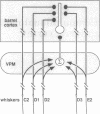
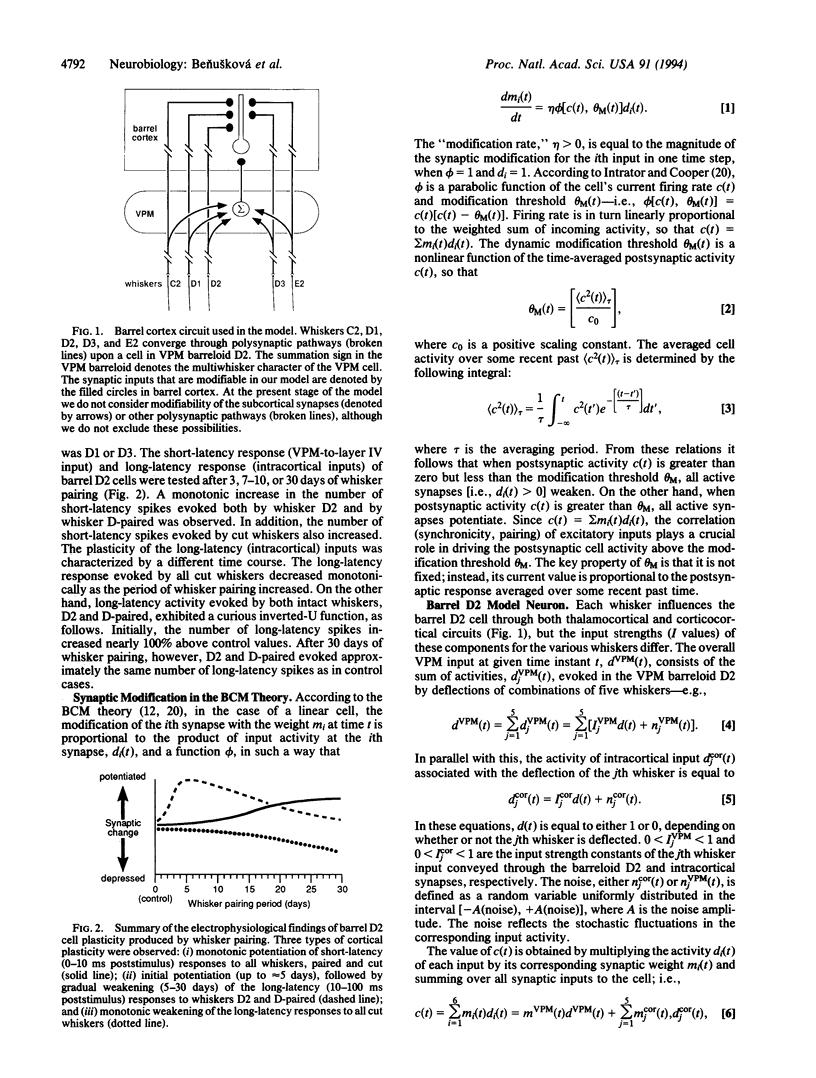
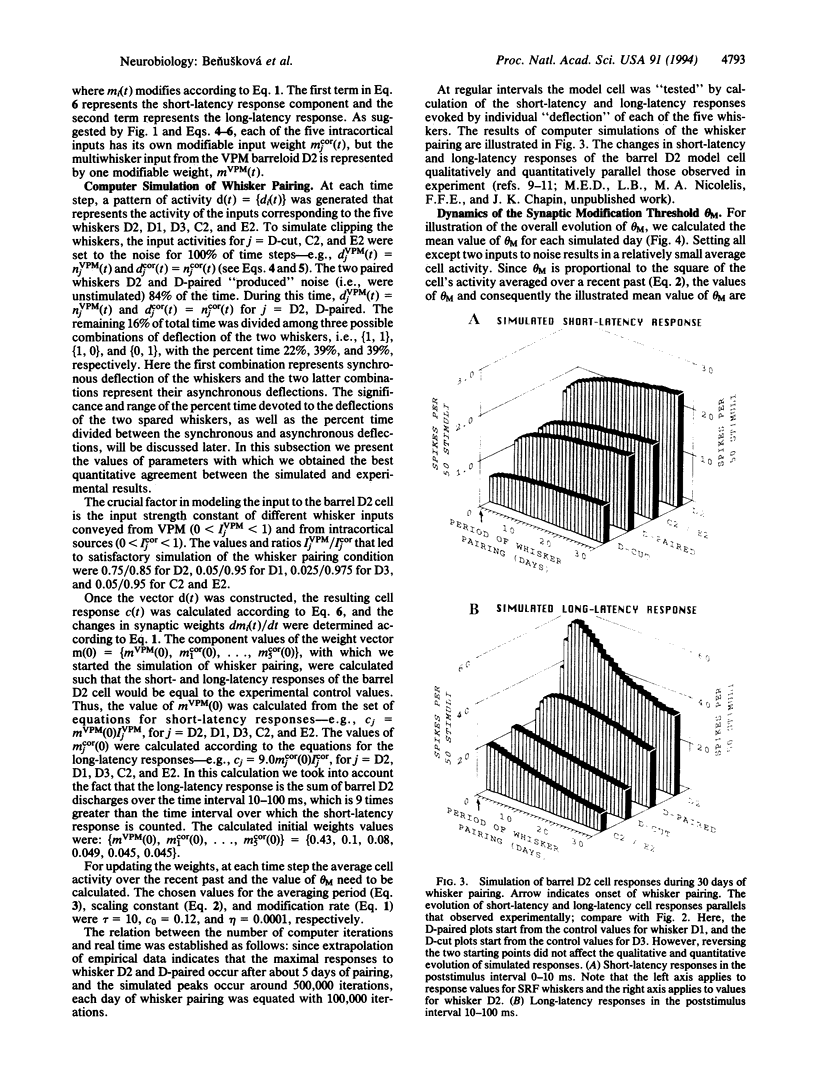
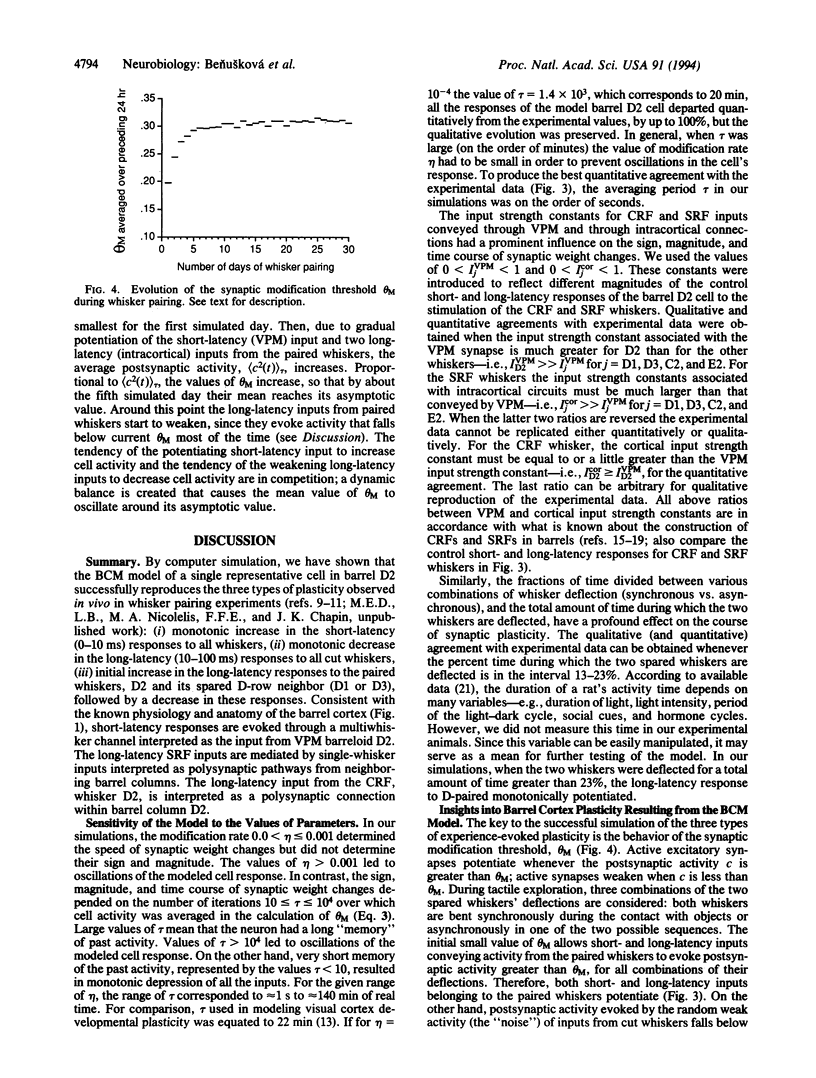
Abstract
Previous electrophysiological experiments have documented the response of neurons in the adult rat somatic sensory ("barrel") cortex to whisker movement after normal experience and after periods of experience with all but two whiskers trimmed close to the face (whisker "pairing"). To better understand how the barrel cortex adapts to changes in the flow of sensory activity, we have developed a computational model of a single representative barrel cell based on the Bienenstock, Cooper, and Munro (BCM) theory of synaptic plasticity. The hallmark of the BCM theory is the dynamic synaptic modification threshold, theta M, which dictates whether a neuron's activity at any given instant will lead to strengthening or weakening of the synapses impinging on it. The threshold theta M is proportional to the neuron's activity averaged over some recent past. Whisker pairing was simulated by setting input activities of the cell to the noise level, except for two inputs that represented untrimmed whiskers. Initially low levels of cell activity, resulting from whisker trimming, led to low values for theta M. As certain synaptic weights potentiated, due to the activity of the paired inputs, the values of theta M increased and after some time their mean reached an asymptotic value. This saturation of theta M led to the depression of some inputs that were originally potentiated. The changes in cell response generated by the model replicated those observed in in vivo experiments. Previously, the BCM theory has explained salient features of developmental experience-dependent plasticity in kitten visual cortex. Our results suggest that the idea of a dynamic synaptic modification threshold, theta M, is general enough to explain plasticity in different species, in different sensory systems, and at different stages of brain maturity.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong-James M., Callahan C. A., Friedman M. A. Thalamo-cortical processing of vibrissal information in the rat. I. Intracortical origins of surround but not centre-receptive fields of layer IV neurones in the rat S1 barrel field cortex. J Comp Neurol. 1991 Jan 8;303(2):193–210. doi: 10.1002/cne.903030203. [DOI] [PubMed] [Google Scholar]
- Armstrong-James M., Callahan C. A. Thalamo-cortical processing of vibrissal information in the rat. II. spatiotemporal convergence in the thalamic ventroposterior medial nucleus (VPm) and its relevance to generation of receptive fields of S1 cortical "barrel" neurones. J Comp Neurol. 1991 Jan 8;303(2):211–224. doi: 10.1002/cne.903030204. [DOI] [PubMed] [Google Scholar]
- Armstrong-James M., Fox K., Das-Gupta A. Flow of excitation within rat barrel cortex on striking a single vibrissa. J Neurophysiol. 1992 Oct;68(4):1345–1358. doi: 10.1152/jn.1992.68.4.1345. [DOI] [PubMed] [Google Scholar]
- Armstrong-James M., Fox K. Spatiotemporal convergence and divergence in the rat S1 "barrel" cortex. J Comp Neurol. 1987 Sep 8;263(2):265–281. doi: 10.1002/cne.902630209. [DOI] [PubMed] [Google Scholar]
- Armstrong-James M., Welker E., Callahan C. A. The contribution of NMDA and non-NMDA receptors to fast and slow transmission of sensory information in the rat SI barrel cortex. J Neurosci. 1993 May;13(5):2149–2160. doi: 10.1523/JNEUROSCI.13-05-02149.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear M. F., Cooper L. N., Ebner F. F. A physiological basis for a theory of synapse modification. Science. 1987 Jul 3;237(4810):42–48. doi: 10.1126/science.3037696. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y., Aniksztejn L., Bregestovski P. Protein kinase C modulation of NMDA currents: an important link for LTP induction. Trends Neurosci. 1992 Sep;15(9):333–339. doi: 10.1016/0166-2236(92)90049-e. [DOI] [PubMed] [Google Scholar]
- Bienenstock E. L., Cooper L. N., Munro P. W. Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J Neurosci. 1982 Jan;2(1):32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C., Van Sluyters R. C. Experimental analysis of amblyopia and strabismus. Br J Ophthalmol. 1974 Mar;58(3):176–182. doi: 10.1136/bjo.58.3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss T. V., Collingridge G. L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993 Jan 7;361(6407):31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Clark S. A., Allard T., Jenkins W. M., Merzenich M. M. Receptive fields in the body-surface map in adult cortex defined by temporally correlated inputs. Nature. 1988 Mar 31;332(6163):444–445. doi: 10.1038/332444a0. [DOI] [PubMed] [Google Scholar]
- Clothiaux E. E., Bear M. F., Cooper L. N. Synaptic plasticity in visual cortex: comparison of theory with experiment. J Neurophysiol. 1991 Nov;66(5):1785–1804. doi: 10.1152/jn.1991.66.5.1785. [DOI] [PubMed] [Google Scholar]
- Diamond M. E., Armstrong-James M., Ebner F. F. Experience-dependent plasticity in adult rat barrel cortex. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):2082–2086. doi: 10.1073/pnas.90.5.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins W. M., Merzenich M. M., Ochs M. T., Allard T., Guíc-Robles E. Functional reorganization of primary somatosensory cortex in adult owl monkeys after behaviorally controlled tactile stimulation. J Neurophysiol. 1990 Jan;63(1):82–104. doi: 10.1152/jn.1990.63.1.82. [DOI] [PubMed] [Google Scholar]
- Jensen K. F., Killackey H. P. Terminal arbors of axons projecting to the somatosensory cortex of the adult rat. I. The normal morphology of specific thalamocortical afferents. J Neurosci. 1987 Nov;7(11):3529–3543. doi: 10.1523/JNEUROSCI.07-11-03529.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killackey H. P. Anatomical evidence for cortical subdivisions based on vertically discrete thalamic projections from the ventral posterior nucleus to cortical barrels in the rat. Brain Res. 1973 Mar 15;51:326–331. doi: 10.1016/0006-8993(73)90383-1. [DOI] [PubMed] [Google Scholar]
- Olson C. R., Freeman R. D. Profile of the sensitive period for monocular deprivation in kittens. Exp Brain Res. 1980;39(1):17–21. doi: 10.1007/BF00237065. [DOI] [PubMed] [Google Scholar]
- Welker C. Microelectrode delineation of fine grain somatotopic organization of (SmI) cerebral neocortex in albino rat. Brain Res. 1971 Mar 5;26(2):259–275. [PubMed] [Google Scholar]
- Woolsey T. A., Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res. 1970 Jan 20;17(2):205–242. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]