Abstract
Objective
Although the role of regulatory B cells (Bregs) in rheumatic disease has gained increasing attention, two lesser-known Breg subsets that express either Foxp3 or transforming growth factor beta (TGFβ) are rarely examined in studies of rheumatic disease. This study investigates the association between the relative proportions of CD19+Foxp3+ and CD19+TGFβ+ Bregs, and clinical indicators of disease severity in rheumatoid arthritis (RA) patients with or without interstitial lung disease (ILD).
Methods
A total of 31 RA patients (14 with and 17 without ILD) and 26 healthy control subjects were included. All subjects did not have other autoimmune disease except RA, tumor, active infection, or a history of related drug administration. Peripheral blood mononuclear cells (PBMCs) were isolated and analyzed by flow cytometry (FCM). The relationship between the relative proportions of CD19+Foxp3+ and CD19+TGFβ+ Bregs and their associations to RA and ILD incidence, as well as disease severity assessed by common clinical indicators, were then examined.
Results
Our analyses revealed RA patients had significantly lower proportions of peripheral CD19+Foxp3+ and CD19+TGFβ+ Bregs as compared to healthy controls. While no association was observed between CD19+Foxp3+ Bregs and ILD incidence, patients with ILD had a substantially lower percentage of CD19+TGFβ+ Bregs compared to RA patients without ILD. In addition, CD19+Foxp3+ Bregs were negatively correlated with erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels in RA patients, whereas CD19+TGFβ+ Bregs were only correlated with CRP in RA patients with ILD. Furthermore, there was a negative association between CD19+Foxp3+ Bregs and disease severity scores, which was not found in analyses with CD19+TGFβ+ Bregs.
Conclusions
The proportions CD19+Foxp3+ and CD19+TGFβ+ Bregs were significantly decreased in RA patients, particularly in those with ILD complications, suggesting that Breg phenotypes may have different functions in the pathogenesis of RA and ILD.
Keywords: Regulatory B cells (Bregs), CD19+Foxp3+ Breg, CD19+TGFβ+ Breg, rheumatoid arthritis (RA), interstitial lung disease (ILD)
Introduction
Rheumatoid arthritis (RA) is one of the most prevalent autoimmune disorders, which is clinically manifested as arthralgia, swelling, limited mobility, and immune dysfunction. RA patients may also present with multisystem and multiorgan involvement and abnormities, such as interstitial lung disease (ILD).
Patients with RA may show numerous immunological abnormalities, including T and B cell dysfunctions that lead to the development of pathogenic autoantibodies. B lymphocytes play important roles in the immune response through their regulatory effects in antibody production. However, several studies have recently characterized a CDl9+CD5+CDld+ B cell sub-population with immunosuppressive effects, termed as regulatory B cells (Bregs) (1,2). The role of Bregs in the immune regulation of rheumatic diseases has received ever-growing attention. Bregs regulate the immune system mainly through producing interleukin 10 (IL-10), transforming growth factor beta 1 (TGFβ1) and other cytokines. It has been proved that IL-l0 plays a vital role in inhibiting inflammatory cytokines secretion, relieving joint swelling and thereby showing obvious immunosuppression in rheumatoid diseases. The considerable benefits of Bregs which produce IL-10 (B10 cells) for treatment of inflammatory and autoimmune diseases have been gradually confirmed. So far, two Breg subsets expressing either Foxp3 or TGFβ have been identified. Previously published studies suggest that the decrease or dysfunction of Bregs may induce rheumatic disorders, such as RA and anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (3,4). It has also been reported that Foxp3+ and TGFβ+ Breg phenotypes have a pivotal role in inducing immunological tolerance in food-induced anaphylaxis. Moreover, TGFβ+ Bregs could increase the CD4+Foxp3+ Treg population in lung tissue, leading to impaired airway eosinophil activity in airway allergic diseases (5,6). Yet, the participation of Foxp3+ and TGFβ+ Breg phenotypes in the pathogenesis of autoimmune diseases, especially in RA and RA related systemic damages is rarely examined. This study aimed to assess the changes in peripheral CD19+Foxp3+ and CD19+TGFβ+ Bregs sub-populations in RA patients with or without ILD. We hypothesized that the relationship between the Breg sub-populations with the development of RA and ILD could provide new thoughts for the treatment of RA patients, particularly those with pulmonary complications.
Methods
Study population
A total of 31 RA patients were included in this study. All RA patients (n=31; 19 female and 12 male with an average age of 45±11.7 years old) were hospitalized in Shengjing Hospital Affiliated to China Medical University between May 2013 and June 2014. Fourteen of the RA subjects (45%) were identified to have ILD as a pulmonary complication. The diagnosis of RA was based on the classification standards proposed by American College of Rheumatology (ACR) (7). In addition, patients in the ILD sub-group met the criteria for ILD as a complication of RA by ACR- European League against Rheumatism (EULAR) in 2013 (8). All patients received initial treatments without hormone-based medications, immunosuppressive agents, and non-steroidal drugs in the first four weeks prior to the study, and had no previous signs of other immunologic diseases, infections or tumors. A contemporary cohort of healthy volunteers (n=26; 15 female and 11 male with an average age of 42±12.17 years) from the Center of Health Examination in Shengjing Hospital Affiliated to China Medical University, were recruited as controls. The two groups did not show statistical differences in age and gender.
This study was approved by the Ethics Committee, Shengjing Hospital Affiliated to China Medical University. All subjects gave written informed consent before they participated in the study.
Subject grouping
Grouping according to presence of ILD
The RA patients were grouped into those with or without ILD. In addition to ACR-EULAR standards, the ILD in these patients also fulfilled the diagnostic criteria recommended by the American Thoracic Society/European Respiratory Society (ATS/ERS) in 2002 (9). The known causes of ILD are drugs, connective tissue disease, infection, and of an idiopathic nature. Specifically, this subgroup of RA patients presented with a variety of respiratory symptoms such as cough, shortness of breath, or exercise-induced dyspnea. Patients with repeated short and weak breath sounds revealed by physical examination, and accompanied by declined pulmonary function and diffusion capacity could be classified as severe ILD. Chest high-resolution computed tomography (HRCT) showed bilateral diffuse ground glass opacity (GGO) or pulmonary reticular opacity.
RA grouping based on disease severity
The disease activity score 28 (DAS28) was used as an indicator to evaluate disease severity in patients with RA. According to the standard (<2.6: remission, <3.2: active disease, DAS28 >5.1: highly-active disease), patients were divided into groups based on DAS28 score (Group 1: 3.2-5.1, and Group 2: ≥5.1).
Sample collection and detection
Blood samples
Peripheral venous blood (5 mL) was collected in all subjects using heparin anticoagulant tubes in the morning before breakfast. Then, the blood samples were processed within 3 hours after the collection.
Extraction of peripheral blood mononuclear cells (PBMCs)
Whole blood and phosphate-buffered saline (PBS) was mixed completely by a ratio of 1:1 (5 mL blood: 5 mL PBS) in centrifuge tubes and 5 mL Ficoll was then added slowly along the tube wall. The mixture was centrifuged at 2,200 g for 22 minutes by density fractionation. After centrifugation, the contents were divided into 5 layers as follows from top to bottom: plasma, PBMCs, separated Ficoll, granular cells, and red blood cells. Mononuclear cells were then collected into glass tubes with disposable straws, resuspended in 6 mL PBS, and centrifuged at 3,000 rpm for 5 minutes. And the cells were washed 3 times following the same procedure. After discarding the supernatant, the cell pellet was resuspended in PBS before being counted under a microscope. All cell densities were adjusted to 1×106 cells/100 μL.
Flow cytometry (FCM)
PBMCs (106 cells in 100 μL PBS) were immunostained at 4 °C in the dark with 5 μL anti-CD19-APC or anti-IgG-APC isotype control (eBioscience, CA, USA). Cells were then resuspended in 1 mL PBS and centrifuged at 1,500 rpm for 5 minutes with a total of 3 washes. For intracellular staining, cells were incubated in 1 mL Fixation/Permeabilization Buffer (concentrated solution with PBS diluted 1:3, eBioscience, CA, USA) at 4 °C in the dark for 30 minutes. Then, 2 mL Permeabilization Wash Buffer (diluted 1:9 in water, eBioscience, CA, USA) was added and the mixtures were centrifuged at 1,500 rpm for 5 minutes. After discarding the supernatant, the cells were resuspended in 100 μL PBS, stained with 5 μL of each anti-Foxp3-FITC, anti-TGFβ-PE, or IgG isotype control, and incubated at 4 °C for 30 minutes in the dark. After staining, the cells were washed twice in 2 mL Permeabilization Wash Buffer and centrifuged at 1,500 rpm for 5 minutes. All the samples were suspended in a total volume of 500 μL PBS prior to detection in a FACS Calibur flow cytometer (BD Biosciences, CA, USA). The data was then analyzed by CellQuest Pro software (BD Biosciences, CA, USA).
Clinical laboratory tests
All patients received routine blood tests, including those for erythrocyte sedimentation rate (ESR), IgG, IgM, IgA, rheumatoid factor (RF), C-reactive protein (CRP), cyclic citrullinated peptide (CCP) antibody, and anti-nuclear antibody (ANA), in the clinical laboratory of Shengjing Hospital. Patients also underwent chest HRCT examinations. If pulmonary interstitial abnormalities presented, lung function tests were performed to confirm the ILD diagnosis.
Statistical analysis
Data was analyzed by SSPS 16.0 (IBM Software, NY, USA). All values were represented by the means ± standard deviation. Differences and data correlation between the groups were determined by two-sided Student’s t-tests and Pearson correlation analysis, respectively. P<0.05 was considered statistical significance.
Results
Relative proportions of CD19+Foxp3+ and CD19+TGFβ+ Bregs in RA patients versus healthy controls
The clinical data of RA patients and healthy controls are provided in Table 1. Interestingly, the percentage of CD19+Foxp3+ Bregs in the peripheral blood of RA patients was significantly reduced compared to that of the healthy control group (3.79%±1.66% vs. 5.70%±2.42% in the CD19+ population, respectively; P=0.01). This trend was also observed found in CD19+TGFβ+ Bregs, which were present at 37.94%±11.29% in RA patients compared to 47.56%±10.07% in healthy controls, respectively (P=0.01; Figures 1,2,3).
Table 1. Basic data of RA patients and healthy control groups.
| Characteristics | RA patients (n=31) | Healthy controls (n=26) |
|---|---|---|
| Age (years) | 45±11.7 | 42±12.2 |
| Sex | ||
| Female | 19 | 15 |
| Male | 12 | 11 |
| Disease duration (years) | 5.0±3.9 | − |
| Patients with ILD | 14 | − |
| Disease severity (DAS28) | − | |
| 3.2-5.1 | 23 | |
| ≥5.1 | 8 |
RA, rheumatoid arthritis; ILD, interstitial lung disease; DAS28, disease activity score. 28.
Figure 1.
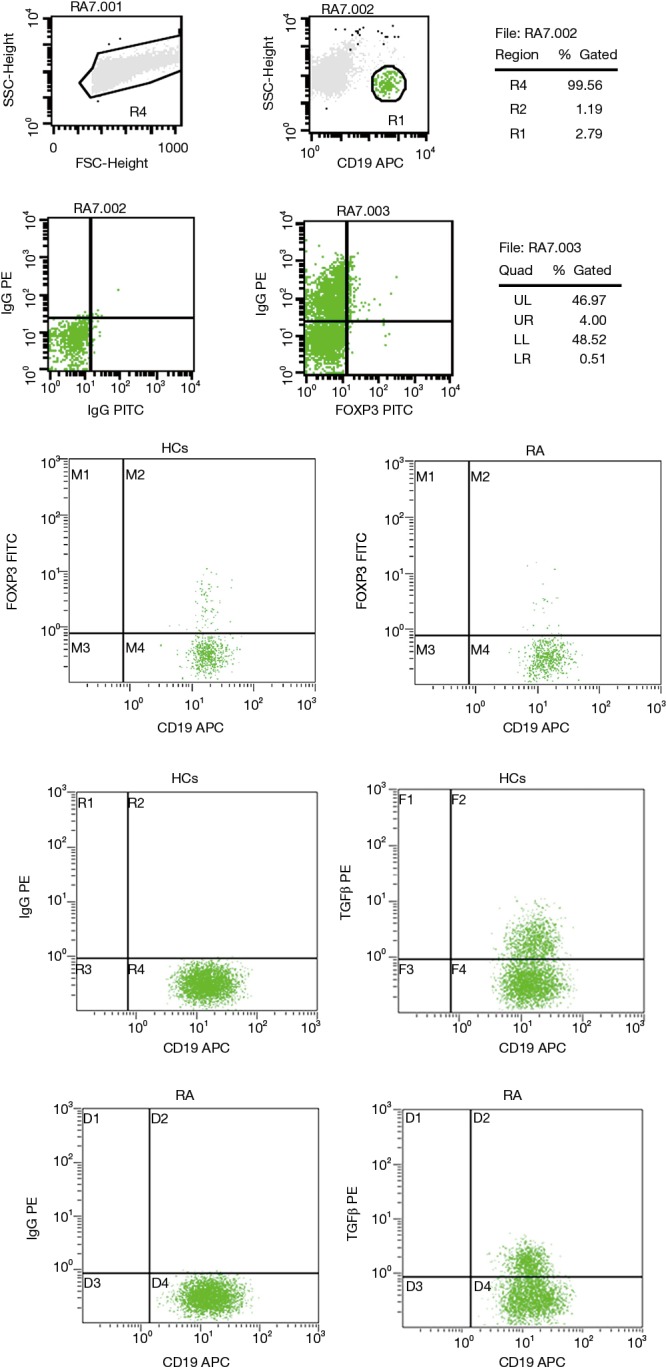
Relative proportions of CD19+Foxp3+ and CD19+TGFβ+ Bregs in RA patients. RA, rheumatoid arthritis; TGFβ, transforming growth factor beta; Bregs, regulatory B cells.
Figure 2.
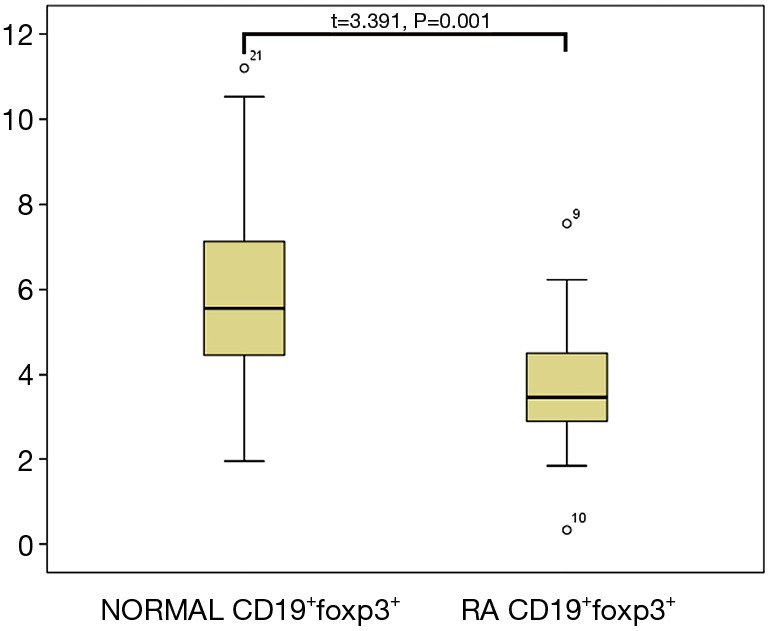
Comparison of the peripheral CD19+Foxp3+ Breg population between RA patients and healthy controls. RA, rheumatoid arthritis; Bregs, regulatory B cells.
Figure 3.
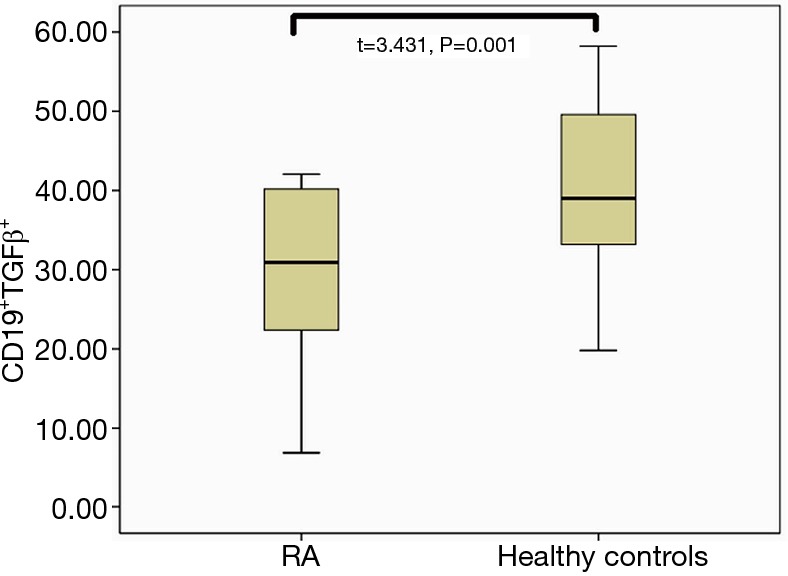
Comparison of the peripheral CD19+TGFβ+ Breg population between RA and control groups. TGFβ, transforming growth factor beta; Breg, regulatory B cell; RA, rheumatoid arthritis.
RA patients with ILD had a smaller CD19+Foxp3+ Breg population which was further suppressed in those with ILD complications
The associations between the CD19+Foxp3+ and CD19+TGFβ+ Breg populations in RA patients with or without ILD were examined (Figures 1,2,3). Notably, the relative population of CD19+Foxp3+ Bregs was decreased significantly in both RA groups, and a greater reduction was observed in those with ILD. Furthermore, no statistical difference was found in the proportion of CD19+TGFβ+ Bregs in RA patients without pulmonary complications compared with those in healthy controls. However, patients with ILD displayed significantly lower proportion CD19+TGFβ+ Bregs compared with the control group (Table 2).
Table 2. Changes in peripheral CD19+Foxp3+ and CD19+TGFβ+ Bregs in RA patients with pulmonary interstitial disease.
| Characteristics | RA (n=31) ILD | Healthy controls (n=26) | Statistics | |
|---|---|---|---|---|
| CD19+Foxp3+ (%) | With | 3.52±1.21 | 5.70±2.42 | t=3.160, P=0.003 |
| Without | 4.09±1.98 | t=2.393, P=0.022 | ||
| CD19+TGFβ+ (%) | With | 32.85±8.46 | 47.56±10.07 | t=4.899, P<0.0001 |
| Without | 41.86±12.18 | t=1.603, P=0.119 | ||
RA, rheumatoid arthritis; ILD, interstitial lung disease; TGFβ, transforming growth factor beta; Bregs, B cells.
CD19+Foxp3+ Bregs were negatively correlated with certain predictors of RA severity
The FCM data was compared with values obtained from clinical blood tests by Pearson’s correlation analysis in order to determine if the suppression found in either Breg population was associated with the indicators of disease severity. The percentage of CD19+Foxp3+ Bregs was negatively correlated with certain disease activity indicators, such as ESR and CRP, particularly in RA patients with ILD (Figure 4). However, the negative correlation was not observed with RF and anti-CCP antibody. In addition, a profound negative correlation was observed between the percentage of CD19+TGFβ+ Bregs and CRP levels in RA patients with ILD (r=0.619, P=0.031).
Figure 4.
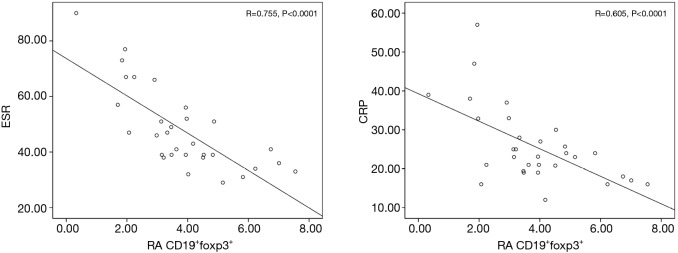
Correlation analysis of CD19+Foxp3+ Bregs with ESR and CRP. ESR, erythrocyte sedimentation rate; Bregs, regulatory B cells; CRP, C-reactive protein.
CD19+Foxp3+ Bregs were negatively correlated with DAS28 scores in RA
Although no statistical differences were found between the CD19+TGFβ+ Breg population and DAS28 scores (t=0.28, P=0.13), the relative proportion of CD19+Foxp3+ Bregs had a significant negative correlation with DAS28 scores in the RA patient group (r=-0.446, P=0.012).
Discussion
The mechanistic role of Bregs in autoimmune disease remained unclear. Madan et al. confirmed that the immunomodulation imparted by Bregs was induced by IL-10 (10). Also, a previous report concluded that B cell-derived IL-10 could control various immune processes by inhibiting IL-12 secretion, regulating the function of T cell (Treg) subsets, and producing immunosuppressive cytokines such as TGFβ (11). Furthermore, the negative regulatory role of Breg cells had been conclusively found in mouse models with a range of inflammatory and immune diseases (12).
RA is a systemic autoimmune disease characterized by chronic polyarticular synovitis, bone and cartilage destruction, and variable progression. The specific pathogenesis of RA is still not clear. ILD is one of the most common clinical complications associated with RA, and usually leads to poor prognosis. Recent studies have found that regulatory Tregs, which have inhibitory functions in immunoregulation, are involved in the onset of both RA and the associated pulmonary complications. The functional participation of Bregs in RA pathogenesis is a central issue in rheumatology research, yet few studies on the relative changes within the peripheral Breg populations in RA patients with ILD currently exist.
A report on the association between ILD and changes in the regulatory Treg compartment has already shown that RA patients with ILD display insufficient Treg counts and have a decreased ability to inhibit pulmonary fibrosis; thus, the effect and function of Tregs have become an important topic in the pathogenesis of pulmonary fibrosis (13,14). Meanwhile, studies in the collagen-induced arthritis (CIA) mouse model reveal that IL-10-secreting Bregs can limit the progression of arthritis (15,16). Then, the progression of rheumatism can be potentially controlled by regulating the immunosuppressive functions of Bregs in modulating inflammation.
The exceedingly small Breg population in human peripheral blood is hard to be detected, and there are few reports on characterizing Bregs in human RA. Recently, relevant research on RA demonstrates that the presence of Bregs (CD19+CD5+CD1d+IL-10+) in the peripheral blood of new-onset RA patients is diminished compared to controls, and is negatively correlated with disease severity (17).
In this study, we performed a correlative analysis to investigate the association between relative proportions of CD19+Foxp3+ and CD19+TGFβ+ Bregs and various clinical factors attributed to disease severity in active RA patients with or without ILD. Our results demonstrate a significant decrease in CD19+Foxp3+ Bregs in RA patients with or without ILD as compared to healthy controls, as well as a reduction of CD19+TGFβ+ Bregs in RA patients with ILD; however, no statistically significant difference was found between RA patients without ILD and healthy controls. The data suggests that CD19+TGFβ+ Bregs play an important role not only in the pathogenesis of RA, but also in the other types of rheumatic diseases. Notably, the decrease in Bregs limits the overall secretion of important anti-inflammatory factors, such as IL-10 and TGFβ, which can weaken the protective immune function. Concurrently, the decrease of Bregs may be responsible for Foxp3+ expression, which likely lead to diminished Treg function, and may play an important role in the onset of disease. Previous studies suggest that Bregs can promote the proliferation of CD4+CD25+Foxp3+ Tregs, and therefore inhibit autoimmune responses with a mutually reinforcing correlation (18). It has been reported that RA patients with pulmonary fibrosis exhibit a reduction in peripheral Tregs, and a positive correlation exists between the Treg and Bregs populations (19). Our study demonstrates a significant reduction in both CD19+Foxp3+ and CD19+TGFβ+ Bregs in RA patients with ILD, indicating a substantial reduction in all Breg subpopulations, not just in those responsible for IL-10 secretion. This decrease may affect the Treg population, and thus cause a series of subsequent reactions that underlie the decreased inhibition of abnormal immune responses.
Bregs play a critical anti-inflammatory role by secreting TGFβ. B lymphocytes activated by lipopolysaccharide (LPS) could secret TGFβ and, in turn, express Fas ligand, that could subsequently promote the apoptosis of Thl-type T lymphocytes and prevent immune-mediated tissue destruction (20). Therefore, it is clear that TGFβ secretion is also one mechanism by which Bregs elicit immunoregulation in RA patients. Furthermore, pulmonary interstitial involvement is one of the most common pathological injuries in RA. Besides, pulmonary fibrosis is often a primary factor in the poor prognosis of RA patients. Our study suggested that the decrease in CD19+Foxp3+ and CD19+TGFβ+ Bregs observed in RA patients tends to prevent the body from protecting damaged tissues and accelerating RA-associated immunopathology, including the development of ILD.
Finally, our correlative analysis of CD19+Foxp3+ and CD19+TGFβ+ Bregs and indicators of RA severity demonstrates an inverse relationship between CD19+Foxp3+ Bregs and ESR/CRP levels, whereas the relative proportion of CD19+TGFβ+ Bregs is only correlated with CRP in RA patients with ILD. The finding indicated that changes within the CD19+TGFβ+ Breg population may play an even more important role in pulmonary damage and may be a significant indicator in the monitoring of potential disease complications. In addition, we also found a negative correction between CD19+Foxp3+ Bregs and DAS scores in RA patients, suggesting that CD19+Foxp3+ Bregs may be important in the onset and severity of RA; however, no correction between CD19+TGFβ+ Bregs and DAS28 scores was noted, which may further indicate that the effect of different Bregs subpopulation varies with diseases and pathological processes.
In conclusion, negative regulatory cells—such as Bregs—play important roles not only in maintaining normal immune homeostasis, but also in the regulation of tissue damage in autoimmunity diseases. Although the percentage of Bregs is low within the human B cell compartment, their immunoregulatory effects worth great attention. The discovery of Bregs may pioneer a new field to research the regulatory mechanisms that govern the immune response. The induction of Breg activation might become an effective method to treat related diseases. And the phenotypic markers discovered in Breg research can be used to predict different therapeutic responses and drug resistance in the treatment of RA.
Acknowledgements
Authors’ contributions: Yingying Guo and Xiaomei Zhang developed the study design and data acquisition. Muting Qin and Xiaofei Wang carried out most of the analyses. Xiaofei Wang conceived and coordinated the study. Yingying Guo, Xiaomei Zhang, Muting Qin and Xiaofei Wang drafted and revised the manuscript. All authors have read and approved the final manuscript.
Funding: This work was supported by The Science Project of Liaoning Province [2014225017] and Shenyang Science Project [F12-277-1-85].
Disclosure: The authors declare no conflict of interest.
References
- 1.Katz SI, Parker D, Turk JL. B-cell suppression of delayed hypersensitivity reactions. Nature 1974;251:550-1. [DOI] [PubMed] [Google Scholar]
- 2.Wolf SD, Dittel BN, Hardardottir F, et al. Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice. J Exp Med 1996;184:2271-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma L, Liu B, Jiang Z, et al. Reduced numbers of regulatory B cells are negatively correlated with disease activity in patients with new-onset rheumatoid arthritis. Clin Rheumatol 2014;33:187-95. [DOI] [PubMed] [Google Scholar]
- 4.Wilde B, Thewissen M, Damoiseaux J, et al. Regulatory B cells in ANCA-associated vasculitis. Ann Rheum Dis 2013;72:1416-9. [DOI] [PubMed] [Google Scholar]
- 5.Noh J, Noh G, Kim HS, et al. Allergen-specific responses of CD19(+)CD5(+)Foxp3(+) regulatory B cells (Bregs) and CD4(+)Foxp3(+) regulatory T cell (Tregs) in immune tolerance of cow milk allergy of late eczematous reactions. Cell Immunol 2012;274:109-14. [DOI] [PubMed] [Google Scholar]
- 6.Natarajan P, Singh A, McNamara JT, et al. Regulatory B cells from hilar lymph nodes of tolerant mice in a murine model of allergic airway disease are CD5+, express TGF-β, and co-localize with CD4+Foxp3+ T cells. Mucosal Immunol 2012;5:691-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569-81. [DOI] [PubMed] [Google Scholar]
- 8.van den Hoogen F, Khanna D, Fransen J, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum 2013;65:2737-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Thoracic Society. European Respiratory Society . American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med 2002;165:277-304. [DOI] [PubMed] [Google Scholar]
- 10.Madan R, Demircik F, Surianarayanan S, et al. Nonredundant roles for B cell-derived IL-10 in immune counter-regulation. J Immunol 2009;183:2312-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cantaert T, Doorenspleet ME, Francosalinas G, et al. Increased numbers of CD5+ B lymphocytes with a regulatory phenotype in spondylarthritis. Arthritis Rheum 2012;64:1859-68. [DOI] [PubMed] [Google Scholar]
- 12.Rieger A, Bar-Or A.B-cell-derived interleukin-10 in autoimmune disease: regulating the regulators. Nat Rev Immunol 2008;8:486-7. [DOI] [PubMed] [Google Scholar]
- 13.Kotsianidis I, Nakou E, Bouchliou I, et al. Global impairment of CD4+CD25+FOXP3+ regulatory T cells in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2009;179:1121-30. [DOI] [PubMed] [Google Scholar]
- 14.Wan Y, Zhang N, Li JP, et al. The imbalance between regulatory T cells and Th17 cells and its effect on pulmonary fibrosis in rats. Acta Medicinae Universitatis Scientiae et Technologiae Huazhong 2013;02
- 15.Evans JG, Chavez-Rueda KA, Eddaoudi A, et al. Novel suppressive function of transitional 2 B cells in experimental arthritis. J Immunol 2007;178:7868-78. [DOI] [PubMed] [Google Scholar]
- 16.Kalampokis I, Yoshizaki A, Tedder TF. IL-10-producing regulatory B cells (B10 cells) in autoimmune disease. Arthritis Res Ther 2013;15:S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma L, Liu B, Jiang Z, et al. Reduced numbers of regulatory B cells are negatively correlated with disease activity in patients with new-onset rheumatoid arthritis. Clin Rheumatol 2014;33:187-95. [DOI] [PubMed] [Google Scholar]
- 18.Ray A, Basu S, Williams CB, et al. A novel IL-10-independent regulatory role for B cells in suppressing autoimmunity by maintenance of regulatory T cells via GITR ligand. J Immunol 2012;188:3188-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang WW, Wang Q, Xie XJ, et al. Marked elevation of CA19-9 in a patient with rheumatoid arthritis-associated pulmonary fibrosis. J Transl Intern Med 2013;1:40-2 [Google Scholar]
- 20.Tian J, Zekzer D, Hanssen L, et al. Lipopolysaccharide-activated B cells down-regulate Th1 immunity and prevent autoimmune diabetes in nonobese diabetic mice. J Immunol 2001;167:1081-9. [DOI] [PubMed] [Google Scholar]


