Abstract
Background
Although high body mass index (BMI) increases risk for developing esophageal adenocarcinoma (EAC), the prognostic influence of BMI is unknown in esophageal squamous carcinoma.
Methods
BMI was calculated using measured height and weight at the first diagnosis and categorized as overweight (25 to 29.9 kg/m2), normal (18.5 to 24.9 kg/m2) or underweight (<18.5 kg/m2). Survival was compared by using the log-rank test on the Kaplan-Meier life table. Multivariate Cox regression analysis was used to evaluate whether BMI was an independent prognostic factor for disease-specific survival (DSS).
Results
Among 1,176 esophageal squamous carcinoma patients, 146 (12.4%) were categorized as overweight, and 277 (23.6%) underweight. More patients in the underweight group had anemia (P=0.001), weight loss (P=0.035) and R1 resection (P<0.001). Less patients in the underweight group received adjuvant chemotherapy (P=0.01). Patients in the overweight group had a higher incidence rate of high blood pressure (P<0.001), diabetes (P<0.001) and coronary artery diseases (P<0.001). Moreover, more patients in the overweight group had a lower TNM stage (P=0.003). In the univariated analysis, high BMI was significantly associated with better DSS (P=0.013).
Conclusions
After adjusting for covariates enrolled for study, high BMI was an independent prognostic factor in weight loss esophageal squamous carcinoma patients.
Keywords: Body mass index (BMI), esophageal squamous carcinoma, overweight, prognostic factor
Introduction
Although the esophageal cancer incidence showed a downtrend in China, especially among urban females, according to the Cancer Statistic Report from the International Agency for Research on Cancer (IARC), there were 462,000 new cases in 2002 and 386,000 deaths worldwide (1). In Guangzhou, a population-based cancer registration during 2000-2002 shown the incidence of esophagus cancer ranked seventh in male and 17th in female (2). As for the mortality rate, it ranked as the fifth leading cause of cancer death in male and 15th in female (2). The impact of high body mass index (BMI) on survival for patients with esophageal carcinoma was contradictory (3-5). A retrospective study found that an elevated BMI was statistically significantly associated with improved overall survival (OS) and disease free survival (DFS) and the authors suspected that the result might be influenced by the migration to lower BMI category of individuals with obvious preoperative weight loss and therefore malnutrition (3).
It is well known that the clinicopathologic characteristics of esophageal carcinoma, including histology, gender, and age distribution vary widely between patients in Eastern and Western countries (6,7). Squamous cell carcinoma is the most common histological type of esophageal cancer in the East, including China (8). Moreover, Chinese patients account for more than half of esophageal cancer patients worldwide (9). The epidemiological evidence of the association between BMI and esophageal cancer is very different for adenocarcinoma (positive association) and squamous cancer cell (inverse association). A systematic review and meta-analysis of prospective observation studies shown that for both men and women, 5 kg/m2 increasing in BMI was strongly associated with esophageal adenocarcinoma (EAC), but not with squamous cancer cell (10). It is essential to explore the prognostic impact of high BMI on patients with esophageal squamous carcinoma stratified by body weight change in China.
Materials and methods
Patients
Study approval was obtained from independent ethics committees at Cancer Center of Sun Yat-Sen University. The study was undertaken in accordance with the ethical standards of the World Medical Association Declaration of Helsinki. Between January 2000 and January 2008, the medical records of 1,191 pathology-proven esophageal squamous carcinoma patients who were diagnosed and received treatment in the Cancer Center of Sun Yat-Sen University were retrospectively analyzed. All the patients included in the analysis met the following criteria: (I) the disease was histologically defined as squamous cell carcinoma; (II) patients with a ≥3 months survival time; (III) patients without a history of other prior malignancy; (IV) the records contained complete information for stage grouping [the sixth edition of the American Joint Committee on Cancer (AJCC) Cancer Staging Manual]; (V) height and weight at the first diagnosis were available.
We excluded patients who had received neoadjuvant chemotherapy or radiotherapy or radical radiotherapy since the TNM stage was unclear in this subgroup of patients. We also excluded patients whose BMI ≥30 since there were only 15 among 1,191 patients whose BMI ≥30. It is hard to make a comparison between obesity and other BMI status because very few patients in the obesity group. Finally, 1,176 patients were included for the analysis. All patients gave written informed consent. Approval from the institutional ethics review boards of our center was obtained before the study.
Treatment procedures
Among the 1,176 patients, 1,025 patients received primary tumor resection and lymph node dissection. The most commonly used surgical approaches were the Ivor-Lewis approach, left thoracotomy, and the cervicothoracoabdominal procedure. The rest 151 patients had unresectable diseases and received palliative chemotherapy. The regimens included taxane, carboplatin and 5-fluorouracil.
Variable definitions
BMI was calculated according to a standardized definition as weight in kilograms divided by height in meters squared. Both the weight and height were recorded at the first diagnosis before any treatment. According to the World Health Organization’s definition, the result of BMI was categorized as obese (BMI ≥30 kg/m2), overweight (BMI 25-29.9 kg/m2), normal weight (BMI 18.5-24.9 kg/m2) and underweight (BMI <18.5 kg/m2). Since only 1.3% (15/1,191) of the patients was in the obese group, we excluded them from the study. In our study, we only analyzed patients in the overweight, normal weight and underweight groups. Weight loss was defined as more than 5% decreasing in the body weight in the last 3 months (11).
Clinical data collected for subsequent analysis included gender (male or female), age, anemia (yes or no), primary tumor site (upper, mid-thoracic and lower part), histologic type (well, moderately differentiated carcinoma or poor + undifferentiated carcinoma), total number of lymph nodes retrieved, pT stage (6th AJCC classification), pN stage (6th AJCC classification), M stage and TNM stage (6th AJCC classification), status of resection margins (R0 or R1), co-morbidities, smoking history, adjuvant chemotherapy and body weight change (Table 1).
Table 1. Demographics and co-morbidities stratified by BMI in patients with esophageal squamous carcinoma.
| Factors | Overweight (n=146) n (%) | Normal weight (n=753) n (%) | Underweight (n=277) n (%) | P value# |
|---|---|---|---|---|
| Sex | 0.41 | |||
| Male | 110 (75.3) | 598 (79.4) | 212 (76.5) | |
| Female | 36 (24.7) | 155 (20.6) | 65 (23.5) | |
| Age (years) (mean ± SD) | 57.1±9.8 | 57.8±9.4 | 58.1±10.1 | 0.60 |
| Anemia | 0.001 | |||
| Yes | 16 (11.0) | 136 (18.1) | 71 (25.6) | |
| No | 130 (89.0) | 617 (81.9) | 206 (74.4) | |
| Location of tumor | 0.04 | |||
| Upper thoracic | 10 (6.8) | 104 (13.8) | 34 (14.4) | |
| Mid-thoracic | 110 (75.3) | 469 (62.3) | 181 (65.3) | |
| Lower thoracic | 26 (17.9) | 180 (23.9) | 62 (20.3) | |
| Histology | <0.001 | |||
| Well | 31 (21.2) | 225 (29.9) | 121 (43.7) | |
| Moderately | 56 (38.4) | 255 (33.9) | 77 (27.8) | |
| Poorly and undifferentiated | 59 (40.4) | 273 (36.3) | 79 (28.5) | |
| The 6th T stage (AJCC) | 0.021 | |||
| T1 | 15 (11.7) | 46 (7.0) | 20 (8.4) | |
| T2 | 33 (25.8) | 139 (21.1) | 34 (14.2) | |
| T3 | 72 (56.3) | 409 (62.2) | 153 (64.0) | |
| T4 | 8 (6.2) | 64 (9.7) | 32 (13.4) | |
| The 6th N stage (AJCC) | 0.56 | |||
| N0 | 60 (46.9) | 277 (42.1) | 106 (44.4) | |
| N1 | 68 (63.1) | 381 (57.9) | 133 (55.6) | |
| The 6th M stage (AJCC) | 0.88 | |||
| M0 | 128 (87.6) | 658 (87.3) | 239 (86.3) | |
| M1 | 18 (12.4) | 95 (12.7) | 38 (13.7) | |
| The 6th TNM stage (AJCC) | 0.003 | |||
| I | 12 (8.2) | 37 (4.9) | 23 (8.3) | |
| IIA | 51 (34.9) | 248 (32.9) | 82 (29.6) | |
| IIB | 19 (13.0) | 77 (10.2) | 10 (3.6) | |
| III | 46 (31.5) | 296 (39.3) | 124 (44.8) | |
| IV | 18 (12.4) | 95 (12.7) | 38 (13.7) | |
| Number of lymph nodes collected in specimen | 10.1±3.4 | 10.4±3.9 | 9.9±3.4 | 0.28 |
| Status of resection margins | <0.001 | |||
| R0 | 123 (96.1) | 628 (95.4) | 222 (92.9) | |
| R1 | 5 (3.9) | 30 (4.6) | 17 (7.1) | |
| High blood pressure | <0.001 | |||
| Yes | 59 (40.4) | 150 (19.9) | 17 (6.1) | |
| No | 87 (59.6) | 603 (80.1) | 260 (93.9) | |
| Diabetes | <0.001 | |||
| Yes | 37 (25.3) | 100 (13.3) | 32 (11.6) | |
| No | 109 (74.7) | 653 (86.7) | 245 (88.4) | |
| Coronary artery diseases | <0.001 | |||
| Yes | 21 (14.5) | 75 (10.0) | 8 (2.9) | |
| No | 125 (85.5) | 678 (90.0) | 269 (97.1) | |
| Cerebrovascular diseases | 0.17 | |||
| Yes | 10 (6.8) | 36 (4.8) | 8 (2.9) | |
| No | 136 (93.2) | 717 (95.2) | 269 (97.1) | |
| COPD | 0.23 | |||
| Yes | 13 (8.8) | 50 (6.6) | 13 (4.7) | |
| No | 133 (91.2) | 703 (93.4) | 264 (95.3) | |
| Hernia | 0.44 | |||
| Yes | 4 (2.7) | 17 (2.3) | 7 (2.5) | |
| No | 142 (97.3) | 736 (97.7) | 270 (97.5) | |
| Smoking history | 0.93 | |||
| Yes | 88 (60.1) | 466 (61.9) | 170 (61.4) | |
| No | 58 (39.9) | 287 (38.1) | 107 (38.6) | |
| Adjuvant chemotherapy | 0.01 | |||
| Yes | 30 (20.5) | 93 (12.4) | 29 (10.5) | |
| No | 116 (79.5) | 660 (87.6) | 248 (89.5) | |
| Body weight | 0.035 | |||
| Weight loss | 57 (39.0) | 346 (45.9) | 144 (52.0) | |
| Weight stable | 89 (61.0) | 407 (54.1) | 133 (48.0) |
Overweight, BMI 25-29.9 kg/m2; normal, BMI 18.5-24.9 kg/m2; underweight, BMI <18.5 kg/m2. BMI, body mass index; AJCC, American Joint Committee on Cancer; TNM, Tumor-Node-Metastasis; COPD, chronic obstructive pulmonary disease. P value#, the comparison of demographics and comorbidities among three different BMI status.
During the study period there had no standardized protocol for postoperative chemotherapy and/or radiotherapy. Adjuvant therapy was suggested to all patients with T3-T4 classification or positive lymph node involvement. Patients with R1 resection were recommended to receive adjuvant radiotherapy. However, only 152 (12.9%) patients completed the adjuvant chemotherapy. In R1 resection, 57.7% (30/52) of patients received the adjuvant radiotherapy. Until December 2012, there were 798 patients died from the disease. The median follow up time for the entire cohort was 52.0 months, ranging from 6 to 140.2 months.
Statistical analysis
All statistical analysis was performed by Statistical Package of Social Sciences 13.0 software. A 2-sided probability value of less than 0.05 was considered statistically significant. The values were presented as the mean ± standard deviation (SD) for continuous data and for these data we used ANOVA. The Pearson χ2 test was used to determine the significance of differences for categorical data. Kaplan-Meier method was used to estimate the 5-year disease-specific survival (DSS) and a log-rank test was used to assess the survival differences. Survival time was measured from the date of diagnosis to the date of death or the last follow-up. The follow-up department in our hospital has updated the state of patients. For patients who remained alive, data were censored at the date of the last contact. Variables showing a trend for association with survival (P<0.05) were selected in the final multivariate Cox proportional hazards model.
Results
Patient demographics
The median age of the 1,176 patients was 57.9±9.8 years. Among them, 920 were male and 256 were female. The overall 5-year survival for the whole group of patients was 31.7%.
Patients characteristics by BMI
Among 1,176 esophageal squamous carcinoma patients, 146 (12.4%) were categorized as overweight, and 277 (23.6%) were underweight. The rest 64.0% of patients had a normal BMI between 18.5 and 25. Table 1 described the cohort characteristics by different BMI categories. The effect of clinical features on survival was also summarized in Table 1. There were no statistical differences among groups in items of sex (P=0.41), age (P=0.60), N stage (P=0.56), M stage (P=0.88), history of cerebrovascular diseases (P=0.17), chronic obstructive pulmonary disease (COPD) (P=0.23) and hernia (P=0.44). Smoking history was similar across the three BMI groups (60.1% vs. 61.9% vs. 61.4%, P=0.93). Patients with overweight were more likely to have a history of high blood pressure, diabetes mellitus and coronary artery diseases compared to patients with normal weight or underweight (P<0.001). Overweight patients were more likely to have a pathologic stage of disease that was I or II than those in the normal weight or underweight group, in whom pathologic stage was more likely to be III or IV (P=0.003). Moreover, patients in the overweight group tended to have an early T stages (P=0.021). More patients in the underweight group had anemia (P=0.001), weight loss (P=0.035) and R1 resection (P<0.001). Less patients in the underweight group received adjuvant chemotherapy (P=0.01). More patients in the overweight group located in the mid-thoracic (P=0.04).
Univariate and multivariate analyses of 5-year DSS in the whole patient group
We used both unvaried and multivariate analyses to evaluate factors relating to 5-year DSS. The 5-year DSS rates were 36.5% for overweight patients and 31.8% for normal weight patients, 28.6% for underweight patients (P=0.008). Almost half of the patients (46.5%) had weight loss. The 5-year DSS was 26.6% and 34.6% for weight loss and weight stable patients, respectively (P=0.016) (Figure 1). Multivariate regression analysis showed that TNM stage, status of resection margins and weight loss status were independent factors for 5-year DSS (P<0.001, <0.001 and 0.023, respectively). While BMI status failed to be an independent prognostic factor.
Figure 1.
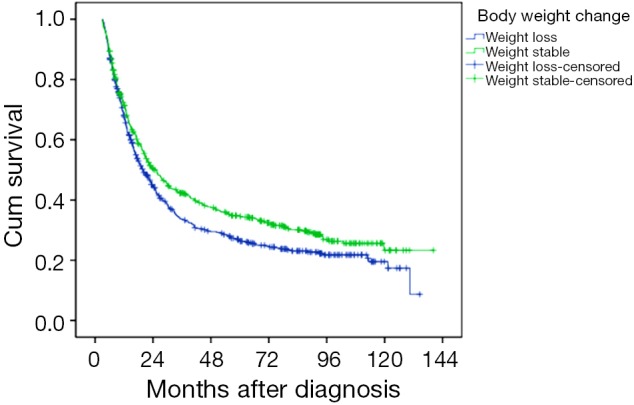
Survival curves of esophageal squamous carcinoma patients according to body weight change status.
Univariate and multivariate analyses of 5-year DSS stratified by the weight loss status
To better understand the effect of body weight change on the prognostic value of BMI, we separated the patients into two subgroups according to the body weight change status, weight loss group and weight stable group. Among weight stable patients (n=629), overweight patients did not have significantly different DSS as compared with normal weight patients or underweight patients (34.3% vs. 33.6% vs. 35.6%, P=0.57; Figure 2A). Among patients with weight loss (n=547), univariate analysis revealed that overweight patients had significantly longer DSS as compared with normal weight and underweight patients (33.6% vs. 27.7% vs. 21.6%, P=0.013; Figure 2B). The items of sex, age, anemia, and type of gastrectomy, histology, number of lymph nodes resected, TNM stage and BMI status were significantly related to 5-year DSS. In multivariable analysis among weight loss patients, overweight was significantly associated with better DSS (P=0.031, hazard ratio was 0.347, 95% confidence interval was 0.178-0.675, Table 2). The other independent prognostic factor in the multivariable analysis was TNM stage and histology (P=0.022 and P=0.028 respectively; Table 2).
Figure 2.
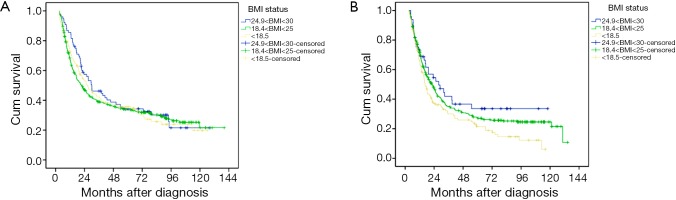
The prognostic impact of BMI on patients with esophageal squamous carcinoma stratified by body weight change: weight stable (A) and weight loss (B). BMI, body mass index.
Table 2. Disease-specific survival according to clinic pathologic variables in univariate and multivariate analysis among weight loss patients (n=547).
| Factors | Univariate analysis (P value) | Multivariate analysis |
|
|---|---|---|---|
| Hazard ratio (95% confidence interval) | P value | ||
| Sex (female) | 0.020 | 0.320 (0.220-1.175) | 0.059 |
| Age | 0.013 | 0.872 (0.630-1.208) | 0.405 |
| Anemia | 0.040 | 0.845 (0.603-1.123) | 0.412 |
| BMI status | 0.013 | 0.347 (0.178-0.675) | 0.031 |
| The 6th TNM stage | <0.001 | 0.084 (0.037-0.191) | 0.022 |
| Number of lymph nodes collected in specimen | 0.036 | 1.087 (0.742-1.591) | 0.669 |
| Status of resection margins | <0.001 | 0.504 (0.408-1.544) | 0.540 |
| Histology | 0.006 | 0.695 (0.163-0.809) | 0.028 |
| Location | 0.233 | ||
| Adjuvant chemotherapy | 0.140 | ||
| Smoking history | 0.505 | ||
| High blood pressure | 0.815 | ||
| Diabetes | 0.160 | ||
| Coronary artery diseases | 0.724 | ||
| Cerebrovascular diseases | 0.342 | ||
| Hernia | 0.581 | ||
| COPD | 0.117 | ||
TNM, Tumor-Node-Metastasis; BMI, body mass index; COPD, chronic obstructive pulmonary disease.
Discussion
The measurement of BMI, as a standard method to detect obese or overweight people, has been widely accepted to have an effect on the development of EAC (12-14). Although in the United States, approximately two-thirds of all adults are obese or overweight, there are less obese people (BMI ≥30 kg/m2) in China, compared with western countries (15). For the esophageal carcinoma patients, as high as 70% of them were overweight or obese in the United States (3), while in our data, only 13.5% of the esophageal squamous cancer patients were overweight or obese. Particularly, obese only accounted for 1.3% of the whole cohort. The percentage of overweight and obese in our dataset is similar with data from the 2002 national nutrition and health survey, which showed that 14.7% of Chinese were overweight (BMI ≥25 kg/m2) and about 2.6% were obese (BMI ≥30 kg/m2) (16). Realizing that obese patients might have a different survival with the overweight patients, we did not combine patients with obese and overweight together for the further analysis. The small number of patients was hard to get a convincing result; we therefore excluded obese patients from the final analysis. Basing on the BMI status, we separated the patients’ cohort into three groups, overweight, normal weight and underweight.
The impact of BMI on the outcome of patients with esophageal carcinoma was inconsistent according to the literatures. Basing on the hypothesis that hyperinsulinemia and other metabolic derangements linked to adiposity might promote tumor growth and progression, obesity had been associated with adverse outcome in other cancers, including the colon and pancreas (17-22). It is reasonable to assume that high BMI might have an adverse impact on the OS since high BMI might be associated with a higher rate of complications, and the hyperinsulinemia and up-regulation of insulin growth factor signaling and pro-inflammatory mediators in high BMI patients may create a more favorable microenvironment for tumor cell survival, proliferation and progression (23). In a large population study (n=778), Yoon et al. found that obesity was independently associated with increased mortality among never smokers (4). However, others studies in patients with EAC had reported that high BMI had a null, or even protective effect on prognosis (3-5). A large study conducted by Trivers et al. shown that patients with a BMI between 25 and 29.9 kg/m2 survived longer than those with a BMI <25 kg/m2 (5). Another study by Melis et al. which included 541 esophageal carcinoma patients found that DFSs were longer in obese patients (3). A recent study showed that high BMI was an independent prognostic factor after curative esophagectomy for ESCC, though it had only 31 patients in high BMI group (24). Meanwhile, another recent study found that preoperative BMI was an independent prognostic factor for survival in oesophageal cancer, but the cutoff values of BMI were according to Asian-specific (25). In univariate analysis of our data, patients with high BMI has a higher 5-year DSS comparing to normal and low BMI patients. However, in the multivariate analysis, the status of BMI failed to be an independent prognostic factor in the whole patient cohort.
To explain the reason of better survival in patients with high BMI in esophageal carcinoma, some author suspected that weight loss would cause patients to be classified in a lower-weight category (3). The impact of body weight change in modulating the association between BMI and mortality may be more pronounced in esophageal carcinoma, since weight loss is common in patients with esophageal carcinoma due to dysphagia. In our study, 46.5% of the patients developed weight loss in the course of disease. Accordingly, the true prognostic effect of BMI on patients with esophageal carcinoma may be better understood only after stratifying by body weight change status. We found that the prognostic impact of high BMI significantly differed on the status of body weight change. There was no significant difference among patients with different BMI status when they had no weight loss. While for patients who lost weight by 5% in the last 3 months, overweight patients had a better DSS compared with patients with normal weight or underweight. After adjusting for co-varieties enrolled for study, high BMI was an independent prognostic factor for patients with weight loss esophageal squamous carcinoma. In a small study including 93 esophagogastric carcinoma patients, Skipworth et al. separated the patients into four groups, no weight loss with BMI >25 kg/m2, no weight loss with BMI <25 kg/m2, weight loss with BMI >25 kg/m2, and weight loss with BMI <25 kg/m2 (26). They found that the difference in survival of up to 3 years did not reach statistical significance among the four groups (26). However, the sample size might be too small to adequately assess the difference.
To better understand the prognostic value of high BMI in the esophageal squamous carcinoma patients, we compared the clinic pathologic characteristics and co-morbidities among these three BMI statuses. As expected, patients in the overweight groups had a higher incidence rate of high blood pressure (P<0.001), diabetes (P<0.001) and coronary artery diseases (P<0.001). The high incidence rate of co-morbidities in overweight patients did not finally lead to a worse outcome. It might be partially because that these co-morbidities could be well controlled by drugs. More patients in the overweight group received adjuvant chemotherapy and fewer patients in this group had anemia and weight loss. It seemed that overweight patients had a better nutrition status and could better tolerate the treatment. In a retrospective study including 301 esophageal cancers, Hayashi et al. found that 44.7% of patients with normal/low BMI had lost weight in the 3 months before surgery whereas only 22.2% of patients with high BMI lost weight (27). The author suspected that the weight loss might correlate with a high tumor burden (27). Moreover, in our data, high BMI certainly appeared to be associated with the diagnosis of early T stage and TNM stage. Hayashi et al. also found that high BMI was associated with a lower baseline stage of esophageal carcinoma (27). Lower stage at diagnosis, better nutrition status and better tolerance to the treatment might lead to an improved OS in the group with high BMI.
The strengths of our study included measurement of BMI values at a uniform time point at the time of diagnosis. Our study population was large and homogeneous with regard to histology subtype. Limitations of our study included its retrospective analysis setting and from a single-institution experience. Although it was a large study population, only 15 patients met the criteria of obese. Moreover, we did not collect data of post-operation complication which might be informative. BMI at diagnosis is already affected by diseases. Many patients would already have lost weight due to the disease prior to being diagnosed. BMI prior to cancer development would be a better measure to explore the relationship between BMI status and esophageal carcinoma.
In summary, this is a large population study including only esophageal squamous carcinoma in a single center in China. It turned out that overweight was independently associated with increased DSS in weight loss but not weight stable esophageal squamous carcinoma patients.
Acknowledgments
The study was conducted in the Medical Oncology department, Sun Yat-sen University, Cancer Center.
This work was supported by National Natural Science Foundation of China grant 30672408, Guangzhou Bureau of Science and Technology grant 2006Z3-E0041 and Sun Yat-sen University 985 Program Initiation Fund (China).
We gratefully thank the staff members in the Department of Medical Oncology and Thoracic Surgery Oncology at Sun Yat-sen University Cancer Center for their suggestion and assistance. We also thank Professor Liu Qing in the epidemiology department for his assist in the statistical analysis.
Disclosure: The authors declare no conflict of interest.
References
- 1.Zeng HM, Zheng RS, Zhang SW, et al. Analysis and prediction of esophageal cancer incidence trend in China. Zhonghua Yu Fang Yi Xue Za Zhi 2012;46:593-7. [PubMed] [Google Scholar]
- 2.Cao KJ, Fan QY, Liu YL, et al. Cancer incidence and mortality in Guangzhou City from 2000 to 2002. Ai Zheng 2008;27:225-30. [PubMed] [Google Scholar]
- 3.Melis M, Weber JM, McLoughlin JM, et al. An elevated body mass index does not reduce survival after esophagectomy for cancer. Ann Surg Oncol 2011;18:824-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoon HH, Lewis MA, Shi Q, et al. Prognostic impact of body mass index stratified by smoking status in patients with esophageal adenocarcinoma. J Clin Oncol 2011;29:4561-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trivers KF, De Roos AJ, Gammon MD, et al. Demographic and lifestyle predictors of survival in patients with esophageal or gastric cancers. Clin Gastroenterol Hepatol 2005;3:225-30. [DOI] [PubMed] [Google Scholar]
- 6.Hongo M, Nagasaki Y, Shoji T.Epidemiology of esophageal cancer: Orient to Occident. Effects of chronology, geography and ethnicity. J Gastroenterol Hepatol 2009;24:729-35. [DOI] [PubMed] [Google Scholar]
- 7.Corley DA, Buffler PA. Oesophageal and gastric cardia adenocarcinomas: analysis of regional variation using the Cancer Incidence in Five Continents database. Int J Epidemiol 2001;30:1415-25. [DOI] [PubMed] [Google Scholar]
- 8.Law S, Wong J.Changing disease burden and management issues for esophageal cancer in the Asia-Pacific region. J Gastroenterol Hepatol 2002;17:374-81. [DOI] [PubMed] [Google Scholar]
- 9.Veeramachaneni NK, Zoole JB, Decker PA, et al. Lymph node analysis in esophageal resection: American College of Surgeons Oncology Group Z0060 trial. Ann Thorac Surg 2008;86:418-21; discussion 421. [DOI] [PubMed] [Google Scholar]
- 10.Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008;371:569-78. [DOI] [PubMed] [Google Scholar]
- 11.Kondrup J, Rasmussen HH, Hamberg O, et al. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr 2003;22:321-36. [DOI] [PubMed] [Google Scholar]
- 12.Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003;349:2241-52. [DOI] [PubMed] [Google Scholar]
- 13.Vaughan TL, Davis S, Kristal A, et al. Obesity, alcohol, and tobacco as risk factors for cancers of the esophagus and gastric cardia: adenocarcinoma versus squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev 1995;4:85-92. [PubMed] [Google Scholar]
- 14.Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 2003;348:1625-38. [DOI] [PubMed] [Google Scholar]
- 15.Moon HG, Ju YT, Jeong CY, et al. Visceral obesity may affect oncologic outcome in patients with colorectal cancer. Ann Surg Oncol 2008;15:1918-22. [DOI] [PubMed] [Google Scholar]
- 16.Wu Y.Overweight and obesity in China. BMJ 2006;333:362-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McWilliams RR, Matsumoto ME, Burch PA, et al. Obesity adversely affects survival in pancreatic cancer patients. Cancer 2010;116:5054-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parr CL, Batty GD, Lam TH, et al. Body-mass index and cancer mortality in the Asia-Pacific Cohort Studies Collaboration: pooled analyses of 424,519 participants. Lancet Oncol 2010;11:741-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geyer SM, Morton LM, Habermann TM, et al. Smoking, alcohol use, obesity, and overall survival from non-Hodgkin lymphoma: a population-based study. Cancer 2010;116:2993-3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sinicrope FA, Foster NR, Sargent DJ, et al. Obesity is an independent prognostic variable in colon cancer survivors. Clin Cancer Res 2010;16:1884-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Becker S, Dossus L, Kaaks R.Obesity related hyperinsulinaemia and hyperglycaemia and cancer development. Arch Physiol Biochem 2009;115:86-96. [DOI] [PubMed] [Google Scholar]
- 22.Komninou D, Ayonote A, Richie JP, Jr, et al. Insulin resistance and its contribution to colon carcinogenesis. Exp Biol Med (Maywood) 2003;228:396-405. [DOI] [PubMed] [Google Scholar]
- 23.Fantuzzi G.Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol 2005;115:911-9; quiz 920. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe M, Ishimoto T, Baba Y, et al. Prognostic impact of body mass index in patients with squamous cell carcinoma of the esophagus. Ann Surg Oncol 2013;20:3984-91. [DOI] [PubMed] [Google Scholar]
- 25.Zhang SS, Yang H, Luo KJ, et al. The impact of body mass index on complication and survival in resected oesophageal cancer: a clinical-based cohort and meta-analysis. Br J Cancer 2013;109:2894-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skipworth J, Foster J, Raptis D, et al. The effect of preoperative weight loss and body mass index on postoperative outcome in patients with esophagogastric carcinoma. Dis Esophagus 2009;22:559-63. [DOI] [PubMed] [Google Scholar]
- 27.Hayashi Y, Correa AM, Hofstetter WL, et al. The influence of high body mass index on the prognosis of patients with esophageal cancer after surgery as primary therapy. Cancer 2010;116:5619-27. [DOI] [PubMed] [Google Scholar]


