Abstract
Background
Endobronchial ultrasound (EBUS) transbronchial needle aspiration (TBNA) is an important diagnostic procedure for the interrogation of mediastinal lymph nodes. There is limited data describing the accuracy & safety of this technique for the diagnosis of tuberculous mediastinal lymphadenitis.
Methods
A multi-centre retrospective study of all EBUS-guided TBNA procedures that referred samples for mycobacteriology was performed. Results were correlated with post-procedural diagnoses after a period of surveillance and cross-checked against relevant statewide tuberculosis (TB) registries, and sensitivity and specificity was calculated. In addition, nucleic acid amplification techniques (NAAT) were assessed, and sensitivity and specificity calculated using positive mycobacterial culture as the reference gold standard.
Results
One hundred and fifty-nine patients underwent EBUS-TBNA and had tissue referred for mycobacterial culture, of which 158 were included in the final analysis. Thirty-nine were ultimately diagnosed with TB (25%). Sensitivity of EBUS-TBNA for microbiologically confirmed tuberculous mediastinal lymphadenitis was 62% (24/39 cases). Specificity was 100%. Negative predictive value (NPV) and diagnostic accuracy for microbiologic diagnosis was 89% [95% confidence intervals (CI), 82-93%] and 91% (95% CI, 84-94%) respectively. For a composite clinicopathologic diagnosis of TB NPV and accuracy were 98% (95% CI, 93-99%) and 98% (95% CI, 95-99%) respectively. Sensitivity for NAAT was 38% (95% CI, 18-65%).
Conclusions
EBUS-TBNA is a safe and well tolerated procedure in the assessment of patients with suspected isolated mediastinal lymphadenitis and demonstrates good sensitivity for a microbiologic diagnosis of isolated mediastinal lymphadenitis. When culture and histological results are combined with high clinical suspicion, EBUS-TBNA demonstrates excellent diagnostic accuracy and NPV for the diagnosis of mediastinal TB lymphadenitis. We suggest EBUS-TBNA should be considered the procedure of choice for patients in whom TB is suspected.
Keywords: Endobronchial ultrasound (EBUS), minimally invasive, bronchoscopy, tuberculosis (TB), mycobacteria, culture, PCR
Introduction
Culture is the gold-standard for diagnosis of mediastinal tuberculous lymphadenitis. All reasonable attempts to achieve microbial diagnosis should be made, as this allows confirmation of the diagnosis and phenotypic drug susceptibility testing which guides antimycobacterial therapy. A majority of patients with mediastinal tuberculous lymphadenitis will have associated lung parenchymal infection (1). Bronchoscopic investigation in such cases is associated with a very high diagnostic sensitivity (2).
In contrast, routine bronchoscopic assessment of patients with isolated tuberculous mediastinal lymphadenitis is a very low yield procedure (3), previously necessitating surgical biopsy (cervical mediastinoscopy) to achieve microbial diagnosis in such cases (4). Mediastinoscopy may be associated with significant potential morbidity/mortality (2%/0.2%) (5,6) and while limited reports indicate high microbiologic diagnostic yield for tuberculosis (TB) following mediastinoscopy (3,7,8), complications specific to TB, such as chronic tuberculous sinus in the endoscopy tract, have been described up to 8% (7).
Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) is a minimally invasive technique allowing sampling of mediastinal lymph nodes via fine needle aspiration under direct sonographic visualisation. It has a low rate of morbidity, and has demonstrated utility in the diagnosis of mediastinal lymphadenopathy secondary to malignancy, lymphoma and sarcoidosis (9-13). Diagnosis of mycobacterial lymph node infection by EBUS-TBNA was first reported in 2009 (14), though to date few studies have reported the accuracy of this technique for the diagnosis of isolated mediastinal tuberculous lymphadenitis. Single centre studies from TB-endemic countries have reported variable results (15-18), and an English multicentre study reported only patients in whom the final diagnosis was confirmed as TB (19). Use of PCR-based diagnostic methods has also been described in one study, though the authors did not describe diagnostic performance of EBUS-TBNA for establishing the diagnosis of TB (20).
Here we present our multi-centre experience of EBUS-TBNA for evaluation of patients with suspected tuberculous lymphadenopathy, and describe the performance of this procedure in this patient population, in particular the sensitivity and diagnostic accuracy of EBUS-TBNA for microbiologic diagnosis of TB.
Materials and methods
A multi-site retrospective study was conducted across four Australian tertiary referral centres in which EBUS-TBNA was performed on a regular basis. Institutional Ethics Committee approval was granted for the performance of this study.
Patient selection and case identification
This study included all patients who had undergone EBUS-guided lymph node aspiration for suspected isolated mediastinal tuberculous mediastinal lymphadenopathy, due to mediastinal lymphadenopathy on CT chest in the absence of parenchymal abnormality (Figure 1). Patients were identified through interrogation of the hospital laboratory databases and bronchoscopists’ individual logbooks. Results from the procedures were recorded, including microscopy for acid-fast bacilli, cytology, mycobacterial cultures, standard bacterial cultures, and, where performed, the results of nucleic acid amplification techniques (NAAT).
Figure 1.
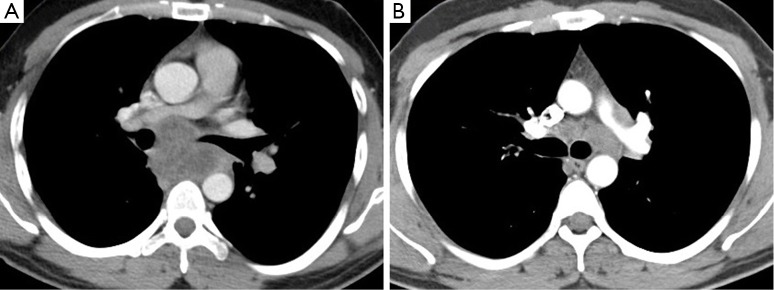
CT chest imaging of patients demonstrating (A) a 28-year-old male with necrotic subcarinal lymph node mass; (B) a 34-year-old male with moderate left lower paratracheal lymphadenopathy. In both cases, EBUS-TBNA demonstrated culture-positive tuberculous lymphadenitis. EBUS-TBNA, endobronchial ultrasound transbronchial needle aspiration.
Performance of EBUS-TBNA
All bronchoscopists were experienced with the procedure. EBUS-TBNA was performed using a dedicated linear array bronchoscope (BF-UC180F-OL8; Olympus, Tokyo, Japan). In all cases, 22-gauge fine needle aspirates were performed from enlarged lymph nodes identified on CT chest. Cytology specimens were prepared as slides without alcohol fixation for microscopy and staining for acid-fast bacilli, as well as cell blocks following centrifugation. As previously described, serial sections were cut and placed on slides before staining with haematoxylin and eosin (21). Further samples were placed in saline and transported directly to laboratories for mycobacterial culture.
Microbiologic testing
Sputum were analysed for the presence of mycobacteria using auramine-rhodamine florescence microscopy, with positives confirmed by Ziehl-Neelsen staining. Culture confirmation of TB was by BacT/ALERT MB mycobacterial detection system (bioMerieux, Australia), or with Lowenstein-Jensen culture media (Thermo Fisher Scientific Inc, USA). NAAT were performed using ProbeTec™ DTB assay (BD, USA), TaqMan real time PCR (Thermo Fisher Scientific Inc, USA) or the Roche Amplicor Mycobacterium Tuberculosis (Roche Diagnostic Systems, Switzerland) test.
Determination of final diagnoses
Post-procedural diagnoses were obtained from the clinical records. Where these were unavailable, or where there was uncertainty regarding the post-procedural diagnosis, the referring physician was contacted to provide contemporaneous clinical information. TB diagnoses must be notified to Health authorities by law. State-wide TB registries were examined to identify patients whose EBUS-TBNA samples were mycobacterial culture negative but who were subsequently diagnosed with TB.
Patients were considered to have had a diagnosis of mediastinal tuberculous lymphadenitis if they met one or more of the following criteria:
A positive culture result for Mycobacterium TB from EBUS-TBNA;
A positive NAAT result with high clinical index of suspicion;
The diagnosis was achieved through culture or biopsy of sites other than mediastinal lymph nodes;
Culture-negative, but high clinical index of suspicion and demonstration of supportive histological changes (granulomatous inflammation) (Figure 2), warranting anti-tuberculous treatment (i.e., highly probable TB, as previously defined (22,23).
Figure 2.
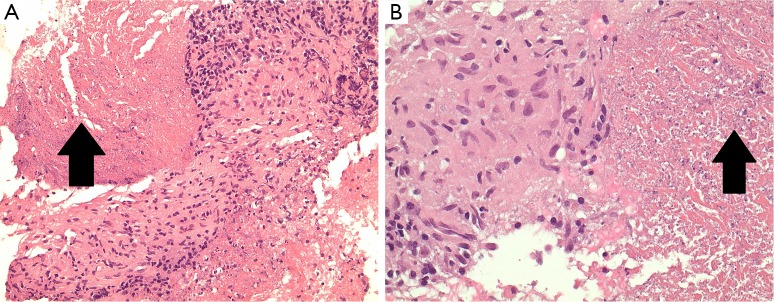
Haematoxylin & eosin images of EBUS-TBNA cell block specimens demonstrating epitheloid histiocytes forming granulomas with necrosis evident (arrows), consistent with necrotizing granulomatous inflammation (A, ×100; B, ×400). EBUS-TBNA, endobronchial ultrasound transbronchial needle aspiration.
Patients were considered not to have had a diagnosis of mediastinal TB lymphadenitis where:
A specific alternate diagnosis was obtained at EBUS-TBNA, and mycobacterial cultures and NAAT were negative;
The post procedural diagnosis after a period of surveillance was not TB. Surveillance was defined as the time between the diagnostic procedure and cross-check against the relevant state TB registry.
Sensitivity, specificity and negative predictive values (NPVs) were calculated, with 95% confidence intervals (CI) calculated using the modified Wald method.
Finally, NAATs were analysed and their results referenced against the gold standard of TB culture.
Results
From January 2010 to November 2013, 159 EBUS-TBNA procedures where specimens were sent for microbiologic analysis were performed. No procedural complications occurred. One case of suspected persistent mediastinal TB lymphadenitis was excluded from the analysis since the patient had been on anti-tuberculous treatment for the preceding 6 months, therefore 158 patients were included in the final analysis. No patients underwent cervical mediastinoscopy or endoscopic ultrasound during the study period. No patients were known to be infected with the human immunodeficiency virus. Post-procedural and final diagnoses are recorded in Table 1. The median time between procedure and cross-check against the relevant TB registry was 532 days (range, 47-1,125 days).
Table 1. Diagnoses following EBUS-TBNA and final diagnoses.
| Final diagnosis (N=158) | EBUS-TBNA diagnosis | Number |
|---|---|---|
| Microbiologically confirmed tuberculosis (N=25) | Culture positive | 23 |
| Culture-negative, NAAT positive | 1 | |
| Necrotising granulomatous inflammationa | 1 | |
| Culture negative tuberculosisb (N=14) | Necrotising granulomatous inflammation | 3 |
| Non-necrotising granulomatous inflammation + clinical history | 9 | |
| Inadequate specimen | 2 | |
| Alternative diagnoses (N=119) | ||
| Sarcoidosis | Non-necrotising granulomatous | 78 |
| Reactive lymphadenopathy | Inflammation | 20 |
| NSCLC | Benign lymphoid material | 12 |
| NSCLC inadequate specimen | 1 | |
| Small cell lung cancer | Small cell lung cancer | 3 |
| Lymphoma | Lymphoma | 2 |
| Metastatic colon cancer | Metastatic colon cancer | 1 |
| Oesophageal cyst | Oesophageal cyst | 1 |
| Mycobacterium avium infection | Mycobacterium avium culture positive | 1 |
Necrotising granulomatous inflammation demonstrated on biopsy of alternative sites; a, endobronchial washings culture-positive for M. tuberculosis; b, see Table 2. EBUS-TBNA, endobronchial ultrasound transbronchial needle aspiration; NAAT, nucleic acid amplification techniques; NSCLC, non-small cell lung cancer.
Thirty-nine patients received a final diagnosis of TB. EBUS-TBNA specimens yielded a microbiologic diagnosis in 24 (62%), with 23 procedures resulting in culture positive MTB and one culture-negative specimen demonstrating NAAT positive result, together with granulomatous inflammation in cytologic specimens. Overall, NAAT testing was performed in 13 patients with microbiologically confirmed TB, with five positive results (38%). NAAT was performed in 16 patients where the final diagnosis was not TB, and in all cases were negative (100%). There were 15 patients with lymph node aspirates that were negative on mycobacterial culture that were treated for mediastinal TB. The diagnosis of mediastinal TB lymphadenitis was supported by cytology in 12 cases. These had granulomatous inflammation from EBUS-TBNA specimens (Figure 2) and a high pre-test probability for MTB infection based on their country of origin and presenting clinical and radiological features. Test and patient characteristics of the culture negative TB cases are recorded in Table 2. There were 119 cases of non-tuberculous mediastinal lymphadenopathy, none of which were notified to the TB registry during the follow-up period. In one of these cases no lymphoid material was aspirated, and a diagnosis of non-small cell lung cancer was subsequently made from a biopsy of an alternative site. Three patients had a diagnosis of TB following non-diagnostic EBUS-TBNA (8% false negative rate)—one patient (2.5% of all patients with final diagnosis of TB) returned a positive culture result from endobronchial washings. In a further two cases the diagnosis was subsequently achieved through demonstration of necrotising granulomatous inflammation following biopsies of alternative sites.
Table 2. Clinical characteristics of patients with culture-negative TB diagnosed by EBUS-TBNA.
| EBUS-TBNA cytology | Interferon gamma release assay | Country of origin | Alternative biopsy | Clinical response to treatment |
|---|---|---|---|---|
| Non-necrotising granulomatous inflammation | Positive | Vietnam | Not performed | Persistent pyrexia responsive to TB treatment |
| Positive | India | Not performed | Mediastinal lymphadenopathy-clinical stability after TB treatment | |
| Negative | India | Not performed | Improvement in mediastinal lymphadenopathy after 3 months of TB treatment | |
| Unknown | India | Not performed | Positive clinical response to treatment | |
| Unknown | India | Not performed | Positive clinical response to treatment | |
| Unknown | Afghanistan | Not performed | Positive clinical response to treatment | |
| Unknown | India | Not performed | Asymptomatic prior to commencing treatment | |
| Unknown | PNG | Not performed | Positive clinical response to treatment | |
| Degenerate inflammatory cells and fibrinoid material | Positive | Afghanistan | Oesophageal stricture subsequently biopsied demonstrating necrotising granulomatous inflammation | Resolution of granulomatous inflammation on repeat gastroscopy; repeat CT demonstrating calcified mediastinal nodes |
| Multinucleate giant cells suspicious for granulomatous inflammation | Positive | India | Not performed | Resolution of mediastinal lymphadenopathy after TB treatment |
| Insufficient specimen for cytology | Negative | India | Right upper lobectomy performed demonstrating necrotising granulomatous inflammation | CXR stable after course of TB treatment |
| Necrotising granulomatous inflammation | Unknown | India | Not performed | Positive clinical response to treatment |
| Unknown | India | Not performed | Positive clinical response to treatment | |
| Unknown | India | Culture positive on bronchial washings | Positive clinical response to treatment | |
| Unknown | Somalia | Not performed | Positive clinical response to treatment |
TB, tuberculosis; EBUS-TBNA, endobronchial ultrasound transbronchial needle aspiration.
The calculated sensitivity for a microbiologic diagnosis of mediastinal TB lymphadenitis from EBUS-TBNA was 62% (24/39 TB cases; 95% CI, 46-75%). If cases where histologic results from EBUS-TBNA allowed a composite clinicopathological diagnosis of highly probable TB were considered to be true positives, the sensitivity for EBUS-guided TBNA increased to 92% (36/39 TB cases; 95% CI, 79-98%). Sensitivity of NAAT was 38% (5/13 cases; 95% CI, 18-65%) in culture confirmed cases in which it was performed, and specificity was 100% (16/16 cases; 95% CI, 83-100%). The NPV in our subjects for culture and MTB-NAAT was 89% (119/134 cases; 95% CI, 82-93%), and for composite clinicopathologic diagnosis of TB the NPV was 98% (119/122; 95% CI, 93-99%). Specificity was 100%. Overall diagnostic accuracy for a microbiologic diagnosis of isolated mediastinal lymphadenitis was 91% (95% CI, 84-94%), and accuracy for a composite clinical and pathological diagnosis was 98% (95% CI, 95-99%).
Discussion
EBUS-TBNA is a minimally invasive technique that has become the procedure of choice for evaluation of mediastinal/hilar lymphadenopathy due to its excellent diagnostic accuracy in staging of lung cancer and evaluation of numerous other processes (11-13). This procedure has an excellent safety profile and cost advantages in comparison to surgical mediastinoscopy (10). Multiple studies have demonstrated diagnostic sensitivity is equivalent to surgical mediastinoscopy (13,24). Complications are rare, with a systematic review reporting no important complications among over 1,600 patients undergoing EBUS-TBNA for evaluation of mediastinal lymphadenopathy (12). Our findings demonstrate excellent efficacy & safety of EBUS-TBNA for diagnosis of suspected isolated mediastinal tuberculous lymphadenitis.
We confirm a high diagnostic sensitivity for microbiologic diagnosis (62%). Just as importantly, our study has confirmed a NPV of 89% for microbiologic diagnosis by EBUS-TBNA, and 98% for a composite clinicopathologic diagnosis in assessment of patients with suspected isolated mediastinal tuberculous lymphadenitis. Our findings also confirmed the very low rate of microbiological diagnosis from routine bronchoscopy in patients with isolated mediastinal tuberculous lymphadenitis (2.5%), suggesting routine bronchoscopy has no role in the investigation of these patients.
Multiple previous reports have confirmed the ability of EBUS-TBNA to diagnose mediastinal mycobacterial infection (9,14,16,25,26) though few have examined diagnostic accuracy, particularly the ability to obtain microbiologic confirmation of TB infection. Only one other multicentre study has been published, with Navani et al. reporting a lower sensitivity of EBUS TBNA for the diagnosis of culture or NAAT confirmed TB lymphadenitis of 47%. Their sensitivity for a composite endpoint of positive culture or positive clinicopathological diagnosis was similar to our results (94%) (27). This study only included patients ultimately diagnosed with TB, rather than outcomes for patients in whom EBUS-TBNA may be used to confirm a clinical suspicion, therefore could not report NPV or diagnostic accuracy.
Other studies from TB-endemic countries describing use of EBUS-TBNA for diagnosis of mediastinal tuberculous lymphadenitis report sensitivity for culture-positive diagnosis from 38% to 46%, and sensitivity for a composite microbiological and clinicopathological diagnosis of 69% to 86% (31/36 TB cases) (15,17). These findings are consistent with studies assessing FNA techniques for TB lymphadenitis, both in mediastinal and extra-thoracic locations (28-31).
Minimally invasive bronchoscopic diagnosis of mediastinal tuberculous lymphadenitis may also be achieved with conventional (“Wang” needle) TBNA, though limited evidence suggest diagnostic sensitivity is significantly lower than for EBUS-TBNA, with reported composite diagnostic sensitivity in HIV-negative patients varying from 65% to 83% (17,32,33) though importantly culture-positivity was markedly lower at 9.5% to 27% (25,33). In addition, conventional TBNA remains underutilised because of inadequate training in the technique (34,35), and concerns regarding nodal movement with respiration (36), and risk of inadvertent vascular puncture (34). Competency may also take considerable time to achieve (34,37). We suggest therefore that both training and procedural outcome factors make EBUS-TBNA the preferable tool for bronchoscopic diagnosis of isolated mediastinal lymphadenitis.
Our study confirms that fine needle aspiration is a useful technique for the investigation of suspected TB lymphadenitis, and further demonstrates that this can be reliably accomplished via a bronchoscopic approach for the diagnosis of mediastinal TB lymphadenitis. For smear negative and extrapulmonary TB NAATs have high specificity but highly variable sensitivity ranging from 42% to 90% (38-42). We found a poor sensitivity (38%) for MTB NAAT in culture-confirmed TB lymphadenitis from EBUS-TBNA sampling, suggesting that culture is still a critical step in diagnosis. However, the rapid confirmation of the diagnosis using NAAT and the possibility for obtaining rapid genotypic susceptibility testing means that NAAT has an increasing role in both the diagnosis and initial management of TB patients.
This study is notable for a number of reasons. Firstly it was performed across four different tertiary teaching hospitals, with different patient demographics, demonstrating the technique is feasible amongst different patient populations. Secondly, we analysed all samples that were sent for mycobacteriology. Given the populations studied, results are likely to be representative of Australian and other Western health care facilities, (predominantly low TB prevalence communities with an incidence of less than 10 per 100,000, and with over 90% of patients being migrants from TB-endemic areas), our results are likely to be generalisable to most developed world patient populations.
This retrospective study has some limitations due to the inherent difficulty of assessing diagnostic tests in which the gold standard (a positive mycobacterial culture result) is an imperfect test. Culture-negative TB is a well recognised clinical entity, particularly in extra-pulmonary disease, and likely reflects differences in sample handling, sampling error, culture techniques, and mycobacterial load. It is possible that some of the patients in whom the post-procedural diagnosis was not TB may ultimately have gone on to develop this condition. However, given the risk of reactivation is greatest in the period shortly after initial infection (43) and given the patients were checked against a relevant TB registry at a median of 532 days after the procedure, we believe this is extremely unlikely. Conversely false positives may have occurred with false positive NAAT tests or as a result of incorrect clinicopathological diagnoses, resulting in unnecessary anti-tuberculous treatment and an underestimation of the sensitivity of this technique in providing a culture-proven diagnosis. It is also possible that a negative culture result from aspirated lymph nodes in patients with TB may not have represented a deficiency in the diagnostic process. Rather, such nodes may have been reactively enlarged secondary to parenchymal lung disease and this too may have reduced the observed sensitivity of the diagnostic technique.
Finally, caution should be exercised when interpreting the results of the NAAT. The test characteristics for TB PCR assays varies considerably between studies and is likely to be highly dependent on the particular assay used (40,44). Recent studies suggest that GeneXpert PCR may have superior sensitivity and specificity for the diagnosis of TB, although its use has not been validated for extra-pulmonary samples (45-48). This assay was not used during the period of our study.
These issues notwithstanding, the results in this study are consistent with the limited reports published previously, and therefore are likely to be reliable estimates of sensitivity and specificity for EBUS TBNA in the diagnosis of mediastinal TB lymphadenitis. Given the poor diagnostic yield from routine bronchoscopy in patients with isolated mediastinal lymphadenitis, EBUS-TBNA should be considered the procedure of choice for patients in whom TB is suspected.
Conclusions
EBUS-TBNA is a safe and well tolerated procedure in the assessment of patients with suspected isolated mediastinal lymphadenitis. The procedure shows a higher sensitivity (62%) for a culture-positive diagnosis of TB than historical reports of conventional TBNA. When culture and histological results are combined with high clinical suspicion, EBUS-TBNA demonstrates excellent diagnostic accuracy (98%) and NPV (98%) for the diagnosis of mediastinal TB lymphadenitis. We suggest EBUS-TBNA should be considered the procedure of choice for patients in whom TB is suspected.
Acknowledgements
Authors’ contributions: J Geake, GA Jenkin and DP Steinfort conceived & designed the study; J Geake, G Hammerschlag, P Nguyen, P Wallbridge, TM Korman, B Jennings, DF Johnson, LB Irving and M Farmer carried out procedure and collected data, J Geake, DF Johnson and DP Steinfort analysed the data, J Geake, GA Jenkin and DP Steinfort wrote the manuscript, DF Johnson & GA Jenkin provided critical expert review, and all authors reviewed and approved the final manuscript.
Disclosure: The authors declare no conflict of interest.
References
- 1.Raviglione MC. Harrison’s Principles of Internal Medicine. In: Fauci A, Braunwald E, Kasper D, et al. eds. Tuberculosis. 17 ed. New York: McGraw-Hill Companies Inc., 2008:1006-20. [Google Scholar]
- 2.Shin JA, Chang YS, Kim TH, et al. Fiberoptic bronchoscopy for the rapid diagnosis of smear-negative pulmonary tuberculosis. BMC Infect Dis 2012;12:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayed AK, Behbehani NA. Diagnosis and treatment of isolated tuberculous mediastinal lymphadenopathy in adults. Eur J Surg 2001;167:334-8. [DOI] [PubMed] [Google Scholar]
- 4.Porte H, Roumilhac D, Eraldi L, et al. The role of mediastinoscopy in the diagnosis of mediastinal lymphadenopathy. Eur J Cardiothorac Surg 1998;13:196-9. [DOI] [PubMed] [Google Scholar]
- 5.Hammoud ZT, Anderson RC, Meyers BF, et al. The current role of mediastinoscopy in the evaluation of thoracic disease. J Thorac Cardiovasc Surg 1999;118:894-9. [DOI] [PubMed] [Google Scholar]
- 6.Reich JM, Brouns MC, O'Connor EA, et al. Mediastinoscopy in patients with presumptive stage I sarcoidosis: a risk/benefit, cost/benefit analysis. Chest 1998;113:147-53. [DOI] [PubMed] [Google Scholar]
- 7.Farrow PR, Jones DA, Stanley PJ, et al. Thoracic lymphadenopathy in Asians resident in the United Kingdom: role of mediastinoscopy in initial diagnosis. Thorax 1985;40:121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salomaa ER, Liippo K, Puhakka HJ, et al. Indispensability of mediastinoscopy in intrathoracic tuberculosis. ORL J Otorhinolaryngol Relat Spec 1992;54:275-7. [DOI] [PubMed] [Google Scholar]
- 9.Steinfort DP, Hew MJ, Irving LB. Bronchoscopic evaluation of the mediastinum using endobronchial ultrasound: a description of the first 216 cases carried out at an Australian tertiary hospital. Intern Med J 2011;41:815-24. [DOI] [PubMed] [Google Scholar]
- 10.Steinfort DP, Liew D, Conron M, et al. Cost-benefit of minimally invasive staging of non-small cell lung cancer: a decision tree sensitivity analysis. J Thorac Oncol 2010;5:1564-70. [DOI] [PubMed] [Google Scholar]
- 11.Tournoy KG, Bolly A, Aerts JG, et al. The value of endoscopic ultrasound after bronchoscopy to diagnose thoracic sarcoidosis. Eur Respir J 2010;35:1329-35. [DOI] [PubMed] [Google Scholar]
- 12.Varela-Lema L, Fernández-Villar A, Ruano-Ravina A.Effectiveness and safety of endobronchial ultrasound-transbronchial needle aspiration: a systematic review. Eur Respir J 2009;33:1156-64. [DOI] [PubMed] [Google Scholar]
- 13.Yasufuku K, Pierre A, Darling G, et al. A prospective controlled trial of endobronchial ultrasound-guided transbronchial needle aspiration compared with mediastinoscopy for mediastinal lymph node staging of lung cancer. J Thorac Cardiovasc Surg 2011;142:1393-400.e1. [DOI] [PubMed]
- 14.Steinfort DP, Johnson DF, Connell TG, et al. Endobronchial ultrasound-guided biopsy in the evaluation of intrathoracic lymphadenopathy in suspected tuberculosis: a minimally invasive technique with a high diagnostic yield. J Infect 2009;58:309-11. [DOI] [PubMed] [Google Scholar]
- 15.Sun J, Teng J, Yang H, et al. Endobronchial ultrasound-guided transbronchial needle aspiration in diagnosing intrathoracic tuberculosis. Ann Thorac Surg 2013;96:2021-7. [DOI] [PubMed] [Google Scholar]
- 16.Madan K, Mohan A, Ayub II, et al. Initial Experience with endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) from a tuberculosis endemic population. J Bronchology Interv Pulmonol 2014;21:208-14. [DOI] [PubMed] [Google Scholar]
- 17.Low SY, Koh MS, Ong TH, et al. Use of endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) in the diagnosis of granulomatous mediastinal lymphadenopathy. Ann Acad Med Singapore 2014;43:250-4. [PubMed] [Google Scholar]
- 18.Senturk A, Arguder E, Hezer H, et al. Rapid diagnosis of mediastinal tuberculosis with polymerase chain reaction evaluation of aspirated material taken by endobronchial ultrasound-guided transbronchial needle aspiration. J Investig Med 2014;62:885-9. [DOI] [PubMed] [Google Scholar]
- 19.Tournoy K.Tuberculosis through the rose tinted spectacles of the EBUS endoscopist: be aware of the bias. Thorax 2012;67:650; author reply 652. [DOI] [PubMed] [Google Scholar]
- 20.Dhasmana DJ, Ross C, Bradley CJ, et al. Performance of Xpert MTB/RIF in the diagnosis of tuberculous mediastinal lymphadenopathy by endobronchial ultrasound. Ann Am Thorac Soc 2014;11:392-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steinfort DP, Russell PA, Tsui A, et al. Interobserver agreement in determining non-small cell lung cancer subtype in specimens acquired by EBUS-TBNA. Eur Respir J 2012;40:699-705. [DOI] [PubMed] [Google Scholar]
- 22.Dosanjh DP, Hinks TS, Innes JA, et al. Improved diagnostic evaluation of suspected tuberculosis. Ann Intern Med 2008;148:325-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson ST, Kaforou M, Brent AJ, et al. Diagnosis of childhood tuberculosis and host RNA expression in Africa. N Engl J Med 2014;370:1712-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ernst A, Anantham D, Eberhardt R, et al. Diagnosis of mediastinal adenopathy-real-time endobronchial ultrasound guided needle aspiration versus mediastinoscopy. J Thorac Oncol 2008;3:577-82. [DOI] [PubMed] [Google Scholar]
- 25.Kuo CH, Lin SM, Lee KY, et al. Algorithmic approach by endobronchial ultrasound-guided transbronchial needle aspiration for isolated intrathoracic lymphadenopathy: a study in a tuberculosis-endemic country. J Formos Med Assoc 2014;113:527-34. [DOI] [PubMed] [Google Scholar]
- 26.Yasufuku K, Nakajima T, Fujiwara T, et al. Utility of endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis of mediastinal masses of unknown etiology. Ann Thorac Surg 2011;91:831-6. [DOI] [PubMed] [Google Scholar]
- 27.Navani N, Molyneaux PL, Breen RA, et al. Utility of endobronchial ultrasound-guided transbronchial needle aspiration in patients with tuberculous intrathoracic lymphadenopathy: a multicentre study. Thorax 2011;66:889-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fritscher-Ravens A, Ghanbari A, Topalidis T, et al. Granulomatous mediastinal adenopathy: can endoscopic ultrasound-guided fine-needle aspiration differentiate between tuberculosis and sarcoidosis? Endoscopy 2011;43:955-61. [DOI] [PubMed] [Google Scholar]
- 29.Handa U, Mundi I, Mohan S.Nodal tuberculosis revisited: a review. J Infect Dev Ctries 2012;6:6-12. [DOI] [PubMed] [Google Scholar]
- 30.Knox J, Lane G, Wong JS, et al. Diagnosis of tuberculous lymphadenitis using fine needle aspiration biopsy. Intern Med J 2012;42:1029-36. [DOI] [PubMed] [Google Scholar]
- 31.Puri R, Mangla R, Eloubeidi M, et al. Diagnostic yield of EUS-guided FNA and cytology in suspected tubercular intra-abdominal lymphadenopathy. Gastrointest Endosc 2012;75:1005-10. [DOI] [PubMed] [Google Scholar]
- 32.Cetinkaya E, Yildiz P, Altin S, et al. Diagnostic value of transbronchial needle aspiration by Wang 22-gauge cytology needle in intrathoracic lymphadenopathy. Chest 2004;125:527-31. [DOI] [PubMed] [Google Scholar]
- 33.Bilaçeroğlu S, Günel O, Eriş N, et al. Transbronchial needle aspiration in diagnosing intrathoracic tuberculous lymphadenitis. Chest 2004;126:259-67. [DOI] [PubMed] [Google Scholar]
- 34.Haponik EF, Shure D. Underutilization of transbronchial needle aspiration: experiences of current pulmonary fellows. Chest 1997;112:251-3. [DOI] [PubMed] [Google Scholar]
- 35.Dabscheck EJ, Hew M, Irving L, et al. Bronchoscopic training and practice in australia and New Zealand is inconsistent with published society guidelines. J Bronchology Interv Pulmonol 2014;21:117-22. [DOI] [PubMed] [Google Scholar]
- 36.Piet AH, Lagerwaard FJ, Kunst PW, et al. Can mediastinal nodal mobility explain the low yield rates for transbronchial needle aspiration without real-time imaging? Chest 2007;131:1783-7. [DOI] [PubMed] [Google Scholar]
- 37.Hsu LH, Liu CC, Ko JS. Education and experience improve the performance of transbronchial needle aspiration: a learning curve at a cancer center. Chest 2004;125:532-40. [DOI] [PubMed] [Google Scholar]
- 38.Chou CH, Hsu HL, Lee LN, et al. Comparison of 2 interferon-gamma assays and Roche Cobas Amplicor Mycobacterium tuberculosis assay for rapid diagnosis of tuberculosis among patients with suspected tuberculosis in Taiwan. J Microbiol Immunol Infect 2009;42:251-7. [PubMed] [Google Scholar]
- 39.Mandell GL, Bennett JE, Dolin R. eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. Philadelphia: Elsevier, 2010. [Google Scholar]
- 40.Mehta PK, Raj A, Singh N, et al. Diagnosis of extrapulmonary tuberculosis by PCR. FEMS Immunol Med Microbiol 2012;66:20-36. [DOI] [PubMed] [Google Scholar]
- 41.Derese Y, Hailu E, Assefa T, et al. Comparison of PCR with standard culture of fine needle aspiration samples in the diagnosis of tuberculosis lymphadenitis. J Infect Dev Ctries 2012;6:53-7. [DOI] [PubMed] [Google Scholar]
- 42.Piersimoni C, Bornigia S, Gherardi G.Performance of a commercial nucleic acid amplification test with extrapulmonary specimens for the diagnosis of tuberculosis. Eur J Clin Microbiol Infect Dis 2012;31:287-93. [DOI] [PubMed] [Google Scholar]
- 43.Horsburgh CR, Jr. Priorities for the treatment of latent tuberculosis infection in the United States. N Engl J Med 2004;350:2060-7. [DOI] [PubMed] [Google Scholar]
- 44.Linasmita P, Srisangkaew S, Wongsuk T, et al. Evaluation of real-time polymerase chain reaction for detection of the 16S ribosomal RNA gene of Mycobacterium tuberculosis and the diagnosis of cervical tuberculous lymphadenitis in a country with a high tuberculosis incidence. Clin Infect Dis 2012;55:313-21. [DOI] [PubMed] [Google Scholar]
- 45.Hillemann D, Rüsch-Gerdes S, Boehme C, et al. Rapid molecular detection of extrapulmonary tuberculosis by the automated GeneXpert MTB/RIF system. J Clin Microbiol 2011;49:1202-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marlowe EM, Novak-Weekley SM, Cumpio J, et al. Evaluation of the Cepheid Xpert MTB/RIF assay for direct detection of Mycobacterium tuberculosis complex in respiratory specimens. J Clin Microbiol 2011;49:1621-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moure R, Muñoz L, Torres M, et al. Rapid detection of Mycobacterium tuberculosis complex and rifampin resistance in smear-negative clinical samples by use of an integrated real-time PCR method. J Clin Microbiol 2011;49:1137-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vuorinen P, Miettinen A, Vuento R, et al. Direct detection of Mycobacterium tuberculosis complex in respiratory specimens by Gen-Probe Amplified Mycobacterium Tuberculosis Direct Test and Roche Amplicor Mycobacterium Tuberculosis Test. J Clin Microbiol 1995;33:1856-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


