Abstract
Although the giant cell tumor of bone is generally classified as a benign tumor it can rarely metastasize and has a potential risk of local recurrence. We want to report about a female patient who suffered from a recurrence of a giant cell tumor of bone after the implantation of a total endoprosthesis of the knee joint. We have treated her with denosumab, which is a receptor activator of nuclear factor kappa-B ligand inhibitor. In this case report we want to present a new option to treat this kind of neoplasm.
Key words: bone, denosumab, tumor
Introduction
The Giant cell tumor of bone is generally a benign tumor that rarely metastasizes. The World Health Organization classifies it as an aggressive, potentially malignant lesion.1 This kind of tumor grows local aggressively and has a potential risk of local recurrence depending on the type of treatment (0-65%).2
The tumor commonly occurs during the second to fourth decades of life3 and it usually originates from the epiphysis of long bones.
If the tumor has a resectable size the standard therapy is an intralesional curettage and polymethylmethacrylate cement to fill the defect.
Active studies have been undertaken in order to find treatment options for patients with previously untreatable tumors. Giant cells have been confirmed to express receptor activator of nuclear factor kappa-B ligand (RANKL), which causes the local aggressive nature of the tumor. It was first reported in 2000 that the inhibition of RANKL could potentially inhibit the destructive process and eliminate the population of giant cells.4
Denosumab is a RANKL inhibitor and has recently shown a therapeutic effect. It is now an option to treat this kind of tumor.5
In 2011 a Phase II study has shown that patients with surgically salvageable and unsalvageable giant cell tumors treated with denosumab, which was well tolerated, had an inhibited disease progression of 99% and had a reduced requirement for surgery.6
We present a case of a female patient who suffered from a recurrence of a giant cell tumor of the tibia 2 years after the implantation of a total endoprosthesis of the knee joint. She was treated with denosumab monthly and is still free of disease.
Case Report
We want to report about a female patient who had already undergone a computed tomography (CT) of the knee joint when she came to our department in August 2010. The patient suffered from arthritis pain but also nocturnal rest pain. In the clinical examination there was no swelling or flush of the knee joint. The CT has shown a solid tumor with a sclerotic border in the epiphysis. A biopsy has shown a giant cell tumor. Intralesional curettage was impossible due to the dimension of the tumor. A resection, revision prosthesis and a cement augmentation were then made. The patient had an undisrupted postoperative healing without any complications (Figures 1 and 2). In February 2012 our patient reported again about recurring, strong pain mainly in the mid-shaft tibia. In the clinical examination she complained about severe local pressure pain in this region. The x-ray has shown a suspicion of a tibial prosthesis loosening due to a recurrent tumor (Figure 3). A recurrent giant cell tumor was confirmed by a biopsy. We then started with denosumab in March 2012, which was given monthly over 2 years.
Figure 1.
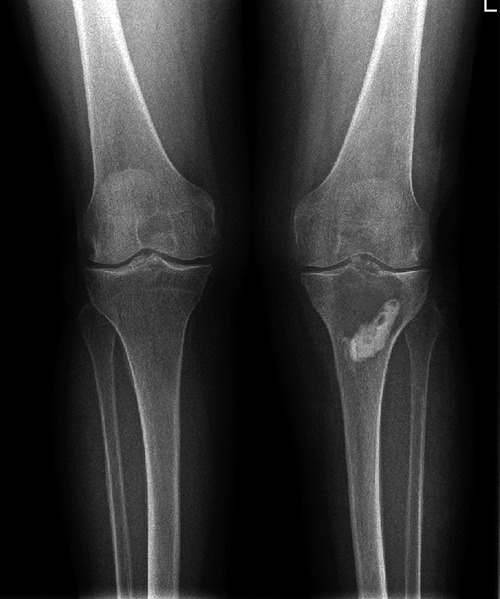
Preoperative x-ray.
Figure 2.
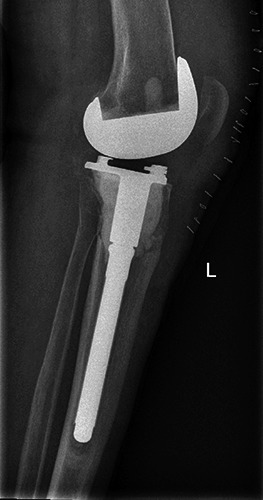
First postoperative x-ray.
Figure 3.
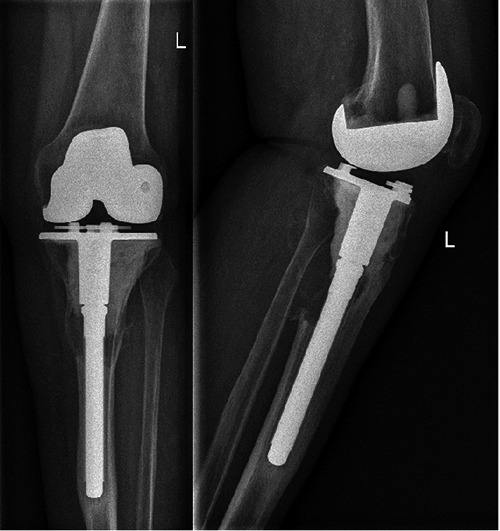
Recurrence of the giant cell tumor of bone.
The periodic radiological imaging has not shown any evidence of disease recurring and the patient tolerated the treatment without any adverse affects (Figure 4).
Figure 4.
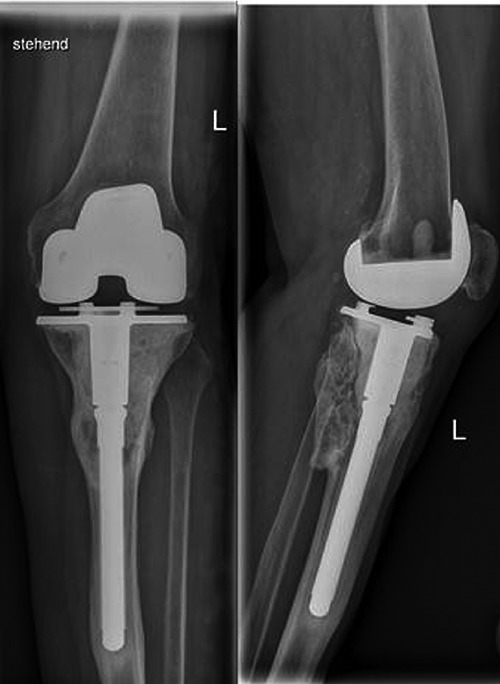
Follow up 24 months after starting the treatment with denosumab.
Discussion and Conclusions
Although the giant cell tumor of bone is a benign tumor it is classified as an aggressive und potentially malignant lesion and can be difficult to resect or to treat with drug therapy. Our case shows on the one hand the difficulty of recurrence and on the other hand the importance of multi-modality management in the treatment of giant cell tumor of bone.
The inhabitation of RANKL and its therapeutic effect to inhibit the destructive process and eliminate the population of giant cells has demonstrated a new way to treat this kind of neoplasm. Also the recent phase II studies bring hope to usher in a new treatment era. However there must be more data available about the long-term effects of denosumab. A recent translational study of the neoplastic cells of giant cell tumor of bone following neoadjuvant denosumab has impressively demonstrated that the cell proliferation is only diminished by denosumab; the cells continued to proliferate in vitro, albeit at a slower rate. On the other hand a complete elimination of RANKL in tumor cells was found after exposure of denosumab.7
Also Lau et al. have shown that zoledronic acid caused in comparison to denosumab apoptosis in two out of three cell lines. Denosumab only had a minimal inhibitory effect in one cell line and did not induce any apoptosis.8
The role of denosumab and other targeted agents is still the subject of ongoing clinical research studies. They may offer a further treatment option for surgically unsalvageable tumor or may prevent patients from severe morbidity.
References
- 1.Schajowicz F, Sissons HA, Sobin LH. The World Health Organization’s histologic classification of bone tumors. A commentary on the second edition. Cancer 1995;75:1208-14. [DOI] [PubMed] [Google Scholar]
- 2.Klenke FM, Wenger DE, Inwards CY, et al. Giant cell tumor of bone: risk factors for recurrence. Clin Orthop Relat Res 2011;469:591-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szendröi M. Giant-cell tumour of bone. J Bone Joint Surg Br 2004;86:5-12. [PubMed] [Google Scholar]
- 4.Huang L, Xu J, Wood DJ, Zheng MH. Gene expression of osteoprotegerin ligand, osteoprotegerin, and receptor activator of NF-kappaB in giant-cell tumor of the bone: possible involvement in tumor cell-induced osteoclast-like cell formation. Am J Pathol 2000;156:761-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas DM, Skubitz KM. Giant cell tumour of bone. Curr Opin Oncol 2009;21:338-44. [DOI] [PubMed] [Google Scholar]
- 6.Blay J, Chawla SP, Martin Broto J, et al. Denosumab safety and efficacy in giant cell tumor of bone (GCTB): interim results from a phase II study. J Clin Oncol 2011;29: abstr10034. [Google Scholar]
- 7.Mak IW, Evaniew N, Popovic S, et al. A translational study of the neoplastic cells of giant cell tumor of bone following neoadjuvant denosumab. J Bone Joint Surg Am 2014;96:e127. [DOI] [PubMed] [Google Scholar]
- 8.Lau CP, Huang L, Wong KC, Kumta SM. Comparison of the anti-tumor effects of denosumab and zoledronic acid on the neoplastic stromal cells of giant cell tumor of bone. Connect Tissue Res 2013;54:439-49. [DOI] [PubMed] [Google Scholar]


