Abstract
Fungal periprosthetic joint infection (PJI) is a rare but devastating complication following total knee arthroplasty (TKA). A standardized procedure regarding an accurate treatment of this serious complication of knee arthroplasty is lacking. In this systematic review, we collected data from 36 studies with a total of 45 reported cases of a TKA complicated by a fungal PJI. Subsequently, an analysis focusing on diagnostic, medicaments and surgical procedures in the pre-, intra- and postoperative period was performed. Candida spp. accounts for about 80% (36 out of 45 cases) of fungal PJIs and is therefore the most frequently reported pathogen. A systemic antifungal therapy was administered in all but one patient whereas a local antifungal therapy, e.g. the use of an impregnated spacer, is of inferior relevance. Resection arthroplasty with delayed re-implantation (two-stage revision) was the surgical treatment of choice. However, in 50% of all reported cases the surgical therapy was heterogeneous. The outcome under a combined therapy was moderate with recurrent fungal PJI in 11 patients and subsequent bacterial PJI as a main complication in 5 patients. In summary, this systematic review integrates data from up to date 45 reported cases of a fungal PJI of a TKA. On the basis of the current literature strategies for the treatment of this devastating complication after TKA are discussed.
Key words: periprosthetic joint infection, fungal, total knee arthroplasty
Introduction
Due to the aging population there is a significant increase regarding the number of total knee arthroplasties (TKA) per year. In this context, periprosthetic joint infection (PJI) following TKA is the most dreaded complication with a reported incidence of 1-4%.1 Gram-positive bacteria, especially staphylococci, account for the majority of these infections.2 In contrast, fungal periprosthetic joint infections are rare but devastating.
Risk factors for the development of fungal infections include immunosuppression, prolonged use of antibiotics, drug abuse, autoimmune diseases such as rheumatoid arthritis and many more.3,4 However, these risk factors are present in only 50% of the affected patients.3,5 In the current medical literature on fungal PJI of a TKA neither a mandatory guideline nor a standardized procedure regarding the diagnostic approach and surgical treatment of this devastating complication is reported. Thus, the objective of the presented review is to systematically analyze the current literature on the management of a fungal PJI after TKA and to derive recommendations for the treatment of this severe complication of knee arthroplasty.
Materials and Methods
Data acquisition
The acquisition of data in this systematic review is based on a thorough search of the present medical literature by use of Medline, PubMed, Embase and Scopus with the search terms fungal, periprosthetic joint infection, PJI, arthroplasty, knee and infection. To ensure accuracy repeated searches have been performed. Additionally, a secondary search was performed on the references obtained from the articles found in the primary search. Using this search algorithm we identified 36 studies involving 45 cases of a fungal PJI of a TKA in the period from 1979 to 2013. Due to the heterogeneity of the studies a meta-analysis could not be performed.
The following data were extracted from the studies: demographics including age, gender, body mass index, smoking habits, concomitant diseases (especially immunity-impairing risk factors such as diabetes mellitus, corticosteroid therapy, malignant disease, and organ transplantation), and prolonged antibiotic treatment.
Case definitions
We defined a fungal PJI of a TKA as definite if proven by detection of a fungal pathogen from joint fluid aspiration or intraoperative samples. Treatment failure was defined as recurrence of the fungal PJI if the patient had already received or was under antimicrobial therapy for a previously diagnosed fungal PJI. Secondary bacterial infection of the respective knee was not regarded as treatment failure if the initial fungal pathogen was no longer detected.
Cases included
This review included medical data from 45 patients (median age 67 years) extracted from 36 studies published between 1979 and 2013. The gender distribution was nearly balanced with 24 female and 21 male patients. Concomitant diseases according to the risk factors for invasive fungal infections were reported in less than 60% of the cases (Supplementary Table S1). Eight patients presented a history of previous or current bacterial PJI.
Preoperative findings
Clinical symptoms at presentation included pain, signs of local and systemic infection (e.g. fever, shivering, Supplementary Table S2). Serological infection parameters (WBC, ESR or CRP) were increased in 25 cases. Radiological evaluation of the prosthesis was reported in 28 of the reported cases. The most common finding was a loosening of the prosthesis followed by osteolysis or local bone destruction (Supplementary Table S2). Preoperative joint aspiration was performed in 32 cases with a positive fungal culture in 26 cases. In 17 cases the authors performed (multiple) re-aspirations to preclude fungal contamination or to increase sensitivity. The respective fungal pathogen reported in Supplementary Table S1 was either detected by preoperative aspiration as mentioned above or confirmed by intraoperative collection of tissue samples. In none of the studies the authors reported microbiological details about the number of cultures, the growth medium or the time of incubation. Candida spp., majorly Candida albicans, has been found in 36 cases as precipitating agent (Figure 1).
Figure 1.
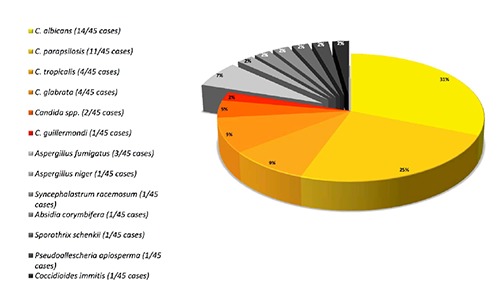
Spectrum of pathogens. Preoperative aspiration of the respective total knee arthroplasty verifies Candida spp. - majorly Candida albicans - as precipitating agent in most of the patients (36 of 45 cases). Aspergillus spp. and other fungal germs are of inferior importance regarding the analyzed studies.
Surgical treatment
The reported initial surgical treatment of fungal TKA infections is heterogeneous (Supplementary Table S3). Resection arthroplasty was the initial intervention for 29 patients. Extensive and radical intraoperative debridement of all infected and necrotic tissue as well as removal of all cement was emphasized by most authors as highly important regarding the outcome. Permanent resection arthroplasty was performed in 1 case whereas 19 cases underwent a delayed re-implantation of the prosthesis (2-stage-procedure) and 9 cases a delayed arthrodesis (Supplementary Table S3).
Intra-articular spacers were used in 13 patients of which 5 had been impregnated with antimicrobial agents (2× tobramycin + vancomycin; 1× teicoplanin; 1× vancomycin + amphotericin B; 1× vancomycin + piperacillin) to prevent bacterial super-infection. As mentioned above, only 3 patients received cement impregnated with antifungal agents. In 5 cases no spacer was implanted following resection arthroplasty.
In three cases without pre- or intraoperative suspicion of a periprosthetic infection a 1-stage-procedure was performed without relapse. Debridement and irrigation with retention of the prosthesis was implemented in 4 cases with 2 patients receiving a continuous suction-irrigation-system with local administration of 200 mg fluconazole per day.
Treatment failures of the 1st therapeutic approach as defined above occurred in 10 cases whereas failure of the 2nd procedure was present in only 2 cases. In these two cases an above-knee amputation with multiple revisions,6 and a resection arthroplasty with consecutive arthrodesis had to be performed.7 Due to recurrence of the fungal periprosthetic joint infection or secondary bacterial PJI an aboveknee amputation was performed in 5 patients (Supplementary Table S3).
Medical therapy
Information about the systemic antifungal therapy is illustrated in Figure 2. Systemic antifungal therapy was administered in all but 1 patient.8 In about half of the cases receiving systemic antifungal medication amphothericin B or fluconazole were administered either orally or intravenously. In descending order, the following drugs have been administered: 5-FC, itraconazole + ketoconazole + voriconazole and caspofungin. A combination of antifungal medication or a sequential antifungal therapy with exchange of medication was present in about 25% of reported cases, respectively. Local antifungal medication during the primary surgical treatment was either applied by implanting an impregnated cement spacer as mentioned above, intraarticular powder (100 mg amphotericing B, amphotericin B + itraconazole) or in a daily lavage (fluconazole 200 mg/d).9-12
Figure 2.
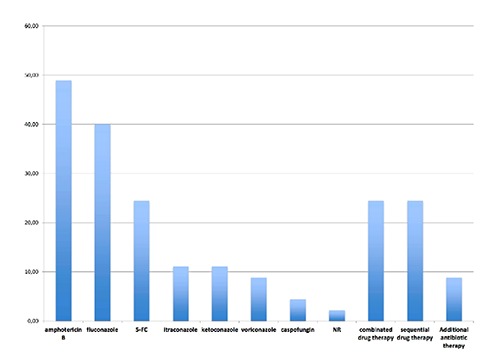
Systemic drug therapy. Systemic antifungal therapy was administered in all but 1 patient. More than 80 percent of the cases receiving systemic antifungal medication received either amphothericin B or fluconazole. In descending order, the following antifungal drugs have been administered: 5-FC, itraconazole + ketoconazole + voriconazole and caspofungin. A combination of antifungal medication or a sequential antifungal therapy with exchange of medication was present in about 25% of reported cases, respectively.
Resection arthroplasty with delayed re-implantation
In 19 cases a 2-stage-approach with delayed re-implantation was applied. In more than half of the reported cases the authors gave no information about the markers used to monitor the infection until re-implantation was initiated. The remaining patients have been monitored by (conjoint) use of clinical, serological and radiographic examination. An aspiration of the respective knee prior to re-implantation was performed in only 4 of the 19 patients. However, at the time of re-implantation in 8 cases the intraoperative cultures were reported negative. In the remaining 11 cases the authors did not report any cultural results. The mean period between resection arthroplasty and re-implantation was 3.3 months with the shortest interval of 8 days,4 and the longest of 6 months.4,11,13,14 At the time of re-implantation a systemic antifungal medication was applied in 5 out of 19 patients. Fluconazole was used in 4 patients and amphotericin B in 1 patient.11,15-18 During reimplantation the administration of a local antifungal medication was not reported in 17 of 19 cases. Wu and Hsu used bone cement impregnated with vancomycin and amphotericin B for revision arthroplasty.11 To prevent bacterial superinfection, Yilmaz et al.19 added teicoplanin to the bone cement whereas Graw et al.17 added low-dose tobramycin to the bone cement.
Outcome
The mean follow-up reported in the implemented studies was 37.4 months with a minimal follow-up of 1.5 months and a maximum follow-up of 240 months.17,18 Death due to an unrelated cause was presented in the studies of Dutronc et al.20 and Koëter et al.21 with the latter reporting a persistent fungal infection in the respective knee. A secondary bacterial infection leading to revision occurred in 5 cases.8,20,22,23 Due to recurrent fungal or secondary bacterial periprosthetic joint infections above-knee amputation was necessary in 5 patients (Supplementary Table S4). In 6 cases no definite statement about the therapeutic outcome was made.
Monitoring during the follow-up phase was accomplished via clinical, radiographic or serological examination. In more than half of the cases the authors did not report how the recurrence or persistence of the respective fungal infection was excluded. Likewise, no aspiration during the postoperative course was indicated.
Discussion
Definition
In the current article a fungal PJI of a TKA was defined as definite if there was a positive cultured proof of fungal germs in the either aspirated or intraoperative retained samples. In a recently published statement of the Musculoskeletal Infection Society an extended definition of PJI was proposed.24 The authors define a PJI if: i) there is a sinus tract communicating with the prosthesis; or ii) a pathogen is isolated by cultures from 2 or more separate tissue or fluid samples obtained from the affected prosthetic joint; or iii) when 4 of the following 6 criteria exist: (a) elevated serum erythrocyte sedimentation rate and serum C-reactive protein (CRP) concentration, (b) elevated synovial white blood cell count, (c) elevated synovial polymorphonuclear percentage (PMN%), (d) presence of purulence in the affected joint, (e) isolation of a microorganism in one culture of periprosthetic tissue or fluid, or (f) greater than 5 neutrophils per high-power field in 5 high-power fields observed from histologic analysis of periprosthetic tissue at 400× magnification. Accordingly, we would propose that the abovementioned definition is suitable for fungal periprosthetic joint infections, too. As fungal PJI often presents with only a mild clinical symptomatic it has to be considered that in a few cases the criteria may be too strict. To enhance sensitivity in case of negative fluid samples and evident clinical symptoms or laboratory signs repeated joint aspirations should be performed.3
Preoperative findings and diagnostic steps
Host factors that have been reported to predispose for fungal PJI are a decrease of cellular immunity, drug-induced immunosuppression, malignancy, injection drug use, chronic/prolonged or inappropriate use of antibiotics, indwelling catheters, diabetes mellitus, rheumatoid arthritis and many others.4,20 The current analysis revealed that in about 50% of the reported cases of a fungal PJI one or more risk factors have been reported (Supplementary Table S1). In this context, it has to be emphasized that a prior PJI with prolonged antibiotic therapy is one of the main risk factors for secondary fungal PJI. Thus, in patients with prior bacterial PJI one has to be aware that mild clinical symptoms such as persistent pain and limitation of ROM in combination with serological signs of infection may indicate a fungal PJI. As mentioned above, in these cases detection of the infectious agent has to be enforced by means of repetitive aspirations or collection of tissue samples.
The analysis of the available literature revealed that the most common clinical symptoms associated with a fungal PJI of a TKA involved pain and signs of local infection such as erythema, swelling or effusion. Signs of systemic infection, i.e. fever or shivering, were only present in 4 patients (<10%, Supplementary Table S2). Due to insufficient data no detailed analysis on the serological parameters could be performed. However, the authors suspect that in case of a fungal PJI standard serological infectious parameters (C-reactive protein, erythrocyte sedimentation rate and white blood cell count) should increase. As mentioned above, (repetitive) aspirations of the respective knee are the most important diagnostic step to verify or exclude a fungal PJI. To optimize the diagnostic process the use of selective fungal media with an adequate incubation time of 5-14 days has to be recommended. Examples of media that promote fungal isolations are ChromAgar Candida and Sabouraud Dextrose Agar.25,26 Especially if suspicion on fungal PJI is high and routine cultures are negative it may be reasonable to employ alternative test methods that are optimized to support the growth of fungi as well as to operatively collect tissue samples.
Medical therapy
The temporary implantation of antibiotic-impregnated PMMA spacers in bacterial periprosthetic joint infections is widely accepted and shows a reduction in the overall rate of late infections.27 In contrast, the topical administration of antifungal agents in fungal PJI is not scientifically proven to date, and there is an ongoing debate on the elution characteristics of impregnated bone cement.28 The release of antifungal medication such as amphotericin B or fluconazole was demonstrated in several in vitro and in vivo studies.29-32 However, it is still unclear if the concentrations are sufficient enough to provide a benefit in the treatment of fungal PJI. The higher resistance of fungi in biofilms may contribute to the loss in therapeutic significance.33 In the reviewed literature a local antifungal medication was either applied by implanting impregnated cement spacers, intra-articular administered powder,9,10 or in a continuous irrigation-system with a daily lavage.11,12 If a local fungal therapy is favored the references analyzed suggest the application of 200 mg of amphotericin B or 800 mg of fluconazole or voriconazole per 40 g bone cement. Furthermore, the addition of antibiotics to cement spacers in the treatment of fungal PJI may lead to a reduction of secondary bacterial joint infections as it was performed in 5 of the 45 patients in the current analysis.
Systemic antifungal therapy is essential in the therapeutic regime of treating fungal PJI and was present in all but one reported case.8 In most cases the application of fluconazole (400-800 mg/d) and amphotericin B (15-35 mg/d) either intravenously or orally produced good results. Combined or sequential antifungal drug therapy was reported in 11 cases, respectively. In addition, the perioperative duration of systemic administration varied from 3 weeks to a lifelong suppressive therapy.
The severity of the disease (sepsis), impairments due to organ failure, previous exposition to antifungal agents, identification of the pathogen, knowledge of the individual susceptibility pattern and patient’s general condition are key facts for the selection of the adequate antimicrobial agent. However, we would recommend a minimum of 6 weeks of intravenous antifungal medication which should be chosen in collaboration with a microbiologist. In cases of two-staged procedures the medication should be stopped prior to re-implantation. If aspirations and laboratory tests without antifungal medication do not indicate a relapse reimplantation can be performed.
Surgical therapy
The reported data regarding the initial surgical treatment of fungal periprosthetic TKA infections is heterogeneous (Supplementary Table S3). In 29 cases removal of the prosthesis, i.e. resection arthroplasty, was performed. Most authors emphasize the need of an extensive and radical debridement of the infected and necrotic tissue as well as the removal of all bone cement. Generally, while choosing the adequate surgical strategy to eliminate the infecting agent the surgeon has to avoid major impairments in the patient’s quality of life, i.e. to keep the patient in a state of sufficient mobility.
Retention of the implant in combination with a suppressive drug therapy or debridement and irrigation failed to produce successful outcomes in a quantitative sufficient manner. Fungal agents are locked in the biofilm and not assailable to the applied medication.33 Thus, even though the debridement was done in a thorough and radical way, fungal residuals will remain in situ if the prosthesis is not removed. However, these therapeutic approaches might be implemented in patients with a limited expectancy of life or operability.
Resection arthroplasty, i.e. the removal of the infected prosthesis, is the recommended surgical procedure in the treatment of fungal PJI. Permanent resection arthroplasty has the main advantage of removing all foreign material from the infected joint, i.e. that there are no surfaces left that might be coated with a biofilm. However, its major drawback is the limitation regarding the postoperative load carrying capacity and range of motion. Thus, this approach is not the first choice in the surgical treatment of fungal infected TKAs. Arthrodesis of the knee, i.e. removal of the prosthesis with (delayed) arthrodesis, has been performed with either intramedullary nails or external fixators. In the analyzed literature, this technique has been performed in 9 patients with only 2 patients suffering from a secondary bacterial infection.20 The recurrence of a fungal PJI was not observed in the postoperative course after resection arthroplasty and (subsequent) arthrodesis. Due to the loss in range of motion arthrodesis of the knee might be favored in patients with a limited demand on mobility or as secondary approach. On the basis of the current literature resection arthroplasty with delayed re-implantation, i.e. the 2-stage-procedure is the recommended treatment option to control fungal infected TKAs. Intercurrent implantation of cement spacers prevents shortening of the circumjacent soft tissue and - with the addition of antimicrobial agents - bacterial superinfection. The duration between removal and re-implantation is indicated with about 3 months. However, before re-implantation a persistent infection has to be excluded by diagnostic testing, i.e. laboratory parameters and joint aspiration with an appropriate incubation interval. The calculated revision rate due to persistent/recurrent fungal infection or a secondary bacterial infection using a 2-stage-procedure as 1st approach was about 30% (6 out of 19 cases).4,20,22,34 Interestingly enough, 2-stage-delayed re-implantation as secondary approach was performed only in one patient with failure of this procedure due to recurrence of the infection.6 To our knowledge, successful one-stage-exchange arthroplasties in fungal PJI of the knee has been reported only by Brooks and Pupparo,9 Simonian et al.35 and Langer et al.363 In these cases, the respective TKA had been changed under suspicion of aseptic loosening with detection of fungal agents in the intraoperative tissue samples. Brooks and Pupparo implemented a tibial exchange in combination with irrigation and debridement as well as topical administration of amphotericin B powder (100 mg).9 In none of the cases a recurrence or reinfection was reported. However, the experience of one-stage-exchange arthroplasties in fungal PJI is rare and needs further evaluation. To conclude, two-stage-re-implantations in fungal PJI may be considered as the gold standard, with a reported infection control rate just under 80%. The use of (impregnated) spacers is still a matter of debate as good outcomes can be achieved without spacers, too. The authors would recommend impregnating the spacer and subsequently the bone cement with standard antibiotics to prevent secondary bacterial infection. However, a restriction in the analysis on resection arthroplasty may result from the fact that it is not clear if the final surgical strategy, e.g. resection arthroplasty and arthrodesis, was the intended strategy before the operation was carried out. In other words, one may speculate that prolonged signs of infection withheld further surgical intervention.
Postoperative therapy and monitoring
In the early postoperative phase the same serological parameters are monitored as in bacterial PJI, i.e. C-reactive protein, erythrocyte sedimentation rate and white blood cell count. To date, there is no specific laboratory test to monitor fungal PJI. It is recommended to collect as many intraoperative tissue samples as possible to i) verify the infectious agent and ii) adapt the medicamentous therapy to the state of resistance. As mentioned above, the clinical presentation of a recurrence or reinfection is mild, i.e. the surgeon has to be vigilant and careful not to oversight the patients symptoms. If there is suspicion of recurrence or reinfection a repeated aspiration of joint fluid or operative biopsy of tissue samples has to be enforced.
Based on the available data, the optimal duration of postoperative antifungal therapy is still unclear. There is no general suggestion to prolong the antifungal agent administration after re-implantation in a 2-stage-exchange procedure.
References
- 1.Adeli B, Parvizi J. Strategies for the prevention of periprosthetic joint infection. J Bone Joint Surg Br 2012;94:42-6. [DOI] [PubMed] [Google Scholar]
- 2.Lentino JR. Infections associated with prosthetic knee and prosthetic hip. Curr Infect Dis Rep 2004;6:388-92. [DOI] [PubMed] [Google Scholar]
- 3.Gebauer M, Frommelt L, Achan P, et al. Management of fungal or atypical periprosthetic joint infections. J Arthroplasty 2014;29:112-4. [DOI] [PubMed] [Google Scholar]
- 4.Phelan DM, Osmon DR, Keating MR, Hanssen AD. Delayed reimplantation arthroplasty for candidal prosthetic joint infection: a report of 4 cases and review of the literature. Clin Infect Dis 2002;34:930-8. [DOI] [PubMed] [Google Scholar]
- 5.Azzam K, Parvizi J, Jungkind D, et al. Microbiological, clinical, and surgical features of fungal prosthetic joint infections: a multi-institutional experience. J Bone Joint Surg Am 2009;91:142-9. [DOI] [PubMed] [Google Scholar]
- 6.Lackner M, De Man FH, Eygendaal D, et al. Severe prosthetic joint infection in an immunocompetent male patient due to a therapy refractory Pseudallescheria apiosperma. Mycoses 2011;54:22-7. [DOI] [PubMed] [Google Scholar]
- 7.Goodman JS, Seibert DG, Reahl GE, Jr, Geckler RW. Fungal infection of prosthetic joints: a report of two cases. J Rheumatol 1983;10:494-5. [PubMed] [Google Scholar]
- 8.Ceffa R, Andreoni S, Borrè S, et al. Mucoraceae infections of antibiotic-loaded cement spacers in the treatment of bacterial infections caused by knee arthroplasty. J Arthroplasty 2002;17:235-8. [DOI] [PubMed] [Google Scholar]
- 9.Brooks DH, Pupparo F. Successful salvage of a primary total knee arthroplasty infected with Candida parapsilosis. J Arthroplasty 1998;13:707-12. [DOI] [PubMed] [Google Scholar]
- 10.Selmon GP, Slater RN, Shepperd JA, Wright EP. Successful 1-stage exchange total knee arthroplasty for fungal infection. J Arthroplasty 1998;13:114-5. [DOI] [PubMed] [Google Scholar]
- 11.Wu MH, Hsu KY. Candidal arthritis in revision knee arthroplasty successfully treated with sequential parenteral-oral fluconazole and amphotericin B-loaded cement spacer. Knee Surg Sports Traumatol Arthrosc 2011;19:273-6. [DOI] [PubMed] [Google Scholar]
- 12.Fukasawa N, Shirakura K. Candida arthritis after total knee arthroplasty: a case of successful treatment without prosthesis removal. Acta Orthop Scand 1997;68:306-7. [DOI] [PubMed] [Google Scholar]
- 13.Hennessy MJ. Infection of a total knee arthroplasty by Candida parapsilosis. A case report of successful treatment by joint reimplantation with a literature review. Am J Knee Surg 1996;9:133-6. [PubMed] [Google Scholar]
- 14.Wyman J, McGough R, Limbird R. Fungal infection of a total knee prosthesis: successful treatment using articulating cement spacers and staged reimplantation. Orthopedics 2002;25:1391-4. [DOI] [PubMed] [Google Scholar]
- 15.Yang SH, Pao JL, Hang YS. Staged reimplantation of total knee arthroplasty after Candida infection. J Arthroplasty 2001;16:529-32. [DOI] [PubMed] [Google Scholar]
- 16.Baumann PA, Cunningham B, Patel NS, Finn HA. Aspergillus fumigatus infection in a mega prosthetic total knee arthroplasty: salvage by staged reimplantation with 5-year follow-up. J Arthroplasty 2001;16:498-503. [DOI] [PubMed] [Google Scholar]
- 17.Graw B, Woolson S, Huddleston JI. Candida infection in total knee arthroplasty with successful reimplantation. J Knee Surg 2010;23:169-74. [DOI] [PubMed] [Google Scholar]
- 18.Yilmaz M, Mete B, Ozaras R, et al. Aspergillus fumigatus infection as a delayed manifestation of prosthetic knee arthroplasty and a review of the literature. Scand J Infect Dis 2011;43:573-8. [DOI] [PubMed] [Google Scholar]
- 19.Darouiche RO, Hamill RJ, Musher DM, et al. Periprosthetic candidal infections following arthroplasty. Rev Infect Dis 1989;11:89-96 Erratum in: Rev Infect Dis 1989;11:510. [DOI] [PubMed] [Google Scholar]
- 20.Dutronc H, Dauchy FA, Cazanave C, et al. Candida prosthetic infections: case series and literature review. Scand J Infect Dis 2010;42:890-5. [DOI] [PubMed] [Google Scholar]
- 21.Koëter S, Jackson RW. Successful total knee arthroplasty in the presence of sporotrichal arthritis. Knee 2006;13:236-7. [DOI] [PubMed] [Google Scholar]
- 22.Badrul B, Ruslan G. Candida albicans infection of a prosthetic knee replacement: a case report. Med J Malaysia 2000;55:93-6. [PubMed] [Google Scholar]
- 23.Levine M, Rehm SJ, Wilde AH. Infection with Candida albicans of a total knee arthroplasty. Case report and review of the literature. Clin Orthop Relat Res 1988:235-9. [PubMed] [Google Scholar]
- 24.Workgroup Convened by the Musculoskeletal Infection Society. New definition for periprosthetic joint infection. J Arthroplasty 2011;26:1136-8. [DOI] [PubMed] [Google Scholar]
- 25.Cuenca-Estrella M, Verweij PE, Arendrup MC, et al. ESCMID guideline for the diagnosis and management of Candida diseases 2012: diagnostic procedures. Clin Microbiol Infect 2012;18:9-18. [DOI] [PubMed] [Google Scholar]
- 26.Schäfer P, Fink B, Sandow D, Frommelt L. Infections in hip and knee arthroplasty: challenges to and chances for the microbiological laboratory. In:Fokter S, ed. Recent advances in arthroplasty. Rijeka: InTech Europe; 2012. pp 439-458. [Google Scholar]
- 27.Hanssen AD, Osmon DR. The use of prophylactic antimicrobial agents during and after hip arthroplasty. Clin Orthop Relat Res 1999:124-38. [DOI] [PubMed] [Google Scholar]
- 28.Goss B, Lutton C, Weinrauch P, et al. Elution and mechanical properties of antifungal bone cement. J Arthroplasty 2007;22:902-8. [DOI] [PubMed] [Google Scholar]
- 29.Buranapanitkit B, Oungbho K, Ingviya N, The efficacy of hydroxyapatite composite impregnated with amphotericin B. Clin Orthop Rel Res 2005;437:236-41. [DOI] [PubMed] [Google Scholar]
- 30.Marra F, Robbins GM, Masri BA, et al. Amphotericin B-loaded bone cement to treat osteomyelitis caused by Candida albicans. Can J Surg 2001:44;383-6. [PMC free article] [PubMed] [Google Scholar]
- 31.Sealy PI, Nguyen C, Tucci M, et al. Delivery of antifungal agents using bioactive and nonbioactive bone cements. Ann Pharmacother 2009;43:1606-15. [DOI] [PubMed] [Google Scholar]
- 32.Silverberg D, Kodali P, Dipersio J, et al. In vitro analysis of antifungal impregnated polymethylmethacrylate bone cement. Clin Orthop Relat Res 2002;403:228-31. [DOI] [PubMed] [Google Scholar]
- 33.Coad BR, Kidd SE, Ellis DH, Griesser HJ. Biomaterials surfaces capable of resisting fungal attachment and biofilm formation. Biotechnol Adv 2014;32:296-307. [DOI] [PubMed] [Google Scholar]
- 34.Gaston G, Ogden J. Candida glabrata periprosthetic infection: a case report and literature review. J Arthroplasty 2004;19:927-30. [DOI] [PubMed] [Google Scholar]
- 35.Simonian PT, Brause BD, Wickiewicz TL. Candida infection after total knee arthroplasty. Management without resection or amphotericin B. J Arthroplasty 1997;12:825-9. [DOI] [PubMed] [Google Scholar]
- 36.Langer P, Kassim RA, Macari GS, Saleh KJ. Aspergillus infection after total knee arthroplasty. Am J Orthop (Belle Mead NJ) 2003;32:402-4. [PubMed] [Google Scholar]


