic, or result in acute hepatitis with varying degree of severity, from mild to fulminant disease. Following resolution of the acute infection, the virus is cleared in the majority of cases. The risk of HBV infection becoming chronic is greater when infection occurs during childhood or prenatally. Chronic infection can be asymptomatic, or evolve into chronic hepatitis of variable severity, cirrhosis, or hepatocellular carcinoma. HBV viral genes encode for several transcripts including; (i) surface antigen (HBsAg); (ii) e antigen (HBeAg); and (iii) core antigen (HBcAg). Host antibodies against HBV antigens can be detected for the diagnosis of HBV infection: acute, post-acute and chronic infections are characterized by different pattern of serological reactivity. Host immune response also plays a major role in HBV-related liver damage, both in the course of acute and chronic hepatitis. Fulminant hepatitis, occurring in 0.1–0.5% of patients, is due to immune-mediated lysis of infected hepatocytes.82 Early diagnosis of HBV is critical for beginning treatment before advanced liver disease develops.
A recombinant HBV vaccine is available; the Advisory Committee on Immunization Practices (ACIP) of the Centers for Diseases Control and Prevention (CDC) recommends that all infants be immunized starting at birth. Immunization of adult at higher risk is also recommended (Table 2a). The U.S. Preventive Services Task Force (USPSTF) recommends against routinely screening the general asymptomatic population for chronic hepatitis B virus infection. However, testing is recommended for certain categories (Table 2b). HBV serological tests can be used to determine if an individual is susceptible to HBV infection, has immunity to HBV infection due to vaccination or prior infection, or has acute or chronic HBV infection. Susceptible individuals are characterized as HBsAg, anti-HBc, and anti-HBs negative. The serological hallmark of current HBV infection is the presence of HBsAg, which is the first serological marker to appear after infection. Acutely infected individuals are positive for HBsAg, anti-HBc IgG and IgM and negative for anti-HBs. Anti-HBc IgG is not a neutralizing antibody. Presence of neutralizing antibodies against the HBsAg, anti-HBs, is suggestive of immunity against HBV developed after recovery from acute HBV infection. Such individuals are also HBsAg negative and anti-HBc IgG positive. Because anti-HBV vaccines are composed of recombinant HBsAg, individuals who have responded successfully to HBV vaccination are also anti-HBs positive and HBsAg negative, but are anti-HBc negative. The failure to clear HBsAg and to develop anti-HBs is associated with chronic HBV infection. Chronic infection is also serologically characterized as anti-HBc IgG positive, and anti-HBc IgM negative. Chronic carriers of HBV comprise a large reservoir for infecting additional cases, and are at risk of developing HBV associated liver diseases. HBcAg is not detectable in serum. IgM anti-HBc is the first antibody to appear after HBV infection, typically within one month after the appearance HBsAg. Detection of IgG anti-HBc is indicative of prior exposure to HBV, regardless of the current HBsAg status. This is referred to as occult infection, and is frequently seen in patients who have hepatitis C virus-related chronic hepatitis.83 It has been suggested that the absence of HBsAg is due to a rearrangement in the HBV genome that interferes with the expression or production of an antigenically modified S protein.84
Table 2.
| a. Recommendations for HBV immunizations of adults. |
|---|
|
| b. Recommendations for testing for chronic HBV infection. |
|---|
|
| c. Interpretation of HBV serology results. | ||||||
|---|---|---|---|---|---|---|
| HBsAg | HBeAg | HBcAb-IgM | HBcAb- IgG |
HBsAb | HBeAb | |
| Naïve | Neg | Neg | Neg | Neg | Neg | Neg |
| Vaccinal immunity | Neg | Neg | Neg | Neg | Pos | Neg |
| Acute infection | Pos | Pos | Pos | Pos | Neg | Neg |
| Natural immunity | Neg | Neg | Neg | Pos | Pos | Neg |
| Chronic infection | Pos | Pos/Neg | Neg | Pos | Neg | Pos/Neg |
| Indeterminate* | Neg | Neg | Neg | Pos | Neg | Neg |
May represent occult infection, but most commonly denotes false positive or, in some cases, resolving or resolved acute infection.
Positive serology for HBeAg is indicative of an active replication cycle of HBV and an increased risk of transmission. It should be noted that a proportion of HBeAg negative patients continue to have HBV replication and liver damage. The seroconversion from HBeAg positivity to anti-HBe positivity typically parallels a decrease in serum HBV DNA levels. This is indicative of a less active viral replication, a decrease in infectivity, and disease remission.
Interpretation of HBV serological markers is summarized in Table 2c. Serology is also useful for identifying patients in various stages of HBV infection. There are three phases for the natural history of chronic hepatitis B virus patients who acquire virus early in life; (i) the immune tolerance phase; (ii) the immune clearance phase; and (iii) the inactive or low replication phase. A fourth phase, when it occurs, can be termed the reactivation phase.82 The immune tolerance phase is typically seen in children and young adults and is associated with high levels of HBV DNA and detectable HBeAg. These patients have minimum disease progression and normal liver enzyme levels. After 20–30 years of infection, the immune clearance phase begins. This phase is characterized by high levels of HBV DNA and detectable HBeAg. Immune-mediated cytotoxicity to infected hepatocytes causes elevated alanine aminotransferase levels. During the third phase, HBV replication greatly diminishes or ceases. This phase is characterized by low levels of HBV DNA, absence of HBeAg, normal alanine aminotransferase levels, and normal or minimal change in liver pathology. The infection can then clear, or persist in the inactive phase, or progress to reactivation phase. Reactivation of HBV may be spontaneous85 or due to immunosuppression,86 and is characterized by moderate levels of HBV DNA, negative HBeAg, elevated alanine aminotransferase levels, and chronic liver inflammation and fibrosis. Annually, 2.2–3.3% of asymptomatic HBsAg carriers will reactivate HBV replication following HBeAg seroconversion.87
HBV serological testing may also play a role in stratifying patient’s response to anti-HBV therapy. HBeAg seroconversion is useful for assessing the efficacy of anti-HBV therapy in patients with chronic hepatitis. HBeAg serology was found to be a better measurement than HBV DNA serum levels for predicting HBeAg seroconversion to anti-HBe in patients treated with peginterferon alpha-2a.88 In a separate small study, low pretreatment HBsAg levels were found to be a better measurement than HBV DNA to predict positive responses to peginterferon and lamivudine combination therapy.89
Finally, serology is useful in typing HBV, although the clinical significance of HBV serotypes is not yet well established. There are four major HBV serotypes (adr, adw, ayr, and ayw) and nine minor serotypes, defined by their antigenic determinants of HBsAg. The relation between HBV serotypes and genotypes has been determined, and there are eight established genotypes (genotypes A through H) with distinct geographical distributions and clinical significance.90 Genotype information may be used to assess the risk of disease development in certain populations. For instance, in Taiwan genotype C (serotype adw2, adrq+, adrq−, ayr) is associated with development of severe liver disease while genotype B (serotype adw2, ayw1) is associated with development of hepatocellular carcinoma in non-cirrhotic patients.91 In India, genotype D (serotype ayw2, ayw3) is associated with hepatocellular carcinoma in young patients and with more severe liver disease than genotype A (serotype adw2, ayw1).92 Genotype information may also be used to predict response to antiviral treatments. For instance; (i) genotypes A and B patients demonstrate a better response to interferon therapy than genotypes C and D;93 (ii) genotype B patients demonstrates a better response to lamivudine therapy than genotype C;94 and (iii) serotype adw patients are associated with a greater risk of developing resistant to lamivudine therapy than serotype ayw.95
Hepatitis C Virus
HCV is an enveloped single-stranded RNA virus of the family Flaviviridae. HCV infection is of global concern. Regional variations in infection prevalence exist. North Africa/Middle East and Central/East Asia are regions of high prevalence, greater than 3.5% of the population,96 with Egypt having the highest prevalence at 14%.97 HCV transmission is primarily parenteral.98 Risk factors for HCV transmission vary between developed and developing countries. In low prevalence areas (developed countries), injecting drug use is the main mode of transmission. In high prevalence areas (developing countries), unsafe medical practices cause a substantial proportion of HCV transmissions, as much as 40% of all HCV infections.99 Together with HBV, HCV is a leading cause of chronic liver disease, cirrhosis, and hepatocellular carcinoma. It is estimated that over 350,000 people worldwide die of HCV-related liver disease each year.100 The U.S. Preventative Services Task Force (USPTF) recommends screening for HCV in persons at high risk for infection (Table 3a) and adults born between 1945 and 1965.
Table 3.
| a. Recommendations for testing for HCV infection. |
|---|
|
| b. Interpretation of HCV infection results. | |||
|---|---|---|---|
| Anti- HCV Ab |
HCV RNA |
Interpretation | Further Action |
| Neg | Not Assessed |
No HCV antibody detected |
If recent exposure is suspected, test for HCV RNA |
| Neg | Pos | Acute Infection |
Provide with appropriate counseling, care and treatment |
| Pos | Not Assessed |
Presumptive HCV infection |
Test for HCV RNA to identify current infection |
| Pos | Pos | Current HCV infection |
Provide with appropriate counseling, care and treatment |
| Pos | Neg | False Positive | Test with another HCV antibody assay to assess false positives |
| Pos | Neg | Resolved Infection |
Follow up with appropriate counseling in certain situations* |
If the subject is suspected to have recent HCV exposure within the past 6 months, or has clinical evidence of HCV infection, or if there is concern with the handling/storage of test specimens.
EIAs are the serological assays of choice for identifying anti-HCV antibodies in serum. Third generation anti-HCV EIAs have a specificity of greater than 99% and have excellent sensitivity when used for HCV-infected immunocompetent patients.101 HCV serological assays are mainly applied in diagnostic screening and epidemiological studies. They also assist in discriminating acute from chronic infection, and in resource limited settings, may find a role in HCV genotyping, although molecular techniques remain fundamental both in clinical practice and molecular epidemiology.
The majority of acute HCV infections are asymptomatic. Positivity for HCV RNA in the absence of anti-HCV antibodies is indicative of acute HCV infection. Interpretations of results for HCV infection, based on CDC guidance,102 are summarized in Table 3b. Patients suspected of having acute HCV infection should be tested for anti-HCV antibodies and HCV RNA. Diagnosis of acute HCV infection may also include documentation of recent seroconversion of HCV antibodies (within the last 6 months).103 If a patient with acute hepatitis is positive for both HCV RNA and anti-HCV antibodies it can become difficult to differentiate between acute HCV infection and chronic HCV infection with superimposed acute process of different etiology.
Resolved infection (15% of cases) is characterized by the presence of anti-HCV antibody and absence of HCV RNA.104 It should be noted that this pattern cannot be differentiated from a false positive EIA result, however, EIAs results should be in any case confirmed by recombinant immunoblot assay. Aviremic, seropositive patients should be retested after a few weeks to confirm that HCV RNA is truly negative and not just temporarily undetectable. Chronic infection is mostly asymptomatic until the development of cirrhosis or end-stage liver disease and is characterized by anti-HCV antibody positivity and HCV RNA positivity. Benign persistent disease with normal/mildly elevated liver enzymes occurs in approximately 70% of cases.105
HCV has been classified into six main genotypes (a seventh has been proposed) and a large number of subtypes. HCV genotype determination must be performed and should occur before treatment begins, as it determines the indication, the duration and the dose of anti-viral therapy, and the monitoring of virological response. Genotype can be determined by a number of molecular approaches including PCR with genotype-specific primers, hybridization techniques, or restriction fragment length polymorphism techniques. In 2013, the FDA approved the first real time PCR HCV genotyping test, which distinguishes types 1, 1a, 1b, 2, 3, 4, and 5. Although clinical practice relies on molecular techniques, when these are not accessible or affordable serology might become a reliable, fast, and inexpensive alternative for subtyping HCV.106 Epitopes encoded by the NS4 region of the HCV genome has shown utility for serologically discriminating HCV genotypes.107 A comparison of two serological methods for determination of HCV genotype has shown sensitivity of 75% and 89% relative to results obtained with a standard genotyping assay.108 Both of these assays had good concordance with the standard genotyping assay. However, for these two assays cross-reactivity amongst HCV genotypes might be responsible for mistyping in 6–8% of the patients. Mixed serological reactivities might also be due to a mixed infection or the recovery from one genotype infection with current viremia from another genotype infection. Serology for determination of HCV genotype may come into more routine use, but molecular assays for genotyping remain standard. Molecular methods remain also the gold standard for assessing treatment efficacy, with sustained virological response being assessed by the absence of HCV RNA in serum 24 weeks after the end of treatment.
Conclusion
Viral infections remain a substantial cause of cancer worldwide, and particularly in developing countries with the least resources to deal with this burden. In developing countries approximately 23% of new cases of cancer are attributable to an infectious agent, compared to 7% in more developed countries.109 Improved serological diagnosis of these viruses will be critical for; (i) diagnosing infection with high specificity and sensitivity; (ii) conducting studies aimed at investigating prevalence, epidemiology, and transmission; and (iii) developing therapeutics to prevent disease burden and monitor treatment. The serological assays for each of the tumor viruses discussed within this article have their own unique technical challenges and challenges in diagnosis application in the clinical setting. Serological assays have evolved over the years from IFA techniques to EIAs/ELISAs to newer multiplexable techniques. With these innovations has generally come increased specificity and sensitivity as well as reproducibility; cost per analyte/sample and processing times have also decreased. Improved serological techniques have given greater insight into host immune response to these viruses over the course of infection and disease. Serological techniques will continue to remain important for conducting epidemiological work and surveying the global health burden associated with tumor viruses. Tumor viruses are a potentially preventable cause of cancer. Prophylactic vaccines against HBV and HPV are now commercially available. Serological techniques may be used to determine suitable target populations for vaccination and assess vaccine efficacy, in particular by determining virus-neutralizing antibody concentrations over time. There are no therapeutic vaccines for virus-associated malignancies. However, tumor viruses are attractive candidates for such vaccines, particularly if viral oncogenes are selectively expressed in tumor tissue. Serodiagnosis will likely also play an important role, along with molecular techniques, in identifying potential new tumor viruses and in assessing the association of a putative tumor virus with a specific cancer.
Acknowledgments
Financial Disclosure Statement: No financial disclosures to report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McLaughlin-Drubin ME, Munger K. Viruses associated with human cancer. Biochimica et biophysica acta. 2008;1782(3):127–150. doi: 10.1016/j.bbadis.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parkin DM. The global health burden of infection-associated cancers in the year 2002. International journal of cancer Journal international du cancer. 2006;118(12):3030–3044. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 3.Humans IWGotEoCRt. Biological agents. Volume 100 B. A review of human carcinogens. IARC monographs on the evaluation of carcinogenic risks to humans / World Health Organization, International Agency for Research on Cancer. 2012;100(Pt B):1–441. [PMC free article] [PubMed] [Google Scholar]
- 4.Price RL, Song J, Bingmer K, Kim TH, Yi JY, Nowicki MO, et al. Cytomegalovirus contributes to glioblastoma in the context of tumor suppressor mutations. Cancer research. 2013;73(11):3441–3450. doi: 10.1158/0008-5472.CAN-12-3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Proceedings of the IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Epstein-Barr Virus and Kaposi's Sarcoma Herpesvirus/Human Herpesvirus 8. Lyon, France, 17–24 June 1997. IARC Monogr Eval Carcinog Risks Hum. 1997;70:1–492. [Google Scholar]
- 6.Cohen JI. Epstein-Barr virus infection. The New England journal of medicine. 2000;343(7):481–492. doi: 10.1056/NEJM200008173430707. [DOI] [PubMed] [Google Scholar]
- 7.Storch GA. Diagnostic virology. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2000;31(3):739–751. doi: 10.1086/314015. [DOI] [PubMed] [Google Scholar]
- 8.Bruu AL, Hjetland R, Holter E, Mortensen L, Natas O, Petterson W, et al. Evaluation of 12 commercial tests for detection of Epstein-Barr virus-specific and heterophile antibodies. Clinical and diagnostic laboratory immunology. 2000;7(3):451–456. doi: 10.1128/cdli.7.3.451-456.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerber MA, Shapiro ED, Ryan RW, Bell GL. Evaluations of enzyme-linked immunosorbent assay procedure for determining specific Epstein-Barr virus serology and of rapid test kits for diagnosis for infectious mononucleosis. Journal of clinical microbiology. 1996;34(12):3240–3241. doi: 10.1128/jcm.34.12.3240-3241.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gutierrez J, Vergara MJ, Piedrola G, Maroto MC. Clinical reliability of IgG, IgA, and IgM antibodies in detecting Epstein-Barr virus at different stages of infection with a commercial nonrecombinant polyantigenic ELISA. Journal of clinical laboratory analysis. 1999;13(2):65–68. doi: 10.1002/(SICI)1098-2825(1999)13:2<65::AID-JCLA4>3.0.CO;2-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rea TD, Ashley RL, Russo JE, Buchwald DS. A systematic study of Epstein-Barr virus serologic assays following acute infection. American journal of clinical pathology. 2002;117(1):156–161. doi: 10.1309/ETK2-L9MG-L6RA-N79Y. [DOI] [PubMed] [Google Scholar]
- 12.Nikoskelainen J, Ablashi DV, Isenberg RA, Neel EU, Miller RG, Stevens DA. Cellular immunity in infectious mononucleosis. II. Specific reactivity to Epstein-Barr Virus antigens and correlation with clinical and hematologic parameters. Journal of immunology. 1978;121(4):1239–1244. [PubMed] [Google Scholar]
- 13.Schooley RT, Dolin R. Epstein-Barr virus (Infectious Mononucleosis) In: Mandell GL, Bennett JE, Dolin R, editors. Principles and Practice of Infectious Diseases. New York, NY: Churchill Livingston; 1990. pp. 1172–1185. [Google Scholar]
- 14.Henle W, Henle G, Andersson J, Ernberg I, Klein G, Horwitz CA, et al. Antibody responses to Epstein-Barr virus-determined nuclear antigen (EBNA)-1 and EBNA-2 in acute and chronic Epstein-Barr virus infection. Proceedings of the National Academy of Sciences of the United States of America. 1987;84(2):570–574. doi: 10.1073/pnas.84.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Binnicker MJ, Jespersen DJ, Harring JA, Rollins LO, Beito EM. Evaluation of a multiplex flow immunoassay for detection of epstein-barr virus-specific antibodies. Clinical and vaccine immunology : CVI. 2008;15(9):1410–1413. doi: 10.1128/CVI.00082-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng P, Chan SH, Soo MY, Liu D, Guan M, Ren EC, et al. Antibody response to Epstein-Barr virus Rta protein in patients with nasopharyngeal carcinoma: a new serologic parameter for diagnosis. Cancer. 2001;92(7):1872–1880. doi: 10.1002/1097-0142(20011001)92:7<1872::aid-cncr1704>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 17.de-The G, Geser A, Day NE, Tukei PM, Williams EH, Beri DP, et al. Epidemiological evidence for causal relationship between Epstein-Barr virus and Burkitt's lymphoma from Ugandan prospective study. Nature. 1978;274(5673):756–761. doi: 10.1038/274756a0. [DOI] [PubMed] [Google Scholar]
- 18.Mueller N, Evans A, Harris NL, Comstock GW, Jellum E, Magnus K, et al. Hodgkin's and disease Epstein-Barr virus. Altered antibody pattern before diagnosis. The New England journal of medicine. 1989;320(11):689–695. doi: 10.1056/NEJM198903163201103. [DOI] [PubMed] [Google Scholar]
- 19.Chakraborty S, Veettil MV, Chandran B. Kaposi's Sarcoma Associated Herpesvirus Entry into Target Cells. Frontiers in microbiology. 2012;3:6. doi: 10.3389/fmicb.2012.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, Babinet P, et al. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood. 1995;86(4):1276–1280. [PubMed] [Google Scholar]
- 21.Wakeham K, Webb EL, Sebina I, Muhangi L, Miley W, Johnson WT, et al. Parasite infection is associated with Kaposi's sarcoma associated herpesvirus (KSHV) in Ugandan women. Infectious agents and cancer. 2011;6(1):15. doi: 10.1186/1750-9378-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vitale F, Briffa DV, Whitby D, Maida I, Grochowska A, Levin A, et al. Kaposi's sarcoma herpes virus and Kaposi's sarcoma in the elderly populations of 3 Mediterranean islands. International journal of cancer Journal international du cancer. 2001;91(4):588–591. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1089>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 23.Martro E, Esteve A, Schulz TF, Sheldon J, Gambus G, Munoz R, et al. Risk factors for human Herpesvirus 8 infection and AIDS-associated Kaposi's sarcoma among men who have sex with men in a European multicentre study. International journal of cancer Journal international du cancer. 2007;120(5):1129–1135. doi: 10.1002/ijc.22281. [DOI] [PubMed] [Google Scholar]
- 24.Gao SJ, Kingsley L, Li M, Zheng W, Parravicini C, Ziegler J, et al. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi’s sarcoma. Nature medicine. 1996;2(8):925–928. doi: 10.1038/nm0896-925. [DOI] [PubMed] [Google Scholar]
- 25.Rainbow L, Platt GM, Simpson GR, Sarid R, Gao SJ, Stoiber H, et al. The 222- to 234-kilodalton latent nuclear protein (LNA) of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) is encoded by orf73 and is a component of the latency-associated nuclear antigen. Journal of virology. 1997;71(8):5915–5921. doi: 10.1128/jvi.71.8.5915-5921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mbisa GL, Miley W, Gamache CJ, Gillette WK, Esposito D, Hopkins R, et al. Detection of antibodies to Kaposi's sarcoma-associated herpesvirus: a new approach using K8.1 ELISA and a newly developed recombinant LANA ELISA. Journal of immunological methods. 2010;356(1–2):39–46. doi: 10.1016/j.jim.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spira TJ, Lam L, Dollard SC, Meng YX, Pau CP, Black JB, et al. Comparison of serologic assays and PCR for diagnosis of human herpesvirus 8 infection. Journal of clinical microbiology. 2000;38(6):2174–2180. doi: 10.1128/jcm.38.6.2174-2180.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mbulaiteye SM, Pfeiffer RM, Engels EA, Marshall V, Bakaki PM, Owor AM, et al. Detection of kaposi sarcoma-associated herpesvirus DNA in saliva and buffy-coat samples from children with sickle cell disease in Uganda. The Journal of infectious diseases. 2004;190(8):1382–1386. doi: 10.1086/424489. [DOI] [PubMed] [Google Scholar]
- 29.Minhas V, Crabtree KL, Chao A, M'Soka TJ, Kankasa C, Bulterys M, et al. Early childhood infection by human herpesvirus 8 in Zambia and the role of human immunodeficiency virus type 1 coinfection in a highly endemic area. American journal of epidemiology. 2008;168(3):311–320. doi: 10.1093/aje/kwn125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quinlivan EB, Wang RX, Stewart PW, Kolmoltri C, Regamey N, Erb P, et al. Longitudinal sero-reactivity to human herpesvirus 8 (KSHV) in the Swiss HIV Cohort 4.7 years before KS. Journal of medical virology. 2001;64(2):157–166. doi: 10.1002/jmv.1031. [DOI] [PubMed] [Google Scholar]
- 31.Burbelo PD, Issa AT, Ching KH, Wyvill KM, Little RF, Iadarola MJ, et al. Distinct profiles of antibodies to Kaposi sarcoma-associated herpesvirus antigens in patients with Kaposi sarcoma, multicentric Castleman disease, and primary effusion lymphoma. J Infect Dis. 2010;201(12):1919–1922. doi: 10.1086/652869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Labo N, Miley W, Marshall V, Gillette W, Esposito D, Bess M, et al. Heterogeneity and Breadth of Host Antibody Response to KSHV Infection Demonstrated by Systematic Analysis of the KSHV Proteome. PLoS Pathog. 2014;10(3):e1004046. doi: 10.1371/journal.ppat.1004046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Jager W, Rijkers GT. Solid-phase and bead-based cytokine immunoassay: a comparison. Methods. 2006;38(4):294–303. doi: 10.1016/j.ymeth.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 34.Laney AS, Dollard SC, Jaffe HW, Offermann MK, Spira TJ, Gunthel CJ, et al. Repeated measures study of human herpesvirus 8 (HHV-8) DNA and antibodies in men seropositive for both HHV-8 and HIV. Aids. 2004;18(13):1819–1826. doi: 10.1097/00002030-200409030-00011. [DOI] [PubMed] [Google Scholar]
- 35.Sitas F, Carrara H, Beral V, Newton R, Reeves G, Bull D, et al. Antibodies against human herpesvirus 8 in black South African patients with cancer. The New England journal of medicine. 1999;340(24):1863–1871. doi: 10.1056/NEJM199906173402403. [DOI] [PubMed] [Google Scholar]
- 36.Gross L. A filterable agent, recovered from Ak leukemic extracts, causing salivary gland carcinomas in C3H mice. Proceedings of the Society for Experimental Biology and Medicine Society for Experimental Biology and Medicine. 1953;83(2):414–421. doi: 10.3181/00379727-83-20376. [DOI] [PubMed] [Google Scholar]
- 37.Stewart SE, Eddy BE, Borgese N. Neoplasms in mice inoculated with a tumor agent carried in tissue culture. Journal of the National Cancer Institute. 1958;20(6):1223–1243. doi: 10.1093/jnci/20.6.1223. [DOI] [PubMed] [Google Scholar]
- 38.Sweet BH, Hilleman MR. The vacuolating virus, S.V. 40. Proceedings of the Society for Experimental Biology and Medicine Society for Experimental Biology and Medicine. 1960;105:420–427. doi: 10.3181/00379727-105-26128. [DOI] [PubMed] [Google Scholar]
- 39.Padgett BL, Walker DL, ZuRhein GM, Eckroade RJ, Dessel BH. Cultivation of papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet. 1971;1(7712):1257–1260. doi: 10.1016/s0140-6736(71)91777-6. [DOI] [PubMed] [Google Scholar]
- 40.Gardner SD, Field AM, Coleman DV, Hulme B. New human papovavirus (B.K.) isolated from urine after renal transplantation. Lancet. 1971;1(7712):1253–1257. doi: 10.1016/s0140-6736(71)91776-4. [DOI] [PubMed] [Google Scholar]
- 41.Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319(5866):1096–1100. doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chun SM, Yun SJ, Lee SC, Won YH, Lee JB. Merkel cell polyomavirus is frequently detected in korean patients with merkel cell carcinoma. Annals of dermatology. 2013;25(2):203–207. doi: 10.5021/ad.2013.25.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khalili K, Del Valle L, Otte J, Weaver M, Gordon J. Human neurotropic polyomavirus, JCV, and its role in carcinogenesis. Oncogene. 2003;22(33):5181–5191. doi: 10.1038/sj.onc.1206559. [DOI] [PubMed] [Google Scholar]
- 44.Theodoropoulos G, Panoussopoulos D, Papaconstantinou I, Gazouli M, Perdiki M, Bramis J, et al. Assessment of JC polyoma virus in colon neoplasms. Diseases of the colon and rectum. 2005;48(1):86–91. doi: 10.1007/s10350-004-0737-2. [DOI] [PubMed] [Google Scholar]
- 45.Walker DL, Padgett BL. The epidemiology of human polyomaviruses. Progress in clinical and biological research. 1983;105:99–106. [PubMed] [Google Scholar]
- 46.Nickeleit V, Klimkait T, Binet IF, Dalquen P, Del Zenero V, Thiel G, et al. Testing for polyomavirus type BK DNA in plasma to identify renal-allograft recipients with viral nephropathy. The New England journal of medicine. 2000;342(18):1309–1315. doi: 10.1056/NEJM200005043421802. [DOI] [PubMed] [Google Scholar]
- 47.Arthur RR, Shah KV, Yolken RH, Charache P. Detection of human papovaviruses BKV and JCV in urines by ELISA. Progress in clinical and biological research. 1983;105:169–176. [PubMed] [Google Scholar]
- 48.Knowles WA, Pipkin P, Andrews N, Vyse A, Minor P, Brown DW, et al. Population-based study of antibody to the human polyomaviruses BKV and JCV and the simian polyomavirus SV40. Journal of medical virology. 2003;71(1):115–123. doi: 10.1002/jmv.10450. [DOI] [PubMed] [Google Scholar]
- 49.Egli A, Infanti L, Dumoulin A, Buser A, Samaridis J, Stebler C, et al. Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors. The Journal of infectious diseases. 2009;199(6):837–846. doi: 10.1086/597126. [DOI] [PubMed] [Google Scholar]
- 50.Hamilton RS, Gravell M, Major EO. Comparison of antibody titers determined by hemagglutination inhibition and enzyme immunoassay for JC virus and BK virus. Journal of clinical microbiology. 2000;38(1):105–109. doi: 10.1128/jcm.38.1.105-109.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Faust H, Andersson K, Ekstrom J, Hortlund M, Robsahm TE, Dillner J. Prospective study of Merkel cell polyomavirus and risk of Merkel cell carcinoma. Int J Cancer. 2014;134(4):844–848. doi: 10.1002/ijc.28419. [DOI] [PubMed] [Google Scholar]
- 52.Paulson KG, Carter JJ, Johnson LG, Cahill KW, Iyer JG, Schrama D, et al. Antibodies to merkel cell polyomavirus T antigen oncoproteins reflect tumor burden in merkel cell carcinoma patients. Cancer research. 2010;70(21):8388–8397. doi: 10.1158/0008-5472.CAN-10-2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferber D. Virology. Monkey virus link to cancer grows stronger. Science. 2002;296(5570):1012–1015. doi: 10.1126/science.296.5570.1012. [DOI] [PubMed] [Google Scholar]
- 54.Shah K, Nathanson N. Human exposure to SV40: review and comment. American journal of epidemiology. 1976;103(1):1–12. doi: 10.1093/oxfordjournals.aje.a112197. [DOI] [PubMed] [Google Scholar]
- 55.Martini F, Corallini A, Balatti V, Sabbioni S, Pancaldi C, Tognon M. Simian virus 40 in humans. Infectious agents and cancer. 2007;2:13. doi: 10.1186/1750-9378-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Engels EA, Viscidi RP, Galloway DA, Carter JJ, Cerhan JR, Davis S, et al. Case-control study of simian virus 40 and non-Hodgkin lymphoma in the United States. Journal of the National Cancer Institute. 2004;96(18):1368–1374. doi: 10.1093/jnci/djh266. [DOI] [PubMed] [Google Scholar]
- 57.Viscidi RP, Rollison DE, Viscidi E, Clayman B, Rubalcaba E, Daniel R, et al. Serological cross-reactivities between antibodies to simian virus 40, BK virus, and JC virus assessed by virus-like-particle-based enzyme immunoassays. Clinical and diagnostic laboratory immunology. 2003;10(2):278–285. doi: 10.1128/CDLI.10.2.278-285.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taronna A, Mazzoni E, Corallini A, Bononi I, Pietrobon S, Guerra G, et al. Serological evidence of an early seroconversion to Simian virus 40 in healthy children and adolescents. PloS one. 2013;8(4):e61182. doi: 10.1371/journal.pone.0061182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.zur Hausen H. Papillomaviruses in human cancers. Proceedings of the Association of American Physicians. 1999;111(6):581–587. doi: 10.1046/j.1525-1381.1999.99723.x. [DOI] [PubMed] [Google Scholar]
- 60.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International journal of cancer Journal international du cancer. 2010;127(12):2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 61.Ault KA. Epidemiology and natural history of human papillomavirus infections in the female genital tract. Infectious diseases in obstetrics and gynecology. 2006;2006(Suppl):40470. doi: 10.1155/IDOG/2006/40470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.D'Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, et al. Case-control study of human papillomavirus and oropharyngeal cancer. The New England journal of medicine. 2007;356(19):1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 63.Harper DM, Franco EL, Wheeler C, Ferris DG, Jenkins D, Schuind A, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet. 2004;364(9447):1757–1765. doi: 10.1016/S0140-6736(04)17398-4. [DOI] [PubMed] [Google Scholar]
- 64.Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. The New England journal of medicine. 1998;338(7):423–428. doi: 10.1056/NEJM199802123380703. [DOI] [PubMed] [Google Scholar]
- 65.de Gruijl TD, Bontkes HJ, Walboomers JM, Schiller JT, Stukart MJ, Groot BS, et al. Immunoglobulin G responses against human papillomavirus type 16 virus-like particles in a prospective nonintervention cohort study of women with cervical intraepithelial neoplasia. Journal of the National Cancer Institute. 1997;89(9):630–638. doi: 10.1093/jnci/89.9.630. [DOI] [PubMed] [Google Scholar]
- 66.Koshiol J, Lindsay L, Pimenta JM, Poole C, Jenkins D, Smith JS. Persistent human papillomavirus infection and cervical neoplasia: a systematic review and meta-analysis. American journal of epidemiology. 2008;168(2):123–137. doi: 10.1093/aje/kwn036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carter JJ, Galloway DA. Humoral immune response to human papillomavirus infection. Clinics in dermatology. 1997;15(2):249–259. doi: 10.1016/s0738-081x(97)00068-0. [DOI] [PubMed] [Google Scholar]
- 68.Pastrana DV, Buck CB, Pang YY, Thompson CD, Castle PE, FitzGerald PC, et al. Reactivity of human sera in a sensitive, high-throughput pseudovirus-based papillomavirus neutralization assay for HPV16 and HPV18. Virology. 2004;321(2):205–216. doi: 10.1016/j.virol.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 69.Silverberg MJ, Schneider MF, Silver B, Anastos KM, Burk RD, Minkoff H, et al. Serological detection of human papillomavirus type 16 infection in human immunodeficiency virus (HIV)-positive and high-risk HIV-negative women. Clinical and vaccine immunology : CVI. 2006;13(4):511–519. doi: 10.1128/CVI.13.4.511-519.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Faust H, Knekt P, Forslund O, Dillner J. Validation of multiplexed human papillomavirus serology using pseudovirions bound to heparin-coated beads. The Journal of general virology. 2010;91(Pt 7):1840–1848. doi: 10.1099/vir.0.019349-0. [DOI] [PubMed] [Google Scholar]
- 71.Villa LL, Ault KA, Giuliano AR, Costa RL, Petta CA, Andrade RP, et al. Immunologic responses following administration of a vaccine targeting human papillomavirus Types 6, 11, 16, and 18. Vaccine. 2006;24(27–28):5571–5583. doi: 10.1016/j.vaccine.2006.04.068. [DOI] [PubMed] [Google Scholar]
- 72.Robbins HA, Kemp TJ, Porras C, Rodriguez AC, Schiffman M, Wacholder S, et al. Comparison of antibody responses to human papillomavirus vaccination as measured by three assays. Frontiers in oncology. 2014;3:328. doi: 10.3389/fonc.2013.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tsukasaki K, Hermine O, Bazarbachi A, Ratner L, Ramos JC, Harrington W, Jr, et al. Definition, prognostic factors, treatment, and response criteria of adult T-cell leukemia-lymphoma: a proposal from an international consensus meeting. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(3):453–459. doi: 10.1200/JCO.2008.18.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Franchini G, Fukumoto R, Fullen JR. T-cell control by human T-cell leukemia/lymphoma virus type 1. International journal of hematology. 2003;78(4):280–296. doi: 10.1007/BF02983552. [DOI] [PubMed] [Google Scholar]
- 75.Matsuoka M, Jeang KT. Human T-cell leukaemia virus type 1 (HTLV-1) infectivity and cellular transformation. Nature reviews Cancer. 2007;7(4):270–280. doi: 10.1038/nrc2111. [DOI] [PubMed] [Google Scholar]
- 76.Thorstensson R, Albert J, Andersson S. Strategies for diagnosis of HTLV-I and -II. Transfusion. 2002;42(6):780–791. doi: 10.1046/j.1537-2995.2002.00114.x. [DOI] [PubMed] [Google Scholar]
- 77.Wiktor SZ, Pate EJ, Weiss SH, Gohd RS, Correa P, Fontham ET, et al. Sensitivity of HTLV-I antibody assays for HTLV-II. Lancet. 1991;338(8765):512–513. doi: 10.1016/0140-6736(91)90585-d. [DOI] [PubMed] [Google Scholar]
- 78.Anderson DW, Epstein JS, Lee TH, Lairmore MD, Saxinger C, Kalyanaraman VS, et al. Serological confirmation of human T-lymphotropic virus type I infection in healthy blood and plasma donors. Blood. 1989;74(7):2585–2591. [PubMed] [Google Scholar]
- 79.Malm K, Kjerstadius T, Andersson S. Evaluation of a new screening assay for HTLV-1 and −2 antibodies for large-scale use. Journal of medical virology. 2010;82(9):1606–1611. doi: 10.1002/jmv.21867. [DOI] [PubMed] [Google Scholar]
- 80.Guidelines for counseling persons infected with human T-lymphotropic virus type I (HTLV-I) and type II (HTLV-II). Centers for Disease Control and Prevention and the U.S.P.H.S. Working Group. Annals of internal medicine. 1993;118(6):448–454. doi: 10.7326/0003-4819-118-6-199303150-00009. [DOI] [PubMed] [Google Scholar]
- 81.Kao JH, Chen DS. Global control of hepatitis B virus infection. The Lancet infectious diseases. 2002;2(7):395–403. doi: 10.1016/s1473-3099(02)00315-8. [DOI] [PubMed] [Google Scholar]
- 82.Kao JH. Diagnosis of hepatitis B virus infection through serological and virological markers. Expert review of gastroenterology & hepatology. 2008;2(4):553–562. doi: 10.1586/17474124.2.4.553. [DOI] [PubMed] [Google Scholar]
- 83.Cacciola I, Pollicino T, Squadrito G, Cerenzia G, Orlando ME, Raimondo G. Occult hepatitis B virus infection in patients with chronic hepatitis C liver disease. The New England journal of medicine. 1999;341(1):22–26. doi: 10.1056/NEJM199907013410104. [DOI] [PubMed] [Google Scholar]
- 84.Brechot C, Thiers V, Kremsdorf D, Nalpas B, Pol S, Paterlini-Brechot P. Persistent hepatitis B virus infection in subjects without hepatitis B surface antigen: clinically significant or purely "occult"? Hepatology. 2001;34(1):194–203. doi: 10.1053/jhep.2001.25172. [DOI] [PubMed] [Google Scholar]
- 85.Chu CM, Liaw YF. Spontaneous relapse of hepatitis in inactive HBsAg carriers. Hepatology international. 2007;1(2):311–315. doi: 10.1007/s12072-007-9002-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lok AS, Liang RH, Chiu EK, Wong KL, Chan TK, Todd D. Reactivation of hepatitis B virus replication in patients receiving cytotoxic therapy. Report of a prospective study. Gastroenterology. 1991;100(1):182–188. doi: 10.1016/0016-5085(91)90599-g. [DOI] [PubMed] [Google Scholar]
- 87.Chu CM, Hung SJ, Lin J, Tai DI, Liaw YF. Natural history of hepatitis B e antigen to antibody seroconversion in patients with normal serum aminotransferase levels. The American journal of medicine. 2004;116(12):829–834. doi: 10.1016/j.amjmed.2003.12.040. [DOI] [PubMed] [Google Scholar]
- 88.Fried MW, Piratvisuth T, Lau GK, Marcellin P, Chow WC, Cooksley G, et al. HBeAg and hepatitis B virus DNA as outcome predictors during therapy with peginterferon alfa-2a for HBeAg-positive chronic hepatitis B. Hepatology. 2008;47(2):428–434. doi: 10.1002/hep.22065. [DOI] [PubMed] [Google Scholar]
- 89.Chan HL, Wong VW, Tse AM, Tse CH, Chim AM, Chan HY, et al. Serum hepatitis B surface antigen quantitation can reflect hepatitis B virus in the liver and predict treatment response. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2007;5(12):1462–1468. doi: 10.1016/j.cgh.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 90.Magnius LO, Norder H. Subtypes, genotypes and molecular epidemiology of the hepatitis B virus as reflected by sequence variability of the S-gene. Intervirology. 1995;38(1–2):24–34. doi: 10.1159/000150411. [DOI] [PubMed] [Google Scholar]
- 91.Kao JH, Chen PJ, Lai MY, Chen DS. Hepatitis B genotypes correlate with clinical outcomes in patients with chronic hepatitis B. Gastroenterology. 2000;118(3):554–559. doi: 10.1016/s0016-5085(00)70261-7. [DOI] [PubMed] [Google Scholar]
- 92.Thakur V, Guptan RC, Kazim SN, Malhotra V, Sarin SK. Profile, spectrum and significance of HBV genotypes in chronic liver disease patients in the Indian subcontinent. Journal of gastroenterology and hepatology. 2002;17(2):165–170. doi: 10.1046/j.1440-1746.2002.02605.x. [DOI] [PubMed] [Google Scholar]
- 93.Kao JH, Wu NH, Chen PJ, Lai MY, Chen DS. Hepatitis B genotypes and the response to interferon therapy. Journal of hepatology. 2000;33(6):998–1002. doi: 10.1016/s0168-8278(00)80135-x. [DOI] [PubMed] [Google Scholar]
- 94.Kao JH, Liu CJ, Chen DS. Hepatitis B viral genotypes and lamivudine resistance. Journal of hepatology. 2002;36(2):303–304. doi: 10.1016/s0168-8278(01)00246-x. [DOI] [PubMed] [Google Scholar]
- 95.Zollner B, Petersen J, Schroter M, Laufs R, Schoder V, Feucht HH. 20-fold increase in risk of lamivudine resistance in hepatitis B virus subtype adw. Lancet. 2001;357(9260):934–935. doi: 10.1016/S0140-6736(00)04219-7. [DOI] [PubMed] [Google Scholar]
- 96.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57(4):1333–1342. doi: 10.1002/hep.26141. [DOI] [PubMed] [Google Scholar]
- 97.Gravitz L. Introduction: a smouldering public-health crisis. Nature. 2011;474(7350):S2–S4. doi: 10.1038/474S2a. [DOI] [PubMed] [Google Scholar]
- 98.Alter MJ. Epidemiology of viral hepatitis and HIV co-infection. Journal of hepatology. 2006;44(1 Suppl):S6–S9. doi: 10.1016/j.jhep.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 99.Hauri AM, Armstrong GL, Hutin YJ. The global burden of disease attributable to contaminated injections given in health care settings. International journal of STD & AIDS. 2004;15(1):7–16. doi: 10.1258/095646204322637182. [DOI] [PubMed] [Google Scholar]
- 100.Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. Journal of hepatology. 2006;45(4):529–538. doi: 10.1016/j.jhep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 101.Colin C, Lanoir D, Touzet S, Meyaud-Kraemer L, Bailly F, Trepo C, et al. Sensitivity and specificity of third-generation hepatitis C virus antibody detection assays: an analysis of the literature. Journal of viral hepatitis. 2001;8(2):87–95. doi: 10.1046/j.1365-2893.2001.00280.x. [DOI] [PubMed] [Google Scholar]
- 102.Getchell JP, Wroblewski KE, DeMaria A, Bean CL, Parker MM, Pandori M, et al. Testing for HCV Infection: An Update of Guidance for Clinicians and Laboratorians. Mmwr-Morbid Mortal W. 2013;62(18):362–365. [PMC free article] [PubMed] [Google Scholar]
- 103.Maheshwari A, Thuluvath PJ. Management of acute hepatitis C. Clinics in liver disease. 2010;14(1):169–176. doi: 10.1016/j.cld.2009.11.007. x. [DOI] [PubMed] [Google Scholar]
- 104.Merkinaite S, Lazarus JV, Gore C. Addressing HCV infection in Europe: reported, estimated and undiagnosed cases. Central European journal of public health. 2008;16(3):106–110. doi: 10.21101/cejph.a3482. [DOI] [PubMed] [Google Scholar]
- 105.Ponde RA. Hidden hazards of HCV transmission. Medical microbiology and immunology. 2011;200(1):7–11. doi: 10.1007/s00430-010-0159-9. [DOI] [PubMed] [Google Scholar]
- 106.Schroter M, Zollner B, Schafer P, Laufs R, Feucht HH. Comparison of three HCV genotyping assays: a serological method as a reliable and inexpensive alternative to PCR based assays. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2001;23(1–2):57–63. doi: 10.1016/s1386-6532(01)00186-x. [DOI] [PubMed] [Google Scholar]
- 107.Pawlotsky JM, Roudot-Thoraval F, Pellet C, Aumont P, Darthuy F, Remire J, et al. Influence of hepatitis C virus (HCV) genotypes on HCV recombinant immunoblot assay patterns. Journal of clinical microbiology. 1995;33(5):1357–1359. doi: 10.1128/jcm.33.5.1357-1359.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pawlotsky JM, Prescott L, Simmonds P, Pellet C, Laurent-Puig P, Labonne C, et al. Serological determination of hepatitis C virus genotype: comparison with a standardized genotyping assay. Journal of clinical microbiology. 1997;35(7):1734–1739. doi: 10.1128/jcm.35.7.1734-1739.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. The lancet oncology. 2012;13(6):607–615. doi: 10.1016/S1470-2045(12)70137-7. [DOI] [PubMed] [Google Scholar]
- 110.Cooper SA, van der Loeff MS, Taylor GP. The neurology of HTLV-1 infection. Practical neurology. 2009;9(1):16–26. doi: 10.1136/jnnp.2008.167155. [DOI] [PubMed] [Google Scholar]
- 111.de Sanjose S, Diaz M, Castellsague X, Clifford G, Bruni L, Munoz N, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. The Lancet infectious diseases. 2007;7(7):453–459. doi: 10.1016/S1473-3099(07)70158-5. [DOI] [PubMed] [Google Scholar]