Abstract
Autoimmune hemolytic anemia (AIHA) is a rare paraneoplastic syndrome associated with ovarian malignancies. We report a case of a 77 year-old female with metastatic ovarian carcinoma who presented with worsening anemia from her baseline, and was found to have a warm autoimmune hemolytic anemia. We performed a literature review and analyzed all 10 cases (including our patient) that have been reported to date, and incorporated the clinical presentation, histology and stage of underlying malignancies, types, treatment, prognosis and mechanisms of AIHA in ovarian carcinoma.
Key words: autoimmune hemolytic anemia, ovarian cancer, paraneoplastic syndrome
Introduction
Paraneoplastic syndrome is a syndrome with constellations of symptoms and signs that are not directly caused by primary or metastatic tumor. Some of these may be attributed to secretion of functional peptides and hormones by cancer cells or immune cross-reactivity between tumor and normal host tissues.1 Given its rarity, it is difficult to determine the prevalence, but one study estimated the prevalence of paraneoplastic syndrome up to 8% in cancer patients.1
Paraneoplastic autoimmune hemolytic anemia (AIHA) is a well-known phenomenon in patients with hematological malignancies, but it is much rare in solid tumors.2 In an analysis of solid tumor cases with paraneoplastic AIHA, majority were secondary to lung, colorectal, renal and Kaposi’s sarcoma.2 On the other hand, paraneoplastic AIHA associated with ovarian malignancy is extremely rare. Herein, we present a case of paraneoplastic autoimmune hemolytic anemia in a patient with ovarian cancer with a literature review of this condition.
Case Report
A 77-year-old female presented to the hospital with worsening of her baseline anemia. Her oncologic history was significant for stage IV ovarian carcinoma, with papillary adenocarcinoma histology. Other significant medical history included ulcerative colitis, which was in remission since past few years. At the time of her ovarian cancer diagnosis, she had a normocytic normochromic anemia with hemoglobin of 11.0 g/dL. In the subsequent months, she underwent chemotherapy with 2 cycles carboplatin/paclitaxel and 4 cycles of carboplatin /docetaxel. Her chemotherapy was intermittently disrupted secondary to worsening pancytopenia, which was a result of cytotoxic effect of chemotherapy resulting in bone marrow suppression.
Subsequently, patient was admitted to the hospital for worsening fatigue, 3 months after her last chemotherapy treatment. She denied any bleeding, dizziness, dyspnea and chest pain. Her physical examination only showed mild conjunctival pallor. Her laboratory investigations revealed hemoglobin of 7.6 g/dL, which was lower than her baseline, MCV of 110 fL and RDW of 34 fL. B12, folic acid and TSH levels were normal. Further tests showed LDH of 338 IU/L, total bilirubin of 2.4 mg/dL with indirect bilirubin 1.9 mg/dL, haptoglobin of 0 mg/dL, reticulocyte of 7.9%, positive direct Coombe’s test and a peripheral smear showing spherocytes and reticulocytes, all suggesting warm autoimmune hemolytic anemia. Her CA-125 during this hospitalization was increased to 359 units/mL compared to 15 a month prior to admission, indicating progression of her underlying ovarian malignancy. Computed tomogrphy scan also confirmed the progression of the disease and showed worsening of hepatic and adrenal metastases. She was treated with oral corticosteroids with minimal improvement, and chemotherapy with doxorubicin was started. One month after, her Hb, reticulocyte count, bilirubin and haptoglobin appear to have improved, correspond to decreased in CA-125 (Figures 1 and 2).
Figure 1.

Graph showing hemoglobin (Hb), reticulocyte, total and indirect bilirubin of the patient 8 months prior to admission until 1 month after admission.
Figure 2.
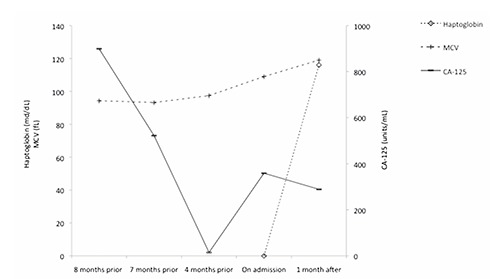
Graph showing haptoglobin, MCV and CA-125 of the patient 8 months prior to admission until 1 month after admission.
Discussion
Paraneoplastic syndromes that have been described associated with ovarian malignancies classified by system included neurological (polyneuropathy, cerebellar generation), connective tissue (dermatomyosistis, polyarteritis nodosa, rheumatoid arthritis, systemic lupus erythematosis, reflex sympathetic dystrophy, palmar fasciitis, digital ischemia), hematological [disseminated intravascular coagulation (DIC), acquired hemolytic anemia, microangiopathic hemolytic anemia, polycythaemia, thrombosis/thrombophlebitis], dermatologic (acanthosis nigricans, leser trelat syndrome, digital ischemia), endocrine (Zollinger-Ellison syndrome, Cushing’s syndrome, nephrotic syndrome, hypercalcemia, hypertension, hypoglycemia).3,4
We hypothesized that our patient’s initial presentation of AIHA was a paraneoplastic phenomenon associated to her underlying ovarian malignancy. Other possible etiologies include drug-induced hemolytic anemia, particularly associated with carboplatin. So far four cases have been reported in the literature.5 However, hemolytic anemia occurred during or shortly after carboplatin administration in all cases.5 Our patient received her last chemotherapy three months prior to her presentation therefore making drug-induced hemolytic anemia unlikely. Similarly AIHA can occur in patients with ulcerative colitis, but it occurs typically in the setting of active colitis. The case in discussion has been in remission from ulcerative colitis since over a decade.6
Paraneoplastic autoimmune hemolytic anemia was initially reported in a benign dermoid cyst of the ovary in 1938, following that the association was described in a patient with pseudomucinous cystadenocarcinoma in 1945.7,8 Subsequently 9 other cases have been reported on the literature to date (Table 1).8-17
Table 1.
Association between ovarian malignancies and autoimmune hemolytic anemia.
| Ref. | Age | Hb (g/dL) | Histology | Metastases | Type of AIHA | Treatment | Timing of anemia | Correlation with disease activity | Cause of death | Survival from cancer diagnosis |
|---|---|---|---|---|---|---|---|---|---|---|
| 9 | 35 | 5 | Pseudomucinous cystadenocarcinom | No | Negative | Tumor control | 1 month prior to cancer diagnosis | - | - | - |
| 10 | 28 | 6.7 | Papillary cystadenocarcinoma | Yes | - | Steroids | 4 months after radiation/7 months after surgery/8 months after chemotherapy | - | Deconditioned, uremia | 13 months |
| 11 | 36 | 4.8 | Anaplastic carcinoma | Yes | Direct/indirect | Steroids | 2 months after cancer diagnosis/radiation/surgery | Positive | Septic shock | 6 months |
| 12 | 54 | 8 | Papillary cystadenocarcinoma | - | Negative | - | 3 months after cancer diagnosis/2 months after radiation | - | - | - |
| 13 | 64 | 4.8 | Epitheliosarcomatoid tumour | No | Direct | - | - | - | - | - |
| 14 | 57 | 6.6 | Papillary cystadenocarcinoma | Yes | Direct/indirect | Tumor control | On diagnosis | Positive | Bilateral pleural effusion/ascites/marrow suppression | 15 months |
| 15 | 29 | 7.3 | Anaplastic carcinoma | Yes | Direct | Tumor control | 1 month prior to cancer diagnosis | Positive | - | - |
| 16 | 56 | 7.4 | Papillary cystadenocarcinoma | Yes | Direct/indirect Coombe’s | Steroids temporary | 28 months after cancer diagnosis /surgery | Positive | Cerebral infarction | 35 months |
| 17 | 75 | 6.8 | Papillary cystadenocarcinoma | Yes | Direct | Steroids/tumor control | Post-chemotherapy | Positive | - | - |
| This case | 77 | 7.6 | Papillary cystadenocarcinoma | Yes | Direct | Steroids/tumor control | Post-chemotherapy | Positive | - | - |
Hb, hemoglobin; AIHA, autoimmune hemolytic anemia.
Presentation of autoimmune hemolytic anemia
In all cases reported including our case, AIHA in the setting of ovarian malignancies appears to be a disease of adult. The age of presentation ranged from 28-77, with a mean age of 51, and our patient is the oldest patient described. Anemia is usually the reason for presentation, and the hemoglobin values can range from 4.8-8.0 mg/dL. AIHA may antedate, postdate or occur concurrently at the time of ovarian malignancies diagnosis. It can also present 2-28 months after definitive treatment. The case in current discussion, presented with AIHA, during the chemotherapy treatments and was the first sign of tumor progression - confirmed by both tumor marker (CA-125) and radiologic finding.
Histology type and stage of the underlying ovarian malignancies
The histology classification of ovarian tumors by the World Health Organization (WHO) is based on the histogenesis of the normal ovary, which can be derived by the surface epithelium, germ cells and mesenchyme.14 Of these, papillary cystadenocarcinoma, which is originated from surface epithelium of ovary, is most commonly associated with AIHA, sixty percent of all reported case.9,11,13,15,16 Two cases of anaplastic carcinoma have also been reported to be associated with AIHA.10,14 All cases except three were found to have metastatic disease either on presentation or later during their course of disease.9,10,13-16
Types of autoimmune hemolytic anemia
AIHA is an immune-mediated disease caused by presence autoantibodies attacking one’s own red blood cells. It can be classified into primary (idiopathic) or secondary to underlying illness. There are usually two types; warm and cold hemolytic anemia.18 They can be differentiated using direct and indirect Coombe’s. In AIHA associated with ovarian malignancies, direct Coombe’s is more frequently found to be positive (7/10 cases). Three of these cases were positive for both direct and indirect Coombe’s test.10,13,15 Coombe’s test was negative in two cases, a hemolytic serum factor was found in one of these.8,11 The types of antibody vary and included anti-Kell, anti-E, anti-I cold agglutinin, IgG and IgA, with no consistent patterns found. Our patent was found to have warm IgG hemolytic anemia. The temporal relationship between paraneoplastic syndrome and cancers are unclear, however there appeared to be correlation seen between the presence of antibodies and underlying disease activity, as in our patient.
Treatment
First line therapy for AIHA is typically corticosteroids. However, the treatment of AIHA in the setting of malignancies are not well established. Although some patients responded to steroids, majority underwent definitive treatment for the underlying malignancies with improvement of hemoglobin.8,13,14,16 Tumor control seems be more effective than the conventional treatment for AIHA. The case in current discussion, responded only modestly to steroids, and responded significantly to treatment of underlying disease, which is treatment of ovarian carcinoma by chemotherapy.
Prognosis
Most patients were in stage IV disease on diagnosis; therefore it is not surprising that their prognosis were poor. Of the 10 cases, 4 patients passed away 6-35 months after their cancer diagnosis.12,13,16,18 Causes of death include deconditioning, uremia, septic shock, cerebral infarction, marrow suppression and complications from chemotherapy.
Mechanism
The mechanisms underlying malignancy associated AIHA are not well understood, and pathogenesis may be different. Studies have suggested that changes in the protein and phospholipid fractions of the RBC membranes in patients with ovarian cancer lead to increases susceptibility to hemolysis.19 It was postulated that CA-125, a glycoprotein measured in ovarian cancer, might have an immunosuppressive effect and inhibit hemolysis, which may explain AIHA after cancer treatment.20 Specially, a study demonstrated that hemolytic serum factor from tumor was found after irradiation.11 In cases where AIHA predate treatment, it may be secondary to antibodies produced as a result of tumor antigen cross-react with normal red blood cells.21
Conclusions
In summary, we highlight the rarity of paraneoplastic AIHA in metastatic ovarian malignancies. Our patient is the oldest described case, and like majority of the cases, her ovarian carcinoma was of papillary cystadenocarcinoma histology. Anemia in patients with ovarian carcinoma may be multifactorial, from bleeding, bone marrow suppression from chemotherapy, or nutritional deficient, but as highlighted by this case AIHA should be considered if the clinical picture suggests hemolysis. Also, new onset of AIHA, may also be related to cancer activity, such as in this case. Early identification may or may not improve mortality, but could potentially improve quality of life.
References
- 1.Pelosof LC, Gerber DE. Paraneoplastic syndromes: an approach to diagnosis and treatment. Mayo Clin Proc 2010;85:838-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Puthenparambil J, Lechner K, Kornek G. Autoimmune hemolytic anemia as a paraneoplastic phenomenon in solid tumors: a critical analysis of 52 cases reported in the literature. Wien Klin Wochenschr 2010;122:229-36. [DOI] [PubMed] [Google Scholar]
- 3.Koyama T, Togashi K, Ueda H, et al. Paraneoplastic syndromes associated with ovarian neoplasms. Int J Clin Oncol 2000;5:79-84. [Google Scholar]
- 4.Hudson CN, Curling M, Potsides P, Lowe DG. Paraneoplastic syndromes in patients with ovarian neoplasia. J R Soc Med 1993;86:202-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haley KM, Russell TB, Boshkov L, et al. Fatal carboplatin-induced immune hemolytic anemia in a child with a brain tumor. J Blood Med 2014;5:55-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giannadaki E, Potamianos S, Roussomoustakaki M, et al. Autoimmune hemolytic anemia and positive Coombs test associated with ulcerative colitis. Am J Gastroenterol 1997;92:1872-4. [PubMed] [Google Scholar]
- 7.West-Watson WN, Young CJ. Failed splenectomy in acholuric jaundice. Br Med J 1938;1:1305-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones E, Tillman C. A case of hemolytic anemia relieved by removal of ovarian tumor. JAMA 1945;128:1225-7. [Google Scholar]
- 9.Norcross JW. Hematologic manifestations of malignant disease. Med Clin North Am 1963;47:345-52. [DOI] [PubMed] [Google Scholar]
- 10.Yam LT, Rudzki C, Busch S, Leithold SL. Ovarian neoplasm associated with autoimmune hemolytic anemia. Am J Obstet Gynecol 1966;95:207-11. [DOI] [PubMed] [Google Scholar]
- 11.Burkert L, Becker G, Pisciotta AV. Ovarian malignancy and hemolytic anemia. Demonstration of a hemolytic serum factor. Ann Intern Med 1970;73:91-3. [DOI] [PubMed] [Google Scholar]
- 12.Andre R, Duhamel G, Najman A, et al. [Autoimmune hemolytic anemia and malignant tumor of the ovary]. Presse Med 1969;77:2133-6 [Article in French]. [PubMed] [Google Scholar]
- 13.Spira MA, Lynch EC. Autoimmune hemolytic anemia and carcinoma: an unusual association. Am J Med 1979;67:753-8. [DOI] [PubMed] [Google Scholar]
- 14.Carreras Vescio LA, Toblli JE, Rey JA, et al. Autoimmune hemolytic anemia associated with an ovarian neoplasm. Medicina (B Aires) 1983;43:415-24. [PubMed] [Google Scholar]
- 15.Tsuda A, Iwabuchi A, Yaguchi M, et al. [The first case of autoimmune hemolytic anemia in a patient with ovarian carcinoma in Japan]. Rinsho Ketsueki 1988;29:227-31 [Article in Japanese] [PubMed] [Google Scholar]
- 16.Morris PG, Swords R, Sukor S, et al. Autoimmune hemolytic anemia associated with ovarian cancer. J Clin Oncol 2008;26:4993-5. [DOI] [PubMed] [Google Scholar]
- 17.Kaku T, Ogawa S, Kawano Y, et al. Histological classification of ovarian cancer. Med Electron Microsc 2003;36:9-17. [DOI] [PubMed] [Google Scholar]
- 18.Gehrs BC, Friedberg RC. Autoimmune hemolytic anemia. Am J Hematol 2002;69:258-71. [DOI] [PubMed] [Google Scholar]
- 19.Kopczynski Z, Kuzniak J, Thielemann A, et al. The biochemical modification of the erythrocyte membranes from women with ovarian cancer. Br J Cancer 1998;78:466-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murdoch WJ, Van Kirk EA, Smedts AM. Complement-inhibiting effect of ovarian cancer antigen CA-125. Cancer Lett 2006;236:54-7. [DOI] [PubMed] [Google Scholar]
- 21.Cobo F, Pereira A, Nomdedeu B, et al. Ovarian dermoid cyst-associated autoimmune hemolytic anemia: a case report with emphasis on pathogenic mechanisms. Am J Clin Pathol 1996;105:567-71. [DOI] [PubMed] [Google Scholar]


