Abstract
Spontaneous haemothorax (SH) is a subcategory of haemothorax that involves the accumulation of blood within the pleural space in the abscence of trauma or other causes. The clinical presentation is variable and includes a rapid progression of symptoms of chest pain and dyspnea that can be life threatening when hemodynamic instability and hypovolemic shock occurs. Despite haemothorax, SH is much less common with data limited to case reports and case series. A literature review has been performed to identify and summarise all potentials causes leading to this clinical entity.
Keywords: Spontaneous haemothorax (SH), coagulopathy, haemopneumothorax
Introduction
Haemothorax is a clinical entity that in most cases can be caused by trauma, coagulopathy, or iatrogenic causes through procedures such as central line insertion, thoracocentesis, pleural biopsies. It is defined as a pleural fluid with hematocrit greater than 50% of the patient’s blood, although in cases of long standing haemothorax due to haemodilution, hematocrit level can be lower mimicking a hemorrhagic exudation. Therefore a hematocrit of 25-50% of the patients blood can raise the suspicion of haemothorax.
Spontaneous haemothorax (SH) is a subcategory of haemothorax that involves the accumulation of blood within the pleural space in the absence of trauma or other causes. The clinical presentation is variable and includes a rapid progression of symptoms of chest pain and dyspnea that can be life threatening when hemodynamic instability and hypovolemic shock occurs.
Despite haemothorax, SH is much less common with data limited to case reports and case series (1).
A literature review has been performed to identify and summarise all potential causes leading to this clinical entity (Table 1).
Table 1. Etiology of SH.
| Category | Etiology |
|---|---|
| Pneumothorax | SHP |
| Coagulopathy | Congenital disease (hemophilia, glanzmann thromboastenia) |
| Drug related | |
| Vascular | AVMs |
| VRD | |
| Aneurysms | |
| EDS type IV | |
| Connective tissue disease | |
| Neoplasia | Angiosarcoma |
| Schwannoma | |
| Thymoma | |
| Vascular tumors | |
| Germ cell tumors | |
| Hepatocellular carcinoma | |
| Lung cancer | |
| Mesothelioma | |
| Miscellaneous | Exostoses |
| Extramedullary hematopoiesis | |
| Endometriosis | |
| Pulmonary sequestration |
SH, spontaneous haemothorax; SHP, spontaneous haemopneumothoax; AVMs, arteriovenous malformations; VRD, von recklinghausen disease; EDS, Ehlers-Danlos syndrome.
Spontaneous haemopneumothorax (SHP)
SHP is the most common cause of haemothorax. Approximately 5% of patients with pneumothorax will have concomitant haemothorax with an amount of blood in the pleural space and with variable clinical presentation (2). The source of bleeding in most cases results from the shearing of the adhesions between the parietal and the visceral pleura. Due to the presence of pneumothorax there is no tamponade from the lung while blood is accumulating in the pleural space under systemic pressure that is about six times higher than in the pulmonary arterial circulations.
Treatment of SHP includes, in addition to fluid resuscitation and blood transfusion, tube thoracostomy for drainage of the haemothorax and re-expansion of the lung.
Following chest drain insertion patients can be potential candidates for surgery via either video-assisted thoracoscopic surgery (VATS) or open thoracotomy. A retrospective review of 24 patients with SHP that compared 11 patients treated with early VATS and 13 treated with initial conservative treatment and subsequent surgery when needed demonstrated that the early VATS group had longer operating time, less preoperative blood loss, less blood transfusion, shorter period of chest tube drainage and shorter length of stay. Surgery in the form of VATS should be considered early in the management of SHP with less postoperative complications and shorter postoperative hospital stay compared also with open thoracotomy (3).
Coagulopathy
Drug related haemothorax
Haemothorax may occur along with the administration of anticoagulant therapy. Blood can be collected in the pleural cavity either as a result of minimal trauma in the chest or spontaneous rupture of small vessels. Bleeding may also occur with the administration of systemic and intrapleural thrombolytics or in the setting of inherited coagulation disorders such as haemophilia (4,5). Haemothorax has been also reported in the setting of plasminogen activator user for venous thrombosis in patient with pneumonia.
Glanzmann thromboastenia
Glanzmann thromboastenia is an autosomal recessive disorder characterized by a lifelong bleeding tendency due to a quantitative and qualitative abnormalities of the platelet integrin αΠbβ3 [glycoprotein (GP) IIb; CD41/IIIa; CD61]. The GP IIb/IIIa is a receptor for fibrinogen, fibronectin, vitronectin, von Willebrand factor and thrombospondin, and mediates platelets aggregation via fibrinogen, firm adhesions and spreading. Common clinical manifestations include purpuric type skin bleeding, epistaxis, gingival bleeding and menorrhagia; haemarthrosis, haematuria, intracranial and visceral hemorrhage are rare but even rarer is SH (6,7). Bleeding could be controlled successfully by platelet transfusing before and after thoracentesis and tube replacement. It is usually self limited once coagulopathy is corrected.
Hemophilia A
Hemophilia A is a life-threatening hemorrhagic disorder caused by the development of an inhibitor against coagulation factor VIII (FVIII). Hemophilia is a X-linked hereditary disorder that consists of a defective and/or deficient FVIII molecule. It affects approximately 1 in 1 million inhabitants and can present as a sudden onset of serious bleeding in patients without a prior history of coagulation disorder (8). Hemophilia A manifests with early muscle and subcutaneous bleeding in 70% of cases. Haemothorax is a very rare event occurring in less than 1% of cases. Treatment of acquired hemophilia requires hemostasis to address hemorrhage and immunosuppressive therapy to suppress production of the FVIII inhibitor. Therapies that activate other coagulation system such as recombinant activated FVII or the active form of prothrombine complex concentrates derived from human plasma are effective (9). Evacuation of the residual haemothorax can be performed safely either with VATS or open thoracotomy.
Vascular
Haemothorax of vascular origin is often due to a rupture of the descending thoracic aorta, initially in the mediastinal and left pleural space due to the proximity of the pleural cavity. Rupture of the thoracic aorta in the right pleural cavity is rare. The ascending thoracic aorta bleeds mainly into the pericardium. Aortic dissection and aneurysm affect patients with risk factors such as hypertension, congenital aortic anomalies (aortic coarctation, bicuspid aortic valve disease). The treatment and the course depend mainly on the severity of the initial clinical manifestation, the type and extent of aortic lesion and the patient history. Radiological investigation are the key for diagnosis: chest X-rays show mediastinal enlargement and pleural effusion. CT scan with contrast is the tool of choice for the diagnosis and the management of patient with suspected haemothorax caused by aneurysm or aortic dissection. For aneurysm, surgery is indicated when patient become symptomatic. In the absence of surgery, radio-clinical strict monitoring is necessary. Dissections of the ascending aorta (Stanford type A) must be operated in an emergency, while decision for surgery of descending aortic dissection (Stanford type B) is made on assessing the case of acute complications. Thoracic drainage in these situations is not recommended because it can lead to hemodynamic instability.
Ehlers-Danlos syndrome (EDS)
EDS forms part of a spectrum of inherited connective tissue disorders that includes osteogenesis imperfecta and has an incidence of 1 in 5,000. It is an inherited disorder of collagen synthesis and is characterized by hyperlaxity of joints. There is also a tendency to bruising and bleeding which is a feature of the vascular type IV EDS. There are six variants of EDS of which type III (joint hypermobility syndrome) is the most frequent. Type IV is an autosomal dominant variant, known as vascular EDS and is rare, accounting for ~10% of the EDS variants. The underlying genetic mutation is that of COL3A1 gene, resulting in abnormalities in type III procollagen production and synthesis (10). Respiratory manifestations of vascular EDS although not always common, have been described and include recurrent hemoptysis, bullae and bleb formation, and spontaneous hemo-pneumothoraces. Most frequent initial manifestations of vascular EDS is arterial dissection or colon rupture. Management of patient with vascular EDS is difficult because there is no specific treatment. Vascular complications are general sudden and catastrophic and gradual vascular dilatation such as seen in Marfan’s syndrome is not a feature in EDS. Patients with hemodynamic instability and rapid rate of bleeding should be managed with surgery performed with open approach.
Von recklinghausen disease (VRD)
Type I neurofibromatosis (NF-1) or VRD is known to cause SH. It is an autosomal dominant disease with an occurrence rate of 1 in 3,000 (11). Its prevalence is similar in all races and sexes, and is usually diagnosed in adulthood, since its clinical characteristics develop throughout life. This entity can affect any organ system, especially connective, nervous and vascular tissues, and is characterized by skin tumors and abnormal cutaneous pigmentation. Two main pathogenetic mechanism have been advocated for vasculopathy associated with VRD: (I) direct vascular invasion from adjacent tumors such as schwannoma, neurofibroma, or neurofibrosarcoma (12); and (II) vascular dysplasia with thickening and concomitant reduced strength of the vessel wall and aneurysm formation (13). Invasive tissue leads to compression of the vasa vasorum with subsequent ischemia and weakness of blood vessels. Small vessel damage is due to dysplasia of the wall due to proliferation of the intimae and muscolaris, loss of the media and fibrosis of the adventitia. This initiates a stenosis of the vessel wall, potentiating vessel wall weakness and friability with subsequent risk of rupture.Vascular dysplasia often causes thickening and concomitant reduced strength of the vessel wall leading to aneurysm formation.
Treatment options are dependent on the patient’s hemodynamic stability. Endovascular embolization is indicated if there is hemodynamic stability. As an alternative, thoracotomy with surgical ligation is indicated in cases of active bleeding, with associated hemodynamic compromise. Regarding the prognosis of NF-1 in patients with haemothorax, the disease mortality is 36% and postoperative mortality is 33%. Recently published cases suggest that coil embolization offers the best results (11).
Rendu-Osler-Weber syndrome
Rendu-Osler-Weber syndrome, also known as hereditary hemorrhagic telangiectasia (HHT), is an autosomal dominant hemorrhagic disorder characterized by multiple cutaneous, systemic, and/or pulmonary arteriovenous malformations (AVMs). Almost invariably, the patients have epistaxis, but bleeding may occur from any AVM site. Bleeding from pulmonary lesions usually occur as haemoptysis and rarely as SH. Only a few fatal cases of such rare presentation of this syndrome have been reported (14). The pulmonary complications of Rendu-Osler-Weber syndrome were first recognized in 1917, 20 years after the first report of pulmonary AVM. For unknown reasons, pulmonary AVMs, which may be congenital or acquired, are more common on the left side and occur most commonly in the left lower lobe. Through these vessels, blood is shunted (usually from branches of the pulmonary artery) into the pulmonary veins. Rupture of an AVM may occur at any age and is not absolutely dependent on the size of the lesion. Rupture and other combination are more common in women. About 15% of patients with Rendu-Osler-Weber syndrome have pulmonary arteriovenous fistulas, and intrabronchial rupture, has been reported in 8% to 25%. Intrapleural rupture with consequent haemothorax is a less common and potentially fatal combination. Once pulmonary AVM has bled, it should be treated aggressively with either surgical resection or embolization as soon as possible to prevent life threatening rupture of the lesion. Until the late 1970s, the treatment of choice for pulmonary AVM was surgical resection or ligation of the arteriovenous fistula. When emergency local excision or segmental resection of the lesion could not be performed due to precarious health conditions or the occurrence of multiple lesions, lobectomy or pneumectomy represents a salvage option. Nowadays, embolotherapy appears to be the preferred treatment modality, since it preserves lung function and minimizes morbidity associated with thoracotomy or lung resection (15).
Neoplasia
Angiosarcoma
Angiosarcoma is a rare malignant vascular tumor that accounts for <2% of al sarcomas. It usually occurs in middle aged patients, and the most common locations of the primary tumor are the skin, heart, liver, spleen, bone and GI tract. Symptoms are closely related to tumor location. The most common metastatic site for angiosarcoma is the lung. Its development can be rapid. Since the pleural effusion is directly related to pleural invasion, it usually has a bloody appearance with high hematocrit value also containing neoplastic cells.
Schwannoma
Schwannoma is a neurogenic tumor arising from the Schwann cells of the neural sheath. It originates commonly in the extremities, head and neck. Most intrathoracic neurogenic tumors originate in the posterior mediastinum, with 5.4% arising in the thoracic wall (16,17). Benign schwannomas rarely manifest as a pleural effusion. It is often impossible to establish whether these tumors are benign or malignant before surgery, but the risk of malignancy for this kind of tumour is very small (2-5%). If the patient has VRD (discussed above) or a history of radiation exposure, the risk of malignancy increases to 10-20%. Magnetic resonance represent a very useful tool to obtain a complete diagnosis.
Thymoma
Thymomas represent a rare cause of SH (18). The cause of spontaneous rupture of a thymoma is obscure. An enlargement of a thymoma may cause rupture and subsequently produce haemomediastinum or haemothorax. In cases of malignant thymoma, rupture may be attributable partly due to tumour invasion to adjacent vital structures. Another mechanism could be spontaneous intratumor hemorrhage without enlargement. VATS surgery has not been accepted completely for thymectomy. As thymomas have a potential malignant behaviour, tumor resection accompanied by total thymectomy is recommended. In addition, tumor dissection from adjacent structures is required.
Hepatocarcinoma
Spontaneous rupture of hepatocellular carcinoma (HCC) is known to be a condition with poor prognosis. The liver is an organ inside the peritoneal cavity, so the rupture of HCC generally causes haemoperitoneum. Among these cases, few reports exist on the rupture of HCC originating from the caudate lobe in which a haematoma is often formed in the omental bursa (also known as the lesser sac). On the other hand, haemothorax is a very unusual presentation of ruptured HCC and is accompanied by a high mortality rate secondary to uncontrollable haemorrhage. This is due to the negative pressure inside the pleural cavity that makes spontaneous haemostasis difficult (19).
The diaphragm is a muscular tissue which separates the thoracic cavity from the abdominal cavity and has three openings: a caval opening, an oesophageal hiatus, and an aortic hiatus. Since the caudate lobe is a section that anatomically comes in contact with the inferior vena cava, HCC and related haematoma could intensely retract the inferior vena cava. Consequently, it is possible that the blood is flowing along the connective tissue sheaths of the inferior vena cava, and, after entering the mediastinum through the caval opening, may ruptures the pleura and flows into the right pleural cavity causing haemothorax.
Miscellaneous
Costal exostoses
Exostosis occurs in the ribs either sporadically or as a manifestation of a genetic disorder known as hereditary multiple exostoses (HME). HME is an autosomal-dominant condition characterised by exostoses that can appear in different skeletal structures. Lesions mainly occur in infants and children, and usually cease enlarging in puberty. HME in the ribs is rare and generally asymptomatic, and approximately 30 cases of intrathoracic complications have been reported. These complications include haemothorax, pneumothorax, diaphragmatic or pericardial lacerations and visceral pleural injury (20).
The exact mechanism of bleeding is not completely clear. It may be due to injury to the visceral pleura and underlying pulmonary parenchyma from direct contact with the sharp exostoses; bleeding may also result from rupture of dilated vessels associated with long term friction between the intrathoracic exostoses and visceral pleura, which also cause pneumothorax. The respiratory motion of the lower lobe is greater than that of the right middle or upper lobes. Thus, pneumothorax may easily occur in both the lower lobe and visceral pleura as a result of lung injury caused by exostoses (21).
Surgical approach with either VATS or thoracotomy is needed to resect the exostoses and prevent recurrence.
Extralobar pulmonary sequestration (EPS)
EPS is an embryonic anomaly. It is considered to be an accessory lobe and was first describe in 1861 by Rokitansky and Rektorik. EPS, an entity in which an abnormal lung segment is enclosed within its own pleural membrane, is completely separated from the tracheobronchial tree and accounts for 25% of all pulmonary sequestrations (22,23). The location, usually related to the left hemidiaphragm, may vary between the lower lobe and the diaphragm within the mediastinum, the lung, pleural and pericardial space or the retroperitoneum. Symptoms are usually ipsilateral chest pain and respiratory failure with sudden onset caused by haemothorax or infarction.
Extramedullary haematopoiesis (EMH)
EMH is a common compensatory mechanism for chronic anaemia, found in patients with haemoglobinopathies such as thalassaemia, sickle cell disease and hereditary spherocytosis (24). These patients are usually asymptomatic. EMH usually manifests in the thorax as multiple posterior mediastinal, paravertebral masses or masses along the lateral margins of the ribs.
B-thalassemia remains the most common cause of EMH, these patients are under a constant hypoxia due to decreased blood oxygen load and defective haemoglobin unload in the periphery. The constant and long lasting hypoxia leads to enhanced rate of erythropoiesis and increased intestinal iron absorption. The former imply a marked bone marrow hyperplasia and extramedullary foci of haematopoiesis, resulting in spleen and liver enlargement or even tumor-like masses in several sites, mainly paravertebral (25). These masses frequently remain asymptomatic but in certain patients may cause different syndromes due to compression of the surrounding structures. The extramedullary foci consist of haemopoietic cells and adipose elements. They are not circumscribed by a capsule. The mass is extremely vascular and contains little fibrous tissue. The structure renders the mass prone to haemorrhage. All the published cases of extramedullary foci bleeding deal with the pleural cavity; the reason is not clear but it may be related to preferential localisation or specific tissue structures rendering them vulnerable to rupture due to thoracic respiratory movements (26).
Endometriosis
SH may result from endometrial implants on the pleural surface and then response to cyclical hormonal changes in menstruating women. Endometrial implantation occurs as a result of migration of endometrial tissue through fenestrations on the diaphragm.
This phenomenon is known as catamenial haemothorax. This entity is usually managed by hormonal therapy designed to limit oestrogen or produce amenorrhoea (27). In case of failure of hormonal therapy, exploration of the pleural space and resection of endometrial implants may be necessary.
Bilateral spontaneous haemothorax (SH)
Bilateral SH represents a very rare entity with only eight cases described in the literature over the last fifty years. A total of 50% of them were related to cardiac angiosarcoma (28). The others were related to subclavian artery aneurysm, endometriosis and iatrogenic coagulopathy. The eighth case was idiopathic. Thus primary or metastatic pleural angiosarcoma should be considered as the main cause of spontaneous bilateral haemothorax (29).
Diagnosis and initial management
In the presence of bloody effusion the first step is to check the haematocrit to confirm a haemothorax identified, as stated above, between 25% to 50%. It is important to consider that an haemothorax can appear like a haemorrhagic effusion with a lower hematocrit due to significant dilution in 3-4 days. Also an enhanced CT of the chest can provide helpful information about the etiology.
For the neoplastic etiologies, cytology of the pleural fluid is useful, but immunochemical markers may increase the yield and degree of confidence. For the endometriosis cases, findings also include haemosiderin, histiocytes but rarely endometrial epithelial cells.
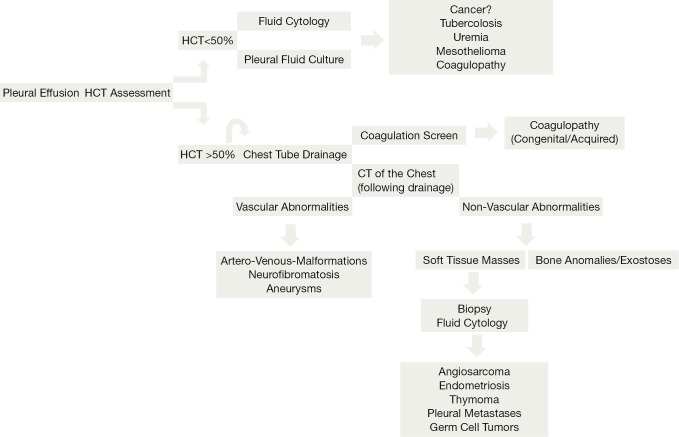
The management is related to the patient stability (Figure 1). Thoracoscopic draining is usually for stable patients. In haemodynamically unstable patients or if the rate of bleeding is more than 500 mL/hr in the first hour with 200-300 mL/hr subsequently, early surgical approach is favoured. Correction of coagulopathy is mandatory in every case of anticoagulant induced bleeding. Embolisation remains a valid option in the treatment of vascular abnormalities. The management of the residual haemothorax is controversial with a growing number favouring early VATS particularly if a significant amount of clot is present to prevent fibrothorax and restrictive physiology.
Figure 1.
Flowchart depicting the process of assessment of a spontaneous haemothorax.
Summary
In this paper we have reviewed the major etiologies of spontaneous (nontraumatic) haemothorax. SH is a rare clinical entity that can lead to potentially life-threatening complications. The thoracic surgeons should be familiar with its multiple causes and management, paying attention to the differential diagnosis and to the management with conservative, thoracoscopic and open surgical approach with attention of etiology specific treatments.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Martinez FJ, Villanueva AG, Pickering R, et al. Spontaneous hemothorax. Report of 6 cases and review of the literature. Medicine (Baltimore) 1992;71:354-68. [PubMed] [Google Scholar]
- 2.Hsu NY, Shih CS, Hsu CP, et al. Spontaneous hemopneumothorax revisited: clinical approach and systemic review of the literature. Ann Thorac Surg 2005;80:1859-63. [DOI] [PubMed] [Google Scholar]
- 3.Chang YT, Dai ZK, Kao EL, et al. Early video-assisted thoracic surgery for primary spontaneous hemopneumothorax. World J Surg 2007;31:19-25. [DOI] [PubMed] [Google Scholar]
- 4.Hsiao CW, Lee SC, Chen JC, et al. Massive spontaneous haemopneumothorax in a patient with haemophilia. ANZ J Surg 2001;71:770-1. [DOI] [PubMed] [Google Scholar]
- 5.Morecroft JA, Lea RE. Haemothorax--a complication of anticoagulation for suspected pulmonary embolism. Br J Clin Pract 1988;42:217-8. [PubMed] [Google Scholar]
- 6.Coller BS, French DL. Hereditary qualitative platelet disorders. In: Beutler E, Lichtman MA, Collier BS, et al. eds. Williams’ Hematology. 6th ed. New York: McGraw-Hill, 2001:1551-60. [Google Scholar]
- 7.Calabrese C, Di Febo G, Areni A, et al. Severe and relapsing upper gastrointestinal bleeding in a patient with Glanzmann’s thrombasthenia. Dig Dis Sci 2000;45:633-6. [DOI] [PubMed] [Google Scholar]
- 8.Franchini M, Gandini G, Di Paolantonio T, et al. Acquired hemophilia A: a concise review. Am J Hematol 2005;80:55-63. [DOI] [PubMed] [Google Scholar]
- 9.Huth-Kühne A, Baudo F, Collins P, et al. International recommendations on the diagnosis and treatment of patients with acquired hemophilia A. Haematologica 2009;94:566-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Purohit N, Marsland D, Roberts N, et al. Haemo-pneumothorax and haemoptysis in a patient with suspected Ehlers-Danlos syndrome. Interact Cardiovasc Thorac Surg 2009;9:130-1. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez-Guzman M, Gallegos-Carrera B, Vicente-Antunes S, et al. Spontaneous Hemothorax in a Patient With von Recklinghausen’s Disease. J Clin Med Res 2014;6:149-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chew DK, Muto PM, Gordon JK, et al. Spontaneous aortic dissection and rupture in a patient with neurofibromatosis. J Vasc Surg 2001;34:364-6. [DOI] [PubMed] [Google Scholar]
- 13.Miura H, Taira O, Uchida O, et al. Spontaneous haemothorax associated with von Recklinghausen’s disease: review of occurrence in Japan. Thorax 1997;52:577-8; discussion 575-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berg AM, Amirbekian S, Mojibian H, et al. Hemothorax due to rupture of pulmonary arteriovenous malformation: an interventional emergency. Chest 2010;137:705-7. [DOI] [PubMed] [Google Scholar]
- 15.White RI, Jr. Pulmonary arteriovenous malformations: how do I embolize? Tech Vasc Interv Radiol 2007;10:283-90. [DOI] [PubMed] [Google Scholar]
- 16.Kara M, Ozkan M, Sak SD, et al. Giant ancient schwannoma of the posterior mediastinum cytologically misdiagnosed as a malignant tumour. A case report. Acta Chir Belg 2002;102:464-6. [DOI] [PubMed] [Google Scholar]
- 17.Varaldo E, Crespi G, Ansaldo GL, et al. Neurinoma originating from the recurrent nerve: report of a case. Surg Today 2008;38:633-4. [DOI] [PubMed] [Google Scholar]
- 18.Iyer A, Malik P, Krishnan R, et al. Ruptured thymoma managed via thoracotomy. Asian Cardiovasc Thorac Ann 2013;21:744-5. [DOI] [PubMed] [Google Scholar]
- 19.Sohara N, Takagi H, Yamada T, et al. Hepatocellular carcinoma complicated by hemothorax. J Gastroenterol 2000;35:240-4. [DOI] [PubMed] [Google Scholar]
- 20.Khosla A, Parry RL. Costal osteochondroma causing pneumothorax in an adolescent: a case report and review of the literature. J Pediatr Surg 2010;45:2250-3. [DOI] [PubMed] [Google Scholar]
- 21.Simansky DA, Paley M, Werczberger A, et al. Exostosis of a rib causing laceration of the diaphragm: diagnosis and management. Ann Thorac Surg 1997;63:856-7. [DOI] [PubMed] [Google Scholar]
- 22.Carter R.Pulmonary sequestration. Ann Thorac Surg 1969;7:68-88. [DOI] [PubMed] [Google Scholar]
- 23.Halkic N, Cuénoud PF, Corthésy ME, et al. Pulmonary sequestration: a review of 26 cases. Eur J Cardiothorac Surg 1998;14:127-33. [DOI] [PubMed] [Google Scholar]
- 24.Chu KA, Lai RS, Lee CH, et al. Intrathoracic extramedullary haematopoiesis complicated by massive haemothorax in alpha-thalassaemia. Thorax 1999;54:466-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chute DJ, Fowler DR. Fatal hemothorax due to rupture of an intrathoracic extramedullary hematopoietic nodule. Am J Forensic Med Pathol 2004;25:74-7. [DOI] [PubMed] [Google Scholar]
- 26.Kupferschmid JP, Shahian DM, Villanueva AG. Massive hemothorax associated with intrathoracic extramedullary hematopoiesis involving the pleura. Chest 1993;103:974-5. [DOI] [PubMed] [Google Scholar]
- 27.Johnson MM. Catamenial pneumothorax and other thoracic manifestations of endometriosis. Clin Chest Med 2004;25:311-9. [DOI] [PubMed] [Google Scholar]
- 28.McCleary AJ. Massive haemothorax secondary to angiosarcoma. Thorax 1994;49:1036-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nomura M, Nakaya Y, Saito K, et al. Hemopneumothorax secondary to multiple cavitary metastasis in angiosarcoma of the scalp. Respiration 1994;61:109-12. [DOI] [PubMed] [Google Scholar]