Abstract
Objective
To investigate the effects of probucol combined with atorvastatin on the serum oxidation index and lipid levels in patients diagnosed with acute coronary syndrome (ACS).
Methods
We randomly assigned 126 ACS patients (77 males and 49 females) to the control group (atorvastatin 20 mg/day, n=62) or the treatment group (atorvastatin 20 mg/day and probucol 750 mg/day, n=64). All the patients were followed up for 12 weeks. As oxidization indices, we measured the serum levels of total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglyceride (TG), oxidized LDL (ox-LDL), and paraoxonase-1 (PON1) before and after treatment. We also monitored the adverse effects of the drugs during the treatment.
Results
At baseline, there were no obvious differences (P>0.05) between the two groups (including age, gender, etc.). After 12 weeks of treatment, the ox-LDL levels in the treatment group were significantly lower while PON1 levels were significantly higher than those in the control group. There were no statistically significant difference between the two groups with respect to the side effects (P<0.05).
Conclusions
The combined use of atorvastatin and probucol in ACS patients could reduce ox-LDL expression and increase PON1 expression more effectively than use atorvastatin alone.
Keywords: Probucol, atorvastatin, acute coronary syndrome (ACS), paraoxonase-1 (PON1), oxidized low-density lipoprotein (ox-LDL)
Introduction
Annually, over one million people in the United States experience a sudden cardiac event as a result of acute coronary syndrome (ACS), with approximately 20 million people affected worldwide (1). Previous studies have found that dyslipidemia, enhanced oxidative stress, and inflammation are involved in the development of atherosclerosis and its related complications, such as the cardiovascular disease (CVD), although the underlying mechanisms remain unknown (2,3). Serum lipids are the most important factors contributing to the occurrence and development of ACS. Moreover, oxidative stress plays an important role in the various stages of thrombosis development (4).
Overwhelming evidence from epidemiological and clinical studies has demonstrated that low-density lipoprotein (LDL) is a key element in the development of atherosclerosis (5). Oxidized LDL (ox-LDL) can induce atherosclerosis by stimulating monocyte infiltration and smooth muscle cell migration and proliferation. Further, it contributes to atherothrombosis by inducing endothelial cell apoptosis and subsequent plaque erosion, impairing the anticoagulant balance in the endothelium, stimulating the tissue factor production of smooth muscle cells, and triggering macrophage apoptosis (6). High-density lipoprotein (HDL) plays an anti-atherogenic role in prevention of atherosclerosis partly due to its anti-oxidative and anti-inflammatory properties by preventing LDL oxidation (3,7). HDL can also transport free cholesterol (FC) from peripheral tissues (including arterial walls) to the blood, with the help of the cell membrane protein ATP-binding Cassette transporter A1 (ABCA1) and the macrophage Scavenger Receptor BI (SR-BI); this mechanism can reverse atherogenesis and is called reverse cholesterol transport (RCT) (8).
The HDL-associated enzyme paraoxonase-1 (PON1) is largely responsible for the antioxidant action of HDL due to its ability to hydrolyze oxidized phospholipids (9). Experiments have shown that knocking out the PON1 gene has pro-inflammatory and pro-atherogenic effects by increasing the levels of ox-LDL, while the trans-gene or over-expression of PON1 is anti-atherogenic by suppressing LDL oxidation and inflammation (10). Lower plasma PON1 activity is associated with increased CVD risk, which may be affected by both genetic polymorphisms and environmental factors, such as pharmaceutical interventions (3).
Probucol is a bisphenolic compound with unique anti-atherogenic properties, including LDL-C-lowering, antioxidant, and anti-inflammatory functions. Although probucol may also significantly decrease the levels of HDL-C, it has been shown to have a strong anti-atherosclerosis effect (11). The mechanism underlying this effect is not entirely clear; however, it is hypothesized to be related to the decreased levels of ox-LDL and the increased levels of PON1 (12). Statins are widely used for lowering plasma LDL and play a pivotal role in the primary prevention of CVD mortality and major cardiac events. It has been suggested that statins may have additional anti-atherogenic effects, such as stabilization of atherosclerotic plaques and inhibition of vascular inflammation and lipid oxidation (13). One of these so-called pleiotropic effects of statins could be a reduction in oxidative stress even before the lipid-lowering effect becomes apparent. These antioxidant functions are considered to be at least partly associated with the beneficial effects that occur very early in the course of statin therapy (14).
Therapy with probucol as well as atorvastatin leads to lipid regulation with antioxidant effects (12). However, little is known regarding the combined use of these two drugs. Whether such combined therapy may act synergistically in antioxidant therapy in patients with ACS has been briefly studied (15). This study aimed to examine the therapeutic effects of the combined use of probucol and atorvastatin on atherosclerosis in ACS patients and discuss the possible therapeutic mechanisms of this combination.
Methods
Subjects
This clinical study was performed in the Cardiology Department of the Chinese PLA General Hospital in Beijing, China. Overall, 126 consecutive patients (77 men and 49 women; mean age, 61.3±8.9 years) who presented with symptoms of acute coronary disease were recruited for this study from December 2010 to July 2011. The patients were admitted based on the history, physical examination, electrocardiogram (ECG) and dynamic ECG, levels of myocardial necrosis markers (CK/CK-MB/cTNT), and coronary angiography for the diagnosis of acute ST-segment elevation myocardial infarction (STEMI), non-ST-segment elevation myocardial infarction (NSTEMI), and unstable angina pectoris (UAP). Informed consent was obtained from all the patients after explaining the nature and the purpose of the study. The study was approved by the Ethical Committee of Chinese PLA General Hospital.
STEMI was diagnosed in patients with chest symptoms suspected of being caused by myocardial infarction (MI) and persisting for at least 20 min within the last 24 h before admission along with ECG findings of ST-segment elevation of ≥1 mm in two or more limb leads, two or more contiguous precordial leads, or left bundle branch block (LBBB). NSTEMI was diagnosed in patients with chest symptoms suspected of being caused by MI and persisting for at least 20 min within the last 24 h before admission; no ST-segment elevation ≥1 mm or LBBB; and elevated levels of the biochemical markers of myocardial necrosis, including cardiac troponin T ≥1.0 nm/dL or creatine phosphokinase MB (CK-MB) two times above the normal range. UA was diagnosed in patients with resting or nocturnal chest pain persisting ≥20 min along with any of the following findings: T-segment depression of ≥0.5 mm, T-wave inversion of ≥3 mm, and serum troponin T ≥1.0 nm/dL. Confirmation of significant stenosis was based on diagnostic imaging, a recent reduction in LV contractions detected by ultrasound echocardiography, or reversible drug or exercise-induced myocardial hypoperfusion on thallium perfusion scintigraphy (5).
Patients with hepatic, endocrine, or renal disorders (serum creatinine level >130 mmol/L); type 2 diabetes mellitus; alcoholism; drug dependence; gallstones; malignancy; pregnancy; or lactation or patients receiving anticoagulant or lipid-lowering therapy were excluded from the study.
Serum specimen preparation
Elbow venous blood was drawn after overnight fasting. Laboratory biochemical tests were performed using these samples. Further, serum samples were separated by low-speed centrifugation (3,000 rpm, 10 min at 4 °C) and aliquots were frozen at −80 °C under nitrogen until measurement. The serum levels of the HDL subfractions HDL2 and HDL3 along with ox-LDL and PON1 were detected using enzyme-linked immunosorbent assay kits.
Patient groups
The 126 ACS patients were divided into odd (group A) and even (group B) groups based on the order of enrollment. Group A (n=62; 37 men and 25 women), received only 20 mg of atorvastatin qd (Lipitor, 20 mg/tablet, Pfizer, Inc, Dalian, China) and were considered the control group. Group B was designated as the treatment group, and patients were given 750 mg of probucol bid (Zhile, 0.125 g/tablet, Qilu Pharmaceutical Co., Ltd, Jinan, China) as well as 20 mg of atorvastatin qd (Lipitor, 20 mg/tablet). In addition to these, both the groups were prescribed conventional nitrate ester and dual anti-platelet drugs. At the beginning of treatment and at follow-up examination at 12 weeks, 12-lead ECGs were obtained and the QT interval was measured, along with hepatic and renal function tests and serum levels of ox-LDL and PON1.
Statistical analysis
Normal data were presented as mean ± standard deviation (SD), and comparisons between the two groups were made using a number of independent-samples t-tests. The parameters before and after treatment in the same group were compared using paired t-tests. For the various measurement data, analysis of variance (ANOVA) was used to test for differences. If homogenous, Fisher’s least significant difference test was used for multiple comparisons between groups. Otherwise, Tamhane’s T2 test was used. For enumeration data, the chi-square test was used. Statistical analysis was performed using SPSS 15.0 for Windows (SPSS Inc., USA). P<0.05 was considered statistically significant.
Results
Characteristics of the study population
The basic characteristics of the two groups are outlined in Table 1. There were no statistically significant differences in terms of age, gender, body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP), heart rate (HR), and serum glucose (GLU), K+, and creatinine (Cr) levels between the two groups (P>0.05).
Table 1. Basic characteristics.
| Subject | Control group (n=62) | Treatment group (n=64) |
|---|---|---|
| Age (years) | 63.7±11.5 | 62.1±12.4 |
| Gender | 37 (male, 59.7%) | 40 (male, 62.5%) |
| BMI (kg/m2) | 29.1±5.2 | 30.4±5.3 |
| SBP (mmHg) | 157.1±13.2 | 158.2±16.7 |
| DBP (mmHg) | 93.4±12.3 | 92.8±11.9 |
| HR (bpm) | 72.6±12.7 | 73.1±13.6 |
| GLU (mmol/L) | 6.1±3.3 | 5.8±3.6 |
| K+ (mmol/L) | 4.1±0.7 | 4.0±0.6 |
| Cr (µmol/L) | 73.7±16.3 | 78.2±17.9 |
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; GLU, serum glucose; K+, serum potassium; Cr, serum creatinine.
The clinical characteristics of the two groups are outlined in Table 2. There were no statistically significant differences in each Type of ACS, culprit vessel, left ventricular ejection fraction (LVEF), and the ratio of hypertension between the two groups (P>0.05).
Table 2. Clinical characteristics.
| Subject | Control group (n=62) | Treatment group (n=64) |
|---|---|---|
| Type of ACS (%) | ||
| NSTEMI | 10 (16.1) | 11 (17.2) |
| STEMI | 12 (19.4) | 17 (26.6) |
| UA | 40 (64.5) | 36 (56.2) |
| Culprit vessel (%) | ||
| LAD | 25 (40.3) | 27 (42.2) |
| RCA | 16 (25.8) | 19 (29.7) |
| LCX | 21 (33.9) | 18 (28.1) |
| LVEF (%) | 50.7±7.3 | 51.2±7.9 |
| Hypertension (%) | 45 (72.6) | 47 (73.4) |
NSTEMI, ST-segment elevation myocardial infarction; NSTEMI, non-ST-segment elevation myocardial infarction; UA, unstable angina pectoris; LAD, left anterior descending artery; RCA, right coronary artery; LCX, left circumflex branch; LVEF, left ventricular ejection fraction.
Serum lipid levels
Table 3 shows the serum lipid levels in the patients before and after treatment. There were no significant differences in the serum lipid levels between the two groups before treatment (P>0.05). After treatment, in the control group, the total cholesterol (TC), triglyceride (TG), and LDL levels were noted to have decreased; the HDL level increased significantly; and the levels of HDL2 and HDL3 as well as the HDL3/HDL2 ratio did not change significantly. In the treatment (combined) group, the TC, TG, and LDL levels were significantly decreased after treatment, being significantly lower than those in the control group; further, the levels of HDL3 and the HDL3/HDL2 ratio increased significantly compared with those in the control group (P<0.05).
Table 3. Serum lipid levels (mean ± SD).
| Parameter | Control group (n=62) |
Treatment group (n=64) |
|||
|---|---|---|---|---|---|
| Before | After | Before | After | ||
| TG (mmol/L) | 1.94±0.54 | 1.64±0.41a | 2.12±0.82 | 1.41±0.38ab | |
| TC (mmol/L) | 4.20±1.00 | 3.85±0.91a | 3.98±0.74 | 3.12±0.36ab | |
| LDL-C (mmol/L) | 2.63±0.58 | 2.39±0.32a | 2.47±0.55 | 2.04±0.23ab | |
| HDL-C (mmol/L) | 1.09±0.19 | 1.17±0.22a | 1.07±0.11 | 0.95±0.07ab | |
| HDL2 (mmol/L) | 0.53±0.14 | 0.51±0.27 | 0.49±0.18 | 0.41±0.22ab | |
| HDL3 (mmol/L) | 0.46±0.10 | 0.48±0.12 | 0.48±0.09 | 0.57±0.11ab | |
| HDL3/HDL2 ratio | 0.89±0.15 | 0.91±0.17 | 1.03±0.20 | 1.53±0.37ab | |
a, post-treatment levels compared with pre-treatment levels, P<0.05; b, significant difference between the two groups, P< 0.05. TG, triglyceride; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; HDL2, HDL2 subfraction; HDL3, HDL3 subfraction.
Serum levels of ox-LDL and PON1
Using the t-test, we compared these two indicators of lipid and antioxidant levels before and after the treatment in each group as well as between the two groups after treatment. The ox-LDL level decreased significantly after treatment as compared to the baseline in both the groups; further, it was significantly lower in the treatment group than in the control group (P<0.05) (Table 4). The PON1 level increased significantly after treatment as compared to the baseline in both the groups; further, it was significantly higher in the treatment group than in the control group (P<0.05).
Table 4. Serum levels of Ox-LDL and PON1.
| Indicator | Control group (n=62) |
Treatment group (n=64) |
|||
|---|---|---|---|---|---|
| Before | After | Before | After | ||
| ox-LDL (mg/L) | 485.4±190.1 | 396.8±130.6a | 454.3±168.1 | 339.3±135.4ab | |
| PON1 (KU/L) | 31.9±13.7 | 38.9±15.3a | 30.1±13.1 | 45.7±18.4ab | |
a, post-treatment levels compared with pre-treatment levels, P<0.05; b, significant difference between the two groups, P<0.05. ox-LDL, oxidized low-density lipoprotein cholesterol; PON1, paraoxonase-1.
The relationship between ox-LDL and PON1
The serum levels of ox-LDL and PON1 showed a significant negative correlation by the Pearson correlation analysis (r≈−0.5) (Figures 1,2,3,4).
Figure 1.
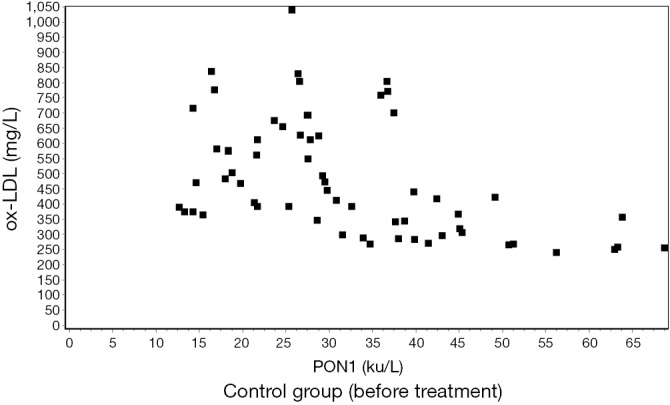
Correlation between ox-LDL and PON1 before treatment in the control group. (r=−0.4650) (P<0.01). ox-LDL, oxidized low-density lipoprotein cholesterol; PON1, paraoxonase-1.
Figure 2.
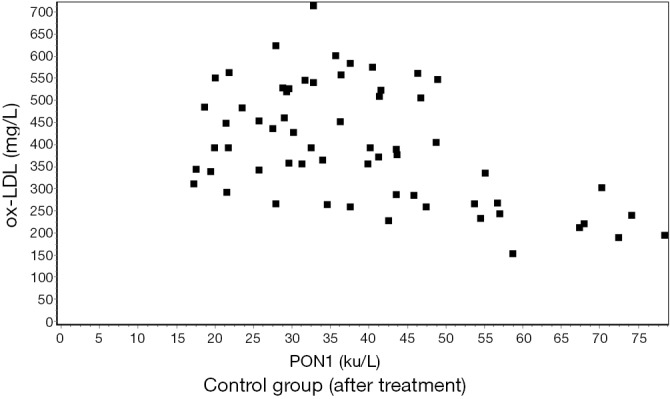
Correlation between ox-LDL and PON1 after treatment in the control group (r=−0.4869) (P<0.01). ox-LDL, oxidized low-density lipoprotein cholesterol; PON1, paraoxonase-1.
Figure 3.
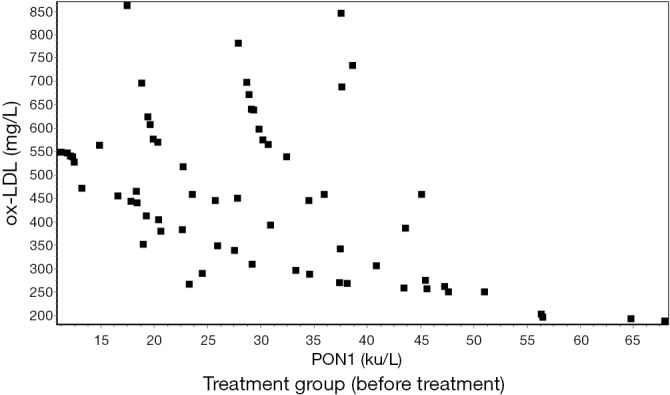
Correlation between ox-LDL and PON1 before treatment in the treatment group. (r=−0.5129) (P<0.01). ox-LDL, oxidized low-density lipoprotein cholesterol; PON1, paraoxonase-1.
Figure 4.
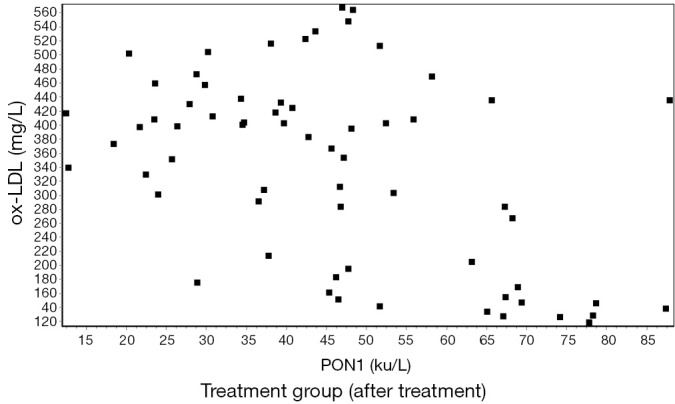
Correlation between ox-LDL and PON1 after treatment in the treatment group (r=−0.4943) (P<0.01). ox-LDL, oxidized low-density lipoprotein cholesterol; PON1, paraoxonase-1.
Follow up and side effects of the therapy
The follow up of these patients involved in the presents study lasts for 12 weeks:
Blood samples were collected at the start and the end of the research respectively; the occurrence of adverse reactions was followed up during this period. No serious effects of the therapy on hepatic or renal function and no evidence of muscular damage were observed. Reversible mild elevation of transaminase in one case in the control group and two cases in the treatment group were observed in the first 2 weeks, which returned to the initial level after treatment at the end of the follow-up. No severe gastrointestinal reaction or prolonged QT interval in the ECG was observed in any of the patients.
Discussion
Atherosclerosis is an inflammatory disease that is associated with oxidative stress and thrombotic agents (16). Oxidative stress is generally considered as the dynamic balance between the production of reactive oxidant species and antioxidant capacity. It has been reported that levels of antioxidants can be affected by various lipoproteins, including LDL (5). Circulating ox-LDL is generally believed to be pro-atherogenic (6). Oxidative modification of LDL in the arterial wall is a complex process involving several biological pathways (17). Ox-LDL can promote the production of chemoattractants, adhesion molecules, and colony stimulating factors for monocytes by endothelial cells. Preliminary adhesion of circulating monocytes to the vascular endothelium and their subsequent transmigration into the arterial intima comprise the early steps of the formation of atherosclerotic lesions. In this pathway, HDL inhibits atherosclerosis by suppressing LDL oxidation and the biological effects of the mildly oxidized LDL on monocyte-endothelial cell interactions (18).
PON1 is an esterase enzyme synthesized by the liver that is associated with HDL in the blood. The antioxidant activity of HDL is largely attributed to PON1 present the HDL molecule (19). PON1 can hydrolyze aromatic carboxylic acid esters, organophosphates, and oxidized phospholipids, and the hydrolysis of oxidized phospholipids by PON1 can destroy the biologically active lipids in the mildly oxidized LDL (9). Since the activity of PON1 is associated with HDL, it possibly protects LDL and HDL from oxidation (18).
Probucol is a bisphenolic compound that was originally synthesized as an antioxidant for its ability to reduce cholesterol levels. However, its utilization has been limited mainly because it leads to considerable reductions in HDL levels (20). Yamashita et al. found that probucol was useful in lowering the risk of cardiovascular events without any significant adverse effects, especially in combination with statins (21). Experiments showed that the effects of multiple anti-atherogenic agents were mainly due to the inhibition of the oxidative modification of LDL. Probucol inhibits foam cell formation from THP-1 cells by suppressing lipid accumulation and by enhancing the release of cholesterol from macrophages (20). Probucol also plays a role in mediating cellular cholesterol efflux and can facilitate reverse cholesterol transport (RCT) from the arterial wall and peripheral tissues to the liver (22).
The development of 3-hydroxy-3-methylglutaryl coenzyme (HMG-CoA) reductase (HMGR) inhibitors or statins has improved the widespread use of lipid-lowering drugs. In addition to the known traditional effects such as lipid regulation, statins can also affect plaque stability, improve the balance of oxidative stress, and reduce the multiple effects of inflammatory reactions (12,23). Oxidative stress and/or weak antioxidant defense systems are considered as important factors in the multi-mechanistic pathogenesis of atherosclerosis. Based on abundant evidences, atorvastatin has been found to exert beneficial cardiovascular effects independent of its lipid-lowering ability, possibly due to its antioxidant properties (24).
The present study aimed to determine whether the treatment of ACS patients using atorvastatin with/without probucol could reduce the levels of ox-LDL and PON1 and explore whether PON1 could regulate the oxidation of LDL. We observed that the levels of ox-LDL were significantly decreased after treatment in both the groups, and the effect was more obvious in the treatment group (P<0.05). We also noted an elevation in the PON1 level after treatment, and this increase was statistically significant in the treatment group (P<0.05). The serum levels of ox-LDL and PON1 showed a significant negative correlation by Pearson’s correlation analysis (r≈−0.5). It has been suggested that atorvastatin may exert additional beneficial effects on atherosclerosis by increasing PON1 activity independent of its cholesterol-lowering properties (25,26). We hypothesized that, apart from facilitating RCT, probucol may increase PON1 activity, especially when used in combination with statins. In the present study, we found that PON1 levels were significantly higher in the treatment group after 12 weeks of combined treatment than that in the control group; this indicated that probucol may play an independent role in the expression of PON1 outside of statins. Hong et al. (27) observed that probucol significantly increased serum PON1 levels and increased the expression of PON1 mRNA. Zhong et al. (12) found that probucol alleviated atherosclerosis by improving the function of HDL. The mechanisms related to this effect included accelerating the process of RCT and improving the anti-inflammatory and antioxidant functions of HDL. PON1 is largely responsible for the antioxidant action of HDL owing to its ability of hydrolyzing oxidized phospholipids (9). Our research found that in the control group, the levels of TC, TG, and LDL decreased; HDL levels increased significantly; the levels of HDL3 and the HDL3/HDL2 ratio did not change significantly. In the treatment group, the levels of TC, TG, HDL, and LDL significantly decreased, while the levels of HDL and HDL3 as well as the HDL3/HDL2 ratio increased significantly. Considering that PON1 activity has been negative correlated with the mean particle size of HDL as well as the HDL2/HDL3 ratio (28), it might be reasonable that probucol reduced HDL levels but increased HDL3 subfraction levels by enhancing PON1 activity. Thus, the therapeutic effects of probucol, despite the reduction in HDL levels, might be attributed to its lipid regulation and antioxidant functions. Long et al. (29) reported that in patients who were administered atorvastatin (10 mg, qd) with/without probucol (500 mg, bid) for 4 weeks, the serum ox-LDL levels declined significantly and PON1 activity increased simultaneously; interestingly, these changes were more significant in the combined treatment group. These findings are consistent with our results. The present study showed that the probucol combined with atorvastatin could inhibit the hydrolysis of PON1 more effectively, and this combination could increase PON1 activity, decrease ox-LDL and HDL oxidation, and produce a synergistic antioxidant effect (30).
The main side effect of statin therapy is liver and muscle toxicity (31,32), and the most common adverse reactions of probucol are gastrointestinal discomfort and prolonged QT intervals in the ECG (21). The present study does not find any serious effect on the hepatic or renal functions, indication of muscle damage, and severe gastrointestinal reaction. Therefore, our results suggest that probucol combined with atorvastatin is a safe and effective treatment for ACS patients. Lastly, the present study suggests that the addition of probucol to atorvastatin has synergistic effects in lowering cholesterol levels and improving antioxidant activity.
Acknowledgements
Funding: This work was supported by the Supporting Fund from the Ministry of Science and Technology of China (2009BAI86B04) (Li Fan).
Disclosure: The authors declare no conflict of interest.
References
- 1.Ge CJ, Lü SZ, Feng LX, et al. Combined effect of atorvastatin and probucol on plasma cystatin C levels and severity of coronary lesion in patients with borderline coronary lesion. Chin Med J (Engl) 2012;125:2472-6. [PubMed] [Google Scholar]
- 2.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005;352:1685-95. [DOI] [PubMed] [Google Scholar]
- 3.Zhou C, Cao J, Shang L, et al. Reduced paraoxonase 1 activity as a marker for severe coronary artery disease. Dis Markers 2013;35:97-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madamanchi NR, Vendrov A, Runge MS. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol 2005;25:29-38. [DOI] [PubMed] [Google Scholar]
- 5.Miyauchi K, Morino Y, Tsukahara K, et al. The PACIFIC (Prevention of AtherothrombotiC Incidents Following Ischemic Coronary attack) Registry: Rationale and design of a 2-year study in patients initially hospitalised with acute coronary syndrome in Japan. Cardiovasc Drugs Ther 2010;24:77-83. [DOI] [PubMed] [Google Scholar]
- 6.Ho E, Karimi Galougahi K, Liu CC, et al. Biological markers of oxidative stress: Applications to cardiovascular research and practice. Redox Biol 2013;1:483-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tabet F, Rye KA. High-density lipoproteins, inflammation and oxidative stress. Clin Sci (Lond) 2009;116:87-98. [DOI] [PubMed] [Google Scholar]
- 8.Mascarenhas-Melo F, Sereno J, Teixeira-Lemos E, et al. Implication of low HDL-c levels in patients with average LDL-c levels: a focus on oxidized LDL, large HDL subpopulation, and adiponectin. Mediators Inflamm 2013;2013:612038. [DOI] [PMC free article] [PubMed]
- 9.Mackness B, Mackness M.Anti-inflammatory properties of paraoxonase-1 in atherosclerosis. Adv Exp Med Biol 2010;660:143-51. [DOI] [PubMed] [Google Scholar]
- 10.Huang Y, Wu Z, Riwanto M, et al. Myeloperoxidase, paraoxonase-1, and HDL form a functional ternary complex. J Clin Invest 2013;123:3815-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inagaki M, Nakagawa-Toyama Y, Nishida M, et al. Effect of probucol on antioxidant properties of HDL in patients with heterozygous familial hypercholesterolemia. J Atheroscler Thromb 2012;19:643-56. [DOI] [PubMed] [Google Scholar]
- 12.Zhong JK, Guo ZG, Li C, et al. Probucol alleviates atherosclerosis and improves high density lipoprotein function. Lipids in Health and Disease 2011;10:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheffer PG, Schindhelm RK, van Verschuer VM, et al. No effect of atorvastatin and simvastatin on oxidative stress in patients at high risk for cardiovascular disease. Neth J Med 2013;71:359-65. [PubMed] [Google Scholar]
- 14.Zhou Q, Liao JK. Pleiotropic effects of statins. - Basic research and clinical perspectives. Circ J 2010;74:818-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yasuda G, Ando D, Hirawa N, et al. Effects of atorvastatin versus probucol on low-density lipoprotein subtype distribution and renal function in hyperlipidemic patients with nondiabetic nephropathy. Ren Fail 2010;32:680-6. [DOI] [PubMed] [Google Scholar]
- 16.Karimi P, Rashtchizadeh N.Oxidative Versus Thrombotic Stimulation of Platelets Differentially activates Signalling Pathways. J Cardiovasc Thorac Res 2013;5:61-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tumova E, Sun W, Jones PH, et al. The impact of rapid weight loss on oxidative stress markers and the expression of the metabolic syndrome in obese individuals. J Obes 2013;2013:729515. [DOI] [PMC free article] [PubMed]
- 18.Shekhanawar M, Shekhanawar SM, Krisnaswamy D, et al. The role of ‘paraoxonase-1 activity’ as an antioxidant in coronary artery diseases. J Clin Diagn Res 2013;7:1284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Durrington PN, Mackness B, Mackness MI. Paraoxonase and atherosclerosis. Arterioscler Thromb Vasc Biol 2001;21:473-80. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto A.A uniqe antilipidemic drug--probucol. J Atheroscler Thromb 2008;15:304-5. [DOI] [PubMed] [Google Scholar]
- 21.Yamashita S, Hbujo H, Arai H, et al. Long-term probucol treatment prevents secondary cardiovascular events: a cohort study of patients with heterozygous familial hypercholesterolemia in Japan. J Atheroscler Thromb 2008;15:292-303. [DOI] [PubMed] [Google Scholar]
- 22.Inagaki M, Nakagawa-Toyama Y, Nishida M, et al. Effect of probucol on antioxidant properties of HDL in patients with heterozygous familial hypercholesterolemia. J Atheroscler Thromb 2012;19:643-56. [DOI] [PubMed] [Google Scholar]
- 23.Sawayama Y, Shimizu C, Maeda N, et al. Effects of probucol and pravastatin on common carotid atherosclerosis in patients with asymptomatic hypercholesterolemia. Fukuoka Atherosclerosis Trial (FAST). J Am Coll Cardiol 2002;39:610-6. [DOI] [PubMed] [Google Scholar]
- 24.Nagila A, Permpongpaiboon T, Tantrarongroj S, et al. Effect of atorvastatin on paraoxonase1 (PON1) and oxidative status. Pharmacol Rep 2009;61:892-8. [DOI] [PubMed] [Google Scholar]
- 25.Yeung AC, Tsao P. Statin therapy: beyond cholesterol lowering and antiinflammatory effects. Circulation 2002;105:2937-8. [DOI] [PubMed] [Google Scholar]
- 26.Kural BV, Orem C, Uydu HA, et al. The effects of lipid-lowering therapy on paraoxonase activities and their relationships with the oxidant-antioxidant system in patients with dyslipidemia. Coron Artery Dis 2004;15:277-83. [DOI] [PubMed] [Google Scholar]
- 27.Hong SC, Zhao SP, Wu ZH. Probucol up-regulates paraoxonase 1 expression in hepatocytes of hypercholesterolemic rabbits. J Cardiovasc Pharmacol 2006;47:77-81. [DOI] [PubMed] [Google Scholar]
- 28.Razavi AE, Ani M, Pourfarzam M, et al. Associations between high density lipoprotein mean particle size and serum paraoxonase-1 activity. J Res Med Sci 2012;17:1020-6. [PMC free article] [PubMed] [Google Scholar]
- 29.Long X, Li XP, Xue YQ, et al. The lipid modulation and antioxidant effect of probucol and atorvastatin combination in patients with acute coronary syndrome. Chinese Journal of Arteriosclerosis 2009;17:933-7. [Google Scholar]
- 30.Harangi M, Mirdamadi HZ, Seres I, et al. Atorvastatin effect on the distribution of high-density lipoprotein subfractions and human paraoxonase activity. Transl Res 2009;153:190-8. [DOI] [PubMed] [Google Scholar]
- 31.Whayne TF, Jr. Problems and possible solutions for therapy with statins. Int J Angiol 2013;22:75-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taha DA, De Moor CH, Barrett DA, et al. Translational insight into statin-induced muscle toxicity: from cell culture to clinical studies. Transl Res 2014;164:85-109. [DOI] [PubMed] [Google Scholar]


